Abstract
Physical exercise is known to be a beneficial factor by increasing the cellular stress tolerance. In ischemic stroke, physical exercise is suggested to both limit the brain injury and facilitate behavioral recovery. In this study we investigated the effect of physical exercise on brain damage following global cerebral ischemia in mice. We aimed to study the effects of 4.5 weeks of forced treadmill running prior to ischemia on neuronal damage, neuroinflammation and its effect on general stress by measuring corticosterone in feces. We subjected C57bl/6 mice (n = 63) to either treadmill running or a sedentary program prior to induction of global ischemia. Anxious, depressive, and cognitive behaviors were analyzed. Stress levels were analyzed using a corticosterone ELISA. Inflammatory and neurological outcomes were analyzed using immunohistochemistry, multiplex electrochemoluminescence ELISA and Western blot. To our surprise, we found that forced treadmill running induced a stress response, with increased anxiety in the Open Field test and increased levels of corticosterone. In accordance, mice subjected to forced exercise prior to ischemia developed larger neuronal damage in the hippocampus and showed higher cytokine levels in the brain and blood compared to non-exercised mice. The extent of neuronal damage correlated with increased corticosterone levels. To compare forced treadmill with voluntary wheel running, we used a different set of mice that exercised freely on running wheels. These mice did not show any anxiety or increased corticosterone levels. Altogether, our results indicate that exercise pre-conditioning may not be beneficial if the animals are forced to run as it can induce a detrimental stress response.
Keywords: Forced exercise, Neuroinflammation, Microglia, Corticosterone, Stress, Cytokines
Highlights
-
•
Enforcement to run results in anxious behavior.
-
•
Mice that are forced to run have elevated levels of corticosterone.
-
•
Enforcement to run results in more neuronal death in hippocampus.
-
•
Corticosterone levels correlates with the neuronal damage in hippocampus.
-
•
Increased corticosterone and anxiety is not seen in mice that run voluntarily.
1. Introduction
Physical exercise is regarded as a promising treatment and complement to pharmacological treatments in several different neurological diseases (Svensson et al., 2014). Exercise can affect both the adrenergic and corticosteroid systems involved in stress response as well as the microglia function and other cells and signaling molecules involved in inflammatory processes (Svensson et al., 2014).
In the central nervous system (CNS), microglial cells are the major source of pro-inflammatory cytokines (Kim and de Vellis, 2005, Carson et al., 2006). In response to neuronal injury, such as stroke, resident microglia are immediately activated and start to proliferate and produce pro-inflammatory cytokines (Banati et al, 1993, Barone et al, 1997). It has been shown in both patients and animal models that cerebral ischemia leads to an inflammatory response, systemically as well as in the brain (Lambertsen et al., 2012). Neuroinflammatory response can further aggravate the neuronal damage and administration of the pro-inflammatory cytokine IL-1β after induction of cerebral ischemia in animal models can exacerbate the brain injury (Yamasaki et al., 1995). Microglial cells or their inflammatory mediator molecules may therefore be suitable targets for treating or preventing deleterious neuroinflammation following brain ischemia (Yenari et al., 2010). Several studies have shown that the levels of pro-inflammatory cytokines decrease, and the levels of anti-inflammatory cytokines increase following physical exercise (Mota et al, 2012, Piao et al, 2013). Indeed, treadmill running in animals has been shown to reduce the microgliosis and inhibit the release of pro-inflammatory cytokines, as well as reducing lesion size and cell death in the hippocampus and prevent short-term memory disturbances following cerebral ischemia (Austin et al, 2014, Lovatel et al, 2014, Sim et al, 2004, Sim et al, 2005).
Out-of-hospital cardiac arrest is a severe complication that can lead to global cerebral ischemia with pronounced neuronal damage. The survival rate is very low, below 10%, and patients that survive are often affected by severe neurological injury and life-long cognitive and motor disabilities (Sasson et al., 2010). In an experimental setting, global cerebral ischemia can be modeled in mice by transient occlusion of the common carotid arteries (Olsson et al., 2003). Global cerebral ischemia results in neuronal cell death in vulnerable brain regions such as hippocampus (Kirino and Sano, 1984, Back et al., 2004) evolving during the first week after the ischemic insult (Bottiger et al., 1998). By altering detrimental neuroinflammatory reactions, physical exercises could make the brain more resistant to ischemic injuries.
On the negative side, exercise can also induce a chronic stress response, which may result in detrimental effects in the event of a brain injury. For example, forced running exercise in rodents can lead to anxiety and increase the levels of the stress hormone corticosterone in serum (Leasure and Jones, 2008, Brown et al, 2007). It has been shown that stress can evoke a pro-inflammatory response in the brain, with increased expression of NLRP3 inflammasome involved in the cleavage and secretion of pro-inflammatory IL-1β (Gadek-Michalska et al, 2013, Frank et al, 2014). Therefore, the overall positive effect of physical exercise in experimental models could potentially be masked by the stress response.
To the best of our knowledge, the effects of treadmill exercise pre-conditioning on stress and neuroinflammatory responses following global cerebral ischemia have not previously been studied in mice. Therefore, the aim of this study was to investigate the effect of pre-conditioning forced treadmill running on stress response, neuroinflammation, neuronal damage and behavioral alterations following global cerebral ischemia. An additional aim was to compare the stress response after forced running with the response after voluntary running.
2. Material and methods
2.1. Animals
All proceedings and animal treatment were in accordance with the guidelines and requirements of the government committee on animal experimentation at Lund University. We used 63 male C57Bl/6 mice, aged 8–10 weeks, weighing 22–27 g that were obtained from Charles River. The mice were housed in standard laboratory cages (3–7 animals per cage), with sawdust bedding and free access to water and food. They were allowed to acclimatize for at least 5 days before testing. The holding room had a 12:12 h light-dark cycle. The mice were weighed at the day of exercise introduction, and thereafter once every second week until the induction of global cerebral ischemia. Thereafter the mice were weighed 3–4 days as well as 10–12 days after ischemia. When the experiment was initiated, the mice were assigned to different groups ensuring an even distribution in body weight and age.
2.2. Experimental outline
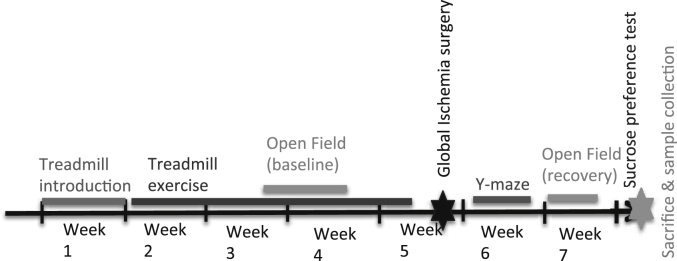
An overview of the experimental outline can be seen in Fig. 1. Briefly, some of the animals were subjected to treadmill running exercise for 4.5 weeks. During the third week of exercise their anxious and motor behavior was assessed once with an Open Field test. After 4.5 weeks of exercise, some of the animals were subjected to ischemia. Behavioral tests were then conducted 5–15 days after ischemia, after which, the animals were sacrificed and samples were collected (15–16 days after ischemia).
Fig. 1.
Experimental Design. Mice in the exercise groups were introduced to treadmill running for a week and thereafter subjected to 3.5 weeks of exercise. During this time an Open Field test was conducted. After 3.5 weeks of exercise some of the exercised and non-exercised mice were subjected to global brain ischemia. The mice were allowed to recover for 3–4 days before Y-maze test was conducted. Then, a new Open Field test was performed followed by a sucrose preference test the night before the mice were sacrificed.
2.3. Running exercise
2.3.1. Treadmill running exercise
Originally, the mice were divided into two different treatment groups, one subjected to exercise and one that was not subjected to any exercise (sedentary). Exercise consisted of 30 min treadmill running at a speed of 25 cm/s with no inclination of the treadmill (5-lane treadmill, Harvard Apparatus, Panlab). The mice were exercised 3 days/week for 4.5 weeks of which the first week was an introduction week with a lower speed and duration of the exercise. On the first day of the introduction, the mice were subjected to 10 min of walking/running at a speed increasing from 5 to 18 cm/s. The second day of the introduction consisted of 20 min of running with speeds up to 25 cm/s. During the third and last day of the introduction, the duration was increased to 30 min, with a speed of 25 cm/s. To motivate the mice to run the researcher pushed them with a small stick if needed. If the mouse refused to run it could be motivated by a transient and light electric stimulation from the grid at the beginning of the treadmill platform. After this, if the mouse persisted in its refusal to run, it was removed from the treadmill and re-introduced to it at a later time point. In this study, all mice without exceptions had to be motivated at least some times by pushing them with the small stick at each day of exercise. Four of our best running mice even had to be motivated with a light electric stimulation at up to three different days. Despite this, several mice refused to run after repeated trials. These mice were excluded from the exercise procedure, and referred to as “bad runners”. Exercise took place in a room separated from the housing room to which all mice were transferred and kept during the exercise procedures in order to minimize environmental confounders among the mice not subjected to the exercise protocol.
2.3.2. Voluntary wheel running
To investigate the effect on stress response resulting from the enforcement to run compared to voluntary running exercise, our main study was complemented with mice subjected to voluntary wheel running. For this, 10 male C57Bl/6 mice at an age of 12 month were used. These mice had the same housing conditions as the mice in the main study, except that they were single caged. Six of these mice had access to low-profile wireless running wheel for mouse (med associates) in their home cages during 6.5 weeks. The running wheels had a wireless connection to a software measuring the distance each mouse ran every day, assuring that all mice with access to running wheels actually run. The remaining four mice were sedentary controls with no access to running wheels in their homecage. After 6.5 weeks of this running intervention, an Open Field test was performed (as described in 2.5.1) and feces samples were collected (as described in 2.6) in order to compare anxiety and corticosterone levels with the sedentary control group.
2.4. Induction of global cerebral ischemia
In order to model cardiac arrest patients with successful cardiopulmonary resuscitation, global ischemia was induced to the mice as previously described (Olsson et al., 2003, Burguillos et al, 2015, Lambertsen et al, 2011, Deierborg et al, 2008). Briefly, the mice were first anesthetized with 5% isofluorane in oxygen. Thereafter, the anesthesia was maintained using 2% isofluorane. A small cut parallel to the trachea was made. The common carotid arteries were then isolated and encircled with a thin silk thread to allow bilateral occlusion using a micro aneurysm clip. Ischemia was induced for 13 min. The wound was then sealed with absorbable stitches before the anesthesia was discontinued. During the surgery, the body temperature was monitored and controlled using a heating pad and an infrared lamp to keep the mice normothermic. The body temperature was maintained around 37.5 °C during the whole procedure. Mice with body temperatures below 35.5 °C a few hours after the intervention were put in an incubator at 34 °C overnight in order to recover. Mice in the sham groups were subjected to the same surgical protocol, except that no occlusion to the common carotid arteries was made. The person performing the surgery was blinded to the animals exercise regimen.
2.5. Behavioral tests
2.5.1. Open Field test
In order to evaluate the locomotion and anxiety levels of the mice subjected to forced treadmill exercise, an Open Field test was conducted before and after induction of ischemia. The test before the induction of ischemia was performed during the third week of exercise. The test was then repeated 12–13 days after the induction of ischemia. The mice were put in an Open Filed arena (Panlab, Barcelona, Spain, 45 cm by 45 cm) and allowed to freely explore it for 10 min. An automated SMART system (Panlab, Barcelona, Spain) was used to measure the velocity of movements, distance moved and time spent in the center and periphery of the box. Spending more time in the periphery was regarded as a sign of anxiety. The box was cleaned first with ethanol and then with water before each mouse was introduced to the Open Field arena. Furthermore, anxious behavior in the Open Field test of mice subjected to voluntary wheel running with age-matched sedentary controls were also analyzed to test if voluntary running results in anxious behavior.
2.5.2. Y-maze spatial memory test
In order to examine any defects in hippocampus-dependent spatial memory, a Y-maze test was performed 5–7 days after induction of ischemia. This test did not show any significant differences between groups. The setup of the test is further described in Supplementary material 1.
2.5.3. Sucrose preference test
Anhedonic behavior of the mice was assessed using a sucrose preference test during the night between day14–15 or 15–16. This test did not show any significant differences between groups. The setup of the test is further described in Supplementary material 1.
2.6. Fecal corticosterone levels
Fecal samples were collected from the Open Field box after conducting the Open Field test performed 12–13 days following induction of ischemia in order to measure the stress levels of the mice. Since anxiety in itself can increase the frequency of defecation, fecal samples from mice that did not defecated during the Open Field test were collected by putting those mice in individual cages until they had defecated. This was done to prevent potential bias that could arise if only the corticosterone levels from those mice that indeed defecated during the test would have been analyzed. Corticosterone levels measured in feces is likely to reflect the level of corticosterone that was found in serum several hours earlier (Kalliokoski et al., 2010). To compare the effect of forced running on the corticosterone levels without ischemic insult as a confounding factor, mice only subjected to sham surgery were used for this test. The feces were stored at −80 °C until analysis. Corticosterone was then extracted and analyzed using a corticosterone ELISA kit (Enzo Life Sciences) as described by Touma et al. (Touma et al., 2003), except that feces was homogenized in 1 ml of 80% Methanol per 100 mg sample. Feces samples from mice subjected to voluntary wheel running with age-matched sedentary controls were also analyzed to investigate if running exercise per see results in corticosterone increase.
2.7. Euthanization and sample collection
The mice were first anesthetized with isofluorane 15–16 days after ischemia. Thereafter, the mice were euthanized and blood samples were collected through cardiac puncture. Blood samples were kept in room temperature for 25 min and then stored on ice a few hours until the samples were centrifuged at 1300 g in 4 °C for 10 min and the serum supernatants were collected and stored at −80 °C until analysis. The brains were dissected out and snap frozen in crushed dry ice and stored at −80 °C until analysis.
2.8. Immunohistochemistry
The brains where sectioned with a Leica CM3050 S Cryostat (Leica Microsystems Nussloch GmbH, Germany). The hippocampus was extracted from −1.20 to −2.20 (according to K. Franklin, G. Paxinos, ‘The Mouse Brain: in Stereotaxic Coordinates’, Academic Press, USA). The brain sections were 30 μm thick and collected into 6 series, with 3 series mounted onto microscope slides and 3 series stored in tubes for preparations of brain homogenates. All sliced samples were stored at −80 °C until used. Sections used for immunohistochemical stainings were fixated with 4% of PFA for 10 min prior to staining procedure.
Viable neurons were stained on hippocampal sections using Mouse-anti-NeuN antibody (1:200, Anti-NeuN, MAB377, ©EMD Millipore Corporation, Billerica, MA, USA). 4′,6-diamidino-2-phenylindole (DAPI) (1:1000, Sigma Aldrich) was used to stain cell nuclei. The number of NeuN positive cells with viable cell morphology was counted in the hippocampal subregions CA1, CA2 and CA3 of each hemisphere from two coronal subsequential sections (bregma −1.2 and 1.4) from each mouse using an epifluorescence microscope (Nikon Eclipse 80i microscope, Europe).
Inflammation and microglial activation was visualized using polyclonal rabbit-anti-Iba1 (1:500, Wako, Japan) and monoclonal rat-anti-galectin-3 (M3/38, 1:300 made in-house, provided by Professor Hakon Leffler) antibodies. To evaluate the overall number and activity of microglia in the hippocampus pictures were captured of the 3 subsequential sections of hippocampus (bregma-1.2 mm, bregma-1.4 mm and bregma-1.6 mm) from each mouse, using the epifluorescence microscope. The extent and intensity of the Iba1 staining was evaluated using ImageJ. The number of galectin-3 positive cells in the hippocampus was counted in the same sections using the epifluorescence microscope.
2.9. Cytokine levels in brain tissue
2.9.1. Brain homogenization protocol
Eight brain slices at the level of the hippocampus from each mouse were homogenized in 100 μl complete lysis buffer (Mesoscale Discovery, Rockville, USA) containing protease inhibitor. The tissue was mechanically dissociated using a syringe plunger. The homogenates were then incubated 20 min on ice and tip-sonicated with 5 pulses for 2–3 s. Samples were thereafter centrifuged at 10,000 g in 4 °C for 20 min before the supernatants were collected. Protein concentrations were determined using the Bradford method and samples were stored at −80 °C until use.
2.9.2. Multiplex cytokine ELISA
The concentrations of different cytokines in the collected serum and homogenized brain samples (25 μl/sample) were measured in the MSD Mouse Proinflammatory V-Plex Plus Kit (IFNγ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, CXCL1, TNFα; K15012C, Mesoscale) using a SECTOR Imager 6000 (Mesoscale Discovery, Rockville, USA) Plate Reader according to the manufacturer's instructions. The recorded data was analyzed using MSD Discovery Workbench software. For the brain homogenate samples the concentrations were normalized to the different protein concentrations measured in the Bradford assay of each homogenized sample.
2.10. Western blot
Eight brain slices at the level of hippocampus from each mouse were homogenized in 160 μl of RIPA buffer (R0278, Sigma-Aldrich) containing protease inhibitor (1% protease inhibitor cocktail, P8340, Sigma-Aldrich) Samples were sonicated twice for 3 s before they were centrifuged at 8000 g for 10 min and the supernatants were collected. Briefly, proteins were loaded on 4–20% Mini-Protean TGX Precast Gels (Bio-Rad) then transferred to Nitrocellulose membranes (Bio-Rad) using Trans-Blot Turbo System (Bio-Rad). The membranes were then blocked with 10% Casein (Sigma-Aldrich) diluted in PBS (tablets, Sigma-Aldrich). After blocking, the membranes were incubated with primary antibodies against NLRP3 (mAb, Adipogen 1:4000) and galectin-3 (M3/38, made in-house, provided by Professor Hakon Leffler, 1:500) at 4 °C over night. The membranes were then incubated with peroxidase secondary antibody (Vector Labs) and the blots were developed using Clarity Western ECL Substrate (Bio-Rad). Protein levels were normalized to beta-actin.
2.11. Exclusions and protocol violations
Unfortunately, the forced treadmill exercise induced stress in the animals to such an extent that severe fighting behavior was induced in their home cages. Therefore, several mice (n = 16) had to be sacrificed during this exercise paradigm, which explains the uneven distribution of mice in the different groups. During the ischemic surgery, several mice (n = 7) also died, as expected. The number of mice in each group during the behavioral tests also decreased due to the expected mortality caused by evolution of secondary neuronal damage during the two weeks following induction of global cerebral ischemia. Furthermore, for the analysis of cytokines, all samples with lack of signal in the SECTOR Imager 6000 plate reader were excluded. This altogether explains why the number of samples in each group varies for each experiment. Thus, the variations in number of samples between the experiments are not due to exclusion of outliers or conscious selection of subgroups.
2.12. Statistical analyses
All statistical analyses were performed in SPSS version 22.0. ANOVA were performed followed by Fisher's post hoc test for all comparisons between groups regarding the immunohistochemical evaluations, Western Blots, behavioral tests and cytokine levels. Two-tailed T-tests were used to compare the levels of corticosterone and anxiety in the two different groups (exercising and non-exercising) during the exercise program. For cytokines IFNγ, IL-6 and IL-10, as well as for galectin-3 and NLRP3, the levels were converted to a logarithmic scale before analysis. The correlations between immunohistochemical data, behavioral data and cytokine and corticosterone levels were analyzed using Pearson's correlation coefficients (r). R-values above 0.7 were regarded as very strong relationships and R-values between 0.4 and 0.69 were regarded as moderate to strong relationships. For survival analysis following ischemic surgery, Kaplan-Meier curves and subsequent log rank tests were performed in order to compare groups. Survival during the ischemic surgery procedure for the exercised and non-exercised groups was evaluated by Fisher's exact test. P-values below 0.05 were considered as statistical significant.
3. Results
3.1. Mice subjected to forced treadmill exercise are more anxious
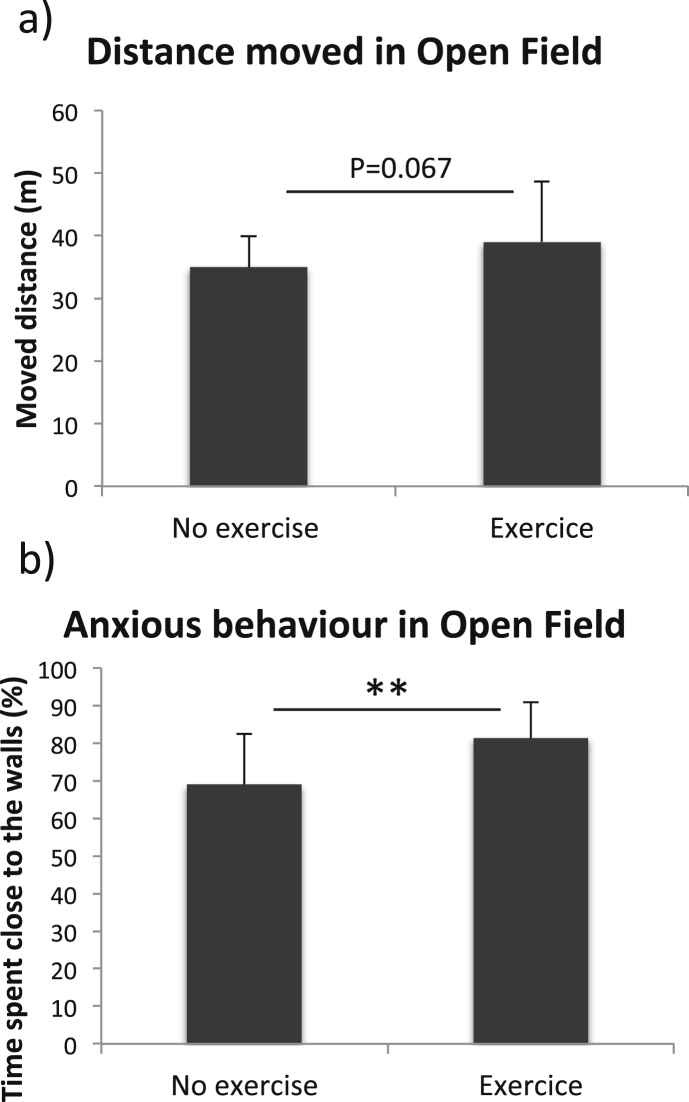
The results from the Open Field tests conducted before the induction of cerebral ischemia can be seen in Fig. 2. At baseline, there were no significant differences in distance moved between mice subjected to exercise and those that were not (Fig. 2a, 3902 ± 962 cm and 3496 ± 502 cm respectively, T-test, p = 0.067). However, exercised mice spent more time close to the walls (Fig. 2b, 81.3 ± 9.6% of time and 69.0 ± 13.6% of time respectively, T-test, p = 0.002) of the Open Field box compared to those who were not subjected to any exercise. This suggests that treadmill exercise by itself could have an impact on anxious behavior. After ischemia, there was no difference between the group subjected to exercise and the group that had not been exercising (data not shown). However, there was a difference between the sham group and the groups subjected to ischemia in the behavior in the Open Field test (see Fig. 1S in Supplementary material 2).
Fig. 2.
Locomotor and anxious behavior in the Open Field test during the exercise program. The distance moved (a) and the anxious behavior (b), measured by time spent close to the walls, in the Open Field test during the exercise program. Bars represent the mean values for each group with error bars indicating the SD. * represents p < 0.05 and ** p < 0.01 in T-test. For non-exercised mice n = 17 and for exercised mice n = 12.
3.2. Treadmill exercise prior to ischemia aggravates the neuronal damage in hippocampus
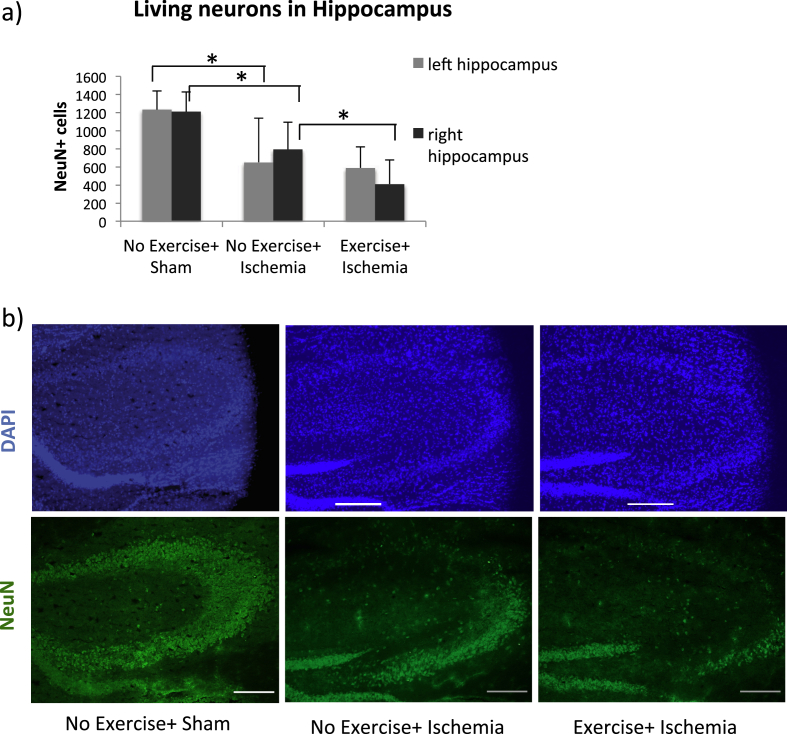
Our model of global brain ischemia is known to be dependent on the patency and lack of posterior communicating artery (Zhen and Dore, 2007, Murakami et al, 1998). Due to heterogeneity in the anatomy of the cerebral vascularization among the mice the experimental induction of ischemia might cause neuronal damage in one or both hemispheres, and the respective hemispheres can therefore be analyzed separately (Olsson et al., 2003, Burguillos et al, 2015). The global brain ischemia caused neuronal damage in the hippocampus both in the left and right hemispheres (Fig. 3a,b 1232 ± 209 and 1210 ± 220 viable-appearing neurons in sham compared to 652 ± 487 and 795 ± 297 living neurons in ischemic mice, ANOVA p = 0.015 and p = 0.001 respectively, Fisher's post hoc test, p = 0.036 and p = 0.03, respectively). Following ischemia, fewer NeuN positive neurons were detected in the right hippocampus of exercised mice compared to those who did not exercise (Fig. 3, Fisher's post hoc test, 411 ± 263 and 795 ± 297 living neurons respectively, p = 0.039). No difference in NeuN positive cells could be found in the left hemispheres between the experimental groups. The mortality during the surgical procedure did not differ between exercising or sedentary mice (Fisher's exact test, p = 0.13, in the ischemic groups 4/12 mice died in the exercising group and 1/17 mice died in the sedentary group). Furthermore, the mortality after ischemia did not differ between exercising or sedentary mice (Log Rank test, p = 0.103, in the sham group, no mice died, and in the ischemic groups 2/8 mice died in the exercising group and 7/16 mice died in the sedentary group).
Fig. 3.
Neuronal damage in hippocampus following global ischemia. The number of NeuN positive neurons in the left and right hippocampus for exercised and non-exercised groups compared to sham (a). Bars represent the mean values for each group, with error bars indicating the SD. * represents p < 0.05 in Fisher's post hoc test. Representative images of NeuN immunohistochemical staining with DAPI background staining in the right hippocampus from each group (b) at 10× magnification. Scale bars represent 200 μm. For sham mice n = 5, non-exercised mice n = 6 and for exercised mice n = 6.
3.3. The increased neuronal damage in the hippocampus cannot be explained by increased microglial activity in the same region
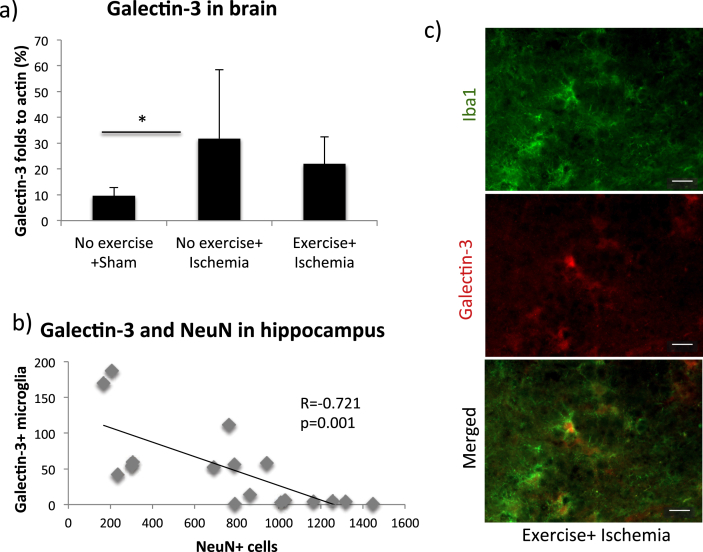
We found no differences in microglial activity between the exercising and non-exercising mice in Iba1 (optical density were 13.7 ± 8.4 and 14.8 ± 8.6 for exercising and non-exercising mice respectively, p = 0.81) or galectin-3 (87 ± 74 and 42 ± 35 positive cells for exercising and non-exercising mice respectively, p = 0.13) immunohistochemical stainings following ischemia that could explain the differences in neuronal survival in the hippocampus.
3.4. Forced treadmill exercise and ischemia induces a stress response that correlates with the degree of neuronal damage in hippocampus
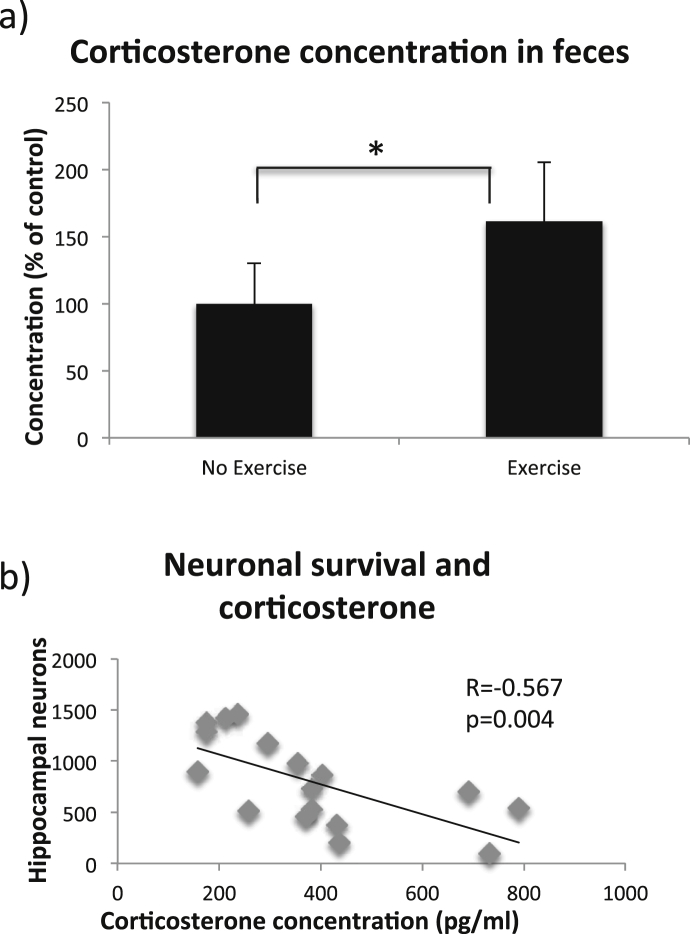
Our behavioral data (Fig. 2) suggest that the mice in the treadmill training groups might be stressed due to the forced exercise. We therefore measured the levels of corticosterone in the feces, which is an established method to measure the stress levels in mice (Kalliokoski et al, 2010, Siswanto et al, 2008). The corticosterone levels in feces collected during the Open Field test are shown in Fig. 4. To compare the effect of forced running on the corticosterone levels without ischemic insult as a confounding factor, mice only subjected to sham surgery are presented in this figure. In these sham groups, corticosterone levels were elevated in mice subjected to forced treadmill running with 61% compared to mice which had not been subjected to any exercise (Fig. 4a, 394 ± 107 pg/ml and 244 ± 73 pg/ml respectively, T-test, p = 0.016). These data suggest that the mice that were exposed to forced treadmill training were profoundly stressed the weeks before the ischemic insult, which can be related to the increased brain damage seen in this group. Indeed, by correlating the corticosterone levels to the number of viable neurons in the hippocampus we found a negative correlation (Fig. 4b, Pearson's R = −0.567, p = 0.004). During the exercise program, there was no significant difference in corticosterone levels between mice shown to be the most unwilling to run and mice that were more willing to run (520 ± 208 pg/ml and 365 ± 147 pg/ml respectively, T-test, p = 0.08, for the mice most unwilling to run n = 6 and for the mice more willing to run n = 12). There was no difference in the levels of corticosterone in feces between those that had been exercising and those that had not been exercising in the group subjected to ischemia (data not shown). However, both groups subjected to ischemia displayed high corticosterone levels, which is most probably due to the effect of global ischemia that has been previously shown (de la Tremblaye et al., 2014).
Fig. 4.
Corticosterone levels during the Open Field test. The levels of corticosterone in feces (a). Bars represent the mean values for each sham group expressed as percentage of non-exercised control, with error bars indicating the SD. * represents p < 0.05 in T-test. For non-exercised mice n = 10 and for exercised mice n = 3. The correlation between the corticosterone concentration following ischemia and the number of surviving neurons in the left hippocampus (b). Each dot corresponds to the measured values from one mouse.
3.5. Ischemia leads to increased levels of NLRP3 and galectin-3 in the brain
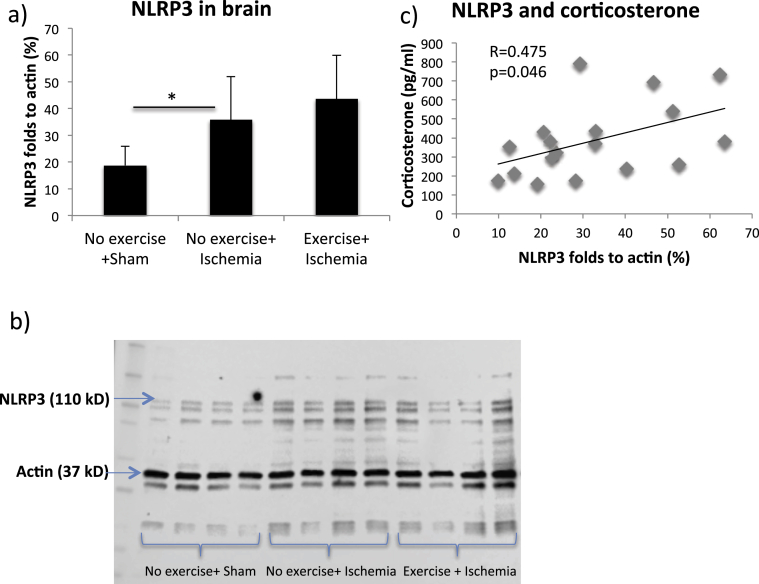
The levels of galectin-3 and NLRP3 in the brain of the different groups were analyzed with Western blots, and can be seen in Fig. 5, Fig. 6a respectively. The levels of NLRP3 and galectin-3 did not significantly differ between exercised and non-exercised mice following ischemia. For galectin-3, no differences were detected in the ANOVA (p = 0.054). However, a difference in galectin-3 was found in Fisher's post hoc test comparing non-exercised ischemic mice to sham (p = 0.038, Fig. 5a). The number of galectin-3 positively stained microglia also correlated negatively with the number of viable NeuN positive neurons in hippocampus (Fig. 5b, Pearson's R = −0.721, p = 0.001). Ischemia induced an increase in NLRP3 (Fig. 6a and b, ANOVA p = 0.027, Fisher's post hoc test p = 0.048) in non-exercised mice compared to sham. The levels of NLRP3 correlated with the levels of corticosterone found in feces (Fig. 6c, Pearson's R = 0.475, p = 0.046) following ischemia. Collectively, this data support our microglial Iba1 immunohistochemistry data, showing increased levels of inflammatory proteins after ischemia, but no significant effect of treadmill exercise prior to ischemia.
Fig. 5.
Inflammation in hippocampus and whole brain following global brain ischemia. The levels of galectin-3 in the brain in exercised and non-exercised groups compared to sham. Bars represent the mean values for each group, with error bars indicating the SD. * represents p < 0.05 in Fisher's post hoc test. For sham mice n = 5, non-exercised mice n = 9 and for exercised mice n = 5. The correlation between the number of galectin-3 positive cells and the number of surviving neurons in the right hippocampus following ischemia (b). Each dot corresponds to the measured values from one mouse. Representative images of galectin-3 and Iba1 staining in the hippocampus (c) at 40× magnification. Scale bars represent 20 μm.
Fig. 6.
Levels of NLRP3 in brain following global ischemia. The levels of NLRP3 in the brain in exercised and non-exercised groups compared to sham (a). Bars represent the mean values for each group, with error bars indicating the SD. * represents p < 0.05 in Fisher's post hoc test. For sham mice n = 5, non-exercised mice n = 9 and for exercised mice n = 5. A picture of the blot with the bands of interest indicated (b). The correlation between the levels of NLRP3 and corticosterone concentration following ischemia (c). Each dot corresponds to the measured values from one mouse.
3.6. Global cerebral ischemia induces a cytokine response that is more pronounced in mice subjected to forced exercise for several cytokines
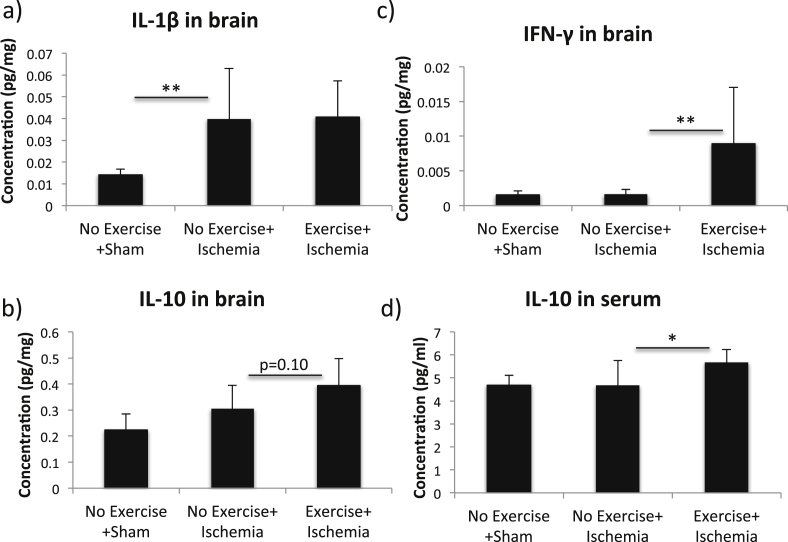
We measured several pro- and anti-inflammatory cytokines in the blood and the brain after global ischemia. The levels of four of the cytokines for which significant differences could be observed between different groups are presented in Fig. 7. The levels of the other cytokines measured in the collected serum and brain tissue from the different groups can be seen in Supplementary material 2, Tables 1 and 2, respectively. Ischemic mice had elevated IL-1β levels in the brain compared to sham (Fig. 7a, 0.040 ± 0.023 and 0.014 ± 0.002 pg/mg protein respectively, ANOVA p = 0.045, Fisher's post hoc test, p = 0.002). No significant difference between non-exercised and exercised mice following ischemia was seen for this specific cytokine.
Fig. 7.
Cytokine levels in serum and brain following global ischemia. Levels of different cytokines in the brain (a–c) and serum (d) for exercised and non-exercised groups compared to sham. Bars represent the mean values for each group, with error bars indicating the SD. * represents p < 0.05, ** represents p < 0.01 and *** represents p < 0.001 in Fisher's post hoc test. For the sham group n = 5, n = 4, n = 5 and n = 4 respectively, for the non-exercised mice with ischemia n = 9, n = 9, n = 9 and n = 8 respectively and for exercised mice with ischemia n = 6, n = 5, n = 6 and n = 6 respectively.
Strikingly, mice that had been subjected to exercise prior to ischemia showed much higher levels of IFNγ in the brain compared to non-exercised mice with ischemia (Fig. 7c, 8.93*10−3 ± 8.1*10−3 and 1.64*10−3± 0.71*10−3 pg/mg protein respectively, Fisher's post hoc test, p = 0.008). For the IL-10 levels in the brain, no significant difference was seen between exercised and non-exercised mice after ischemia (Fig. 7b, 0.39 ± 0.10 and 0.31 ± 0.09 pg/mg protein respectively, p = 0.10). No difference was seen in the ANOVA (p = 0.084) for the serum levels of IL-10. However, higher levels of IL-10 were observed in serum from exercised mice compared to non-exercised mice following ischemia in Fisher's post hoc test (Fig. 7d, 5.68 ± 0.55 and 4.68 ± 1.08 pg/ml respectively, p = 0.042).
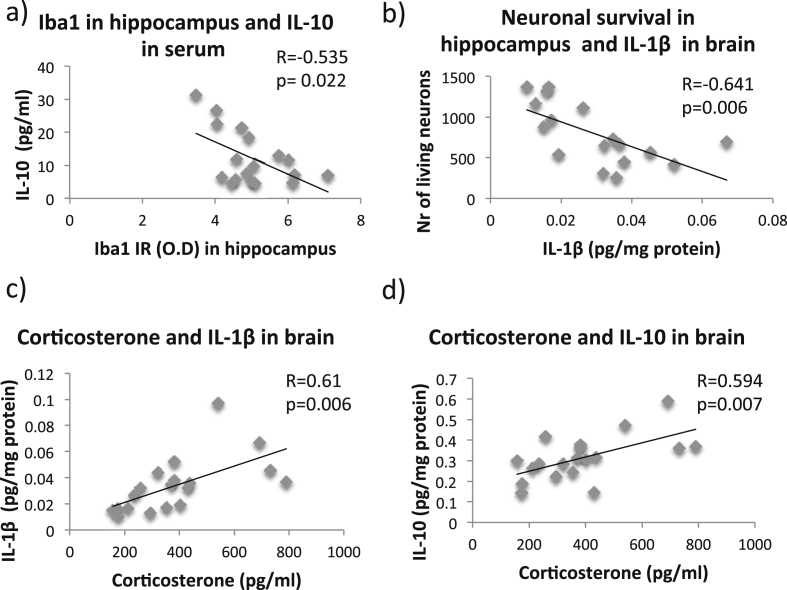
The concentration of IL-10 in serum correlated negatively with the immunoreactivity of the Iba1 staining in the hippocampus (Fig. 8a, Pearson R = −0.535, p = 0.022). The amount of surviving NeuN positive cells in the hippocampus showed a negative correlation with the levels of several different pro-inflammatory cytokines such as IL-1β (Fig. 8b, Pearson R = −0.641, p = 0.006) in the brain. The corticosterone levels also correlated with the levels of IL-1β (Fig. 8c, Pearson R = 0.61, p = 0.006) and IL-10 (Fig. 8d, Pearson R = 0.594, p = 0.007) in the brain. In summary, our data suggest an expected, negative association between pro-inflammatory cytokines and neuronal survival (Fig. 8b) and also a positive correlation between cytokine levels in the brain and corticosterone levels in feces indicating that the stress caused by the forced exercise affects the neuroinflammation in the injured brain.
Fig. 8.
Correlations between cytokines, microglial activation, neuronal survival and corticosterone. Correlations between immunoreactivity of Iba1 in the hippocampus and IL-10 in serum (a), neuronal survival and IL-1β in the brain (b), corticosterone levels after ischemia and IL-1β in the brain (c) and corticosterone levels after ischemia and IL-10 in the brain (d). Each dot corresponds to the measured values from one mouse.
3.7. Global ischemia does not induce cognitive dysfunctions or anhedonic behavior
There were no differences between the different groups in the Y-maze test and the sucrose preference test (See Supplementary Material 2, Table 3).
3.8. Voluntary wheel running does not induce a stress response
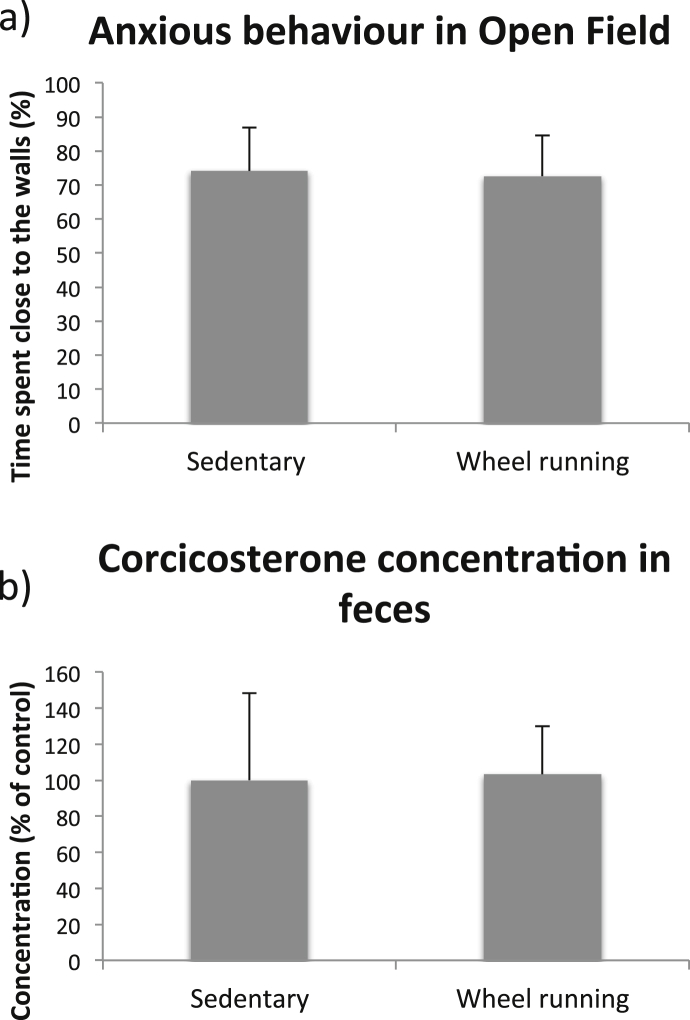
Mice subjected to voluntary wheel running did not display increased anxious behavior compared to their age-matched sedentary controls (Fig. 9a, 74.2 ± 12.8% of time and 72.6 ± 12.0% of time respectively, T-test, p = 0.86). As can be seen in Fig. 9b, the corticosterone levels in feces from wheel running mice did not differ from their age-matched sedentary controls. Taken together, we found that running exercise per se does not cause a stress response with increased anxiety and corticosterone levels.
Fig. 9.
Anxious behavior and fecal corticosterone levels after voluntary wheel running. Anxious behavior in the Open Field test in mice subjected to voluntary wheel running (a). Bars represent the mean values for each group, with error bars indicating the SD. For sedentary control mice n = 4 and for wheel running mice n = 4. The levels of corticosterone in feces in mice subjected to voluntary wheel running(b). Bars represent the mean values for each group expressed as percentage of the sedentary control group, with error bars indicating the SD. For sedentary control mice n = 4 and for wheel running mice n = 6.
4. Discussion
In the present study, we investigated the potential preventive effect of physical exercise prior to global cerebral ischemia in mice by subjecting one group of mice to a four-week long treadmill running program prior to induction of global cerebral ischemia. Our main findings show that forced treadmill exercise induced a stress response that can counteract the anti-inflammatory effects of exercise, and lead to increased neuronal damage in a mouse model of global cerebral ischemia. In the Open Field test during the exercise period prior to ischemic onset, we observed that exercised mice were significantly more anxious, spending more time close to the walls of the Open Field arena, compared to the non-exercised group. These results confirm our observations during the running procedure, where the mice gave the impression of being anxious and stressed during and after the training. Indeed, we lost several mice (n = 16) during the training period due to fighting between the mice that were housed together. Also, many of the mice showed an increasing unwillingness to run and were therefore excluded from the exercise group. Mice that were most unwilling to run also seemed to have higher levels of the stress hormone corticosterone in feces collected during the Open Field test conducted during this exercise period, even though this difference was not statistically significant (data not shown). Importantly, we found that the forced running itself significantly increased the corticosterone levels. These stress levels represent the status of the mice the weeks before they were subjected to ischemia, suggesting that stress prior to an ischemic insult is detrimental. Indeed, the potential of stress hormones such as corticosterone to potentiate the neuronal damage in hippocampus following global cerebral ischemia was shown already in the 1980s (Sapolsky and Pulsinelli, 1985). Examination of the brain samples from our mice also showed that exercised mice had fewer surviving neurons in the hippocampus compared to non-exercised mice following ischemia. Furthermore, higher corticosterone levels correlated with fewer surviving neurons, indicating that the reduced neuronal survival in the exercised mice may be due to the increased stress caused by the enforcement to run. This conclusion was further strengthened by the fact that this increase in corticosterone levels where not observed in mice subjected to voluntary wheel running when compared to age-matched sedentary littermate controls.
When it comes to the effect of exercise on the level of inflammation following ischemia, our results demonstrate the complexity of the immune response following exercise and cerebral ischemia. Galectin-3 is a pro-inflammatory immunomodulator expressed by activated microglia. It has been suggested to sustain microglial activation through binding and activating the toll-like receptor 4 (Burguillos et al., 2015). Interestingly, we found a strong negative correlation between galectin-3 positive cells in the hippocampus and the number of viable hippocampal neurons (NeuN), suggesting that the presence of galectin-3 positive microglia is associated with increased neuronal cell death.
Previous studies in rats have shown that running exercise can reduce microgliosis if the rats are exercised after ischemic induction (Lovatel et al., 2014). However, no microglial effect of exercise prior to ischemic induction was observed. In our study, following ischemia, exercised mice had a subtle increase in the levels of the anti-inflammatory cytokine IL-10 in serum compared to non-exercised mice. Increased levels of IL-10 is a well-known anti-inflammatory effect of exercise (Pedersen and Febbraio, 2008) and can provide neuroprotection after cerebral ischemia (Ooboshi et al., 2005). Though, we did not find an altered level of IL-10 in the brain.
However, ischemic exercised mice had a 5 times elevation of the pro-inflammatory IFNγ in the brain compared to non-exercised counterparts. As IFNγ is a canonical cytokine known to induce a pro-inflammatory phenotype in microglia (Prajeeth et al, 2014, Papageorgiou et al, 2016), it is tempting to speculate that this could in part explain the increased neuronal damage seen in the exercised mice compared to the corresponding non-exercised mice following ischemic insult. The effect of exercise on the levels of IFNγ has not yet been elucidated, several studies have shown that exercise can decrease as well as increases the levels of IFNγ (Tuon et al, 2015, Nichol et al, 2008). However, in mice, IFNγ has been shown to increase in response to stress (Fuertig et al., 2015). This suggests the possibility that the elevated levels of IFNγ observed in our mice may be caused by the stress associated with the exercise rather than the exercise per se.
Our data confirm the complex relationship between different cytokines, where functions are dependent on the inflammatory context and possible interplay between the different cytokines, and where the effect of pro-inflammatory cytokines can be counterbalanced by high levels of anti-inflammatory cytokines. It is also possible that exercise and stress can alter the inflammatory interplay between CNS and the periphery. The connection between the various cytokines involved in neuroinflammation and their importance in the context of physical exercise remains to be elucidated. A limitation of this study is that we measured the cytokines fairly late, and only at one time-point in the process, which made it impossible to determine their temporal dynamics.
We further show that high levels of pro-inflammatory cytokines, such as IL-1β, in the brain is correlated with significantly lower number surviving neurons in hippocampus following ischemia, suggesting that these cytokines are related to a negative impact on neuronal survival. This negative impact of pro-inflammatory cytokines on neuronal survival has been shown in ischemic rats previously (Li et al., 2014). In our study, mice with high levels of corticosterone also had higher levels of cytokines, such as IL-1β and IL-10, in the brain. These results are in accordance with other studies where it has been shown that stress can evoke a pro-inflammatory response in the brain, with increased expression of NLRP3 inflammasome involved in the cleavage and secretion of pro-inflammatory IL-1β (Gadek-Michalska et al, 2013, Frank et al, 2014). Recently, it has been shown that a stress-induced increase in corticosterone levels lowers the threshold for microglia to release pro-inflammatory cytokines (Dey et al., 2014). Increased stress has even been suggested to exacerbate neuronal cell death by increasing neuroinflammation, which in turn is neurotoxic (de Pablos et al., 2014). Indeed, several studies have shown that stress increase the vulnerability to inflammation resulting in increased microglial activation and neuronal damage in different brain regions such as hippocampus (Espinosa-Oliva et al., 2011), substantia nigra (de Pablos et al., 2014) and prefrontal cortex (de Pablos et al., 2006). Furthermore, the same studies also showed that these effects of stress were dependent on glucocorticoid receptor signaling. In our study we did not detect any differences between exercised and non-exercised mice in the levels of IL-1β and NLRP3 in the brain, suggesting that the link between stress and the inflammatory response may also involve other mechanisms. However, one possible confounding factor is that the corticosterone samples were collected more than a week prior to collecting the samples used for measuring cytokine levels, NLRP3 and assessing brain damage.
The forced treadmill running clearly induced a stress response that could explain the negative effects on the brains of the mice in our study. The reason why many studies, including ours, are based on forced treadmill running as a method for studying the effects of exercise is that this method can be standardized compared to, for example, voluntary wheel running. With treadmills, the intensity, duration and timing of the exercise can be standardized so that all mice in the same exercise group are subjected to exactly the same amount of exercise. This is not possible when using voluntary wheel running as the wheel is placed in the home cages, allowing the mice to run as much, fast and often as they want. However, one advantage of using voluntary wheel running is that it does not include the stressful element of forced exercise, and that the mice can choose to run during the night, which in itself is less stressful as they are nocturnal animals. However, for the voluntary wheel running, we choose to cage the mice separately in order to better control the distance run by each mouse. It has been shown in other study that social isolation per see could be very stressful to the mice, as it is used in standardized stress protocols (de Pablos et al, 2014, Espinosa-Oliva et al, 2011). However, our single caged mice did not display any signs of anxiety in the Open Field test compared to the behavior we use to observe in our mice. Our treadmill study is not alone in demonstrating that forced exercise can have negative effects due to stress. Indeed, other studies have shown that forced treadmill running causes anxiety and stress in form of increased levels of corticosterone, effects that were not observed in animals subjected to voluntary wheel running (Leasure and Jones, 2008, Ke et al, 2011, Griesbach et al, 2012). Interestingly, another study showed that it might not be the enforcement to run per se that induces stress, but rather the fact that being pushed if not running (Greenwood et al., 2013). In this study, there were no differences in stress induction between voluntary wheel running and motorized, forced wheel running. However, the animals subjected to forced treadmill running, being pushed by foot shocks when not running, were more stressed. In fact, forced treadmill running is also used as a model to induce and study stress responses in mice (Hong et al., 2015). Furthermore, Zheng and coauthors showed that following experimental stroke in rats, forced treadmill running leads to reduced motor recovery compared to rats subjected to voluntary wheel running (Ke et al., 2011). It has also been shown that forced and voluntary exercise can have different effects on inflammatory response, where forced exercise increases and voluntary exercise decreases levels of pro-inflammatory cytokines after injury in mice (Cook et al., 2013). Moreover, it has been shown that voluntary running leads to higher levels of BDNF compared to forced running (Uysal et al, 2015, Griesbach et al, 2014). However, there are also studies comparing the effect of forced versus voluntary running showing that both regimens can be of equal beneficial effect when it comes to improving cognition, neuronal survival and BDNF levels in different models of brain injury (Lin et al, 2015a, Lin et al, 2015b).
Furthermore, it has been shown that forced exercise can activate a stress response similar to restraint stress, reducing the ability to cope with an oxidative challenge. For example, forced running has been shown to increase the cardiac infarct size in rats (Mancardi et al., 2009), and failed to improve recovery after experimental stroke in rats (Auriat et al., 2006). However, several studies have shown beneficial effects of forced exercise on the recovery from cerebral hypoperfusion (Cechetti et al., 2012) as well as experimental stroke (Park et al., 2012).
5. Conclusions
This study shows that forced treadmill running induces stress, which can cause increased neuronal damage following global cerebral ischemia in mice. We detected 5-fold increased IFNγ levels in ischemic mice that had been subjected to treadmill running, even though we found a small increase in serum IL-10 levels. This stress response with increased anxiety and corticosterone levels were not seen in mice exposed to voluntary wheel running. It is therefore tempting to conclude that running exercise can have beneficial neuroinflammatory effects, but that the stress induced by the enforcement to run is disadvantageous and exceeds the beneficial effects. Our study highlights the importance of taking into account the stress that the enforcement of running exercise can induce, which can counteract the otherwise positive effects of physical exercise. Hence, it is important to monitor chronic stress in experimental animals to be able to infer the right conclusions about the effects of physical exercise.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work has been supported by the Strategic Research Area MultiPark at Lund University, Lund, Sweden, the Swedish Research Council grant no. 2012-2229, by the A.E. Berger Foundation, Swedish Brain Foundation, Crafoord Foundation, Gyllenstiernska Krapperup Foundation, Bergvall Foundation, G&J Kock Foundation, Swedish National Stroke Foundation, Swedish Parkinson Foundation, Stohnes Foundation and the Royal Physiographic Society. We are grateful to Hakon Leffler for supplying us with the galectin-3 antibody.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ynstr.2016.09.002.
Contributor Information
Martina Svensson, Email: martina.svensson@med.lu.se.
Philip Rosvall, Email: philip.a.rosvall@gmail.com.
Antonio Boza-Serrano, Email: antonio.boza_serrano@med.lu.se.
Emelie Andersson, Email: emelie.andersson@med.lu.se.
Jan Lexell, Email: jan.lexell@med.lu.se.
Tomas Deierborg, Email: tomas.deierborg@med.lu.se.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Auriat A.M. Forced exercise does not improve recovery after hemorrhagic stroke in rats. Brain Res. 2006;1109(1):183–191. doi: 10.1016/j.brainres.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Austin M.W. Aerobic exercise effects on neuroprotection and brain repair following stroke: a systematic review and perspective. Neurosci. Res. 2014;87:8–15. doi: 10.1016/j.neures.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Back T., Hemmen T., Schuler O.G. Lesion evolution in cerebral ischemia. J. Neurol. 2004;251(4):388–397. doi: 10.1007/s00415-004-0399-y. [DOI] [PubMed] [Google Scholar]
- Banati R.B. Cytotoxicity of microglia. Glia. 1993;7(1):111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- Barone F.C. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28(6):1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Bottiger B.W. Neuronal stress response and neuronal cell damage after cardiocirculatory arrest in rats. J. Cereb. Blood Flow Metab. 1998;18(10):1077–1087. doi: 10.1097/00004647-199810000-00004. [DOI] [PubMed] [Google Scholar]
- Brown D.A. Short-term treadmill running in the rat: what kind of stressor is it? J. Appl. Physiol. 2007;103(6):1979–1985. doi: 10.1152/japplphysiol.00706.2007. 1985. [DOI] [PubMed] [Google Scholar]
- Burguillos M.A. Microglia-secreted Galectin-3 acts as a toll-like receptor 4 ligand and contributes to microglial activation. Cell Rep. 2015;10(9):1626–1638. doi: 10.1016/j.celrep.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Carson M.J., Thrash J.C., Walter B. The cellular response in neuroinflammation: the role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin. Neurosci. Res. 2006;6(5):237–245. doi: 10.1016/j.cnr.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetti F. Forced treadmill exercise prevents oxidative stress and memory deficits following chronic cerebral hypoperfusion in the rat. Neurobiol. Learn Mem. 2012;97(1):90–96. doi: 10.1016/j.nlm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Cook M.D. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav. Immun. 2013;33:46–56. doi: 10.1016/j.bbi.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deierborg T. Overexpression of UCP2 protects thalamic neurons following global ischemia in the mouse. J. Cereb. Blood Flow Metab. 2008;28(6):1186–1195. doi: 10.1038/jcbfm.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A. Glucocorticoid sensitization of microglia in a genetic mouse model of obesity and diabetes. J. Neuroimmunol. 2014;269(1–2):20–27. doi: 10.1016/j.jneuroim.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Oliva A.M. Stress is critical for LPS-induced activation of microglia and damage in the rat hippocampus. Neurobiol. Aging. 2011;32(1):85–102. doi: 10.1016/j.neurobiolaging.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Frank M.G. Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology. 2014;40:191–200. doi: 10.1016/j.psyneuen.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertig R. Mouse chronic social stress increases blood and brain kynurenine pathway activity and fear behaviour: both effects are reversed by inhibition of indoleamine 2,3-dioxygenase. Brain Behav. Immun. 2015;54:59–72. doi: 10.1016/j.bbi.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Gadek-Michalska A. Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol. Rep. 2013;65(6):1655–1662. doi: 10.1016/s1734-1140(13)71527-5. [DOI] [PubMed] [Google Scholar]
- Greenwood B.N. Exercise-induced stress resistance is independent of exercise controllability and the medial prefrontal cortex. Eur. J. Neurosci. 2013;37(3):469–478. doi: 10.1111/ejn.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbach G.S. Differential effects of voluntary and forced exercise on stress responses after traumatic brain injury. J. Neurotrauma. 2012;29(7):1426–1433. doi: 10.1089/neu.2011.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbach G.S. Recovery of stress response coincides with responsiveness to voluntary exercise after traumatic brain injury. J. Neurotrauma. 2014;31(7):674–682. doi: 10.1089/neu.2013.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.S. The traditional drug Gongjin-Dan ameliorates chronic fatigue in a forced-stress mouse exercise model. J. Ethnopharmacol. 2015;168:268–278. doi: 10.1016/j.jep.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Kalliokoski O. Distribution and time course of corticosterone excretion in faeces and urine of female mice with varying systemic concentrations. Gen. Comp. Endocrinol. 2010;168(3):450–454. doi: 10.1016/j.ygcen.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Ke Z. The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: a rat brain ischemia model. PLoS One. 2011;6(2):e16643. doi: 10.1371/journal.pone.0016643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.U., de Vellis J. Microglia in health and disease. J. Neurosci. Res. 2005;81(3):302–313. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
- Kirino T., Sano K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984;62(3):201–208. doi: 10.1007/BF00691853. [DOI] [PubMed] [Google Scholar]
- Lambertsen K. Differences in origin of reactive microglia in bone marrow chimeric mouse and rat after transient global ischemia. J. Neuropathol. Exp. Neurol. 2011;70(6):481–494. doi: 10.1097/NEN.0b013e31821db3aa. [DOI] [PubMed] [Google Scholar]
- Lambertsen K.L., Biber K., Finsen B. Inflammatory cytokines in experimental and human stroke. J. Cereb. Blood Flow Metab. 2012;32(9):1677–1698. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure J.L., Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156(3):456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Li S.J. The role of TNF-alpha, IL-6, IL-10, and GDNF in neuronal apoptosis in neonatal rat with hypoxic-ischemic encephalopathy. Eur. Rev. Med. Pharmacol. Sci. 2014;18(6):905–909. [PubMed] [Google Scholar]
- Lin Y. Involuntary, forced and voluntary exercises are equally capable of inducing hippocampal plasticity and the recovery of cognitive function after stroke. Neurol. Res. 2015;37(10):893–901. doi: 10.1179/1743132815Y.0000000074. [DOI] [PubMed] [Google Scholar]
- Lin Y. Involuntary, forced and voluntary exercises equally attenuate neurocognitive deficits in vascular dementia by the BDNF-pCREB mediated pathway. Neurochem. Res. 2015;40(9):1839–1848. doi: 10.1007/s11064-015-1673-3. [DOI] [PubMed] [Google Scholar]
- Lovatel G.A. Long-term effects of pre and post-ischemic exercise following global cerebral ischemia on astrocyte and microglia functions in hippocampus from Wistar rats. Brain Res. 2014;1587:119–126. doi: 10.1016/j.brainres.2014.08.068. [DOI] [PubMed] [Google Scholar]
- Mancardi D. Omega 3 has a beneficial effect on ischemia/reperfusion injury, but cannot reverse the effect of stressful forced exercise. Nutr. Metab. Cardiovasc Dis. 2009;19(1):20–26. doi: 10.1016/j.numecd.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Mota B.C. Exercise pre-conditioning reduces brain inflammation and protects against toxicity induced by traumatic brain injury: behavioral and neurochemical approach. Neurotox. Res. 2012;21(2):175–184. doi: 10.1007/s12640-011-9257-8. [DOI] [PubMed] [Google Scholar]
- Murakami K. The development of a new mouse model of global ischemia: focus on the relationships between ischemia duration, anesthesia, cerebral vasculature, and neuronal injury following global ischemia in mice. Brain Res. 1998;780(2):304–310. doi: 10.1016/s0006-8993(97)01217-1. [DOI] [PubMed] [Google Scholar]
- Nichol K.E. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J. Neuroinflamm. 2008;5:13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson T., Wieloch T., Smith M.L. Brain damage in a mouse model of global cerebral ischemia. Effect of NMDA receptor blockade. Brain Res. 2003;982(2):260–269. doi: 10.1016/s0006-8993(03)03014-2. [DOI] [PubMed] [Google Scholar]
- Ooboshi H. Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation. 2005;111(7):913–919. doi: 10.1161/01.CIR.0000155622.68580.DC. [DOI] [PubMed] [Google Scholar]
- de Pablos R.M. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J. Neurosci. 2006;26(21):5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pablos R.M. Chronic stress enhances microglia activation and exacerbates death of nigral dopaminergic neurons under conditions of inflammation. J. Neuroinflamm. 2014;11:34. doi: 10.1186/1742-2094-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou I.E. TLR4-activated microglia require IFN-gamma to induce severe neuronal dysfunction and death in situ. Proc. Natl. Acad. Sci. U. S. A. 2016;113(1):212–217. doi: 10.1073/pnas.1513853113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. Forced exercise enhances functional recovery after focal cerebral ischemia in spontaneously hypertensive rats. Brain Sci. 2012;2(4):483–503. doi: 10.3390/brainsci2040483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen B.K., Febbraio M.A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 2008;88(4):1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Piao C.S. Late exercise reduces neuroinflammation and cognitive dysfunction after traumatic brain injury. Neurobiol. Dis. 2013;54:252–263. doi: 10.1016/j.nbd.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajeeth C.K. Effector molecules released by Th1 but not Th17 cells drive an M1 response in microglia. Brain Behav. Immun. 2014;37:248–259. doi: 10.1016/j.bbi.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M., Pulsinelli W.A. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science. 1985;229(4720):1397–1400. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- Sasson C. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ. Cardiovasc Qual. Outcomes. 2010;3(1):63–81. doi: 10.1161/CIRCOUTCOMES.109.889576. [DOI] [PubMed] [Google Scholar]
- Sim Y.J. Treadmill exercise improves short-term memory by suppressing ischemia-induced apoptosis of neuronal cells in gerbils. Neurosci. Lett. 2004;372(3):256–261. doi: 10.1016/j.neulet.2004.09.060. [DOI] [PubMed] [Google Scholar]
- Sim Y.J. Long-term treadmill exercise overcomes ischemia-induced apoptotic neuronal cell death in gerbils. Physiol. Behav. 2005;84(5):733–738. doi: 10.1016/j.physbeh.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Siswanto H. Corticosterone concentrations in blood and excretion in faeces after ACTH administration in male Sprague-Dawley rats. In Vivo. 2008;22(4):435–440. [PubMed] [Google Scholar]
- Svensson M., Lexell J., Deierborg T. Effects of physical exercise on neuroinflammation, neuroplasticity, neurodegeneration, and behavior: what we can learn from animal models in clinical settings. Neurorehabil Neural Repair. 2014;29(6):577–589. doi: 10.1177/1545968314562108. [DOI] [PubMed] [Google Scholar]
- Touma C. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen. Comp. Endocrinol. 2003;130(3):267–278. doi: 10.1016/s0016-6480(02)00620-2. [DOI] [PubMed] [Google Scholar]
- de la Tremblaye P.B. Evidence of lasting dysregulation of neuroendocrine and HPA axis function following global cerebral ischemia in male rats and the effect of Antalarmin on plasma corticosterone level. Horm. Behav. 2014;65(3):273–284. doi: 10.1016/j.yhbeh.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Tuon T. Physical training regulates mitochondrial parameters and neuroinflammatory mechanisms in an experimental model of Parkinson's disease. Oxid. Med. Cell Longev. 2015;2015:261809. doi: 10.1155/2015/261809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal N. Effects of voluntary and involuntary exercise on cognitive functions, and VEGF and BDNF levels in adolescent rats. Biotech. Histochem. 2015;90(1):55–68. doi: 10.3109/10520295.2014.946968. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26(4):676–680. doi: 10.1161/01.str.26.4.676. discussion 681. [DOI] [PubMed] [Google Scholar]
- Yenari M.A., Kauppinen T.M., Swanson R.A. Microglial activation in stroke: therapeutic targets. Neurotherapeutics. 2010;7(4):378–391. doi: 10.1016/j.nurt.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen G., Dore S. Optimized protocol to reduce variable outcomes for the bilateral common carotid artery occlusion model in mice. J. Neurosci. Methods. 2007;166(1):73–80. doi: 10.1016/j.jneumeth.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.