Abstract
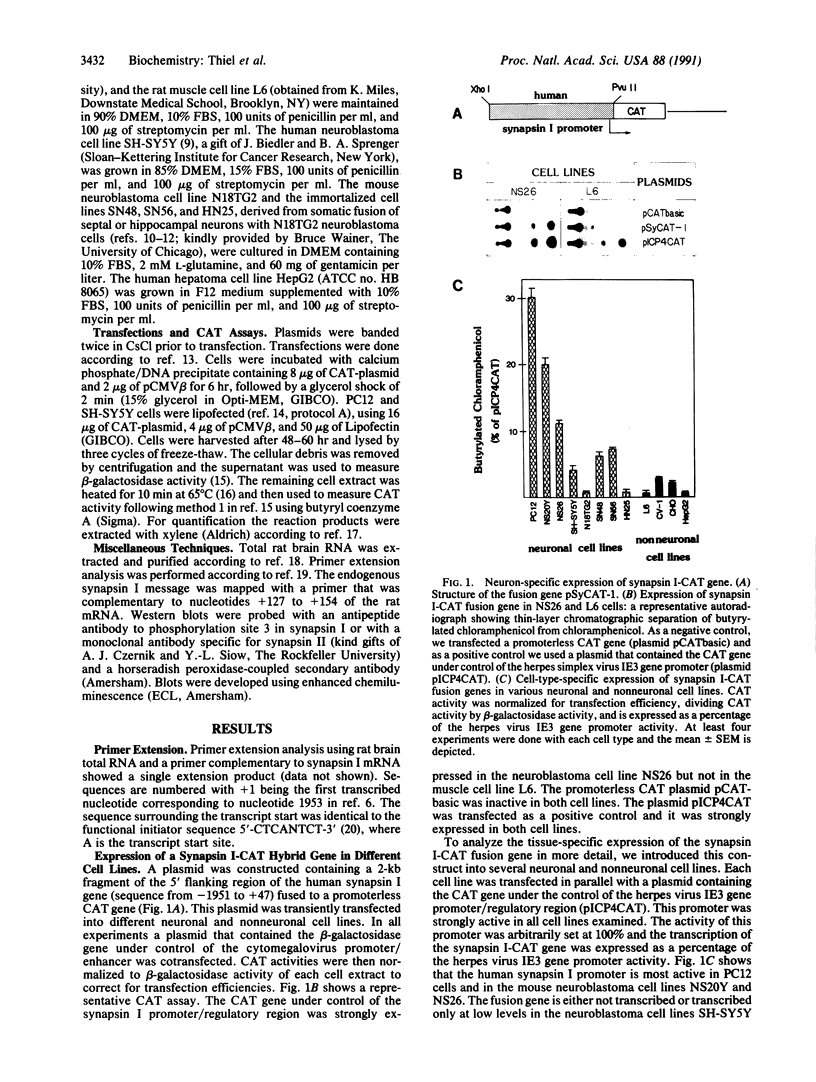
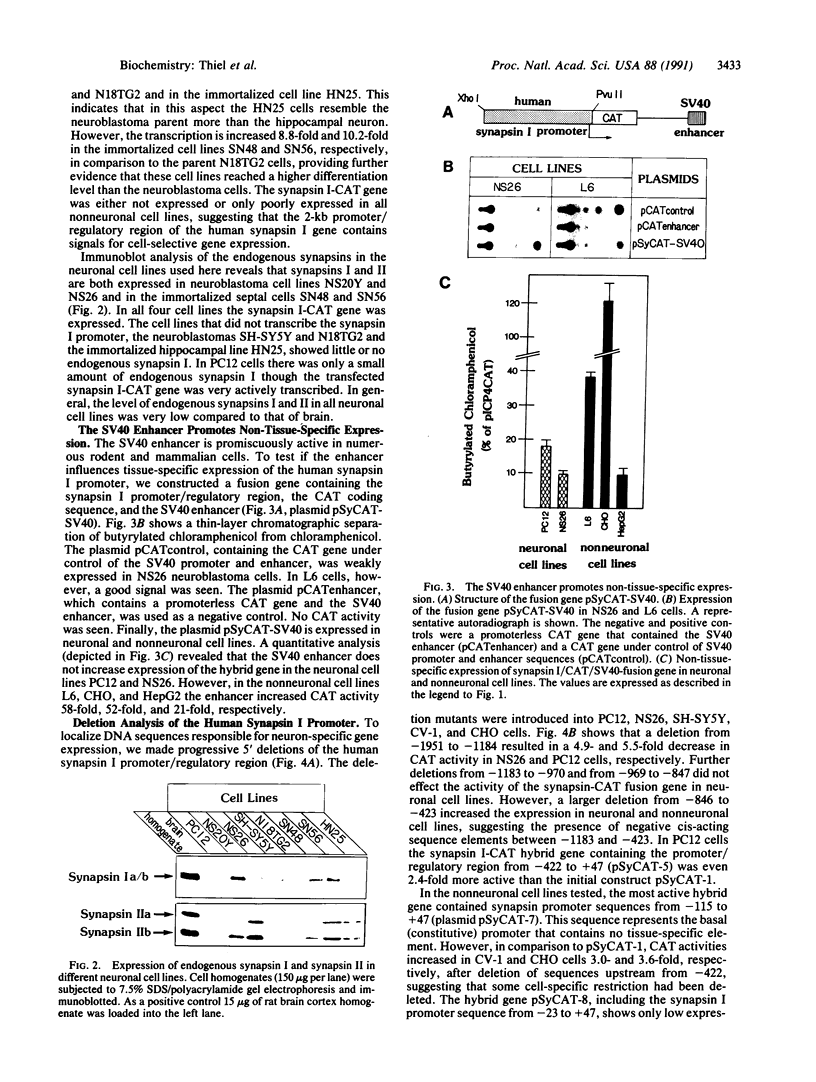
Synapsin Ia and synapsin Ib are abundant synaptic vesicle proteins that are derived by differential splicing from a single gene. To identify control elements directing the neuronal expression of synapsins Ia/b, we functionally analyzed the promoter region of the human synapsin I gene. A hybrid gene was constructed containing 2 kilobases of 5' flanking sequence from the synapsin I gene fused to the bacterial gene chloramphenicol acetyltransferase and transfected into 12 different neuronal and nonneuronal cell lines. In general, expression of the chimeric reporter gene showed excellent correlation with endogenous expression of synapsin I in different neuronal cell lines, whereas transcription was low in all nonneuronal cell lines examined. The addition of the simian virus 40 enhancer promoted non-tissue-specific expression. Deletion mutagenesis of the synapsin I promoter revealed the presence of positive and negative sequence elements. A basal (constitutive) promoter that directs reporter gene expression in neuronal and nonneuronal cell lines was mapped to the region -115 to +47. The promoter region from -422 to -22 contains positive elements that upon fusion with the herpes simplex virus thymidine kinase promoter potentiate its transcription in PC12 and neuroblastoma cells but not in Chinese hamster ovary cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biedler J. L., Helson L., Spengler B. A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973 Nov;33(11):2643–2652. [PubMed] [Google Scholar]
- Bähler M., Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987 Apr 16;326(6114):704–707. doi: 10.1038/326704a0. [DOI] [PubMed] [Google Scholar]
- Cato A. C., Miksicek R., Schütz G., Arnemann J., Beato M. The hormone regulatory element of mouse mammary tumour virus mediates progesterone induction. EMBO J. 1986 Sep;5(9):2237–2240. doi: 10.1002/j.1460-2075.1986.tb04490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Benfenati F., Valtorta F., Greengard P. The synapsins. Annu Rev Cell Biol. 1990;6:433–460. doi: 10.1146/annurev.cb.06.110190.002245. [DOI] [PubMed] [Google Scholar]
- Hammond D. N., Wainer B. H., Tonsgard J. H., Heller A. Neuronal properties of clonal hybrid cell lines derived from central cholinergic neurons. Science. 1986 Dec 5;234(4781):1237–1240. doi: 10.1126/science.3775382. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Lee H. J., Hammond D. N., Large T. H., Roback J. D., Sim J. A., Brown D. A., Otten U. H., Wainer B. H. Neuronal properties and trophic activities of immortalized hippocampal cells from embryonic and young adult mice. J Neurosci. 1990 Jun;10(6):1779–1787. doi: 10.1523/JNEUROSCI.10-06-01779.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Hammond D. N., Large T. H., Wainer B. H. Immortalized young adult neurons from the septal region: generation and characterization. Brain Res Dev Brain Res. 1990 Mar 1;52(1-2):219–228. doi: 10.1016/0165-3806(90)90238-t. [DOI] [PubMed] [Google Scholar]
- Petrucci T. C., Morrow J. S. Synapsin I: an actin-bundling protein under phosphorylation control. J Cell Biol. 1987 Sep;105(3):1355–1363. doi: 10.1083/jcb.105.3.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerwald A., Hoesche C., Oschwald R., Kilimann M. W. The 5'-flanking region of the synapsin I gene. A G+C-rich, TATA- and CAAT-less, phylogenetically conserved sequence with cell type-specific promoter function. J Biol Chem. 1990 Sep 5;265(25):14932–14937. [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Sleigh M. J. A nonchromatographic assay for expression of the chloramphenicol acetyltransferase gene in eucaryotic cells. Anal Biochem. 1986 Jul;156(1):251–256. doi: 10.1016/0003-2697(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Schmidt M. C., Berk A. J., Baltimore D. Transcriptional activation by Sp1 as directed through TATA or initiator: specific requirement for mammalian transcription factor IID. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4509–4513. doi: 10.1073/pnas.87.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C., Czernik A. J., Kao H. T., Takei K., Johnston P. A., Horiuchi A., Kanazir S. D., Wagner M. A., Perin M. S., De Camilli P. Synapsins: mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science. 1989 Sep 29;245(4925):1474–1480. doi: 10.1126/science.2506642. [DOI] [PubMed] [Google Scholar]
- Südhof T. C. The structure of the human synapsin I gene and protein. J Biol Chem. 1990 May 15;265(14):7849–7852. [PubMed] [Google Scholar]
- Südhof T. C., Van der Westhuyzen D. R., Goldstein J. L., Brown M. S., Russell D. W. Three direct repeats and a TATA-like sequence are required for regulated expression of the human low density lipoprotein receptor gene. J Biol Chem. 1987 Aug 5;262(22):10773–10779. [PubMed] [Google Scholar]
- Thiel G., Südhof T. C., Greengard P. Synapsin II. Mapping of a domain in the NH2-terminal region which binds to small synaptic vesicles. J Biol Chem. 1990 Sep 25;265(27):16527–16533. [PubMed] [Google Scholar]
- van der Eb A. J., Graham F. L. Assay of transforming activity of tumor virus DNA. Methods Enzymol. 1980;65(1):826–839. doi: 10.1016/s0076-6879(80)65077-0. [DOI] [PubMed] [Google Scholar]










