Abstract
Stress responses in humans can be attenuated by exogenous oxytocin administration, and these stress-buffering properties may be moderated by social factors. Yet, the influence of acute stressors on circulating endogenous oxytocin levels have been inconsistent, and limited information is available concerning the influence of social support in moderating this relationship. In the current investigation, undergraduate women (N = 67) were assessed in the Trier Social Stress Test (TSST) with either social support available from a close female friend or no social support being available. An additional set of women served as controls. The TSST elicited marked elevations of state anxiety and negative emotions, which were largely attenuated among women who received social support. Furthermore, baseline oxytocin levels were inversely related to women's general feelings of distrust, as well as basal plasma cortisol levels. Despite these associations, oxytocin levels were unaffected by the TSST, and this was the case irrespective of oral contraceptive use or estrogen levels. In contrast, plasma cortisol elevations were elicited by the psychosocial stressor, but only in women using oral contraceptives, an effect that was prevented when social support was available. Taken together, these data provisionally suggest that changes in plasma oxytocin might not accompany the stress attenuating effects of social support on cortisol levels. Moreover, as plasma oxytocin might not reliably reflect brain oxytocin levels, the linkage between oxytocin and prosocial behaviors remains tenuous.
Keywords: Cortisol, Distrust, Oxytocin, Stress, Social support
Highlights
-
•
Baseline oxytocin levels were inversely related to distrust and cortisol levels.
-
•
Empathy from a friend correlated with baseline levels of oxytocin among participants.
-
•
Social support attenuated psychosocial and cortisol stress responses.
-
•
Oxytocin levels were unaffected by the stressor or social support from a friend.
-
•
Cortisol responses following the stressor varied with oral contraceptive use.
1. Introduction
Oxytocin, a neuropeptide produced in the hypothalamus, has gained considerable attention given its presumed role in prosocial behaviors (Donaldson and Young, 2008). In this regard, endogenous oxytocin levels have been associated with trust behaviors (Zak et al., 2005, Kéri et al., 2009), maternal sensitivity (Feldman et al., 2012) and empathy towards strangers (Barraza and Zak, 2009). Furthermore, administration of oxytocin by nasal spray promotes enhanced generosity (Zak et al., 2007), trust (Kosfeld et al., 2005), empathy (Domes et al., 2007), positive communication (Ditzen et al., 2009), and helping behavior (Riem et al., 2013).
Through interactions with other biological systems, oxytocin can modulate stress responses, such as cortisol and cytokine reactivity, and may thus be germane to stress-related psychological disorders (McQuaid et al., 2014). For instance, intranasal oxytocin attenuated salivary cortisol elevations elicited by a physical stressor (Cardoso et al., 2013), limited the cortisol rise elicited by social ostracism (Linnen et al., 2012) and that associated with couple conflict (Ditzen et al., 2009). As encouraging as these data seem, a recent meta-analysis indicated a modest non-significant effect of intranasal oxytocin in attenuating cortisol levels during stressful laboratory tasks. However, a dampening effect of oxytocin on cortisol was apparent in those studies that involved a challenging laboratory task that elicited a robust stimulation of the HPA-axis as well as in studies involving clinical populations (Cardoso et al., 2014).
It is likely that social interactions and social support are important components of the stress-attenuating effects of oxytocin. Social support and enhanced connectedness is strongly associated with improved physical (Uchino, 2006) and mental health (Cruwys et al., 2013), and provides an important method of dealing with stressors, thereby attenuating the propensity for illness development (Holt-Lunstad et al., 2008). In this regard, social support diminished cortisol and activation of the dorsal anterior cingulate cortex elicited by a psychosocial stressor (Eisenberger et al., 2007). Furthermore, in males who received social support coupled with intranasal oxytocin prior to a laboratory stressor (Trier Social Stress Test; TSST), a less pronounced cortisol rise occurred compared to individuals who received either social support, oxytocin or neither of these treatments (Heinrichs et al., 2003). Following a social stressor, children who were able to see or hear their mothers, had higher oxytocin levels and cortisol levels were diminished compared to children that had no-contact with their mothers (Seltzer et al., 2010). To be sure, the effectiveness of social support in attenuating stressor responses may vary as a function of the quality of the support provided, or the closeness and empathy of the person providing support (Holt-Lunstad et al., 2007, Holt-Lunstad et al., 2008).
There have been several studies that assessed the influence of intranasal oxytocin on stress responses, but relatively few reports examined the effect of acute stressors on endogenous oxytocin levels in women under ordinary conditions (i.e. in the absence of lactation and mother-infant bonding). Moreover, the data that have been reported concerning oxytocin release in response to acute psychosocial stressors have been inconsistent (Taylor et al., 2006, Ditzen et al., 2007, Seltzer et al., 2010, Pierrehumbert et al., 2012), possibly being related to various procedural differences, including the way in which oxytocin levels were determined. In particular, in some studies oxytocin was measured without first extracting the hormone from blood, and thus molecules in addition to oxytocin might have been tagged, yielding exceptionally high levels, likely reflecting oxytocin together with other factors that were present (see Szeto et al., 2011, for a discussion of this topic).
The overarching aim of the current investigation was to determine, whether plasma oxytocin levels among women, would increase in response to a psychosocial stressor. Women were chosen for the current investigation as it has been suggested that plasma oxytocin might be most relevant to women, and vasopressin to men (Taylor et al., 2010). This may be particularly relevant as estrogen regulates oxytocin functioning (i.e. transcription of oxytocin as well as its receptor; Choleris et al., 2008). Psychosocial responses and biological variations, including cortisol, oxytocin, and estradiol levels, were assessed in women at baseline and following the TSST. Efforts were made to eliminate factors that could confound the potential effects of stressors on oxytocin levels. For instance, in the current study, using females, the effects of a stressor were assessed on circulating oxytocin in properly extracted samples. Furthermore, as self-reported menstrual phase may be unreliable, and estrogen can regulate oxytocin functioning; (Choleris et al., 2008), estradiol levels were also assessed. As well, in the present study, the impact of support from a close female friend was assessed, considering that social support coming from a male may be less effective (Glynn et al., 1999). It was hypothesized that having social support from a close female friend would attenuate anxiety, negative mood outcomes as well as cortisol stress responses. However, it was uncertain whether or not oxytocin levels would be expected to rise in response to the stressor or social support manipulation. Conflicting findings were reported in this regard, with some indicating that oxytocin either does not increase in response to a stressor irrespective of social support (Taylor et al., 2006, Ditzen et al., 2007), increases in response to a stressor if social support is also present (Seltzer et al., 2010), or increases in response to a stressor in the absence of support (Pierrehumbert et al., 2012). Given these disparate findings, in the present investigation we also examined whether qualities of the individual providing support, including empathy, would be related to the participants stress responses. Conceivably, elevated empathy among individuals providing support may affect stress responses among participants, resulting in attenuated stressor effects.
2. Methods
2.1. Participants
The study included female undergraduate students (N = 67) from Carleton University ranging in age from 17 to 30 years (Mage = 19.37, SD = 2.08) that were recruited using the online SONA system. Participants represented an ethnically diverse sample comprising White (50.7%, n = 34), Black (19.4%, n = 13) Arab/West Asian (9.0%, n = 6) Asian (6.0%, n = 4), Latin American/Hispanic (4.5%, n = 3), South Asian (2.0%, n = 3), South East Asian (1.5%, n = 1), Aboriginal (1.5%, n = 1), and other (1.0%, n = 3). In addition to these participants, data relevant to several psychosocial factors were collected from the friends who provided social support during the laboratory session (n = 18). This group of individuals ranged in age from 17 to 21 years with a mean age of 18.78 (SD = 1.17) and comprised an ethnically diverse sample that included White (61.1%, n = 11), Black (16.7%, n = 3), Asian (5.6%, n = 1), South Asian (5.6%, n = 1), South East Asian (5.6%, n = 1), and other (5.6%, n = 1).
2.2. General procedure
The current study was conducted in two phases. Participants first completed a brief on-line pre-screening questionnaire (Part 1) that determined eligibility for the laboratory session that comprised the TSST and a blood draw (Part 2). The on-line pre-screening questionnaire assessed the presence of a number of exclusion criteria, such as medical conditions or medications that may influence hormone functioning, as well as issues surrounding blood sampling (e.g. a fear of needles, previous history of nausea or fainting during blood collection, or difficulty with veins). Eligible participants were then randomly assigned to one of three conditions: stress-social support (participants were asked to bring a friend to a laboratory session, n = 18), stress-no support (participants arrived alone to the laboratory session, n = 23) or controls (no stress, no friend, n = 26). To avoid a selection bias, all participants who stated they could bring a close females friend were randomly assigned to one of the three conditions, including that in which no friend was present.
2.2.1. Laboratory session
All procedures in this study were approved by the Carleton University Ethics Committee for Psychological Research. Laboratory sessions were conducted between 1300 and 1730 h, and women were asked not to eat, drink (with the exception of water) or smoke for at least an hour before arriving to the session. Once informed consent was signed, participants filled out a number of questionnaires assessing demographic information (including information on oral contraceptive use and menstrual cycle phase), depressive symptomatology and a relationship closeness measure (related to the friend they brought with them to the laboratory session). This allowed participants 30 min to habituate to the laboratory environment. Upon completion of these measures, a registered nurse inserted a catheter into the participants’ non-dominant arm for blood collection. Participants were then asked to relax for 10 min to habituate to the catheter. Following the relaxation period, participants in the stress conditions were instructed that they would be given 10 min to prepare for an employment task comprising a five-minute speech and five-minute mental arithmetic task in front of a panel of graduate student judges. In addition, participants were told they were being videotaped during the psychosocial stressor.
During the 10-min preparatory period, participants in the stress-no support condition prepared for the stressor alone, whereas participants within the stress-social support condition had their friend present. The friends were instructed to provide emotional and/or instrumental support to the participant. Immediately following the 10 min preparatory period, participants in the two stressor conditions underwent the TSST. Participants in the control condition were asked to complete an employment task, which comprised writing down their strengths and past work/volunteer experience on a form. Immediately following the TSST or control task, participants completed questionnaires including focused on stressor appraisal, state anxiety and affect scores. Furthermore, approximately 30 min following this, the general distrust, and empathy scales were completed.
Blood, which was continuously being drawn through a Dakmed ambulatory pump, was sampled at five time-points during the session, which included 15 min before the TSST or the written employment task (controls), immediately before the stressor/tasks began, and then at five, 15 and 30 min following completion of the TSST or the written employment task.
Upon arrival to the experimental session, the friend was led to a nearby room in which they signed an informed consent, and were asked to complete a questionnaire booklet assessing basic demographic information, depressive symptomatology, a closeness of relationship measure, and empathy scores. They were told about the stressful nature of the employment task (their friend would undergo), and told that they would be assisting the participant during the 10 min preparatory period. They were asked to provide as much support as possible to their friend. Once the preparatory period was complete, participants were asked to return to a nearby room to complete the remainder of the questionnaires. They were not present when the participant was being tested (instructions adapted from Heinrichs et al., 2003).
2.3. Measures
2.3.1. Depression
The 21-item Beck Depression Inventory (BDI) was used as a measure of depressive symptomatology (Beck et al., 1961). Each item comprises one of four options, ranging from low to high depressive symptomatology. Total scores were calculated by summing across all items, (Chronbach's α = .88).
2.3.2. Closeness of relationship
Inclusion of other in the self scale (IOSS) (Aron et al., 1992) was used to assess closeness of the friend and the participant. This assessment provides a graphical representation of seven separate Venn-like diagrams, in which participants are asked to choose one, where increasing overlap between two circles represents a greater degree of closeness.
2.3.3. Distrust and cynicism
An 8-item Distrust and Cynicism Scale (derived from the Cook-Medley Hostility Scale) was used to measure general feelings of cynical distrust (Greenglass and Julkunen, 1989, Greenglass and Julkunen, 1991). Each item can be rated from 0 (completely disagree) to 3 (completely agree). A total score was calculated by taking the mean across all items (α = .82). Items such as; ‘It is safer to trust nobody’ can be found in this scale.
2.3.4. Empathy
The 28 item Interpersonal Reactivity Index (Davis, 1983) was used to assess total empathy scores. Each item ranges from 0 (does not describe me well) to 4 (describes me well), with higher scores reflecting greater empathy. Items such as, ‘I am often quite touched by things that I see happen,’ can be found in this scale. Total empathy scores were created using a mean across all items (α = .82).
2.3.5. Stress appraisals
The 28-item Stress Appraisal Measure (SAM) (Peacock and Wong, 1990) comprises multiple subscales representing appraisals of threat, challenge, centrality, stressfulness, controllability and uncontrollability. In the present study, only the four items that represent the subscale for stressfulness were used. These items were measured on a five-point scale ranging from 1 (not at all) to 5 (extremely), with higher scores indicating higher levels of perceived stress. A mean across all items created a total stress appraisal score (α = .95).
2.3.6. Anxiety
State anxiety was assessed using the Spielberger State Trait Anxiety Inventory (STAI) (Spielberger, 1983). The 20-item state anxiety scale was used to measure current feelings of anxiety following the TSST. Items ranged from 1 (not at all) to 4 (very much), where higher scores indicate greater state anxiety. Total scores were obtained by summing across all items (α = .95).
2.3.7. Mood
The 41-item Positive and Negative Affect Schedule (PANAS) (Watson et al., 1988) assessed the presence and severity of positive and negative affective states following the TSST. Responses ranged on a six-point scale from 0 (not at all) to 6 (extremely). Various mood-state subscales were calculated including sadness (α = .91), anger (α = .94), fear (α = .93) and shame (α = .96). Total negative affect scores were calculated by a mean across items (α = .94).
2.3.8. Blood collection
Blood samples were collected continuously, at a low draw rate, (2.08 mL/5 min) into chilled EDTA coated (for plasma) and Serum separator (for serum) vacutainer tubes. Separate chilled EDTA tubes were used for the collection of oxytocin, which contained aprotinin. Samples were taken at an increased draw rate (6.9 mL/5 min) 15 min before the TSST or the employment task (controls), immediately before the stressor/tasks began, and then at five, 15 and 30 min post-task. Approximately 2.76 mL of plasma and serum were collected for each of the time-points of interest. Following collection, samples were centrifuged for 15 min at 4 °C and 2100 g, plasma and serum were immediately aliquoted into Eppendorf tubes and frozen at −80 °C.
2.3.9. Plasma cortisol
Plasma cortisol was determined in duplicate by a radioimmuno assay (RIA) using the 125I kit obtained from ICN Biomedicals Inc., Irvine, CA. The assays were performed according to the manufacturer's instructions. The intra-assay variability was less than 8% and the minimum detectable concentration was .17 μg/dL.
2.3.10. Plasma oxytocin
Prior to the assay, oxytocin was extracted as recommended following the procedure manual from Enzo Life Science Inc., (Farmingdale, NY). For the extraction procedure, 1 mL of plasma was used and samples were evaporated with nitrogen gas, following which samples were stored at −20 °C until the assay. Plasma oxytocin concentrations were determined through an ELISA kit obtained from Enzo Life Science Inc., according to the manufacturer's instructions. The intra-assay variability was less than 12%. Furthermore, according to the manufacturer's kit information, cross reactivity for arginine vasopressin was less than .02% and the minimum detectable was 3.75 pg/mL.
2.3.11. Serum estradiol
Serum estradiol was determined through an ELISA kit obtained from Invitrogen (Camarillo, CA). This assay was performed according to the manufacturer's instructions. The intra-assay variability was less than 5% and the minimum detectable concentration was 5 ± 2pgmL.
Of the 67 participants, there were four individuals from whom blood samples could not be obtained due to complications with small veins. As well, in some instances participants did not have five valid samples for hormone detection (e.g., failure of blood collection equipment, collapsed vein or not enough blood could be obtained), thus they were removed from any repeated measures analyses and as such the n's per group will vary.
3. Statistical analyses
Statistical analyses were performed using SPSS for Windows 18.0 (SPSS Science, Chicago, Illinois, USA). Analyses assessing the influence of the TSST on stress appraisals and state anxiety were performed using one-way analyses of variance (ANOVA) (Stressor Condition: controls, stress-support, stress-no support). A MANOVA was used to determine the effects of the TSST condition on mood outcomes, including sadness, anger, fear, shame and negative affect. Oxytocin was analyzed using a 3 (Stressor Condition) x 5 (Time: 5 time-points) mixed measures ANOVA with Time serving as the within-group factor. For cortisol assessment, an area under the curve (AUC) analysis with respect to increase was performed using the formula proposed by Pruessner et al. (2003) with Stressor Condition and Oral contraceptives (yes vs. no) serving as the between groups factors. Follow-up comparisons comprised t-tests with a Bonferonni correction to maintain the alpha level at .05. Additionally, Pearson's correlation coefficients were determined between cortisol, oxytocin, and distrust levels. Pearson's correlation coefficients were also determined between the friends' psychosocial scores and the participants' psychosocial and biological scores.
4. Results
4.1. Psychosocial responses
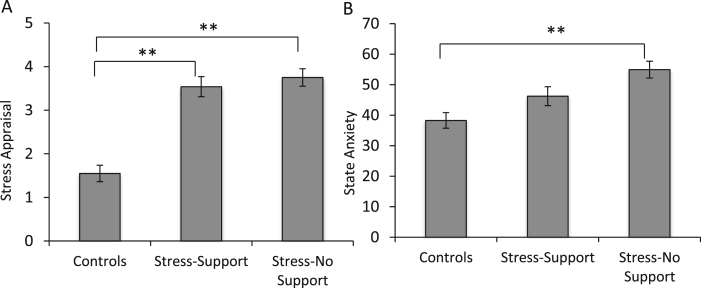
As expected, prior to the TSST, there were no differences between individuals in the control, stress-support, or stress-no support conditions on levels of depressive symptoms, F (2, 64) = .21, p = .81. Following the TSST, stress appraisals varied as a function of the Stressor Condition, F (2, 64) = 37.08, p < .001, η2 = .54. As shown in Fig. 1A, individuals in both the stress-support and the stress-no support conditions reported elevated stressfulness compared to controls, p < .001 and p < .001, respectively. State anxiety also differed with the Stressor Condition, F (2, 64) = 9.94, p < .001, η2 = .24, such that individuals who were stressed but did not receive support exhibited elevated state anxiety compared to controls, p < .001, an effect not apparent among the stressed individuals who received social support p = .15 (Fig. 1B).
Fig. 1.
Stress appraisal scores (A) and State anxiety levels (B) among the controls (n = 26), stress-support (n = 18) and stress-no support (n = 23) following the TSST. Data represents means ± S.E.M. **p < .001.
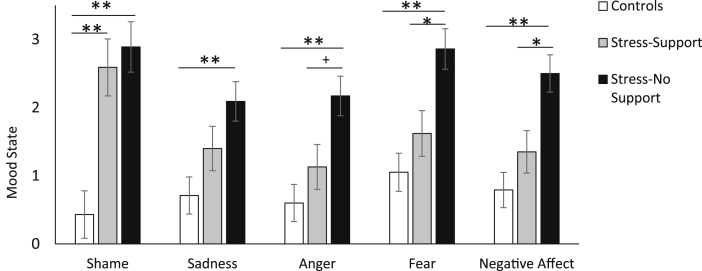
A significant MANOVA revealed differences in affective states following the TSST as a function of the Stressor Condition, F (10, 122) = 4.77, p < .001, η2 = .28. As shown in Fig. 2, there was an overall effect of stress for feelings of shame, F (2, 64) = 13.88, p < .001, η2 = .30, such that higher levels of shame occurred among both the stress-no support and stress-support conditions compared to controls, p < .001 and p = .001 respectively. Levels of sadness also differed between Stressor Conditions, F (2, 64) = 6.10, p = .004, η2 = .16. Specifically, the stressed-no support group displayed elevated sadness compared to controls, p < .001, whereas individuals who were stressed but received support did not differ from controls, p = .32. Feelings of anger differed between Stressor Conditions, F (2, 64) = 7.90, p = .001, η2 = .20, such that individuals in the stress-no support condition reported more anger compared to controls, p = .001, an effect not apparent among individuals in the stress-support condition. Further, as shown in Fig. 2, anger tended to be somewhat higher in the stress-no support compared to the stress-support condition, although this only approached significance, p = .06. Fear and negative affect followed a similar trend, F (2, 64) = 10.21, p < .001, η2 = .24 and F (2, 64) = 10.54, p < .001, η2 = .25 respectively. In these instances, individuals who were stressed and had no support displayed elevated fear and negative affect compared to controls, p's = .001 and their stress-support counterparts, p's = .02.
Fig. 2.
Affective states including shame, sadness, anger, fear and overall negative affect among the controls (n = 26), stress-support (n = 18) and stress-no support (n = 23) conditions following the TSST. Data represents means ± S.E.M. + p = .06, *p < .05, **p ≤ .001.
4.2. Friends' responses
An analysis was conducted to assess the extent to which the participants and the friends they brought to the laboratory shared the closeness they felt toward each other. Unexpectedly, the correlation of perceived closeness between the friend and the participant, indicated that friends' perceptions of closeness and participants' perceptions of closeness with each other were unrelated, r (15) = .02, p = .93. Furthermore, friends' perceptions of closeness were not related to participants' psychosocial responses to the TSST, including state anxiety r (15) = .15, p = .56, stress appraisals, r (15) = .17, p = .50, and any of the participants' biological scores at baseline or in response to the TSST. As well, the participants' perceptions of closeness also did not significantly correlate with their psychosocial or biological stress responses and similarly, the friends' level of empathy did not relate to the participants' state anxiety, r (16) = −.05, p = .86, or stress appraisal, r (16) = −.02, p = .93 scores.
Interestingly, empathy scores of the friend were inversely related to the participants' cortisol levels at both baseline, r (15) = −.60, p = .01, and following the TSST, r (15) = −.50, p = .04. As well, a relationship was found between the friends' level of empathy and the participants' basal oxytocin levels, such that higher empathy from the friend was associated with elevated oxytocin levels at baseline among the participants, r (15) = .56, p = .02. A similar association was not observed in relation to oxytocin scores following the TSST.
4.3. Biological responses
It was first of interest to examine the relationships between baseline oxytocin levels with depression and distrust scores. As shown in Table 1, depressive scores were not significantly associated with oxytocin, however, a significant inverse relationship was found between distrust and oxytocin, r (59) = −.28, p = .03. As well, oxytocin at baseline was inversely related to baseline cortisol levels, r (55) = .28, p = .04. This correlation was not found to be significant when run separately according to Stressor condition.
Table 1.
Zero-order Pearson correlations between depressive symptoms, distrust and baseline oxytocin, cortisol and estradiol levels.
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| 1. Depressive symptoms | – | – | – | – | – |
| 2. Distrust | .55** | – | – | – | – |
| 3. Oxytocin | −.15 | −.28* | – | – | – |
| 4. Cortisol | .16 | .15 | −.28* | – | – |
| 5. Estradiol | .05 | −.05 | .05 | −.24 | – |
**p < .001; *p < .05.
4.3.1. Oxytocin, cortisol and estradiol
Although estrogen is known to influence oxytocin transcription, as shown in Table 1, no relationship was found between levels of oxytocin and estradiol at baseline. Almost half of the females in the current experiment reported using oral contraceptives (n = 30), whereas 36 females were not taking oral contraceptives. As expected, females taking oral contraceptives (M = 40.31, SE = 8.26) displayed reduced serum estradiol levels compared to females not taking oral contraceptives, (M = 73.84, SE = 8.47), t (1, 52) = 2.83, p = .007. Similar to estradiol, oxytocin levels at baseline also did not vary based on whether females were using oral contraceptives or not, F (2, 59) = .35, p = .55. Therefore, for further oxytocin analyses, neither oral contraceptives nor estrogen were controlled for. There were no initial difference between controls (M = 10.54 ± 4.98), the stress-support (M = 10.97 ± 1.11) or stress-no support (M = 12.06 ± .96) groups on baseline levels of oxytocin, F (2, 59) = .65, p = .53. Furthermore, over the course of the TSST or the written employment task (for controls), oxytocin levels did not significantly increase, although there was a trend in this direction, F (4, 180) = 2.14, p = .08, η2 = .05. Additionally, oxytocin levels in response to the TSST did not vary by Stressor Condition, F (8, 180) = .40, p = .92. In addition to this analysis, AUC analyses were also conducted for oxytocin with respect to ground (full area beneath the oxytocin scores, irrespective of whether or not oxytocin rose) and with respect to increase (change of oxytocin levels). In neither instance were differences found between conditions.
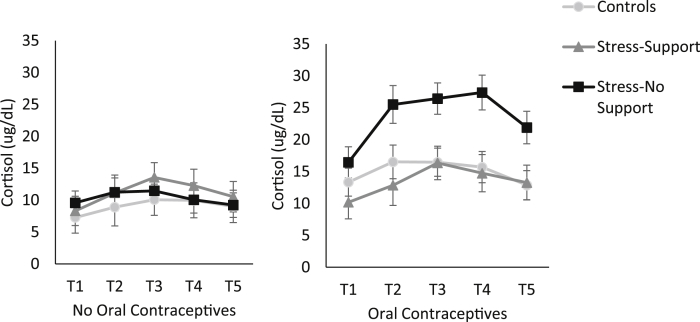
Oral contraceptive users displayed elevated baseline cortisol levels (M = 14.19, SE = 1.61) compared to individuals not taking oral contraceptives (M = 8.62, SE = 1.15), t (1, 55) = −2.88, p = .006. However, cortisol levels did not vary as a function of menstrual phase or smoking. Thus, oral contraceptive use was included as a variable in subsequent analyses relating to cortisol. Individuals not taking oral contraceptives included, (control, n = 8), (stress-support, n = 9) and (stress-no support, n = 14), and individuals who were taking oral contraceptives included, (control, n = 10), (stress-support, n = 7), and (stress-no support, n = 8). An analysis of area under the curve (AUC) indicated that although there was no main effect of Stressor Condition on cortisol, F (2, 50) = 2.22, p = .12, η2 = . 08, there was a main effect of Oral contraceptives, F (2, 64) = 6.50, p = .014, η2 = .12, with a larger AUC being present among individuals who were taking oral contraceptives (mean difference = 154.27). Additionally, cortisol varied as a function of the Stressor Condition × Oral contraceptives interaction, F (2, 50) = 5.44, p = .007, η2 = .18. As shown in Fig. 3, and confirmed by the follow-up tests of the simple effects comprising this interaction, among individuals not using oral contraceptives (left hand panel), cortisol did not vary in relation to the TSST. However, for individuals using oral contraceptives, the stress-no support group displayed a larger cortisol AUC compared to controls (mean difference = 358.86), p = .004. Further, while there were no differences between controls and individuals who were stressed with support available, the AUC tended to be greater in the stress-no support group compared to the stress-support group (mean difference = 266.77), p = .071. Cortisol was also examined through an individual-peak analysis, which accounts for the fact that individuals display peak cortisol levels at different times following a stressor, and the results were identical.
Fig. 3.
Cortisol levels in plasma (μg/dL) collected at five time points including 15 min prior to the TSST (T1), immediately before the TSST began (T2) and five minutes (T3), 15 min (T4) and 30 min (T5) following the TSST. The graph represents individuals who were not taking oral contraceptives (left panel) in the control (n = 8), stress-support (n = 9) and stress-no support (n = 14), conditions as well as the individuals in the control (n = 10), stress-support (n = 7), and stress-no support (n = 8), conditions who were taking oral contraceptives (right panel). Data represents means ± S.E.M.
5. Discussion
In the current study, the TSST increased stressor appraisals irrespective of social support being available, whereas state anxiety scores following the TSST were only elevated among individuals who were stressed and did not receive support. In essence, although participants in both conditions were equally cognizant of the stressor, social support buffered the anxiety associated with the TSST. Similarly, relative to controls, individuals who were stressed and did not receive support displayed high levels of anger, fear, and sadness as well as overall negative affect. In contrast, stressed individuals who received support did not exhibit elevations of these emotions, again pointing to the stress-buffering effects of social support (Cohen and Wills, 1985). Interestingly, despite the effectiveness of social support in acting against some of the negative emotions promoted by the TSST, feelings of shame were not diminished by support. It is not immediately apparent why shame was not attenuated; however, such feelings may be a uniquely powerful emotion (relative to the others measured), being a threat to the ‘social self’, and might thus not be amenable to being appreciably diminished by social support (Gruenewald et al., 2004).
Although the availability of social support was accompanied by reductions of several negative emotions, the independent assessments made by participants and their friends regarding their closeness were not consistent with one another (i.e., participants' perceived closeness to the friend was not necessarily shared). Likewise, the degree of closeness and/or empathy expressed by the friend was not related to anxiety or negative affect elicited by the TSST among participants, and similarly, the participants' perceived closeness to the friend was unrelated to their feelings of anxiety or negative affect. It has been predicted that relationship quality is an important component of support efficacy (Holt-Lunstad et al., 2007). However, in the present investigation social support appeared to buffer against anxiety and negative emotionality irrespective of perceived closeness. In effect, simply because individuals did not share their perceived closeness, this may not have had any bearing on the effectiveness of the support provided. The measure of closeness adopted (Inclusion of other in the self) is one that has been frequently used and is considered to be a valid measure of closeness (Tropp and Wright, 2001).
5.1. Basal oxytocin
Given the presumed relationship between oxytocin and prosocial behaviors, and that oxytocin was also implicated in inferring the mood states of others (Domes et al., 2007), it is interesting that having a friend present with generally high levels of empathy was related to elevated oxytocin levels among participants. Although it is tempting to suggest that the higher empathy of the friend caused an oxytocin release, the correlational nature of the data preclude this conclusion. Empathy has been found to be related to endogenous oxytocin release, particularly among females (Barraza and Zak, 2009), and intranasal oxytocin promotes empathetic behaviors (Domes et al., 2007), but it has yet to be shown that empathy of one individual may be related to oxytocin levels in a close other. This finding, being the first of this nature to be reported, certainly needs to be replicated.
Lower baseline oxytocin levels were related to higher levels of distrust, which is consistent with the reported elevated plasma oxytocin levels associated with trust behaviors in a monetary game (Zak et al., 2005) as well as trust-related interactions involved in sharing secrets (Kéri et al., 2009). Furthermore, salivary oxytocin correlated positively with trust upon arriving to a laboratory testing condition for a second time (Tops et al., 2013). Consistent with these reports, intranasal oxytocin administration increased trust-related behaviors in a monetary game (Kosfeld et al., 2005), and trust with confidential information (Mikolajczak et al., 2010). Unlike the studies showing a relationship between endogenous oxytocin and trust (Zak et al., 2005, Kéri et al., 2009), however, such a relationship has not always been reported (Christensen et al., 2014). It was suggested that these discrepant results may be due to the procedure in the former studies not including the extraction of oxytocin from samples prior to the assay being conducted and hence the presence of oxytocin may have been contaminated by other factors being assayed concurrently (Christensen et al., 2014). In the current study, the relation between oxytocin and basal levels of general distrust was found in samples where the hormone was extracted prior to the assay, although this does not imply that oxytocin scores would be linked to trust or distrust following an experimental manipulation.
Consistent with reports that oxytocin administration reduces cortisol levels (Ditzen et al., 2009, Linnen et al., 2012, Cardoso et al., 2013), baseline oxytocin was inversely related to circulating cortisol. Although oxytocin is thought to contribute to affiliative/social approach behaviors by diminishing fear/anxiety (Taylor, 2006), it is uncertain whether the oxytocin link to cortisol was tied to prosocial behaviors.
5.2. Oxytocin and cortisol in relation to post-stressor responses
Although studies in animals have indicated that oxytocin elevations are elicited by acute stressors (Neumann, 2002, Danevova et al., 2013), such effects are less consistently observed in humans. Cortisol treatment in humans is known to increase oxytocin levels (Tops et al., 2012), and thus it might have been expected that a stressor would elicit a similar outcome. In this regard, it was shown that the TSST elicited a rapid increase in salivary OT (Jong et al., 2015). That said, as oxytocin measurements in the saliva and blood may not be measuring the same thing (McCullough et al., 2013), it is premature to base conclusions that incorporate oxytocin variations elicited by stressor in these two different products. However, as previously observed (Taylor et al., 2006, Ditzen et al., 2007), plasma oxytocin levels were unaffected by the TSST, and this was the case in the current manuscript regardless of circulating estradiol levels or whether women were using oral contraceptives. It is also important to consider that oxytocin is released in a pulsatile fashion and is a dynamic process (Ludwig and Leng, 2006) and thus, it is possible that the time of collection did not capture changes in this hormone. This said, blood for each sample was collected over a two minute period, and samples were taken at three post-stressor intervals, thus making this possibility less likely.
Cortisol profiles among women taking oral contraceptives, as in earlier reports (Kirschbaum et al., 1999), were distinct from non-oral contraceptive users. Compared to females not using oral contraceptives, those taking oral contraceptives in the current study displayed elevated baseline plasma cortisol levels as well as greater plasma cortisol responses to the TSST. Indeed, it was only among women using oral contraceptives that the TSST effectively increased plasma cortisol. Although it has been reported that oral contraceptive users displayed a blunted cortisol response, this only occurred when cortisol was measured in saliva, whereas an appreciable increase (55%) was apparent in blood (Kirschbaum et al., 1999). Thus, the present findings in blood are consistent with those previously reported, and reinforce the distinction between salivary ‘free’ cortisol and total cortisol levels in plasma (which mainly comprises ‘bound’ rather than free cortisol). It was suggested that among oral contraceptive users, estrogen stimulates corticosteroid-binding globulin (CBG) synthesis, resulting in reduced free bioactive cortisol (Kajantie, 2008) and enhanced bound cortisol. This aside, it was surprising that cortisol levels among females not taking oral contraceptives were largely unaffected by the TSST. The high baseline estradiol levels among females in the current study who were not taking oral contraceptives, may have contributed to the lack of cortisol change in response to the TSST. In this regard, it has been observed that women with high estrogen levels (corresponding to the luteal phase of the cycle), display significantly increased free cortisol levels in response to stressors (i.e., within saliva), but blunted blood cortisol stress responses (Kirschbaum et al., 1999). Furthermore, it may be significant that although the TSST is a potent stressor, its effects on cortisol are typically more pronounced among males (Kajantie and Phillips, 2006). This could be related to differences of sex hormones, but it could also be linked to the finding that males tend to display greater responses to achievement-oriented stressors, such as mathematics and verbal tasks, whereas women show a greater reactivity to rejection related stressors (Stroud et al., 2002).
Among oral contraceptive users in the present study, social support attenuated the cortisol rise in response to the TSST that was otherwise evident among the individuals who did not receive support. It is thought that one potential mechanism by which social support may buffer cortisol stress responses is through hypothalamic oxytocin release, as treatment with an oxytocin receptor antagonist blocked the social buffering effects in rodents (Smith and Wang, 2014). Likewise, female children that received some form of support from their mothers displayed elevated oxytocin and attenuated cortisol responses to the TSST (Seltzer et al., 2010). Thus, although it might have been expected in the current investigation that the females receiving social support from a close friend would display elevated oxytocin levels following the stressor, this hormone was unaffected by the social support manipulation. Given that plasma oxytocin might not reflect changes in brain oxytocin, our findings do not necessarily suggest that brain oxytocin levels are unimportant in the buffering effects of support (Landgraf and Neumann, 2004, Leng and Ludwig, 2015). In essence, central oxytocin release could buffer HPA axis responses to stressors, but levels of this hormone in the periphery might not adequately reflect oxytocin changes in brain.
There were several limitations associated with the current investigation. The sample size was admittedly modest, but it is unlikely that increasing the sample size would have had any bearing on the lack of an oxytocin rise in response to the stressor or the absence of a cortisol rise among women not using oral contraceptives as in both instances, the stressor did not elicit even a hint of a hormonal change. We cannot exclude the possibility that, in this instance, the TSST was not stressful enough to activate the HPA axis among all participants. However, as already indicated, the cortisol measure was determined in blood, and it is uncertain how the stressor and use of oral contraceptives might have interacted in affecting free cortisol in saliva. Ideally, cortisol levels should have been determined from both saliva and blood. It may also be the case that the lack of hormonal change was due to the timing of the baseline sample. In this regard, participants' baseline sample was drawn 10 min following needle insertion (40 min after arrival to the laboratory) and therefore, hormone levels may have already increased as a result of the anxiety associated with the needle prick. Ideally, a longer length of time should have transpired between needle insertion and the baseline sample; however, as the continuous blood draw was already 70 min, this was not considered feasible. In this regard it would have also been advantageous to have extended the blood draw in order to capture the cortisol decline and return to baseline levels, however, once more this was unfortunately not feasible. Furthermore, a group was not included in the present study in which support was available in the absence of a stressor manipulation. Thus, it was not possible to examine the influence of social support on oxytocin levels over time in the absence of a stressor. Likewise, it would have been advantageous to include a manipulation in which a neutral party was present (as opposed to a friend) who could have acted as a distractor and thus modified the stress response. In this way it could be determined whether the buffering effects observed with respect to cortisol were linked to a close friend being present prior to the actual TSST or whether simply having another person present could have acted in this capacity, possibly by distracting participants.
These caveats notwithstanding, the current study addressed several important issues concerning endogenous oxytocin functioning in relation to stressful experiences. Only a limited number of studies have included females in research pertaining to stress and oxytocin, particularly those studies in which intranasal oxytocin was administered (owing to complications relating to hormonal fluctuations across the menstrual cycle). Yet, increasing evidence has pointed to differential actions (or correlations) of endogenous and exogenous oxytocin on various behaviors among men and women (Taylor et al., 2010, Hoge et al., 2014), thus suggesting that this hormone may potentially serve different functions in men and women. The present investigation, in women, suggested that basal oxytocin and cortisol levels are related, and oxytocin is linked to attitudes, such as distrust and empathy. A psychosocial stressor in the form of the TSST, did not affect plasma oxytocin levels, but among women using oral contraceptives the stressor increased circulating cortisol. This outcome could be buffered by social support, and once again this occurred despite oxytocin levels in plasma being unaffected by this manipulation. This is not altogether surprising as plasma oxytocin might not be an accurate reflection of oxytocin functioning within stress-relevant brain regions (Landgraf and Neumann, 2004, Leng and Ludwig, 2015). Certainly, it needs to be determined whether circulating oxytocin is an ideal measure to assess the linkage to the stress-attenuating properties of social support. This said, studies in animals have made it fairly clear that central oxytocin release is fundamental to the positive effects of social support on stress responses (Smith and Wang, 2014), but studies in humans have typically relied on plasma oxytocin to provide data relevant to prosocial behaviors. In this regard, efforts have been made to assess the effects of intranasal oxytocin treatment, but it is uncertain how much of the hormone actually reaches the brain. Nonetheless, in humans, this approach is a reasonable option at the moment, particularly when coupled with analyses of polymorphisms related to oxytocin or its receptor.
Role of funding source
This research was supported by the Canadian Institutes of Health Research (CIHR) and the Natural Sciences and Engineering Research Council of Canada (NSERC). The funding sources had no further role in the study or in the writing of this article.
Conflict of interest
All authors report no conflicts of interest in the conduct or in the reporting of this research.
Acknowledgments
H.A. holds a Canadian Research Chair in Neuroscience. R.J.M. is supported by the CIHR Frederick Banting and Charles Best Canada Graduate Scholarship. The assistance of Dr. Barbara Woodside and Jerzy Kulczycki is very much appreciated.
Footnotes
Parenthetically, although not predicted a priori, given that oral contraceptive use affected cortisol responses to the TSST, we also assessed state anxiety under these conditions. This analysis revealed that state anxiety varied as a function of the Oral contraceptive × Stress condition interaction, F (2, 60) = 4.95, p = .01, η2 = .14. The simple effects comprising this interaction revealed that state anxiety scores did not differ among the stressor groups for individuals not taking oral contraceptives. In contrast, among women using oral contraceptives, individuals in the stress-no support condition displayed significantly elevated state anxiety scores compared to controls, p < .001 and the stress-support condition, p = .001.
References
- Aron A., Aron E.N., Smollan D. Inclusion of other in the self scale and the structure of interpersonal closeness. J. Pers. Soc. Psychol. 1992;63(4):596–612. [Google Scholar]
- Barraza J.A., Zak P.J. Empathy toward strangers triggers oxytocin release and subsequent generosity. Ann. N.Y. Acad. Sci. 2009;1167(1):182–189. doi: 10.1111/j.1749-6632.2009.04504.x. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Cardoso C., Kingdon D., Ellenbogen M.A. A meta-analytic review of the impact of intranasal oxytocin administration on cortisol concentrations during laboratory tasks: moderation by method and mental health. Psychoneuroendocrinology. 2014;49:161–170. doi: 10.1016/j.psyneuen.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Cardoso C., Ellenbogen M.A., Orlando M.A., Bacon S.L., Joober R. Intranasal oxytocin attenuates the cortisol response to physical stress: a dose-response study. Psychoneuroendocrinology. 2013;38:399–407. doi: 10.1016/j.psyneuen.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Choleris E., Devidze N., Kavaliers M., Pfaff D.W. Steroidal/neuropeptide interactions in hypothalamus and amygdala related to social anxiety. Prog. Brain Res. 2008;170:291–303. doi: 10.1016/S0079-6123(08)00424-X. [DOI] [PubMed] [Google Scholar]
- Christensen J.C., Shiyanov P.A., Estepp J.R., Schlager J.J. Lack of association between human plasma oxytocin and interpersonal trust in a prisoner's dilemma paradigm. PLoS One. 2014;9(12):e116172. doi: 10.1371/journal.pone.0116172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Wills T.A. Stress, social support, and the buffering hypothesis. Psychol. Bull. 1985;98(2):310. [PubMed] [Google Scholar]
- Cruwys T., Dingle G.A., Haslam C., Haslam A.S., Jetten J., Morton T.A. Social group memberships protect against future depression, alleviate depression symptoms and prevent depression relapse. Soc. Sci. Med. 2013;98:179–186. doi: 10.1016/j.socscimed.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Danevova V., Kvetnansky R., Jezova D. Kinetics of oxytocin response to repeated restraint stress and/or chronic cold exposure. Horm. Metab. Res. 2013:45,845–45,848. doi: 10.1055/s-0033-1348265. [DOI] [PubMed] [Google Scholar]
- Davis M.H. Measuring individual differences in empathy: evidence for a multidimensional approach. J. Pers. Soc. Psychol. 1983;44:113–126. [Google Scholar]
- Ditzen B., Neumann I.D., Bodenmann G., von Dawans B., Turner R.A., Ehlert U., Heinrichs M. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology. 2007;32:565–574. doi: 10.1016/j.psyneuen.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Ditzen B., Schaer M., Gabriel B., Bodenmann G., Ehlert U., Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol. Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Michel A., Berger C., Herpertz S.C. Oxytocin improves “mind-reading” in humans. Biol. Psychiatry. 2007;61(6):731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Donaldson Z.R., Young L.J. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Taylor S.E., Gable S.L., Hilmert C.J., Lieberman M.D. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35(4):1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R., Zagoory-Sharon O., Weisman O., Schneiderman I., Gordon I., Maoz R. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol. Psychiatry. 2012;72(3):175–181. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Glynn L.M., Christenfeld N., Gerin W. Gender, social support, and cardiovascular responses to stress. Psychosom. Med. 1999;61:234–242. doi: 10.1097/00006842-199903000-00016. [DOI] [PubMed] [Google Scholar]
- Greenglass E.R., Julkunen J. Construct validity and sex differences in Cook-Medley hostility. Pers. Individ. Dif. 1989;10:209–218. [Google Scholar]
- Greenglass E.R., Julkunen J. Cook-Medley hostility, anger, and the Type A behaviour pattern in Finland. Psychol. Rep. 1991;68:1059–1066. doi: 10.2466/pr0.1991.68.3c.1059. [DOI] [PubMed] [Google Scholar]
- Gruenewald T.L., Kemeny M.E., Aziz N., Fahey J.L. Acute threat to the social self: shame, social self-esteem, and cortisol activity. Psychosom. Med. 2004;66(6):915–924. doi: 10.1097/01.psy.0000143639.61693.ef. [DOI] [PubMed] [Google Scholar]
- Heinrichs M., Baumgartner T., Kirschbaum C., Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hoge E.A., Anderson E., Lawson E.A., Bui E., Fischer L.E., Khadge S.D. Gender moderates the effect of oxytocin on social judgments. Hum. Psychopharm. Clin. 2014;29(3):299–304. doi: 10.1002/hup.2402. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J., Birmingham W., Jones B.Q. Is there something unique about marriage? the relative impact of marital status, relationship quality, and network social support on ambulatory blood pressure and mental health. Ann. Behav. Med. 2008;35(2):239–244. doi: 10.1007/s12160-008-9018-y. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J., Uchino B.N., Smith T.W., Hicks A. On the importance of relationship quality: the impact of ambivalence in friendships on cardiovascular functioning. Ann. Behav. Med. 2007;33(3):278–290. doi: 10.1007/BF02879910. [DOI] [PubMed] [Google Scholar]
- Jong T.R., Menon R., Bludau A., Grund T., Biermeier V., Klampfl S.M. Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: the Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology. 2015;62:381–388. doi: 10.1016/j.psyneuen.2015.08.027. [DOI] [PubMed] [Google Scholar]
- Kajantie E. Physiological stress response, estrogen, and the male–female mortality gap. Curr. Dir. Psychol. Sci. 2008;17(5):348–352. [Google Scholar]
- Kajantie E., Phillips D.I. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31(2):151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kéri S., Kiss I., Kelemen O. Sharing secrets: oxytocin and trust in schizophrenia. Soc. Neurosci. 2009;4(4):287–293. doi: 10.1080/17470910802319710. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Kudielka B.M., Gaab J., Schommer N.C., Hellhammer D.H. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kosfeld M., Heinrichs M., Zak P.J., Fischbacher U., Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Landgraf R., Neumann I.D. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004;25(3):150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Leng G., Ludwig M. Intranasal oxytocin: myths and delusions. Biol. Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.05.003. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Ludwig M., Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Linnen A.-M., Ellenbogen M.A., Cardoso C., Joober R. Intranasal oxytocin and salivary cortisol concentrations during social rejection in university students. Stress. 2012;15:393–402. doi: 10.3109/10253890.2011.631154. [DOI] [PubMed] [Google Scholar]
- McCullough M.E., Churchland P.S., Mendez A.J. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev. 2013;37:1285–1492. doi: 10.1016/j.neubiorev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- McQuaid R.J., McInnis O.A., Abizaid A., Anisman H. Making room for oxytocin in understanding depression. Neurosci. Biobehav. Rev. 2014;45:305–322. doi: 10.1016/j.neubiorev.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Mikolajczak M., Pinon N., Lane A., de Timary P., Luminet O. Oxytocin not only increases trust when money is at stake, but also when confidential information is in the balance. Biol. Psychol. 2010;85(1):182–184. doi: 10.1016/j.biopsycho.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Neumann I.D. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog. Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- Peacock E.J., Wong P.T. The Stress Appraisal Measure (SAM): a multidimensional approach to cognitive appraisal. Stress Med. 1990;6:227–236. [Google Scholar]
- Pierrehumbert B., Torrisi R., Ansermet F., Borghini A., Halfon O. Adult attachment representations predict cortisol and oxytocin responses to stress. Attach. Hum. Dev. 2012;14:453–576. doi: 10.1080/14616734.2012.706394. [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Kirschbaum C., Meinlschmid G., Hellhammer D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Riem M.M., Bakermans-Kranenburg M.J., Huffmeijer R., van IJzendoorn M.H. Does intranasal oxytocin promote prosocial behavior to an excluded fellow player? A randomized-controlled trial with cyberball. Psychoneuroendocrinology. 2013;38(8):1418–1425. doi: 10.1016/j.psyneuen.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Seltzer L.J., Ziegler T.E., Pollak S.D. Social vocalizations can release oxytocin in humans. Proc. Biol. Sci. 2010;277:2661–2666. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the State-trait Anxiety Inventory (Form Y) [Google Scholar]
- Smith A.S., Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psychiatry. 2014;76(4):281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud L.R., Salovey P., Epel E.S. Sex differences in stress responses: social rejection versus achievement stress. Biol. Psychiatry. 2002;52(4):318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Szeto A., McCabe P.M., Nation D.A., Tabak B.A., Rossetti M.A., McCullough M.E. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom. Med. 2011;73(5):393. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.E. Tend and befriend biobehavioral bases of affiliation under stress. Curr. Dir. Psychol. Sci. 2006;15:273–277. [Google Scholar]
- Taylor S.E., Gonzaga G.C., Klein L.C., Hu P., Greendale G.A., Seeman T.E. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom. Med. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Taylor S.E., Saphire-Bernstein S., Seeman T.E. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychol. Sci. 2010;21:3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Tropp L.R., Wright S.C. Ingroup identification as the inclusion of ingroup in the self. Pers. Soc. Psychol. Bull. 2001;27:585–600. [Google Scholar]
- Tops M., Buisman-Pijlman F.T., Boksem M.A., Wijers A.A., Korf J. Cortisol-induced increases of plasma oxytocin levels predict decreased immediate free recall of unpleasant words. Front. Psychiatry. 2012;3:43. doi: 10.3389/fpsyt.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tops M., Huffmeijer R., Linting M., Grewen K.M., Light K.C., Koole S.L. The role of oxytocin in familiarization-habituation responses to social novelty. Front. Psychol. 2013;4:761. doi: 10.3389/fpsyg.2013.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino B.N. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J. Behav. Med. 2006;29(4):377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Per. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Zak P.J., Kurzban R., Matzner W.T. Oxytocin is associated with human trustworthiness. Horm. Behav. 2005;48(5):522–527. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Zak P.J., Stanton A.A., Ahmadi S. Oxytocin increases generosity in humans. PLoS One. 2007;2(11):e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]