Abstract
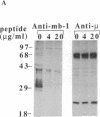
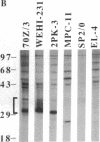
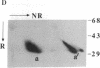
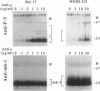
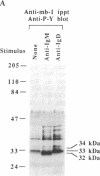
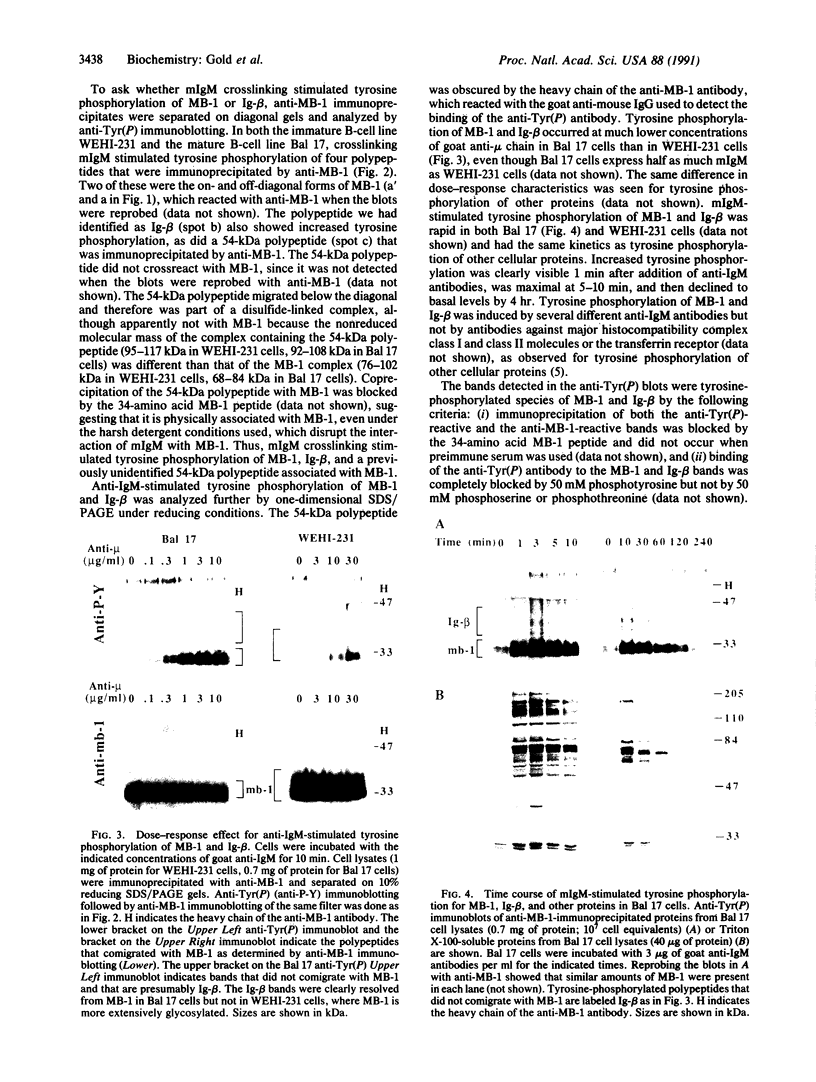
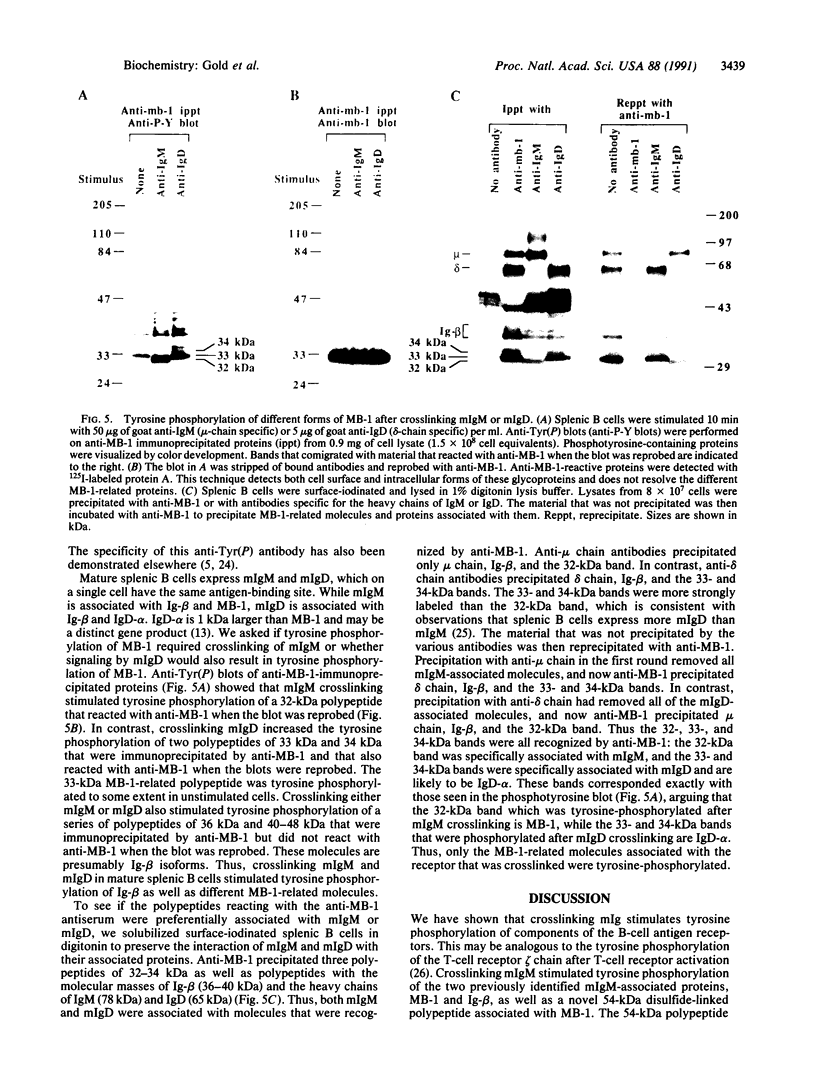
Crosslinking membrane immunoglobulin (mIg), the B-cell antigen receptor, stimulates tyrosine phosphorylation of a number of proteins. Since many receptors are phosphorylated after ligand binding, we asked if components of the mIg receptor complexes were tyrosine-phosphorylated after mIg crosslinking. Both mIgM and mIgD are noncovalently associated with at least two other proteins. mIgM is associated with the MB-1 protein, which is disulfide-linked to a protein designated Ig-beta. mIgD is not associated with MB-1 but is with IgD-alpha, which is also disulfide-linked to Ig-beta. Using immunoprecipitation with a specific anti-MB-1 antiserum followed by anti-phosphotyrosine immunoblotting, we found that crosslinking mIgM stimulated tyrosine phosphorylation of MB-1, Ig-beta, and a previously unidentified 54-kDa polypeptide associated with MB-1. In mature splenic B cells that express both mIgM and mIgD, mIgM crosslinking stimulated tyrosine phosphorylation of the 32-kDa MB-1 protein, whereas mIgD crosslinking stimulated tyrosine phosphorylation of MB-1-related proteins of 33 and 34 kDa. The 32-kDa MB-1 protein was only associated with mIgM, whereas the 33- and 34-kDa MB-1-related proteins were specifically associated with mIgD and are most likely IgD-alpha. Thus, crosslinking either mIgM or mIgD stimulated tyrosine phosphorylation only of the MB-1-related proteins associated with that receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baniyash M., Garcia-Morales P., Luong E., Samelson L. E., Klausner R. D. The T cell antigen receptor zeta chain is tyrosine phosphorylated upon activation. J Biol Chem. 1988 Dec 5;263(34):18225–18230. [PubMed] [Google Scholar]
- Bijsterbosch M. K., Meade C. J., Turner G. A., Klaus G. G. B lymphocyte receptors and polyphosphoinositide degradation. Cell. 1985 Jul;41(3):999–1006. doi: 10.1016/s0092-8674(85)80080-5. [DOI] [PubMed] [Google Scholar]
- Burgess T. L., Craik C. S., Kelly R. B. The exocrine protein trypsinogen is targeted into the secretory granules of an endocrine cell line: studies by gene transfer. J Cell Biol. 1985 Aug;101(2):639–645. doi: 10.1083/jcb.101.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier J., Chen Z. Z., Pasternak J., Ransom J., Sandoval V., Pickles H. Ligand-induced desensitization of B-cell membrane immunoglobulin-mediated Ca2+ mobilization and protein kinase C translocation. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6493–6497. doi: 10.1073/pnas.85.17.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. S., Cambier J. C. B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide linked, inducibly phosphorylated glycoprotein complex. EMBO J. 1990 Feb;9(2):441–448. doi: 10.1002/j.1460-2075.1990.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Sefton B. M. Protein tyrosine phosphorylation is induced in murine B lymphocytes in response to stimulation with anti-immunoglobulin. EMBO J. 1990 Jul;9(7):2125–2131. doi: 10.1002/j.1460-2075.1990.tb07381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Z., Stall A. M., Herzenberg L. A., Herzenberg L. A. Differences in glycoprotein complexes associated with IgM and IgD on normal murine B cells potentially enable transduction of different signals. EMBO J. 1990 Jul;9(7):2117–2124. doi: 10.1002/j.1460-2075.1990.tb07380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall K. M., Cambier J. C. B cell activation. VIII. Membrane immunoglobulins transduce signals via activation of phosphatidylinositol hydrolysis. J Immunol. 1984 Dec;133(6):3382–3386. [PubMed] [Google Scholar]
- DeFranco A. L. Molecular aspects of B-lymphocyte activation. Annu Rev Cell Biol. 1987;3:143–178. doi: 10.1146/annurev.cb.03.110187.001043. [DOI] [PubMed] [Google Scholar]
- Fahey K. A., DeFranco A. L. Cross-linking membrane IgM induces production of inositol trisphosphate and inositol tetrakisphosphate in WEHI-231 B lymphoma cells. J Immunol. 1987 Jun 1;138(11):3935–3942. [PubMed] [Google Scholar]
- Gold M. R., Jakway J. P., DeFranco A. L. Involvement of a guanine-nucleotide-binding component in membrane IgM-stimulated phosphoinositide breakdown. J Immunol. 1987 Dec 1;139(11):3604–3613. [PubMed] [Google Scholar]
- Gold M. R., Law D. A., DeFranco A. L. Stimulation of protein tyrosine phosphorylation by the B-lymphocyte antigen receptor. Nature. 1990 Jun 28;345(6278):810–813. doi: 10.1038/345810a0. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Adelstein S., Lavoie T. B., Smith-Gill S. J., Brink R. A., Pritchard-Briscoe H., Wotherspoon J. S., Loblay R. H., Raphael K. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988 Aug 25;334(6184):676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Harnett M. M., Klaus G. G. G protein coupling of antigen receptor-stimulated polyphosphoinositide hydrolysis in B cells. J Immunol. 1988 May 1;140(9):3135–3139. [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Havran W. L., DiGiusto D. L., Cambier J. C. mIgM:mIgD ratios on B cells: mean mIgD expression exceeds mIgM by 10-fold on most splenic B cells. J Immunol. 1984 Apr;132(4):1712–1716. [PubMed] [Google Scholar]
- Hombach J., Tsubata T., Leclercq L., Stappert H., Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990 Feb 22;343(6260):760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Morrison D. K., Wong G., McCormick F., Williams L. T. PDGF beta-receptor stimulates tyrosine phosphorylation of GAP and association of GAP with a signaling complex. Cell. 1990 Apr 6;61(1):125–133. doi: 10.1016/0092-8674(90)90220-9. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Kanellopoulos-Langevin C., Merwin R. M., Sachs D. H., Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979 Feb;122(2):549–554. [PubMed] [Google Scholar]
- Morrison D. K., Browning P. J., White M. F., Roberts T. M. Tyrosine phosphorylations in vivo associated with v-fms transformation. Mol Cell Biol. 1988 Jan;8(1):176–185. doi: 10.1128/mcb.8.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Nemazee D. A., Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989 Feb 9;337(6207):562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. Cellular mechanisms of immunologic tolerance. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- Oettgen H. C., Pettey C. L., Maloy W. L., Terhorst C. A T3-like protein complex associated with the antigen receptor on murine T cells. Nature. 1986 Mar 20;320(6059):272–275. doi: 10.1038/320272a0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N., Kashiwamura S., Kimoto M., Thalmann P., Melchers F. B lymphocyte lineage-restricted expression of mb-1, a gene with CD3-like structural properties. EMBO J. 1988 Nov;7(11):3457–3464. doi: 10.1002/j.1460-2075.1988.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987 Mar 27;48(6):913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Strasser R. H., Benovic J. L., Daniel K., Lefkowitz R. J. Phosphorylation/dephosphorylation of the beta-adrenergic receptor regulates its functional coupling to adenylate cyclase and subcellular distribution. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9408–9412. doi: 10.1073/pnas.83.24.9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. A., Lau A. L., Cunningham D. D. Selective radiolabeling of cell surface proteins to a high specific activity. Biochemistry. 1987 Feb 10;26(3):743–750. doi: 10.1021/bi00377a014. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Warner N. L., Daley M. J., Richey J., Spellman C. Flow cytometry analysis of murine B cell lymphoma differentiation. Immunol Rev. 1979;48:197–243. doi: 10.1111/j.1600-065x.1979.tb00304.x. [DOI] [PubMed] [Google Scholar]
- Wienands J., Hombach J., Radbruch A., Riesterer C., Reth M. Molecular components of the B cell antigen receptor complex of class IgD differ partly from those of IgM. EMBO J. 1990 Feb;9(2):449–455. doi: 10.1002/j.1460-2075.1990.tb08130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]