Abstract
Repeated bouts of a major stressor such as social defeat are well known to induce a depression phenotype in male rats. Despite strong evidence and acknowledgement that women have a two-fold lifetime greater risk of developing major depression compared to men, the inclusion of female rats in studies employing social defeat are very rare; their absence is attributed to less aggressive interactions. This study sought to compare in male and female rats the impact of repeated social defeat, three times per week for four weeks, on the development of changes in sleep architecture and continuity, sucrose preference as a measure of anhedonia, changes in body weight, and basal plasma corticosterone levels. We found significant reductions in rapid eye movement sleep (REMS) during the light phase in both females and males, and significant increases in numbers of vigilance state transitions during the early dark phase in females but not in males. Additionally, females exhibited significantly greater reductions in sucrose intake than males. On the other hand, no sex differences in significantly elevated basal corticosterone levels were evident, and only the males exhibited changes in body weight. Taken together these findings suggest that the inclusion of female rats in studies of social defeat may offer greater insights in studies of stress and depression.
1. Introduction
“The most common stressors in humans are of a psychological or social nature” (Hollis et al., 2011), and severe life events often precede onset of depression (Bangasser and Valentino, 2014, Hollis and Kabbaj, 2014, Stroud et al., 2008). Vulnerability to depression may be increased in situations reflective of loss of power or social standing as well as from an experience of defeat (Carvalho et al., 2013). Although stress in rats can in no way fully depict the human biobehavioral phenomenon of depression, social defeat has been useful for studying individual stress responsiveness and is cautiously acknowledged as a valid model for human depression (Chaouloff, 2013, Hollis and Kabbaj, 2014, Koolhaas et al., 2013). Biobehavioral commonalities between melancholic or endogenous depression in humans and social defeat in rats include anhedonia, operationalized as a reduced preference for sucrose water; alterations in sleep architecture or continuity; changes in weight, and hypothalamic-pituitary-adrenal (HPA) axis dysregulation (American Psychiatric Association, 2013, Becker et al., 2008, Kamphuis et al., 2015, Patki et al., 2013, Razzoli et al., 2007, Rygula et al., 2006, Stetler and Miller, 2011). The administration of an antidepressant has been shown to significantly reduce the social defeat-induced anhedonia (Becker et al., 2008, Rygula et al., 2006) and HPA axis changes (Becker et al., 2008). More recent studies have shown social defeat to result in a negative cognitive processing bias, pessimism (Papciak et al., 2013) and greater sensitivity to pain resulting from hind paw formalin injection (Rivat et al., 2010).
A major limitation of the rodent social defeat literature is our lack of knowledge of sex differences in responses to this stressor given the vast majority of studies are undertaken in male rats because females exhibit a reduced level of aggression (Chaouloff, 2013, Hollis and Kabbaj, 2014). Yet women have a two-fold lifetime greater risk of developing major depression compared to men (Altemus et al., 2014, Ferrari et al., 2013, Kessler and Bromet, 2013); thus, the omission of social defeat studies in female rats is a scientific gap. Furthermore, a limited number of papers report the impact of social defeat on sleep architecture or continuity; no studies including females were evident. Poor sleep may be antecedent to depression or co-morbid with depression (Gold et al., 2015, Palagini et al., 2013, Pillai et al., 2011). This study sought to determine whether the consequences of social defeat observed in male rats are also evident in females, particularly with regard to changes in sleep architecture and continuity, anhedonia, changes in body weight and basal levels of plasma corticosterone.
2. Materials and methods
2.1. Experimental design
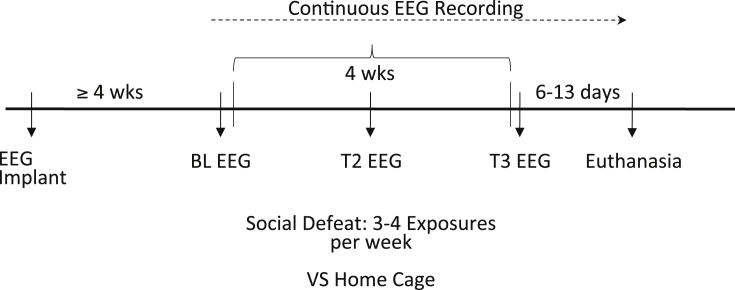
A 2 × 2 factorial design was employed to accomplish the objectives of this study: male versus female and intruder versus home cage control. Fig. 1 depicts the procedural time course for this study.
Fig. 1.
Time course of study procedures.
2.2. Animals
Fischer 344 rats from Harlan Laboratories (Indianapolis, IN) were purchased to maintain a breeding colony for the F344 rats used as intruders in this study. Cohorts of age-matched mature offspring were entered into study at 4–12 months at the time of the first social defeat encounter; age was included as a covariate in the analyses. Three cohorts were specifically bred for random distribution into the four groups comprising this 2 × 2 design. Nine additional cohorts were accrued from the unperturbed control group of a larger study seeking to learn whether early pain affects behavioral responses to social defeat and pain at maturity. The timing of perturbations was consistent across studies. Table 1 includes the numbers of animals employed for each outcome included in this report.
Table 1.
Numbers of animals included in each outcome.
| Outcome | Males |
Females |
||
|---|---|---|---|---|
| Intruder | Home | Intruder | Home | |
| Sleep – EEG instrumented | 8 | 4 | 7 | 8 |
| Sucrose preference | 10 | 10 | 11 | 12 |
| Body weight | 15 | 12 | 16 | 17 |
| Corticosterone | 8 | 5 | 6 | 6 |
Note: vertical columns are not additive as any single animal contributes data to more than one outcome.
Animals had free access to food and water except only water was available for the 4 h before surgery. The vivarium was maintained at 22 ± 1 °C on a 12/12 light–dark cycle such that all testing, surgery and manipulations were accomplished during the dark phase. Long Evans Outbred rats were purchased from Harlan Laboratories to be used as residents for the social defeat paradigm. All animals were weighed weekly to monitor general health. All experimental protocols were approved by the Johns Hopkins University Institutional Animal and Care and Use Committee.
2.3. Surgery
Implantation of telemetric transmitters for electroencephalogram (EEG) and electromyogram (EMG) recording was accomplished under isoflurane anesthesia. After shave and betadine preparation, animals were injected with 50 mg/kg ampicillin subcutaneously. A small incision through the skin and abdominal musculature on the right side just below the thorax was made and the transmitter unit inserted (Model TL11M2-F40-EET, Data Sciences International, St. Paul, MN). After immobilization in a stereotaxic frame, an incision was made through the scalp and neck and the skull was cleared of tissue using 2% hydrogen peroxide and dehydrated using 70% ethyl alcohol. The tunneled electrodes were attached to steel screws threaded into the skull at coordinates 2.0 mm lateral to the central suture and 2.0 mm anterior to both lambda and bregma, and stabilized with dental acrylic. EMG wires were inserted and anchored at the dorsal nuchal muscle. The abdominal skin and muscle layers were sutured with 5-0 monofilament wire and the scalp and neck incision with 3-0 Braunamid polyamide thread. Before the discontinuation of isoflurane, animals were injected subcutaneously with 1.5 mg/kg meloxicam and 10 mg/kg morphine in a slow release suspension (SRS) in separate injections, and 2% lidocaine ointment was applied to both suture lines. Animals recovered for 4 weeks before entering into the protocol. The SRS is comprised of mannide monooleate (Arlacel A, Sigma, St. Louis, MO), light mineral oil and normal saline (6.7%, 40% and 53.3% by volume, respectively) (Page et al., 1993).
Each female Long Evans resident for the social defeat paradigm underwent surgery to separate the ovary from the uterine horn bilaterally, rendering them sterile but preserving normal hormonal cycling. Under isoflurane anesthesia, the abdomen was shaved and prepared with betadine, and ampicillin, 50 mg/kg was administered subcutaneously. A 2 cm midline incision was made through the skin and muscle layer, and both uterine horns were ligated with 3-0 Braunamid polyamide thread just below the ovary and the separated ovary remained in the abdomen. The abdomen was irrigated and the skin and muscle layers sutured with 5-0 monofilament wire. Before discontinuing the isoflurane, animals were injected subcutaneously with 1.5 mg/kg meloxicam and 10 mg/kg morphine SRS in separate injections. Sutures were removed 10–12 days later, after which each female was introduced into the cage of a single male Long Evans retired breeder for a minimum of 4 weeks before being used in the social defeat paradigm.
2.4. Vigilance state determination
Biometric signals were recorded in freely moving rats using the ICELUS acquisition system (M.R. Opp) from baseline before the social defeat paradigm was initiated through euthanasia. Wakefulness (WAKE), non-REM sleep (NREMS) and REM sleep (REMS) were scored in 12-s epochs using the ICELUS rodent sleep scoring software developed by M.R. Opp. WAKE was determined by low amplitude mixed frequency EEG accompanied by high EMG activity. NREMS was characterized by high absolute EEG amplitude (≥100 μV), slow frequency EEG (0.5–4.5 Hz), and low EMG activity. REMS was determined by low amplitude EEG (6–9 Hz) and very little to no EMG activity. Epochs with movement artifacts or electrical noise were excluded from analysis of slow wave activity, and epochs with more than one vigilance state were assigned to the state occupying 50% or more of the epoch.
Sleep architecture was analyzed over a 24 h period at three times: (1) 24 h before the first social defeat bout, baseline (BL); (2) immediately following a social defeat encounter at the mid-point of the series, Time 2 (T2); and (3) immediately following the final social defeat encounter, Time 3 (T3). Investigators processing data were blinded to animal group/sex at the time of sleep analysis.
2.5. Social defeat paradigm
Male and female F344 rats were intruders in the social defeat paradigm. Each Long Evans resident pair was comprised of a retired breeder male and younger sterile female having been together for 4 weeks or more to enhance male territoriality. Social defeat encounters were undertaken during the dark phase and in a room illuminated by red light. For all encounters, the resident cage lid was removed, and the walls were increased to 100 cm using a wire mesh enclosure. For each male social defeat session, the male F344 intruder was introduced into the resident cage containing the Long Evans male and female residents. For each female social defeat session, the female F344 intruder was introduced into the resident cage immediately following removal of the male Long Evans resident. Animals were observed carefully and when males were attacked 5 times or defeated, evidenced by being pinned on its back under the resident for 5 s (Grant and Mackintosh, 1963, Koolhaas et al., 2013), they were covered with a protective mesh enclosure in the resident cage throughout the remainder of the 1 h encounter. Upon being attacked 10 times or pinned for 5 s, females were similarly protected in a mesh enclosure. We observed female attacks to be less aggressive compared to males, reducing the need for the protection of the mesh enclosure. Social defeat encounters were chronic: 3–4 times per week for 4 weeks totaling an average of 14 encounters (range 12–16). F344 intruder rats did not face the same Long Evans resident(s) for more than two encounters during this period. The number of encounters in which an F344 animal was attacked or pinned was recorded.
2.6. Sucrose preference
One week before the first social defeat session, each animal received two bottles, both with 0.5% sucrose solution. After 48 h exposure to sucrose in both bottles, one bottle was switched to plain water and baseline sucrose preference was established over the remaining 5 days, 24 h per day. For the remainder of the protocol, Bottles were weighed and fresh sucrose solution and water were provided 3 times per week, and to control for placement preference, the positioning of the bottles was rotated with each change. Sucrose preference was calculated as a percentage of total liquid intake and analyzed via 1-week intervals.
2.7. Corticosterone
Blood was taken at the time of euthanasia in the resting animal at 6 h after lights off via cardiac puncture under rapid isoflurane anesthesia within 180 s of contact, from cage retrieval to blood withdrawal. Following heparinization (20 units/mL) and centrifugation, plasma was aliquoted and frozen at −80 °C for batch assay. Corticosterone levels were measured in duplicate using enzyme immunoassay (EIA) procedures per the manufacturer's protocol (Enzo Life Sciences Plymouth Meeting, PA, USA). Only animals from the three cohorts specifically bred for random distribution into the 2 × 2 design were used for measuring corticosterone levels. Euthanasia was completed between 6 and 13 days following the final social defeat encounter.
2.8. Data analysis
Change in vigilance states over three time points (baseline, social defeat series midpoint, and endpoint), were evaluated using Repeated Measures Analysis of Variance (RM-ANOVA), reflecting the within animal comparisons necessary to address individual variability in sleep architecture noted by others (Ahmed et al., 2011). After RM-ANOVA was established as statistically significant, post-hoc comparisons were made using paired t-tests with Bonferroni correction, α ≤ 0.017. Repeated Measures Analyses of Covariance (RM-ANCOVA, controlling for age) was used to test for possible intruder or sex differences in sucrose preference and body weight over the four-week course of the social defeat paradigm. To meet the assumption of normality of the dependent variables required for RM-ANCOVA, the weekly percentage sucrose consumed and body weights were Log 10 transformed prior to analysis. Similarly, possible intruder or sex differences in plasma corticosterone were tested using ANCOVA, controlling for age and time from final intruder bout to euthanasia; again, outcomes were Log 10 transformed to meet the assumption of normality.
3. Results
3.1. Sleep
Social defeat resulted in two notable changes in sleep: the number of vigilance state transitions was altered in female intruder rats during the early dark phase, and REMS was suppressed in male and female intruder rats, largely during the light phase.
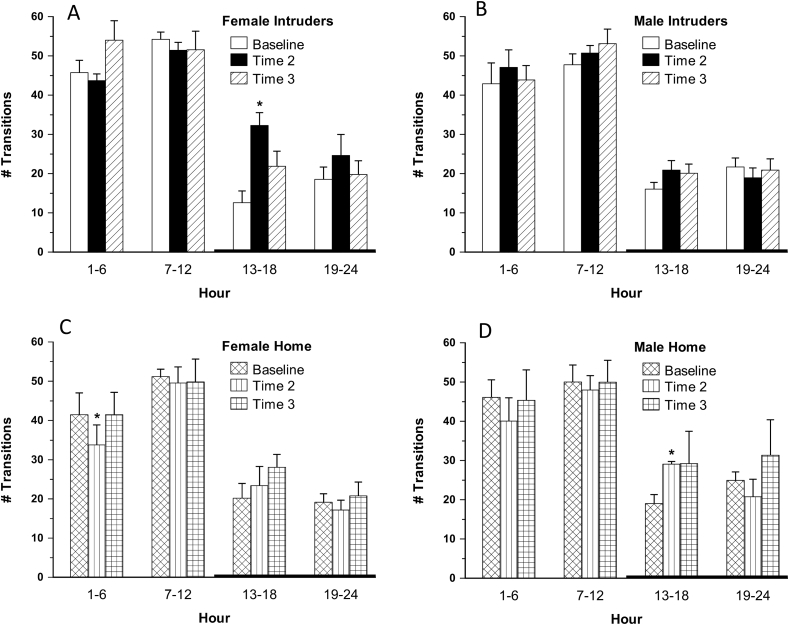
Female intruders exhibited a 2.5-fold increase in the number of vigilance state transitions at T2 during the early dark phase, hours 13–18, [F(1,6) = 19.546, p < 0.05]; male intruders did not exhibit this pattern (Fig. 2). Significant increases in NREM bouts (2-fold) and WAKE bouts (2.1-fold) were also evident during early dark phase hours 13–18 at T2 in the females [Table 2, F(1,6) = 7.064 and 7.879, NREM and WAKE bouts, respectively, p < 0.05], perhaps contributing to the observed increase in vigilance state transitions. Given that WAKE accounts for 80–90% of sleep during the early dark phase, that there was no significant intruder associated impact on WAKE% is not surprising. On the other hand, early dark phase findings of a significant decrease in WAKE duration seems consistent with consequences of sleep fragmentation (Table 2) (see Table 3).
Fig. 2.
A–D: Number of arousal state transitions in female intruders (A), male intruders (B), female home (C), and male home (D) animals at baseline (BL); immediately following the midpoint social defeat encounter (T2); and immediately following the final social defeat encounter (T3). Error bars are ± SEM; *versus BL: F(1,6) = 19.546, F(1,7) = 14.959, and F(1,3) = 42.768, p < 0.01, respectively for female intruder, and female and male home; n = 7, 8, 8, and 4, A-D respectively.
Table 2.
Sleep architecture parameters from male and female intruder animals at baseline (BL), immediately following a social defeat encounter at the mid-point of the series (T2), and immediately following the final social defeat encounter (T3), mean ± SEM.
| Time | Hours | Number of bouts per hour |
Bout duration (min) |
||||
|---|---|---|---|---|---|---|---|
| NREM | REMS | WAKE | NREM | REMS | WAKE | ||
| Female intruder | |||||||
| BL | 1–6 Light | 7.9 ± 0.5 | 1.8 ± 0.5 | 6.5 ± 0.3 | 3.6 ± 0.2 | 0.8 ± 0.2 | 6.8 ± 1.5 |
| 7–12 Light | 6.2 ± 0.7 | 1.6 ± 0.5 | 5.9 ± 0.6 | 1.6 ± 0.2 | 0.7 ± 0.1 | 13.9 ± 2.0 | |
| 13–18 Dark | 2.4 ± 0.7 | 0.1 ± 0.1 | 2.0 ± 0.5 | 0.9 ± 0.2 | 0.2 ± 0.1 | 35.4 ± 4.7 | |
| 19–24 Dark | 3.4 ± 0.8 | 0.6 ± 0.3 | 2.8 ± 0.5 | 1.6 ± 0.2 | 0.3 ± 0.1 | 30.6 ± 4.9 | |
| T2 | 1–6 Light | 8.2 ± 0.5 | 1.0 ± 0.4a | 6.5 ± 0.4 | 3.5 ± 0.4 | 0.6 ± 0.2 | 11.5 ± 5.8 |
| 7–12 Light | 7.5 ± 0.6 | 0.8 ± 0.3 | 6.4 ± 0.5 | 1.9 ± 0.2 | 0.5 ± 0.2 | 15.3 ± 6.9 | |
| 13–18 Dark | 4.9 ± 0.7 | 0.5 ± 0.2 | 4.3 ± 0.6 | 1.8 ± 0.2 | 0.3 ± 0.1 | 16.0 ± 2.4a | |
| 19–24 Dark | 3.9 ± 0.2 | 0.3 ± 0.1 | 3.2 ± 0.2 | 1.5 ± 0.1 | 0.2 ± 0.1 | 24.3 ± 2.5 | |
| T3 | 1–6 Light | 8.5 ± 0.5 | 1.4 ± 0.4 | 6.8 ± 0.4 | 3.2 ± 0.4 | 0.8 ± 0.3 | 4.8 ± 0.9 |
| 7–12 Light | 6.4 ± 1.1 | 0.9 ± 0.3a | 5.8 ± 0.8 | 1.5 ± 0.2 | 0.7 ± 0.2 | 12.4 ± 3.9 | |
| 13–18 Dark | 4.1 ± 0.9 | 0.8 ± 0.8 | 3.5 ± 0.7 | 1.8 ± 0.4 | 0.4 ± 0.2 | 23.2 ± 6.1 | |
| 19–24 Dark | 3.3 ± 0.5 | 0.5 ± 0.2 | 2.8 ± 0.5 | 1.5 ± 0.2 | 0.3 ± 0.1 | 27.7 ± 3.2 | |
| Male Intruder | |||||||
| BL | 1–6 Light | 7.9 ± 0.6 | 1.9 ± 0.5 | 6.4 ± 0.5 | 2.9 ± 0.3 | 1.2 ± 0.2 | 7.8 ± 2.2 |
| 7–12 Light | 8.0 ± 0.8 | 1.6 ± 0.4 | 6.9 ± 0.6 | 1.8 ± 0.2 | 1.1 ± 0.3 | 8.7 ± 1.8 | |
| 13–18 Dark | 2.9 ± 0.3 | 0.6 ± 0.1 | 2.6 ± 0.2 | 1.4 ± 0.2 | 0.4 ± 0.1 | 30.9 ± 3.2 | |
| 19–24 Dark | 4.5 ± 0.4 | 1.2 ± 0.3 | 4.0 ± 0.4 | 2.3 ± 0.2 | 0.8 ± 0.1 | 21.6 ± 3.1 | |
| T2 | 1–6 Light | 8.7 ± 0.5 | 0.7 ± 0.2a | 6.9 ± 0.4 | 3.1 ± 0.3 | 0.5 ± 0.2a | 4.8 ± 0.5 |
| 7–12 Light | 7.7 ± 0.7 | 0.5 ± 0.2a | 6.6 ± 0.4 | 1.6 ± 0.1 | 0.4 ± 0.1a | 9.3 ± 1.7 | |
| 13–18 Dark | 4.2 ± 0.4a | 0.5 ± 0.1 | 3.5 ± 0.4 | 1.5 ± 0.1 | 0.3 ± 0.1 | 24.8 ± 1.8 | |
| 19–24 Dark | 3.9 ± 0.4 | 0.5 ± 0.2 | 3.3 ± 0.3 | 2.1 ± 0.2 | 0.3 ± 0.1a | 23.3 ± 2.5 | |
| T3 | 1–6 Light | 8.8 ± 0.6 | 1.3 ± 0.3 | 6.7 ± 0.4 | 2.9 ± 0.1 | 0.6 ± 0.2 | 6.7 ± 2.2 |
| 7–12 Light | 8.5 ± 0.5 | 1.0 ± 0.4 | 6.7 ± 0.4 | 1.8 ± 0.2 | 0.6 ± 0.2 | 6.8 ± 0.8 | |
| 13–18 Dark | 4.0 ± 0.4 | 0.6 ± 0.2 | 3.2 ± 0.3 | 1.3 ± 0.2 | 0.5 ± 0.1 | 27.9 ± 3.4 | |
| 19–24 Dark | 4.3 ± 0.4 | 0.9 ± 0.3 | 3.3 ± 0.3 | 2.3 ± 0.2 | 0.5 ± 0.1 | 24.4 ± 3.1 | |
Different from baseline (BL), p ≤ 0.017, Bonferroni Correction, at the matching time in the light:dark cycle.
Table 3.
Percentage of recording time spent in vigilance states.
| Time | Hours | NREM |
WAKE |
||
|---|---|---|---|---|---|
| Females | Males | Females | Males | ||
| BL | 1–6 Light | 49.1 ± 2.6 | 42.4 ± 3.1 | 47.2 ± 2.3 | 52.7 ± 4.3 |
| 7–12 Light | 26.1 ± 2.5 | 30.1 ± 3.8 | 70.3 ± 3.1 | 65.8 ± 4.8 | |
| 13–18 Dark | 8.2 ± 2.4 | 12.0 ± 1.0 | 91.6 ± 2.5 | 86.8 ± 1.2 | |
| 19–24 Dark | 16.8 ± 3.1 | 25.2 ± 2.1 | 81.9 ± 3.6 | 72.1 ± 2.4 | |
| T2 | 1–6 Light | 49.5 ± 5.0 | 46.6 ± 2.8 | 48.5 ± 5.2 | 52.1 ± 2.9 |
| 7–12 Light | 29.2 ± 2.7 | 27.6 ± 2.6 | 69.3 ± 3.1 | 71.4 ± 2.8 | |
| 13–18 Dark | 16.7 ± 3.1 | 15.9 ± 1.8 | 82.5 ± 3.4 | 83.0 ± 1.9 | |
| 19–24 Dark | 16.2 ± 2.0 | 19.5 ± 2.3 | 83.1 ± 2.2 | 79.6 ± 2.4 | |
| T3 | 1–6 Light | 47.5 ± 5.2 | 46.8 ± 2.2 | 49.1 ± 6.0 | 50.7 ± 2.0 |
| 7–12 Light | 23.7 ± 3.7 | 31.5 ± 3.0 | 74.2 ± 4.3 | 66.3 ± 3.6 | |
| 13–18 Dark | 17.7 ± 4.6 | 15.3 ± 1.7 | 80.9 ± 5.1 | 83.4 ± 1.9 | |
| 19–24 Dark | 14.7 ± 3.4 | 23.9 ± 1.7 | 84.5 ± 3.7 | 74.3 ± 2.3 | |
No pairings reached statistical significance.
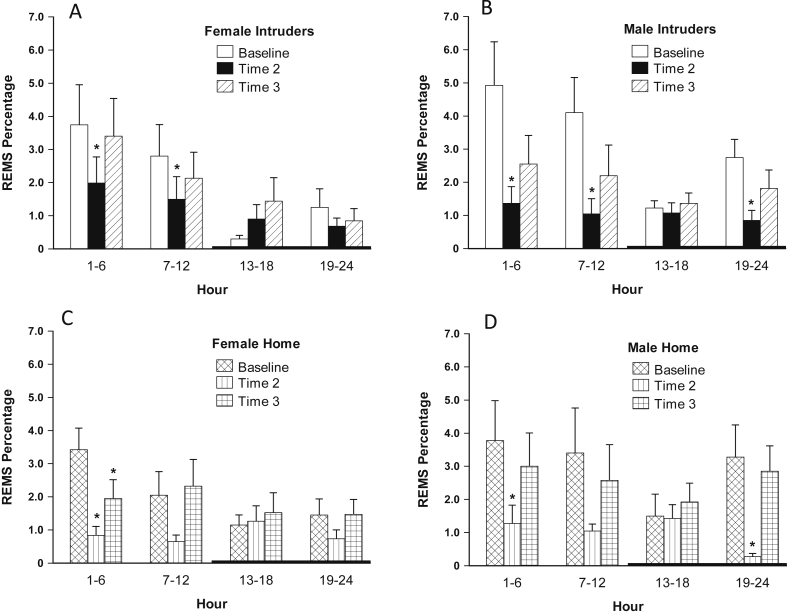
Both male and female intruders exhibited significantly reduced REMS at T2 throughout the light phase, hours 1–12, including bouts and percentage (Table 2 and Fig. 3, respectively). Males also exhibited reduced REMS during the latter dark phase, hours 19–24 [REMS%: F(1,7) = 12.642, 12.281, and 15.409 for hours 1–6, 7–12 and 19–24, respectively, p ≤ 0.01].
Fig. 3.
A–D: Percent REMS in female intruders (A), male intruders (B), female home (C), and male home (D) animals at baseline (BL); immediately following the midpoint social defeat encounter (T2); and immediately following the final social defeat encounter (T3). Error bars are ± SEM; *versus BL: A: F(1,6) = 7.378, and 6.621, p < 0.05 for hours 1–6 and 7–12, respectively; B: F(1,7) = 12.642, 12.281 and 15.409, p < 0.01 for hours 1–6, 7–12 and 19–24, respectively; C: F(1,7) = 14.46 and 14.795, p < 0.01 for hours 1–6; D: F(1,3) = 13.736 and 11.157, p < 0.05 for hours 1–6 and 19–24, respectively; n = 7, 8, 8, and 4, A-D, respectively.
3.2. Sucrose preference
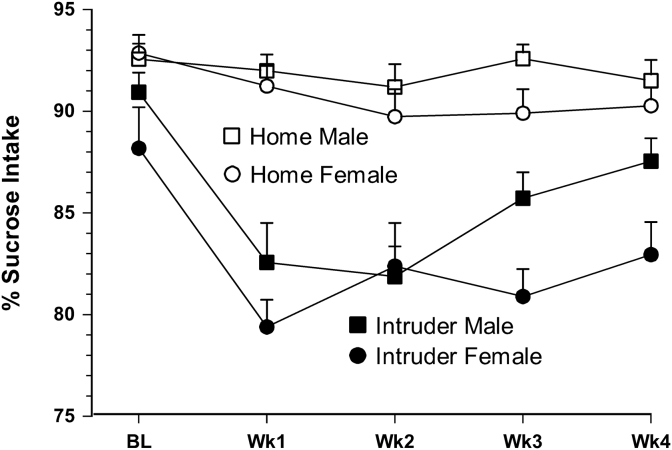
Animals undergoing social defeat exhibited a 10% reduction in sucrose preference during the first week of the intruder paradigm, which was largely maintained in the females and diminished by one-half in the males over the 4-week course of social defeat [F(1,41) = 81.321, p < 0.001]. Additionally, females exhibited significantly less sucrose intake overall [F(1,41) = 6.039,p < 0.05] as well as among the intruder animals [F(1,19) = 6.178, p < 0.05]. Age at study entry was a significant covariate only in the full model [p = 0.039], but not when controlling for sex differences [p > 0.05]. Fig. 4 depicts these relationships.
Fig. 4.
Percent sucrose intake among intruder versus home control animals at baseline (BL) through the 4 weeks of the intruder paradigm. Social defeat animals exhibited a significant reduction in sucrose intake throughout the paradigm (p < 0.01) and females exhibited less sucrose intake overall (p = 0.019); Error bars are ± SEM. Numbers provided in Table 1.
3.3. Weight
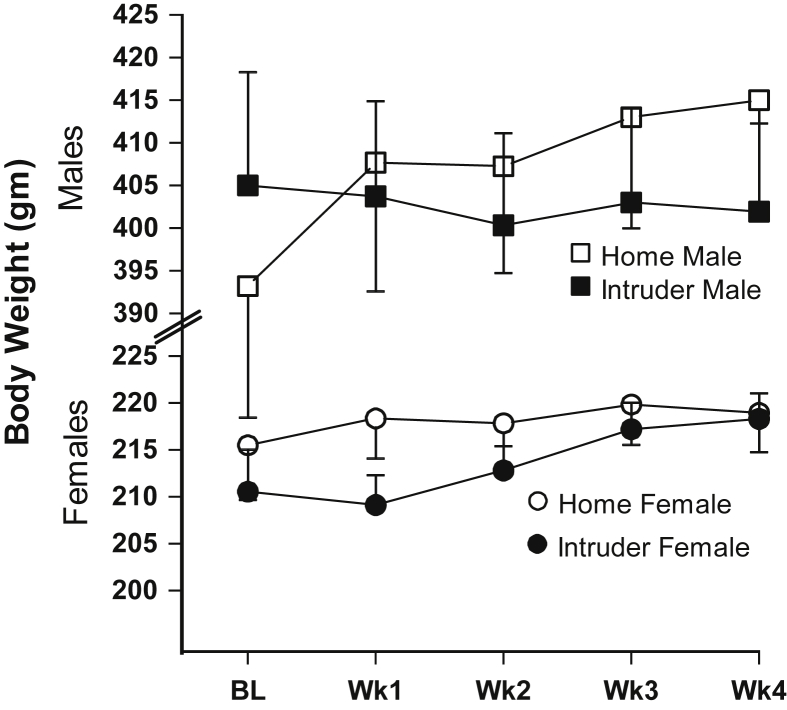
There were sex differences in body weights of rats upon entry in this study, with male rats weighing more than female rats. Unlike home cage comparison animals, the intruder males did not gain weight during the 4 week social defeat protocol [F(1,25) = 4.456, p < 0.05]; age at study entry was a significant covariate, p < 0.01. There were no body weight differences between intruder and home females. Fig. 5 depicts these relationships.
Fig. 5.
Body weight changes over the social defeat paradigm. Social defeat resulted in statistically significant weight differences in only the males, p < 0.05; Error bars are ± SEM. Numbers provided in Table 1.
3.4. Plasma corticosterone
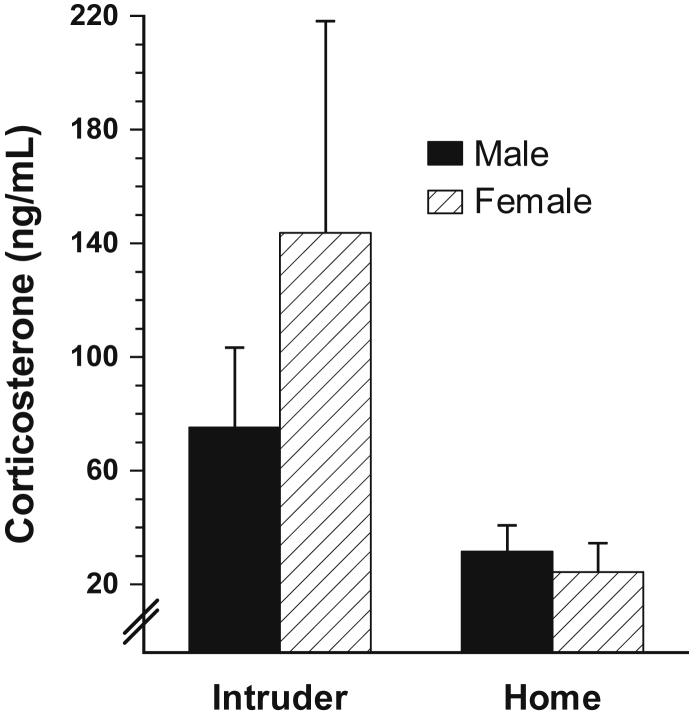
Plasma corticosterone levels were elevated in intruder animals that were unperturbed at the time of euthanasia [F(1,23) = 6.136, p < 0.05, Fig. 6]; there were no effects of age, sex or time from final intruder bout to euthanasia [p > 0.05].
Fig. 6.
Mid-dark phase resting plasma corticosterone levels in animals undergoing social defeat, intruder, versus animals remaining in their home cage during this time. Social defeat animals exhibited significantly greater plasma corticosterone levels, p < 0.05; Error bars are ± SEM. Numbers provided in Table 1.
4. Discussion
To our knowledge this is the first report examining the impact of chronic and repeated social defeat exposures on sleep, anhedonia and basal corticosterone levels in both male and female rats. We observed sex differences in sleep architecture and continuity in response to our 4-week 3-times per week social defeat paradigm, the induction of anhedonia in the first week of social defeat exposures, and elevated corticosterone levels in the resting animal suggesting increased HPA axis basal tone.
The intruder F344 females exhibited a significant and large, 2.5-fold, increase in numbers of vigilance state transitions, a measure of sleep fragmentation, which was concentrated during the early dark period, hours 13–18. The intruder males did not exhibit this outcome (Fig. 2). Two-fold and statistically significant increases in NREM and WAKE bouts at this time likely contributed to the increase in vigilance state transitions evident in the females. Additionally, that WAKE bouts durations were significantly decreased and of 2-fold magnitude, suggests that sleep fragmentation may have been accompanied by greater sleep pressure during these early dark hours (Table 2). These findings may reflect a hypersomnia, which has been identified in clinical states of depression (American Psychiatric Association, 2013, Armitage, 2007, Dauvilliers et al., 2013).
Both males and females exhibited significant reductions in REMS% during the light period at T2, hours 1–12; and males only during the latter half of the dark period, hours 19–24 (Fig. 3). These reductions in REMS% are accompanied by reductions in REMS bouts in both males and females; however, only the males exhibited a double hit of reduced REMS bouts as well as REMS bout duration (Table 2).
What underlies the diminished magnitude of sleep architecture differences at T3 compared to T2 would be only speculative; however, it is important to note that the patterns of T2 and T3 differences from baseline varied. It would seem that some compensatory adjustment occurred over the course of 4 weeks in most sleep outcomes. Indeed, there appears to be some recovery of sucrose preference in the intruder males over time (Fig. 4).
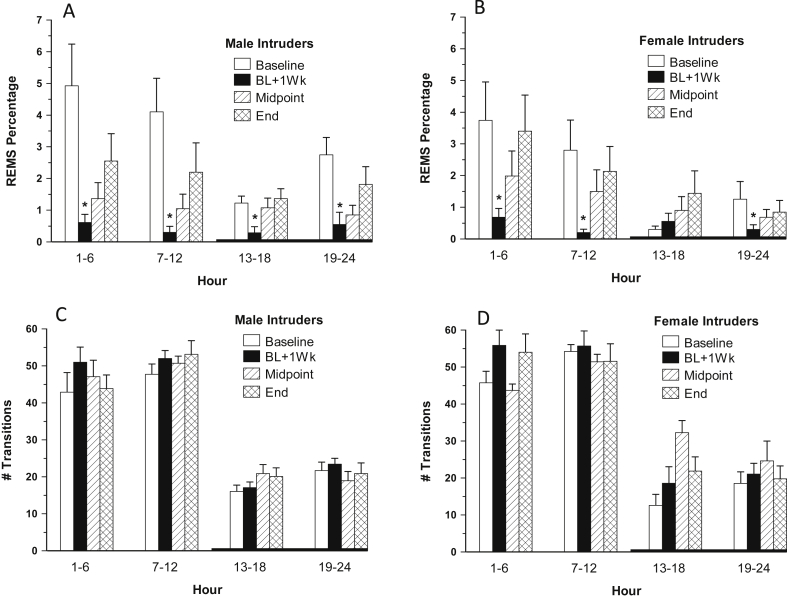
Several issues regarding sleep measurement and analysis warrant comment. First, findings are based upon longitudinal within animal comparison of sleep vigilance states and architecture given the individual variability in sleep architecture noted by others (Ahmed et al., 2011). In this study EEG/EMG biopotentials were also continuously acquired in non-intruder, home animals, 4 males and 8 females. Second, we hypothesize that the environment likely impacted the sleep of both home and intruder animals. Whereas the acquired biopotentials used for analysis of baseline sleep outcomes were from the weekend day before intruder exposures were to begin, the selection of biopotentials used for analyzing changes at the midpoint and endpoint, T2 and T3, were anchored to the designated intruder exposure. A second line of experiments requiring sleep recording were being conducted during this time, and active animal handling was occurring between hours 1–6 and 19–24. These realities beget three points regarding sleep outcomes in this study: (1) in both intruder and home animals, baseline recordings biopotentials were acquired in a quiet and undisturbed environment; (2) the evidence regarding the consequences of sleep fragmentation during hours 13–18 was also acquired during a daily period of quiet and non-disturbance (1:00–7:00 AM), so sleep architecture changes during this period are cautiously attributable to the condition of the animals; and (3) these explanations only partially hold with regard to the REMS outcomes, particularly at T2 related to the small n for home males, 4, and the matching suppression patterns between the intruder and home animals. Indeed, the analysis of an additional 24 h of recordings obtained 4–5 days post intruder paradigm initiation showed a substantial and statistically significant decrement in REMS% from baseline among all but the female intruders during hours 13–18 (Fig. 7A and B). This finding may reflect the fact that intruder and home animals were housed in the same room and that some “contagion” of the intruder effect may be evident in REMS%, known to be highly susceptible to disruption (Kamphuis et al., 2015) and promoted as empathy in recent pain literature (e.g., Langford et al., 2006, Mogil, 2015). The findings of the additional post intruder time point regarding sleep transitions are consistent with the midpoint and endpoint measures [Fig. 7C and D; F(3,39) = 2.96, p < 0.05], particularly considering that the additional sleep analysis focused upon sleep that was more than 24 h following the most recent intruder bout, unlike the analyses comprising the midpoint and endpoint which immediately followed an intruder bout. Also important in these considerations is that only the intruder animals exhibited anhedonia and greater plasma corticosterone levels.
Fig. 7.
A–D: REMS% and # transitions in male and female intruders at baseline; 4–5 days following intruder paradigm initiation (BL + 1Wk); immediately following the midpoint social defeat encounter (T2); and immediately following the final social defeat encounter (T3). (A) male intruders REMS%; (B) female intruders REMS%; (C) male intruders # transitions; and (D) female intruders # transitions at baseline Error bars are ± SEM; *versus BL; n = 8 males and 7 females.
Previous studies investigating the impact of social defeat on sleep architecture used paradigms that were of shorter duration ranging from a single encounter during lights off (Meerlo et al., 1997) and lights on (Kamphuis et al., 2015, Meerlo et al., 2001) to 2 consecutive days during the dark phase (Kinn Rod et al., 2014, Kinn et al., 2008). Sleep architecture analyses were followed up to 21 days post social defeat showing fragmentation of slow wave sleep (Kinn et al., 2008 c.f., however, Kinn Rod et al., 2014) and increased slow wave activity (Kamphuis et al., 2015, Meerlo et al., 1997, Meerlo et al., 2001) with significantly reduced REMS% in the early hours following defeat (Kamphuis et al., 2015). Our finding of decreases in REMS% during lights on is consistent with these latter findings of Kamphuis et al. (Kamphuis et al., 2015). To our knowledge, the current study is the first to examine chronic changes in sleep from before the first intruder exposure, baseline, at the two-week midpoint of the social defeat paradigm and through the final intruder exposure at 4 weeks in both males and females.
Sleep disturbance is evident in 50–70% of individuals afflicted with major depressive disorder (Armitage, 2007, Palagini et al., 2013) and women report more disruptions in sleep compared to men (Bangasser and Valentino, 2014). Insomnia or poor sleep is a key criterion for the diagnosis of major depression (American Psychiatric Association, 2013) and is likely causally related to major depression (Roth et al., 2007, Staner, 2010). HPA axis dysregulation and the accompanying hyperarousal impacts sleep architecture evidenced by impaired sleep continuity, reductions in NREMS, and disinhibition of REMS (Palagini et al., 2013, Pillai et al., 2011). Our findings of altered sleep continuity in the females are consistent with observations in humans with major depression, though our findings regarding REMS differ from the human phenomenon.
Females figured prominently in the social defeat paradigm used herein. To our knowledge, ours is the first study in which the female resident remained in the cage with the resident male for the male social defeat encounters. Our intent was to enhance the territoriality of the resident male, and indeed, the females often were near their male partner during attacks and defeats. None of our analyses showed a relationship between number of defeats or attacks and outcomes among the intruders. Intruder males were defeated and placed in a mesh protective barrier in a significantly greater number of encounters compared to intruder females, 10.2 versus 0.75 (±0.57 and ± 0.34, SEM), respectively [t = 14.81, p < 0.001]. On the other hand, females attacked the intruding female significantly more compared to males exhibiting this aggressive behavior, 7.2 versus 1.0 (±0.84 and ± 0.33, SEM), respectively [t = 6.52, p < 0.001]. That males exhibited greater aggression evidenced by defeating the intruding male is a clear explanation for these differences in defeats versus attacks. Despite this difference, female intruders exhibited significantly greater anhedonia than did the males.
Anhedonia is a commonly measured behavioral consequence of social defeat and is evident in virtually all social defeat studies monitoring for sucrose preference. Our findings are consistent with the literature in showing a significant reduction in the preferential consumption of sucrose water in males as well as females (Andre et al., 2005, Becker et al., 2008, Bourke and Neigh, 2012, Patki et al., 2013, Rygula et al., 2005, Rygula et al., 2008). Our findings showed females to exhibit significantly greater decrements of sucrose intake than the males. Consistent with previous studies, body weight gain was significantly reduced among the intruder males (Andre et al., 2005, Becker et al., 2008). Intruder females did not exhibit reductions in body weight, consistent with findings in females following 6 daily social defeat encounters (Bourke and Neigh, 2012). This latter finding is not surprising as the females were close to their maximum weight upon entry into the study, and males continue to gain weight through maturity.
Our findings of elevated plasma corticosterone levels in the resting intruders during the latter half of the dark phase suggests an elevated basal HPA axis tone that is consistent with findings from similar intruder paradigms undertaken in only males (Andre et al., 2005, Becker et al., 2008, Kieran et al., 2010, Wood et al., 2010). The sample collection interval from the final social defeat to blood withdrawal in the resting animal has ranged from 24 h (Kieran et al., 2010, Haller et al., 1999) to 4 or more days (Andre et al., 2005, Becker et al., 2008, Wood et al., 2010). Haller et al. showed elevated basal corticosterone levels in males but not in females at 24 h after the completion of a 4-day 4 h per day intruder paradigm (Haller et al., 1999). It has been suggested that stress related increases in HPA axis activation contributes to sleep fragmentation (Pillai et al., 2011, Steiger and Kimura, 2010).
Finally, it is important to point out that, compared to other inbred and outbred strains, the F344 rat strain is a high stress responsive strain both emotionally (Carobrez and Bertoglio, 2005) and in HPA axis responsiveness to stress (Page et al., 2014, Sternberg et al., 1992). Given that other studies of social defeat have largely used Wistar (e.g., Kieran et al., 2010, Kinn et al., 2008, Rygula et al., 2005, Rygula et al., 2008) or Sprague Dawley (Andre et al., 2005, Becker et al., 2008) rats as intruders, it will be important to validate these findings in other strains.
5. Conclusions
In summary, this study shows that both male and female intruder rats exhibit significant alterations in sleep architecture and continuity from baseline to 2- and 4-weeks subsequent to the initiation of a chronic social defeat paradigm. Also new is evidence of sex differences in sleep architecture consequences, whereas females exhibit significant increases in vigilance state transitions during the early dark hours, both sexes exhibit reductions in REMS during the light phase and only males during the latter hours of the dark phase. Anhedonia and elevated baseline plasma corticosterone levels were also statistically significantly different in both males and females, validating the similar and highly stressful nature of repeated social defeat encounters. Collectively, these findings suggest important and clinically relevant findings are obtained when females are included in studies of stress and depression.
Acknowledgements
This work was supported by NIH grants HL108332 and NR012294. We wish to thank the many hands assisting with this protocol: Amanda Kuhs, Elizabeth Schenning and Amanda Brightman.
Contributor Information
Gayle G. Page, Email: gpage1@jhu.edu.
Mark R. Opp, Email: mopp@u.washington.edu.
Sharon L. Kozachik, Email: skozach1@jhu.edu.
References
- Ahmed S., Meng H., Liu T., Sutton B.C., Opp M.R., Borjign J., Wang M.M. Ischemic stroke selectively inhibits REM sleep of rats. Exp. Neurol. 2011;232:168–175. doi: 10.1016/j.expneurol.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemus M., Sarvaiya N., Epperson C.N. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 2014;35:320–330. doi: 10.1016/j.yfrne.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Association; Washington DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. DSM-5. Ref Type: Generic. [Google Scholar]
- Andre J., Zeau B., Pohl M., Cesselin F., Benoliel J.J., Becker C. Involvement of cholecystokininergic systems in anxiety-induced hyperalgesia in male rats: behavioral and biochemical studies. J. Neurosci. 2005;25:7896–7904. doi: 10.1523/JNEUROSCI.0743-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr. Scand. 2007;115:104–115. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C., Zeau B., Rivat C., Blugeot A., Hamon M., Benoliel J.J. Repeated social defeat-induced depression-like behavioral and biological alterations in rats: involvement of cholecystokinin. Mol. Psychiatry. 2008;13:1079–1092. doi: 10.1038/sj.mp.4002097. [DOI] [PubMed] [Google Scholar]
- Bourke C.H., Neigh G.N. Exposure to repeated maternal aggression induces depressive-like behavior and increases startle in adult female rats. Behav. Brain Res. 2012;227:270–275. doi: 10.1016/j.bbr.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carobrez A.P., Bertoglio L.J. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci. Biobehav Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Carvalho S., Pinto-Gouveia J., Pimentel P., Maia D., Gilbert P., Mota-Pereira J. Entrapment and defeat perceptions in depressive symptomatology: through an evolutionary approach. Psychiatry. 2013;76:53–67. doi: 10.1521/psyc.2013.76.1.53. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Social stress models in depression research: what do they tell us? Cell Tissue Res. 2013;354:179–190. doi: 10.1007/s00441-013-1606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvilliers Y., Lopez R., Ohayon M., Bayard S. Hypersomnia and depressive symptoms: methodological and clinical aspects. BMC Med. 2013;11 doi: 10.1186/1741-7015-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A.J., Charlson F.J., Norman R.E., Patten S.B., Freedman G., Murray C.J.L., Vos T., Whiteford H.A. Burden of depressive disorders by country, sex, age and year: findings from the Global Burden of Disease Study 2010. PLoS Med. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold P.W., Machado-Vieira R., Pavlatou M.G. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. Neural Plast. 2015;2015 doi: 10.1155/2015/581976. Article ID 581976, http://dx.doi.org/10.1155/2015/581976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant E.C., Mackintosh J.H. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21:246–259. [Google Scholar]
- Haller J., Fuchs E., Halász J., Makara G. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Res. Bull. 1999;50:33–39. doi: 10.1016/s0361-9230(99)00087-8. [DOI] [PubMed] [Google Scholar]
- Hollis F., Duclot F., Gunjan A., Kabbaj M. Individual differences in the effect of social defeat on anhedonia and histone acetylation in the rat hippocampus. Horm. Behav. 2011;59:331–337. doi: 10.1016/j.yhbeh.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis F., Kabbaj M. Social defeat as an animal model for depression. ILAR J. 2014;55:221–232. doi: 10.1093/ilar/ilu002. [DOI] [PubMed] [Google Scholar]
- Kamphuis J., Lancel M., Koolhaas J.M., Meerlo P. Deep sleep after social stress: NREM sleep slow-wave activity is enhanced in both winners and losers of a conflict. Brain Behav. Immun. 2015;47:149–154. doi: 10.1016/j.bbi.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Bromet E. The epidemiology of depression across cultures. Annu. Rev. Public Health. 2013;34:119–138. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieran N., Ou X.M., Iyo A.H. Chronic social defeat downregulates the 5-HT1A receptor but not Freud-1 or NUDR in the rat prefrontal cortex. Neurosci. Lett. 2010;469:380–384. doi: 10.1016/j.neulet.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinn Rod A.M., Murison R., Mrdalj J., Milde A.M., Jellestad F.K., Øvernes L.A., Grοnli J. Effects of social defeat on sleep and behaviour: importance of the confrontational behaviour. Physiol. Behav. 2014;127:54–63. doi: 10.1016/j.physbeh.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Kinn A.M., Grønli J., Eldbjørg F., Kuipers S., Ursin R., Murison R., Portenoy R.K. A double exposure to social defeat induces sub-chronic effects on sleep and open field behavior in rats. Physiol. Behav. 2008;95:553–561. doi: 10.1016/j.physbeh.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Koolhaas J.M., Coppens C.M., De Boer S.F., Buwalda B., Meerlo P., Timmermans P.J.A. The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J. Vis. Exp. 2013;77:e4367. doi: 10.3791/4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D.J., Crager S.E., Shehzad Z., Smith S.B., Sotocinal S.G., Levenstadt J.S., Chanda M.L., Levitin D.J., Mogil J.S. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- Meerlo P., de Bruin E.A., Strijkstra A.M., Daan S. A social conflict increases EEG slow-wave activity during subsequent sleep. Physiol. Behav. 2001;73:331–335. doi: 10.1016/s0031-9384(01)00451-6. [DOI] [PubMed] [Google Scholar]
- Meerlo P., Pragt B.J., Daan S. Social stress induces high intensity sleep in rats. Neurosci. Lett. 1997;225:41–44. doi: 10.1016/s0304-3940(97)00180-8. [DOI] [PubMed] [Google Scholar]
- Mogil J.S. Social modulation of and by pain in humans and rodents. Pain. 2015;156:S35–S41. doi: 10.1097/01.j.pain.0000460341.62094.77. [DOI] [PubMed] [Google Scholar]
- Page G.G., Ben-Eliyahu S., Yirmiya R., Liebeskind J.C. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain. 1993;54:21–28. doi: 10.1016/0304-3959(93)90095-7. [DOI] [PubMed] [Google Scholar]
- Page G.G., Opp M.R., Kozachik S.L. Reduced sleep, stress responsivity, and female sex contribute to persistent inflammation-induced mechanical hypersensitivity in rats. Brain Behav. Immun. 2014;40:244–251. doi: 10.1016/j.bbi.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Palagini L., Baglioni C., Ciapparelli A., Gemignani A., Riemann D. REM sleep dysregulation in depression: state fo the art. Sleep. Med. Rev. 2013;17:377–390. doi: 10.1016/j.smrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Papciak J., Popik P., Fuchs E., Rygula R. Chronic psychosocial stress makes rats more 'pessimistic' in the ambiguous-cue interpretation paradigm. Behav. Brain Res. 2013;256:305–310. doi: 10.1016/j.bbr.2013.08.036. [DOI] [PubMed] [Google Scholar]
- Patki G., Solanki N., Atrooz F., Allam F., Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013;1539:73–86. doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai V., Kalmbach D.A., Ciesla J.A. A meta-analysis of electroencephalographic sleep in depression: evidence for genetic biomarkers. Biol. Psychiatry. 2011;70:912–919. doi: 10.1016/j.biopsych.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Razzoli M., Carboni L., Guidi A., Gerrard P., Arban R. Social defeat-induced contextual conditioning differentially imprints behavioral and adrenal activity: a time-course study in the rat. Physiol. Behav. 2007;92:734–740. doi: 10.1016/j.physbeh.2007.05.063. [DOI] [PubMed] [Google Scholar]
- Rivat C., Becker C., Blugeot A., Zeau B., Mauborgne A., Pohl M., Benoliel J.J. Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain. 2010;150:358–368. doi: 10.1016/j.pain.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Roth T., Roehrs T.A., Pies R. Insomnia: pathophysiology and implications for treatment. Sleep. Med. Rev. 2007;11:71–79. doi: 10.1016/j.smrv.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Rygula R., Abumaria N., Flügge G., Fuchs E., Rüther E., Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav. Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Rygula R., Abumaria N., Flügge G., Hiemke C., Fuchs E., Rüther E., Havemann-Reinecke U. Citalopram counteracts depressive-like symptoms evoked by chronic social stress in rats. Behav. Pharmacol. 2006;17:19–29. doi: 10.1097/01.fbp.0000186631.53851.71. [DOI] [PubMed] [Google Scholar]
- Rygula R., Abumaria N., Havemann-Reinecke U., Rüther E., Hiemke C., Zernig G., Fuchs E., Flügge G. Pharmacological validation of a chronic social stress model of depression in rats: effects of reboxetine, haloperidol and diazepam. Behav. Pharmacol. 2008;19:183–196. doi: 10.1097/FBP.0b013e3282fe8871. [DOI] [PubMed] [Google Scholar]
- Staner L. Comorbidity of insomnia and depression. Sleep. Med. Rev. 2010;14:35–46. doi: 10.1016/j.smrv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Steiger A., Kimura M. Wake and sleep EEG provide biomarkers in depression. J. Psychiatric Res. 2010;44:242–252. doi: 10.1016/j.jpsychires.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Sternberg E.M., Glowa J.R., Smith M.A., Calogero A.E., Listwak S.C., Aksentijevich S., Chrousos G.P., Wilder R.L., Gold P.W. Corticotropin releasing hormone related behavioral and neuroendocrine responses to stress in Lewis and Fischer rats. Brain Res. 1992;570 doi: 10.1016/0006-8993(92)90563-o. 54–60RE. [DOI] [PubMed] [Google Scholar]
- Stetler C., Miller G.E. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom. Med. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Stroud C.B., Davila J., Moyer A. The relationship between stress and depression in first onsets versus recurrences: a meta-analytic review. J. Abnorm Psychol. 2008;117:206–213. doi: 10.1037/0021-843X.117.1.206. [DOI] [PubMed] [Google Scholar]
- Wood S.K., Walker H.E., Valentino R.J., Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]