Abstract
There is a growing emphasis on the relationship between the complexity and diversity of the microorganisms that inhabit our gut (human gastrointestinal microbiota) and health/disease, including brain health and disorders of the central nervous system. The microbiota-gut-brain axis is a dynamic matrix of tissues and organs including the brain, glands, gut, immune cells and gastrointestinal microbiota that communicate in a complex multidirectional manner to maintain homeostasis. Changes in this environment can lead to a broad spectrum of physiological and behavioural effects including hypothalamic-pituitary-adrenal (HPA) axis activation, and altered activity of neurotransmitter systems and immune function. While an appropriate, co-ordinated physiological response, such as an immune or stress response are necessary for survival, a dysfunctional response can be detrimental to the host contributing to the development of a number of CNS disorders.
In this review, the involvement of the gastrointestinal microbiota in stress-mediated and immune-mediated modulation of neuroendocrine, immune and neurotransmitter systems and the consequential behaviour is considered. We also focus on the mechanisms by which commensal gut microbiota can regulate neuroinflammation and further aim to exploit our understanding of their role in stress-related disorders as a consequence of neuroinflammatory processes.
1. Introduction
1.1. The microbiome
The human gut plays host to a multitude of microorganisms (bacteria, archaea, yeasts, single-celled eukaryotes, as well as helminth parasites and viruses, including bacteriophage) collectively referred to as the microbiota (Eckburg et al., 2005, Gaci et al., 2014, Scarpellini et al., 2015, Williamson et al., 2015). The gut microbiota forms part of a complex network termed the microbiota-gut-brain axis along with the enteric nervous system, sympathetic and parasympathetic divisions of the autonomic nervous system, and neuroendocrine and neuroimmune components of the central nervous system (Cryan and Dinan, 2015b, Mayer et al., 2014, Rhee et al., 2009). The composition of the core bacteria that we harbour throughout our adult life is established early in our first few years of life and are shaped by a number of factors including mode of delivery (vaginal or C-section), whether we are breastfed or bottlefed, diet, medication (in particular antibiotic medication), and exposure to viral or bacterial infections and stress (Borre et al., 2014b). It is worth noting that the critical development period in which the seeding of our core microbiota and the development of the bacterial community in our gut, occurs in parallel with the growth, maturation and sprouting of neurons in the young brain (Borre et al., 2014a, Borre et al., 2014b), and a similar profile is evident in old age where a decline in microbiota complexity and diversity occurs in parallel with a decrease in neuronal complexity (Biagi et al., 2012). With an estimated mass of 1–2 kg, numbering in their trillions (Frank and Pace, 2008) and together possessing 100 times the number of genes in the human genome (Kurokawa et al., 2007), these microbes can influence virtually all aspects of human physiology and biology, through interactions with their host (Allez et al., 2007, Burokas et al., 2015b; Cryan and Dinan, 2012, Cryan and Dinan, 2015b, Dinan et al., 2015, Mayer et al., 2014, Mayer et al., 2015, Moloney et al., 2014, Sampson and Mazmanian, 2015, Williamson et al., 2015). This interaction between the bacteria and host is mutually beneficial with the bacteria involved in energy regulation, gut barrier function, protection from pathogens, and immune system function amongst others (Bengmark, 2013, Borre et al., 2014a, Burokas et al., 2015b, Collins et al., 2012, Cryan and Dinan, 2015a), while the host provides the nutrients and environment in which the bacteria can thrive. Recently, the gastrointestinal microbiota has also emerged as a key regulator of centrally mediated events including stress and neuroinflammation (Cryan and Dinan, 2015a, Dinan and Cryan, 2012, El Aidy et al., 2015, El Aidy et al., 2014, Moloney et al., 2014, Sampson and Mazmanian, 2015). A recent hypothesis is that this neuronal development is mediated by microbiota-governed maturation and activation of central microglia. The concept of the intestinal microbiota as a key regulator of the stress and/or immune response, facilitating adaption or habituation to these conditions, and protecting against the development of stress-related and/or neuroinflammation-related disease and dysfunction will be discussed. We will first focus on the interaction of stress and neuroinflammation, and then outline the role of gastrointestinal microbiota in the stress/immune response.
1.2. The stress response
Stress may be defined as a complex dynamic condition in which the homeostasis, or the steady state internal milieu of an organism is disturbed or threatened (De Kloet et al., 1994, McEwen et al., 2015, Wilder, 1995). At some stage in their lifespan, all organisms are exposed to factors that surpass a homeostatic threshold, resulting in a stress response, be they physical, psychological or immunological in nature. Evolution has equipped most organisms with the biological machinery necessary to mount a defensive response to acute stressful stimulus, and to restore homeostatic balance once the stress or perceived threat has subsided. The co-ordination of this defensive response is governed by a pathway linking the hypothalamus, the pituitary gland and the adrenal glands, (the hypothalamic-pituitary-adrenal (HPA) axis), ultimately resulting in the release of behaviour-altering chemicals including glucocorticoids, mineralocorticoids and catecholamines. The activity of the HPA axis is regulated by multiple afferent sympathetic, parasympathetic, and limbic circuits (e.g., amygdala, hippocampus, and medial prefrontal cortex) innervating either directly or indirectly the paraventricular nucleus (PVN) of the hypothalamus (Smith and Vale, 2006). Under normal conditions, HPA axis activity exhibits a continuous oscillatory activity synchronised with circadian and ultradian rhythms (Dallman et al., 1994, Dickmeis et al., 2013, Lightman, 2008, Tsigos and Chrousos, 2002). The sympathetic nervous system (SNS) and HPA axis activation are the principal neurotransmitter and neuroendocrine components of the response to stress, respectively (Mayer, 2000). In response to stress, the SNS is responsible for recruiting increased catecholamine levels in systemic circulation and tissues, while concomitantly, corticosterone releasing factor (CRF) is released from paraventricular neurons of the hypothalamus, which then stimulate the release of adrenocorticotrophic hormone (ACTH) from the anterior pituitary, which travels in the systemic circulation to induce the synthesis and release of glucocorticoids from the adrenal cortex: cortisol in humans and corticosterone in animals. The primary function of SNS and HPA axis activation is to prime the body for ‘fight or flight’ response by increasing blood sugar through gluconeogenesis, suppression of the immune system (cytokine suppression) and enhanced fat and protein metabolism (Mayer, 2000). In response to a stressful stimulus, glucocorticoids pass into the brain more readily and mediate their effects through high affinity mineralocorticoid receptors and lower affinity glucocorticoid receptors: the latter being activated at higher stress intensity. Receptors sensitive to glucocorticoids are expressed throughout the CNS including in the very same brain regions, and neuronal circuits, involved in the mounting of a stress-mediated neuroendocrine response, and thus an autoregulatory feedback loop is in place to prevent over-activation of this response once the stressor is removed. Chronic exposure to stress and subsequent glucocorticoid production results in various adverse side effects such as osteoporosis, diabetes, hypertension, dyslipidemia, and even neurodegeneration (Howell and Muglia, 2006, Kleiman and Tuckermann, 2007). Stress is also one of the most significant risk factors for irritable bowel syndrome (Dinan et al., 2010, Elsenbruch, 2011, Mayer, 2000). On the other hand, a deficient or blunted HPA axis is commonly observed in the clinic in a wide range of autoimmune and inflammatory diseases. Glucocorticoids also strongly influence the phenotype, survival and functions of monocytes, macrophages and glial cells and their receptors are ubiquitously expressed on practically every type of immune cell (Bellavance and Rivest, 2014, Sierra et al., 2008).
2. Stress & neuroimmune function
2.1. Microglia –key regulators of stress & neuroinflammation
In the CNS, the microglia are the innate sentinel immune cells that can detect subtle changes in molecules in their locality (Kim and de Vellis, 2005, Tremblay et al., 2011), and ultimately are responsible for neuroinflammatory processes (Kettenmann et al., 2011, Ransohoff et al., 2015, Yamasaki et al., 2014). These resident cells compromising 5–12% of total brain cells, are highly ramified and extremely plastic, and once activated can release a number of cytokines and chemokines, express numerous antigenic markers, regulate neurotransmitters and undergo extreme morphological changes (Pocock and Kettenmann, 2007). Cytokines, together with neurotransmitters and hormones, play a key role in the maintenance of neuro-immune-endocrine system homeostasis (Alboni and Maggi, 2015, Schafer and Stevens, 2015). The cytokine system (constituted by cytokines, their receptors and regulators of their activity) are expressed throughout the brain and their expression is regulated thoughout the lifespan from brain development until aging. Almost all populations of cells in the brain are susceptible to modulation by the cytokine system. Under normal physiological conditions, cytokines typically participate in brain development and plasticity by translating environmental cues into molecular signals, and are criticial in the maintenance of healthy brain function and plasticity (Beattie et al., 2002, Bilbo and Schwarz, 2009, Bilbo and Schwarz, 2012). Cytokines and chemokines also play a key role in the repair of damaged tissue and the restoration of homeostasis (Charo and Ransohoff, 2006), and in signalling between immune cells. Cytokines are generally classed as pro-inflammatory or anti-inflammatory which facilitate or inhibit inflammatory processes respectively. Interleukin (IL)-1β, IL-6 and tumour necrosis factor α (TNFα) are amongst the most widely investigated proinflammatory cytokines, whilst IL-4 and IL-10 are well-known anti-inflammatory cytokines. With the exception of IFN-gamma mRNA, transcripts for the other cytokines were found to be constitutively present in the brain (Pitossi et al., 1997). The mobilisation of these anti- and pro-inflammatory cytokines and chemical messengers are beneficial to the host by allowing the body to defend against infection or tissue damage. However, a delicate balance between pro-inflammatory or anti-inflammatory alterations needs to be tightly regulated, as an uncontrolled and sustained response can have significant inflammatory collateral damage and detrimental effects on mood and behaviour.
2.2. Reciprocal involvement of stress and immune systems
Acknowledging the intricate nature of the stress and neuroinflammatory responses, it is not surprising to note that glucocorticoid receptors are highly expressed on microglia throughout the brain (Sierra et al., 2008), while integration of stress-induced signalling is facilitated in the brain by microglia-mediated neuroinflammatory signalling (Wohleb et al., 2014b). Under stressful situations, neuroendocrine pathways alter peripheral and central immune responses, leading to monocyte priming and trafficking, subsequent alterations in microglia phenotype and, ultimately resulting in neuroinflammation (Delpech et al., 2015, Frank et al., 2007, Frank et al., 2012, Johnson et al., 2005, Tynan et al., 2010, Wohleb et al., 2014b), which can subsequently lead to a number of psychiatric disorders, including stress, anxiety and depression (Hodes et al., 2015, Miller, 1998). The release of cytokines following microglia activation have been reported to have a causal role in behavioural phenotype in stress models (Blandino et al., 2013, Hinwood et al., 2012, Hinwood et al., 2013, Kreisel et al., 2014, Wohleb et al., 2013). Intriguingly, stress-induced microglia activation occurs in discrete brain regions, and in specific neuronal circuits, heavily involved in stress-mediated neuroendocrine activation. Accordingly, strong evidence exists directly linking cytokines with HPA axis activity (Bellavance and Rivest, 2012, Bellavance and Rivest, 2014, Jara et al., 2006, Mastorakos and Ilias, 2000, Mastorakos and Ilias, 2006, Mulla and Buckingham, 1999, Savastano et al., 1994). An immunogenic insult can also trigger HPA axis activity through immunomodulators traversing the chorus plexus (Kunis et al., 2013) and BBB (Capuron and Miller, 2011, Turrin and Rivest, 2004). The choroid plexus, whose activity is governed by resident CD4+ IFNγ-producing helper T-cells, is generally considered as a gate for leukocyte trafficking to the CNS. An increase in circulating immunomodulators triggers an opening of this gate, facilitating neuroinflammation, and the mounting of a stress response. Depending on the intensity and nature of the insult, an increase in systemic immunomodulators may also cause neuroinflammation indirectly by relaying their information via cytokines from endothelial cells of capillaries at the blood brain barrier (Verma et al., 2006), or via vagal nerve stimulation by peripheral inflammatory mediators (Eskandari et al., 2003). Nerve endings of the vagus nerve can detect the release of inflammatory mediators from the gut, and communicate this sensory information to the brainstem, resulting in efferent cholinergic vagal suppression of peripheral inflammatory cytokines, such as TNF α and IL-1β in the spleen (Rosas-Ballina and Tracey, 2009, Tracey, 2007). Thus, in addition to their fundamental physiological significance independently, a greater appreciation of the reciprocal interaction of stress and immune responses may prove useful in understanding the aetiology of stress- and neuroinflammatory-related disorders and in developing new approaches for their treatment. Aberrant regulation of neuroendocrine, and immune responses are believed to play a key role in the precipitation, maintenance, and/or exacerbation of a number of neuroinflammatory- and stress-related disorders including anxiety, depression, Alzheimer's disease, multiple sclerosis, arthritis and autism amongst others.
3. Microbiota-gut-brain axis governing immune and stress response
3.1. Gastrointestinal microbiota-mediated modulation of behavioural response to stress
Preclinical studies have illuminated our understanding of the role of the microbiota in behavioural responses, however for the purpose of this review we will focus on behavioural responses to stress and neuroinflammation. It has been shown that different types of psychological stress including maternal separation, chronic social defeat, restraint conditions, crowding, heat stress, and acoustic stress can alter the composition of gastrointestinal microbiota (Bailey, 2014, Bailey et al., 2011, Bharwani et al., 2015, De Palma et al., 2015, De Palma et al., 2014, Hsiao et al., 2013, Moloney et al., 2014), as can stress-related disorders such as irritable bowel syndrome (IBS) (Jeffery et al., 2012, Shankar et al., 2015). As such, a number of experimental models and conditions have been employed to interrogate the role of the microbiota in stress behaviour including prebiotic and probiotic intervention, antibiotic administration, faecal transplantation and the use of germ-free and specific pathogen free animals.
Studies have shown that animals raised in a sterile environment from birth, and as such, without gastrointestinal bacteria (germ-free), exhibit an exaggerated hypothalamic-pituitary-adrenal axis activity with elevated ACTH and corticosterone levels in response to a mild novel arena stressor (Clarke et al., 2013) and in the more stressful restraint test (Sudo, 2012, Sudo et al., 2004), which normalised after colonisation with commensal bacteria from control mice. Furthermore, germ-free animals display an anxiolytic phenotype in mildly stressful novel and aversive environments including elevated plus maze, light/dark box and open field test (Clarke et al., 2013, De Palma et al., 2015, Neufeld et al., 2011a, Neufeld et al., 2011b). Recolonisation of germ-free mice with commensal bacteria in early life was sufficient to reverse some of the observed behavioural and neurochemical effects although only in males (Clarke et al., 2013). In contrast, a recent study using a different strain of mice demonstrated that short-term colonisation with intragastic inoculation of commensal bacteria (Bifidobacterium infantis and Blautia coccoides) reduced anxiety-like behaviour as compared with the germ-free controls (Nishino et al., 2013). In a separate study using stress-sensitive germ-free rats, an increased corticosterone response was reported in germ-free animals following open field test exposure (Crumeyrolle-Arias et al., 2014). Taken together, these data suggest that the relationship between gut microbiota and stress- and/or anxiety-related phenotype may be subject to temporal, sex, strain and species dependent factors.
An alternate approach to investigating the role of the gastrointestinal microbiota on stress biochemistry and behaviour is to diminish stable core bacteria using antibiotic interventions. However, preclinical and clinical studies investigating the effects of antibiotic administration on changes in neuroendocrine levels in response to stress are surprisingly lacking (Ait-Belgnaoui et al., 2012, Beijers et al., 2010, Desbonnet et al., 2015b, Matsuo et al., 2009, O'Mahony et al., 2014, Reber et al., 2011), yet they support the finding that antibiotic administration was able to prevent stress-mediated changes in behaviour and immunosuppression (Ait-Belgnaoui et al., 2012, Reber et al., 2011). A separate study where disruption of bacterial colonisation was mitigated by vancomycin reported decreased microbiota diversity and an increased sensitivity to visceral pain (O'Mahony et al., 2014). Whilst antibiotic use in healthy infants disrupts the diversity and complexity of commensal bacteria, it has also been postulated to exacerbate inflammatory responses in subjects with a pre-existing susceptibility to inflammatory disorders such as Crohn's disease (Gevers et al., 2014). However, as a general concept, the use of antibiotics alone to treat stress-mediated disorders in clinical trials has not been evaluated.
Allying with the premise that gastrointestinal bacteria can impact on emotional behaviour and stress response, a number of studies have investigated the effect of prebiotic and probiotic intervention in preclinical stress models. Maternal separation is a useful translational animal model to represent early life stress in humans, with long term changes in behaviour and gastrointestinal microbiota complexity/diversity in adulthood in rats (Golubeva et al., 2015). In this model, Bifidobacterium infantis intervention attenuated immune changes, and normalised the behavioural phenotype without restoring HPA axis activity in one study (Desbonnet et al., 2010), while in a similar model, probiotics reduced the stress-induced increase in corticosterone (Gareau et al., 2007). In a separate study, maternal probiotic intervention (Bifidobacterium animalis subspecies lactis BB-12 and Propionibacterium jensenii 702) induced activation of neonatal stress pathways and improved the immune environment of stressed animals and protected, in part, against stress-induced disturbances in adult gut microflora (Barouei et al., 2012). Furthermore, maternal separation was shown to exacerbate visceral hypersensitivity in mice (Moloney et al., 2012, Tramullas et al., 2014) and rats genetically predisposed to display an anxious phenotype (Hyland et al., 2015), an effect that was reversible with probiotic administration of Bifidobacterium infantis 35624 (McKernan et al., 2010).
Probiotic treatments (Bifidobacterium longum 1714, and Bifidobacterium breve 1205; lactobacillus strain) were also successful in reversing behavioural alterations without altering neuroendocrine output in restraint and food deprivation tests (Palomar et al., 2014, Savignac et al., 2014). In the forced swim test, 28 day Lactobacillus rhamnosus (JB-1) administration reduced stress-induced corticosterone levels, an effect that was abolished with vagotomy, suggesting that a functional neural relay from gut to brain is necessary for governing stress response (Bravo et al., 2011). Another study demonstrated that the HPA axis response to acute stress was attenuated by lactobacillus farciminis (Ait-Belgnaoui et al., 2012). Recently, we have demonstrated that a combination of the prebiotics fructooligosaccharide (FOS) and galactooligosaccharide (GOS) attenuated the corticosterone response to acute swim stress and attenuated anxiety related behaviour in naïve mice, and further demonstrated a protective effect of this cocktail on stress and anxiety parameters in chronic social defeat (Burokas et al., 2015a, Burokas et al., 2015b). Similarly, a prebiotic cocktail of non-digestible oligosaccharides demonstrated anxiolytic activity as well as an immunosuppressive effect in response to LPS (Savignac et al., 2015).
In a clinical setting, the number of studies investigating the effects of prebiotic or probiotic supplements on stress behaviour and output are limited. Using cortisol as an index of stress response, the probiotics Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 (Messaoudi et al., 2011a, Messaoudi et al., 2011b) and galactooligosaccharide prebiotic (Schmidt et al., 2015) were effective in boosting the subjects resilience to stress and improved emotional responses in healthy subjects. Lactic acid-producing bacteria have been shown to alleviate abdominal discomfort and IBS symptoms in clinical settings (Clarke et al., 2012). Furthermore, ingestion of a probiotic cocktail was shown to alter brain activity and informational processing of emotional material in a functional magnetic resonance imaging brain scan study (Tillisch et al., 2013), as well as reducing negative thoughts associated with sad mood (Steenbergen et al., 2015) reinforcing the role for gastrointestinal microbiota in stress and emotional responses. However, the precise mechanisms that orchestrate a functionally relevant communication between the microbiota and the stress response are yet to be elucidated.
3.2. Regulation of the neuroinflammatory response by gastrointestinal microbiota
The gut microbiota influences the relative populations, migration and function of various subsets of immune cells (Dorrestein et al., 2014), and a spate of recent publications have demonstrated how the microbial population of the gut can modulate innate and adaptive immune responses at mucosal surfaces during infection, inflammation and autoimmunity (Cassel et al., 2008, El Aidy et al., 2014, Kamada et al., 2013, Mazmanian et al., 2005, Round and Mazmanian, 2009). More recently, changes in crosstalk between the intestinal epithelium, the intestinal immune system and gut microbes has been recognised for its ability to modulate systemic immunity (Belkaid and Naik, 2013). However, until very recently, the interaction between gastrointestinal microbiota and CNS-restricted immune cells had not been investigated.
Along with their established role in host defence, the constitutively active microglia are critically involved in neuronal events at various stages in development and adulthood, including synaptic remodelling to improve neuronal network signalling (Schafer et al., 2012, Schafer and Stevens, 2015). Despite the seclusion of the microglia as a consequence of BBB protection, a recent comprehensive study (Erny et al., 2015) has highlighted a critical role for gastrointestinal microbiota in microglia maturation, morphology and immunological function. Under normal homeostatic conditions, a diverse gastrointestinal microbiota is necessary for the maintenance of microglia in a healthy functional state. In contrast, the absence of a complex host microbiota (as evidenced by germ free models, antibiotic suppression of microbiota or use of specific pathogen free (SPF) mice) led to increased microglial populations; defects in microglia maturation, activation state and differentiation; alterations to microglia morphology (with longer processes and increased branching, terminal points and clubbing at synaptic boutons); and a compromised immune response to bacterial or viral infection (Erny et al., 2015). The observed alterations in microglial phenotype were reversed with recolonisation of gut microbiota, following 6 weeks co-habitation of germ-free mice with control mice. The findings from this study have redefined the ideology that the relationship between our microbiota, immune system and neurodevelopment is moot in adulthood; as a healthy, and diverse gastrointestinal microbiota is essential for the continuous preservation of healthy microglia and proper brain function throughout our lifespans (Cryan and Dinan, 2015a). It should also be noted that minocycline, a semi-synthetic, second-generation tetracycline analogue can effectively cross the blood–brain barrier and has been reported to exert neuroprotective effects in cerebral ischaemia, traumatic brain injury, amyotrophic lateral sclerosis, Parkinson's disease, kainic acid treatment, Huntington’ disease, multiple sclerosis and Alzheimers in various preclinical animal models (Kim and Suh, 2009); reportedly through suppression of microglia activation (Tikka and Koistinaho, 2001). However, minocycline has a dual action as an antibiotic and thus may be influencing centrally-mediated events indirectly through microbiota depletion, where it acts on both gram positive and negative bacteria.
4. Potential mechanisms of action of gastrointestinal microbiota on stress and immune systems
Gastrointestinal microorganisms are by their very definition constrained to the gut, and interact with the host tissue along the alimentary canal as well as with their cohabitating microbial consortia. The epithelial layer of the gut has many functions including regulating the absorption of nutrients and fluid from the lumen of the intestines and to serve as a physical barrier to invading pathogens or harmful substances (Farhadi et al., 2003). This monolayer is interspersed with complex protein structures known as tight junction proteins (Madara, 1998), establishing a barrier to prevent diffusion of fluids and solvents between adjacent epithelial cells (Ivanov and Naydenov, 2013). Another important function of the intestines is to limit the contact of intestinal microbiota with the visceral tissue, which it does by secreting a protective viscous mucous layer from goblet cells of the epithelium. The exchange of molecules through the mucous layer and epithelium serve to facilitate communication between the gut and the immune system through the recognition of self and non-self antigens (Fasano and Shea-Donohue, 2005), and thus to prime the immune system to identify potentially harmful pathogens.
It is evident that the gastrointestinal microbiota plays a key role in HPA axis activity and immune response, but the question remains; how are the microbiota of the gut modulating physiological response at a central level? A number of mechanisms have been suggested, however the precise mechanism or mechanisms of action are, as yet, unresolved. Crosstalk in the bi-directional microbiome-gut-brain axis, in homeostatic conditions, and in response to changes in environment, likely involve dynamic molecules with multiple effector mechanisms that can traverse different anatomical environments and communicate within their local environment to mediate a complex co-ordinated response on multiple physiological systems. Numerous molecular candidates have been proposed including neurotransmitters, neuropeptides, endocrine hormones and immunomodulators.
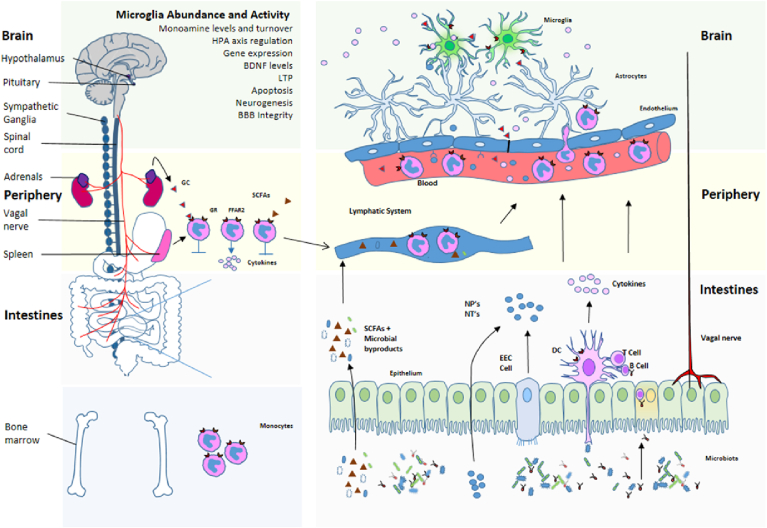
This final section will consider the likely involvement of the microbiota in mediating or modulating stress and neuroinflammation, and their associated behavioural responses. This section is necessarily speculative as there is a paucity of studies conclusively linking microbiota-mediated changes locally in the gut and periphery with central effects. A schematic for the mechanisms by which microbiota can influence stress and neuroinflammation is provided in Fig. 1.
Fig. 1.
Schematic for microbiota regulation of neuroinflammation and HPA axis activity. Communication within the microbiota-gut-brain axis involves the complex co-ordination of a number of factors and systems. The microbiota can govern events in the periphery and CNS by various means of communication including vagal nerve activation, cytokine production, neuropeptide and neurotransmitter release, SCFA release and microbial by-products, and by utilising the lymphatic and systemic circulation. Once these signals penetrate the blood brain barrier and reach the brain, they can influence the maturation and activation state of the microglia. Once activated, microglia play a key role in immune surveillance, synaptic pruning and clearance of debris. They also facilitate a number of everyday functions in the brain, including the regulation of HPA axis activation state. The release of glucocorticoids (cortisol) as a consequence of HPA axis activation can in turn regulate the activation state of brain microglia, as well as influence cytokine release and trafficking of monocytes from the periphery to the brain. HPA Hypothalamic-Pituitary-Adrenal; BDNF Brain derived neurotrophic factor; LTP Long term potentiation; BBB Blood-brain barrier; GC Glucocorticoids; GR Glucocorticoid receptor; FFAR Free fatty acid receptor; SCFA Short chain fatty acid; NP Neuropeptide; NT Neurotransmitter; DC Dendritic cell; EEC Enteroendocrine cell.
4.1. The environmental niche of the gastrointestinal microbiota
To better understand the mechanisms by which these commensal microbes influence our daily lives, one must first understand their environment. The gastrointestinal tract can govern microbial density by neurally controlling gut motility, gut permeability, release of gut hormones and immune function via vagal and pelvic connections between the central nervous system (CNS), and enteric nervous system (ENS) and sympathetic prevertebral ganglia. The luminal-mucosal interface of the intestinal tract is the first relevant location where host-microorganism interactions occur and is highly dependent on immune response. The mucosal surface layers the epithelial lining of the gut, which contains predominantly enterocytes, secretory cells and gut associated lymphoid tissue (GALT) (Pott and Hornef, 2012). The enterocytes express innate immune receptors and can release cytokines and chemokines, while the GALT utilise lymphocytes to mount a more specific immune response if the case is warranted. The secretory cells are involved in mucus release from goblet cells, the control of bacterial diversity by antimicrobial secretion from Paneth cells and in neuroendocrine control via the release of substances from enteroendocrine cells such as ghrelin, somatostatin, cholecystokinin, peptide YY and serotonin amongst others (Cani et al., 2013). Thus the gastrointestinal microbiota have had to adapt to survival in a somewhat hostile environment where the host intestinal barrier is on constant immunological surveillance.
4.2. Physical communication between the microbiota and the epithelium
The polysaccharide coating of gastrointestinal bacteria protects them from degradation but also serves to identify the numerous and diverse bacteria to the host. This allows the host protective immune surveillance system to monitor and regulate commensal bacteria, and to identify invading pathogens. Evidence suggests that a disruption to homeostatic bacterial diversity could trigger an increase in immune mediators in the gut, as a consequence of surplus numbers of certain cell wall components, such as peptidoglycans (Royet et al., 2011). Epithelial pattern recognition receptors (PRRs) recognise molecular patterns unique to bacteria and other microorganisms (pathogen associated molecular patterns, or PAMPs) (Duerkop et al., 2009, Vaishnava et al., 2008a) of which the Toll-like receptor family are the most studied, and once activated can recruit inflammatory mediators, cytokine production and chemokine-mediated recruitment of acute inflammatory cells (Takeda and Akira, 2004, Vaishnava et al., 2008b). This in turn helps regulate the number and diversity of bacteria in the gut. However, in response to invading pathogens or as a consequence of chronically high inflammatory tone, prolonged cytokine production can weaken the integrity of the intestinal barrier and mucosal layer, facilitating an increase in plasma lipopolysaccharide (LPS) levels as a consequence of increased bacteria infiltration across the gut (Souza et al., 2004). This concept that the “leaky gut” may facilitate communication between the microbiota and inflammatory tone has gained traction, and may be causal in the chronic low-grade inflammation often observed in mood disorders, such as depression (Kelly et al., 2015).
4.3. Biochemical communication between the microbiota and the epithelium
It has been estimated that there are greater than 100 species (Qin et al., 2010) and more than 7000 (Ley et al., 2008) strains of bacteria in the average human gut, largely dominated by Bacteriodetes and Firmicute phylotypes with less prevalence of Proteobacteria, Actinobacteria, Fusobacteria and Verrucomicrobia (Eckburg et al., 2005). Indeed, the different regions of the gut vary in terms of the indigenous microbiota diversity (Human Microbiome Project, 2012, Lozupone et al., 2012). These commensal bacteria are capable of synthesising and releasing many neurotransmitters and neuromodulators themselves, or evoke enteroendocrine cells into the synthesis and release of neuropeptides. For example, Lactobacillus and Bifidobacterium species can produce gamma-aminobutyric acid (GABA): Escheridia, Bacillus and Saccharomyces spp. can produce norepinephrine: Candida, Streptococcus, Escheridia and Enterococcus spp. can produce 5-HT: Bacillus can produce dopamine: and Lactobacillus can produce acetylcholine (Lyte, 2013, Lyte, 2014). These microbially synthesised neurotransmitters can cross the mucosal layer of the intestines, and possibly mediate effects locally in the gut, or enter the bloodstream and impact on physiological events in the brain.
Approximately 95% of serotonin in the body is compartmentalised in the gut, predominantly in enterochromaffin cells of the mucosa and in nerve terminals of the enteric nervous system. Studies using germ-free mice have implicated the gastrointestinal microbiota in impacting on tryptophan metabolism, the precursor to serotonin (Clarke et al., 2013). It seems likely that the secretion of these neurotransmitter molecules in the intestinal lumen may induce epithelial cells to release molecules that can in turn modulate enteric neural communication, or perhaps these neurotransmitters can pass through the intestinal barrier and mediate effects locally in the gut. Evidence to support a role for gastrointestinal microbiota in neural communication between the brain and gut is provided by studies whereby probiotic-mediated reduction in stress-response was ablated by vagotomy (Bravo et al., 2011).
Gastrointestinal bacteria can also secrete bioactive chemicals such as bacteriocins, bile acids, choline and short chain fatty acids (SCFAs) that are integral to host health and disease. Bacteriocins are antibiotic proteins that inhibit the growth of other bacteria in the vicinity, while SCFAs including butyric acid, acetic acid, proprionic acid and lactic acid are bacterial metabolites derived from the fermentation of polysaccharides (Cummings and Macfarlane, 1997). These fatty acids can enter the blood and activate free fatty acid receptors (FFARs), or mediate epigenetic events (Kasubuchi et al., 2015) and have been reported to have neuroactive properties (Russell et al., 2013). Indeed, deficits in BBB permeability of germ-free animals was partly reversible with colonisation of SCFA-producing commensal bacteria (Braniste et al., 2014). Similarly, deficits in germ-free animals in microglia density, morphology and maturity were reversible with administration of a SCFA mix (sodium proprionate, sodium butyrate, and sodium acetate) in the drinking water (Erny et al., 2015).
4.4. Consequences of microbial interaction
Under homeostatic conditions there exists a healthy, resting inflammatory tone where the microbiota stimulate cytokines and chemokines release, which in turn regulates local levels of bacteria in the gut. As well as influencing local immune responses at the epithelium, microbiota can synthesise and release neurotransmitters and SCFAs, as well as influence the release of neuropeptides and hormones from enteroendocrine cells of the intestines. Gut peptides such as ghrelin, gastrin, orexin, galanin, cholecystokinin, leptin and neuropeptide Y are thought to influence peripheral neural communication and can also act centrally to influence behaviour. Current hypotheses suggest that these circulating cytokines, chemokines, endocrine messengers and microbial by-products can infiltrate the blood and lymphatic systems, or influence neural messages carried by the vagal and spinal afferent neurons to impact on centrally-mediated events, including regulation of HPA axis activity and neuroinflammation.
4.5. Penetrating the blood brain barrier
Until recently, the central nervous system was considered an immune privileged entity lacking a lymphatic drainage system (Aspelund et al., 2015, Louveau et al., 2015). Despite the observation of this lymphatic drainage system in the brain, the migration of immune cells, cytokines, chemokines, endocrine messengers and microbial by-products is still tightly regulated by the blood brain barrier (BBB), a highly capillarised structure composed of astrocytes and endothelial cells, amongst others. The permeability of this barrier can be compromised in certain physiological conditions, including in response to stress, infection or injury, allowing the influx and egress of messenger molecules to influence the homeostatic environment in the brain. Anatomical loci where these compounds can enter the CNS include the choroid plexus, meninges, perivascular space and parenchymal tissue (Ransohoff and Engelhardt, 2012), and each of these stations is under vigilant surveillance by resident macrophage and microglia (Nayak et al., 2012). These cells are the first cells to detect changes in their environment, and once activated recruit help locally within the brain but also by recruiting monocyte trafficking to the brain to aid in defence and cell debris clearing. As with the epithelium of the gut, pattern recognition receptors (PRRs) on these immune cells recognise microbial by-products, while changes in the environment as a consequence of cytokines, chemokines and endocrine messengers ultimately result in neuroinflammation, and trafficking of monocytes to the brain. Whilst the multimodal influence of microbiota on physiological events at the level of the CNS has gained credence, more recently there has been a shift to a stronger role for microbiota in regulating neuroinflammation via intervention in the recruitment of local immune regulators from the periphery to the brain.
4.6. Peripheral influence of microbiota regulating neuroinflammation and HPA axis
Emerging evidence implicates a novel neuroimmune circuit involving microglia activation and altered sympathetic neural tone to the peripheral immune system to recruit inflammatory monocytes to the brain (Wohleb et al., 2014b), and further reinforce stress-related behaviours. The trafficking of these monocytes to the brain appeared to be governed by TNF α-mediated microglia activation (D'Mello et al., 2009) and was reversible by probiotic intervention with VSL#3 (D'Mello et al., 2015). Indeed, the mediation of certain behavioural phenotypes as a consequence of prolonged HPA activation, involves the trafficking of monocyte from the spleen (Wohleb et al., 2014a, Wohleb et al., 2014b), a tissue with high density of FFAR2 receptors (Erny et al., 2015). These FFAR2 receptors are Gi/Go G-protein coupled receptors and have been localised to lymphocytes (Nilsson et al., 2003). SCFAs, the natural ligands for FFARs, were shown to reverse the detrimental effects on microglia density, morphology and maturity in the absence of a complex microbiota (Erny et al., 2015). Taken together these findings suggest that gastrointestinal microbiota may govern centrally mediated events indirectly through regulation of monocyte trafficking to the brain and subsequent microglia activation and HPA axis activity, possibly via SCFA-mediated mechanisms.
5. Neuroinflammation, stress and microbiota at critical time windows
5.1. Early life
Childhood and adolescence represent the most dynamic periods of change in our gastrointestinal microbiome, as well as our neuronal development. The formation of our central nervous system require a complex and well-orchestrated series of events including neurogenesis, axonal and dendritic growth, synaptogenesis, and refinement of these connections. The first two weeks of postnatal development are a period of remarkable plasticity with new synapses being actively formed and remodelled (Tremblay et al., 2011). Initially, far more synaptic connections than necessary are created, and by a microglia-governed process termed ‘pruning’ (Hong et al., 2016, Paolicelli et al., 2011, Bilimoria and Stevens, 2015, Schafer and Stevens, 2015), a large number of these immature synapses are permanently eliminated while a subset of synapses are maintained and strengthened (Schafer et al., 2012, Schafer et al., 2013). During and shortly after birth, infants are expose to microbes originating predominantly from the mother. Initially, the mode of delivery (C-section versus vaginally born) will influence the very first seeding of the gastrointestinal microbiota, followed thereafter by whether the infant is breast- or bottle-fed (Borre et al., 2014a, Borre et al., 2014b, O'Mahony et al., 2015). Given the evidence presented herein, it is tempting to speculate that the microbes that populate our gut in early life influence our neural development via microglia activation. As such, disruptions (such as stress or antibiotic medication) to our microbiota at these critical periods of dynamic microbiota-host interactions have the potential to profoundly alter gut-brain signalling, affect health throughout life and increase the risk of (or lead to) neurodevelopmental disorders. A recent comprehensive review (Ganguly and Brenhouse, 2015) has looked at the effect of early life adversity on neuroinflammation, and implicates a differential role for microglia at different stages in life to subsequent stress exposure, as well as in various psychopathologies.
5.1.1. Adolescence
Subsequent to the initial colonisation at birth and through infancy, the microbiota continues to develop throughout childhood and adolescence. Currently, there is a lack of studies investigating the effects of hormonal changes at puberty on the diversity and complexity of commensal gut bacteria, or whether any such changes are sexually dimorphic. At this developmental stage, distinct changes in neuronal architecture and function occur in adolescence that facilitate the maturation of behavioural, social and cognitive skills (Burnett et al., 2011). It is also a period of increased behavioural and susceptibility to various psychiatric vulnerabilities, including schizophrenia, substance abuse, irritable bowel and mood disorders (O'Connor and Cryan, 2014). Adolescence is generally considered to begin with the onset of puberty and ends as one takes on adult social roles (Dahl, 2004, Spear, 2004). Human structural imaging studies have demonstrated a loss of gray matter during adolescence (Sowell et al., 1999, Sowell et al., 2001), with gray-matter reductions in portions of the temporal lobe and cortical regions (dorsal PFC) occurring in late adolescence (Raznahan et al., 2011, Sowell et al., 1999, Sowell et al., 2004). Preclinical studies suggest that these changes are related to pruning of synapses during this developmental period (Rakic et al., 1986, Rakic et al., 1994, Rakic, 1999), most likely via microglial interaction (Hong et al., 2016; Paolicelli et al., 2011; Bilimoria and Stevens, 2015; Schafer and Stevens, 2015). This process of remodelling continues beyond adolescence into the third decade of life before stabilising at adult levels (Petanjek et al., 2011). Further preclinical evidence using antibiotic depletion of the microbiota during adolescence has also demonstrated a role for the microbiota in anxiety and cognitive behaviours at this developmental time window, as well as regulating neuromodulators of the microbiota-gut-brain axis (Desbonnet et al., 2015a).
5.2. Adulthood and aging
As adulthood approaches, the gut microbiota stabilises and becomes more diverse (Palmer et al., 2007), and can resist detrimental environmental elements such as antibiotics and stress by restoring its diverse and stable ‘normal’ core microbiota (Rajilic-Stojanovic et al., 2012). However, as we age, our microbiota diversity and stability decline (Biagi et al., 2010), and there is a concomitant increase in number, size and activation of microglia (von Bernhardi et al., 2015) that can lead to an increased inflammatory tone termed ‘inflammaging’ (Franceschi et al., 2007). There is high heterogeneity of microglia in various neurodegenerative diseases, including Alzheimers disease and those phenotypes share common characteristics with aging (Bachstetter et al., 2015). In normal aging, close crosstalk with astrocytes, neurons and other brain cells, serve crucial functions as the scavenger system of the CNS, providing beneficial functions as tissue repair in the CNS. However, chronic, dysregulated activation of microglia can lead to increased inflammatory tone ultimately resulting in malfunction and damage of brain cells. What drives this dysregulation is not fully understood, and as yet, the role of the microbiota in this has not been fully investigated.
6. Concluding remarks
The gastrointestinal microbiota has recently emerged as a key facilitator of stress adaption and immune response in the body. At the behavioural level, gastrointestinal content and complexity can impact on anxiety and fear responses, and can reduce behavioural despair or anhedonia. The evidence presented herein suggests that resilience to stress- and immune-related disorders and dysfunction of stress- and immune-systems may be dependent on the diversity and complexity of gastrointestinal microbiota. The studies investigating the influence of gastrointestinal microbiota have been largely preclinical in nature, with the findings in clinical cohorts being diagnostic in nature, and further studies with pre- and pro-biotic interventions are warranted. The ultimate goal is to exploit our understanding of the role of gastrointestinal microbiota in fundamental physiological and pathophysiological processes to better understand and treat a range of stress- and immune-related disorders. The studies covered in this review illustrate the numerous complex relationships between gastrointestinal microbiota and the stress and immune responses. However, commensal microbiota-mediated causality of the physiological and behaviour responses to stress and neuroinflammation is still unconfirmed.
Acknowledgements
This work was supported by Science Foundation Ireland in the form of a centre grant (Alimentary Pharmabiotic Centre; Grant No. SFI/12/RC/2273).
References
- Ait-Belgnaoui A., Durand H., Cartier C., Chaumaz G., Eutamene H., Ferrier L., Houdeau E., Fioramonti J., Bueno L., Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37(11):1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Alboni S., Maggi L. Editorial: cytokines as players of neuronal plasticity and sensitivity to environment in healthy and pathological brain. Front. Cell Neurosci. 2015;9:508. doi: 10.3389/fncel.2015.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allez M., Tieng V., Nakazawa A., Treton X., Pacault V., Dulphy N., Caillat-Zucman S., Paul P., Gornet J.M., Douay C., Ravet S., Tamouza R., Charron D., Lemann M., Mayer L., Toubert A. CD4+NKG2D+ T cells in Crohn's disease mediate inflammatory and cytotoxic responses through MICA interactions. Gastroenterology. 2007;132(7):2346–2358. doi: 10.1053/j.gastro.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Aspelund A., Antila S., Proulx S.T., Karlsen T.V., Karaman S., Detmar M., Wiig H., Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015;212(7):991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter A.D., Van Eldik L.J., Schmitt F.A., Neltner J.H., Ighodaro E.T., Webster S.J., Patel E., Abner E.L., Kryscio R.J., Nelson P.T. Disease-related microglia heterogeneity in the hippocampus of Alzheimer's disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol. Commun. 2015;3:32. doi: 10.1186/s40478-015-0209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M.T. Influence of stressor-induced nervous system activation on the intestinal microbiota and the importance for immunomodulation. Adv. Exp. Med. Biol. 2014;817:255–276. doi: 10.1007/978-1-4939-0897-4_12. [DOI] [PubMed] [Google Scholar]
- Bailey M.T., Dowd S.E., Galley J.D., Hufnagle A.R., Allen R.G., Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011;25(3):397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouei J., Moussavi M., Hodgson D.M. Effect of maternal probiotic intervention on HPA axis, immunity and gut microbiota in a rat model of irritable bowel syndrome. PLoS One. 2012;7(10):e46051. doi: 10.1371/journal.pone.0046051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie E.C., Stellwagen D., Morishita W., Bresnahan J.C., Ha B.K., Von Zastrow M., Beattie M.S., Malenka R.C. Control of synaptic strength by glial TNFalpha. Science. 2002;295(5563):2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Beijers R., Jansen J., Riksen-Walraven M., de Weerth C. Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics. 2010;126(2):e401–e409. doi: 10.1542/peds.2009-3226. [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Naik S. Compartmentalized and systemic control of tissue immunity by commensals. Nat. Immunol. 2013;14(7):646–653. doi: 10.1038/ni.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavance M.A., Rivest S. The HPA - immune Axis and the immunomodulatory actions of glucocorticoids in the brain. Front. Immunol. 2014;5:136. doi: 10.3389/fimmu.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavance M.A., Rivest S. The neuroendocrine control of the innate immune system in health and brain diseases. Immunol. Rev. 2012;248(1):36–55. doi: 10.1111/j.1600-065X.2012.01129.x. [DOI] [PubMed] [Google Scholar]
- Bengmark S. Gut microbiota, immune development and function. Pharmacol. Res. 2013;69(1):87–113. doi: 10.1016/j.phrs.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Bharwani A., Mian M.F., Foster J.A., Surette M.G., Bienenstock J., Forsythe P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology. 2015;63:217–227. doi: 10.1016/j.psyneuen.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Biagi E., Candela M., Fairweather-Tait S., Franceschi C., Brigidi P. Aging of the human metaorganism: the microbial counterpart. Age (Dordr) 2012;34(1):247–267. doi: 10.1007/s11357-011-9217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi E., Nylund L., Candela M., Ostan R., Bucci L., Pini E., Nikkila J., Monti D., Satokari R., Franceschi C., Brigidi P., De Vos W. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5(5):e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo S.D., Schwarz J.M. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front. Behav. Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo S.D., Schwarz J.M. The immune system and developmental programming of brain and behavior. Front. Neuroendocrinol. 2012;33(3):267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilimoria P.M., Stevens B. Microglia function during brain development: new insights from animal models. Brain Res. 2015;1617:7–17. doi: 10.1016/j.brainres.2014.11.032. [DOI] [PubMed] [Google Scholar]
- Blandino P., Jr., Hueston C.M., Barnum C.J., Bishop C., Deak T. The impact of ventral noradrenergic bundle lesions on increased IL-1 in the PVN and hormonal responses to stress in male sprague dawley rats. Endocrinology. 2013;154(7):2489–2500. doi: 10.1210/en.2013-1075. [DOI] [PubMed] [Google Scholar]
- Borre Y.E., Moloney R.D., Clarke G., Dinan T.G., Cryan J.F. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv. Exp. Med. Biol. 2014;817:373–403. doi: 10.1007/978-1-4939-0897-4_17. [DOI] [PubMed] [Google Scholar]
- Borre Y.E., O'Keeffe G.W., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol. Med. 2014;20(9):509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Toth M., Korecka A., Bakocevic N., Ng L.G., Kundu P., Gulyas B., Halldin C., Hultenby K., Nilsson H., Hebert H., Volpe B.T., Diamond B., Pettersson S. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6(263) doi: 10.1126/scitranslmed.3009759. 263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U. S. A. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S., Sebastian C., Cohen Kadosh K., Blakemore S.J. The social brain in adolescence: evidence from functional magnetic resonance imaging and behavioural studies. Neurosci. Biobehav Rev. 2011;35(8):1654–1664. doi: 10.1016/j.neubiorev.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burokas A., Moloney R.D., Arboleya S., Stanton C., Dinan T.G., Cryan J. Poster Session Presented at 45th Annual Conference for Society for Neuroscience, Oct 17-21, Washington, DC, USA. 2015. Targeting the microbiota-gut-brain axis with prebiotics in mice: a novel strategy for stress-related disorders. [Google Scholar]
- Burokas A., Moloney R.D., Dinan T.G., Cryan J.F. Microbiota regulation of the Mammalian gut-brain axis. Adv. Appl. Microbiol. 2015;91:1–62. doi: 10.1016/bs.aambs.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Cani P.D., Everard A., Duparc T. Gut microbiota, enteroendocrine functions and metabolism. Curr. Opin. Pharmacol. 2013;13(6):935–940. doi: 10.1016/j.coph.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Capuron L., Miller A.H. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol. Ther. 2011;130(2):226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel S.L., Sutterwala F.S., Flavell R.A. The tiny conductor: immune regulation via commensal organisms. Cell Host Microbe. 2008;3(6):340–341. doi: 10.1016/j.chom.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Charo I.F., Ransohoff R.M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 2006;354(6):610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Clarke G., Cryan J.F., Dinan T.G., Quigley E.M. Review article: probiotics for the treatment of irritable bowel syndrome–focus on lactic acid bacteria. Aliment. Pharmacol. Ther. 2012;35(4):403–413. doi: 10.1111/j.1365-2036.2011.04965.x. [DOI] [PubMed] [Google Scholar]
- Clarke G., Grenham S., Scully P., Fitzgerald P., Moloney R.D., Shanahan F., Dinan T.G., Cryan J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry. 2013;18(6):666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Collins S.M., Surette M., Bercik P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012;10(11):735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- Crumeyrolle-Arias M., Jaglin M., Bruneau A., Vancassel S., Cardona A., Dauge V., Naudon L., Rabot S. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207–217. doi: 10.1016/j.psyneuen.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Cryan J.F., Dinan T.G. Gut microbiota: microbiota and neuroimmune signalling-Metchnikoff to microglia. Nat. Rev. Gastroenterol. Hepatol. 2015;12(9):494–496. doi: 10.1038/nrgastro.2015.127. [DOI] [PubMed] [Google Scholar]
- Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Cryan J.F., Dinan T.G. More than a gut feeling: the microbiota regulates neurodevelopment and behavior. Neuropsychopharmacology. 2015;40(1):241–242. doi: 10.1038/npp.2014.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J.H., Macfarlane G.T. Role of intestinal bacteria in nutrient metabolism. JPEN J. Parenter. Enter. Nutr. 1997;21(6):357–365. doi: 10.1177/0148607197021006357. [DOI] [PubMed] [Google Scholar]
- D'Mello C., Le T., Swain M.G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci. 2009;29(7):2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello C., Ronaghan N., Zaheer R., Dicay M., Le T., MacNaughton W.K., Surrette M.G., Swain M.G. Probiotics improve inflammation-associated sickness behavior by altering communication between the peripheral immune system and the brain. J. Neurosci. 2015;35(30):10821–10830. doi: 10.1523/JNEUROSCI.0575-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R.E. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann. N. Y. Acad. Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dallman M.F., Akana S.F., Levin N., Walker C.D., Bradbury M.J., Suemaru S., Scribner K.S. Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Ann. N. Y. Acad. Sci. 1994;746:22–31. doi: 10.1111/j.1749-6632.1994.tb39206.x. discussion 31-2, 64-7. [DOI] [PubMed] [Google Scholar]
- De Kloet E.R., Oitzl M.S., Schobitz B. Cytokines and the brain corticosteroid receptor balance: relevance to pathophysiology of neuroendocrine-immune communication. Psychoneuroendocrinology. 1994;19(2):121–134. doi: 10.1016/0306-4530(94)90002-7. [DOI] [PubMed] [Google Scholar]
- De Palma G., Blennerhassett P., Lu J., Deng Y., Park A.J., Green W., Denou E., Silva M.A., Santacruz A., Sanz Y., Surette M.G., Verdu E.F., Collins S.M., Bercik P. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat. Commun. 2015;6:7735. doi: 10.1038/ncomms8735. [DOI] [PubMed] [Google Scholar]
- De Palma G., Collins S.M., Bercik P., Verdu E.F. The microbiota-gut-brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both? J. Physiol. 2014;592(Pt 14):2989–2997. doi: 10.1113/jphysiol.2014.273995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpech J.C., Madore C., Nadjar A., Joffre C., Wohleb E.S., Laye S. Microglia in neuronal plasticity: influence of stress. Neuropharmacology. 2015;96(Pt A):19–28. doi: 10.1016/j.neuropharm.2014.12.034. [DOI] [PubMed] [Google Scholar]
- Desbonnet L., Clarke G., O'Sullivan O., Cotter P.D., Dinan T.G., Cryan J.F. Re: gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav. Immun. 2015;50:335–336. doi: 10.1016/j.bbi.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Desbonnet L., Clarke G., Traplin A., O'Sullivan O., Crispie F., Moloney R.D., Cotter P.D., Dinan T.G., Cryan J.F. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav. Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Desbonnet L., Garrett L., Clarke G., Kiely B., Cryan J.F., Dinan T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170(4):1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Dickmeis T., Weger B.D., Weger M. The circadian clock and glucocorticoids–interactions across many time scales. Mol. Cell Endocrinol. 2013;380(1–2):2–15. doi: 10.1016/j.mce.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Dinan T.G., Cryan J., Shanahan F., Keeling P.W., Quigley E.M. IBS: an epigenetic perspective. Nat. Rev. Gastroenterol. Hepatol. 2010;7(8):465–471. doi: 10.1038/nrgastro.2010.99. [DOI] [PubMed] [Google Scholar]
- Dinan T.G., Cryan J.F. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37(9):1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Dinan T.G., Stilling R.M., Stanton C., Cryan J.F. Collective unconscious: how gut microbes shape human behavior. J. Psychiatr. Res. 2015;63:1–9. doi: 10.1016/j.jpsychires.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Dorrestein P.C., Mazmanian S.K., Knight R. Finding the missing links among metabolites, microbes, and the host. Immunity. 2014;40(6):824–832. doi: 10.1016/j.immuni.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerkop B.A., Vaishnava S., Hooper L.V. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31(3):368–376. doi: 10.1016/j.immuni.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M., Gill S.R., Nelson K.E., Relman D.A. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Aidy S., Dinan T.G., Cryan J.F. Gut microbiota: the conductor in the orchestra of immune-neuroendocrine communication. Clin. Ther. 2015;37(5):954–967. doi: 10.1016/j.clinthera.2015.03.002. [DOI] [PubMed] [Google Scholar]
- El Aidy S., Dinan T.G., Cryan J.F. Immune modulation of the brain-gut-microbe axis. Front. Microbiol. 2014;5:146. doi: 10.3389/fmicb.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav. Immun. 2011;25(3):386–394. doi: 10.1016/j.bbi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Erny D., Hrabe de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., Keren-Shaul H., Mahlakoiv T., Jakobshagen K., Buch T., Schwierzeck V., Utermohlen O., Chun E., Garrett W.S., McCoy K.D., Diefenbach A., Staeheli P., Stecher B., Amit I., Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari F., Webster J.I., Sternberg E.M. Neural immune pathways and their connection to inflammatory diseases. Arthritis Res. Ther. 2003;5(6):251–265. doi: 10.1186/ar1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadi A., Banan A., Fields J., Keshavarzian A. Intestinal barrier: an interface between health and disease. J. Gastroenterol. Hepatol. 2003;18(5):479–497. doi: 10.1046/j.1440-1746.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- Fasano A., Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005;2(9):416–422. doi: 10.1038/ncpgasthep0259. [DOI] [PubMed] [Google Scholar]
- Franceschi C., Capri M., Monti D., Giunta S., Olivieri F., Sevini F., Panourgia M.P., Invidia L., Celani L., Scurti M., Cevenini E., Castellani G.C., Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Frank D.N., Pace N.R. Gastrointestinal microbiology enters the metagenomics era. Curr. Opin. Gastroenterol. 2008;24(1):4–10. doi: 10.1097/MOG.0b013e3282f2b0e8. [DOI] [PubMed] [Google Scholar]
- Frank M.G., Baratta M.V., Sprunger D.B., Watkins L.R., Maier S.F. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav. Immun. 2007;21(1):47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Frank M.G., Thompson B.M., Watkins L.R., Maier S.F. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav. Immun. 2012;26(2):337–345. doi: 10.1016/j.bbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaci N., Borrel G., Tottey W., O'Toole P.W., Brugere J.F. Archaea and the human gut: new beginning of an old story. World J. Gastroenterol. 2014;20(43):16062–16078. doi: 10.3748/wjg.v20.i43.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly P., Brenhouse H.C. Broken or maladaptive? Altered trajectories in neuroinflammation and behavior after early life adversity. Dev. Cogn. Neurosci. 2015;11:18–30. doi: 10.1016/j.dcn.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau M.G., Jury J., MacQueen G., Sherman P.M., Perdue M.H. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56(11):1522–1528. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers D., Kugathasan S., Denson L.A., Vazquez-Baeza Y., Van Treuren W., Ren B., Schwager E., Knights D., Song S.J., Yassour M., Morgan X.C., Kostic A.D., Luo C., Gonzalez A., McDonald D., Haberman Y., Walters T., Baker S., Rosh J., Stephens M., Heyman M., Markowitz J., Baldassano R., Griffiths A., Sylvester F., Mack D., Kim S., Crandall W., Hyams J., Huttenhower C., Knight R., Xavier R.J. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe. 2014;15(3):382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubeva A.V., Crampton S., Desbonnet L., Edge D., O'Sullivan O., Lomasney K.W., Zhdanov A.V., Crispie F., Moloney R.D., Borre Y.E., Cotter P.D., Hyland N.P., O'Halloran K.D., Dinan T.G., O'Keeffe G.W., Cryan J.F. Prenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology. 2015;60:58–74. doi: 10.1016/j.psyneuen.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Hinwood M., Morandini J., Day T.A., Walker F.R. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb. Cortex. 2012;22(6):1442–1454. doi: 10.1093/cercor/bhr229. [DOI] [PubMed] [Google Scholar]
- Hinwood M., Tynan R.J., Charnley J.L., Beynon S.B., Day T.A., Walker F.R. Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cereb. Cortex. 2013;23(8):1784–1797. doi: 10.1093/cercor/bhs151. [DOI] [PubMed] [Google Scholar]
- Hodes G.E., Kana V., Menard C., Merad M., Russo S.J. Neuroimmune mechanisms of depression. Nat. Neurosci. 2015;18(10):1386–1393. doi: 10.1038/nn.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Dissing-Olesen L., Stevens B. New insights on the role of microglia in synaptic pruning in health and disease. Curr. Opin. Neurobiol. 2016;36:128–134. doi: 10.1016/j.conb.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell M.P., Muglia L.J. Effects of genetically altered brain glucocorticoid receptor action on behavior and adrenal axis regulation in mice. Front. Neuroendocrinol. 2006;27(3):275–284. doi: 10.1016/j.yfrne.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., Codelli J.A., Chow J., Reisman S.E., Petrosino J.F., Patterson P.H., Mazmanian S.K. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland N.P., O'Mahony S.M., O'Malley D., O'Mahony C.M., Dinan T.G., Cryan J.F. Early-life stress selectively affects gastrointestinal but not behavioral responses in a genetic model of brain-gut axis dysfunction. Neurogastroenterol. Motil. 2015;27(1):105–113. doi: 10.1111/nmo.12486. [DOI] [PubMed] [Google Scholar]
- Ivanov A.I., Naydenov N.G. Dynamics and regulation of epithelial adherens junctions: recent discoveries and controversies. Int. Rev. Cell Mol. Biol. 2013;303:27–99. doi: 10.1016/B978-0-12-407697-6.00002-7. [DOI] [PubMed] [Google Scholar]
- Jara L.J., Navarro C., Medina G., Vera-Lastra O., Blanco F. Immune-neuroendocrine interactions and autoimmune diseases. Clin. Dev. Immunol. 2006;13(2–4):109–123. doi: 10.1080/17402520600877059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery I.B., Quigley E.M., Ohman L., Simren M., O'Toole P.W. The microbiota link to irritable bowel syndrome: an emerging story. Gut Microbes. 2012;3(6):572–576. doi: 10.4161/gmic.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.D., Campisi J., Sharkey C.M., Kennedy S.L., Nickerson M., Greenwood B.N., Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135(4):1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Kamada N., Seo S.U., Chen G.Y., Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013;13(5):321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- Kasubuchi M., Hasegawa S., Hiramatsu T., Ichimura A., Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7(4):2839–2849. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J.R., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G., Hyland N.P. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H., Hanisch U.K., Noda M., Verkhratsky A. Physiology of microglia. Physiol. Rev. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kim H.S., Suh Y.H. Minocycline and neurodegenerative diseases. Behav. Brain Res. 2009;196(2):168–179. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Kim S.U., de Vellis J. Microglia in health and disease. J. Neurosci. Res. 2005;81(3):302–313. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
- Kleiman A., Tuckermann J.P. Glucocorticoid receptor action in beneficial and side effects of steroid therapy: lessons from conditional knockout mice. Mol. Cell Endocrinol. 2007;275(1–2):98–108. doi: 10.1016/j.mce.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Kreisel T., Frank M.G., Licht T., Reshef R., Ben-Menachem-Zidon O., Baratta M.V., Maier S.F., Yirmiya R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol. Psychiatry. 2014;19(6):699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- Kunis G., Baruch K., Rosenzweig N., Kertser A., Miller O., Berkutzki T., Schwartz M. IFN-gamma-dependent activation of the brain's choroid plexus for CNS immune surveillance and repair. Brain. 2013;136(Pt 11):3427–3440. doi: 10.1093/brain/awt259. [DOI] [PubMed] [Google Scholar]
- Kurokawa K., Itoh T., Kuwahara T., Oshima K., Toh H., Toyoda A., Takami H., Morita H., Sharma V.K., Srivastava T.P., Taylor T.D., Noguchi H., Mori H., Ogura Y., Ehrlich D.S., Itoh K., Takagi T., Sakaki Y., Hayashi T., Hattori M. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007;14(4):169–181. doi: 10.1093/dnares/dsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R.E., Hamady M., Lozupone C., Turnbaugh P.J., Ramey R.R., Bircher J.S., Schlegel M.L., Tucker T.A., Schrenzel M.D., Knight R., Gordon J.I. Evolution of mammals and their gut microbes. Science. 2008;320(5883):1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman S.L. The neuroendocrinology of stress: a never ending story. J. Neuroendocrinol. 2008;20(6):880–884. doi: 10.1111/j.1365-2826.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- Louveau A., Smirnov I., Keyes T.J., Eccles J.D., Rouhani S.J., Peske J.D., Derecki N.C., Castle D., Mandell J.W., Lee K.S., Harris T.H., Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M. Microbial endocrinology and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014;817:3–24. doi: 10.1007/978-1-4939-0897-4_1. [DOI] [PubMed] [Google Scholar]
- Lyte M. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013;9(11):e1003726. doi: 10.1371/journal.ppat.1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J.L. Regulation of the movement of solutes across tight junctions. Annu. Rev. Physiol. 1998;60:143–159. doi: 10.1146/annurev.physiol.60.1.143. [DOI] [PubMed] [Google Scholar]
- Mastorakos G., Ilias I. Interleukin-6: a cytokine and/or a major modulator of the response to somatic stress. Ann. N. Y. Acad. Sci. 2006;1088:373–381. doi: 10.1196/annals.1366.021. [DOI] [PubMed] [Google Scholar]
- Mastorakos G., Ilias I. Relationship between interleukin-6 (IL-6) and hypothalamic-pituitary-adrenal axis hormones in rheumatoid arthritis. Z Rheumatol. 2000;59(Suppl. 2) doi: 10.1007/s003930070023. II/75–9. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Zhang X., Ono Y., Nagatomi R. Acute stress-induced colonic tissue HSP70 expression requires commensal bacterial components and intrinsic glucocorticoid. Brain Behav. Immun. 2009;23(1):108–115. doi: 10.1016/j.bbi.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Mayer E.A. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47(6):861–869. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E.A., Knight R., Mazmanian S.K., Cryan J.F., Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J. Neurosci. 2014;34(46):15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E.A., Tillisch K., Gupta A. Gut/brain axis and the microbiota. J. Clin. Invest. 2015;125(3):926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian S.K., Liu C.H., Tzianabos A.O., Kasper D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Gray J.D., Nasca C. 60 YEARS OF NEUROENDOCRINOLOGY: redefining neuroendocrinology: stress, sex and cognitive and emotional regulation. J. Endocrinol. 2015;226(2):T67–T83. doi: 10.1530/JOE-15-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan D.P., Fitzgerald P., Dinan T.G., Cryan J.F. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol. Motil. 2010;22(9) doi: 10.1111/j.1365-2982.2010.01520.x. 1029–35, e268. [DOI] [PubMed] [Google Scholar]
- Messaoudi M., Lalonde R., Violle N., Javelot H., Desor D., Nejdi A., Bisson J.F., Rougeot C., Pichelin M., Cazaubiel M., Cazaubiel J.M. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011;105(5):755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- Messaoudi M., Violle N., Bisson J.F., Desor D., Javelot H., Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2(4):256–261. doi: 10.4161/gmic.2.4.16108. [DOI] [PubMed] [Google Scholar]
- Miller A.H. Neuroendocrine and immune system interactions in stress and depression. Psychiatr. Clin. North Am. 1998;21(2):443–463. doi: 10.1016/s0193-953x(05)70015-0. [DOI] [PubMed] [Google Scholar]
- Moloney R.D., Desbonnet L., Clarke G., Dinan T.G., Cryan J.F. The microbiome: stress, health and disease. Mamm. Genome. 2014;25(1–2):49–74. doi: 10.1007/s00335-013-9488-5. [DOI] [PubMed] [Google Scholar]
- Moloney R.D., O'Leary O.F., Felice D., Bettler B., Dinan T.G., Cryan J.F. Early-life stress induces visceral hypersensitivity in mice. Neurosci. Lett. 2012;512(2):99–102. doi: 10.1016/j.neulet.2012.01.066. [DOI] [PubMed] [Google Scholar]
- Mulla A., Buckingham J.C. Regulation of the hypothalamo-pituitary-adrenal axis by cytokines. Baillieres Best. Pract. Res. Clin. Endocrinol. Metab. 1999;13(4):503–521. doi: 10.1053/beem.1999.0041. [DOI] [PubMed] [Google Scholar]
- Nayak D., Zinselmeyer B.H., Corps K.N., McGavern D.B. In vivo dynamics of innate immune sentinels in the CNS. Intravital. 2012;1(2):95–106. doi: 10.4161/intv.22823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld K.A., Kang N., Bienenstock J., Foster J.A. Effects of intestinal microbiota on anxiety-like behavior. Commun. Integr. Biol. 2011;4(4):492–494. doi: 10.4161/cib.4.4.15702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld K.M., Kang N., Bienenstock J., Foster J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011;23(3) doi: 10.1111/j.1365-2982.2010.01620.x. 255–64, e119. [DOI] [PubMed] [Google Scholar]
- Nilsson N.E., Kotarsky K., Owman C., Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 2003;303(4):1047–1052. doi: 10.1016/s0006-291x(03)00488-1. [DOI] [PubMed] [Google Scholar]
- Nishino R., Mikami K., Takahashi H., Tomonaga S., Furuse M., Hiramoto T., Aiba Y., Koga Y., Sudo N. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol. Motil. 2013;25(6):521–528. doi: 10.1111/nmo.12110. [DOI] [PubMed] [Google Scholar]
- O'Connor R.M., Cryan J.F. Adolescent brain vulnerability and psychopathology through the generations: role of diet and dopamine. Biol. Psychiatry. 2014;75(1):4–6. doi: 10.1016/j.biopsych.2013.10.022. [DOI] [PubMed] [Google Scholar]
- O'Mahony S.M., Clarke G., Dinan T.G., Cryan J.F. Early-life adversity and brain development: is the microbiome a missing piece of the puzzle? Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.09.068. [DOI] [PubMed] [Google Scholar]
- O'Mahony S.M., Felice V.D., Nally K., Savignac H.M., Claesson M.J., Scully P., Woznicki J., Hyland N.P., Shanahan F., Quigley E.M., Marchesi J.R., O'Toole P.W., Dinan T.G., Cryan J.F. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience. 2014;277:885–901. doi: 10.1016/j.neuroscience.2014.07.054. [DOI] [PubMed] [Google Scholar]
- Palmer C., Bik E.M., DiGiulio D.B., Relman D.A., Brown P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5(7):e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomar M.M., Maldonado Galdeano C., Perdigon G. Influence of a probiotic lactobacillus strain on the intestinal ecosystem in a stress model mouse. Brain Behav. Immun. 2014;35:77–85. doi: 10.1016/j.bbi.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira T.A., Guiducci E., Dumas L., Ragozzino D., Gross C.T. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Petanjek Z., Judas M., Simic G., Rasin M.R., Uylings H.B., Rakic P., Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2011;108(32):13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitossi F., del Rey A., Kabiersch A., Besedovsky H. Induction of cytokine transcripts in the central nervous system and pituitary following peripheral administration of endotoxin to mice. J. Neurosci. Res. 1997;48(4):287–298. doi: 10.1002/(sici)1097-4547(19970515)48:4<287::aid-jnr1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Pocock J.M., Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30(10):527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Pott J., Hornef M. Innate immune signalling at the intestinal epithelium in homeostasis and disease. EMBO Rep. 2012;13(8):684–698. doi: 10.1038/embor.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., Mende D.R., Li J., Xu J., Li S., Li D., Cao J., Wang B., Liang H., Zheng H., Xie Y., Tap J., Lepage P., Bertalan M., Batto J.M., Hansen T., Le Paslier D., Linneberg A., Nielsen H.B., Pelletier E., Renault P., Sicheritz-Ponten T., Turner K., Zhu H., Yu C., Li S., Jian M., Zhou Y., Li Y., Zhang X., Li S., Qin N., Yang H., Wang J., Brunak S., Dore J., Guarner F., Kristiansen K., Pedersen O., Parkhill J., Weissenbach J., Meta H.I.T.C., Bork P., Ehrlich S.D., Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajilic-Stojanovic M., Heilig H.G., Tims S., Zoetendal E.G., de Vos W.M. Long-term monitoring of the human intestinal microbiota composition. Environ. Microbiol. 2012 doi: 10.1111/1462-2920.12023. [DOI] [PubMed] [Google Scholar]
- Rakic P., Bourgeois J.P., Eckenhoff M.F., Zecevic N., Goldman-Rakic P.S. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232(4747):232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Rakic P., Bourgeois J.P., Goldman-Rakic P.S. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog. Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- Rakic P.T. The importance of being well placed and having the right connections. Ann. N. Y. Acad. Sci. 1999;882:90–106. doi: 10.1111/j.1749-6632.1999.tb08536.x. discussion 128–34. [DOI] [PubMed] [Google Scholar]
- Ransohoff R.M., Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012;12(9):623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- Ransohoff R.M., Schafer D., Vincent A., Blachere N.E., Bar-Or A. Neuroinflammation: ways in which the immune system affects the brain. Neurotherapeutics. 2015 doi: 10.1007/s13311-015-0385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D., Clasen L., Gogtay N., Giedd J.N. How does your cortex grow? J. Neurosci. 2011;31(19):7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber S.O., Peters S., Slattery D.A., Hofmann C., Scholmerich J., Neumann I.D., Obermeier F. Mucosal immunosuppression and epithelial barrier defects are key events in murine psychosocial stress-induced colitis. Brain Behav. Immun. 2011;25(6):1153–1161. doi: 10.1016/j.bbi.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Rhee S.H., Pothoulakis C., Mayer E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009;6(5):306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M., Tracey K.J. Cholinergic control of inflammation. J. Intern Med. 2009;265(6):663–679. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet J., Gupta D., Dziarski R. Peptidoglycan recognition proteins: modulators of the microbiome and inflammation. Nat. Rev. Immunol. 2011;11(12):837–851. doi: 10.1038/nri3089. [DOI] [PubMed] [Google Scholar]
- Russell W.R., Hoyles L., Flint H.J., Dumas M.E. Colonic bacterial metabolites and human health. Curr. Opin. Microbiol. 2013;16(3):246–254. doi: 10.1016/j.mib.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Sampson T.R., Mazmanian S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17(5):565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savastano S., Tommaselli A.P., Valentino R., Scarpitta M.T., D'Amore G., Luciano A., Covelli V., Lombardi G. Hypothalamic-pituitary-adrenal axis and immune system. Acta Neurol. (Napoli) 1994;16(4):206–213. [PubMed] [Google Scholar]
- Savignac H.M., Couch Y., Stratford M., Bannerman D.M., Tzortzis G., Anthony D.C., Burnet P.W. Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL-1-beta levels in male mice. Brain Behav. Immun. 2015 doi: 10.1016/j.bbi.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savignac H.M., Kiely B., Dinan T.G., Cryan J.F. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil. 2014;26(11):1615–1627. doi: 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- Scarpellini E., Ianiro G., Attili F., Bassanelli C., De Santis A., Gasbarrini A. The human gut microbiota and virome: potential therapeutic implications. Dig. Liver Dis. 2015 doi: 10.1016/j.dld.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D.P., Lehrman E.K., Kautzman A.G., Koyama R., Mardinly A.R., Yamasaki R., Ransohoff R.M., Greenberg M.E., Barres B.A., Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D.P., Lehrman E.K., Stevens B. The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61(1):24–36. doi: 10.1002/glia.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D.P., Stevens B. Microglia function in Central nervous system development and plasticity. Cold Spring Harb. Perspect. Biol. 2015 doi: 10.1101/cshperspect.a020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K., Cowen P.J., Harmer C.J., Tzortzis G., Errington S., Burnet P.W. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacol. Berl. 2015;232(10):1793–1801. doi: 10.1007/s00213-014-3810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar V., Homer D., Rigsbee L., Khamis H.J., Michail S., Raymer M., Reo N.V., Paliy O. The networks of human gut microbe-metabolite associations are different between health and irritable bowel syndrome. ISME J. 2015;9(8):1899–1903. doi: 10.1038/ismej.2014.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A., Gottfried-Blackmore A., Milner T.A., McEwen B.S., Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56(6):659–674. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Vale W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza D.G., Vieira A.T., Soares A.C., Pinho V., Nicoli J.R., Vieira L.Q., Teixeira M.M. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J. Immunol. 2004;173(6):4137–4146. doi: 10.4049/jimmunol.173.6.4137. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Holmes C.J., Jernigan T.L., Toga A.W. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat. Neurosci. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Tessner K.D., Toga A.W. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J. Neurosci. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Toga A.W. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10(4):372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Spear L.P. Adolescent brain development and animal models. Ann. N. Y. Acad. Sci. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Steenbergen L., Sellaro R., van Hemert S., Bosch J.A., Colzato L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015;48:258–264. doi: 10.1016/j.bbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Sudo N. Role of microbiome in regulating the HPA axis and its relevance to allergy. Chem. Immunol. Allergy. 2012;98:163–175. doi: 10.1159/000336510. [DOI] [PubMed] [Google Scholar]
- Sudo N., Chida Y., Aiba Y., Sonoda J., Oyama N., Yu X.N., Kubo C., Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Akira S. Microbial recognition by Toll-like receptors. J. Dermatol Sci. 2004;34(2):73–82. doi: 10.1016/j.jdermsci.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Tikka T.M., Koistinaho J.E. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J. Immunol. 2001;166(12):7527–7533. doi: 10.4049/jimmunol.166.12.7527. [DOI] [PubMed] [Google Scholar]
- Tillisch K., Labus J., Kilpatrick L., Jiang Z., Stains J., Ebrat B., Guyonnet D., Legrain-Raspaud S., Trotin B., Naliboff B., Mayer E.A. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144(7):1394–1401. doi: 10.1053/j.gastro.2013.02.043. 1401 e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K.J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 2007;117(2):289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramullas M., Finger B.C., Moloney R.D., Golubeva A.V., Moloney G., Dinan T.G., Cryan J.F. Toll-like receptor 4 regulates chronic stress-induced visceral pain in mice. Biol. Psychiatry. 2014;76(4):340–348. doi: 10.1016/j.biopsych.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Tremblay M.E., Stevens B., Sierra A., Wake H., Bessis A., Nimmerjahn A. The role of microglia in the healthy brain. J. Neurosci. 2011;31(45):16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C., Chrousos G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002;53(4):865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Turrin N.P., Rivest S. Unraveling the molecular details involved in the intimate link between the immune and neuroendocrine systems. Exp. Biol. Med. (Maywood) 2004;229(10):996–1006. doi: 10.1177/153537020422901003. [DOI] [PubMed] [Google Scholar]
- Tynan R.J., Naicker S., Hinwood M., Nalivaiko E., Buller K.M., Pow D.V., Day T.A., Walker F.R. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav. Immun. 2010;24(7):1058–1068. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Vaishnava S., Behrendt C.L., Hooper L.V. Innate immune responses to commensal bacteria in the gut epithelium. J. Pediatr. Gastroenterol. Nutr. 2008;1(46 Suppl):E10–E11. doi: 10.1097/01.mpg.0000313823.93841.65. [DOI] [PubMed] [Google Scholar]
- Vaishnava S., Behrendt C.L., Ismail A.S., Eckmann L., Hooper L.V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. U. S. A. 2008;105(52):20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S., Nakaoke R., Dohgu S., Banks W.A. Release of cytokines by brain endothelial cells: a polarized response to lipopolysaccharide. Brain Behav. Immun. 2006;20(5):449–455. doi: 10.1016/j.bbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- von Bernhardi R., Eugenin-Von Bernhardi L., Eugenin J. Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 2015;7:124. doi: 10.3389/fnagi.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder R.L. Neuroendocrine-immune system interactions and autoimmunity. Annu. Rev. Immunol. 1995;13:307–338. doi: 10.1146/annurev.iy.13.040195.001515. [DOI] [PubMed] [Google Scholar]
- Williamson L.L., McKenney E.A., Holzknecht Z.E., Belliveau C., Rawls J.F., Poulton S., Parker W., Bilbo S.D. Got worms? Perinatal exposure to helminths prevents persistent immune sensitization and cognitive dysfunction induced by early-life infection. Brain Behav. Immun. 2015 doi: 10.1016/j.bbi.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Wohleb E.S., McKim D.B., Shea D.T., Powell N.D., Tarr A.J., Sheridan J.F., Godbout J.P. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol. Psychiatry. 2014;75(12):970–981. doi: 10.1016/j.biopsych.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb E.S., McKim D.B., Sheridan J.F., Godbout J.P. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front. Neurosci. 2014;8:447. doi: 10.3389/fnins.2014.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb E.S., Powell N.D., Godbout J.P., Sheridan J.F. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J. Neurosci. 2013;33(34):13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R., Lu H., Butovsky O., Ohno N., Rietsch A.M., Cialic R., Wu P.M., Doykan C.E., Lin J., Cotleur A.C., Kidd G., Zorlu M.M., Sun N., Hu W., Liu L., Lee J.C., Taylor S.E., Uehlein L., Dixon D., Gu J., Floruta C.M., Zhu M., Charo I.F., Weiner H.L., Ransohoff R.M. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 2014;211(8):1533–1549. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]