Abstract
Humans often make decisions in stressful situations, for example when the stakes are high and the potential consequences severe, or when the clock is ticking and the task demand is overwhelming. In response, a whole train of biological responses to stress has evolved to allow organisms to make a fight-or-flight response. When under stress, fast and effortless heuristics may dominate over slow and demanding deliberation in making decisions under uncertainty. Here, I review evidence from behavioral studies and neuroimaging research on decision making under stress and propose that stress elicits a switch from an analytic reasoning system to intuitive processes, and predict that this switch is associated with diminished activity in the prefrontal executive control regions and exaggerated activity in subcortical reactive emotion brain areas. Previous studies have shown that when stressed, individuals tend to make more habitual responses than goal-directed choices, be less likely to adjust their initial judgment, and rely more on gut feelings in social situations. It is possible that stress influences the arbitration between the emotion responses in subcortical regions and deliberative processes in the prefrontal cortex, so that final decisions are based on unexamined innate responses. Future research may further test this ‘stress induced deliberation-to-intuition’ (SIDI) model and examine its underlying neural mechanisms.
Keywords: Stress, Decision making, Cortisol
Sometimes when people are under stress, they hate to think, and it's the time when they most need to think.
Bill Clinton
1. Introduction
Stressful situations are not uncommon in everyday life, experienced for example by a doctor in the emergency room, a police officer in action, or a financial trader on a London trading floor. Individuals sometimes need to make important decisions when the stakes are high and when not enough information or cognitive resources are available to guarantee a sound choice. However, the high pressure may dramatically change decision making strategies, leading to different choices than would be made without such pressure. For example, individuals may approach situations differently depending on whether decisions are easy to make without far reaching consequences or life-altering and ambiguous. Although it is vital to understand decision making under stress, the majority of previous studies on decision making are carried out in non-stressful contexts. Only recent years have witnessed a remarkable burgeoning of decision-making research related to stress. But many findings in this field are mixed, leaving the specific effects of stress on judgment and decision making relatively unclear. The purpose of the current review is to summarize evidence from both human and animal studies on decisions under stress and to elucidate the neurobiological mechanisms underlying strategy shifting in the context of stress induced decision making.
Theories on decision making have proposed that there are two routes to making decisions: a fast route labeled System 1 and a slow route labeled System 2 (Kahneman, 2011, Evans, 2008, Gilovich et al., 2002, Evans and Stanovich, 2013). System 1 operates quickly and automatically with little effort. It activates our innate and instinctive responses to stimuli. For example, whenever a snake is detected or believed to be out there, instinctive fear is aroused and avoidance behavior is initiated without much thought. Such genetically hard-wired responses can enhance our ability to cope with vital environmental challenges of the type experienced during most of human history. Prolonged practice and experience also produce involuntary actions or habits. On the other hand, System 2 runs slowly and in an effortful manner, requiring complex computation. The pros and cons associated with each option are calculated and compared until an optimal choice can be made. Comparing both systems, System 2 is thought to be an evolutionarily more recent system and can flexibly check, modify, and override the decisions from System 1 (Tversky & Kahneman, 1983).
Although evidences showing that stress modulates decision making is accumulating (Starcke and Brand, 2012, Galvan and Rahdar, 2013, Morgado et al., 2015), there is currently no theoretical framework to explain why stress should influence decisions in certain ways. The evolutionary perspective on stress posits that the stress response has been shaped by natural selection to increase the ability of organisms to cope with situations that require action or defense (Cannon, 1914). When organisms are faced with possible damage or a loss of resources and a “fight-or-flight” response is required, they can express protective features that allow them to survive adverse conditions and help them mitigate the harmful effects of environmental stresses (Nesse & Young, 2000). For example, when under stress, organisms show increases in heart rate and contractility that speed circulation, increases in the rate and depth of breathing that speed gas exchange, increased sweating that cools the body and makes it slippery, increased glucose synthesis associated with spikes in energy, a shunt of blood from gut and skin to muscles, and greater muscle tension that increases strength and endurance (Graham, 1953).
Based on the evolutionary accounts of stress and the dual-system theories of judgment and decision making, it is reasonable to predict that stress promotes evolutionarily rooted intuitive responses in System 1. These intuitive responses are fast and require fewer cognitive resources to execute than in System 2. In normal situations, the intuition system may initiate some default action tendency and the reasoning system checks whether such a tendency is compatible with the current goals and environment. That is, intuition proposes first and reasoning decides whether to approve or to modify it. When under stress, the reasoning system may not check these response tendencies and instead allow individuals to rely on these rigid default actions in response to environmental challenges.
Grounded on this ‘intuition proposes, reasoning decides’ type of dual-system, I propose that when under stress, intuitive responses may bypass the examination of reasoning and reach the threshold to become final decisions. Thus, stressed individuals may fall back more on intuition and involve less amounts of conscious reasoning. Because they are intuitive, automatic processes ought to lead to premature choices, and stress may exacerbate decision biases via shifting decision strategies from deliberation to intuition, labeled as “stress induced deliberation-to-intuition” (SIDI). Intuition is a cognitive process that has multiple layers of meanings and implications. In the SIDI model, intuition refers to an automatic, habitual, and evolutionarily based decision making process, corresponding to System 1. Deliberation refers to System 2, which is a slow, goal-directed, and reasoning based process. This dual system account complements the previous theory that stress promotes a shift from flexible cognitive to rather rigid habit memory systems (Schwabe & Wolf, 2013). It is also consistent with the idea that stress impairs prefrontal cortex functions such as working memory and attention regulation, switching from thoughtful ‘top-down’ control by the PFC to ‘bottom-up’ control by the amygdala and related subcortical structures (Arnsten, 2009). Here, I extend these ideas by explicitly proposing that stress favors intuition versus reasoning in the decision making domain and thus leads to decision biases in some circumstances. The effects of stress on human behaviors are multifaceted (Staal, 2004), including long-term memory (Schwabe and Wolf, 2013, Schwabe et al., 2012, Joëls et al., 2006), working memory (Arnsten, 2009), and learning (Joëls et al., 2006). In this review, I focus only on the effect of stress on reward-based decision making in humans and discuss how the existing empirical findings support the SIDI hypothesis.
2. Stressors potentiate decision biases
Reward-based decision making refers to the process of examining reward magnitudes, probabilities, and risks, comparing options, and choosing a course of action. In the next section, I summarize the findings concerning stress and different decision making components, including the encoding of decision parameters (e.g. reward/punishment processing and risk analysis), executive control, and social decision making (see Table 1).
Table 1.
Effects of acute and chronic stress on decision making.
| Category | Study | Results | Decision-making paradigm |
|---|---|---|---|
| Reward | Bogdan & Pizzagalli, 2006 | Hyposensitive to reward | Signal detection task |
| Elman et al., 2009 | Hyposensitive to reward | Wheel of fortune–type task | |
| Ossewaarde et al., 2011 | Hyposensitive to reward | Monetary incentive delay task | |
| Lighthall et al., 2012 | Greater reward collection and faster responses in males Less reward collection and slower responses in females |
Balloon analogue risk task | |
| Nikolova et al., 2012 | Hyposensitive to reward | Number guessing paradigm | |
| Porcelli et al., 2012 | Hyposensitive to reward# | Card guessing task | |
| Oei et al., 2014 | Hyposensitive to reward | Masked sexual stimuli | |
| Montoya et al., 2014 | Hyposensitive to reward | Monetary incentive delay task | |
| Lewis et al., 2014 | Hypersensitive to reward magnitude | Pavlovian conditioning task | |
| Punishment and threat | Petzold et al., 2010 | Reduced use of negative feedback | Probabilistic learning task |
| Robinson et al., 2013 | Increased aversive prediction error signals | The “What's in the box?” task | |
| Cavanagh et al., 2011 | Better punishment learning | Probabilistic learning task | |
| Gullo and Stieger, 2011 | Increasing sensitivity to losses | Iowa Gambling Task | |
| Roelofs et al., 2007 | Vigilant to the angry faces | Angry and happy faces task | |
| van Wingen et al., 2011 | Increased reactivity to threat | Angry and happy faces task | |
| Akinola and Mendes, 2012 | Heightened sensitivity to potential danger | Shooting targets task | |
| Jackson et al., 2006 | Facilitate fear conditioning in males Inhibit fear conditioning in females |
Fear conditioning | |
| Stark et al., 2006 | Facilitate fear conditioning in males Inhibit fear conditioning in females |
Fear conditioning | |
| Tabbert et al., 2010 | Enhance fear responses in females | Fear conditioning | |
| Merz et al., 2012 | No effect on fear conditioning/extinction | Fear conditioning | |
| Klucken et al., 2013 | Weak gene and stressful life events interaction | Fear conditioning | |
| Merz et al., 2013a | Facilitate fear conditioning in men and women taking oral contraceptives | Fear conditioning | |
| Merz et al., 2013b | Impaired fear conditioning in men and facilitated fear conditioning in women taking oral contraceptives | Fear conditioning | |
| Antov et al., 2013 | Attenuate fear conditioning after 2nd wave and facilitate fear conditioning after 1st wave in men | Fear conditioning | |
| Merz et al., 2014 | Attenuates fear retrieval in men | Fear conditioning | |
| Risk | Preston et al., 2007 | Risk seeking in males Risk averse in females |
Iowa gambling task |
| van den Bos et al., 2009 | Risk seeking in males U shape in females |
Iowa gambling task | |
| Mather et al., 2009 | Risk averse in elderly | Iowa gambling task | |
| Zhang et al., 2011 | Poorer performance only in formerly heroin-dependent patients | Iowa gambling task | |
| Starcke et al., 2008 | Risk seeking | Game of Dice Task | |
| Pabst et al., 2013b | Risk seeking | Game of Dice Task | |
| Pabst et al., 2013a | Reduced reflection effect in loss framing | Game of Dice task | |
| Gathmann et al., 2014 | Risk seeking | Game of Dice Task | |
| Lighthall et al., 2009 | Risk seeking in males Risk averse in females |
Balloon analogue risk task | |
| Kandasamy et al., 2014 | Risk averse | Financial choices in the field | |
| Porcelli and Delgado, 2009 | Enhanced reflection effect | Financial decision-making task | |
| Cueva et al., 2015 | Risk seeking | Asset trading game | |
| Haushofer et al., 2013 | No effect on delay discounting | Delay discounting task | |
| Cognitive control | Raio et al., 2013 | Impaired emotion regulation | Fear learning |
| Schwabe and Wolf, 2009 | More habitual choices | Instrumental learning | |
| Schwabe et al., 2011 | More habitual choices | Instrumental learning | |
| Dias-Ferreira et al., 2009∗ | More habitual choices | Instrumental learning | |
| Soares et al., 2012∗ | More habitual choices | Instrumental learning | |
| Kassam et al., 2009 | More habitual choices | Anchor and adjustment task | |
| Maier et al., 2015 | More immediate gratification | Food choice task | |
| Seehagen et al., 2015 | More habitual responses in infants | Instrumental learning task. | |
| Margittai et al., 2015a | More intuitive thinking | cognitive reflection test | |
| Prosocial tendency | Takahashi et al., 2007 | Give more | Dictator's game |
| Vinkers et al., 2013 | Give less | Dictator's game | |
| von Dawans et al., 2012 | Share more | Trust game | |
| McGinley et al., 2010 | Greater anonymous prosocial tendency Less costly prosocial tendency |
Survey | |
| Starcke et al., 2012 | Less utilitarian | Moral dilemmas | |
| Youssef et al., 2012 | Less utilitarian | Moral dilemmas | |
| Starcke et al., 2011 | More egoistic | Everyday Moral Decision-Making | |
| Leder et al., 2013 | Reduced emtalizing | Beauty contest game | |
| Smeets et al., 2009 | Enhanced metalizing in males Reduced metalizing in females |
Movie for the Assessment of Social Cognition | |
| Margittai et al., 2015b | Increased generosity towards close but not distant others | Social discounting task |
∗Animal studies.
#Another possibility is hypersensitivity to punishment.
2.1. Altered encoding of decision parameters
2.1.1. Reward sensitivity
Weighing positive and negative aspects of decision options is the first important step in decision making. It has been proposed that stress triggers additional reward salience (STARS), which may contribute to a stress induced risk preference change (Mather & Lighthall, 2012). It is argued that because stress sensitizes dopamine release in reward-processing brain regions (Pruessner et al., 2004), acute stress may enhance selection of previously rewarding outcomes but impair avoidance of previously negative outcomes. Indeed, there is some empirical evidence of reward hypersensitivity under stress. In one study, cortisol responders in the stress group, compared to the no stress group, exhibited differential activity in the ventral striatum to cues predicting high versus low reward (Lewis et al., 2014). In another, a cold pressor stress enhanced learning about cues that predicted positive outcomes in both younger and older adults (Lighthall et al., 2013). It has also been found that behavioral preference for sexual rewards increased with long-term cortisol exposure (2 months pre-test cortisol derived from a hair sample) (Chumbley et al., 2014). However, most studies have shown that acute stress actually reduces reward responsiveness. Using the same probabilistic stimulus selection task (PSST) used in the Lighthall et al. (2013) study, Berghorst et al. (2013) found that stress induced by threat of shock selectively reduced reward sensitivity with no influence on punishment processing in highly stress-reactive individuals (Berghorst et al., 2013). Other research showed that stress induced by threat of shock impaired reward responsiveness, particularly in individuals with anhedonic symptoms (Bogdan & Pizzagalli, 2006); regression analyses indicated that self-report measures of anhedonia predicted stress-induced hedonic deficits even after controlling for anxiety symptoms (Bogdan & Pizzagalli, 2006). This is consistent with findings showing that individuals with posttraumatic stress disorder exhibit smaller bilateral striatal activations in response to gains, which are in turn associated with more self-reported motivational and social deficits (Elman et al., 2009). Participants under stress also show decreased differential responses to reward and punishment in the dorsal striatum and OFC (Porcelli et al., 2012). However, whether such an effect is driven by the reduced sensitivity to reward, enhanced responses to punishment, or both, remains unknown. Consistent with these stress studies on reward processing, in a randomized within-subject design, Montoya et al. found that administration of a high dose of cortisol (40 mg) down-regulated activity in the brain regions involved in reward-related behavior in male participants (Montoya et al., 2014). This study provides direct evidence that cortisol acts on brain's reward circuit, suggesting that stress may modulate reward processing via cortisol.
Gender differences and individuals' temperament are also important modulators of stress related reward sensitivity and decision-making. It was found that stress led to greater reward collection and faster decision speed in males but less reward collection and slower decision speed in females (Lighthall et al., 2012). One study on gender showed that psychological stress, elicited by strongly aversive movie clips, resulted in a significant decrease in reward-related responses in the medial prefrontal cortex (mPFC) without affecting ventral striatal responses in women (Ossewaarde et al., 2011). Individual differences research has shown that higher levels of life stress are associated with lower positive affect for participants with relatively low, but not for those with high, reward-related ventral striatum reactivity (Nikolova et al., 2012), and high cortisol levels are related to stronger ventral striatum activation (Oei et al., 2014).
Taken together, findings regarding whether stress increases or decreases reward sensitivity are still mixed. The SIDI account also does not make a simple prediction regarding the direction of reward sensitivity changes under stress. Whether stress amplifies or diminishes reward sensitivity may be task dependent.
2.1.2. Punishment and threat sensitivity
Previous research has shown that individuals tend to strongly prefer avoiding losses to acquiring gains, known as loss aversion (Kahneman & Tversky, 1984). Loss aversion might be the result of an evolutionary process selecting for the evolutionary most advantageous risk attitude (McDermott et al., 2008). According to the SIDI model, which emphasizes evolutionary adaptedness of decision-making, stress should potentiate loss aversion. In line with this idea, it has been found that during times of chronic stress, glucocorticoids, acting through the amygdala and hippocampus, promote selective attention to mostly negative precedents and produce a tendency to find threat and risk where none exist (Korte, 2001, Sapolsky, 2000). Thus, stress may facilitate the propensity to form threat-related associations. Stress also has been shown to significantly increase ventral striatum aversive (but not appetitive) prediction error signals in a learning task (Robinson et al., 2013). Amygdala and insula reactivity to biologically salient stimuli were also exaggerated in a group of combat exposed individuals, suggesting that severe stress exposure sensitizes amygdala and insula reactivity (van Wingen et al., 2011). Under stress as a result of social evaluative threat, low trait-level punishment sensitivity was related to a tendency towards better reward learning and poorer punishment learning; the opposite pattern was found in highly punishment sensitive individuals (Cavanagh et al., 2011). Stress has also been shown to restore decision-making deficits in heavy drinkers by increasing sensitivity to losses (relative to gains) in the Iowa Gambling Task (IGT) (Gullo & Stieger, 2011). Only one study found that stress reduces use of negative feedback in reward-based learning tasks (Petzold et al., 2010). Interestingly, one study found that individuals with lower chronic cortisol displayed stronger loss aversion, whereas individuals with higher endogenous cortisol weighted losses and gains more equally (Chumbley et al., 2014), suggesting that cortisol reduces oversensitivity to potential losses. Thus, in the domain of monetary negative feedback processing, the majority of studies consistently demonstrate that stress potentiates punishment detection, supporting the SIDI account.
Learning that certain environment stimuli predict aversive outcomes promotes survival in the face of present and future threats (Maren, 2001). Stress may operate as an orchestrated defense that makes innate fight or flight decisions to help animals adapt to threat (Cannon, 1932, McNaughton and Corr, 2004). Consistent with this notion, the SIDI model predicts that stress elevates the sensitivity to potential threat in general. Studies with individuals who underwent extreme stress in everyday life seem to support this. It was found that in soldiers, combat stress increased amygdala and insula reactivity to biologically salient stimuli (i.e. angry and fearful face stimuli) (van Wingen et al., 2011). Such enhanced vigilance to the angry faces was also found in high cortisol responders in a sample of healthy volunteers (Roelofs et al., 2007). Police officers who had larger cortisol increases to the social-stress task subsequently made fewer errors in a threat-related decision making task in which they were deciding whether or not to shoot targets, suggesting that stress may exacerbate vigilance for threat cues (Akinola & Mendes, 2012).
In additional to the primary physiological fight-or-flight responses to stress for both males and females, it has been proposed that females respond to stress by engaging in nurturing activities designed to benefit both mother and offspring (the tending pattern) and by affiliating with social groups to reduce risk (the befriending pattern) (Taylor et al., 2000, Taylor, 2006). The SIDI model accords with the ‘tend and befriend’ hypothesis and proposes that stress sharpens threat sensitivity in males in order for the individuals to get ready to engage in fight-or-flight) and reduces threat sensitivity in females to allow for being tending and befriending. Using the fear conditioning paradigm, several studies found gender-dependent effects of stress on fear acquisition and fear extinction. In a typical fear-conditioning procedure, an initially neutral conditioned stimulus (CS) acquires emotional properties through predictive pairings with an aversive unconditioned stimulus (US, e.g. an electric shock). During the fear acquisition phase, it was found that stress led to higher conditioned responses in males (Zorawski et al., 2005, Zorawski et al., 2006, Jackson et al., 2006, Merz et al., 2013a, Antov et al., 2013), but impaired conditioned responses in females (Jackson et al., 2006). Neuroimaging studies using cortisol application, however, revealed reduced conditioned responses in men (Stark et al., 2006), but enhanced conditioned responses in women in several brain structures (Stark et al., 2006, Tabbert et al., 2010). This discrepancy might be due to the fact that exogenous cortisol levels may actually inhibit the stress-induced activation of the hypothalamus–pituitary–adrenal (HPA) axis. During the extinction phase when no aversive stimulation was administered, women taking oral contraceptives exhibited higher conditioned responses than men and women in the luteal phase (Merz et al., 2012), although no effect of cortisol or sex hormones on fear acquisition was found in this study (Merz et al., 2012). Stress impaired the neuronal correlates of fear learning and expression in men, but facilitated them in oral contraceptives women (Merz et al., 2013b). During the retrieval phase, exposure to stress attenuated fear retrieval in healthy men (Merz et al., 2014). Although stressful life events (SLEs) did not have a main effect on fear conditioning, a weak 5-HTTLPR genotype by SLEs interaction on fear learning was found (Klucken et al., 2013). These studies suggest that how stress modulates fear learning depends on sex, current sex hormone availability, and genotypes. Taken together, stress seems to boost learning from negative feedback and threat in general, but it also exerts fine-tuned modulation on behaviors depending on the evolutionary demands of male and female Homo sapiens.
2.1.3. Risk analysis
Decision makers may fall back on automatized reactions to risk under the influence of disruptive stress. Previous research identified the reflection bias, the reversal of risk-attitude when the sign of the outcomes changes (Kahneman & Tversky, 1979). This bias emphasizes the inconsistency in risk attitude (risk aversion in the domain of gains but risk seeking in the domain of losses, The SIDI model predicts that stress amplifies this reflection effect since it might be evolutionally adaptive to change risk preference according to domains. Consistent with this prediction, using a novel financial decision-making task combined with ice-cold water induced stress, it was found that the reflection effect was significantly increased under stress (Porcelli & Delgado, 2009). Stress potentially exacerbates behavioral biases in decision-making by inducing more conservative choices for those who are generally risk-averse and more risky choices for those who tend to be risk-seekers. However, using a more complex Game of Dice Task (GDT), it was found that stress reduced the effect of loss framing, such that stressed participants showed less risky behavior compared with non-stressed participants (Pabst et al., 2013b). More studies are needed to further examine how stress modulates domain-specific risk sensitivity.
In the domain of mixed gains and losses, previous research on the relationship between stress and risk sensitivity has also yielded conflicting results. It has been demonstrated that cortisol levels in a group of male traders in London were significantly and positively correlated with financial uncertainty, which was measured by the variance of economic return and the expected variance of the market (Coates & Herbert, 2008). Thus, financial uncertainty elicits heightened stress, which may in turn shift individuals' risk preferences. In non-traders, recent research found that individual and aggregate levels of endogenous cortisol predict subsequent risk-taking and administered cortisol shifted investment towards riskier assets (Cueva et al., 2015). Indeed, using a double-blind, placebo-controlled, cross-over protocol, the same research group raised cortisol levels in volunteers over eight days and found that participants became more risk-averse (Kandasamy et al., 2014). It seems that stress response calibrates risk taking to the circumstances, so that individuals avoid risks in times of prolonged uncertainty, such as a financial crisis. Another study found that stress reduced older adults' but not younger adults' risk taking in a computer-based driving game (Mather et al., 2009). Thus, stress can lead to risk aversion at least in some circumstances in elderly.
On the other hand, it is argued that men should be more likely to take greater risk after stress, analogous to a ‘‘fight’’ response to stress during competition for territory or other valuable resources, whereas women should be more likely to be conservative after stress, avoiding endangerment of the lives of dependent offspring (Taylor et al., 2000). Consistent with this view, several studies have found gender differences in response to stress. In one study, acutely stressed (anticipating a public speech) men were risk-taking, whereas acutely stressed women were risk-aversive (Preston et al., 2007). Similar gender by stress results were found in research using the Balloon Analogue Risk Task in which risk taking is associated with more reward collection (Lighthall et al., 2012, Lighthall et al., 2009). Exposure to cold pressor stress (immerse a hand for 1 min into ice water) also increased neural response to the risky decision task in the dorsal striatum (putamen) and anterior insula among men, but decreased the response in these regions among women (Lighthall et al., 2012). In the IGT, the more (salivary) cortisol levels were elevated after the TSST the poorer the subsequent performance in the IGT in male subjects, whereas in females, slightly elevated levels of cortisol after the TSST improved IGT performance and highly elevated levels decreased IGT performance (van den Bos et al., 2009). However, a recent study found only a small but non-significant decrease in performance in the IGT task in healthy male subjects (Zhang et al., 2011). The inconsistent findings regarding the effect of stress on risk taking in males might be due to culture difference (western vs. eastern), which has not been examined yet.
The complexity of tasks also plays an important role in modulating the effects of stress on decision making strategies. Using the Game of Dice Task, a decision-making task with explicit and stable rules that taps both executive functioning and feedback learning, it was found that stress can lead to disadvantageous decision making (Starcke et al., 2008). Recent studies showed that stressed participants in the single-task group made riskier decisions compared with nonstressed controls, but the effects of stress and a secondary task cancelled each other out (Pabst et al., 2013a, Gathmann et al., 2014). The authors interpreted these results as showing that stress evokes a shift from serial to parallel processing. Thus, task complexity may determine how much executive control is available for making decisions and thus modulate how stress may influence decision-making.
These findings, taken together, suggest that stress may amplify risk sensitivity depending on the domain (win/loss), gender, and task complexity. They indicate that the SIDI model needs to make more detailed predictions regarding the relationship between stress and risk sensitivity, taking into account contextual situation factors and individual differences.
2.2. Diminished cognitive control
People usually generate judgments and attitudes through automatic processes and then use controlled processes to make necessary adjustments (Kahneman & Tversky, 1979). When first being asked “Is the population of Chicago more or less than 200,000?”, participants automatically anchor their answer on 200,000 when later asked “What is Chicago's population?”. Adjustment away from self-generated anchors requires the expenditure of mental effort. If mental resources are diminished, such fine-tuned adjustments may be compromised, according to the SIDI model. Indeed, a previous study found that under stress, individuals were more likely to make decisions before all available alternatives had been systematically considered, even if no time constraint for the performance of the task was imposed (Keinan, 1987). This study indicates that stress makes decision makers more impulsive and more likely to make unexamined responses. Recently, Kassam et al. found that challenge stress, which individuals appraise as demanding but manageable, improves adjustment, whereas threat stress, which individuals perceive as a situational demand that outweighs resources, reduces adjustment (Kassam et al., 2009). This study demonstrates that how stress is perceived (challenge or threat) dictates how it influences decision making.
Cognitive control not only inhibits the tendency to make premature responses but also regulates emotional responses to stimuli. A large body of work has shown that responses to emotionally salient stimuli can be flexibly changed and controlled through cognitive regulation (Nolen-Hoeksema, 2012). Recruiting cognitive strategies to deliberately change the way a stimulus is evaluated, either by reinterpreting (i.e., reappraising) its meaning or focusing on its more positive aspects, has proven effective at reducing the subjective, physiological, and neural components of emotional arousal. It is specifically under stressful conditions that individuals may benefit most from such deliberate forms of emotion regulation. However, the SIDI would predict that the efficacy of cognitive regulation attempts after stress exposure would be reduced due to the diminished cognitive control ability under stress. Indeed, regulation training has been shown to produce robust fear reduction in non-stressed participants but not stressed participants (Raio et al., 2013). A recent neuroimaging study also found that acute stress impairs self-control in goal-directed choice by reducing connectivity between ventromedial PFC and dorsolateral PFC regions linked to self-control success (Maier et al., 2015). These results highlight critical limitations of this technique to control affective responses under stress; other techniques such as emotion regulation strategies may also have limited power to overcome stress-biased decisions. If stress markedly impairs the cognitive regulation of emotion, it is less likely than individuals can exert cognitive control to overcome stress induced decision biases.
Another crucial function of cognitive control is to keep individuals on the track of pursuing goals. In the ever-changing environment, individuals need to adjust their behaviors according to the goals they want to accomplish. Such goal-directed flexible behaviors demand an effortful control and monitoring of the response. To increase response efficiency, recurring decision processes can be automated to form a rule or a habit. The goal-directed system learns action-outcome associations (Dickinson, 1985), and is believed to be mediated by prefrontal cortex areas and dorsomedial striatum (Balleine & O'Doherty, 2010). By contrast, the habitual system learns stimulus–response associations regardless of outcomes, and is supported by posterolateral putamen (Balleine and O'Doherty, 2010, Yin and Knowlton, 2006). A recent study in humans showed that stressed individuals continued to perform the action associated with a particular outcome even after this outcome had been devalued, accompanied by a significant decrease in explicit knowledge of action-outcome contingencies (Schwabe & Wolf, 2009). The β-adrenoceptor antagonist propranolol, which blocks the action of adrenaline and noradrenline, blocked this stress-induced bias toward habit behavior, suggesting that noradrenergic activation plays a crucial role in such stress elicited decision strategy switching (Schwabe et al., 2011). Importantly, a longitudinal assessment of the stressed individuals showed that both the structural and functional changes triggered by stress are reversible and that decisions become goal-directed again (Soares et al., 2012). The link between neuroticism and distress has been shown to be strong in individuals with high perseverative response tendency (i.e., less switching across consecutive trials), suggesting a relationship between distress and response preservation (Robinson et al., 2006). A recent psychopharmacological study found that cortisol impaired performance in the cognitive reflection test (CRT) by biasing responses toward intuitive but incorrect answers (Margittai et al., 2015a). The profound influence of stress on individuals' ability to adjust their behavior to changing circumstances can even be found in infants. Recent evidence showed that 15-mo-old infants exposed to stress kept performing a previously effective action, even after the action suddenly became ineffective, suggesting that stressed human infants tend to perform habitual behavior rigidly (Seehagen et al., 2015). These findings echo the well-established stress-induced switching from goal-directed to habitual control of action in rodents (see a more comprehensive review in Ref. (Schwabe & Wolf, 2011). Chronic stress caused rats to become insensitive to changes in outcome value (Graham et al., 2010), accompanied by atrophy of the medial prefrontal cortex and caudate and hypertrophy of the putamen (Dias-Ferreira et al., 2009). Stressed rats also relied more on habit memory rather than cognitive memory to guide their actions (Elliott & Packard, 2008). Taken together, evidence from both human studies and animal research point to the possibility that stress biases decision-making strategies by shifting from goal directed decisions to habitual choices (Schwabe & Wolf, 2011).
To summarize, previous studies suggest that cognitive control is diminished under stress, leading to premature decision-making. Without optimal cognitive control, decision makers are less likely to do fine-tuned adjustments, exhibit weakened cognitive regulation of emotion, and also often fail to choose goal-directed actions. In accord with the SIDI model, stressed individuals are more likely to fall back to their emotional and habitual responses.
2.3. Social decisions under stress
The deliberation to intuition framework for understanding decision making under stress does not entail the assumption that stress promotes prosocial or antisocial responses. Rather, it predicts that stress facilitates spontaneous and innate responses in social situations. Such responses should be adapted in ancestral human environments, thus are ‘ecologically rational’ (Hammerstein and Hagen, 2005, Gigerenzer and Selten, 2002). Rand et al. have shown that individuals are more cooperative and altruistic when they have to make choices quickly under time pressure compared with conditions in which they are given enough time to do analytic calculation (Rand et al., 2012, Rand et al., 2014). These findings seem to suggest that our first responses are prosocial actions. However, whether such a conclusion can be generalized to other social contexts awaits further exploration. If individuals' innate tendency is to cooperate, the SIDI hypothesis would hold that stress promotes cooperation in social situations. The majority of studies on stress and prosociality supports this prediction (Buchanan & Preston, 2014), across a range of experimental paradigms. In a study using the dictator game, responders under social evaluation stress allocated significantly more money than controls (Takahashi et al., 2007). Using the trust game, it was found that male participants who experienced acute social stress engaged in substantially more prosocial behavior (trust, trustworthiness, and sharing) compared with participants in a control condition (von Dawans et al., 2012). The authors reasoned that stress triggers social approach behavior, which operates as a potent stress buffering strategy in humans, thereby providing evidence for the tend-and-befriend hypothesis and also extend this model to male individuals. Social closeness also modulate prosocial behaviors in males such that stressed males only showed increased generosity towards close but not distant others (Margittai et al., 2015b). Survey results have also shown that higher levels of acculturative stress are linked to greater anonymous prosocial tendencies and with fewer costly (altruistic) prosocial tendencies (McGinley et al., 2010). In the domain of moral decisions, recent studies using complex moral dilemmas have shown that persons under stress show significantly fewer utilitarian responses compared to control subjects (Youssef et al., 2012, Starcke et al., 2012).
However, contrary to the above findings that stress promotes prosocial behaviors, another study found that stress decreased men's tendency to reject unfair offers in the ultimatum game (UG) and reduced the amount of money allocated to the other participant in the dictator game (Vinkers et al., 2013). Research using the Everyday Moral Decision-Making Task (EMDM), which seeks to distinguish between altruistic and egotistical behavior, has shown that the cortisol stress response was associated with egoistic decision-making in high-emotional situations (Starcke et al., 2011). These contradictory findings might be due to differing methods used to probe prosociality. For example, in the UG, rejecting unfair offers is usually interpreted as a costly prosocial behavior since such altruistic punishment costs individuals the potential earning that they could have otherwise earned (Fehr & Gachter, 2002). However, they are many social motives may drive such behavior (Pillutla and Murnighan, 1996, Sanfey et al., 2003). Recent studies also showed that the tendency to reject unfair offers in the UG is not correlated with individuals' tendencies to exhibit various prosocial behaviors in other situations (Yamagishi et al., 2012). Also, how individuals believe they will act in hypothetical moral decision situations may differ dramatically from how they actually act in the real world situations (Ajzen et al., 2004, FeldmanHall et al., 2012). Another possibility is that the link between stress and prosocial tendency is modulated by social closeness. A recent study found that men tested 20 min after stressor onset indeed showed increased generosity towards close but not distant others compared to non-stressed men or men tested 90 min after stressor onset (Margittai et al., 2015b). Thus, the proposal tendency elicited by stress may be limited to close friends or family members but not extended to strangers. Nevertheless, majority of these findings do provide support for the notion that stress leads to prosocial behaviors (Buchanan & Preston, 2014).
Stress also influences individuals' ability to mentalize. In the beauty contest game, which is designed to measure the depth of reasoning, participants under stress chose higher numbers than non-stressed participants, indicating less strategizing (Leder et al., 2013). In this game, entrants are asked to pick a number between 0 and 100, with the winner of the contest being the person that is closest to 2/3 the average number picked for all contestants. The lowest, 'Level 1′ players believe that all other players choose randomly and therefore choose 33 (2/3 of 50). Similarly, Level 2 players would choose 22 (2/3 of 33). The smaller the number players choose, the higher level of strategic thinking they are. A recent study found that stress exposure influences individuals' mind reading abilities, assessed by Movie for the Assessment of Social Cognition (MASC). High cortisol responses led to elevated MASC scores in men but reduced MASC scores among women (Smeets et al., 2009). These results partially support the idea of sex differences in biobehavioral stress responses, with men engaging in fight-or-flight responses and women showing tending and befriending behavior (Smeets et al., 2009). The SIDI model may also explain such a gender difference. It is possible that in certain situations, the default action for men to respond to threat is to fight rather than flee. Thus, stress may enhance men's metalizing ability in order to initiate attack. Overall, there are only few studies examining the effects of stress on social decisions, although social decisions are the most important choices we make in our daily life. The lack of neuroimaging data in this field also hinders our understanding of the neural mechanisms mediating stress and social decision making.
3. Neural mechanisms for stress induced deliberation-to-intuition
How human brains respond to stress is a topic that has been investigated extensively in both animal studies and human research, see reviews in Refs. (Schwabe and Wolf, 2013, Arnsten, 2009, Roozendaal et al., 2009, Kim and Diamond, 2002). The stressful event leads to activation of two biological systems: the autonomic nervous system (ANS), involving release of (nor)adrenaline, and the hypothalamo-pituitary-adreanal (HPA) axis (Arnsten, 2009). Immediate fast responses are mostly mediated by the sympathetic nervous system (SNS) and the associated release of epinephrine from the adrenal medulla (Joels & Baram, 2009). A more delayed response-release of cortisol emanates from the adrenal cortex (de Kloet et al., 2005, Droste et al., 2008). This is initiated by neural signals to the hypothalamus, which releases corticotropin-releasing hormone (CRH), resulting in secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland on the bottom of the brain. The ACTH induces cortisol synthesis and release from the adrenal gland. Stress evokes high concentrations of catecholamines such as noradrenalin and dopamine, and an increased concentration of the glucocorticoid cortisol. The changes of these hormones may alter functioning of neural correlates of decision making, such as the dorsal PFC, ventromedial PFC/anterior cingulate cortex, striatum, hippocampus, and amygdala. The SIDI model predicts the existence of two neural circuits that support System 1 and System 2 respectively. Stress should enhance the System 1 intuition related neural activity (e.g., in subcortical regions) and decrease System 2 reasoning associated brain activity (e.g., in prefrontal cortex). Here, I selectively review neuroimaging studies that examine both the prefrontal control system and subcortical emotional response system in relation to stress.
Previous neuroimaging research has demonstrated a shift in activity from the newly developed prefrontal cortex to phylogenetically older midbrain regions when threat stress draws closer. Using a Pac-Man like computer game in which volunteers were pursued by a virtual predator, Mobbs et al. found that as the virtual predator grew closer, brain activity shifted from the ventromedial prefrontal cortex to the periaqueductal gray (Mobbs et al., 2007). In another study, phylogenetic threat was introduced by making participants believe that a tarantula was placed close to their foot, and the experience of fear coincided with augmented activity in a cascade of fear-related brain networks including the periaqueductal gray, amygdala, and bed nucleus, as well as diminished activity in the orbitofrontal cortex and posterior cingulate cortex (Mobbs et al., 2010). In a series of studies, social evaluative threat was also shown to cause activity increases in a more dorsal pregenual cingulate region, whose activity was coupled with heart rate increases; conversely, social evaluative threat caused activity decreases in a right ventromedial/medial orbital region, which were coupled with heart rate increases (Wager et al., 2009, Wager et al., 2009). Numerous studies have identified structural and functional connectivity between prefrontal cortex and amygdala, a key region in emotion processing (Kim et al., 2011, Kober et al., 2008). Functional coupling between the prefrontal cortex and the key reward region striatum is also well established (van den Bos et al., 2012, Haber and Knutson, 2010).
If stress interferes with cognitive systems, it is plausible that reduced cognitive control ability would lead to an exaggerated reliance on lower-level automatized systems (Masicampo & Baumeister, 2008). A recent study, using both PET and fMRI, demonstrated that in the Montreal Imaging Stress Task (MIST), stress induced significant deactivation of the limbic system including hippocampus, hypothalamus, medio-orbitofrontal cortex and ACC in subjects who reacted to the stressor with increased cortisol (Pruessner et al., 2008). Large-scale network analysis has provided similar results. In a recent study, responsiveness and interconnectivity within a network including cortical (frontoinsular, dorsal ACC, inferotemporal, and temporoparietal) and subcortical (amygdala, thalamus, hypothalamus, and midbrain) regions increased during exposure to a fear-related acute stressor (Hermans et al., 2011). Importantly, b-adrenergic receptor blockade, but not cortisol synthesis inhibition, diminished the increase, suggesting that the neuromodulator noradrenaline drives this network reorganization. This study not only identified two key networks involved in stress but also highlighted the importance of the balance between the two networks. Recently, these researchers proposed that there are two brain networks, salience (e.g. emotional reactivity) vs. executive control (e.g. working memory), in governing stress (Hermans et al., 2014). The two stress-related networks play differential roles in the neurobiology of decision making under stress and may be analogous to the System 1 and System 2 framework in the SIDI model.
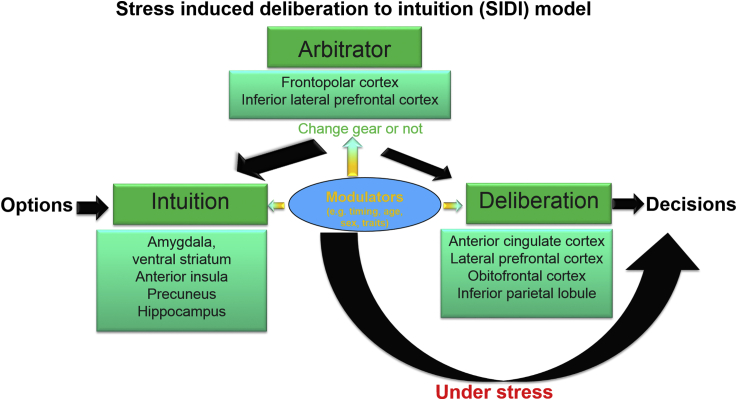
Taken together, these neuroimaging studies show that stress diminishes activity in prefrontal cortex and augments activity in subcortical regions including amygdala, hippocampus, and midbrain. This activity profile supports the notion that stress evokes a switch from deliberation that is supported mainly by prefrontal cortex to intuition that involves phylogenetically older brain regions such as subcortical areas (see Fig. 1).
Fig. 1.
The stress induced deliberation to intuition (SIDI) model.
4. A stress induced deliberation-to-intuition (SIDI) model
This perspective, referred to as the stress induced deliberation-to-intuition model, posits that decision making under stress is influenced by a combination of immature cognitive control and heightened intuitive response tendency, which are tied to the prefrontal reasoning system and the subcortical intuition related regions. It is acknowledged that not all evidence mentioned above fits the simple dichotomy the SIDI model assumes. The findings in this field are often contradictory and complex. For instance, studies often yield mixed results regarding whether stress increases or decreases sensitivity to reward. There are several methodological caveats that may account for such a discrepancy, such as the time between stress induction and task performance (Schwabe & Wolf, 2014), the number of subjects, and the power of the stress induction task used (Allen et al., 2014). Moreover, the stress effects are modulated by age (Galvan & Rahdar, 2013), gender, personalities (Lempert et al., 2012, Richards et al., 2014), the nature of the experimental tasks, appraisal of threat (van Wingen et al., 2011), individuals' basal cortical level, and cortisol responses during stress (Roelofs et al., 2005). The effects of stress on decision-making are also multidimensional, ranging from reward/punishment sensitivity and risk tendency to adjustment and emotion regulation. Although previous studies tried to isolate these dimensions, it is worth noting that stress may influence some or all of these decision-making stages. However, despite all the factors that complicate the relationship between stress and decision making, it is also worth noting that the majority of studies do provide convergent evidence that stressed individuals are more sensitive to threat/punishment, less likely to exert cognitive control to examine their responses, and more prosocial in social contexts. At the neural level, accumulating evidence suggests that stress diminishes activity in the evolutionally new prefrontal cortex and exaggerates evolution rooted subcortical regions. Thus, both behavioral and neuroimaging studies so far support the SIDI model, although further investigation of stress and decision making is urgently needed.
The SIDI can serve as a simple but useful tool to guide further research on stress and decision-making. Several hypotheses can be generated based on the SIDI model. First, a large number of decision biases are believed to be a result of the imbalance between System 1 and System 2. The SIDI model would predict that stress potentiates these biases. In addition to the several biases mentioned before (e.g., the framing effect and reflection effect), there are many other biases that should be examined to further test the SIDI model, such as social conformity (Huang et al., 2014, Asch, 1955), the decoy bias (Hu and Yu, 2014, Huber et al., 1982), the default bias (Yu et al., 2010), delay discounting (Yu, 2012, Mischel et al., 1989, Haushofer et al., 2013, Haushofer and Fehr, 2014), and availability (Tversky & Kahneman, 1974). For example, it is reasonable to predict that individuals would be more vulnerable to social influence if they rely more on System 1 to make decisions, as the SIDI model posits. Moreover, the SIDI may also predict how stress interacts with psychiatric disorders such as addiction, depression, and bipolar mood disorder (Zhang et al., 2011, Deckers et al., 2014). For example, stress may amplify the decision-making deficits in addictive patients (Zhang et al., 2011). Stress may also interact with aging to determine decision making in the elderly, whose prefrontal cortex based cognitive control ability is already compromised (Peavy et al., 2009). The SIDI model would also predict that the effect of stress on decisions should mimic effects found in patients with prefrontal lesions or individuals after brain stimulation, which diminishes activity in cognitive control related brain regions.
4.1. Parallel-competitive or default-interventionist
The distinction between deliberative and intuitive systems has enjoyed considerable popularity in social cognition and decision making domains (Kahneman, 2011, Evans, 2008, Gilovich et al., 2002, Evans and Stanovich, 2013, Evans, 2003), although it has also been challenged (Kruglanski & Gigerenzer, 2011). Across different sub-fields of psychology, a diverse set of dual-process models have been proposed, including analytic vs. heuristic (Kahneman, 2011, Evans, 2008, Evans and Stanovich, 2013, Evans, 2003), conscious vs. unconscious (Dijksterhuis et al., 2006, Lassiter et al., 2009), associative versus rule based (Ashby & O'Brien, 2005), goal-directed vs. habitual (Dickinson, 1985), cognitive vs. affective (Bechara et al., 1997), and reflective (C-system) vs. reflexive (X-system) models (Lieberman, 2007). Recently, there have been discussions about how stress modulates leaning and decision making through the lens of duality models. Based on research with non-human animals, Arnsten posited that stress impairs PFC function and strengthens functions mediated by amygdala and other subcortical regions, thus creating a ‘vicious cycle’ (Arnsten, 2009). In response to stress, the amygdala activates stress pathways in the hypothalamus and brainstem, which produces high levels of catecholamine release (e.g. noradrenaline and dopamine). On the one hand, this disrupts the top-down control by the PFC and allows the bottom-up control by sensory cortices to be dominating. On the other hand, high levels of catecholamine strengthen amygdala functioning which biases individuals towards reflexive and habitual responding (Arnsten, 2009). All of these remain plausible hypotheses awaiting further tests in humans. For example, whether stress always exerts opposite influence on PFC and amygdala system or stress only alters one system and leaves the other intact remains to be examined. Schwabe and Wolf proposed that stress influences instrumental behavior by favors habitual over goal-directed memory systems (Schwabe and Wolf, 2013, Schwabe and Wolf, 2011). Extending previous these accounts, the current SIDI model is not limited to specific forms of decision making (e.g. spatial navigation and instrumental learning) and attempts to integrate decision parameters encoding, risk evaluation, cognitive control, and social emotions. The SIDI model extends these proposals by integrating a range of decision-making components that allow more precise predictions of behavior. Importantly, the proposed model describes how the two systems interact at various stages of processing in a synergistic or antagonistic fashion. The SIDI model differs from previous accounts on stress and decision-making on how the two systems interact. Previous accounts seem to take the parallel-competitive forms of dual-process theory, based on the notion that two systems work in parallel and produce two forms of decisions that may lead to competing attempts to control the behaviors. The SIDI model corresponds more closely to the default-interventionist assumption that intuition system supplies rapid default responses (intuition proposes) and deliberation system may approve or intervene upon (deliberation decides) (Evans, 2008). The key differences between the two approaches relate to the order and dominance of different cognitive processes. Whether the two systems operate in parallel or the intuition system is fast and the deliberation is slow to decide can be tested using high temporal resolution neuroimaging methods combined with computational models such as granger causality analysis (Goebel et al., 2003). How the PFC-mediated deliberation system exerts control over the subcortical-based intuition system can also be delineated using functional connectivity analysis methods such dynamic causal modeling (Friston et al., 2003). The challenge for researchers in this field is to delineate the exact neuropsychological processes of the switching from deliberation to intuition system and tease apart factors that modulate such switching. Neuroimaging methods can be applied to elucidate the biopsychological processes of decision making under stress.
4.2. Arbitration between intuitive and deliberative processes
The arbitrary between intuitive and deliberative responses has not been directly investigated in the context of stress. One possibility is that there is an arbitrator that keeps track of the degree of reliability of the two systems and uses this information in order to proportionately allocate behavioral control. Thus, the arbitrator modulates both systems. Another possibility is that the arbitrator deems that the intuition system should be relied on, it allows all decisions bypass the deliberation processes. In this case, the arbitrator modulates only one of the two systems due to reasons of computational efficiency. A recent computational neuroimaging study provides evidence for the existence in the human brain of an arbitrator mechanism that determines the extent to which model-based (goal-directed) and model-free (habitual) learning systems control behavior (Lee et al., 2014). Lee et al., found that inferior lateral prefrontal and frontopolar cortex encode both reliability signals of both systems and the output of a comparison between those signals, implicating these regions in the arbitration process (Lee et al., 2014). Importantly, instead of modulating either model-based or model-free systems depending on which one has the most reliable estimate, the controller appears to work by selectively gating the model-free habitual system. Whether stress works in a similar way in determining which system should control behavior remain to be examined using computational modelling and neuroimaging methods.
4.3. Consequences of intuition are context-dependent
Although some believe that System 2 is superior to System 1 and always leads to rational decisions, this is not always the case, especially for experts. For example, the recognition-primed decision model proposes that people use their experience in the form of patterns (Klein, 1999). These patterns help the decision makers to recognize the relevant cues, identify plausible goals, and remember typical types of reactions in certain type of situation. When people need to make a decision, they can quickly match the situation to the patterns they have learned and experienced in the past. Doing this allows people to successfully make rapid decisions without comparing options. Thus, if the first workable option turns out to be satisfactory and deliberative analysis does not produce a significantly better one, System 1 can produce even better and more effective outcomes than System 2 (Dijksterhuis et al., 2006, Reyna, 2004, Johnson and Raab, 2003). For example, chess experts rapidly retrieve a schema that usually provides a solution to the current problem rather than exhaustively comparing all options (Chase & Simon, 1973). Intuitive responses are not necessarily associated with disadvantageous outcomes and more cognitive control is not always more adaptive. Whether a response or tendency is advantageous or not is context dependent. Risk seeking may be advantageous if the task is structured to favour risk-seeking strategies. Thus, stress does not necessarily degrade decision quality, and it can improve decisions in some circumstances. The SIDI model is agnostic with respect to whether the stress induced changes in decision making strategies produce desirable or undesirable consequences. Intuitive judgments may turn out to be optimal. It is worth noting that the SIDI model does not imply that stress is always detrimental for decision-making. In certain circumstances, certain levels of stress might be beneficial for decision makers. Moreover, it might not be obvious that which responses are our rapid intuitive responses and which are not, especially in social contexts. Thus, without a clear definition of what an innate response is, the argument that stress promotes intuitive processes is a circular one. More likely than not, innate responses are context dependent. Humans may have default tendency to be risk taking in certain situations and be risk aversion in other situations (e.g. the reflection bias). It is arbitrary to simply assign a general tendency (e.g. risking taking, loss aversion, and prosocial) as the default or an innate response. Research on stress should be integrated with studies in which time constraints, physical fatigue, hunger, and cognitive loads are manipulated in order to better define what intuitive responses are. Otherwise, the hypotheses of the SIDI are derived post-hoc and therefore represent a common factor to many stress phenomena.
4.4. Modulators of decision making under stress
First, stress level is a matter of degree. Intuitively, too much as well as too little stress is often detrimental for cognitive performance. In most studies mentioned above, researchers assume that the elicited stress levels are well above the optimal level. If individuals reach the ultimatum level of stress that they cannot handle, they may crash and give up. Lack of stress, such as boredom and apathy, may also hinder individuals' performance due to lack of motivation. Thus, the relationship between he stress level and performance might be inverted U-shape (Yu, 2015). Second, the time dependency of cortisol effects might also modulate when and how stress shapes behaviors. Previous studies have revealed time-dependent effects on working memory processing, emotional memory, and brain function in general (Henckens et al., 2011, Joels et al., 2011). The rapid, non-genomic effects of cortisol in combination with noradrenaline increase subcortical and decrease prefrontal functioning, whereas the aftermath of stress is associated with upregulated prefrontal functioning (Hermans et al., 2011, Hermans et al., 2014). The SIDI model applies to the immediate rapid effect of stress and may make opposite predictions for the late or recovery stage of stress. Third, reaction time was not reported or discussed in most of previous studies. The SIDI model would predict that when under time pressure, the greater the psychological stress, the greater the tendency to make a premature choice (hence the shorter RT) or seek premature closure. Such hypothesis awaits future empirical research to test. Fourth, different ways in which stress is induced in the laboratory (using social evaluation, cold-pressor tasks, pharmacological methods, and stressful life events) may also impact the stress effect. For example, cold-presssor tasks may produce physical challenges whereas social evaluation tasks may tap more into psychological and social stress, although they all elevate cortical levels. The difference between acute and chronic stress should also be taken into consideration. Individuals may develop various compensatory strategies and coping responses to deal with stress over a long time period. Environmental factors such as social support and societal interventions may also play a role in determining the effects of chronic stress on behaviors. Finally, individuals' characteristics such as age (Galvan & Rahdar, 2013), gender, and personalities (Lempert et al., 2012, Richards et al., 2014) may also interact with stress and determine to a large degree how stress modulates decision-making. Facing similar stressful events, some may cope well but others may develop psychological disorders such as anxiety or even posttraumatic stress disorder (PTSD), depending on individuals' life experience and personal traits.
At this stage, the distinction between deliberation and intuition is admittedly rather simplistic. The relationship between stress and decision making might be quite complicated. It is hard to develop a model that can explain all existing findings in the literature given the complexity of this issue. Despite that the SIDI model may have oversimplified the evidence or overlooked inconsistencies in the literature, the framework offers a useful model for understanding how stress modulates decision making in humans. Specially, in the absence of an alternative theoretical account, the SIDI model provides a useful guideline for the formulation of specific and testable hypotheses and an integrated account for the observed effects of stress on behavioral outcomes and the structural and functional neural changes in the human brain. There are findings that run counter to predictions derived from the SIDI model, suggesting that more nuanced analyses and theory modification are needed. The SIDI model yields specific and testable predictions. Future research may add the detailed quantitative and computational component into this model. Further specification of the model will become possible as the field develops new advanced research methods and refined ways to integrate behavioral and neuroscientific sources of evidence.
5. Concluding remarks
The current review provides an overview of the recent research on how stress shapes decision-making. Stress may interfere in this competition between emotion and cognitive functioning and thereby impair decision-making. The framework presented in this review serves as a new way to revive the discussion on how stress influences decisions and may encourage researchers to adopt a more theory-based and hypothesis-driven approach to their investigations. Observing some behavior effects of stress in isolation is not sufficient to allow us to make conclusions about how stress perpetrates decision-making processes in general. The current review provides a relatively comprehensive summary of stress and economic decision-making and proposes that stress may potentiate decision biases. The stress induced deliberation-to-intuition model provides testable hypotheses and can guide future research in this direction. Although what constitutes intuitive decisions is debatable, integrating findings from studies that manipulate reasoning abilities may inform research on stress and decision-making. This field is still in its infancy and requires more research to fully understand the phenomenology of decision-making under stress. Understanding how stress biases decisions would be highly useful to those people working or living under extreme stress, such as military soldiers in the war zone, plane pilots, and emergency responders.
Declaration of conflicting interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Acknowledgments
This work was supported by the National University of Singapore Grant WBS R-581-000-166-133 to RY. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Ajzen I., Brown T.C., Carvajal F. Explaining the discrepancy between intentions and actions: the case of hypothetical bias in contingent valuation. Personal. Soc. Psychol. Bull. 2004;30(9):1108–1121. doi: 10.1177/0146167204264079. [DOI] [PubMed] [Google Scholar]
- Akinola M., Mendes W.B. Stress-induced cortisol facilitates threat-related decision making among police officers. Behav. Neurosci. 2012;126(1):167–174. doi: 10.1037/a0026657. [DOI] [PubMed] [Google Scholar]
- Allen A.P., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G. Biological and psychological markers of stress in humans: focus on the Trier Social Stress Test. Neurosci. Biobehav. Rev. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Antov M.I., Wölk C., Stockhorst U. Differential impact of the first and second wave of a stress response on subsequent fear conditioning in healthy men. Biol. Psychol. 2013;94(2):456–468. doi: 10.1016/j.biopsycho.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch S.E. Opinions and social pressure. Read. Soc. Anim. 1955:17–26. [Google Scholar]
- Ashby F.G., O'Brien J.B. Category learning and multiple memory systems. Trends Cogn. Sci. 2005;9(2):83–89. doi: 10.1016/j.tics.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Balleine B.W., O'Doherty J.P. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2010;35(1):48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Tranel D., Damasio A.R. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304):1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Berghorst L.H., Bogdan R., Frank M.J., Pizzagalli D.A. Acute stress selectively reduces reward sensitivity. Front. Hum. Neurosci. 2013;7:133. doi: 10.3389/fnhum.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R., Pizzagalli D.A. Acute stress reduces reward responsiveness: implications for depression. Biol. psychiatry. 2006;60(10):1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T.W., Preston S.D. Stress leads to prosocial action in immediate need situations. Front. Behav. Neurosci. 2014;8:5. doi: 10.3389/fnbeh.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon W.B. The emergency function of the adrenal medulla in pain and the major emotions. Am. J. Physiol. 1914;33:356–372. [Google Scholar]
- Cannon W.B. 1932. The Wisdom of the Body. [Google Scholar]
- Cavanagh J.F., Frank M.J., Allen J.J. Social stress reactivity alters reward and punishment learning. Soc. Cogn. Affect. Neurosci. 2011;6(3):311–320. doi: 10.1093/scan/nsq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase W.G., Simon H.A. Perception in chess. Cogn. Psychol. 1973;4(1):55–81. [Google Scholar]
- Chumbley J.R., Hulme O., Kochli H., Russell E., Van Uum S., D A.P. Stress and reward: long term cortisol exposure predicts the strength of sexual preference. Physiol. Behav. 2014;131:33–40. doi: 10.1016/j.physbeh.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Chumbley J.R., Krajbich I., Engelmann J.B., Russell E., Van Uum S., Koren G. Endogenous cortisol predicts decreased loss aversion in young men. Psychol. Sci. 2014;25(11):2102–2105. doi: 10.1177/0956797614546555. [DOI] [PubMed] [Google Scholar]
- Coates J.M., Herbert J. Endogenous steroids and financial risk taking on a London trading floor. Proc. Natl. Acad. Sci. U. S. A. 2008;105(16):6167–6172. doi: 10.1073/pnas.0704025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueva C., Roberts R.E., Spencer T., Rani N., Tempest M., Tobler P.N. Cortisol and testosterone increase financial risk taking and may destabilize markets. Sci. Rep. 2015;5:11206. doi: 10.1038/srep11206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet E.R., Joels M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Deckers J.W., Lobbestael J., van Wingen G.A., Kessels R.P., Arntz A., Egger J.I. The influence of stress on social cognition in patients with borderline personality disorder. Psychoneuroendocrinology. 2014;52C:119–129. doi: 10.1016/j.psyneuen.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E., Sousa J.C., Melo I., Morgado P., Mesquita A.R., Cerqueira J.J. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325(5940):621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Dickinson A. Actions and habits: the development of behavioural autonomy. Philos. Trans. R. Soc. Lond. B, Biol. Sci. 1985;308(1135):67–78. [Google Scholar]
- Dijksterhuis A., Bos M.W., Nordgren L.F., van Baaren R.B. On making the right choice: the deliberation-without-attention effect. Science. 2006;311(5763):1005–1007. doi: 10.1126/science.1121629. [DOI] [PubMed] [Google Scholar]
- Droste S.K., de Groote L., Atkinson H.C., Lightman S.L., Reul J.M., Linthorst A.C. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology. 2008;149(7):3244–3253. doi: 10.1210/en.2008-0103. [DOI] [PubMed] [Google Scholar]
- Elliott A.E., Packard M.G. Intra-amygdala anxiogenic drug infusion prior to retrieval biases rats towards the use of habit memory. Neurobiol. Learn. Mem. 2008;90(4):616–623. doi: 10.1016/j.nlm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Elman I., Lowen S., Frederick B.B., Chi W., Becerra L., Pitman R.K. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biol. psychiatry. 2009;66(12):1083–1090. doi: 10.1016/j.biopsych.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J.S.B., Stanovich K.E. Dual-process theories of higher cognition advancing the debate. Perspect. Psychol. Sci. 2013;8(3):223–241. doi: 10.1177/1745691612460685. [DOI] [PubMed] [Google Scholar]
- Evans J.S. In two minds: dual-process accounts of reasoning. Trends Cogn. Sci. 2003;7(10):454–459. doi: 10.1016/j.tics.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Evans J.S. Dual-processing accounts of reasoning, judgment, and social cognition. Annu. Rev. Psychol. 2008;59:255–278. doi: 10.1146/annurev.psych.59.103006.093629. [DOI] [PubMed] [Google Scholar]
- Fehr E., Gachter S. Altruistic punishment in humans. Nature. 2002;415(6868):137–140. doi: 10.1038/415137a. [DOI] [PubMed] [Google Scholar]
- FeldmanHall O., Mobbs D., Evans D., Hiscox L., Navrady L., Dalgleish T. What we say and what we do: the relationship between real and hypothetical moral choices. Cognition. 2012;123(3):434–441. doi: 10.1016/j.cognition.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Harrison L., Penny W. Dynamic causal modelling. NeuroImage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Galvan A., Rahdar A. The neurobiological effects of stress on adolescent decision making. Neuroscience. 2013;249:223–231. doi: 10.1016/j.neuroscience.2012.09.074. [DOI] [PubMed] [Google Scholar]
- Gathmann B., Schulte F.P., Maderwald S., Pawlikowski M., Starcke K., Schafer L.C. Stress and decision making: neural correlates of the interaction between stress, executive functions, and decision making under risk. Exp. Brain Res. 2014;232(3):957–973. doi: 10.1007/s00221-013-3808-6. [DOI] [PubMed] [Google Scholar]
- Gigerenzer G., Selten R. Mit Press; 2002. Bounded Rationality: the Adaptive Toolbox. [Google Scholar]
- Gilovich T., Griffin D., Kahneman D. Cambridge University Press; 2002. Heuristics and Biases: the Psychology of Intuitive Judgment. [Google Scholar]
- Goebel R., Roebroeck A., Kim D.S., Formisano E. Investigating directed cortical interactions in time-resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Magn. Reson. Imaging. 2003;21(10):1251–1261. doi: 10.1016/j.mri.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Graham L.K., Yoon T., Kim J.J. Stress impairs optimal behavior in a water foraging choice task in rats. Learn Mem. 2010;17(1):1–4. doi: 10.1101/lm.1605510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B.F. Neuroendocrine components in the physiological response to stress. Ann. N. Y. Acad. Sci. 1953;56(2):184–199. doi: 10.1111/j.1749-6632.1953.tb30216.x. [DOI] [PubMed] [Google Scholar]
- Gullo M.J., Stieger A.A. Anticipatory stress restores decision-making deficits in heavy drinkers by increasing sensitivity to losses. Drug Alcohol Depend. 2011;117(2–3):204–210. doi: 10.1016/j.drugalcdep.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerstein P., Hagen E.H. The second wave of evolutionary economics in biology. Trends Ecol. Evol. 2005;20(11):604–609. doi: 10.1016/j.tree.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Haushofer J., Fehr E. On the psychology of poverty. Science. 2014;344(6186):862–867. doi: 10.1126/science.1232491. [DOI] [PubMed] [Google Scholar]
- Haushofer J., Cornelisse S., Seinstra M., Fehr E., Joels M., Kalenscher T. No effects of psychosocial stress on intertemporal choice. PLoS One. 2013;8(11):e78597. doi: 10.1371/journal.pone.0078597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens M.J., van Wingen G.A., Joels M., Fernandez G. Time-dependent corticosteroid modulation of prefrontal working memory processing. Proc. Natl. Acad. Sci. U. S. A. 2011;108(14):5801–5806. doi: 10.1073/pnas.1019128108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E.J., van Marle H.J., Ossewaarde L., Henckens M.J., Qin S., van Kesteren M.T. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334(6059):1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Hermans E.J., Henckens M.J., Joels M., Fernandez G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014;37(6):304–314. doi: 10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Hu J., Yu R. The neural correlates of the decoy effect in decisions. Front. Behav. Neurosci. 2014;8:271. doi: 10.3389/fnbeh.2014.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Kendrick K.M., Yu R. Conformity to the opinions of other people lasts for no more than 3 days. Psychol. Sci. 2014;25(7):1388–1393. doi: 10.1177/0956797614532104. [DOI] [PubMed] [Google Scholar]
- Huber J., Payne J.W., Puto C. Adding asymmetrically dominated alternatives: violations of regularity and the similarity hypothesis. J. Consum. Res. 1982:90–98. [Google Scholar]
- Jackson E.D., Payne J.D., Nadel L., Jacobs W.J. Stress differentially modulates fear conditioning in healthy men and women. Biol. psychiatry. 2006;59(6):516–522. doi: 10.1016/j.biopsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Joels M., Baram T.Z. The neuro-symphony of stress. Nat. Rev. Neurosci. 2009;10(6):459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M., Pu Z., Wiegert O., Oitzl M.S., Krugers H.J. Learning under stress: how does it work? Trends Cogn. Sci. 2006;10(4):152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Joels M., Fernandez G., Roozendaal B. Stress and emotional memory: a matter of timing. Trends Cogn. Sci. 2011;15(6):280–288. doi: 10.1016/j.tics.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Johnson J.G., Raab M. Take the first: option-generation and resulting choices. Organ. Behav. Hum. Decis. Process. 2003;91(2):215–229. [Google Scholar]
- Kahneman D., Tversky A. Prospect theory: an analysis of decision under risk. Econom. J. Econ. Soc. 1979:263–291. [Google Scholar]
- Kahneman D., Tversky A. Choices, values, and frames. Am. Psychol. 1984;39(4):341. [Google Scholar]
- Kahneman D. Macmillan; 2011. Thinking, Fast and Slow. [Google Scholar]
- Kandasamy N., Hardy B., Page L., Schaffner M., Graggaber J., Powlson A.S. Cortisol shifts financial risk preferences. Proc. Natl. Acad. Sci. U. S. A. 2014;111(9):3608–3613. doi: 10.1073/pnas.1317908111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassam K.S., Koslov K., Mendes W.B. Decisions under distress: stress profiles influence anchoring and adjustment. Psychol. Sci. 2009;20(11):1394–1399. doi: 10.1111/j.1467-9280.2009.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinan G. Decision making under stress: scanning of alternatives under controllable and uncontrollable threats. J. Personal. Soc. Psychol. 1987;52(3):639. doi: 10.1037//0022-3514.52.3.639. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Diamond D.M. The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 2002;3(6):453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Loucks R.A., Palmer A.L., Brown A.C., Solomon K.M., Marchante A.N. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav. Brain Res. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G.A. MIT press; 1999. Sources of Power: How People Make Decisions. [Google Scholar]
- Klucken T., Alexander N., Schweckendiek J., Merz C.J., Kagerer S., Osinsky R. Individual differences in neural correlates of fear conditioning as a function of 5-HTTLPR and stressful life events. Soc. Cogn. Affect. Neurosci. 2013;8(3):318–325. doi: 10.1093/scan/nss005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H., Barrett L.F., Joseph J., Bliss-Moreau E., Lindquist K., Wager T.D. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte S.M. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci. Biobehav. Rev. 2001;25(2):117–142. doi: 10.1016/s0149-7634(01)00002-1. [DOI] [PubMed] [Google Scholar]
- Kruglanski A.W., Gigerenzer G. Intuitive and deliberate judgments are based on common principles. Psychol. Rev. 2011;118(1):97–109. doi: 10.1037/a0020762. [DOI] [PubMed] [Google Scholar]
- Lassiter G.D., Lindberg M.J., Gonzalez-Vallejo C., Bellezza F.S., Phillips N.D. The deliberation-without-attention effect: evidence for an artifactual interpretation. Psychol. Sci. 2009;20(6):671–675. doi: 10.1111/j.1467-9280.2009.02347.x. [DOI] [PubMed] [Google Scholar]
- Leder J., Hausser J.A., Mojzisch A. Stress and strategic decision-making in the beauty contest game. Psychoneuroendocrinology. 2013;38(9):1503–1511. doi: 10.1016/j.psyneuen.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Lee S.W., Shimojo S., O'Doherty J.P. Neural computations underlying arbitration between model-based and model-free learning. Neuron. 2014;81(3):687–699. doi: 10.1016/j.neuron.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempert K.M., Porcelli A.J., Delgado M.R., Tricomi E. Individual differences in delay discounting under acute stress: the role of trait perceived stress. Front. Psychol. 2012;3:251. doi: 10.3389/fpsyg.2012.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A.H., Porcelli A.J., Delgado M.R. The effects of acute stress exposure on striatal activity during Pavlovian conditioning with monetary gains and losses. Front. Behav. Neurosci. 2014;8:179. doi: 10.3389/fnbeh.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M.D. Social cognitive neuroscience: a review of core processes. Annu. Rev. Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lighthall N.R., Mather M., Gorlick M.A. Acute stress increases sex differences in risk seeking in the balloon analogue risk task. PLoS One. 2009;4(7):e6002. doi: 10.1371/journal.pone.0006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall N.R., Sakaki M., Vasunilashorn S., Nga L., Somayajula S., Chen E.Y. Gender differences in reward-related decision processing under stress. Soc. Cogn. Affect. Neurosci. 2012;7(4):476–484. doi: 10.1093/scan/nsr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall N.R., Gorlick M.A., Schoeke A., Frank M.J., Mather M. Stress modulates reinforcement learning in younger and older adults. Psychol. Aging. 2013;28(1):35–46. doi: 10.1037/a0029823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S.U., Makwana A.B., Hare T.A. Acute Stress impairs self-control in goal-directed choice by altering multiple functional connections within the brain's decision circuits. Neuron. 2015;87(3):621–631. doi: 10.1016/j.neuron.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Margittai Z., Nave G., Strombach T., van Wingerden M., Schwabe L., Kalenscher T. Exogenous cortisol causes a shift from deliberative to intuitive thinking. Psychoneuroendocrinology. 2015;64:131–135. doi: 10.1016/j.psyneuen.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Margittai Z., Strombach T., van Wingerden M., Joels M., Schwabe L., Kalenscher T. A friend in need: Time-dependent effects of stress on social discounting in men. Hormones Behav. 2015;73:75–82. doi: 10.1016/j.yhbeh.2015.05.019. [DOI] [PubMed] [Google Scholar]
- Masicampo E.J., Baumeister R.F. Toward a physiology of dual-process reasoning and judgment: lemonade, willpower, and expensive rule-based analysis. Psychol. Sci. 2008;19(3):255–260. doi: 10.1111/j.1467-9280.2008.02077.x. [DOI] [PubMed] [Google Scholar]
- Mather M., Lighthall N.R. Both risk and reward are processed differently in decisions made under stress. Curr. Dir. Psychol. Sci. 2012;21(2):36–41. doi: 10.1177/0963721411429452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M., Gorlick M.A., Lighthall N.R. To brake or accelerate when the light turns yellow? Stress reduces older adults' risk taking in a driving game. Psychol. Sci. 2009;20(2):174–176. doi: 10.1111/j.1467-9280.2009.02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott R., Fowler J.H., Smirnov O. On the evolutionary origin of prospect theory preferences. J. Polit. 2008;70(02):335–350. [Google Scholar]
- McGinley M., Carlo G., Crockett L.J., Raffaelli M., Stone R.A., Iturbide M.I. Stressed and helping: the relations among acculturative stress, gender, and prosocial tendencies in Mexican Americans. J. Soc. Psychol. 2010;150(1):34–56. doi: 10.1080/00224540903365323. [DOI] [PubMed] [Google Scholar]
- McNaughton N., Corr P.J. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci. Biobehav. Rev. 2004;28(3):285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Merz C.J., Tabbert K., Schweckendiek J., Klucken T., Vaitl D., Stark R. Neuronal correlates of extinction learning are modulated by sex hormones. Soc. Cogn. Affect. Neurosci. 2012;7(7):819–830. doi: 10.1093/scan/nsr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz C.J., Stark R., Vaitl D., Tabbert K., Wolf O.T. Stress hormones are associated with the neuronal correlates of instructed fear conditioning. Biol. Psychol. 2013;92(1):82–89. doi: 10.1016/j.biopsycho.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Merz C.J., Wolf O.T., Schweckendiek J., Klucken T., Vaitl D., Stark R. Stress differentially affects fear conditioning in men and women. Psychoneuroendocrinology. 2013;38(11):2529–2541. doi: 10.1016/j.psyneuen.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Merz C.J., Hamacher-Dang T.C., Wolf O.T. Exposure to stress attenuates fear retrieval in healthy men. Psychoneuroendocrinology. 2014;41:89–96. doi: 10.1016/j.psyneuen.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Mischel W., Shoda Y., Rodriguez M.I. Delay of gratification in children. Science. 1989;244(4907):933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Mobbs D., Petrovic P., Marchant J.L., Hassabis D., Weiskopf N., Seymour B. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317(5841):1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D., Yu R., Rowe J.B., Eich H., FeldmanHall O., Dalgleish T. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc. Natl. Acad. Sci. U. S. A. 2010;107(47):20582–20586. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya E.R., Bos P.A., Terburg D., Rosenberger L.A., van Honk J. Cortisol administration induces global down-regulation of the brain's reward circuitry. Psychoneuroendocrinology. 2014;47:31–42. doi: 10.1016/j.psyneuen.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Morgado P., Sousa N., Cerqueira J.J. The impact of stress in decision making in the context of uncertainty. J. Neurosci. Res. 2015;93(6):839–847. doi: 10.1002/jnr.23521. [DOI] [PubMed] [Google Scholar]
- Nesse R.M., Young E.A. Evolutionary origins and functions of the stress response. Encycl. stress. 2000;2:79–84. [Google Scholar]
- Nikolova Y.S., Bogdan R., Brigidi B.D., Hariri A.R. Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biol. psychiatry. 2012;72(2):157–163. doi: 10.1016/j.biopsych.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Emotion regulation and psychopathology: the role of gender. Annu. Rev. Clin. Psychol. 2012;8:161–187. doi: 10.1146/annurev-clinpsy-032511-143109. [DOI] [PubMed] [Google Scholar]
- Oei N.Y., Both S., van Heemst D., van der Grond J. Acute stress-induced cortisol elevations mediate reward system activity during subconscious processing of sexual stimuli. Psychoneuroendocrinology. 2014;39:111–120. doi: 10.1016/j.psyneuen.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Ossewaarde L., Qin S., Van Marle H.J., van Wingen G.A., Fernandez G., Hermans E.J. Stress-induced reduction in reward-related prefrontal cortex function. NeuroImage. 2011;55(1):345–352. doi: 10.1016/j.neuroimage.2010.11.068. [DOI] [PubMed] [Google Scholar]
- Pabst S., Schoofs D., Pawlikowski M., Brand M., Wolf O.T. Paradoxical effects of stress and an executive task on decisions under risk. Behav. Neurosci. 2013;127(3):369–379. doi: 10.1037/a0032334. [DOI] [PubMed] [Google Scholar]
- Pabst S., Brand M., Wolf O.T. Stress effects on framed decisions: there are differences for gains and losses. Front. Behav. Neurosci. 2013;7:142. doi: 10.3389/fnbeh.2013.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy G.M., Salmon D.P., Jacobson M.W., Hervey A., Gamst A.C., Wolfson T. Effects of chronic stress on memory decline in cognitively normal and mildly impaired older adults. Am. J. Psychiatry. 2009;166(12):1384–1391. doi: 10.1176/appi.ajp.2009.09040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold A., Plessow F., Goschke T., Kirschbaum C. Stress reduces use of negative feedback in a feedback-based learning task. Behav. Neurosci. 2010;124(2):248–255. doi: 10.1037/a0018930. [DOI] [PubMed] [Google Scholar]
- Pillutla M.M., Murnighan J.K. Unfairness, anger, and spite: Emotional rejections of ultimatum offers. Organ. Behav. Hum. Decis. Process. 1996;68(3):208–224. [Google Scholar]
- Porcelli A.J., Delgado M.R. Acute stress modulates risk taking in financial decision making. Psychol. Sci. 2009;20(3):278–283. doi: 10.1111/j.1467-9280.2009.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli A.J., Lewis A.H., Delgado M.R. Acute stress influences neural circuits of reward processing. Front. Neurosci. 2012;6:157. doi: 10.3389/fnins.2012.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston S.D., Buchanan T.W., Stansfield R.B., Bechara A. Effects of anticipatory stress on decision making in a gambling task. Behav. Neurosci. 2007;121(2):257–263. doi: 10.1037/0735-7044.121.2.257. [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Champagne F., Meaney M.J., Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J. Neurosci. Off. J. Soc. Neurosci. 2004;24(11):2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner J.C., Dedovic K., Khalili-Mahani N., Engert V., Pruessner M., Buss C. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol. psychiatry. 2008;63(2):234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Raio C.M., Orederu T.A., Palazzolo L., Shurick A.A., Phelps E.A. Cognitive emotion regulation fails the stress test. Proc. Natl. Acad. Sci. U. S. A. 2013;110(37):15139–15144. doi: 10.1073/pnas.1305706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand D.G., Greene J.D., Nowak M.A. Spontaneous giving and calculated greed. Nature. 2012;489(7416):427–430. doi: 10.1038/nature11467. [DOI] [PubMed] [Google Scholar]
- Rand D.G., Peysakhovich A., Kraft-Todd G.T., Newman G.E., Wurzbacher O., Nowak M.A. Social heuristics shape intuitive cooperation. Nat. Commun. 2014;5:3677. doi: 10.1038/ncomms4677. [DOI] [PubMed] [Google Scholar]
- Reyna V.F. How people make decisions that involve risk a dual-processes approach. Curr. Dir. Psychol. Sci. 2004;13(2):60–66. [Google Scholar]
- Richards J.M., Patel N., Daniele-Zegarelli T., MacPherson L., Lejuez C.W., Ernst M. Social anxiety, acute social stress, and reward parameters interact to predict risky decision-making among adolescents. J. anxiety Disord. 2014;29C:25–34. doi: 10.1016/j.janxdis.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.D., Wilkowski B.M., Kirkeby B.S., Meier B.P. Stuck in a rut: perseverative response tendencies and the neuroticism-distress relationship. J. Exp. Psychol. General. 2006;135(1):78–91. doi: 10.1037/0096-3445.135.1.78. [DOI] [PubMed] [Google Scholar]
- Robinson O.J., Overstreet C., Charney D.R., Vytal K., Grillon C. Stress increases aversive prediction error signal in the ventral striatum. Proc. Natl. Acad. Sci. U. S. A. 2013;110(10):4129–4133. doi: 10.1073/pnas.1213923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs K., Elzinga B.M., Rotteveel M. The effects of stress-induced cortisol responses on approach-avoidance behavior. Psychoneuroendocrinology. 2005;30(7):665–677. doi: 10.1016/j.psyneuen.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Roelofs K., Bakvis P., Hermans E.J., van Pelt J., van Honk J. The effects of social stress and cortisol responses on the preconscious selective attention to social threat. Biol. Psychol. 2007;75(1):1–7. doi: 10.1016/j.biopsycho.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., McEwen B.S., Chattarji S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009;10(6):423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Sanfey A.G., Rilling J.K., Aronson J.A., Nystrom L.E., Cohen J.D. The neural basis of economic decision-making in the ultimatum game. Science. 2003;300(5626):1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch. general psychiatry. 2000;57(10):925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Schwabe L., Wolf O.T. Stress prompts habit behavior in humans. J. Neurosci. Off. J. Soc. Neurosci. 2009;29(22):7191–7198. doi: 10.1523/JNEUROSCI.0979-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L., Wolf O.T. Stress-induced modulation of instrumental behavior: from goal-directed to habitual control of action. Behav. Brain Res. 2011;219(2):321–328. doi: 10.1016/j.bbr.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Schwabe L., Wolf O.T. Stress and multiple memory systems: from ‘thinking’ to ‘doing’. Trends Cogn. Sci. 2013;17(2):60–68. doi: 10.1016/j.tics.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Schwabe L., Wolf O.T. Timing matters: temporal dynamics of stress effects on memory retrieval. Cogn. Affect. Behav. Neurosci. 2014;14(3):1041–1048. doi: 10.3758/s13415-014-0256-0. [DOI] [PubMed] [Google Scholar]
- Schwabe L., Hoffken O., Tegenthoff M., Wolf O.T. Preventing the stress-induced shift from goal-directed to habit action with a beta-adrenergic antagonist. J. Neurosci. Off. J. Soc. Neurosci. 2011;31(47):17317–17325. doi: 10.1523/JNEUROSCI.3304-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L., Joels M., Roozendaal B., Wolf O.T., Oitzl M.S. Stress effects on memory: an update and integration. Neurosci. Biobehav. Rev. 2012;36(7):1740–1749. doi: 10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Seehagen S., Schneider S., Rudolph J., Ernst S., Zmyj N. Stress impairs cognitive flexibility in infants. Proc. Natl. Acad. Sci. U. S. A. 2015;112(41):12882–12886. doi: 10.1073/pnas.1508345112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets T., Dziobek I., Wolf O.T. Social cognition under stress: differential effects of stress-induced cortisol elevations in healthy young men and women. Hormones Behav. 2009;55(4):507–513. doi: 10.1016/j.yhbeh.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Soares J.M., Sampaio A., Ferreira L.M., Santos N.C., Marques F., Palha J.A. Stress-induced changes in human decision-making are reversible. Transl. psychiatry. 2012;2:e131. doi: 10.1038/tp.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal M.A. NASA Technical Memorandum; 2004. Stress, cognition, and human performance: a literature review and conceptual framework; p. 212824. [Google Scholar]
- Starcke K., Brand M. Decision making under stress: a selective review. Neurosci. Biobehav. Rev. 2012;36(4):1228–1248. doi: 10.1016/j.neubiorev.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Starcke K., Wolf O.T., Markowitsch H.J., Brand M. Anticipatory stress influences decision making under explicit risk conditions. Behav. Neurosci. 2008;122(6):1352–1360. doi: 10.1037/a0013281. [DOI] [PubMed] [Google Scholar]
- Starcke K., Polzer C., Wolf O.T., Brand M. Does stress alter everyday moral decision-making? Psychoneuroendocrinology. 2011;36(2):210–219. doi: 10.1016/j.psyneuen.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Starcke K., Ludwig A.C., Brand M. Anticipatory stress interferes with utilitarian moral judgment. Judgm. Decis. Mak. 2012;7(1):61–68. [Google Scholar]
- Stark R., Wolf O.T., Tabbert K., Kagerer S., Zimmermann M., Kirsch P. Influence of the stress hormone cortisol on fear conditioning in humans: evidence for sex differences in the response of the prefrontal cortex. NeuroImage. 2006;32(3):1290–1298. doi: 10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Tabbert K., Merz C.J., Klucken T., Schweckendiek J., Vaitl D., Wolf O.T. Cortisol enhances neural differentiation during fear acquisition and extinction in contingency aware young women. Neurobiol. Learn. Mem. 2010;94(3):392–401. doi: 10.1016/j.nlm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Ikeda K., Hasegawa T. Social evaluation-induced amylase elevation and economic decision-making in the dictator game in humans. Neuro Endocrinol. Lett. 2007;28(5):662–665. [PubMed] [Google Scholar]
- Taylor S.E., Klein L.C., Lewis B.P., Gruenewald T.L., Gurung R.A., Updegraff J.A. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol. Rev. 2000;107(3):411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Taylor S.E. Tend and befriend biobehavioral bases of affiliation under stress. Curr. Dir. Psychol. Sci. 2006;15(6):273–277. [Google Scholar]
- Tversky A., Kahneman D. Judgment under Uncertainty: heuristics and biases. Science. 1974;185(4157):1124–1131. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- Tversky A., Kahneman D. Extensional versus intuitive reasoning: the conjunction fallacy in probability judgment. Psychol. Rev. 1983;90(4):293. doi: 10.3389/fpsyg.2015.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos R., Harteveld M., Stoop H. Stress and decision-making in humans: performance is related to cortisol reactivity, albeit differently in men and women. Psychoneuroendocrinology. 2009;34(10):1449–1458. doi: 10.1016/j.psyneuen.2009.04.016. [DOI] [PubMed] [Google Scholar]
- van den Bos W., Cohen M.X., Kahnt T., Crone E.A. Striatum-medial prefrontal cortex connectivity predicts developmental changes in reinforcement learning. Cereb. Cortex. 2012;22(6):1247–1255. doi: 10.1093/cercor/bhr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen G.A., Geuze E., Vermetten E., Fernandez G. Perceived threat predicts the neural sequelae of combat stress. Mol. psychiatry. 2011;16(6):664–671. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkers C.H., Zorn J.V., Cornelisse S., Koot S., Houtepen L.C., Olivier B. Time-dependent changes in altruistic punishment following stress. Psychoneuroendocrinology. 2013;38(9):1467–1475. doi: 10.1016/j.psyneuen.2012.12.012. [DOI] [PubMed] [Google Scholar]
- von Dawans B., Fischbacher U., Kirschbaum C., Fehr E., Heinrichs M. The social dimension of stress reactivity: acute stress increases prosocial behavior in humans. Psychol. Sci. 2012;23(6):651–660. doi: 10.1177/0956797611431576. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Waugh C.E., Lindquist M., Noll D.C., Fredrickson B.L., Taylor S.F. Brain mediators of cardiovascular responses to social threat: part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage. 2009;47(3):821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., van Ast V.A., Hughes B.L., Davidson M.L., Lindquist M.A., Ochsner K.N. Brain mediators of cardiovascular responses to social threat, part II: prefrontal-subcortical pathways and relationship with anxiety. NeuroImage. 2009;47(3):836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi T., Horita Y., Mifune N., Hashimoto H., Li Y., Shinada M. Rejection of unfair offers in the ultimatum game is no evidence of strong reciprocity. Proc. Natl. Acad. Sci. U. S. A. 2012;109(50):20364–20368. doi: 10.1073/pnas.1212126109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H.H., Knowlton B.J. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Youssef F.F., Dookeeram K., Basdeo V., Francis E., Doman M., Mamed D. Stress alters personal moral decision making. Psychoneuroendocrinology. 2012;37(4):491–498. doi: 10.1016/j.psyneuen.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Yu R., Mobbs D., Seymour B., Calder A.J. Insula and striatum mediate the default bias. J. Neurosci. Off. J. Soc. Neurosci. 2010;30(44):14702–14707. doi: 10.1523/JNEUROSCI.3772-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R. Regional white matter volumes correlate with delay discounting. PLoS One. 2012;7(2):e32595. doi: 10.1371/journal.pone.0032595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R. Choking under pressure: the neuropsychological mechanisms of incentive-induced performance decrements. Front. Behav. Neurosci. 2015;9:19. doi: 10.3389/fnbeh.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.L., Shi J., Zhao L.Y., Sun L.L., Wang J., Wang G.B. Effects of stress on decision-making deficits in formerly heroin-dependent patients after different durations of abstinence. Am. J. psychiatry. 2011;168(6):610–616. doi: 10.1176/appi.ajp.2010.10040499. [DOI] [PubMed] [Google Scholar]
- Zorawski M., Cook C.A., Kuhn C.M., LaBar K.S. Sex, stress, and fear: individual differences in conditioned learning. Cogn. Affect. Behav. Neurosci. 2005;5(2):191–201. doi: 10.3758/cabn.5.2.191. [DOI] [PubMed] [Google Scholar]
- Zorawski M., Blanding N.Q., Kuhn C.M., LaBar K.S. Effects of stress and sex on acquisition and consolidation of human fear conditioning. Learn Mem. 2006;13(4):441–450. doi: 10.1101/lm.189106. [DOI] [PMC free article] [PubMed] [Google Scholar]