Abstract
In visual search tasks, neglect patients tend to explore and repeatedly re-cancel stimuli on the ipsilesional side, as if they did not realize that they had previously examined the rightward locations favoured by their lateral bias. The aim of this study was to explore the hypothesis that a spatial working memory deficit explains these ipsilesional re-cancellation errors in neglect patients. For the first time, we evaluated spatial working memory and re-cancellation through separate and independent tasks in a group of patients with right hemisphere damage and a diagnosis of left neglect. Results showed impaired spatial working memory in neglect patients. Compared to the control group, neglect patients cancelled fewer targets and made more re-cancellations both on the left side and on the right side. The spatial working memory deficit appears to be related to re-cancellations, but only for some neglect patients. Alternative interpretations of re-exploration of space are discussed.
Keywords: Adult; Aged; Perceptual Disorders; Space Perception; Spatial Memory; Attention; Female; Humans; Male; Memory Disorders; Memory, Short-Term; Middle Aged; Neuropsychological Tests
1. Introduction
Spatial neglect is a heterogeneous syndrome characterized by an inability to orient, to report or to respond to stimuli arising in the hemispace contralateral to a brain lesion (Heilman et al. 1984). Neglect patients tend to explore mainly ipsilesional locations and ignore events or objects localized on their contralesional side. Attentional disturbances play a major role in left neglect, including an attentional lateral bias towards the right and difficulty disengaging attention from items on the right side (for a review, see Bartolomeo et al. 2012). Recently, it has been suggested that impairments in spatial working memory (SWM) might exacerbate the neglect syndrome (e.g. Kristjánsson and Vuilleumier 2010; Malhotra et al. 2004; Pisella et al. 2004). In keeping with this hypothesis, several functional imaging studies in healthy subjects support the idea that the right posterior parietal cortex, frequently damaged in spatial neglect and related to attentional networks (Bartolomeo et al. 2007), is important in tasks requiring SWM (Awh and Jonides 2001; Corbetta and Shulman 2011).
To investigate SWM deficits in visual neglect, Malhotra et al. (2004, 2005) used a modified version of the Corsi test. To avoid contamination of performance by perceptual or attentional deficits, all stimuli were presented in a vertical array. The participants’ tasks were to recall the presented locations in the correct order, or to judge whether a specific presented location was part of the sequence. Neglect patients demonstrated a non-lateralized impairment of SWM on both tasks (span < 2). However, Kristjánsson and Vuilleumier (2010) found that the SWM deficit was more pronounced for the left than for the right side, suggesting that this deficit might be more lateralized than previously argued. To ensure that this spatial deficit did not result from a general impairment in visuo-SWM, Pisella et al. (2004) used a change detection task in which a matrix containing four objects which varied in location, colour and shape was presented. In separate sessions, participants were required to detect changes in a particular attribute of one of the objects. Neglect patients demonstrated a memory impairment for spatial location, but not for colour or shape, consistent with the hypothesis of a specific impairment of SWM in visual neglect (see Danckert and Ferber 2006, for a review).
Several studies have also shown that a SWM deficit might exacerbate neglect, as reflected in the patients’ tendency to repeatedly search through items located on the right, as if they did not realize that they had previously examined the rightward locations favored by their lateral bias (Husain et al. 2001; Mannan et al. 2005; Wojciulik et al. 2001). This spatial re-exploration behaviour is particularly evident in cancellation tasks in which no marks are left on the processed targets (Husain et al. 2001; Mannan et al. 2005; Wojciulik et al. 2001). As Driver and Husain (2002) pointed out, the visible cancellation task does not involve SWM, since the targets that have already been found are noticeably marked. Conversely, the invisible cancellation task requires remembering the already processed targets to ensure effective visual search.
Other studies have investigated the cognitive mechanisms involved in space re-exploration, and more specifically the role of SWM and stimulus salience in re-cancellation. For example, Parton et al. (2006) used several cancellation tasks in which visual salience was manipulated after a target had been cancelled. They found that the presence or absence of visual feedback influenced the degree of neglect and the amount of re-cancellation, these being particularly marked in conditions without visual feedback. In addition, these pathological behaviours seemed limited to SWM: frequent re-cancellations were observed when the targets differed only in their spatial location, and not when each target was a different object (Wojciulik et al. 2001). Together, these results suggest that attentional capture by salient visible marks cannot completely account for pathological search in spatial neglect. By contrast, SWM does appear to be involved in recursive search behaviour, exacerbating the initial lateral bias in invisible cancellation tasks.
Husain et al. (2001), Mannan et al. (2005) more specifically studied the role of SWM in the pathological search behaviours of neglect patients using a “search-and-click” method, which corresponds to an “invisible” visual search task. The subjects’ task was to find targets (Ts) among distractors (Ls), and to press a response button only when they judged that the stimulus they were fixating on was a “new” target that they had not yet found. The analysis of the click responses showed that many neglect patients treated previously fixated targets as new discoveries, thus demonstrating a deficit in keeping these previously examined locations in memory. In addition, re-cancellation (re-clicking) behaviour was correlated with the severity of neglect. Together, these results led the authors to conclude that SWM deficits can exacerbate spatial neglect, because right-sided stimuli are repeatedly reexamined as if being searched for the first time. As a result, left-sided targets continue to be ignored. Ronchi et al. (2009) directly investigated the relationship between SWM (evaluated using traditional and modified Corsi tests) and re-cancellation in a visible cancellation task. They found that impaired SWM was not related to re-cancellation, but that it was related to left spatial neglect. However, the use of a visual feedback in the cancellation task in this study could explain this failure to find a relationship.
In sum, these studies suggest that the combination of attentional bias and a SWM deficit may explain recursive search and omission behaviours in visual neglect (Husain and Rorden 2003). However, in the majority of these studies, the measure of SWM was the rate of re-cancellations (re-clicks) in the invisible search task, without any direct assessment of SWM using independent tasks. Thus, it is difficult to conclude that SWM is involved in re-cancellation on the basis of these results, because the same measure was used to assess both SWM and re-cancellations.
Thus, the putatively direct link between re-cancellation and SWM in invisible cancellation tasks—which make greater demands on SWM (Driver and Husain 2002; see also Wojciulik et al. 2001, 2004)—has not yet been investigated. The purpose of the present study was to test the extent to which impaired SWM can explain re-cancellations in unilateral neglect, by separately assessing SWM and re-cancellation. If a deficit of SWM is indeed closely related to the pathological behaviours shown by neglect patients, it should significantly correlate with re-cancellation, especially in invisible cancellation tasks.
2. Methods
2.1. Participants
Fourteen right brain-damaged patients suffering from left spatial neglect (mean age, 60.00 years; SD, 16.24) and 14 elderly age-matched controls without neurological impairment (mean age, 59.21; SD, 15.60) participated in this study. Spatial neglect was assessed using the Batteried’ Evaluation de la Négligence (Azouvi et al. 2002). Patients were considered to have left neglect if they had poor performance on two or more clinical tests. The aetiology of the lesions was vascular in 12 patients and neoplastic in two patients.1 Exclusion criteria were bilateral lesions, evidence of previous neurological diseases, or psychiatric disorders. The mean interval between stroke onset and experimental testing was 142.14 days (range: 29–409 days). Neither significant age differences, t(26) = .13, p = .90, nor difference in years of education, t(26) = .32, p = .75, were found between the neglect and control group.
The volume and location of the brain lesion (available for 13 out of 14 neglect patients) was assessed on the basis of a computed tomographic (CT) or magnetic resonance imaging (MRI) scan of the patient’s brain. The anatomical data were normalized to MNI space using the Clinical toolbox(Rorden et al. 2012). This toolbox, implemented in SPM8, has two advantages over the default spatial normalization implemented in SPM. On the one hand, it uses a template based on elderly people’s scans, which is more suitable for analysing our data. On the other hand, it implements a cost function masking method, wherein regions identified as unusual (i.e. the lesions) do not contribute to the normalization transforms. As a consequence, the nonlinear components employed in normalization do not decrease the calculated size of a brain lesion or distort the local healthy tissue. The lesions were then manually traced on the normalized structural image using PMOD software (http://www.pmod.com/technologies/index.html).
All participants gave informed consent in accordance with the local research ethics committee. The patients’ demographic, neurological and clinical data are summarized in Table 1.
Table 1.
Demographic, neurological, and clinical data on neglect patients
| Patient | Sex/age/education | Days post stroke onset | Aetiology/lesion site | Bells cancellation (left/right found targets, max = 15/15) | Bells cancellation (time in seconds) | Letter Cancellation (left/right found targets, max = 30/30) | Line bisection (mm of rightward deviation for 20 cm lines) | Landscape drawing score (Odgen) | Reading (left/right words, max = 61/55) |
|---|---|---|---|---|---|---|---|---|---|
| P1 | M/29/8 | 29 | N/FTP | 0/9* | 60 | 0/19* | +31* | NA | 6/43* |
| P2 | M/64/17 | 261 | I/Ins (sylvian) | 12/14* | 127 | 10/29* | +12* | 1* | 61/55 |
| P3 | M/51/16 | 187 | N/F | 14/14 | 272* | 20/29* | −1.5 | 1* | NA |
| P4 | F/53/15 | 409 | H/Ic (sylvian) | 15/15 | 290* | 30/30 | +2.5 | 1* | 61/55 |
| P5 | F/68/6 | 137 | I/F Th | 10/14* | 60 | 21/27* | −1 | 0 | 61/55 |
| P6 | F/54/10 | 106 | I + H/T | 14/13 | 230* | 30/30 | +12.5* | 1* | 61/55 |
| P7 | F/84/10 | 253 | I/TO Ic | 15/10 | 225* | 24/21* | +34.5* | 0 | 51/55* |
| P8 | F/70/6 | 183 | I/TP (sylvian) | 11/13 | 211* | NA | +9* | 0 | 61/55 |
| P9 | M/46/20 | 84 | I/rolandic | 13/15 | 284* | 28/29* | +10* | 0 | 61/55 |
| P10 | M/75/12 | 41 | I/FP Bg ic | 0/13* | 50 | 0/10 | +13.5* | NA | 61/55 |
| P11 | M/88/16 | 29 | I + H/FO | 12/15* | 159 | 24/29* | +1.5 | 0 | 61/55 |
| P12 | F/53/6 | 78 | I/P Ins (sylvian) | 0/5* | 72 | NA | +39* | 4* | 0/37* |
| P13 | M/62/10 | 159 | I/P Ins (sylvian) | 14/15 | 102 | 30/30 | +15.5* | 1* | 60/55* |
| P14 | M/43/14 | 34 | I + H/PF (sylvian) | 1/14* | 58 | 10/23* | −4.5 | 0 | 60/55* |
I ischemic, H haemorrhagic, N neoplastic, F frontal, P parietal, T temporal, O occipital, Th thalamus, Ins insula, Bg basal ganglia, ic internal capsule, NA not available. For line bisection, positive values indicate rightward deviations and negative values indicate leftward deviation. Score for landscape drawing indicates the number of omitted left-sided details. Asterisks denote pathological performance
2.2. Procedure
All tasks were administered on a touchscreen (Shuttle X50V2; 32.7 × 39.1) viewed from a distance of approximately 50 cm. These computerized tasks were programmed with the Toolbook software (version 9.0). All participants were seated in front of the computer screen, with its vertical midline aligned with the body midline.
2.2.1. Spatial working memory
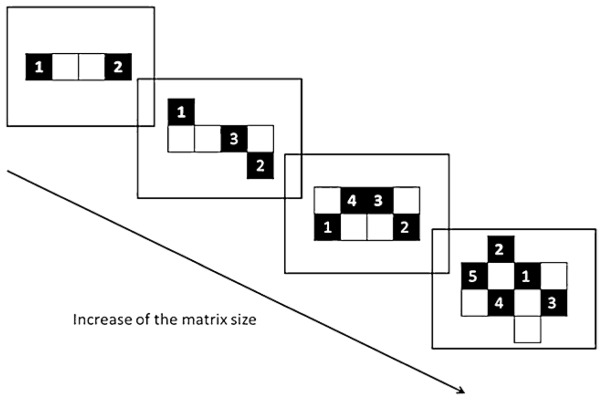
Because the spatial memory abilities measured through performance on traditional Corsi blocks test might be underestimated due to the patients’ failure to encode leftward locations, many studies have used a vertical version of the Corsi test (e.g. Malhotra et al. 2005). However, the vertical version is not exempt from potential problems. For example, it could promote the involvement of verbal working memory even more than the traditional version. In addition, the spatial dimension (and the complexity) of the pattern is much reduced in the vertical version compared to the traditional task. For these reasons, we decided to develop a new procedure in order to both circumvent the potential limitations of the vertical Corsi task and the influence of the neglect on the span measure with the traditional Corsi task. SWM was assessed using a computerized version of the Corsi test in which the grids progressed in size vertically from the smallest to the largest while keeping the horizontal basis (four squares, see Figure 1) constant throughout testing. The procedure used to determine the patient’s span (and the stop criterion) was aimed to limit the impact of the patient’s inability to encode leftward locations on the span calculation; the central point of this procedure was that the stop criterion was not based on the patient’s ability to recall the whole pattern (as it is traditionally the case), but instead on the patient’s inability to improve his/her recall when presented with new patterns comprising more targets on their non-neglected side (see below for details).
Fig. 1.
Schematic drawing of the sequence of events in the spatial working memory task (NB: the numbers were not shown to the subject)
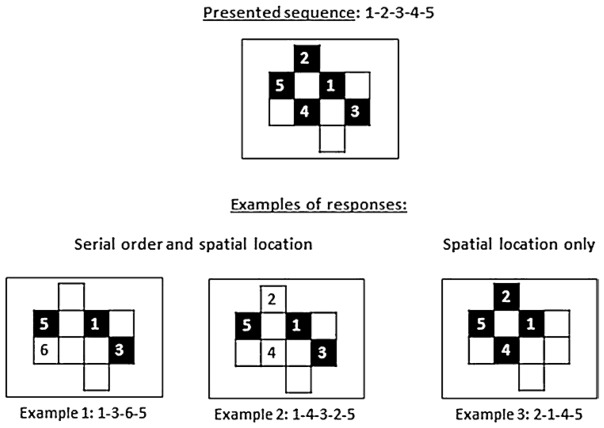
Each trial consisted in the presentation of a matrix pattern in which half of the black squares were shown sequentially, one square per second, with an inter-stimulus interval of one second. No square was highlighted twice during a sequence. There were four trials for each level of complexity; over these four trials, the number of targets presented was equivalently distributed in the left and right halves of the screen. After each trial, an empty matrix of the same size was presented on the touchscreen and the participant’s task was to reproduce the sequence by touching the screen, with no time limit. A beep prompted the participant to recall the sequence. The task ended when the participant was no longer able to improve his/her performance, i.e. when the number of items correctly recalled did not increase from one level of difficulty to the next. In order to estimate SWM capacity as precisely as possible, we used two different scoring procedures (see Figure 2): (1) one based on both the serial order and spatial location of the stimuli (e.g. for the sequence 1-2-3-4-5, participants who answered 1-3-6-5 or 1-4-3-2-5 would both obtain a score of 3, because in each case, the locations 1, 3, and 5 were reproduced in the correct order—in the first case, with an omission between 1 and 3 and an error between 3 and 5, and in the second case, with an inversion of the locations of 2 and 4) and (2) a less strict procedure taking into account only the locations of the stimuli (e.g. for the sequence 1-2-3-4-5, the response 2-1-4-5 would yield a score of 4, because the locations 2, 1, 4, and 5 were all part of the presented sequence, regardless of their order, see Fig. 2). Our aim with these procedures was to avoid underestimating the patients’ span because of their difficulty in processing stimuli presented on the left side. The subject’s score (spatial span) corresponded to the highest score obtained at least three times out of all trials presented. Once this highest score was obtained, a last series of four trials, corresponding to the next level of difficulty, was administered. The span value was decreased or increased by .25 depending on performance on each of the four trials in this final series (plus .25 if performance on a trial was greater than the span, minus .25 if it was less than the span). Furthermore, in order to control for possible difficulties in programming leftward hand movements (see Bartolomeo et al. 1998), we administered a separate task in which no motor response was required. On this task, participants were instructed to respond by verbally selecting digits randomly placed on the matrix.
Fig. 2.
Examples of responses in the two scoring modes. Responses counting for one point are represented by a black square. Examples 1 and 2 illustrate the first scoring mode; example 3 illustrates the second scoring mode (see text)
2.2.2. Cancellation tasks
Targets consisted of 32 white outline circles (1.5 cm diameter) with an equal number of stimuli on the left and on the right side of the screen, without distractors (Toba et al. 2009). The visual salience of the targets was manipulated across two experimental conditions, with visual feedback (visible condition) or without visual feedback (invisible condition). Participants were instructed to cancel each circle once. In the visible condition, detected targets were marked by increasing their visual salience on the black screen (the outline circles were filled with white). In the invisible cancellation task, no mark was left on the detected targets. In this condition, which was intended to increase memory load, we stressed that each target had to be cancelled only once and that the participants had to remember the targets they had already cancelled. The experiment ended when the participant judged that all the targets had been touched. There was no time limit. Mannan et al. (2005) proposed to differentiate between “immediate” perseverative behaviour (re-touching that could be associated with an inhibition deficit) and “delayed” re-cancellations (with visits to other targets in the interim, associated with a SWM impairment). In the present study, the number of immediate re-cancellations was very low, with four patients committing only one immediate re-cancellation. Thus, we considered only delayed re-cancellations.
3. Results
Since the data were not normally distributed and the variances were not homogeneous, we used nonparametric tests.
3.1. Spatial working memory
Control subjects achieved better performance than neglect patients in the SWM task, U = 13, Z = −3.90, p < .001: neglect patients had a mean spatial span of 3.46 (SD = 1.12), while the mean of the control group was 5.23 (SD = .80). A less strict scoring mode based only on target locations led to the same outcome, U = 19, Z = −3.65, p < .001. As expected, there was a laterality effect in neglect patients, with fewer correct responses on the left side than on the right side, T = 1, Z = 3.23, p = .001, while there was no such asymmetry in the control group, T = 46, Z = .41, p = .68. On the task without a manual motor response, neglect patients’ performance was generally similar to their performance on the motor task, T = 20.5, Z = .71, p = .47. Some patients had a greater spatial span on the control task; consequently, their best score was used in correlation analyses. Spatial span for each patient is reported in Table 2.
Table 2.
Performances on spatial and cancellation tasks for neglect patients in each condition
| Patient | Span score | Omission (%)
|
Re-cancellation (%)
|
||
|---|---|---|---|---|---|
| Visible | Invisible | Visible | Invisible | ||
| P1 | 3.25 | 9.38 | 50 | 0 | 31.25 |
| P2 | 5 | 0 | 34.38 | 0 | 14.29 |
| P3 | 2 | 0 | 3.13 | 0 | 6.45 |
| P4 | 3.5 | 0 | 3.13 | 3.13 | 12.9 |
| P5 | 5 | 0 | 6.25 | 0 | 0 |
| P6 | 2.5 | 0 | 9.38 | 0 | 0 |
| P7 | 3 | 0 | 21.88 | 0 | 16 |
| P8 | 4 | 0 | 12.50 | 0 | 7.14 |
| P9 | 3 | 0 | 9.38 | 0 | 6.90 |
| P10 | 1.75 | 3.13 | 0 | 0 | 28.13 |
| P11 | 5 | 0 | 15.63 | 0 | 7.41 |
| P12 | 2.25 | 50 | 53.13 | 0 | 53.33 |
| P13 | 4 | 0 | 9.38 | 0 | 20.69 |
| P14 | 4.25 | 3.13 | 9.38 | 0 | 10.34 |
3.2. Cancellation tasks
The percentage of omissions [(number of omissions/number of targets presented) X 100] and the percentage of re-cancellations [(number of targets with one additional delayed mark/number of targets cancelled) X 100] were determined for each participant in both conditions. Omissions and re-cancellation percentages for each neglect patient in both conditions are reported in Table 2.
In the visible condition, neglect patients omitted more targets than controls, U = 70, Z = 2.08, p = .04. As expected, neglect patients demonstrated a tendency to omit more targets on the left side of the screen (9%) than on the right side (1%), T = 0, Z = 1.83, p = .07. In this condition, there was only one re-cancellation by a single patient (all ps> .05). In the invisible condition, the two groups of participants made significantly more omissions than in the visible cancellation task (all ps< .05). Neglect patients omitted more targets on the left side (24%) than on the right side (10%), although this difference was not statistically significant, T = 38, Z = .91, p = .36. Compared to the control group, neglect patients cancelled fewer targets, U = 19, Z = 3.69, p < .001, and made more re-cancellations, U = 17, Z = 3.9, p < .001, both on the left, U = 48, Z = 2.65, p = .007, and the right side, U = 30, Z = 3.52, p < .001. These results are in accordance with a clear tendency to re-cancel previously found targets in neglect patients. However, the laterality of the stimuli did not modulate the omission and re-cancellation in the neglect group.
3.3. Correlation analysis
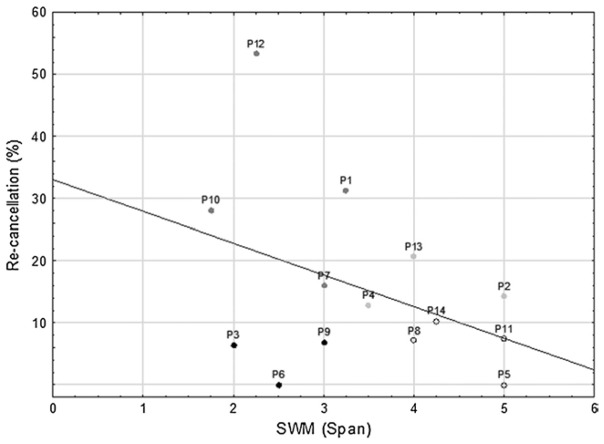
The relationship between SWM and re-cancellations was explored through Kendall rank order correlation analyses, a nonparametric equivalent to Pearson’s correlation. The Kendall coefficient (τ) is equivalent to the Pearson correlation coefficient in terms of effect size (the “r family”; Ellis 2010, p.11). Since in the visible task, there was a floor effect in the rate of re-cancellations in both groups (only one re-cancellation by a single patient) and no correlation analysis was performed for this condition. A one-tailed significance level of .05 was adopted for all analyses. Given that rejection of H1 does not necessarily correspond to acceptance of H0, effect sizes must be taken into account. Following Cohen (1988), for correlation analyses we considered effect sizes lower than the benchmark value of .30 as “small effects”. Only in this case does the acceptance of H0 become plausible when H1 is rejected. In the neglect group, spatial memory span was not correlated with the percentage of re-cancellations in the invisible condition, τ= −.15, p = .46. Inspection of individual patterns of performance (Figure 3) demonstrated that there was an association between impaired SWM and high re-cancellation percentage in four patients (P1, P7, P10, and P12), three patients showed only a high re-cancellation percentage with normal spatial span (P2, P4, and P13), and three other patients showed the reverse pattern, with little or no re-cancellation despite a low spatial span (P3, P6, and P9)2.
Fig. 3.
Scatterplot showing individual patients’ performance on the visual search task (number of re-cancellations in the invisible condition) and the Corsi test (spatial span). Black dots patients with decreased span but little or no re-cancellation; light grey dots patients with normal spatial span and a high re-cancellation rate; dark grey dots patients with decreased span and high re-cancellation rate
For omissions, there was no significant relationship with SWM, either in the visible condition, τ = −.27, p = .17; or in the invisible condition, τ = .18, p = .38. No additional correlation was found (all τs< .16, all ps> .05) when scores from the less strict scoring mode (based only on position) were analysed. We also examined whether spatial neglect of left-sided targets was associated with re-cancellation: in neglect patients, there was a nearly significant positive correlation in the invisible condition, τ = .36, p = .07; while in control subjects, the correlation was negative, τ= −.42, p = .04.
3.4. Anatomical correlates of high re-cancellation rates
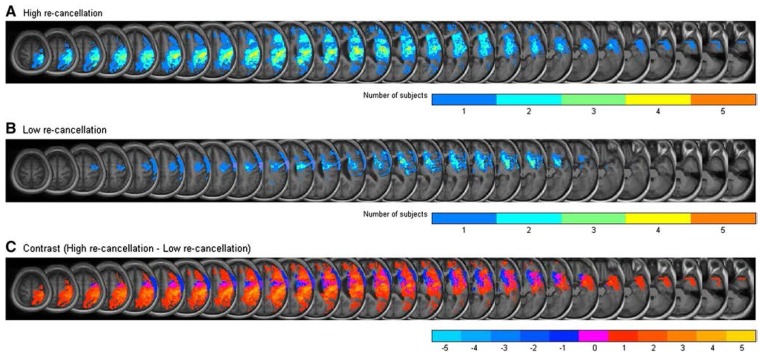
To assess the potential impact of lesion volume and location on re-cancellation, lesion maps were used to compare the neglect patients with the highest (P1, P2, P4, P10, P12, P13) versus the lowest (P3, P5, P6, P8, P9, P11, P14) percentage of re-cancellations. The proportion of lesioned tissue appeared to be larger in patients with high re-cancellation rates compared to patients with the lowest re-cancellation rates, U= 4, Z = 2.36, p = .02. This may indicate that additional structures were affected in re-cancelling patients. Two kinds of maps were computed: lesion overlap maps identified structures that were commonly damaged in each patient subgroup (Figure 4A and 4B), and contrast maps were obtained by subtracting the superimposed lesions of one subgroup from those of the other subgroup, revealing the areas of damage most distinctive of each subgroup (Figure 4C). The former were computed by overlapping the lesion areas (in the normalized MNI space) of all the subjects in each group, while the latter was calculated as the difference (subtraction) between the overlap maps of the two groups. In order to determine the locations of damaged areas, the resulting plot was superimposed on an MNI template (shown in black and white, in contrast with the regions of interest which are shown in different colours according to the number of lesions overlapped). Both kinds of maps were computed on MATLAB from the isolated lesions extracted with PMOD as described in Sect. 2.1. This analysis is similar to that of Karnath et al. (2005).
Fig. 4.
Overlay lesion plots of the subjects with high re-cancellation rate (a) and low re-cancellation rate (b). The number of overlapping lesions is illustrated by different colours coding increasing frequencies from blue (1 patient) to orange (all 5 patients). c Contrast maps subtracting superimposed lesions of patients in each subgroup. The number of patients demonstrating brain damage in a specific location after subtraction of the other subgroup is illustrated by different colour coding (in blue shades for lesions specific to the low re-cancellation subgroup and in yellow shades for lesions specific to the high re-cancellation subgroup, with magenta representing areas with equal lesion frequency in the two subgroups)
Contrast maps revealed that the right insula (MNI coordinates of the centre of the lesion: x = 46, y = −6, z = 15) was damaged in five out of six patients with the highest re-cancellation percentage, but spared in the subgroup of patients with the lowest re-cancellation percentage (Figure 4C). The only patient (P4) with a high re-cancellation percentage whose brain damage spared the insula had a haemorrhagic lesion. In contrast, there was no area that was specifically damaged in the low re-cancellation subgroup, but spared in the high re-cancellation subgroup.
4. Discussion
The present study aimed to examine the role of SWM in the re-exploration behaviour typically observed in spatial neglect. In order to check whether re-cancellation errors are related to SWM deficits, we separately evaluated SWM and re-cancellation using specific tasks, at variance with previous studies in which SWM was estimated through the rate of re-cancellations.
As expected, we observed SWM deficits in our neglect patients, confirming the results of previous studies (e.g. Kristjánsson and Vuilleumier 2010; Malhotra et al. 2005). Furthermore, our span estimate for the neglect patients was not influenced by a motor exploration deficit, which was controlled through an additional visuo-spatial task involving verbal responses instead of motor ones.
In cancellation tasks, we observed that both neglect patients and controls made more omissions in the invisible cancellation task than in the visible one. However, neglect patients cancelled fewer targets than controls, and contrary to controls, they re-cancelled a substantial number of targets. These results confirm that neglect patients can demonstrate re-cancellation, perhaps because they cannot maintain in memory the locations of targets that they have already found in the invisible cancellation task (Husain et al. 2001; Mannan et al. 2005; Wojciulik et al. 2001). Indeed, a number of studies have shown that SWM is involved in visual search tasks (e.g. Beck et al. 2006; Kennard et al. 2008; Oh and Kim 2004; Peterson et al. 2001; Woodman and Luck 2004). For instance, Peterson et al. (2001) suggested that memory and attention interact during visual search because memory can guide attention. However, re-exploration of space in healthy subjects would be the consequence of inadequate processing of the target on the first examination, and not of the forgetting of already examined targets.
In our study, the correlation between the measure of SWM and re-cancellation (τ= −.15, p = .46) corresponded to an effect size which can be considered as “small” (Cohen 1988). Our results suggest that in the present sample of neglect patients, impaired SWM was not the main predictor of re-cancellation in the invisible task. Despite this, perusal of individual data shows that SWM did seem to be related to re-cancellation for some neglect patients (P1, P7, P10, and P12). Otherwise, the observation that some patients showed a normal spatial span and a high re-cancellation rate (P2, P4, and P13), or a decreased span but little or no re-cancellation (P3, P6, and P9) (see Figure 2), rules out simple accounts based on differences in task difficulty. Importantly, it seems unlikely that P3, P6 and P9 had failed the spatial memory task primarily because of neglect, because they had a similar rate of errors on the left side (34%) and on the right side (25%). Thus, these results confirm that visual neglect is a heterogeneous condition, resulting from the interaction of several elementary deficits which can be different among patients (Bartolomeo 2014). This heterogeneity, indicating that visual neglect might be characterized by both spatial and non-spatial deficits, also highlights the need to consider each patient individually in devising a programme for rehabilitation. This is consistent with the idea that combining different therapeutic approaches could lead to additive benefits with regard to visual neglect symptoms, as suggested by Saevarsson et al. (2010, 2011). In addition, re-cancellations and omissions were more numerous in the invisible condition than in the visible one. Thus, magnetic attraction of attention (Gainotti et al. 1991; Mark et al. 1988) also did not seem to contribute to re-cancellation in the present group. Indeed, the visible marks made on the ipsilesional side appeared to actually help the patients search for contralesional targets (Cristinzio et al. 2009; Wojciulik et al. 2001).
The possibility remains that the Corsi test taps into different aspects of spatial memory than those involved in re-cancellation. Moreover, this possibility leaves open the problem of how to assess SWM independently of re-cancellation. Such an independent assessment seems necessary in order to explore the relationship between SWM and re-cancellation. The aim of the present approach was to avoid the potential circularity of assessing SWM through re-cancellation, which was also the pattern of performance in need of an explanation.
Finally, the correlation between re-cancellations and omissions approached significance. This may suggest that re-cancellation is influenced by the severity of neglect and/or by the attentional bias (for similar result, see Na et al. 1999; Nys et al. 2006), which may lead to a highly inefficient visual search strategy in neglect patients. Patients tended to repeatedly process the same stimuli, but this behaviour did not improve their ability to detect the targets, regardless of their degree of SWM deficit.
How can the re-cancellation observed in neglect patients be explained other than in terms of spatial memory impairment? Mannan et al. (2005) suggested that these behaviours in neglect patients could be related to different cognitive impairments depending on the location of the lesion: re-cancellation in patients with parietal damage may be due to working memory impairments, while in frontal patients, these behaviours could be related to executive deficits.
Executive functions may be crucial to efficient search planning (Mark et al. 2004; Olk and Harvey 2006; Weintraub and Mesulam 1988) or to the inhibition of return to previously processed targets (Bourgeois et al. 2012). In the cancellation task, visual search requires the patients to sequentially examine their environment, but also to inhibit responses to previously found targets or distractors (Mark et al. 2004). Difficulties planning a visual search associated with an attentional impairment could exacerbate neglect behaviours (i.e. both omissions and re-cancellations), leading to repetitive searching towards the right side of space and thus to failures to explore the left space. In support of this assumption, Weintraub and Mesulam (1988) observed that neglect patients tend to explore the visual environment erratically, without implementing research strategies. Importantly, the erratic search during visual exploration observed in neglect patients does not appear to be related to neglect behaviour (i.e. left omissions) per se, but rather to delayed re-cancellation (Mark et al. 2004; Ronchi et al. 2012). This defective search strategy could contribute to increase the severity of neglect signs. In this context, an alternative and plausible explanation (that should be explored in future research) to the lack of relationship that we observed could be related to the greater involvement of executive function in the invisible visual search task compared to the spatial working memory task.
In our study, the nature of the task allows us to determine whether each participant used a specific strategy to cancel the targets (far to near, by rows, or columns). For exploratory purposes, we used a method based on Cartesian (x, y) coordinates to evaluate the relationship between the spatial organization of cancellations (Best r, Mark et al. 2004 for details) and pathological behaviours (omissions and re-cancellations) in our neglect patients. Spatial organization was negatively related to omissions (τ = −.50, p = .01); there was a numerical tendency towards a relationship with re-cancellations (τ = −.35, p = .08). Further investigations will be necessary to determine the relative contribution of search planning and SWM in the re-exploration of space in visual neglect.
Examination of lesion location indicated that the right insula was commonly damaged in most patients with frequent re-cancellation, while this region was spared in patients with infrequent re-cancellation. As the small number of patients does not allow any statistical comparison, the lesion presented by patients with high re-cancellation levels was identified by means of contrasts between lesion overlap maps of the two patient subgroups (Karnath et al. 2005). It has been suggested that the right insula belongs to a ventral frontoparietal network that supports the reorientation of attention, detection, and arousal. Impairment of these spatial and non-spatial mechanisms following lesion of the ventral frontoparietal network may contribute to spatial neglect by reducing interaction between the ventral and dorsal networks (the latter being more directly involved in spatial processes; Corbetta and Shulman 2002). Moreover, a recent model proposed the involvement of the insula (mainly the anterior part) in the detection of salient events, particularly to mark such events in time and space for additional processing (Menon and Uddin 2010). Damage to this region could explain the re-cancellation observed in neglect patients. However, the present anatomical results should be taken with caution, given the inherent problems with using the lesion overlapping method to infer the critical locus of lesion in cognitive deficits (Bartolomeo 2011).
In conclusion, although our results provide evidence of impaired SWM in visual neglect, this deficit was not able to predict the degree of neglect or the number of re-cancellations in all neglect patients. Further investigations are needed to better explore the reasons for inefficient visual search (e.g. planning, inhibition of return, and SWM) and the lesional correlates of re-cancellations in left neglect patients.
Footnotes
The exclusion of non-vascular patients did not change the results.
Lesion volume appeared to be greater in the four neglect patients with high re-cancellation rates and spatial working memory deficits than in the other neglect patients. The neuropsychological evaluation did not reveal a particular distinct pattern of impairment in these four patients in comparison to the other patients. Additional anatomical analyses on subgroups of patients also did not uncover any particular lesion sites related to their cognitive profiles.
References
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Azouvi P, et al. Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. J Neurol Neurosurg Psychiatry. 2002;73:160–166. doi: 10.1136/jnnp.73.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomeo P. The quest for the ‘critical lesion site’ in cognitive deficits: problems and perspectives. Cortex. 2011;47:1010–1012. doi: 10.1016/j.cortex.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P. Attention disorders after right brain damage: living in halved worlds. Springer-Verlag; London: 2014. [Google Scholar]
- Bartolomeo P, D’Erme P, Perri R, Gainotti G. Perception and action in hemispatial neglect. Neuropsychologia. 1998;36:227–237. doi: 10.1016/s0028-3932(97)00104-8. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P, Thiebaut de Schotten M, Chica AB. Brain networks of visuospatial attention and their disruption in visual neglect. Front Hum Neurosci. 2012;6:110. doi: 10.3389/fnhum.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomeo P, Thiebaut de Schotten M, Doricchi F. Left unilateral neglect as a disconnection syndrome. Cereb Cortex. 2007;17:2479–2490. doi: 10.1093/cercor/bhl181. [DOI] [PubMed] [Google Scholar]
- Beck MR, Peterson MS, Vomela M. Memory for where, but not what, is used during visual search. J Exp Psychol Hum Percept Perform. 2006;32:235–250. doi: 10.1037/0096-1523.32.2.235. [DOI] [PubMed] [Google Scholar]
- Bourgeois A, Chica AB, Migliaccio R, Thiebaut de Schotten M, Bartolomeo P. Cortical control of inhibition of return: evidence from patients with inferior parietal damage and visual neglect. Neuropsychologia. 2012;50:800–809. doi: 10.1016/j.neuropsychologia.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Cohen JD. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum; London: 1988. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci. 2011;34:569–599. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristinzio C, Bourlon C, Pradat-Diehl P, Trojano L, Grossi D, Chokron S, Bartolomeo P. Representational neglect in “invisible” drawing from memory. Cortex. 2009;45:313–317. doi: 10.1016/j.cortex.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Danckert J, Ferber S. Revisiting unilateral neglect. Neuropsychologia. 2006;44:987–1006. doi: 10.1016/j.neuropsychologia.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Driver J, Husain M. The role of spatial working memory deficits in pathological search by neglect patients. In: Karnath HO, Milner D, Vallar G, editors. The cognitive and neural basis of spatial neglect. Oxford University Press; Oxford: 2002. pp. 351–362. [Google Scholar]
- Ellis PD. The essential guide to effect sizes: statistical power, meta-analysis, and the interpretation of research results. Cambridge University Press; Cambridge: 2010. [Google Scholar]
- Gainotti G, D’Erme P, Bartolomeo P. Early orientation of attention toward the half space ipsilateral to the lesion in patients with unilateral brain damage. J Neurol Neurosurg Psychiatry. 1991;54:1082–1089. doi: 10.1136/jnnp.54.12.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. Oxford University Press; New York: 1984. pp. 243–293. [Google Scholar]
- Husain M, Mannan S, Hodgson T, Wojciulik E, Driver J, Kennard C. Impaired spatial working memory across saccades contributes to abnormal search in parietal neglect. Brain. 2001;124:941–952. doi: 10.1093/brain/124.5.941. [DOI] [PubMed] [Google Scholar]
- Husain M, Rorden C. Non-spatially lateralized mechanisms in hemispatial neglect. Nat Rev Neurosci. 2003;4:26–36. doi: 10.1038/nrn1005. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Baier B, Nagele T. Awareness of the functioning of one’s own limbs mediated by the insular cortex? J Neurosci. 2005;25:7134–7138. doi: 10.1523/JNEUROSCI.1590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennard C, Sumner P, Rees G, Mannan SK, Anderson EJ. A role for spatial and nonspatial working memory processes in visual search. Exp Psychol (formerly “Zeitschrift für Experimentelle Psychologie”) 2008;55:301–312. doi: 10.1027/1618-3169.55.5.301. [DOI] [PubMed] [Google Scholar]
- Kristjánsson Á, Vuilleumier P. Disruption of spatial memory in visual search in the left visual field in patients with hemispatial neglect. Vis Res. 2010;50:1426–1435. doi: 10.1016/j.visres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Malhotra P, Mannan S, Driver J, Husain M. Impaired spatial working memory: one component of the visual neglect syndrome? Cortex. 2004;40:667–676. doi: 10.1016/s0010-9452(08)70163-1. [DOI] [PubMed] [Google Scholar]
- Malhotra P, et al. Spatial working memory capacity in unilateral neglect. Brain. 2005;128:424–435. doi: 10.1093/brain/awh372. [DOI] [PubMed] [Google Scholar]
- Mannan S, Dominic M, Hodgson T, Driver J, Kennard C, Husain M. Revisiting previously searched locations in visual neglect: role of right parietal and frontal lesions in misjudging old Locations as new. J Cogn Neurosci. 2005;17:340–354. doi: 10.1162/0898929053124983. [DOI] [PubMed] [Google Scholar]
- Mark VW, Kooistra CA, Heilman KM. Hemispatial neglect affected by non neglected stimuli. Neurology. 1988;38:1207–1213. doi: 10.1212/wnl.38.8.1207. [DOI] [PubMed] [Google Scholar]
- Mark VW, Woods AJ, Ball KK, Roth DL, Mennemeier M. Disorganized search on cancellation is not a consequence of neglect. Neurology. 2004;63:78–84. doi: 10.1212/01.wnl.0000131947.08670.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na DL, Adair JC, Kang Y, Chung CS, Lee KH, Heilman KM. Motor perseverative behavior on a line cancellation task. Neurology. 1999:52. doi: 10.1212/wnl.52.8.1569. [DOI] [PubMed] [Google Scholar]
- Nys GM, van Zandvoort MJ, van der Worp HB, Kappelle LJ, de Haan EH. Neuropsychological and neuroanatomical correlates of perseverative responses in subacute stroke. Brain. 2006;129:2148–2157. doi: 10.1093/brain/awl199. [DOI] [PubMed] [Google Scholar]
- Oh SH, Kim MS. The role of spatial working memory in visual search efficiency. Psychon Bull Rev. 2004;11:275–281. doi: 10.3758/bf03196570. [DOI] [PubMed] [Google Scholar]
- Olk B, Harvey M. Characterizing exploration behavior in spatial neglect: omissions and repetitive search. Brain Res. 2006;1118:106–115. doi: 10.1016/j.brainres.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Parton P, Malholtra P, Nachev P, Ames D, Ball J, Chataway J, Husain M. Space re-exploration in hemispatial neglect. Cogn Neurosci and Neuropsychol. 2006;17:833–836. doi: 10.1097/01.wnr.0000220130.86349.a7. [DOI] [PubMed] [Google Scholar]
- Peterson MS, Kramer AF, Wang RF, Irwin DE, McCarley JS. Visual search has memory. Psychol Sci. 2001;12:287–292. doi: 10.1111/1467-9280.00353. [DOI] [PubMed] [Google Scholar]
- Pisella L, Berberovic N, Mattingley JB. Impaired working memory for location but not for colour or shape in visual neglect: a comparison of parietal and non-parietal lesions. Cortex. 2004;40:379–390. doi: 10.1016/s0010-9452(08)70132-1. [DOI] [PubMed] [Google Scholar]
- Ronchi R, Posteraro L, Fortis P, Bricolo E, Vallar G. Perseveration in left spatial neglect: drawing and cancellation tasks. Cortex. 2009;45:300–312. doi: 10.1016/j.cortex.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Ronchi R, Algeri L, Chiapella L, Spada MS, Vallar G. Spatial neglect and perseveration in visuomotor exploration. Neuropsychology. 2012;26:588–603. doi: 10.1037/a0029216. [DOI] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61:957–965. doi: 10.1016/j.neuroimage.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saevarsson S, Kristjánsson Á, Halsband U. Strenght in numbers: combining neck vibration and prism adaptation produces additive therapeutic effects in unilateral neglect. Neuropsychol Rehabil. 2010;20:704–724. doi: 10.1080/09602011003737087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saevarsson S, Halsband U, Kristjánsson Á. Designing rehabilitation programs for neglect: could 2 be more than 1+1? Applied Neuropsychology. 2011;18:95–106. doi: 10.1080/09084282.2010.547774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba MN, Ciaraffa F, Pradat-Diehl P, Blanchard M, Loeper-Jeny C, Gainotti G, Bartolomeo P. Components of visual neglect: attentional bias versus spatial working memory impairment. Paper presented at the 4th international conference on spatial cognition; Rome, Italy. Sept.2009. [Google Scholar]
- Weintraub S, Mesulam MM. Visual hemispatial inattention: stimulus parameters and exploratory strategies. J Neurol Neurosurg Psychiatry. 1988;51:1481–1988. doi: 10.1136/jnnp.51.12.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciulik E, Husain M, Clarke K, Driver J. Spatial working memory deficit in unilateral neglect. Neuropsychologia. 2001;39:390–396. doi: 10.1016/s0028-3932(00)00131-7. http://dx.doi.org/10.1016/S0028-3932%2800%2900131-7. [DOI] [PubMed] [Google Scholar]
- Wojciulik E, Rorden C, Clarke K, Husain M, Driver J. Group study of an “undercover” test for visuospatial neglect: invisible cancellation can reveal more neglect than standard cancellation. J Neurol Neurosurg Psychiatry. 2004;75:1356–1358. doi: 10.1136/jnnp.2003.021931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Visual search is slowed when visuospatial working memory is occupied. Psychon Bull Rev. 2004;11:269–274. doi: 10.3758/bf03196569. [DOI] [PubMed] [Google Scholar]