Abstract
There is growing evidence of a relationship between inflammation and psychiatric illness. In particular, the cytokine Interleukin-6 (IL-6) has been linked to stress-related disorders such as depression and anxiety. Here we discuss evidence from preclinical and clinical studies examining the role of IL-6 in mood disorders. We focus on the functional role of peripheral and central release of IL-6 on the development of stress susceptibility and depression-associated behavior. By examining the contribution of both peripheral and central IL-6 to manifestations of stress-related symptomatology, we hope to broaden the way the field thinks about diagnosing and treating mood disorders.
It is estimated that approximately 30–60% of patients with depression are not responsive to available antidepressant treatments (Krishnan and Nestler, 2008). High rates of treatment resistance may be due to heterogeneity in biological mechanisms of depression, such as increased inflammation, that are unaltered by standard antidepressants. Despite numerous correlative studies showing increased inflammation in depression, we still know little about the mechanisms through which inflammation may trigger depression or whether inflammation is simply a consequence of the experience of depression. There is growing evidence that depression alters both the brain and the body of the individual. Many patients with Major Depressive Disorder (MDD) have higher levels of multiple inflammatory markers, including the cytokine Interleukin 6 (IL-6) (Maes et al., 1995 Bob et al., 2010, Dowlati et al., 2010, Hodes et al., 2014). This cytokine is a small multifunctional protein (Tanaka and Kishimoto, 2014), that can be released from a myriad of tissues including white blood cells, endothelial cells, epithelial cells, adipose tissue, astrocytes, microglia and neurons (Coppack, 2001 Spooren et al., 2011, Rossi et al., 2015). IL-6 is primarily categorized as a pro-inflammatory cytokine, but it also has anti-inflammatory properties (Wolf et al., 2014). Recent research in both preclinical (Hodes et al., 2014) and clinical models (Khandaker et al., 2014, Hsu et al., 2015) has suggested a functional role for IL-6 in the development of depression and a potential for targeting it to treat depression in humans. Here we discuss current research examining the contribution of IL-6 to depression and stress-related behavior.
1. IL-6 signaling and its role in inflammation
IL-6 belongs to a family of proteins that use GP130 as a signal transducer. These include Interleukins 11, 27, and 31, ciliary inhibitory factor, leukemia inhibitory factors, cardiotrophin-1, neuropoietin, neurotrophin-1/B-cell stimulating factor 3 and oncostatin M (Scheller et al., 2011, Murakami and Hirano, 2012). IL-6 signaling is complex and can result in both inflammatory and anti-inflammatory cascades depending upon the presence of either IL-6 receptor (IL-6R) or the membrane bound gp130 signal transducer, which are expressed at very different frequencies within specific cell types throughout the body.
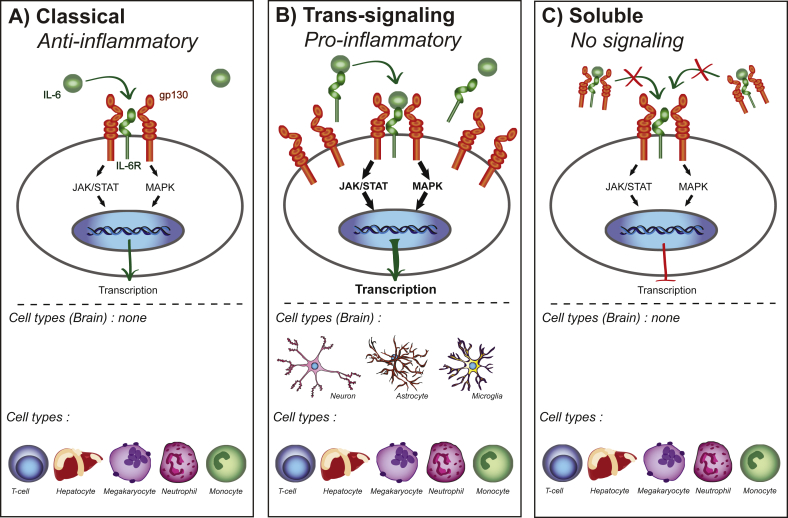
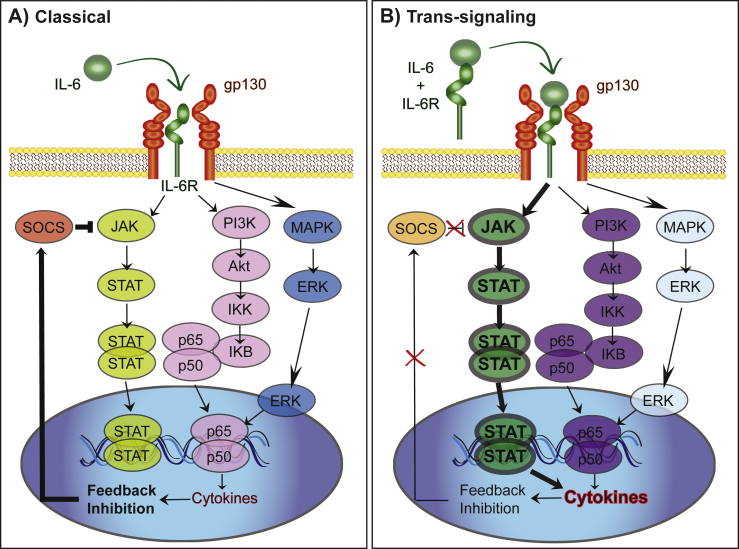
Classical IL-6 signaling (Fig. 1a) is thought to be anti-inflammatory (Wolf et al., 2014) and occurs through binding of IL-6 to the membrane bound cell surface receptor. Classical IL-6 signaling only occurs on some subsets of T cells, hepatocytes, megakaryocytes, neutrophils and monocytes (Scheller et al., 2011). Additionally, IL-6 engages pro-inflammatory trans-signaling (Fig. 1b) in which the soluble form of the IL-6 receptor (sIL-6R) is shed from the membrane bound receptors (Lust et al., 1992, Mullberg et al., 1993). The sIL-6R binds to IL-6 and is transported to any cell type that expresses gp130 on its surface (Wolf et al., 2014). While most soluble receptors, such as the soluble receptor for tumor necrosis factor alpha (TNFα) result in antagonistic action by competing for the ligand, the sIL-6R is agonistic and increases the types of cells through which IL-6 can signal. In both classical and trans-signaling, the IL-6/IL-6R/gp130 complex activates intracellular signaling through the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway and the mitogen-activated protein kinase (MAPK) pathway. There is evidence that an imbalance away from the MAPK pathway via removal of regulation by suppressor of cytokine signaling 3 (SOCS3) towards the pro-inflammatory STAT3 signaling pathway contributes to autoimmune disease (Tanaka and Kishimoto, 2014) and therefore may also be a target for stress susceptibility (Fig. 2). Another method through which circulating levels of IL-6 and its downstream mechanisms are altered is via the soluble form of gp130. While sIL-6R acts as an agonist, the soluble form of gp130 acts as an antagonist sequestering IL-6 and sIL-6R in blood (Wolf et al., 2014, Garcia-Oscos et al., 2015), thereby stopping IL-6 from activating trans-signaling but not classical signaling (Fig. 1c). Further research is needed to determine whether stress alters soluble gp130 and its potential use as an antidepressant.
Fig. 1.
Types of IL-6 signaling. A. Classical signaling only occurs in a few cells types found in the periphery. In classical signaling both the IL-6 receptor and gp130 signal transducer are membrane bound. IL-6 binds to the receptor leading transcription that is thought to be anti-inflammatory. B. IL-6 trans-signaling can occur in any cell type that has membrane bound gp130, all brain IL-6 signaling is thought to be trans-signaling. IL-6 bound to sIL-6R activates signaling through membrane bound gp130. Trans-signaling is thought to be pro-inflammatory in part though its ability to activate more gp130 signal transducers compared to classical IL-6 signaling. C. Blockade of IL-6 signaling through soluble gp130. A soluble form of gp130 can bind sIL-6R/IL-6 complexes and block trans-signaling. Soluble gp130 does not block classical IL-6 signaling.
Fig. 2.
Molecular profiles of classical IL-6 vs. trans-signaling. A. Classical IL-6 signaling leads to activation of the JAK/STAT, AKT and MAPK pathways to alter transcription. Release of cytokines leads to feedback inhibition through SOCS. B. Trans-signaling creates a shift towards greater JAK/STAT signaling than MAPK through suppression of SOCS and alterations in NFkB. This lack of feedback in concert with the increase in activation of JAK/STAT lead to greater transcription of cytokines and a pro-inflammatory state.
A number of transcription factors directly regulate the IL-6 gene including nuclear factor kappa B (NFκB), cAMP response element binding protein (CREB), activator protein 1 (AP-1) and nuclear factor for interleukin 6 (NF-IL6) (Dendorfer et al., 1994, Spooren et al., 2011). The binding of NFκB to the wild type IL-6 promoter in a variety of human cell types is necessary and sufficient to regulate IL-6 (Libermann and Baltimore, 1990 Zhang et al., 1990, Ray and Prefontaine, 1994). Through trans-repression, glucocorticoid receptors (GR) can block the ability of NFκB to act as a transcription factor, potentially comprising a method through which stress modulates IL-6 levels (Ray and Prefontaine, 1994, De Bosscher et al., 2000). Therefore, disruptions in the sensitivity of GR within the body could lead to an increased inflammatory response, independent of cortisol levels.
IL-6 has many functions within the immune system depending upon which type of tissue it is acting upon, thereby contributing to its role as both an inflammatory and anti-inflammatory cytokine. IL-6 was originally discovered as a B cell stimulating factor, but was also identified as a liver cell growth and stimulating factor (Tanaka and Kishimoto, 2014) that can act to differentiate B-cells into immunoglobulin producing plasma cells. IL-6 can also act on T helper cells (Th/CD4+ cells), shifting naïve Th cells into the pro-inflammatory Th17 subtype that suppresses the ability of TGF-β to induce anti-inflammatory T-regulatory (Treg) cells (Murakami and Hirano, 2012). Thus, activation of IL-6 leads to a pro-inflammatory dominance in the proportion of Th17: Treg cells. Furthermore, the presence of IL-17 released from Th17 cells activates a positive feedback loop through NFκB-IL-6 signaling, which is considered an IL-6 amplifier (Murakami and Hirano, 2012).
Within the peripheral and central nervous systems, IL-6 can act as a neuronal growth factor leading to neurite development and nerve regeneration (Spooren et al., 2011). However, this is highly dependent upon circulating IL-6 levels, the type of neuron studied, as well as age and inflammatory state of the animal. For example, low concentrations of IL-6 in vitro reduced the survival of serotonin neurons but had little effect on dopaminergic neurons, whereas higher concentrations of IL-6 were detrimental only to dopaminergic neurons (Jarskog et al., 1997). Furthermore, low concentrations of IL-6 from conditioned media of endotoxin-stimulated astrocytes actually promoted the survival of dopaminergic neurons, whereas high concentrations attenuated survival (Li et al., 2009).
There are numerous ways that chronic alterations in IL-6 levels, both within the periphery and the brain, may contribute to depression symptomatology. Because IL-6 acts on so many different target tissues throughout the body, dysregulation of this particular cytokine can precipitate a multitude of events relevant to depression. While it is unlikely that IL-6 is acting alone to trigger the symptoms of depression, blocking the effects of IL-6 can prevent further escalation of inflammatory responses, as will be discussed in more detail below.
2. Relationship between IL-6 and major depressive disorder
Two recent meta analyses indicated that IL-6 is the most consistently elevated cytokine in the blood of patients with MDD (Dowlati et al., 2010, Haapakoski et al., 2015), corroborating emerging evidence that IL-6 levels might serve as a predictive biomarker. In antidepressant non-responders, peripheral levels of IL-6 positively correlate with symptom severity (Lanquillon et al., 2000). In healthy subjects undergoing psychosocial distress, low peripheral IL-6 levels can predict earlier resolution of negative mood (Virtanen et al., 2015). Individual differences in the IL-6 response to adverse conditions may have a genetic basis. Polymorphism of a single nucleotide on the IL-6 promoter (SNP rs1800795) is thought to contribute to a heightened risk of inflammation in individuals that are exposed to adverse socio-economic environments (Cole et al., 2010). This occurs via β-adrenergic activation of the erythroid transcription factor (GATA 1), a mediator of red blood cell development and maturation. Another polymorphism on the IL-6R gene (rs 8192284) leads to a functional amino acid change altering the proteolytic cleavage site that changes circulating levels of sIL-6r (Tanaka and Kishimoto, 2014). It is possible that these genetic differences may contribute to an individual's stress sensitivity that may inform future bioassay development.
There are implications that sex differences in the immune system may contribute to the greater incidence of depression in women (Kessler et al., 1993). The menstrual cycle alters circulating levels of cytokines in healthy women with increases found in sIL-6R, TNFα and IL-4 during the luteal phase (O'Brien et al., 2007b), which may have implications for pre-menstrual dysphoric disorder. A surge in the female gonadal hormones estrogen and progesterone correlated with elevations of circulating levels of IL-4 and TNFα, respectively. Women also had higher circulating levels of sIL-6R and TNFα than males during both stages of the cycle (O'Brien et al., 2007b). Women are more sensitive to depression and social disconnection induced by exposure to the endotoxin lipopolysaccharide (LPS) (Moieni et al., 2015). In women but not men, the change in TNFα and IL-6 following LPS administration positively correlated with increased feelings of social isolation, but there were no sex differences between males and females in the circulating levels of IL-6 or TNFα in response to LPS (Moieni et al., 2015). Later in life, post-menopausal women seem to have greater basal levels of IL-6 and a larger IL-6 stress response than age matched men (Endrighi et al., 2016), suggesting that the relationship between gonadal hormones and cytokines is complicated and unlikely to be directly correlative.
In the early 90's, Maes, Smith and colleagues examined the relationship between the peripheral immune system and depression (Maes et al., 1992) and put forth a theory suggesting that immune dysregulation contributed to behavioral symptoms of the illness (Smith, 1991, Maes, 1995, Maes, 1999). In support of this hypothesis, there have been numerous studies in patients with various inflammatory conditions including rheumatoid arthritis (Joaquim and Appenzeller, 2015 Matcham et al., 2015, Ryan and McGuire, 2015), multiple sclerosis (Hoang et al., 2016; Kallaur et al., 2015, Marrie et al., 2015), and psoriasis (Walker et al., 2011 Rabin et al., 2012, McDonough et al., 2014), demonstrating a relationship between inflammation and depressed mood. However, the relationship between depression and inflammation in humans is largely confounded by the likelihood that the symptoms of the disease themselves contribute to mood changes.
A recent longitudinal study in humans provided the first evidence that peripheral inflammation predates the occurrence of depression. Children with higher circulating levels of IL-6 at age 9 were at a 10% greater risk of developing MDD by age 18 than the general population or children with low levels of IL-6 (Khandaker et al., 2014). While these clinical data are promising, the best way to directly explore the functional relationship between IL-6 and depression is through stress based animal models of depression.
3. Peripheral IL-6 contributes to stress sensitivity
Within preclinical research, the basis for a functional relationship between depression-like behavior and inflammation originated from studies examining sickness response to systemic administration of LPS (Bluthe et al., 1992, Dantzer et al., 2008), which triggers pro-inflammatory cytokine release. Following a single systemic LPS injection, rodents exhibit decreased self care, social interaction, locomotor activity and feeding over the subsequent 24 h period (Bluthe et al., 1992). Furthermore, LPS injection in male rats produces increased anhedonia as measured by decreased preference for saccharin and decreased sexual behavior (Yirmiya, 1996). However, as the expression of these behaviors is tied to inflammatory activation by LPS and subsides following a return to baseline, this syndrome is considered sickness behavior rather than a valid model of depression (Dantzer et al., 2008).
Stress based preclinical models of depression and anxiety demonstrate that IL-6 is elevated following the onset of depression-associated behaviors. Rodents exposed to chronic mild stress (CMS), a series of stressors presented in an unpredictable manner over time, express anhedonia and increased circulating levels of pro-inflammatory cytokines including IL-6 (Pan et al., 2006, Mutlu et al., 2012). It should be noted some studies have found no significant change (Farooq et al., 2012) or even a decrease in peripheral IL-6 (Mormede et al., 2002) following CMS. However, both of these studies found increased brain levels of other inflammatory markers and may reflect a time-dependent shift from peripheral to central cytokine activation or even potentially transport of the peripheral cytokines into the brain (Mormede et al., 2002, Farooq et al., 2012). Peripheral and hippocampal levels of IL-6 were increased in a rodent model of seasonal affective disorder in which depression-like behavior was induced by 4 weeks of constant darkness (Monje et al., 2011). IL-6 knockout mice were resistant to the development of a depression-like phenotype following exposure to constant darkness, suggesting a functional role for IL-6 in stress susceptibility (Monje et al., 2011).
Not every individual exposed to prolonged or extreme stress develops a psychiatric disorder (Russo et al., 2012). In humans, resilience has been defined as the ability to actively adapt to stressful experience and avoid the negative social, biological and psychological consequences of exposure (Russo et al., 2012, Pfau and Russo, 2015). Within the context of animal models, we define resilience as an active coping mechanism that allows the animal to avoid the deleterious physiological and/or behavioral effects of chronic stress (Hodes et al., 2015, Pfau and Russo, 2015). In contrast to resilience, we define susceptibility as a passive coping response that results in maladaptive behavioral and biological consequences (Hodes et al., 2015). Our group has recently demonstrated that differences in IL-6 levels in the innate peripheral immune system predict vulnerability to repeated social defeat stress (RSDS) (Hodes et al., 2014), a resident intruder-based stress model. In this model, experimental mice are placed into the home cage of a larger, sexually experienced, aggressive mouse each day for 10 days. The larger mouse quickly establishes dominance through physical interaction (Golden et al., 2011). Following RSDS, approximately two thirds of mice exhibit depression-like phenotypes measured by social avoidance, anhedonia, disruptions of the circadian system, and metabolic changes (Krishnan et al., 2007) along with increased activation of pro-inflammatory immune markers such as IL-6 (Hodes et al., 2014).
We found that IL-6 is elevated to a greater degree in the blood of susceptible mice than resilient mice and that this elevation occurs within 20 min of the first social defeat. Even though there were no baseline differences before mice were exposed to stress, we discovered that the animals that later become susceptible had higher numbers of circulating leukocytes and that those cells released more IL-6 when stimulated via LPS ex vivo (Hodes et al., 2014). To examine whether these individual differences in the peripheral immune system are causal to the development of stress susceptibility, bone marrow (BM)-derived hematopoietic stem cells (HSCs) were removed from stress susceptible mice releasing high IL-6 or from IL-6 knockout (IL-6−/−) mice and transplanted into wild type mice whose own peripheral immune cells were lethally irradiated. Lead shielding protected the brains of these animals, preserving microglia. Stress-susceptible BM chimeras exhibited increased social avoidance behavior after exposure to subthreshold RSDS. IL-6−/− BM chimeras, as well as those treated with a systemic IL-6 monoclonal antibody, were resilient to RSDS, suggesting that reduced production of IL-6 by circulating immune cells contributes to depression behaviors. We also replicated these findings in a purely emotional stress paradigm with no physical component, demonstrating that this is not simply a peripheral response to physical trauma (Hodes et al., 2014). Recently, a second study in rats exposed to a version of RSDS also found higher circulating levels of pro-inflammatory cytokines, including IL-6, only in the blood of animals that showed submissive behavior during social interaction with an aggressor (Wood et al., 2015).
Data from a non-social stress based model, learned helplessness (LH), corroborates a functional role for IL-6 in the development of stress susceptibility. Here, subjects are exposed to a controllable or uncontrollable stress, such as shock, and the ability to actively escape a subsequent stressor is measured. Only approximately 20% of animals that undergo the uncontrollable stress are susceptible and develop LH, whereas the remaining responders are resilient and do not show escape deficits. Susceptible animals display anhedonia and increased levels of circulating IL-6 (Yang et al., 2015), while animals exposed to controllable stressors or resilient animals do not show these same perturbations (Yang et al., 2015). Furthermore, IL-6 knockout mice are resilient to acute stress models, including the forced swim test (FST), tail suspension test (TST) and LH (Chourbaji et al., 2006). Together, these studies indicate that peripheral IL-6 has a functional role in the development of depression-like behaviors. One possibility is that IL-6 in the periphery is targeting receptors in the brain although the detailed mechanisms of how this would occur need to be elucidated.
4. Central IL-6 contributes to stress sensitivity
Clinical literature provides evidence of increased IL-6 in the brain of patients with depression. Elevations of IL-6 in the cerebral spinal fluid (CSF) were found in older women with depression (Kern et al., 2014), in patients with either depression or schizophrenia (Sasayama et al., 2013), suicide attempters (Lindqvist et al., 2009) and women experiencing post-partum depression (Boufidou et al., 2009). Interestingly, studies that examined both plasma and CSF levels of IL-6 did not find correlations between the measures, suggesting that peripheral IL-6 levels do not necessarily directly reflect central IL-6 levels (Boufidou et al., 2009 Lindqvist et al., 2009, Sasayama et al., 2013).
In animal models, increased levels of IL-6 have also been found in many areas of the brain. Maternal deprivation leads to increased levels of pro-inflammatory cytokines, including IL-6 in the CSF of rats (Reus et al., 2015). Increased IL-6 mRNA is found in microglia isolated directly from the brains of mice that have undergone a variant of repeated social defeat stress (Ramirez et al., 2015). Treatment with the antidepressant imipramine blocked social avoidance behavior and reduced microglia IL-6 in animals exposed to stress or those given a systemic injection of LPS (Ramirez et al., 2015). Increased IL-6 protein was reported in the hippocampus of rats that underwent chronic unpredictable stress (Tianzhu et al., 2014) and was attenuated by chronic treatment with the antidepressant fluoxetine or treatment with an alternative medicine Cordycepin, a derivative of adenosine extracted from fungi shown to have antidepressant efficacy (Li et al., 2015). Within the prefrontal cortex, increased levels of IL-6 protein were reported in rats exposed to uncontrollable shock that demonstrated LH behavior, but not in animals that were resilient to the same stressor (Sukoff Rizzo et al., 2012). There has also been some indication of sex differences in vulnerability to stress mediated by IL-6 (Tonelli et al., 2008). The authors (Tonelli et al., 2008) found that repeated intra-nasal LPS administration in rats led to greater immobility in the FST in females than males and this coincided with increased IL-6 transcription in the hippocampus of females only.
Intracranial injection of IL-6 was pro-depressant across a behavioral test battery in mice independent of sickness behavior (Sukoff Rizzo et al., 2012). Intracranial injection of IL-6 increased protein levels of IL-6 in a number of brain areas including frontal cortex, hippocampus and hypothalamus. Additionally, transgenic mice that overexpressed IL-6 centrally also demonstrated increased immobility behavior on the FST and TST similar to the animals that received intra-cranial injections of IL-6. Both IL-6 antibody and soluble gp130 were able to block the effects of IL-6 infusion on immobility (Sukoff Rizzo et al., 2012). Work examining enhancement of susceptibility to a virally induced mouse model of multiple sclerosis by social stress (Meagher et al., 2007) has also demonstrated that a centrally administered IL-6 antibody blocked the stress induced increase in severity of symptoms. Additionally, central injection of IL-6 in the absence of social stress was sufficient to increase symptom severity (Meagher et al., 2007).
Recent research suggests that IL-6 may act through trans-signaling to increase the synaptic inhibition/excitation (E/I) ratio on prefrontal cortical neurons (Garcia-Oscos et al., 2015). This effect on E/I ratio was blocked by vagal nerve stimulation activating the “anti-inflammatory reflex.” Intriguingly, systemic administration of IL-6 was found to decrease extracellular dopamine levels in the nucleus accumbens (NAc), an effect that was further potentiated by a mild stress (Song et al., 1999) suggesting that peripheral sources of IL-6 alter activity of brain reward circuitry. In addition, some of the actions of central expression of IL-6 are likely mediated by its effects on astrogliosis, microgliosis and blood brain barrier integrity (Spooren et al., 2011), potentially resulting in greater peripheral infiltration into the brain.
Within the brain, the interactions between NFκB and IL-6 may also contribute to depression-associated behavior through effects on synaptic plasticity (Christoffel et al., 2011a, Russo and Nestler, 2013). Inhibitor of κB kinase (IκK), which contributes to increased NFκB signaling, is elevated in the NAc of susceptible mice that undergo repeated social defeat stress (Christoffel et al., 2011b, Christoffel et al., 2012). Over-expression of a constitutively active form of IκK leads to a number of depression- and anxiety-like behaviors including increased social avoidance following a sub-threshold stress, passive coping in the FST, increased anhedonia measured by decreased sucrose preference (Christoffel et al., 2012) and increased exploratory based anxiety behavior measured in an open field. Social avoidance behavior is linked to an IκK-dependent increase in excitatory synaptic plasticity within the NAc (Christoffel et al., 2011b, Christoffel et al., 2012, Golden et al., 2013), however it remains to be determined whether NFκB is activated upstream through IL-6 to exert its effects on NAc plasticity or depression-like behavior.
5. IL-6 and anti-inflammatory agents as a treatment for depression
The interest in peripheral IL-6 as a mechanism for depression has the potential to change how we treat mental disorders. A number of humanized monoclonal antibody therapies are currently undergoing clinical trials for treatment of mood disorders. Biologics including humanized and chimeric IL-6 receptor antibodies (Tocilizumab) or IL-6 antibodies (Siltuximab/Sirukumab) are currently in clinical trials or are already FDA approved to treat inflammatory illnesses including rheumatoid arthritis, and Castleman's syndrome (Venkiteshwaran, 2009, Williams, 2013). A recent trial of Sirukumab conducted in patients with rheumatoid arthritis showed a significant alleviation of depressive symptoms (Smolen et al., 2014). These types of therapies are now being considered for the treatment of primary unipolar and bipolar depression (Brietzke et al., 2011).
Traditional antidepressants have yielded mixed results regarding their anti-inflammatory properties. Some studies report that antidepressants reduce systemic inflammation (Sluzewska et al., 1995, Hannestad et al., 2011), whereas some report no effects (Maes et al., 1995, Jazayeri et al., 2010). The type of antidepressant and dose may contribute to the variability of the effects of antidepressants on cytokines. High doses of tricyclic antidepressants have actually been shown to increase stimulated IL-6 release ex-vivo in the blood of both patients with depression and healthy controls (Kubera et al., 2004). Additionally, low doses of fluoxetine when presented in combination with 5-hydroxytryptophan also increased IL-6 release under the same circumstances. Fluoxetine by itself did not significantly alter levels of IL-6 released over 48 h at either dose (Kubera et al., 2004). Meta-analysis of in vivo studies demonstrated that SSRIs, but not tricyclic antidepressants, reduced circulating levels of IL-6 and IL-1β (Hannestad et al., 2011). Sex of the patient also likely contributes to some of the variation in treatment response as men, but not women, given serotonin-norepinephrine reuptake inhibitors had increased levels of IL-6 and c-reactive protein (CRP) whereas SSRIs seemed to decrease IL-6 levels in men only (Vogelzangs et al., 2012). It is interesting to note that tricyclic antidepressants did not alter IL-6 in either males or females, but they did increase CRP in both (Vogelzangs et al., 2012).
Treatment resistant status of patients may also contribute to the variation found in studies examining the effects of traditional antidepressants on cytokines. This suggests the potential utility of cytokine and cytokine receptor bio-assays to predict antidepressant response. Increased peripheral levels of IL-6 prior to treatment are an indicator of poor response (Lanquillon et al., 2000, O'Brien et al., 2007a). Other cytokines and their receptors have also been implicated in treatment resistance. Higher circulating levels of TNFα (O'Brien et al., 2007a, Eller et al., 2008), macrophage inhibiting factor and IL-1B were found in leukocytes of patients that did not respond to antidepressants (Audet and Anisman, 2013, Cattaneo et al., 2013). The same study found a reduction in IL-6 mRNA following treatment in antidepressant responders (Cattaneo et al., 2013). High basal CRP prior to treatment has also been associated with positive treatment response to antidepressants (Harley et al., 2010). Additionally, treatment of patients suffering depression following heart attacks found that antidepressant behavioral response was linked heavily to an increase in soluble TNFα receptor 1 as well as a small reduction in circulating levels of sIL-6R (Tulner et al., 2011).
Animal models of depression are starting to be used to determine which cytokines may relate to antidepressant treatment response. Transgenic overexpression of IL-6 in the frontal cortex and hippocampus of mice blunted the antidepressant response to fluoxetine (Sukoff Rizzo et al., 2012) and this effect could be recapitulated using intra-cranial infusion of IL-6. Another model of antidepressant resistance generated by treating rats chronically with adrenocorticotropic hormone (ACTH) prior to antidepressant treatment with ketamine (Walker et al., 2015) suggested a relationship between circulating levels of TNFα and CRP but not IL-6 in responsiveness to treatment. In humans, a growing literature suggests that ketamine, decreases pro inflammatory cytokine levels (De Kock et al., 2013). Together, studies in humans and animal models present strong evidence that inflammation is altered in a subset of depressed subjects and identify IL-6 as a novel target for MDD treatment.
6. Conclusion
Given the pleiotropic nature of IL-6, its perturbation by chronic stress or disease could easily result in numerous peripheral and central consequences. While this makes IL-6 an interesting biomarker and potential therapeutic target for mood related disorders, it also raises the possibility of potential side effects. Therefore, while we should test the ability of antibodies to sequester IL-6 and prevent it from acting in the brain, we must also continue more nuanced research into the mechanisms contributing to the increased circulating levels of IL-6 observed in stressed animals and humans with depression. Currently, a large body of evidence suggests that chronic increases of IL-6 in both the brain and body are implicated in stress susceptibility. However, we lack understanding of the mechanisms by which IL-6 signaling and its molecular components may contribute to depression manifestation. By studying the interface of peripheral cytokines and CNS cellular processes contributing to depression, we may be able to develop a new class of therapeutics to treat mood disorders by sequestering and preventing these peripherally-derived inflammatory cytokines from acting on mood circuits in the brain.
Acknowledgments
The authors thank Madeline Pfau for her assistance with editing the manuscript. We would like to acknowledge the following support: R01MH104559, R01MH090264 and R21MH099562 to SJR. Brain and behavior research foundation NARSAD young investigator award to GEH. This publication was supported by Grant Number P50 AT008661-01, titled “Dietary Botanicals in the Preservation of Cognitive and Psychological Resilience,” from the National Center for Complementary and Integrative Health (NCCIH) and the Office of Dietary Supplements (ODS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCIH, ODS, or the National Institutes of Health.
References
- Audet M.C., Anisman H. Interplay between pro-inflammatory cytokines and growth factors in depressive illnesses. Front. Cell Neurosci. 2013;7:68. doi: 10.3389/fncel.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthe R.M., Dantzer R., Kelley K.W. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992;573:318–320. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- Bob P., Raboch J., Maes M., Susta M., Pavlat J., Jasova D., Vevera J., Uhrova J., Benakova H., Zima T. Depression, traumatic stress and interleukin-6. J. Affect Disord. 2010;120:231–234. doi: 10.1016/j.jad.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Boufidou F., Lambrinoudaki I., Argeitis J., Zervas I.M., Pliatsika P., Leonardou A.A., Petropoulos G., Hasiakos D., Papadias K., Nikolaou C. CSF and plasma cytokines at delivery and postpartum mood disturbances. J. Affect Disord. 2009;115:287–292. doi: 10.1016/j.jad.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Brietzke E., Scheinberg M., Lafer B. Therapeutic potential of interleukin-6 antagonism in bipolar disorder. Med. Hypotheses. 2011;76:21–23. doi: 10.1016/j.mehy.2010.08.021. [DOI] [PubMed] [Google Scholar]
- Cattaneo A., Gennarelli M., Uher R., Breen G., Farmer A., Aitchison K.J., Craig I.W., Anacker C., Zunsztain P.A., McGuffin P., Pariante C.M. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline 'predictors' and longitudinal 'targets'. Neuropsychopharmacology. 2013;38:377–385. doi: 10.1038/npp.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji S., Urani A., Inta I., Sanchis-Segura C., Brandwein C., Zink M., Schwaninger M., Gass P. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol. Dis. 2006;23:587–594. doi: 10.1016/j.nbd.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Christoffel D.J., Golden S.A., Russo S.J. Structural and synaptic plasticity in stress-related disorders. Rev. Neurosci. 2011;22:535–549. doi: 10.1515/RNS.2011.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel D.J., Golden S.A., Heshmati M., Graham A., Birnbaum S., Neve R.L., Hodes G.E., Russo S.J. Effects of inhibitor of kappaB kinase activity in the nucleus accumbens on emotional behavior. Neuropsychopharmacology. 2012;37:2615–2623. doi: 10.1038/npp.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel D.J., Golden S.A., Dumitriu D., Robison A.J., Janssen W.G., Ahn H.F., Krishnan V., Reyes C.M., Han M.H., Ables J.L., Eisch A.J., Dietz D.M., Ferguson D., Neve R.L., Greengard P., Kim Y., Morrison J.H., Russo S.J. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J. Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S.W., Arevalo J.M., Takahashi R., Sloan E.K., Lutgendorf S.K., Sood A.K., Sheridan J.F., Seeman T.E. Computational identification of gene-social environment interaction at the human IL6 locus. Proc. Natl. Acad. Sci. U. S. A. 2010;107:5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppack S.W. Pro-inflammatory cytokines and adipose tissue. Proc. Nutr. Soc. 2001;60:349–356. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bosscher K., Vanden Berghe W., Vermeulen L., Plaisance S., Boone E., Haegeman G. Glucocorticoids repress NF-kappaB-driven genes by disturbing the interaction of p65 with the basal transcription machinery, irrespective of coactivator levels in the cell. Proc. Natl. Acad. Sci. U. S. A. 2000;97:3919–3924. doi: 10.1073/pnas.97.8.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kock M., Loix S., Lavand'homme P. Ketamine and peripheral inflammation. CNS Neurosci. Ther. 2013;19:403–410. doi: 10.1111/cns.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendorfer U., Oettgen P., Libermann T.A. Multiple regulatory elements in the interleukin-6 gene mediate induction by prostaglandins, cyclic AMP, and lipopolysaccharide. Mol. Cell Biol. 1994;14:4443–4454. doi: 10.1128/mcb.14.7.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctot K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Eller T., Vasar V., Shlik J., Maron E. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:445–450. doi: 10.1016/j.pnpbp.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Endrighi R., Hamer M., Steptoe A. Post-menopausal women exhibit greater interleukin-6 responses to mental stress than older men. Ann. Behav. Med. 2016 doi: 10.1007/s12160-016-9783-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq R.K., Isingrini E., Tanti A., Le Guisquet A.M., Arlicot N., Minier F., Leman S., Chalon S., Belzung C., Camus V. Is unpredictable chronic mild stress (UCMS) a reliable model to study depression-induced neuroinflammation? Behav. Brain Res. 2012;231:130–137. doi: 10.1016/j.bbr.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Garcia-Oscos F., Pena D., Housini M., Cheng D., Lopez D., Borland M.S., Salgado-Delgado R., Salgado H., D'Mello S., Kilgard M.P., Rose-John S., Atzori M. Vagal nerve stimulation blocks interleukin 6-dependent synaptic hyperexcitability induced by lipopolysaccharide-induced acute stress in the rodent prefrontal cortex. Brain Behav. Immun. 2015;43:149–158. doi: 10.1016/j.bbi.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S.A., Covington H.E., 3rd, Berton O., Russo S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S.A., Christoffel D.J., Heshmati M., Hodes G.E., Magida J., Davis K., Cahill M.E., Dias C., Ribeiro E., Ables J.L., Kennedy P.J., Robison A.J., Gonzalez-Maeso J., Neve R.L., Turecki G., Ghose S., Tamminga C.A., Russo S.J. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat. Med. 2013;19:337–344. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapakoski R., Mathieu J., Ebmeier K.P., Alenius H., Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J., DellaGioia N., Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley J., Luty S., Carter J., Mulder R., Joyce P. Elevated C-reactive protein in depression: a predictor of good long-term outcome with antidepressants and poor outcome with psychotherapy. J. Psychopharmacol. 2010;24:625–626. doi: 10.1177/0269881109102770. [DOI] [PubMed] [Google Scholar]
- Hoang H., Laursen B., Stenager E.N., Stenager E. Psychiatric co-morbidity in multiple sclerosis: the risk of depression and anxiety before and after MS diagnosis. Mult. Scler. 2016 Mar;22(3):347–353. doi: 10.1177/1352458515588973. [DOI] [PubMed] [Google Scholar]
- Hodes G.E., Kana V., Menard C., Merad M., Russo S.J. Neuroimmune mechanisms of depression. Nat. Neurosci. 2015;18:1386–1393. doi: 10.1038/nn.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes G.E. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. U. S. A. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu B., Wang D., Sun Y., Salvadore G., Singh J., Curran M., Caers I., Drevets W., Wittenberg G., Chen G. SAT0182 improvement in measures of depressed mood and anhedonia, and fatigue, in a randomized, placebo-controlled, phase 2 study of sirukumab, a human anti-interleukin-6 antibody, in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2015;74 720.723-721. [Google Scholar]
- Jarskog L.F., Xiao H., Wilkie M.B., Lauder J.M., Gilmore J.H. Cytokine regulation of embryonic rat dopamine and serotonin neuronal survival in vitro. Int. J. Dev. Neurosci. 1997;15:711–716. doi: 10.1016/s0736-5748(97)00029-4. [DOI] [PubMed] [Google Scholar]
- Jazayeri S., Keshavarz S.A., Tehrani-Doost M., Djalali M., Hosseini M., Amini H., Chamari M., Djazayery A. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatry Res. 2010;178:112–115. doi: 10.1016/j.psychres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Joaquim A.F., Appenzeller S. Neuropsychiatric manifestations in rheumatoid arthritis. Autoimmun. Rev. 2015;14:1116–1122. doi: 10.1016/j.autrev.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Kallaur A.P., Lopes J., Oliveira S.R., Simao A.N., Reiche E.M., de Almeida E.R., Morimoto H.K., de Pereira W.L., Alfieri D.F., Borelli S.D., Kaimen-Maciel D.R., Maes M. Immune-inflammatory and oxidative and nitrosative stress biomarkers of depression symptoms in subjects with multiple sclerosis: increased peripheral inflammation but less acute neuroinflammation. Mol. Neurobiol. 24 September 2015:1–12. doi: 10.1007/s12035-015-9443-4. [DOI] [PubMed] [Google Scholar]
- Kern S., Skoog I., Borjesson-Hanson A., Blennow K., Zetterberg H., Ostling S., Kern J., Gudmundsson P., Marlow T., Rosengren L., Waern M. Higher CSF interleukin-6 and CSF interleukin-8 in current depression in older women. Results from a population-based sample. Brain Behav. Immun. 2014;41:55–58. doi: 10.1016/j.bbi.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., McGonagle K.A., Swartz M., Blazer D.G., Nelson C.B. Sex and depression in the national comorbidity survey. I: lifetime prevalence, chronicity and recurrence. J. Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Khandaker G.M., Pearson R.M., Zammit S., Lewis G., Jones P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71:1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Nestler E.J. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Kubera M., Kenis G., Bosmans E., Kajta M., Basta-Kaim A., Scharpe S., Budziszewska B., Maes M. Stimulatory effect of antidepressants on the production of IL-6. Int. Immunopharmacol. 2004;4:185–192. doi: 10.1016/j.intimp.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Lanquillon S., Krieg J.C., Bening-Abu-Shach U., Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Li B., Hou Y., Zhu M., Bao H., Nie J., Zhang G.Y., Shan L., Yao Y., Du K., Yang H., Li M., Zheng B., Xu X., Xiao C., Du J. 3'-Deoxyadenosine (Cordycepin) produces a rapid and robust antidepressant effect via enhancing prefrontal AMPA receptor signaling pathway. Int. J. Neuropsychopharmacol. 2015 Oct 6 doi: 10.1093/ijnp/pyv112. pii: pyv112. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.Z., Bai L.M., Yang Y.P., Luo W.F., Hu W.D., Chen J.P., Mao C.J., Liu C.F. Effects of IL-6 secreted from astrocytes on the survival of dopaminergic neurons in lipopolysaccharide-induced inflammation. Neurosci. Res. 2009;65:252–258. doi: 10.1016/j.neures.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Libermann T.A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell Biol. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D., Janelidze S., Hagell P., Erhardt S., Samuelsson M., Minthon L., Hansson O., Bjorkqvist M., Traskman-Bendz L., Brundin L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol. Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Lust J.A., Donovan K.A., Kline M.P., Greipp P.R., Kyle R.A., Maihle N.J. Isolation of an mRNA encoding a soluble form of the human interleukin-6 receptor. Cytokine. 1992;4:96–100. doi: 10.1016/1043-4666(92)90043-q. [DOI] [PubMed] [Google Scholar]
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- Maes M. Major depression and activation of the inflammatory response system. Adv. Exp. Med. Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- Maes M., Meltzer H.Y., Bosmans E., Bergmans R., Vandoolaeghe E., Ranjan R., Desnyder R. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J. Affect Disord. 1995;34:301–309. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- Maes M., Van der Planken M., Stevens W.J., Peeters D., DeClerck L.S., Bridts C.H., Schotte C., Cosyns P. Leukocytosis, monocytosis and neutrophilia: hallmarks of severe depression. J. Psychiatr. Res. 1992;26:125–134. doi: 10.1016/0022-3956(92)90004-8. [DOI] [PubMed] [Google Scholar]
- Marrie R.A., Fisk J.D., Tremlett H., Wolfson C., Warren S., Tennakoon A., Leung S., Patten S.B., Epidemiology CTit, impact of comorbidity on multiple S Differences in the burden of psychiatric comorbidity in MS vs the general population. Neurology. 2015 Dec 1;85(22):1972–1979. doi: 10.1212/WNL.0000000000002174. Epub 2015 Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcham F., Norton S., Scott D.L., Steer S., Hotopf M. Rheumatology; Oxford: 2015. Symptoms of Depression and Anxiety Predict Treatment Response and Long-term Physical Health Outcomes in Rheumatoid Arthritis: Secondary Analysis of a Randomized Controlled Trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough E., Ayearst R., Eder L., Chandran V., Rosen C.F., Thavaneswaran A., Gladman D.D. Depression and anxiety in psoriatic disease: prevalence and associated factors. J. Rheumatol. 2014;41:887–896. doi: 10.3899/jrheum.130797. [DOI] [PubMed] [Google Scholar]
- Meagher M.W., Johnson R.R., Young E.E., Vichaya E.G., Lunt S., Hardin E.A., Connor M.A., Welsh C.J. Interleukin-6 as a mechanism for the adverse effects of social stress on acute Theiler's virus infection. Brain Behav. Immun. 2007;21:1083–1095. doi: 10.1016/j.bbi.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M., Irwin M.R., Jevtic I., Olmstead R., Breen E.C., Eisenberger N.I. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology. 2015;40:1709–1716. doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje F.J., Cabatic M., Divisch I., Kim E.J., Herkner K.R., Binder B.R., Pollak D.D. Constant darkness induces IL-6-dependent depression-like behavior through the NF-kappaB signaling pathway. J. Neurosci. 2011;31:9075–9083. doi: 10.1523/JNEUROSCI.1537-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormede C., Castanon N., Medina C., Moze E., Lestage J., Neveu P.J., Dantzer R. Chronic mild stress in mice decreases peripheral cytokine and increases central cytokine expression independently of IL-10 regulation of the cytokine network. Neuroimmunomodulation. 2002;10:359–366. doi: 10.1159/000071477. [DOI] [PubMed] [Google Scholar]
- Mullberg J., Schooltink H., Stoyan T., Gunther M., Graeve L., Buse G., Mackiewicz A., Heinrich P.C., Rose-John S. The soluble interleukin-6 receptor is generated by shedding. Eur. J. Immunol. 1993;23:473–480. doi: 10.1002/eji.1830230226. [DOI] [PubMed] [Google Scholar]
- Murakami M., Hirano T. The pathological and physiological roles of IL-6 amplifier activation. Int. J. Biol. Sci. 2012;8:1267–1280. doi: 10.7150/ijbs.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu O., Gumuslu E., Ulak G., Celikyurt I.K., Kokturk S., Kir H.M., Akar F., Erden F. Effects of fluoxetine, tianeptine and olanzapine on unpredictable chronic mild stress-induced depression-like behavior in mice. Life Sci. 2012;91:1252–1262. doi: 10.1016/j.lfs.2012.09.023. [DOI] [PubMed] [Google Scholar]
- O'Brien S.M., Scully P., Fitzgerald P., Scott L.V., Dinan T.G. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J. Psychiatr. Res. 2007;41:326–331. doi: 10.1016/j.jpsychires.2006.05.013. [DOI] [PubMed] [Google Scholar]
- O'Brien S.M., Fitzgerald P., Scully P., Landers A., Scott L.V., Dinan T.G. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14:84–90. doi: 10.1159/000107423. [DOI] [PubMed] [Google Scholar]
- Pan Y., Zhang W.Y., Xia X., Kong L.D. Effects of icariin on hypothalamic-pituitary-adrenal axis action and cytokine levels in stressed Sprague-Dawley rats. Biol. Pharm. Bull. 2006;29:2399–2403. doi: 10.1248/bpb.29.2399. [DOI] [PubMed] [Google Scholar]
- Pfau M.L., Russo S.J. Peripheral and central mechanisms of stress resilience. Neurobiol. Stress. 2015;1:66–79. doi: 10.1016/j.ynstr.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin F., Bhuiyan S.I., Islam T., Haque M.A., Islam M.A. Psychiatric and psychological comorbidities in patients with psoriasis- a review. Mymensingh Med. J. 2012;21:780–786. [PubMed] [Google Scholar]
- Ramirez K., Shea D.T., McKim D.B., Reader B.F., Sheridan J.F. Imipramine attenuates neuroinflammatory signaling and reverses stress-induced social avoidance. Brain Behav. Immun. 2015;46:212–220. doi: 10.1016/j.bbi.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Prefontaine K.E. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc. Natl. Acad. Sci. U. S. A. 1994;91:752–756. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reus G.Z., Nacif M.P., Abelaira H.M., Tomaz D.B., dos Santos M.A., Carlessi A.S., da Luz J.R., Goncalves R.C., Vuolo F., Dal-Pizzol F., Carvalho A.F., Quevedo J. Ketamine ameliorates depressive-like behaviors and immune alterations in adult rats following maternal deprivation. Neurosci. Lett. 2015;584:83–87. doi: 10.1016/j.neulet.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Rossi J.F., Lu Z.Y., Jourdan M., Klein B. Interleukin-6 as a therapeutic target. Clin. Cancer Res. 2015;21:1248–1257. doi: 10.1158/1078-0432.CCR-14-2291. [DOI] [PubMed] [Google Scholar]
- Russo S.J., Nestler E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo S.J., Murrough J.W., Han M.H., Charney D.S., Nestler E.J. Neurobiology of resilience. Nat. Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S., McGuire B. Psychological predictors of pain severity, pain interference, depression, and anxiety in rheumatoid arthritis patients with chronic pain. Br. J. Health Psychol. 2015 Nov 3 doi: 10.1111/bjhp.12171. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Sasayama D., Hattori K., Wakabayashi C., Teraishi T., Hori H., Ota M., Yoshida S., Arima K., Higuchi T., Amano N., Kunugi H. Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J. Psychiatr. Res. 2013;47:401–406. doi: 10.1016/j.jpsychires.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. biophys. acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Sluzewska A., Rybakowski J.K., Laciak M., Mackiewicz A., Sobieska M., Wiktorowicz K. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Ann. N. Y. Acad. Sci. 1995;762:474–476. doi: 10.1111/j.1749-6632.1995.tb32372.x. [DOI] [PubMed] [Google Scholar]
- Smith R.S. The macrophage theory of depression. Med. Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- Smolen J.S., Weinblatt M.E., Sheng S., Zhuang Y., Hsu B. Sirukumab, a human anti-interleukin-6 monoclonal antibody: a randomised, 2-part (proof-of-concept and dose-finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Ann. Rheum. Dis. 2014;73:1616–1625. doi: 10.1136/annrheumdis-2013-205137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Merali Z., Anisman H. Variations of nucleus accumbens dopamine and serotonin following systemic interleukin-1, interleukin-2 or interleukin-6 treatment. Neuroscience. 1999;88:823–836. doi: 10.1016/s0306-4522(98)00271-1. [DOI] [PubMed] [Google Scholar]
- Spooren A., Kolmus K., Laureys G., Clinckers R., De Keyser J., Haegeman G., Gerlo S. Interleukin-6, a mental cytokine. Brain Res. Rev. 2011;67:157–183. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Sukoff Rizzo S.J., Neal S.J., Hughes Z.A., Beyna M., Rosenzweig-Lipson S., Moss S.J., Brandon N.J. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl. Psychiatry. 2012;2:e199. doi: 10.1038/tp.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol. Res. 2014;2:288–294. doi: 10.1158/2326-6066.CIR-14-0022. [DOI] [PubMed] [Google Scholar]
- Tianzhu Z., Shihai Y., Juan D. Antidepressant-like effects of cordycepin in a mice model of chronic unpredictable mild stress. Evid. Based Complement. Altern. Med. 2014;2014:438506. doi: 10.1155/2014/438506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli L.H., Holmes A., Postolache T.T. Intranasal immune challenge induces sex-dependent depressive-like behavior and cytokine expression in the brain. Neuropsychopharmacology. 2008;33:1038–1048. doi: 10.1038/sj.npp.1301488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulner D.M., Smith O.R., Schins A., de Jonge P., Quere M., Delanghe J.R., Crijns H.J., den Boer J.A., Korf J., Honig A. Antidepressive effect of mirtazapine in post-myocardial infarction depression is associated with soluble TNF-R1 increase: data from the MIND-IT. Neuropsychobiology. 2011;63:169–176. doi: 10.1159/000321624. [DOI] [PubMed] [Google Scholar]
- Venkiteshwaran A. Tocilizumab. MAbs. 2009;1:432–438. doi: 10.4161/mabs.1.5.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen M., Shipley M.J., Batty G.D., Hamer M., Allan C.L., Lowe G.D., Ebmeier K.P., Akbaraly T.N., Alenius H., Haapakoski R., Singh-Manoux A., Kivimaki M. Interleukin-6 as a predictor of symptom resolution in psychological distress: a cohort study. Psychol. Med. 2015:1–8. doi: 10.1017/S0033291715000070. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N., Duivis H.E., Beekman A.T., Kluft C., Neuteboom J., Hoogendijk W., Smit J.H., de Jonge P., Penninx B.W. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl. Psychiatry. 2012;2:e79. doi: 10.1038/tp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A.J., Foley B.M., Sutor S.L., McGillivray J.A., Frye M.A., Tye S.J. Peripheral proinflammatory markers associated with ketamine response in a preclinical model of antidepressant-resistance. Behav. Brain Res. 2015;293:198–202. doi: 10.1016/j.bbr.2015.07.026. [DOI] [PubMed] [Google Scholar]
- Walker J.R., Graff L.A., Dutz J.P., Bernstein C.N. Psychiatric disorders in patients with immune-mediated inflammatory diseases: prevalence, association with disease activity, and overall patient well-being. J. Rheumatol. Suppl. 2011;88:31–35. doi: 10.3899/jrheum.110900. [DOI] [PubMed] [Google Scholar]
- Williams S.C. First IL-6-blocking drug nears approval for rare blood disorder. Nat. Med. 2013;19:1193. doi: 10.1038/nm1013-1193. [DOI] [PubMed] [Google Scholar]
- Wolf J., Rose-John S., Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70:11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Wood S.K., Wood C.S., Lombard C.M., Lee C.S., Zhang X.Y., Finnell J.E., Valentino R.J. Inflammatory factors mediate vulnerability to a social stress-induced depressive-like phenotype in passive coping rats. Biol. Psychiatry. 2015;78:38–48. doi: 10.1016/j.biopsych.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Shirayama Y., Zhang J.C., Ren Q., Hashimoto K. Peripheral interleukin-6 promotes resilience versus susceptibility to inescapable electric stress. Acta Neuropsychiatr. 2015:1–5. doi: 10.1017/neu.2015.36. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y.H., Lin J.X., Vilcek J. Interleukin-6 induction by tumor necrosis factor and interleukin-1 in human fibroblasts involves activation of a nuclear factor binding to a kappa B-like sequence. Mol. Cell Biol. 1990;10:3818–3823. doi: 10.1128/mcb.10.7.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]