Abstract
Dogs are the hosts for a wide helminth spectrum including tapeworms, flatworms, and nematodes. These parasites affect the dog health and cause morbidity and mortality, especially in young and old animals. Some species, as Toxocara canis, Ancylostoma caninum, Dipylidium caninum, and Echinococcus spp. are well-known zoonotic parasites worldwide, resulting in high public health risks. Poor data about canine helminth species and prevalence are available in Russia, mainly due to the absence of official guidelines for the control of dog parasites. Moreover, the consequent low quality of veterinary monitoring and use of preventive measures, the high rate of environmental contamination by dog feces and the increase of stray dog populations, make the control of the environmental contamination by dog helminths very difficult in this country. This paper reviews the knowledge on canine helminth fauna and prevalence in Russia. Practical aspects related to diagnosis, treatment, and control of parasitic diseases of dogs in Russia are discussed.
Keywords: dog, helminth infections, Russia, zoonosis
Introduction
Dogs are the most popular pets worldwide and are infested with many parasites, which may represent a health risk for humans, especially children, the elderly and the immune-compromised [1-3]. For instance, Dipylidium caninum, Echinococcus granulosus, Ancylostoma spp., and Toxocara canis are common parasites of dogs that can affect humans in different countries around the world. Knowledge about parasite species in domestic dogs, prevalence and intensity of helminth infestations in dog populations, transmission of canine parasites and the seasonal dynamics of parasite infestations are essential for control and prevention of helminthosis in domestic animals and humans.
Investigation of free-roaming dog populations as part of urban ecology is a major key for the solution of many ecological problems in industrial ecosystems [4,5].
In Russia, 40-50% of household owners keep a dog, and the total dog population amounts to 30 million [6]. Therefore, the growing number of owned dogs in urban areas in Russia has also been accompanied by substantial increases in the stray dog population. For example, the stray dog population amounts to 12,300 specimens in Kazan [7], 9500 in Novosibirsk (http://laboratorium.narod.ru/gelm.html) and 10,000 in Omsk [8]. Stray dogs do not receive medical attention and never, or rarely, receive anti-parasitic treatments. Thus, they represent a major source of spread of gastrointestinal helminth eggs, which are harmful for people [9,10]. This article is a compendium on helminth infestations in dogs in the Russian Federation, with particular focus on zoonoses.
The Russian Federation
The Russian Federation is the largest country in the world, covering 7,125,200 km2 (6,612,100 sq. miles). Russia consists of seven basic climate zones. The humid continental climate predominates in all parts of the country: European Russia, in the south of West Siberia and in the south of the Russian Far East, including the cities of Moscow and Saint Petersburg, except for the tundra and the extreme southeast.
The Russian Federation is divided into eight large Federal Districts: Northwestern, South, Central, Volga, North Caucasian Federal district, Siberian, Urals, and Far East Federal District. There is lack of data about canine helminth communities and prevalence. The North Caucasian District [11-17] and Central Federal District [18-25] are the most commonly investigated areas. Sporadic reports are published from the Siberian [26-28], Volga [29-31], Ural [32], and Northwest districts [33]. A single report was published from the Far East Federal District [34] in the last 10 years (Tables-1-3 and Figure-1). The greatest number of helminth species was registered in the North-Caucasian Federal District, followed by the Volga Federal District (17 species and 11 species, respectively).
Table 1.
Fauna of gastrointestinal helminths of domestic dogs in Russia.
| Phylum | Class | Order | Family | Species | Method | Region |
|---|---|---|---|---|---|---|
| Platyhelminthes | Trematoda | Plagiorchiida | Opistorchiidae | M. bilis | AU | North Caucasian Federal District |
| M. xanthosomus | AU | North Caucasian Federal District | ||||
| O. felineus | AU | North Caucasian Federal District, Siberian Federal District, Siberian Federal District, Ural Federal District | ||||
| C. sinensis | AU | Far Eastern Federal District | ||||
| Dicrocoeliidae | D. lanceatum | AU | North Caucasian Federal District | |||
| Strigeidida | Diplostomatidae | A. alata | AU | Central Federal District, Siberian Federal District, Volga Federal District, North Caucasian Federal District | ||
| Echinostomida | Echinostomatidae | E. perfoliatus | AU | Volga Federal District | ||
| Cestode | Cyclophyllidea | Dipylidiidae | D. caninum | AU; CE | North Caucasian Federal District, Central Federal District, Siberian Federal District, Ural Federal District, Volga Federal District, Siberian Federal District, Far East Federal District | |
| E. granulosus | AU; CE | North Caucasian Federal District, Ural Federal District, Siberian Federal District, Volga Federal District | ||||
| Mesocestoididae | M. lineatus | AU | North Caucasian Federal District, Central Federal District | |||
| Taeniidae | T. multiceps (syn. M.multiceps) | AU; CE | Siberian Federal District, North Caucasian Federal District, Siberian Federal District | |||
| T. hydatigena | AU; CE | North Caucasian Federal District, Central Federal District, Siberian Federal District, Kazakhstan, Volga Federal District | ||||
| T. ovis | AU | North Caucasian Federal District | ||||
| T. pisiformis | AU | North Caucasian Federal District, Central Federal District | ||||
| Pseudophyllidea | Diphyllobothriidae | D. latum | AU, CE | Central Federal District, Urals Federal District, Volga Federal District, Ural Federal District | ||
| Nematoda | Secernentea | Ascaridida | Ascarididae | T. canis | AU, CE | Central Federal District, North Caucasian Federal District, North-West Federal District, Volga Federal District, Siberian Federal District, Far East Federal District, Urals Federal District |
| T. leonina | AU, CE | North Caucasian Federal District, Central Federal District, Siberian Federal District, Volga Federal District, Ural Federal District, Far East Federal District | ||||
| Rhabditida | Strongyloididae | S. stercoralis | AU | Central Federal District | ||
| Strongylida | Ancylostomatidae | A. caninum | AU, CE | Siberian Federal District, Volga Federal District, North-Caucasian Federal District, Central Federal District, Far East Federal District | ||
| U. stenocephala | AU, CE | Ural Federal District, Volga Federal District, Far East Federal District, North-Caucasian Federal District, Central Federal District, Siberian Federal District | ||||
| Trichurida | Trichuridae | T. vulpis (syn T. vulpis) | AU, CE | North Caucasian Federal District, Central Federal District |
AU=Autopsy method, CE=Coproscopically examination method, M. bilis=Methorchis bilis, M. xanthosomus=Methorchis xanthosomus, O. felineus=Opisthorchis felineus, C. sinensis=Clonorchis sinensis, D. lanceatum=Dicrocoelium lanceatum, A. alata=Alaria alata, E. perfoliatus=Echinochasmus perfoliatus, D. caninum=Dipylidium caninum, E. granulosus=Echinococcus granulosus, M. lineatus=Mesocestoides lineatus, T. multiceps=Taenia multiceps, T. hydatigena=Taenia hydatigena, T. ovis=Taenia ovis, T. pisiformis=Taenia pisiformis, D. latum=Diphyllobothrium latum, T. canis=Toxocara canis, T. leonina=Toxascaris leonina, S. stercoralis=Strongyloides stercoralis, A. caninum=Ancylostoma caninum, U. stenocephala=Uncinaria stenocephala, T. vulpis=Trichuris vulpis, T. vulpis=Trychocephalus vulpis, M. multiceps=Multiceps multiceps
Table 2.
Prevalence and intensity (min and max intensity rates or mean intensity) data of dogs’ gastrointestinal helminths based on autopsy examination.
| Region | Dagestan [16] | Kursk [21] | Altai [27] | North Caucasus [17] | Voronezh [20] | Caucasian mineral waters [14] | Ivanovo [18,19] | Kabardino- Balkarian Republic [12] | Moscow [24] |
|---|---|---|---|---|---|---|---|---|---|
| Total number of investigated dogs | n=320 | n=67 | n=72 dogs+826 fecal samples | n=35 | n=12 | n=385 | n=173 | n=17 | n=86 |
| D. lanceatum | 9.3% 12.4±1.3 | - | - | - | - | - | - | - | - |
| A. alata | 8.7% 2.6±0.2 | - | 2.18% | 16.5% | 18.2% | 11.1% 9-12 | 20.2% | 29.4% | 6.6% 12.6 for 1.5-3 years old dogs |
| T. hydatigena | 66.5% 5.7±0.5 | - | 2.66% | - | - | 20.2% 3-5 | 5.2% | 29.4% | - |
| E. granulosus | 66.8% 203.8±1.4 | - | 1.09% | 80-100% | - | 34.6% 11-246 | - | 76.5% | - |
| T. ovis | 16.5% 2.0±0.1 | - | 1.45% | - | - | - | - | 35.3% | - |
| M. lineatus | 13.7% 2.4±0.2 | - | - | - | - | - | 1.7% | 23.5% | - |
| D. caninum | 26.2% 4.1±0.3 | 10.4% | 38.01% | 26% 12.8 | 72.7% | 34.2% 5-33 | 68.2% | 61.5% | 100% 5.8-19.8 (in dogs aged 1-6 months; 7-12 months and dogs 1.5-3 years old) |
| T. canis | 81.8% 39.4±0.4 | 38.8% | 43.95% | 30.5% 12.8 | - | 72.2% 6-49 | 53.7% | 70.6% | 6.6-100% 1.5-38.8 (in dogs aged 1-6 months; 7-12 months and dogs 1.5-3 years old) |
| T. leonina | 57.5% 12.6±0.8 | 7.46% | 39.95% | - | - | 35.8% 3-19 | 22.5% | 41.2% | 100% 8.8-189 (in dogs aged 7-12 months and dogs 1.5-3 years old) |
| A. caninum | 27.8% 23.6±1.0 | - | 2.06% | - | - | 62.3% 7-52 | 12.7% | 53% | 50-100% 7.8-8.9 (in dogs aged 7-12 months and dogs 1.5-3 years old) |
| U. stenocephala | 23.4% 19.2±1.2 | - | 16.34% | 46.3% 4.6-5.3% | 100% 18.5 | 30.9 8-91 | 57.7% | 41.2% | 100% 12.8-36.8 (in dogs aged 1-6 months; 7-12 months and dogs 1.5-3 years old) |
| O. felineus | 6.5% 3.0±0.2 | - | 5.6% | - | - | - | - | - | - |
| M. xanthosomus | 6.5% 4.1±0.3 | - | - | - | - | - | - | 23.5% | - |
| T. vulpis | - | 8.95% | - | - | - | - | - | - | - |
| S. stercoralis | - | 4.47% | - | - | - | - | 6.3% | - | 100% 18.6-22.8 (in dogs aged 1-6 month and dogs 1.5-3 years old) |
| D. latum | - | - | - | - | - | - | 1.15% | - | - |
| M. bilis | - | - | - | - | - | 10.1% 4-17 | - | - | - |
| T. pisiformis | 32.8% 3.1±0.2 | 2.98% | 1.09% | - | - | 12.5% 2-8 | 2.8% | 15.4% | - |
| Taenia multiceps | 22.5 2.1±0.2 | - | 0.85% | - | - | 10.5% 3-8 | - | 35.3% | - |
M. bilis=Methorchis bilis, M. xanthosomus=Methorchis xanthosomus, O. felineus=Opisthorchis felineus, D. lanceatum=Dicrocoelium lanceatum, A. alata=Alaria alata, D. caninum=Dipylidium caninum, E. granulosus=Echinococcus granulosus, M. lineatus=Mesocestoides lineatus, T. multiceps=Taenia multiceps, T. hydatigena=Taenia hydatigena, T. ovis=Taenia ovis, T. pisiformis=Taenia pisiformis, D. latum=Diphyllobothrium latum, T. canis=Toxocara canis, T. leonina=Toxascaris leonina, S. stercoralis=Strongyloides stercoralis, A. caninum=Ancylostoma caninum, U. stenocephala=Uncinaria stenocephala, T. vulpis=Trichuris vulpis, T. vulpis=Trychocephalus vulpis
Table 3.
Prevalence data (%) of gastrointestinal helminths based on coproscopically examinations.
| City | Total number of investigated dogs | Method | T. hydatigena | D. caninum | E. granulosus | Opisthorchiidae | A. caninum | T. canis | T. leonina | U. stenocephala | T. vulpis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Machachkala [15] | 42 | Fulleborn’s method | 33.3 | 26.1 | 16.6 | - | - | 61.9 | 38 | 26.1 | - |
| Kursk [21] | 32 | Fulleborn’s method | - | 12.5 | - | - | - | 18.7 | - | - | - |
| Voronezh [25] | 587 | Darling’s method | Taenia spp. 2.13 | 19.15 | - | 0.71 | - | 33.3 | 19.15 | 19.86 | 7.09 |
| Barnaul [28] | 1019 | Fulleborn’s method; Kotelnikov- Chrenov’s method; Goryachev’s method | - | 16.3 | - | 5.3 (n=150) | 0.49 | 39.8 | 24.9 | 10.1 | - |
| Kazan [31] | - | Fulleborn’s method; Kotelnikov- Chrenov’s method; Kotelnikov- Varenichev’s method | Taenia spp. 4.8 | 11.1 | - | 3.2 | 3.2 | 46 | 28.5 | - | - |
| Vladikavkaz [11] | 179 | Fulleborn’s method | - | 6.45 | - | - | 9.68 | 12.9 | 1.08 | - | - |
| Moscow [23] | 367 | Floatation method | - | - | - | - | - | 33.4 | 10.2 | 27.3 | - |
| Novosibirsk [26] | 3564 | Fulleborn’s method; Kotelnikov- Chrenov’s method | Taenia spp. 0.59-1.87 | 4.68-9.42* | - | 0.35-3.91* | - | 9.38-30.38* | 3.77-6.94* | 1.21-1.31* | 0.14-2.93* |
| Saratov [29] | 1563 | Fulleborn’s method | - | 8.9 | - | - | 1.2 | 63.6 | 7.4 | 2.9 | - |
| Krasnodar [22] | 689 | Fulleborn’s method | T. pisiformis 0.58 | 4.35 | - | - | 1.31 | 12.77 | 7.69 | 1.01 | 1.31 |
| Vladivostok [34] | 97 | Fulleborn’s method; Sedimentation method | Taenia sp. 2.1 | 2.1 | - | - | 10.3 | 1.03 | - | 4.1 | - |
Min and max rates. T. hydatigena=Taenia hydatigena, D. caninum=Dipylidium caninum, E. granulosus=Echinococcus granulosus, A. caninum=Ancylostoma caninum, T. canis=Toxocara canis, T. leonina=Toxascaris leonina, U. stenocephala=Uncinaria stenocephala, T. vulpis=Trichuris vulpis
Figure-1.
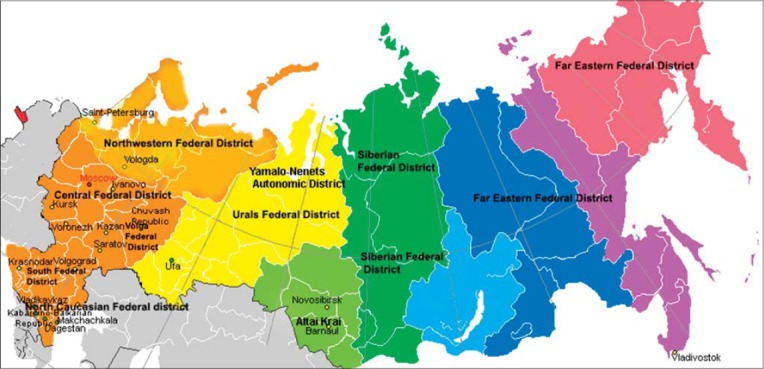
Map of researched area.
Helminthes of dogs in Russia
In Russia, dogs may be infested with a large number of helminths including cestodes, trematodes, and nematodes. The canine gastrointestinal helminth fauna includes 21 species (Table-1). There are eight species of tapeworms, from the following families: Taeniidae (4), Dipylidiidae (2), Mesocestoididae (1), and Diphyllobothriidae (1). Roundworms are made up of six species from the families Ascarididae (2), Ancylostomatidae (2), Strongiloididae (1), and Trichuridae (1). Flatworms include seven species from the families Opisthorchiidae (4), Dicrocoeliidae (1), and Diplostomatidae (1). Some species, such as Toxascaris leonina, T. canis, D. caninum, E. granulosus and A. caninum, are frequently found in dogs from different Russian regions (Tables-1-3). Other helminths such as Mesocestoides lineatus (Goeze, 1782), Metorchis bilis (Braun, 1890), Metorchis xanthosomus (Creplin, 1846), Dicrocoelium lanceatum (Stiles et Hassal, 1896), Diphyllobothrium latum, and Strongyloides stercoralis have only been sporadically reported in Russia. Clonorchis sinensis Looss, 1907 is an endemic species from the Asian Region; it was found in dogs from the Russian Far East [35]. Among Taenia species found in dogs from Russia, the most common parasites are Taenia hydatigena, followed by Taenia multiceps. Two species, Taenia ovis and Taenia pisiformis, have been found in the North Caucasian District only.
Diagnosis of Canine Helminth Parasites
The diagnosis of helminth parasites affecting dogs is made by coprological examination methods, Strongyloides larva detection techniques and post-mortem examination. Helminth eggs are usually detected in feces by ordinary coprologycal techniques such as Fulleborn’s method and Darling’s method; these methods are present low sensitivity for some helminth species and result in the underestimation of the real prevalence of some parasites [36-38]. Some flotation and sedimentation techniques are used only in Russia. For instance, Kotelnikov-Varenichev’s and Kotelnikov-Chrenov’s methods are centrifugal flotation techniques, which have high sensitivity for many helminth species [39-42]. Goryachev’s sedimentation technique proposed for detecting Opisthorchis eggs is also use in some studies (Table-4) [43]. However, other coprological examination methods using worldwide are not used in Russia. For example, TF-test® designed for detecting human gastrointestinal parasites [44] is frequency used for detecting helminth eggs in canine feces [45]. Some comparative study showed that the centrifugal flotation technique was more sensitive than centrifugal sedimentation and TF-test® for recovery Ancylostoma spp., T. canis, Trichuris vulpis eggs in canine feces [45]. Another method is Willis technique has high sensitivity for T. canis eggs in canine feces [46]. Moreover, recent study showed that this method performed better than the centrifugal flotation techniques and Hoffman-Pons-Janer technique for detecting Ancylostoma spp. in dog feces [47].
Table 4.
Comparison of coproscopically examination method using in Russia.
| Method | Solution (Specific gravidity) | Technique | Sensitivity |
|---|---|---|---|
| Fulleborn [36] | NaCl (1.2) | Flotation | Good sensitivity for Toxocara, Toxascaris and Trichuris eggs which frequently appear in canine faeces |
| Darling [37,38] | NaCl + C3H8O3 (1.21) | Flotation-sedimentation | Low sensitivity for flatworms and Diphyllobothrium eggs identification |
| Goryachev [43] | NaCl (1.2) | Sedimentation | Use only for Opisthorchis eggs detection |
| Kotelnikov-Varenichev [39-41] | ZnCl2 (1.82) | Centrifugation flotation | High sensitivity for Toxocara, Toxascaris and Trichuris eggs, flatworms and cestode eggs |
| Kotelnikov-Chrenov [41,42] | NH4NO3 (1.28) | High sensitivity for flatworm eggs, Taenia eggs and nematode eggs |
Traditional Baermann’s and modified Baermann-Orlov’s methods are used for S. stercoralis larva detection from canine feces [48,49]. Zink sulfate flotation technique which is sensitive for S. stercoralis larva is not used in Russia [50].
Necropsy examination is performed according to the standard procedures [49,51]; methods of total and part helminthological examination suggested by Skrjabin [52] used in parasitological study.
Prevalence of Helminth Infections in Dogs in Russia
Data obtained from reports in different regions showed broad prevalence rate fluctuations. The prevalence depends on climate, living conditions, and quality of veterinary care [53,54]. Many reports did not include data about the total prevalence of gastrointestinal parasites in dogs. However, individual prevalence rates for different parasites were greater than 50% in 37% of studies. High prevalence rates and a broad parasite spectrum were found in studies using the necropsy method (Table-2).
Overall, nine parasites species were found in studies using coproscopic examination methods. Some species, such as M. xanthosomus, M. bilis, S. stercolaris, M. lineatus and D. latum, were not found on fecal examination. In some cases, it was also difficult to distinguish species of eggs Opisthorchiidae family [55] and eggs genus Taenia based only on morphological characters [56].
The most common species in Russia was T. canis, followed by D. caninum, T. leonina, and Uncinaria stenocephala (Tables-2 and 3). A. caninum was found in 52.3% of studies conducted in areas with a continental or temperate climate, where there are warm summers and high humidity, as these conditions are optimal for A. caninum larval development [57].
Flatworms of the genus Metorchis (Looss, 1899) are worldwide parasites of Cyprinidae fishes, and infest fish-eating mammals. In Russia, M. xanthosomus was found in Dagestan and Kabardino-Balkaria Republic, while M. bilis was found in Caucasian Minerals Water (Table-2). It is interesting that these species were found separately in different regions since both species have common intermediate hosts. The first host is the mollusk Bithynia tentaculata L. 1758 living in the Palearctic zone, except the North zone [58]. The second hosts are Cyprinidae fishes [59].
The cestode M. lineatus has a worldwide distribution. It has been found in Europe [60], the Middle East [61], Africa [62], North, and South America [63]. Adult worms live in small intestine of carnivorous mammals including fox, wolves, dogs, cats, coyotes, raccoons, and lynxes [64]. One case of the peritoneal larval stage was recorded in dogs from Germany [65]. This species spreads proglottids via the feces so it cannot be found with flotation methods. In Russia, dogs infested with M. lineatus were found in Dagestan with a low prevalence rate [16].
Dogs and People: Problem of Parasite Zoonoses
Most parasites species found in dogs from Russia have zoonotic potential. T. canis is the most common canine intestinal endoparasites worldwide. Humans are infected by Toxocara via ingestion of embryonated eggs in contaminated soil [66]; however, pet hair can also contain embryonated eggs [67]. The first importance reports about human toxocariasis in Russia were published in 1961-1962 [68,69]. Only in 1988 did the connection between the source of toxocariasis in dogs and nosoareal of toxocariasis in humans appear [70]. Recently, the problem of toxocariasis in humans and dogs has been highlighted worldwide. In the Russian Federation, toxocariasis has frequency appeared in children, especially in children with allergic diseases (31-47% of children with allergic diseases) [71]. Ocular toxocariasis is frequently recorded in children, whereas visceral toxocariasis is more frequently recorded in adults. Since 1991, Toxocara infestation rates in people have increased. For example, Toxocara infestation was recorded in 0.03 per 100,000 people in 1991 and in 2.32 per 100,000 people in 2012.
However, toxocariasis was recorded in 2.1 per 100,000 people from 2008 to 2012 [72]. Infestation rates are broadly variable in different regions and in people of different age groups. Toxocara prevalence in people from Russia is 5.4% in Moscow and Tula, 7.4% in Dagestan and 6% in the Irkutsk District [73]. The Toxocara prevalence was 16.7% in children from the Altay Region, whereas toxocariasis was recorded 6 times more frequently in adults from the Krasnodar region. E. granulosus is a widespread parasite, which has a major medical, veterinarian and socioeconomic cost. The adult parasite stage occurs in the canine small intestine, and people are infested by ingestion of contaminated food [74] or by direct contact with contaminated dogs that retain eggs on their coats [75]. Echinococcosis in humans is the most serious parasitic disease, as a fatal outcome is recorded in 2-23% of cases [76]. Higher prevalence rates appear in China, western and southern Russia, Southwestern Europe, South Africa and Central and South America [76]. The Orenburgskii Region is the most problematic territory in Russia, as echinococcosis was registered in 3.4±0.4 per 100,000 people [77]. Recently, DNA-based studies showed that E. granulosus comprise 10 genotipes which have been distinguished in different species [78]. E. granulosus s. s. (G1-G3), E. canadensis (G6, G8 and G10) were found in Russia [79].
People are infrequently infested with D. caninum, which occurs through ingestion feline fleas infected with tapeworms [80,81]. D. caninum infestation was also registered in Russia. Dipylidiasis was recorded in humans from the Orenburgskii Region [82], Moscow [83], and the Kabardino-Balkarian Republik [84].
Liver flukes of the genera Clonorchis Loos, 1907, Metorchis, and Opisthorchis Blanchard 1895, in the family Opisthorchiidae, exploit freshwater snails and fish as the first and second intermediate hosts, respectively. The final hosts, fish-eating birds and mammals, including dogs and humans, are infected by eating fish harboring infective metacercariae [85]. Feces of dogs infested with Opisthorchis felineus and Metorchis spp. are major sources of water contamination [86]. The largest infestation center is located in the Ob-Irtysh basin and includes 10 regions of Russia and Kazachstan. The infestation rate is 51-82% in humans. The other intensive infestation center is Chulym River in the Krasnoyarsk region; the prevalence of O. felineus in people is 70-80% [87].
M. bilis is also found in fish from the Ob-Irtysh basin. The prevalence rate in people from West Siberia is 28.1% [88].
The flatworm C. sinensis is endemic to the Far Eastern region and it was also found in dogs and people from China and Korea [86,89]. C. sinensis is frequently found in people from the Russian Far East [90].
The tapeworm M. lineatus has major veterinary importance, and occasionally, it has been found in people [91]. In Russia, cases of M. lineatus infestation in humans have not been reported, however dog feces containing proglottids are major sources of environmental contamination.
D. latum is a common parasite of fish-eating mammals. Fecal contamination of water is a source of D. latum spread. In the last 10 years, only one report regarding canine infestation from Ivanovo was published. A big center of infestation located in Russia is Baikal Lake. Infestation rates in humans from the Irkutsk Region were 9.6 cases per 100,000 people [92].
Another flatworm, Alaria alata, has specific veterinary importance [93], however, several reports about human larval alariosis were published since 1973 [94,95]. In Russia, A. alata was found in dogs, foxes, wolves and badgers in the Vladimir, Ivanovo and Moscow regions, and in the Volgograd and Astrachan regions, in the North Caucasian District. Prevalence rates were 38.4-48.6% in farm dogs, 46.1-59.3% in stray dogs and 100% in wolves and foxes [96].
Control and Prevention
Zoonosis is the major veterinary and medical problem. Zoonotic infestations include well-known parasite species such as T. canis and E. granulosus, which have a worldwide distribution.
However, there are no official guidelines for the control of endoparasite infestations in dogs, such as that provided by the Companion Animal Parasite Council (CAPC: http://www.capcvet.org/) in the United States and the European Scientific Counsel Companion Animal Parasites (ESCCAP: http://www.esccap.org/) in Europe.
There is scant information about problems of veterinary epidemiology in Russia. Two guidelines for the sanitary and veterinary rules were published by the Veterinary Department of the Ministry of Agriculture of the Russian Federation (http://docs.cntd.ru/document/1200050554) with State Sanitary and Epidemiological Supervision of the Russian Federation (http://rospotrebnadzor.ru/documents/details.php?ELEMENT_ID=2890). Veterinarians do not have a native source of information for parasite epidemiology, life cycles or control measures [97].
Parasitology monitoring is provided irregularly. Poor living conditions and lack of anti-parasitic medication causes environmental contamination with helminth eggs [98]. Environmental contamination of helminth eggs is a big problem in many urban and rural areas in Russia, especially in agricultural areas, where feces are used for fertilizing. Currently, basic methods for dog helminth infections prevention include regular deworming of domestic animals, control of environmental contamination (avoid contamination of canine feces in public places), and spread of information about zoonotic parasites [99]. Moreover, control of food quality and pet diets help to prevent parasite infestations. For example, to prevent C. sinensis and O. felineus infestation in dogs, it is recommend to avoid the feeding of fresh cyprinid fishes [99].
Many dog owners cannot afford preventive measures and will act only when a life-threatening problem is affecting their animals. Furthermore, there are a large number of free-roaming dogs populations in the Russian cities. Government is not able to manage these animals due to the lack of adequate infrastructure and trained personnel to conduct an effective long-term population control program. As a result, pet dogs and cats are usually endangered by a wide range of parasites that may cause disease in them and eventually in their human counterpart.
Conclusions
The close contact between pets and humans may involuntarily represent a hazard for humans. Therefore, to avoid the potential risks associated with owning a pet, it is fundamental to maintain pets in good health and protect them from zoonotic pathogens.
Therefore, veterinary practitioners and medical physicians should work together toward improving the well-being and general health of both animals and humans.
Authors’ Contributions
MTV and AVE participated in the draft and revision of the manuscript. Both authors read and approved the final manuscript.
Acknowledgments
The authors are grateful to Far Eastern Federal University (FEFU), Russia for providing access for international and Russian scientist database.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Abere T, Bogale B, Melaku A. Gastrointestinal helminth parasites of pet and stray dogs as a potential risk for human health in Bahir Dar town, North-Western Ethiopia. Vet. World. 2013;6:388–392. [Google Scholar]
- 2.Neves D, Lobo L, Simoes P.B, Cardoso L. Frequency of intestinal parasites in pet dogs from an urban area (Greater Oporto, northern Portugal) Vet. Parasitol. 2014;200:295–298. doi: 10.1016/j.vetpar.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Overgaauw P.A, van Zutphen L, Hoek D, Yaya F.O, Roelfsema J, Pinelli E, van Knapen F, Kortbeek L.M. Zoonotic parasites in fecal samples and fur from dogs and cats in the Netherlands. Vet. Parasitol. 2009;163:115–122. doi: 10.1016/j.vetpar.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 4.Massei G, Fooks A.R, Horton D.L, Callaby R, Sharma K, Dhakal I.P, Dahal U. Free roaming dogs in Central Nepal: Demographics, health and public knowledge, attitudes and practices. Zoonoses Public Health. 2016 doi: 10.1111/zph.12280. DOI:10.1111/zph.12280. [DOI] [PubMed] [Google Scholar]
- 5.Garde E, Acosta-Jamett G, Bronsvoort B.M. Review of the risks of some canine zoonoses from free-roaming dogs in the post-disaster setting of Latin America. Animals. 2013;3:855–865. [Google Scholar]
- 6.Shmerlina I. Sobaki i ih vladel’cy. (Dogs and their owners) Soc.’naja Real’nost’. 2007;10:35. [Google Scholar]
- 7.Shamsuvaleeva J.S, Rahimov I.I. Osobennosti jekologii bezdomnyh sobak vuslovijah goroda Kazani i ego okrestnostej (Ecological characters of free-roaming dogs populations in Kazan) Kazan: ZAO “Novoe znanie”; 2013. p. 168. [Google Scholar]
- 8.Berezina E.S. Ecological characters of dogs populations in towns. Ecological groups, size of population, population structure, communication. Vet. Patol. 2002;1:132–135. [Google Scholar]
- 9.Beiromvand M, Akhlaghi L, Massom S.H.F, Meamar A.R, Motevalian A, Oormazdi H, Razmjou E. Prevalence of zoonotic intestinal parasites in domestic and stray dogs in a rural area of Iran. Prev. Vet. Med. 2013;109:162–167. doi: 10.1016/j.prevetmed.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Deplazes P, van Knapen F, Schweiger A, Overgaauw P.A. Role of pet dogs and cats in the transmission of helminthic zoonoses in Europe, with a focus on echinococcosis and toxocarosis. Vet. Parasitol. 2011;182:41–53. doi: 10.1016/j.vetpar.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Bagaeva U.V, Bocharova M.M. Epizootic situation on helminthosis of dogs in Northern Ossetia. Russ. J. Parasitol. 2008;4:26–30. [Google Scholar]
- 12.Bittirov A.M, Mantaeva S.S, Shihalieva M.A, Sarbasheva M.M, Bidzhiev A.Z, Golubev A.A, Akieva O.M. Jepizootologicheskaja ocenka gel’mintov sobak i dikih psovyh v Kabardino-Balkarii (Epizootological evaluation of dogs and wild canis helminthes in Kabarino-Balkarian Republic) Agrarnaja Nauk. 2012;9:31–32. [Google Scholar]
- 13.Gadzhiev I.G, Ataev A.M, Gazimagomedov M.G. Fauna of helminthes of domestic and wild Canidae in the plane zone of Dagestan. Russ. J. Parasitol. 2010;4:12–15. [Google Scholar]
- 14.Kolesnikov V.I, Popov O.V. Fauna of helminths of dogs in region the caucasus mineral waters. Nauchno Proizvodstvennyj Zhurnal Ovcy, Kozy, Sherstjanoe Delo. 2012;4:49–52. [Google Scholar]
- 15.Musaev M.C, Kurochkina K.G, Machieva B.M. Helminthes of dogs in Makhachkala city. Russ. J. Parasitol. 2012;3:22–24. [Google Scholar]
- 16.Trunova A.S. Specific structure of helminths of dogs in the plain zone of Dagestan. Russ. J. Parasitol. 2008;3:1–5. [Google Scholar]
- 17.Zhuravlev A.S. Osnovnye gel “mintozy sobak v regione Severnogo Kavkaza. (Major dogs’ helminthosis in North Caucasian Region) Vestn. Krasnojarskogo Gosudarstvennogo Agrarnogo Univ. 2008;5:257–259. [Google Scholar]
- 18.Kryuchkova E.N, Abalihin B.G, Egorov S.V, Sokolov E.A, Balandina V.N, Egorov D.S. Parasitiases of carnivorous pets of Ivanovo Region’s urban populations. Vestn. Kostromskogo Gosudarstvennogo Univ. Im N. A. Nekrasova. 2014;6:41–44. [Google Scholar]
- 19.Balandina V.N, Kruchkova E.N, Abalihin B.G, Sokolov E.A, Belyaev D.K. Heminth fauna in dogs of urban populations in the Ivanovo region. Teor. Prak. Parazitarnyh Boleznej Zhivotnyh. 2014;15:43–44. [Google Scholar]
- 20.Nikulin P.I, Romashov B.V. Helminthes of domestic carnivores in Voronezh region. Russ. J. Parasitol. 2011;1:32–39. [Google Scholar]
- 21.Puzenko S.V, Malysheva N.S. Distribution of helminthosis of carnivorous in Kursk area. Russ. J. Parasitol. 2012;3:77–80. [Google Scholar]
- 22.Vlasenko J.I. Associacii gel’ mintov plotojadnyh i ih lechenie v ravninnoj zone Krasnodarskogo kraja (Association of helminths of carnivorous and its treatment in flat zone in Krasnodarskii Region) Trud. KubGAU. 2007;5:147–150. [Google Scholar]
- 23.Zubov A.V. Age dynamics of infection of dogs by intestinal parasites in Central region of Russia. Russ. J. Parasitol. 2008;2:1–4. [Google Scholar]
- 24.Trusova A.V, Korenskova E.V, Zubov A.B. Parasitofauna from dogs in Moscow and Moscow region. Russ. J. Parasitol. 2008;4:16–18. [Google Scholar]
- 25.Menyaylova I.S, Gaponov S.P. Intestinal invasion of carnivores in Voronezh. Russ. J. Parasitol. 2012;2:30–33. [Google Scholar]
- 26.Bortsova M.S, Bortsov S.A, Zubareva I.M. Associacii parazitov zheludochno-kishechnogo trakta sobak v uslovijah megapolisa (Associations of parasites in gastrointestinal tract of dogs under conditions of megapolis) Sibirskij Vestn. sel’skohozjajstvennoj Nauk. 2007;9:131–133. [Google Scholar]
- 27.Luneva N.A. Helminthoses of dogs at the Altaisk Territory. Teor. Prak. Parazitarnyh Boleznej Zhivotnyh. 2014;15:138–139. [Google Scholar]
- 28.Ponamarev N.M, Luneva N.A. Helminth fauna in dogs in the city of Barnaul. Vestn. Altajskogo Gosudarstvennogo Agrarnogo Univ. 2013;3:62–63. [Google Scholar]
- 29.Sidorkin V.A, Kashkovskaja L.M, Gorbunov A.B. Kishechnye gel’mintozy sobak g. Saratova (Gasthrointestinalhelmints in dogs in Saratov) Veterinarija. 2008;4:30–32. [Google Scholar]
- 30.Kosyayev N.I, Farkhutdinova A.F. Helminthisism epizootic situation in dogs of Chuvash republic. Uchenye Zap. Kazanskoj gosudarstvennoj Akad. Vet. Med. im N. Je. Baumana. 2012;209:175–179. [Google Scholar]
- 31.Timerbaeva R.R, Kornishina M.D, Shageeva A.R. Epizootological situation in helminthiasis in domestic carnivores conditions of Kazan. Uchenye Zap. Kazanskoj Gosudarstvennoj Akad. Vet. Med. Im N. Je. Baumana. 2012;211:143–145. [Google Scholar]
- 32.Arhipov I.A, Zejnalov O.A, Kokorina L.M, Avdanina D.A, Lihotina S.V. Rasprostranenie gel’ mintozov sobak i koshek v Rossii i primenenie prazitela dlja bor’ by s nimi (Spreading of helminths in dogs and cats in Russia and usage a Prazitelfor its control) Ross. Vet. Zhurnal. Melkie Domashnie i Dikie Zhivotnye. 2005;2:26–30. [Google Scholar]
- 33.Anisimova E, Poloz S, Subbotin A. Gel’minty hishhnyh mlekopitajushhih (semejstvo Canidae, Fischer 1817) V estestvennyh uslovijah i na zverofermah (Helminths of carnivorous (Canidae)) in nature and farms. Belarus: Nauka, Litres; 2014. p. 9. [Google Scholar]
- 34.Moskvina T.V, Zheleznova L.V. A survey on endoparasites and ectoparasites in domestic dogs and cats in Vladivostok, Russia 2014. Vet. Parasitol. Regional Stud. Rep. 2016;1:31–34. doi: 10.1016/j.vprsr.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Skrjabin K.I, Pod’yapolskaya V.P, Shulc R.C. Rabota 60-j sojuznoj gel’ mintologicheskoj jekspediciii v Dal’nevostochnom krae. (Research of 60th allied expedition in Far East Region) Russ. Zhurnal Trop. Med. 1929;1:36. [Google Scholar]
- 36.Połozowski A, Zawadzki W, Nowak M. Comparison of two fecal flotation techniques for diagnostic of internal parasites infections in swine and dogs. E. J. P. A. U. 2006;9:39. [Google Scholar]
- 37.Semashko N.A Gel’mintologicheskie metody issledovanija (Helminths detection methods. Bol’shaja Medicinskaja Jenciklopedija (Big Medical Encyclopedia) Vol. 6. Moscow: Directmedia; 2013. pp. 443–446. [Google Scholar]
- 38.Becker A.C, Kraemer A, Epe C, Strube C. Sensitivity and efficiency of selected coproscopical methods-sedimentation, combined zinc sulfate sedimentation-flotation, and McMaster method. Parasitol. Res. 2016;113:2401–2406. doi: 10.1007/s00436-016-5003-8. [DOI] [PubMed] [Google Scholar]
- 39.Lutfullin M.H, Latypov D.G, Nikiforov P.G. Gel’mintoovoskopicheskaja diagnostika trematodozov i puti uluchshenija ee jeffektivnosti, (Gelmintoovoskopicheskaya trematodozov diagnosis and ways of improving its efficiency). Uchenye Zapiski Kazanskoj Gosudarstvennoj Akad. Vet. Med. N. Je. Baumana. 2010;201:75–80. [Google Scholar]
- 40.Dolbin D.A, Lutfullin M.H, Hayrullin R.M. Universal coproscopy diagnosis of intestinal parasites. Uchenye Zapiski Kazanskoj Gosudarstvennoj Akad. Vet. Med. N. Je. Baumana. 2011;207:190–195. [Google Scholar]
- 41.Timerbaeva R.R, Idrisov A.A, Lutfullin M.H. Comparative efficacy of helminthovoscopic methods of diagnosis of swine helminthoses. Teor. Prak. Parazitarnyh Boleznej Zhivotnyh. 2014;15:314–317. [Google Scholar]
- 42.Kotelnikov G.A, Chrenov V.M. Metodicheskie rekomendacii po diagnostike naibolee rasprostranennyh gel’mintozov sel’skohozjajstvennyh zhivotnyh (Guidelines for Diagnostic Frequency Helminthiasis in Farm Animals) Moscow. 1980 [Google Scholar]
- 43.Goryachev P.P. K metodike koprologicheskogo analiza na opistorhoz (Coprological examination method for opisthorchosis detection) Trud. Omsk. Med. In-ta. 1949;11:191–193. [Google Scholar]
- 44.Gomes J.F, Hoshino-Shimizu S, Dias L.C, Araujo A.J, Castilho V.L, Neves F.A. Evaluation of a novel kit (TF-Test) for the diagnosis of intestinal parasitic infections. J. Clin. Lab. Anal. 2004;18:132–138. doi: 10.1002/jcla.20011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katagiri S, Oliveira-Sequeira T.C. Comparison of three concentration methods for the recovery of canine intestinal parasites from stool samples. Exp. Parasitol. 2010;126:214–216. doi: 10.1016/j.exppara.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 46.Táparo C.V, Perri S.H.V, Serrano A.C.M, Ishizaki M.N, Costa T.P, Amarante A.F.T, Bresciani K.D.S. Comparison between coproparasitological techniques for the diagnosis of helminth eggs or protozoa oocysts in dogs. Rev. Bras. Parasitol. Vet. 2006;15:1–5. [PubMed] [Google Scholar]
- 47.Mandarino-Pereira A, de Souza F.S, Lopes C.W, Pereira M.J. Prevalence of parasites in soil and dog feces according to diagnostic tests. Vet. Parasitol. 2010;170:176–181. doi: 10.1016/j.vetpar.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Lima J.P, Delgado P.G. Diagnosis of strongyloidiasis: Importance of Baermann’s method. Am. J. Dig. Dis. 1961;6:899–904. doi: 10.1007/BF02231086. [DOI] [PubMed] [Google Scholar]
- 49.Tret’yakov A.M, Evdokimov P.I, Shabaev V.A. Laboratornaja Diagnostika Parazitarnyh Zabolevanij Zhivotnyh. (Laboratory Diagnostic of Parasites Diseases in animals) Ulan-Ude: FGOU VPO BGSHA. 2006 [Google Scholar]
- 50.Truant A.L, Elliott S.H, Kelly M.T, Smith J.H. Comparison of formalin-ethyl ether sedimentation, formalin-ethyl acetate sedimentation, and zinc sulfate flotation techniques for detection of intestinal parasites. J. Clin. Microbiol. 1981;13:882–884. doi: 10.1128/jcm.13.5.882-884.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martínez-Carrasco C, Berriatua E, Garijo M, Martínez J, Alonso F.D, de Ybáñez R.R. Epidemiological study of non-systemic parasitism in dogs in Southeast mediterranean Spain assessed by coprological and post-mortem examination. Zoonoses Public Health. 2007;54:195–203. doi: 10.1111/j.1863-2378.2007.01047.x. [DOI] [PubMed] [Google Scholar]
- 52.Skrjabin K.I. Metod polnyh gel’mintologicheskih vskrytij pozvonochnyh, vkljuchaja cheloveka. (Full helminthologycal autopsy method for vertebrates) Moscow: MGU; 1928. [Google Scholar]
- 53.Katagiri S, Oliveira-Sequeira T.C.G. Prevalence of dog intestinal parasites and risk perception of zoonotic infection by dog owners in Sao Paulo State, Brazil. Zoonoses Public Health. 2008;55:406–413. doi: 10.1111/j.1863-2378.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 54.Inpankaew T, Traub R, Thompson R.C, Sukthana Y. Canine parasitic zoonoses in Bangkok temples. Southeast Asian J. Trop. Med. Public Health. 2007;38:247–255. [PubMed] [Google Scholar]
- 55.Kang S, Sultana T, Loktev V.B, Wongratanacheewin S, Sohn W.M, Eom K.S, Park J.K. Molecular identification and phylogenetic analysis of nuclear rDNA sequences among three opisthorchid liver fluke species (Opisthorchiidae: Trematoda) Parasitol. Int. 2008;57:191–197. doi: 10.1016/j.parint.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 56.Allan J.C, Craig P.S, Noval J.G, Mencos F, Liu D, Wang Y, Wen H, Zhou P, Stringer R, Rogan M, Zeyhle E. Coproantigen detection for immunodiagnosis of echinococcosis and taeniasis in dogs and humans. Parasitology. 1992;104:347–355. doi: 10.1017/s0031182000061801. [DOI] [PubMed] [Google Scholar]
- 57.Croese J. Seasonal influence on human enteric infection by Ancylostoma caninum. Am. J. Trop. Med. Hyg. 1995;53:158–161. doi: 10.4269/ajtmh.1995.53.158. [DOI] [PubMed] [Google Scholar]
- 58.Fedorov V.G. K izmenchivosti rakovin Bithynia tentaculata (Mollusca, Gastropoda) (Variability of shell Bithynia tentaculata (Mollusca, Gastropoda)) Al’manah Sovrem. Nauk. Obrazovanija. 2012;5:146–150. [Google Scholar]
- 59.Ilyinskikh Y.N, Novitsky V.V, Ilyinskikh N.N, Lepyokhin A.V. Opisthorchis felineus (Rivolta 1884) and Metorchis bilis (Braun 1890) infections in population of some regions of the Ob river basin. Parasitologya. 2007;41:55–64. [PubMed] [Google Scholar]
- 60.Dalimi A, Sattari A, Motamedi G. A study on intestinalhelminths of dogs, foxes and jackals in the western part of Iran. Vet. Parasitol. 2006;142:129–133. doi: 10.1016/j.vetpar.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 61.Loos-Frank B. Cestodes of the genus Mesocestoides (Mesocestoididae) from carnivores in Israel. Isr. J. Zool. 1990;37:3–13. [Google Scholar]
- 62.Reid W.A, Reardon M.J. Mesocestoides in the baboon and its development in laboratory animals. J. Med. Primatol. 1976;5:345–352. doi: 10.1159/000459989. [DOI] [PubMed] [Google Scholar]
- 63.Nolan T.J, Smith G. Time series analysis of the prevalence of endoparasitic infections in cats and dogs presented to a veterinary teaching hospital. Vet. Parasitol. 1985;59:87–96. doi: 10.1016/0304-4017(94)00742-u. [DOI] [PubMed] [Google Scholar]
- 64.Razmashkin D.A. Species affiliation of metacercaria of the genus Metorchis (Trematoda, Opisthorchidae) from fishes in Western Siberia. Parazitologiia. 1978;12:68–78. [PubMed] [Google Scholar]
- 65.Wirtherle N, Wiemann A, Ottenjann M, Linzmann H, van der Grinten E, Kohn B, Gruber A.D, Clausen P.H. First case of canine peritoneal larval cestodosis caused by Mesocestoides lineatus in Germany. Parasitol. Int. 2007;56:317–320. doi: 10.1016/j.parint.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 66.Fan C.K, Liao C.W, Cheng Y.C. Factors affecting disease manifestation of toxocarosis in humans: Genetics and environment. Vet. Parasitol. 2013;193:342–352. doi: 10.1016/j.vetpar.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 67.Öge H, Öge S, Özbakış G, Gürcan S. Comparison of Toxocara eggs in hair and faecal samples from owned dogs and cats collected in Ankara, Turkey. Vet. Parasitol. 2014;206:227–231. doi: 10.1016/j.vetpar.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 68.Drobinskij I.R. O toksokaroze u koshek i cheloveka (Toxocariasis in cats and humans) Tr. Kishinev Gos Med. In-ta. 1961;14:177–186. [Google Scholar]
- 69.Lejkina E.S. Zabolevanija cheloveka, vyzvannye migraciej lichinok nematod zhivotnyh (obzor zarubezhnoj literatury) (Human diseases caused by larva migrans (rewiev) Med. Parazitol. 1962;1:100–104. [Google Scholar]
- 70.Lysenko A.J, Alekseeva M.I, Avdjuhina T.I, Kuprijanova N.J, Cuckiridze N.P, Sokol’skaja G.M. Geografija toksokaroza v SSSR. Soobshhenie 2. Nozoareal (Toxocarosis geography in SSSR. Second report. Nosological area) Med. Parazitol. 1988;4:78–80. [PubMed] [Google Scholar]
- 71.Berezina E.S. Osobennosti rasprostranenija toksokaroza v populjacijah sobak i cheloveka, (Characters of toxocarosis spreading in humans and dogs popultions) Vet. Patol. 2006;3:45–56. [Google Scholar]
- 72.Shishkanova L.V, Tverdohlebova T.I, Ermakova L.A, Dumbadze O.S, Shirinyan A.A, Nagorny S.A. Toxocara canis infection at the South of Russia. Teor. Prak. Parazitarnyh Boleznej Zhivotnyh. 2014;15:356–358. [Google Scholar]
- 73.Zamaziy T.N, Zdor O.A. Peculiarities of epidemiology and clinical course of toxocariasis in contemporary conditions. Int. Med. J. 2005;1:133–135. [Google Scholar]
- 74.Adanir R, Tasci F. Prevalence of helminth eggs in raw vegetables consumed in Burdur, Turkey. Food Control. 2013;31:482–484. [Google Scholar]
- 75.Eckert T.J, Deplazes P. Biological, epidemiological, and clinical aspect of Echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eckert J, Gemmell M.A, Meslin F.X, Pawłowski Z.S. WHO/OIE. Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Paris, France: World Organisation for Animal Health; 2001. [Google Scholar]
- 77.Korneyev A.G, Trishin M.V, Solovykh V.V, Krivulya Y.S, Bozhenova I.V. Echinococcosis in the Orenburg region: Epidemiological, immunological and taxonomic aspects. Aktual’naja Infektologija. 2014;4:46–49. [Google Scholar]
- 78.McManus D.P. Current status of the genetics and molecular taxonomy of Echinococcus species. Parasitology. 2013;140:1617–1623. doi: 10.1017/S0031182013000802. [DOI] [PubMed] [Google Scholar]
- 79.Konyaev S.V, Yanagida T, Nakao M, Ingovatova G.M, Shoykhet Y.N, Bondarev A.Y, Odnokurtsev V.A, Loskutova K.S, Lukmanova G.I, Dokuchaev N.E, Spiridonov S, Alshinecky M.V, Sivkova T.N, Andreyanov O.N, Abramov S.A, Krivolapov A.V, Kaepenko S.V, Lopatina N.V, Dupal T.A, Sako Y, Ito A. Genetic diversity of Echinococcu s spp. in Russia. Parasitology. 2013;140:1637–1647. doi: 10.1017/S0031182013001340. [DOI] [PubMed] [Google Scholar]
- 80.Bartsocas C.S, Von Graevenitz A, Blodgett F. Dipylidium infection in a 6-month-old infant. J. Pediatr. 1977;69:814–815. doi: 10.1016/s0022-3476(66)80132-4. [DOI] [PubMed] [Google Scholar]
- 81.Jackson D, Crozier W.J, Andersen S.E, Giles W, Bowen T.E. Dipylidiasis in a 57-year-old woman. Med. J. Aust. 1977;2:740–741. doi: 10.5694/j.1326-5377.1977.tb99255.x. [DOI] [PubMed] [Google Scholar]
- 82.Chmeleva L.A, Kovaleva L.N. Dva sluchaja dipilidioza v Orenburgskoj oblasti, (Two cases of dipilidiasis in Orenburg Region) Med. Parazitol. 1968;37:243. [PubMed] [Google Scholar]
- 83.Romanenko N.A. Jepidemiologija dipilidioza detej v megapolise Moskva (Epidemiology of dipilidiasis in children from Moscow) Gig. Sanit. 2003;5:54–57. [Google Scholar]
- 84.Sarbasheva M.M, Bittirova A.A, Atabieva Z.A, Bittirov A.M, Bittirov A.M. Regional epidemiology of human cestodiasis in the Kabardino-Balkar Republic. Jepidemiologija i Infekcionnye Bolezni. 2012;6:35–37. [Google Scholar]
- 85.King S, Scholz T. Trematodes of the family Opisthorchiidae: A minireview. Korean J. Parasitol. 2001;39:209–211. doi: 10.3347/kjp.2001.39.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lun Z.R, Gasser R.B, Lai D.H, Li A.X, Zhu X.Q, Yu X.B, Fang Y.Y. Clonorchiasis: A key foodborne zoonosis in China. Lancet Infect. Dis. 2005;5:31–41. doi: 10.1016/S1473-3099(04)01252-6. [DOI] [PubMed] [Google Scholar]
- 87.Nikolaeva N, Nikolaeva L, Gigileva N. Opistorhoz (Jepidemiologija, Klinika, Diagnostika, Lechenie), (Opistorchosis (Epidemiology, clinic, diagnostics and treatment)) Vrach. 2005;7:17–20. [Google Scholar]
- 88.Ilyinskikh Y.N, Novitsky V.V, Ilyinskikh N.N, Lepyokhin A.V. Assessment of the incidence of Opisthorchis felineus (Rivolta 1884) and Metorchis bilis (Braun 1890) infections in population of several regions of the West Siberia. Bjulleten” Sibirskoj Med. 2006;5:18–22. [Google Scholar]
- 89.Lin R.Q, Tang J.D, Zhou D.H, Song H.Q, Huang S.Y, Chen J.X, Chen M.X, Zhang H, Zhu X.Q, Zhou X.N. Prevalence of Clonorchis sinensis infection in dogs and cats in subtropical Southern China. Parasit. Vectors. 2011;4:180. doi: 10.1186/1756-3305-4-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Besprozvannyh V.V, Ermolenko A.V, Rumjanceva E.E, Voronok V.M, Bartkova A.D. Klonorhis (Clonorchis sinensis) i klonorhoz v Primorskom krae (Clonorchis (Clonorchis sinensis) and clonorchiasis in Prymorsky region) Vladivostok: Dal’nauka; 2013. [Google Scholar]
- 91.Savchenkov M.F, Chumachenko I.G, Turchinova D.A. Diphyllobothriasis in Baikalsky region (epidemiologic observation) Sibirskij Med. Zhurnal. 2008;78:88–90. [Google Scholar]
- 92.Fuentes M.V, Gala M.T, Puchades N, Malone J.B. Short report a new case report of human mesocestoides infection in the United States. Am. J. Trop. Med. Hyg. 2003;68:566–567. doi: 10.4269/ajtmh.2003.68.566. [DOI] [PubMed] [Google Scholar]
- 93.Petrov Y.F, Krjuchkova E.N, Trusova A.V. The methodical position on preventive maintenance of alariosis of carnivores in Russian Federation. Russ. J. Parasitol. 2004;2:118–120. [Google Scholar]
- 94.Kramer M.H, Eberhard M.L, Blankenberg T.A. Respiratory symptoms and subcutaneous granuloma caused by mesocercariae: A case report. Am. J. Trop. Med. Hyg. 1996;55:447–448. doi: 10.4269/ajtmh.1996.55.447. [DOI] [PubMed] [Google Scholar]
- 95.Shea M, Maberley A.L, Walters J, Freeman R.S, Fallis A.M. Intraretinal larval trematode. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1973;77:784–791. [PubMed] [Google Scholar]
- 96.Petrov Y.F, Kryuchkova E.N, Trusova A.V, Gudkova A.Y, Shachbiev C.C. Biology of Alaria alata and epizootology of alariose of carnivora in the European part of Russia. Vet. Cubani. 2011;3:4–5. [Google Scholar]
- 97.Makarov V.V. Zoonozes. Vet. Patol. 2004;3:7–9. [Google Scholar]
- 98.Gorohov V.V, Peshkov R.A, Gorochova E.V. Toxocarosis as ecological problem. Vet. Patol. 2009;1:10–12. [Google Scholar]
- 99.Schuster R.K, Heidrich J, Pauly A, Nöckler K. Liver flukes in dogs and treatment with praziquantel. Vet. Parasitol. 2007;150:362–365. doi: 10.1016/j.vetpar.2007.09.016. [DOI] [PubMed] [Google Scholar]


