Abstract
Proteins released from Mycobacterium tuberculosis (Mtb) during late logarithmic growth phase are often considered candidate components of immunogenic or autolysis markers. One such protein is isocitrate dehydrogenase (ICD), a key regulatory enzyme in the citric acid cycle. We have evaluated the immunogenic properties of two isoforms of Mtb ICD and compared them with the control antigens heat-shock protein 60 and purified protein derivative (PPD). PPD lacks the sensitivity to distinguish between bacillus Calmette–Guérin (BCG)-vaccinated and tuberculosis (TB)-infected populations, and, therefore, epidemiological relevance of PPD in BCG-vaccinated regions is debatable. We show that Mtb ICDs elicit a strong B cell response in TB-infected populations and can differentiate between healthy BCG-vaccinated populations and those with TB. The study population (n = 215) was categorized into different groups, namely, patients with fresh infection (n = 42), relapsed TB cases (n = 32), patients with extrapulmonary TB (n = 35), clinically healthy donors (n = 44), nontuberculous mycobacteria patients (n = 30), and non-TB patients (culture negative for acid-fast bacteria but carrying other infections, n = 32). The Mtb ICDs showed statistically significant antigenic distinction between healthy BCG-vaccinated controls and TB patients (P < 0.0001) and those with other infections. Although extrapulmonary infections could not be discriminated from healthy controls by heat-shock protein 60 (P = 0.2177), interestingly, the Mtb ICDs could significantly (P < 0.0001) do so. Our results highlight the immunodominant, immunosensitive, and immunospecific nature of Mtb ICDs and point to an unusual property of this tricarboxylic acid energy cycle enzyme.
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), remains a major threat to the human population, being responsible for ≈2–3 millions deaths every year worldwide (1–3). The secret of the pathogen's success is its ability to escape the host immune system and remain undetected in lungs for decades. In only 10% of infected people, the number being higher in immunocompromised patients, does TB erupt as a full-blown disease (4). Delay in diagnosis and treatment impedes the downstream management and control of the disease. With the increasing emergence of multidrug resistant strains and coinfection with HIV, the problem is further compounded (5–7). Early diagnosis, therefore, is a matter of utmost concern not just for TB disease management but also for epidemiological investigations (8). Current diagnostic tools for TB often lack sensitivity and can be time consuming. TB diagnosis in developing countries largely banks on tuberculin skin tests and staining and culture methods. The epidemiological relevance of the tuberculin test with purified protein derivative (PPD) is questionable in areas where bacillus Calmette–Guérin vaccination is compulsory because PPD is not sensitive enough to distinguish between vaccinated and infected individuals (9). Microscopic determination of the bacilli in the sputum samples is a direct way of examining pulmonary TB (5). This method, however, requires high titers of bacilli (5,000–10,000 per ml) in sputum, a condition seen only in full-blown TB patients. Culture techniques can detect very low titers but are time-consuming, taking ≈3–6 weeks (10).
The importance of the major extracellular proteins of the pathogen as candidate components of a subunit vaccine has been reported earlier (11). Discovery of the RD1 locus in the Mtb genome, encoding mainly the proteins actively secreted by mycobacteria into the culture medium, such as CFP-10 and ESAT-6, has further encouraged immunological tests as an adjunct to conventional diagnosis (12–15). Proteins that are released from Mtb during late logarithmic growth phase, such as superoxide dismutase and isocitrate dehydrogenase (ICD), are used as autolysis markers (16). The use of ICD as a potential antigen for serodiagnosis along with malate dehydrogenase has been suggested (17, 18). The Mtb genome carries two isoforms of ICD, Mtb ICD-1 and Mtb ICD-2. Multiple sequence alignment revealed a closer similarity of Mtb ICD-1 to eukaryotic NADP+-dependent ICDs, whereas Mtb ICD-2 groups with bacterial ICDs (unpublished data). We have evaluated the utility of ICDs as immunogenic markers for TB through the detection of anti-Mtb ICD antibodies in sera of different well characterized categories of TB patients through enzyme-linked immunosorbent assays. We describe the sensitivity and specificity of ICDs to distinguish TB patients from those vaccinated with bacillus Calmette–Guérin and from those patients infected with nontuberculous mycobacteria (NTM) or other pathogens by means of the conventional antigens, heat-shock protein 60 (HSP 60) (19) and PPD.
Materials and Methods
Cloning, Expression, and Purification of Mtb ICD-1 and Mtb ICD-2. The ORFs corresponding to Mtb ICD-1 (Rv3339c, 1.230 kb) and Mtb ICD-2 (Rv0066c, 2.238 kb) were PCR-amplified from the genomic DNA of H37Rv. BamHI and HindIII restriction sites were incorporated in the 5′ end of forward and reverse primers, respectively, for both Mtb ICD-1 and Mtb ICD-2. The primers and parameters for thermal cycle amplification have been tabulated in Table 1. The amplicons carrying the full-length Mtb ICD-1 and Mtb ICD-2 were cloned at the BamHI and HindIII sites of the expression vector pRSET-A (Invitrogen) with six N-terminal histidine sequence tags. The generated constructs setAicd1 and setAicd2 were further transformed into the BL21 (DE3) strain of Escherichia coli. The clones were confirmed by sequencing with the T7 promoter primer on an Applied Biosystems Prism 377 DNA sequencer.
Table 1. PCR primers and thermal cycle parameters for amplification of Mtb ICD-1 and ICD-2.
| Primer | Sequence | PCR parameters | Amplicon size |
|---|---|---|---|
| Mtb icd-1 | |||
| forward | ggatccATGTCCAACGCACCCAAGATA | 94°C for 2 h | ≈1.2 kb |
| reverse | aagcttCTAATTGGCCAGCTCCTTTTC | 35 cycles: | |
| 94°C for 30 min, | |||
| 50°C for 1 h, | |||
| 72°C for 3 h, | |||
| 72°C for 7 h | |||
| Mtb icd-2 | |||
| forward | AGCTTggatccATGAGCGCCGAACAGCC | 94°C for 2 h | ≈2.23 kb |
| reverse | CATGGaagcttTCAGCCTTGGACAGCCT | 10 cycles: | |
| 94°C for 30 min, | |||
| 50°C for 30 min, | |||
| 72°C for 3.5 h | |||
| 25 cycles: | |||
| 94°C for 30 min, | |||
| 72°C for 3.5 h, | |||
| 72°C for 7 h |
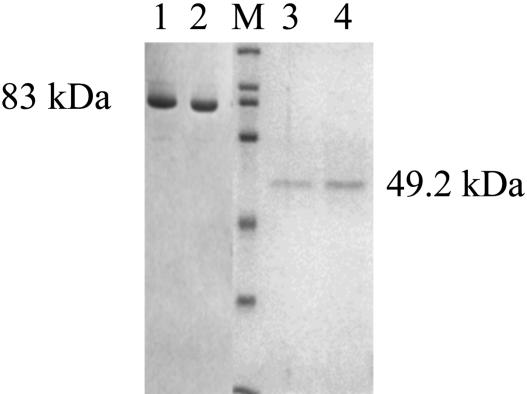
The genes were overexpressed in the pRSET-A/E. coli BL-21 (DE3) expression system. The overexpressed His-tagged recombinant protein was purified by Ni2+-nitrilotriacetate affinity chromatography. The cells transformed with the constructs were grown in Terrific Broth containing ampicillin (100 μg/ml) to an OD600 of 0.4–0.5 at 37°C, cooled to 27°C, induced with 0.1 mM isopropyl β-d-thiogalactoside, and grown overnight at 27°C. The cells were lysed by sonication, followed by centrifugation at 16,000 × g for 30 min at 4°C. The clear lysate was loaded onto a Ni2+-nitrilotriacetic acid column, which was then washed with 50 mM NaH2PO4/300 mM NaCl/20 mM imidazole, pH 8. The protein was eluted in the same buffer supplemented with 200 mM imidazole. The proteins were 90–95% pure as seen on 10% SDS/PAGE followed by Coomassie blue staining (Fig. 1). The purified recombinant proteins were dialyzed against 20 mM Tris·HCl, pH 7.5, with 100 mM NaCl and 3% glycerol and quantified by using Bradford reagent (20).
Fig. 1.
Affinity purification of Mtb ICD-1 and Mtb ICD-2. His-tagged recombinant protein was purified by nickel column chromatography under native conditions and stained with Coomassie blue after 10% SDS/PAGE. Lanes: 1 and 2, Mtb ICD-2; M, protein molecular mass markers (200, 116, 97, 66, 45, 31, and 21.5 kDa); 3 and 4, Mtb ICD-1.
Human Sera. The study population (n = 215) comprised the Mtb-infected human sample population reporting to the Mahavir Hospital and Research Centre and the Central JALMA Institute for Leprosy. These populations were categorized into three groups, namely Group 1 (n = 42 patients), Group 2 (n = 32 patients), and Group 3 (n = 35 patients). In addition to the above, 44 clinically healthy donors, 30 NTM cases, and 32 non-TB patients who were proven culture-negative for acid-fast bacteria were also included as controls in this study. Group 1 comprised patients with fresh infection with no history of TB treatment. Group 2 comprised patients with relapsed cases, i.e., those who were treated earlier for TB but whose symptoms reemerged after the completion of the treatment. Group 3 included patients with extrapulmonary TB. Patients from Groups 1 and 2 were diagnosed by sputum examination (acid-fast bacillus smear positive and negative), whereas the extrapulmonary cases were confirmed by tissue biopsy. Clinically healthy donors were Mycobacterium bovis bacillus Calmette–Guérin-vaccinated and had no symptoms of TB at the time of sera collection. Randomly picked individuals from the population of healthy controls were subjected to a PCR test for TB and were found to be PCR-negative. Mycobacteria other than Mycobacterium leprae that are not included in the Mtb complex are referred to as NTMs (21). However, the group referred to as NTMs in this study included sera collected from patients infected with NTM species (n = 14), such as Mycobacterium avium, Mycobacterium xenopi, and Mycobacterium fortuitum, as well as sera from patients with M. leprae infection (n = 16). The non-TB patient category included infected individuals who were tested negative for acid-fast bacteria by staining and culture-based techniques. These patients were also negative for HIV and hepatitis B virus. These randomly picked patients were suffering from pneumonia, lower respiratory infections, septicemia, urinary tract infections, gastrointestinal infections, cirrhosis, or fever of unknown origin. The study population had no sex or age bias. This study was approved by the Institutional Ethics Committee.
Immunosorbent Assays. ELISAs were performed to check the B cell immune response in humans to the Mtb ICD-1 and ICD-2 proteins and control antigens HSP 60 and PPD. The HSP 60 used was Mtb HSP65/GroEL. In brief, the 96-well microtiter plates (Costar) were coated with ≈500 ng of either control antigens or recombinant Mtb ICD-1 and Mtb ICD-2. The plates were incubated overnight at 4°C, washed three times with PBS, and blocked with 100 μl of blocking buffer (2% BSA in PBS) for 2 h at 37°C. The plates were then washed three times with wash buffer PBST (0.05% Tween 20 in 1× PBS). The Mtb-infected human sera belonging to different clinical groups were diluted 200 times in blocking buffer (1% BSA in PBS). Serum (50 μl) was added to antigen-coated wells and then incubated for 1 h at 37°C. The plates were thoroughly washed with PBST and further incubated with anti-human IgG-horseradish peroxidase (Sigma) at 37°C for 1 h. Horseradish peroxidase activity was detected by using a chromogenic substance, o-phenylenediamine tetrahydrochloride (Sigma), in citrate-phosphate buffer (pH 5.4) and H2O2 (Qualigens Fine Chemicals, Ahmedabad, India) as 1 μl/ml. The reactions were terminated by using 0.5 M H2SO4, and the absorbance values were measured at 492 nm in an ELISA reader (Bio-Rad). Each ELISA was repeated at least twice with some randomly picked serum samples tested three times for confirmation, with and without replicates for each sample within individual ELISA.
Data Analysis. Student's t test was performed to compare the means of two variable groups, healthy and infected classes, by using the online scientific calculator of GraphPad (www.graphpad.com/quickcalcs/ttest1.cfm) to calculate means, SEM, and P values.
Results
Expression and Purification of Mtb ICD-1 and Mtb ICD-2. The overexpressed N-terminal His-tagged Mtb ICD-1 was purified to 95% homogeneity on a nickel affinity column (Fig. 1). The molecular mass of the recombinant ICD-1 was determined to be 49.2 kDa. The purification was carried out under native conditions from soluble fractions with an yield of 3.25 mg of protein per 500 ml of start culture. Similarly Mtb ICD-2, an 83-kDa protein, was purified to 90–95% homogeneity (Fig. 1) with a yield of ≈20.4 mg per 1,000 ml of start culture.
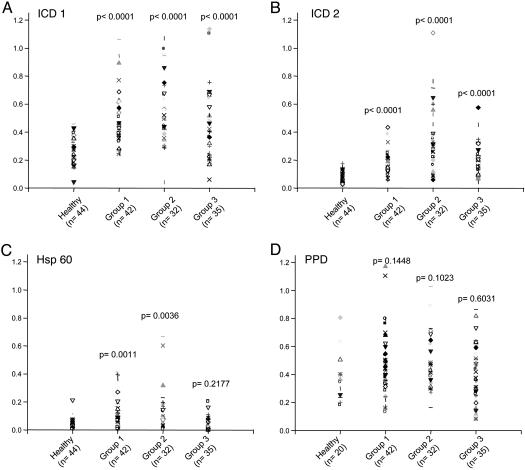
Mtb ICD-1 and Mtb ICD-2 Show High Reactivity to Patient Sera As Opposed to Bacillus Calmette–Guérin-Vaccinated Healthy Controls. Humoral immune responses directed against the Mtb ICD-1 and Mtb ICD-2 were compared among patients with TB and bacillus Calmette–Guérin-vaccinated healthy controls (Fig. 2 A and B). The recombinant proteins were used to screen the infected and healthy sera by ELISA using anti-human IgG-horseradish peroxidase as a conjugate. The sera were also tested against Mtb HSP 60 and the PPD (Fig. 2 C and D). The immunoreactivity of ICD-1, ICD-2, HSP 60, and PPD was statistically analyzed and compared with respect to both infected and healthy sera. These data demonstrate that sera of all of the infected patients mounted a statistically significant (P < 0.0001) antibody response against recombinant Mtb ICD-1 and Mtb ICD-2 proteins as compared with that of the healthy controls. PPD, on the other hand, reacted against both healthy and TB-infected sera. It is interesting to note that, compared with ICD-1 and ICD-2 (P < 0.0001), the difference in the reactivity of PPD to total infected and healthy sera was negligible and statistically insignificant (P = 0.2301). Because PPD, a mixture of proteins, showed statistically insignificant discrimination between healthy populations and different categories of infected populations (Fig. 2D), the reactivities of the recombinant proteins were compared with the B cell response to Mtb HSP 60 (Fig. 2C) in different categories of patients. The difference between reactivity to HSP 60 between TB patients and healthy controls was statistically not quite significant (P = 0.0645).
Fig. 2.
Mtb ICD-1 and ICD-2 show high B cell reactivity to sera from TB-infected patients from different groups as opposed to bacillus Calmette–Guérin-vaccinated healthy controls. The humoral immune responses directed against the recombinant proteins Mtb ICD-1 (A) and Mtb ICD-2 (B) and control antigens HSP 60 (C) and PPD (D) were compared among different categories of patients and healthy controls. Group 1, fresh infections; Group 2, relapsed infection; Group 3, extrapulmonary TB. The respective sample numbers and P values are shown.
A correlation between reactivity against Mtb ICD-1 and Mtb ICD-2 in patient sera with the state of disease, fresh or relapse, was attempted by comparing the antibody responses to Mtb ICD-1 and Mtb ICD-2 of various clinical categories (Fig. 2 A and B, respectively). Mtb ICD-1 failed to discriminate between fresh, relapsed, and extrapulmonary TB cases because no significant differences in immunoreactivity in different patient groups were observed (Fig. 2 A). Yet compared with bacillus Calmette–Guérin-vaccinated healthy controls each category yielded P values <0.0001, indicating that Mtb ICD-1 can differentiate substantially between bacillus Calmette–Guérin-vaccinated healthy populations and any category of Mtb-infected patients, pulmonary or nonpulmonary. Mtb ICD-2, on the other hand, could also discriminate relapsed cases from both fresh infections (P < 0.0001) and extrapulmonary infections (P = 0.0003). Like Mtb ICD-1, Mtb ICD-2 could also distinguish substantially between bacillus Calmette–Guérin-vaccinated healthy population and any category of Mtb-infected patients. Surprisingly, HSP 60, although it could discriminate Groups 1 and 2 from healthy controls (P = 0.0011 and 0.0036, respectively), failed to distinguish the extrapulmonary infections from bacillus Calmette–Guérin-vaccinated healthy controls (P = 0.2177). These results demonstrate that (i) recombinant Mtb ICD-1 and ICD-2 proteins could differentiate sera from TB-infected patients by means of healthy bacillus Calmette–Guérin-vaccinated controls, (ii) the extrapulmonary infections that could not be distinguished from healthy controls by HSP 60 could be significantly identified and categorized by Mtb ICDs, and (iii) Mtb ICD-2 mounted a stronger antibody response in relapsed cases and could significantly discriminate them from Group 1 and Group 3. These proteins, which have an apparently important metabolic role, are thus able to elicit a strong B cell response as a function of the TB infection.
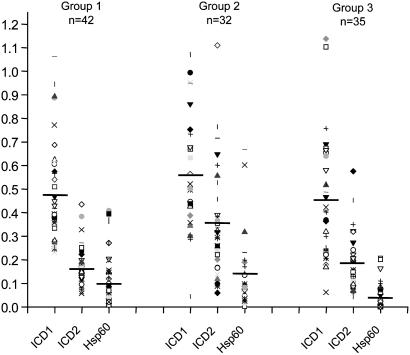
Immunodominace of ICDs over HSP 60. We compared the immunogenicity of ICDs over HSP 60. Humoral response to HSP 60 in all of the three categories of TB patients was tested and compared with those to ICDs (Fig. 3). The data clearly indicate that the mean reactivity (represented by the horizontal bands in Fig. 3) of HSP 60 in all of the classes of patient sera was much lower than in either ICD-1 or ICD-2 (Fig. 3). Thus, ICDs are immunodominant and serologically more sensitive than HSP 60. The mean values for ICD-1 in Groups 1, 2, and 3 were 0.481, 0.565, and 0.457, respectively, whereas those for ICD-2 were 0.165, 0.362, and 0.188, respectively. It is therefore apparent that ICD-1 elicited a stronger response in all of the three categories of patients tested than ICD-2. The data also confirm the discriminatory power of ICD-2 for relapsed case as compared with other categories.
Fig. 3.
Mtb ICDs are more immunogenic than HSP 60. The ELISA reactivity to Mtb ICD-1, Mtb ICD-2, and control antigen HSP 60 was compared in different patient groups. Horizontal bands represent the mean reactivity or average levels of humoral response in each category.
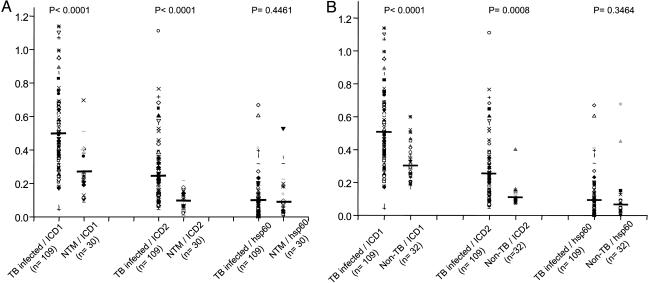
Immunospecificity of Mtb ICDs. The potential of Mtb ICDs to specifically distinguish between TB, NTMs, and non-TB patient sera (those essentially culture-negative for acid-fast bacteria but harboring other pathogens) was tested by examining the crossreactivity of the recombinant proteins with NTMs and non-TB patient sera. Thirty NTMs and 32 non-TB patient sera were tested for their immunogenic response against Mtb ICD-1, Mtb ICD-2, and HSP 60. The data were statistically analyzed to check whether ICDs could significantly distinguish between TB-infected patients and NTMs or non-TB patients. Fig. 4 shows that ICDs could significantly distinguish TB-infected sera from NTMs (P < 0.0001) and non-TB patients (P < 0.0001 for ICD-1 and P = 0.0008 for ICD-2), thus ruling out any crossreactivity with NTMs and other bacterial pathogens tested. HSP 60 appeared to react more broadly to the population under study and could not differentiate between TB infections and NTMs (P = 0.4461) or non-TB cases (P = 0.3464) significantly. That HSP 60 reacted broadly was apparent by calculating the average reactivity for each group (infected, NTMs, and non-TB), for which reactivity to HSP 60 remained almost the same (Fig. 4). The mean reactivity for HSP 60 is in contrast to the mean humoral response to ICD-1 and ICD-2, for which a distinctly higher response was seen in TB-infected sera as compared with NTMs or non-TB cases.
Fig. 4.
Mtb ICDs could significantly distinguish TB-infected sera from NTMs and non-TB patient sera. Recombinant Mtb ICD-1 and Mtb ICD-2 and HSP 60 were tested against sera of NTM (A) and non-TB (B) patients. The respective humoral responses were compared with those for TB-infected sera, the P values for which are shown. HSP 60 could not distinguish TB-infected patients from either NTM or non-TB patients significantly. Horizontal bands represent the mean reactivity in each category.
Discussion
The main objective of our study was to evaluate Mtb ICD-1 and Mtb ICD-2 in terms of their immune features as compared with the control antigens, HSP 60 and PPD, that are frequently used for diagnosis of TB. ICDs serve as markers of autolysis (16, 17) and are among the secretory proteins released during the late logarithmic phase. Although earlier efforts have pointed to the antigenic potential of Mtb ICDs (17), the present study is systematic investigation of their potential as an immune marker.
The cases of TB were identified and enrolled based on their history of treatment as fresh infections, relapsed cases, and extrapulmonary infections. The categorization of patients largely depended on the treatment history dictated by the patients or their family members. As evident from Fig. 2, there is little doubt about the ability of either Mtb ICD-1 or Mtb ICD-2 to elicit a strong B cell response, irrespective of patient category. When compared with Mtb ICD-2, Mtb ICD-1 was more antigenic (Figs. 2 and 3). It would be interesting to explore this disparity. A comparative analysis of ELISA reactivities among different categories of patients for Mtb ICDs (Fig. 2 A and B) revealed higher reactivity in Group 2 as compared with fresh (Group 1) and extrapulmonary (Group 3) infections. More specifically, the antigenic response in this category of patients to ICD 2 was significantly higher than that in Group 1 and Group 3. Because these patients had undergone treatment earlier, a high number of autolysed infected macrophages and autolysed pathogens could possibly explain the comparative high-antibody response against Mtb ICDs in this category. Drugs, like isoniazid, are known to affect the cell envelope architecture of mycobacteria and, hence, the increase in the production of the secreted proteins (22).
Comparative immunoreactivity of Mtb ICD-1, Mtb ICD-2, and HSP 60 clearly indicates that the antigenic distinction between healthy and TB patients is statistically significant for both Mtb ICD-1 and Mtb ICD-2 (P < 0.0001) but not quite so for HSP 60 (P = 0.0645). Earlier reports have shown crossreactive epitopes between microbial HSP 60/65 and human HSP 60, which often serve as autoimmune targets in conditions like atherosclerosis (19). The crossreactive epitopes probably could explain the broader reactivity of HSP 60 to healthy and infected sera. Because negligible antibody responses were obtained in the bacillus Calmette–Guérin-vaccinated healthy control group as compared with TB-infected patients and because a statistically significant difference in the immunoreactivity between infected and healthy sera was observed, it can be argued that Mtb ICD-1 and Mtb ICD-2 can be used for diagnosis of Mtb infection even in areas where bacillus Calmette–Guérin vaccination is routinely followed. The poor performance of PPD can be attributed to its nonspecific immune reaction in bacillus Calmette–Guérin-vaccinated healthy controls. Interestingly, the extrapulmonary TB cases (Group 3) in the study population did not show any significant humoral response to HSP 60 to distinguish them from healthy controls. On the other hand, Group 3 patients mounted a very significant B cell response to ICD-1 and ICD-2, separating them from bacillus Calmette–Guérin-vaccinated healthy controls.
Immunodominance is a parameter that we defined to compare the antibody titers against the tested proteins, i.e., ICD-1, ICD-2, and HSP 60, in the patient sera. Our data clearly showed that ICD-1 was most antigenic and mounted a very strong B cell response in all of the patient categories, followed by ICD-2 and HSP 60 (Fig. 3). Having shown that Mtb ICDs elicit a B cell response much higher than HSP 60, we checked for immune specificity of these proteins. Crossreaction with sera of NTMs and non-TB patients is one of the critical parameters that needed to be checked before establishing any antigenic marker for possible serological studies in Mtb. Mtb complex, including M. bovis and Mycobacterium africanum, is responsible for more illness worldwide than any other bacteria. However, there are >82 recognized species of mycobacteria that occasionally infect mammalian hosts. These species, referred to as NTMs, are omnipresent in the environment, and most species are either nonpathogenic for humans or are rarely associated with disease, except for a few, like M. avium, that are opportunistic pathogens, more frequently associated with immunocompromised patients (23, 24). The clinical significance of many NTMs remains unclear; however, it is important to check the crossreactivity of Mtb antigens with this group of mycobacteria. Our experiments could establish that Mtb ICDs do not crossreact with either NTMs or non-TB patient sera (Fig. 4).
The existing diagnostic tests for TB, even to this day, largely depend on tuberculin skin tests and staining and culture techniques. These methods are slow, cumbersome, and lack sensitivity and specificity in bacillus Calmette–Guérin-vaccinated cases. As more and more recombinant antigens are being tested (25–31), serological methods are likely to be favored over others. ELISA per se is unlikely to replace the current TB diagnosis; however, in combination or parallel with other rapid PCR-based diagnostic techniques, ELISA can largely improve the accuracy and rapidity of TB diagnosis for an effective disease management. Our data reveal the antigenic potential of recombinant Mtb ICD-1 and also present a systematic study on immunogenicity of recombinant Mtb ICD-2. Mtb ICD-1 and Mtb ICD-2 can be further analyzed for their pathogen-specific antigenic epitopes. Given their important role in the energy cycle, an evaluation of these two Mtb enzymes as possible drug targets is necessary. That such important enzymes can also have strong antigenic attributes that enable them to significantly discriminate between bacillus Calmette–Guérin-vaccinated healthy controls and TB patients and at the same time TB from other pathogenic infections is a very exciting proposition, possibly pointing to their immunomodulatory function.
Acknowledgments
We thank Dr. Shekhar Mande (Centre for DNA Fingerprinting and Diagnostics) for providing purified recombinant Mtb HSP 65/GroEL. This work was supported by research grants from the Council of Scientific and Industrial Research and the Department of Biotechnology, Government of India (to S.E.H.). S.B. was supported by a Senior Research Fellowship from the Council of Scientific and Industrial Research.
Abbreviations: Mtb, Mycobacterium tuberculosis; TB, tuberculosis; ICD, isocitrate dehydrogenase; PPD, purified protein derivative; HSP 60, heat-shock protein 60; NTM, nontuberculous mycobacteria.
References
- 1.Dye, C., Scheele, S., Dolin, P., Pathania, V. & Raviglione, M. C. (1999) J. Am. Med. Assoc. 282, 677–686. [DOI] [PubMed] [Google Scholar]
- 2.Bloom, B. R. & Murray, C. J. L. (1992) Science 257, 1055–1064. [DOI] [PubMed] [Google Scholar]
- 3.Chakhaiyar, P. & Hasnain, S., E. (2004) Med. Prin. Prac. 13, 177–184. [DOI] [PubMed] [Google Scholar]
- 4.Helmuth, L. (2000) Science 289, 1123–1125. [DOI] [PubMed] [Google Scholar]
- 5.Dye, C., Espinal, M. A., Watt, C. J., Mbiaga, C. & Williams, B. G. (2002) J. Infect. Dis. 185, 1197–1202. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqi, N., Shamim, M., Hussain, S., Choudhary, R. K., Ahmed, N., Prachee, Banerjee, S., Savithri, G. R., Alam, M., Pathak, N., et al. (2002) Antimicrob. Agents Chemother. 46, 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed, N., Caviedes, L., Alam, M., Rao, K. R., Sangal, V., Sheen, P., Gilman, R. H. & Hasnain, S. E. (2003) J. Clin. Microbiol. 41, 1712–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed, N., Alam, M., RajenderRao, K., Kauser, F., Ashok Kumar, N., Qazi, N., N., Sangal, V., Sharma, V., D., Das, R., Katoch, V., M., et al. (2004) J. Clin. Microbiol. 42, 3240–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roche, P. W., Triccas, J .A., Avery, D. T., Fifis, T., Billman-Jacobe, H. & Britton, W. J. (1994) J. Infect. Dis. 170, 1326–1330. [DOI] [PubMed] [Google Scholar]
- 10.Laidlaw, M. (1989) in Practical Medical Microbiology, eds. Colle, J. G., Duguid, J. P., Fraser, A. G. & Marimon, B, P. (Churchill Livingstone, New York), pp. 399–416.
- 11.Horwitz, M. A., Lee, B. W., Dillon, B. J. & Harth, G. (1995) Proc. Natl. Acad. Sci. USA 92, 1530–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mustafa, A. S. (2002) Mol. Immunol. 39, 113–119. [DOI] [PubMed] [Google Scholar]
- 13.Louise, R., Skjot, V., Agger, E. M. & Andersen, P. (2001) Scand. J. Infect. Dis. 33, 643–647. [DOI] [PubMed] [Google Scholar]
- 14.Trajkovic, V., Natarajan, K. & Sharma, P. (2004) Microbes Infect. 6, 513–519. [DOI] [PubMed] [Google Scholar]
- 15.Mori, T., Sakatani, M., Yamagishi, F., Takashima, T., Kawabe, Y., Nagao, K., Shigeto, E., Harada, N., Mitarai, S., Okada, M., et al. (2004) Am. J. Respir. Crit. Care Med. 170, 59–64. [DOI] [PubMed] [Google Scholar]
- 16.Anderson, P., Askgaard. D., Ljungqvist, L., Bennedsen, J. & Heron, I. (1991) Infect. Immun. 59, 1905–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohman, R. & Ridell, M. (1996) Tuberc. Lung Dis. 77, 454–461. [DOI] [PubMed] [Google Scholar]
- 18.Florio, W., Bottai, D., Batoni, G., Esin, S., Pardini, M., Maisetta, G. & Campa, M. (2002) Clin. Diagn. Lab. Immunol. 9, 846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perschinka, H., Mayr, M., Millonig, G., Mayerl, C., van der Zee, R., Morrison, S. G., Morrison, R. P., Xu, Q. & Wick, G. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 1060–1065. [DOI] [PubMed] [Google Scholar]
- 20.Bradford, M. M. (1976) Analyt. Biochem. 72, 248–252. [DOI] [PubMed] [Google Scholar]
- 21.Saiman, L. (2004) Paediatr. Respir. Rev. 221–223.
- 22.Bardou, F., Quemard, A., Dupont, M. A., Horn, C., Marchal, G. & Daffe, M. (1996) Antimicrob. Agents Chemother. 40, 2459–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiratsuch, H. & Basson, M. D. (2003) Am. J. Surg. 186, 547–551. [DOI] [PubMed] [Google Scholar]
- 24.Hadad, D. J., Palaci, M., Pignatari, A. C., Lewi, D. S., Machado, M. A., Telles, M. A., Martins, M. C., Ueki, S.Y., Vasconcelos, G. M. & Palhares, M. C. (2004) Epidemiol. Infect. 132, 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choudhary, R. K. Mukhopadhyay, S., Chakhaiyar, P., Sharma, N., Murthy, K. J. R., Katoch, V. M. & Hasnain, S. E. (2003) Infect. Immun. 71, 6338–6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramalingam, B., UmaDevi, K. R. & Raja, A. (2003) Scand. J. Infect. Dis. 35, 234–239. [DOI] [PubMed] [Google Scholar]
- 27.Brusasca, P. N., Peters, R. L., Motzel, S. L., Klein, H. J. & Gennaro, M. L. (2003) Comp. Med. 53, 165–172. [PubMed] [Google Scholar]
- 28.Maekura, R., Kohno, H., Hirotani, A., Okuda, Y., Ito, M., Ogura, T. & Yano, I. (2003) J. Clin. Microbiol. 41, 1322–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins, M. D., Conde, M. B., Martins, M. & Kritski, A. L. (2003) Chest 123, 107–112. [DOI] [PubMed] [Google Scholar]
- 30.Maekura, R., Okuda, Y., Nakagawa, M., Hiraga, T., Yokota, S., Ito, M., Yano, I., Kohno, H., Wada M., Abe, C., et al. (2001) J. Clin. Microbiol. 39, 3603–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakhaiyar, P., Nagalakshmi, Y., Aruna, B., Murthy, K., J., R, Katoch, V., M. & Hasnain, S., E. (2004) J. Infect. Dis., in press. [DOI] [PubMed]