Abstract
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a powerful arteriogenic factor in the hypoperfused rat brain. To test the pathophysiological relevance of this response, the influence of GM-CSF on brain energy state was investigated in a model of hemodynamic stroke. Sprague-Dawley rats were submitted to three-vessel (bilateral vertebral and unilateral common carotid artery) occlusion (3-VO) to induce unilaterally accentuated brain hypoperfusion. One week later, hemodynamic stroke was induced by additional lowering of arterial blood pressure. Experiments were terminated by in situ freezing of the brain. ATP was measured in cryostat sections by using a bioluminescence method. The use of 3-VO, in combination with 15 min of hypotension of 50, 40, or 30 mmHg, did not produce disturbances of energy metabolism, however, focal areas of ATP depletion were unilaterally detected after 3-VO, in combination with 15 min of hypotension of 20 mmHg. Treating such animals with GM-CSF (40 μg·kg-1·d-1) during the 1-week interval between 3-VO and induced hypotension significantly reduced the hemispheric volume of energy depletion from 48.8 ± 44.2% (untreated group, n = 10) to 15.8 ± 17.4% (treated group, n = 8, P = 0.033). GM-CSF-induced arteriogenesis is another approach to protect the brain against ischemic injury.
Keywords: collateral circulation, cerebrovascular circulation, prevention of stroke, brain vessels, rat
In a recent investigation, we introduced three-vessel occlusion (3-VO) as a model of unilaterally accentuated nonfatal hypoperfusion of the rat brain (1). By coagulation of both vertebral arteries and unilateral ligation of one common carotid artery, a state of subliminal hypoperfusion of the brain is produced that leads to exhaustion of the hemodynamic reserve, but not to morphological lesions or functional neurological deficits. As blood supply in this model is redistributed across the ipsilateral posterior cerebral artery, arteriogenesis, the active outward remodelling of preexisting collateral pathways (2) results in the expansion of the posterior cerebral artery by a factor of two, and the gradual improvement of the hemodynamic reserve, as measured by the CO2 reactivity of blood flow.
In the hindlimb or the coronary system, arteriogenesis may be stimulated by infusion of different chemoor cytokines such as monocyte chemoattractant protein 1 (MCP-1) (3), fibroblast growth factors (FGFs) (4-7), or granulocyte-macrophage colony-stimulating factor (GM-CSF) (2). In the 3-VO model, GM-CSF also markedly accelerated arteriogenesis, as reflected by the faster expansion of the diameter of the posterior cerebral artery and normalization of the hemodynamic reserve within 1 week after onset of treatment (8). GM-CSF-induced arteriogenesis may therefore protect the brain against stroke by improvement of collateral blood supply.
To test this hypothesis, we developed a model of hemodynamic stroke. In this model, 3-VO is combined with systemic hypotension that, on the side of carotid artery occlusion, results in further lowering of blood flow below the threshold of energy failure.
In the following, we describe the development of this model and we present evidence that GM-CSF-induced arteriogenesis does in fact significantly reduce brain injury in this experimental model.
Materials and Methods
Animal Groups. Experiments were carried out in male Sprague-Dawley rats (290-410 g) with permission of state authorities according to the German Law for Protection of Animals, and the National Institute of Health Guidelines for Care and Use of Laboratory Animals. Animals were housed under diurnal lighting conditions and allowed food and water ad libitum.
To produce nonlethal chronic hypoperfusion, 78 animals were submitted to 3-VO. A total of 12 animals died during 3-VO, and 13 animals were rejected because of intermittent infections, weight loss, or technical problems. The remaining 53 animals were used for two experiments: in the first experiment (n = 19), rats were submitted to 15 or 30 min of hypotension at 50, 40, 30, and 20 mm Hg, respectively (three to seven animals per group). In the second experiment (n = 34), animals were divided into two groups: one group (n = 16) was treated with daily injections of GM-CSF (40 μg·kg-1·d-1; treated group), and the other group (n = 18) received daily injections of 0.2 ml of saline (untreated group).
Surgical Procedures. Anesthesia was induced with 4% halothane and maintained with 0.8-1.5% halothane in 70/30% nitrous oxide/oxygen. Rectal temperature was kept at 37.0 ± 0.5°C by using a feedback-controlled heating system.
The 3-VO procedure was performed as described by Busch et al. (1). Vertebral arteries were electrocoagulated bilaterally by a paravertebral approach. The common carotid artery was exposed unilaterally on the left side by ventral cervical incision and was ligated. Wounds were closed after surgery and infiltrated with a local anaesthetic (Bupivacain 0.25%). After recovery from anesthesia, animals were returned in pairs of two to their cages with free access to food and water, and were allowed to move freely. Before induction of hypotension, animals were reanesthetized, and the skull was exposed bilaterally for blood flow monitoring by using transcranial laser-Doppler flowmetry (LDF) (PeriSoft, PeriMed, Järfälla, Sweden). The laser probes were placed directly on the bone. The animals were tracheotomized, paralyzed with pancuronium bromide (0.2 mg·kg-1·h-1) and mechanically ventilated with a small animal respirator. Both femoral arteries were catheterized, one for measurement of blood pressure and the other one for blood sampling and induction of hemorrhagic hypotension. Blood gases were analyzed by using a Ciba Corning blood gas analyzer (Ciba Corning Diagnostics, Fernwald, Germany), and hypotension was started as soon as blood values were in the normal range. First, a single dose of papaverine (5 mg·kg-1) was injected into the femoral artery. When blood pressure began to decline, blood was withdrawn rapidly into a heparinized syringe. A stop watch was started when the desired value of blood pressure was reached. Blood pressure was held at the desired value by steady withdrawal or reinfusion of blood. During hypotension, blood gases and pH were measured twice. Experiments were terminated by “funnel freezing,” i.e., by inactivating cerebral enzyme activity in vivo by pouring liquid nitrogen into a funnel attached to the head (9). Brains were removed in a cold box cabinet (-20°C) and were cut into 20-μm coronal cryostat sections at -20°C. Sections were mounted on coverslips for ATP bioluminescence, and on object holders for histology.
For measurement of cerebral blood flow (CBF), catheters were additionally inserted into both femoral veins and the coccygeal arter y. The procedure of i.v. infusion by iodo[14C]antipyrine (40 μCi per animal; 1 Ci = 37 GBq) was as described by Sakurada et al. (10). During the infusion time of 1 minute, arterial blood samples were taken every 7-10 sec. Arterial circulation was stopped after 1 min by an i.v. bolus of 2 ml of saturated potassium chloride solution, and heads were immediately frozen in liquid nitrogen. Brains were removed in a cold box cabinet (-20°C), cut into 20-μm coronal cryostat sections at -20°C, and sections were mounted on object holders. The 14C radioactivity of blood was measured in a Wallac 1410 liquid scintillation counter (Wallac Distribution, Freiburg, Germany).
Histology and Regional Measurement of ATP. For histological evaluation of brain injury, sections were stained with hematoxylin/eosin. Pictorial measurements of ATP were performed on coronal cryostat sections by using ATP-specific bioluminescence (11). This technique allows the immediate detection of regional ATP depletion, which inevitably leads to brain infarction if flow reduction persists (12). Bioluminescence images were taken with a SensiCam digital camera system (Pro CCD Imaging, Kelheim, Germany), analyzed by using nih image software (imagemg 1.55), and calibrated by taking small tissue samples from both hemispheres in the area of the parietal cortex for quantitative enzymatic analysis. ATP depletion was defined as the decline to <0.5 μmol·g-1, and the volume of energy failure was calculated by integration at six coronal levels with appropriate edema correction (13). Values are given in mm3 and in percent of contralateral hemisphere. Incidence maps of ATP depletion were prepared at the level of the striatum.
Measurement of CBF by Autoradiography. Brain sections and corresponding 14C standards (Amersham Pharmacia International, Buckinghamshire, U.K.) were exposed to autoradiographic films (Hyperfilm-βmax, Amersham Pharmacia International) for 10 days. Optical densities of brain sections and 14C standards were measured (nih image software, imagemg 1.55) and CBF was calculated according to Sakurada et al. (10).
Statistical Analysis. All data are given as means ± SD. Changes of blood pressure and laser-Doppler flow during hypotension were expressed as the percentage of the mean baseline value. Group differences were analyzed for statistical significance by using the Mann-Whitney U test. Statistical significance was assumed for P < 0.05.
Results
Experimental Model of Hemodynamic Stroke. To establish a suitable model for testing arteriogenesis-induced stroke prevention, we combined 3-VO with increasing severities of hypotension. Hypotension was induced 1 week after 3-VO and was maintained for 15 or 30 min before freezing brains in situ.
Before hypotension, all physiological variables were in the normal range. When blood pressure was lowered, arterial pCO2, and to a lesser degree, arterial pO2, declined. Arterial pH remained in the normal range (7.35 ± 0.1 units), except at 30 min of 30 mmHg hypotension, where it slightly declined to 7.11 (Table 1).
Table 1. General physiological variables before and during hypotension.
| Normotension
|
Hypotension
|
|||||||
|---|---|---|---|---|---|---|---|---|
| SAP, mmHg | pO2, mmHg | pCO2, mmHg | pH | SAP, mmHg | pO2, mmHg | pCO2, mmHg | pH | |
| Experiment I | ||||||||
| 50 mmHg, 15 min (n = 7) | 112 ± 12 | 148 ± 34 | 44 ± 4 | 7.37 ± 0.03 | 49 ± 1 | 155 ± 42 | 27 ± 5 | 7.30 ± 0.10 |
| 40 mmHg, 15 min (n = 3) | 100 ± 13 | 145 ± 17 | 40 ± 5 | 7.37 ± 0.02 | 39 ± 1 | 139 ± 23 | 31 ± 1 | 7.36 ± 0.03 |
| 30 mmHg, 15 min (n = 3) | 120 ± 9 | 119 ± 6 | 37 ± 3 | 7.40 ± 0.02 | 29 ± 1 | 107 ± 19 | 22 ± 3 | 7.32 ± 0.02 |
| 20 mmHg, 15 min (n = 3) | 110 ± 17 | 144 ± 42 | 42 ± 1 | 7.44 ± 0.04 | 19 ± 0 | 110 ± 36 | 19 ± 5 | 7.29 ± 0.08 |
| 30 mmHg, 30 min (n = 3) | 103 ± 21 | 112 ± 8 | 45 ± 3 | 7.41 ± 0.05 | 29 ± 0 | 106 ± 21 | 29 ± 15 | 7.11 ± 0.23 |
| Experiment II | ||||||||
| Untreated (n = 14) | 109 ± 6 | 140 ± 41 | 40 ± 5 | 7.41 ± 0.03 | 20 ± 0 | 105 ± 29 | 21 ± 5 | 7.29 ± 0.06 |
| GM-CSF-treated (n = 12) | 120 ± 8 | 146 ± 43 | 41 ± 6 | 7.42 ± 0.03 | 20 ± 0 | 105 ± 22 | 17 ± 2* | 7.31 ± 0.10 |
Values are means ± SD. SAP, systemic arterial pressure; pO2, pCO2, and pH, arterial blood measurements.
Significantly different from untreated group (P < 0.05).
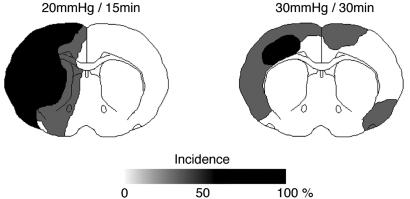
During hypotension, laser-Doppler flow was markedly reduced (Table 2). At systemic arterial pressure down to 40 mmHg, flow reduction was similar in both hemispheres, but at 30 and 20 mmHg, the ipsilateral hemisphere (with respect to common carotid artery occlusion) was more severely affected (Fig. 1). Imaging of ATP content after 15 min of hypotension did not reveal any abnormalities down to 30 mm Hg, but at 20 mmHg, multifocal areas of ATP depletion were detected in 38.2 ± 33.4% of the ipsilateral, but not the contralateral, hemisphere (Table 2). Focal areas of ATP depletion were also detected when hypotension of 30 mmHg was maintained for 30 min, but in this situation, contralateral alterations were also present (Fig. 2). We therefore decided to combine 3-VO with a 15-min period of 20 mmHg hypotension to induce unilateral brain infarction.
Table 2. Changes in laser-Doppler flow and energy metabolism during hypotension.
| LDF ipsi, percent of control | LDF contra, percent of control | ATP depletion volume, percent of ipsilateral hemisphere | |
|---|---|---|---|
| Experiment I | |||
| 50 mmHg (n = 7) | 64.6 ± 22.5 | 48.6 ± 13.3 | 0.0 |
| 40 mmHg (n = 3) | 55.0 ± 16.5 | 55.3 ± 8.0 | 0.0 |
| 30 mmHg (n = 3) | 21.3 ± 6.5 | 32.7 ± 9.1 | 0.0 |
| 20 mmHg (n = 3) | 18.0 ± 9.9 | 30.0 ± 5.2 | 38.2 ± 33.4 |
| Experiment II | |||
| Untreated (n = 10) | 14.5 ± 5.0 | 40.0 ± 12.2 | 48.8 ± 44.2 |
| GM-CSF-treated (n = 8) | 17.7 ± 8.3 | 25.5 ± 11.6* | 15.8 ± 17.4* |
Values are means ± SD; ipsi, ipsilateral (left) cortex; contra, contralateral (right) cortex.
Significantly different from untreated group (P < 0.05).
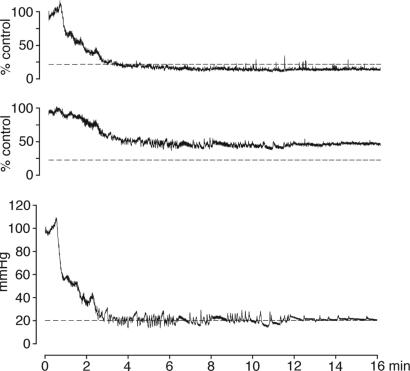
Fig. 1.
Recording of CBF from ipsilateral (Top) and contralateral (Middle) parietal cortex by LDF during 3-VO in combination with hemorrhagic hypotension (Bottom). During hypotension, LDF decline is more pronounced in the ipsilateral hemisphere, i.e., on the side of carotid artery occlusion (20% flow level and 20 mmHg pressure level are indicated by the dotted lines).
Fig. 2.
Incidence maps of ATP depletion at the end of hemorrhagic hypotension in combination with 3-VO. Comparison of 15 min of hypotension at 20 mmHg (Left) with 30 min of hypotension at 30 mmHg (Right). Areas of disturbed metabolism are outlined on coronal cryostat sections of three animals per group and superimposed to demonstrate the regional incidence of energy failure. Note the strict lateralization of energy failure in the left hemisphere during hypotension at 20 mmHg.
GM-CSF Treatment for the Alleviation of Hemodynamic Stroke. During the 1-week interval between 3-VO and the induction of hypotension, arteriogenesis was induced in 16 rats by injections of 40 μg·kg-1·d-1 GM-CSF (treated group). Eighteen animals received injections of Ringer's solution (untreated group). Before induction of hypotension, blood pressure and blood gases were in the normal range in both groups. During hypotension, blood pressure, arterial pO2, and arterial pH did not differ between groups, but the decline of arterial pCO2 was more pronounced in the treated animals (Table 1). Ipsilateral laser-Doppler flow was slightly, but not significantly, more reduced in the untreated animals, but the flow decline in the opposite hemisphere was more pronounced in the treated animals (Table 2).
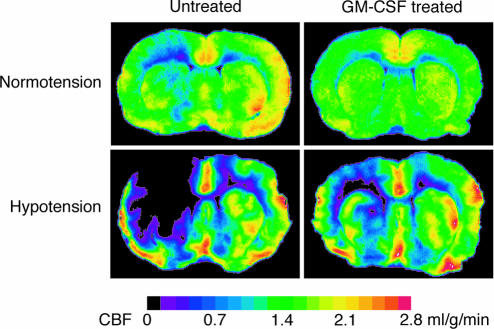
Quantitative measurements of CBF were carried out by iodo[14C]antipyrine autoradiography (Fig. 3). In untreated animals, hypotension led to the significant reduction of ipsilateral CBF in all regions except for the hypothalamus and the cortex supplied by the anterior cerebral artery. In the treated animals, hypotension-induced reduction of ipsilateral CBF was less pronounced and reached no statistical significance. During hypotension, treated animals exhibited significantly higher flow values in the ipsilateral hypothalamus and the cortex supplied by the posterior cerebral artery as compared with untreated animals (Tables 3 and 4).
Fig. 3.
Effect of 15 min of hemorrhagic hypotension on CBF at 1 week after 3-VO. Comparison of untreated animals with animals treated with GM-CSF (40 μg·kg-1·d-1). Representative iodo[14C]antipyrine autoradiograms of brain cryostat sections at the level of caudate-putamen obtained either at normotension (above) or at the end of 15 min of hypotension (below). Note the lesser reduction of blood flow in the left hemisphere of treated animals.
Table 3. CBF measured by iodo[14C]antipyrine autoradiography (ml·g-1 · min-1).
| ACA cortex
|
MCA cortex
|
PCA cortex
|
||||
|---|---|---|---|---|---|---|
| ipsi | contra | ipsi | contra | ipsi | contra | |
| Untreated group | ||||||
| Normotension (n = 4) | 1.68 ± 0.96 | 1.67 ± 0.89 | 0.75 ± 0.45 | 1.62 ± 0.87 | 0.53 ± 0.31 | 0.88 ± 0.28 |
| Hypotension (n = 4) | 0.73 ± 0.33 | 0.81 ± 0.34 | 0.20 ± 0.09* | 1.06 ± 0.31 | 0.12 ± 0.04* | 0.55 ± 0.33 |
| GM-CSF-treated group | ||||||
| Normotension (n = 4) | 1.81 ± 1.03 | 1.64 ± 0.77 | 0.92 ± 0.39 | 1.14 ± 0.35 | 0.64 ± 0.25 | 0.94 ± 0.25 |
| Hypotension (n = 4) | 1.51 ± 0.41 | 1.67 ± 0.28† | 0.66 ± 0.32 | 1.84 ± 0.23† | 0.43 ± 0.17† | 1.10 ± 0.26 |
Values are means ± SD. ACA, anterior cerebral artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; ipsi, ipsilateral; contra, contralateral.
Significantly different from normotension (P < 0.05).
Significantly different from untreated (P < 0.05).
Table 4. CBF measured by iodo[14C]antipyrine autoradiography (ml·g-1·min-1).
| Caudate-putamen
|
Hippocampus
|
Hypothalamus
|
||||
|---|---|---|---|---|---|---|
| ipsi | contra | ipsi | contra | ipsi | contra | |
| Untreated group | ||||||
| Normotension (n = 4) | 0.72 ± 0.43 | 1.40 ± 0.72 | 0.99 ± 0.23 | 0.91 ± 0.61 | 0.53 ± 0.18 | 1.09 ± 0.78 |
| Hypotension (n = 4) | 0.25 ± 0.12* | 1.05 ± 0.49 | 0.11 ± 0.05* | 0.52 ± 0.26 | 0.40 ± 0.12 | 1.06 ± 0.51 |
| GM-CSF-treated group | ||||||
| Normotension (n = 4) | 1.04 ± 0.54 | 1.47 ± 0.71 | 0.61 ± 0.25 | 0.85 ± 0.35 | 0.56 ± 0.18 | 0.82 ± 0.31 |
| Hypotension (n = 4) | 0.72 ± 0.28 | 1.82 ± 0.23 | 0.35 ± 0.22 | 1.16 ± 0.21 | 0.80 ± 0.12† | 1.54 ± 0.19 |
Values are means ± SD. ipsi, ipsilateral; contra, contralateral.
Significantly different from normotension (P < 0.05).
Significantly different from untreated (P < 0.05).
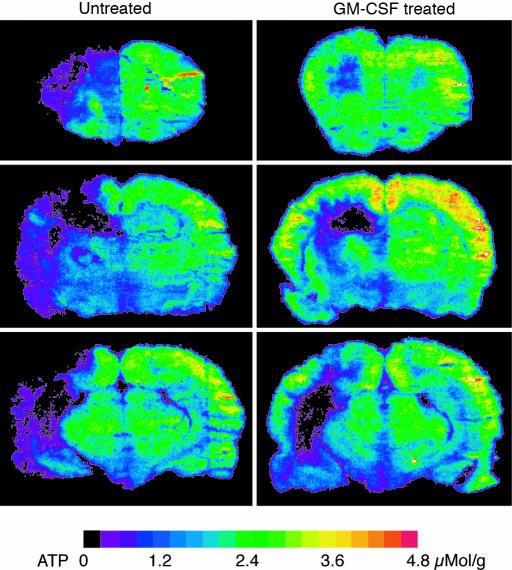
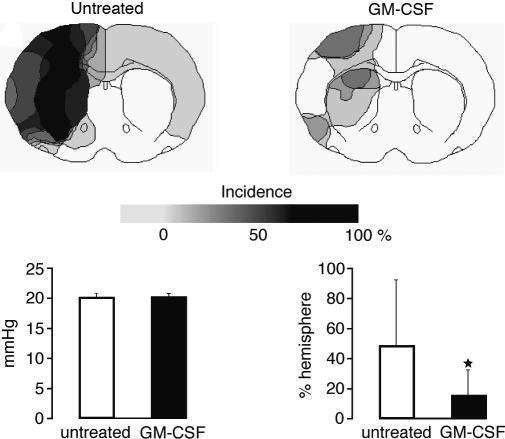
Coronal ATP images prepared at the end of the 15-min hypotension period showed large areas of ATP depletion in all untreated animals. In the GM-CSF-treated group, in contrast, areas of ATP depletion were distinctly smaller (Fig. 4). Volumetric measurements revealed a significant reduction of the hemispheric ATP depletion volume from 48.8 ± 44.2% in the untreated to 15.8 ± 17.4% in the treated group (P < 0.05, Fig. 5). Remarkably, in two treated animals, ATP depletion was completely prevented, and in two others it was <2% of the ipsilateral hemisphere. The difference in the severity of hemodynamic injury was not due to the slightly higher blood pressure in treated animals before the induction of hypotension (120 ± 8 vs. 109 ± 6 mmHg, P < 0.001). When ATP depletion was referred to the blood pressure difference before and during hypotension, treated animals exhibited energy failure in only 0.8 ± 0.9 mm3 brain tissue per mmHg of blood pressure fall as compared with 3.1 ± 2.5 mm3 per mmHg (P < 0.05) in the untreated rats.
Fig. 4.
Effect of 15 min of hypotension on ATP content at 1 week after 3-VO. Comparison of untreated animals with animals treated with GM-CSF (40 μg·kg-1·d-1). ATP-dependent bioluminescence was measured during hypotension at three coronal levels, passing through the frontal part of caudateputamen, dorsal hippocampus, and globus pallidus. Note the marked reduction of energy failure in the treated animal.
Fig. 5.
Incidence maps of ATP depletion in animals submitted to 3-VO in combination with 15 min of hemorrhagic hypotension at 20 mmHg (for explanation, see Fig. 2). Comparison of untreated (n = 10) with GM-CSF-treated animals (n = 8). Note the marked reduction of areas with ATP depletion, despite similar blood pressure levels during hypotension (Left Lower). Calculation of injury volume by integration of ATP-depleted areas at six coronal levels reveals significant (*, P < 0.05) volume reduction after treatment (means ± SD, Right Lower).
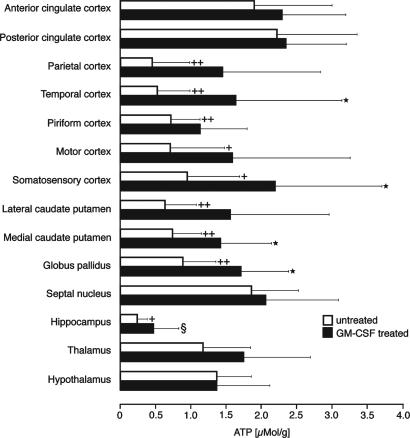
The markedly protective effect of GM-CSF was also reflected by the quantitative measurements of regional ATP content (Fig. 6). In the untreated group, significant reductions of ATP (compared with the opposite hemisphere) were present in the caudate putamen, globus pallidus, hippocampus, motor, somatosensory, parietal, temporal, and piriform cortex. In the GM-CSF-treated group, significant disturbances of energy metabolism were restricted to the hippocampus. As a consequence, treated rats exhibited significantly higher ATP values than untreated animals in the medial caudate putamen, globus pallidus, somatosensory, and temporal cortex (Fig. 6). In all of the other areas, disturbances were too scattered to reveal significant differences.
Fig. 6.
Measurements of regional ATP content (means ± SD) by quantitative bioluminescence imaging in various cortical and subcortical regions of the hemisphere ipsilateral to the left common carotid artery occlusion. White bars, untreated animals (n = 10); black bars, GM-CSF-treated animals (n = 8). The P values were calculated by Student's t test and were assigned in the following manner: +, P < 0.005; ++, P < 0.001 next to the white bar designates comparison between ipsilateral and contralateral regions of untreated animals; §, P < 0.005 next to the black bars indicates comparison between ipsilateral and contralateral regions of GM-CSF-treated animals; *, P < 0.05 next to the black bars represents difference between ipsilateral GM-CSF-treated and ipsilateral untreated animals.
Histological staining of brain sections sampled at the end of the 15-min hypotension period did not reveal morphological lesions in any of the treated or untreated animals, which is not surprising because the duration of hypotension was too short to produce structural changes. However, the use of ATP imaging allowed the early detection of energy failure, which, in the GM-CSF-treated animals was significantly reduced. Energy failure may be reversed if blood flow is instantaneously restored; however, in the absence of adequate recirculation, brain infarction inevitably ensues. Incidentally, the absence of morphological changes confirms our previous observation that 3-VO per se does not result in morphological lesions and excludes prehypotension differences between the two experimental groups.
Discussion
Arteriogenesis, the active outward remodelling of preexisting collateral pathways, has been documented after peripheral and coronary artery occlusion (2, 14), and recently, also in the brain after 3-VO (1). The functional significance of brain arteriogenesis is reflected by an improvement of the hemodynamic reserve, as demonstrated by the gradual recovery of CO2 reactivity, which, shortly after 3-VO, is completely suppressed (1). Under conditions of permanently increased vascular shear stress, arteriogenesis proceeds spontaneously, but the speed of vascular growth can be markedly accelerated by various cytokines, notably by GM-CSF (8).
In the present investigation, we demonstrate for the first time, to our knowledge, that in the hypoperfused brain this treatment leads within 1 week to highly significant protection against hemodynamic stroke, evoked by the combination of 3-VO and induced hypotension. This model replicates the pathophysiology of patients with extracranial vascular occlusion who suffer stroke during transient periods of hypotension (15), e.g., after antihypertensive treatment or during the circadian blood pressure fall in the early morning (16-18). Obviously, the hemorrhagic blood pressure decline to 20 mmHg used here goes far beyond the level of blood pressure fluctuations responsible for hemodynamic stroke in patients. However, it has to be considered that in the healthy rat 3-VO does not reduce the hemispheric blood perfusion pressure to the same extent as advanced cerebral vascular occlusion under clinical conditions.
Our observation of GM-CSF-induced brain protection against energy failure reflects the metabolic situation at the end of the 15-min period of hypotension and confirms the functional importance of the previously observed improvement of the cerebrovascular reserve (8). The beneficial effect of arteriogenesis is also supported by the quantitative blood flow measurements, which, in the treated animals, revealed a significantly lesser flow decline during induced hypotension. The failure to demonstrate these changes by LDF is not at variance to these observations because this method detects only relative changes in respect to the prehypotension baseline but not the absolute flow differences between treated and untreated animals.
An interesting side observation of our study was the absence of a therapeutic effect of GM-CSF on hippocampal energy failure. It is well known that hippocampus is more sensitive to ischemia than other parts of the brain, but this selective vulnerability is attributed to a cascade of molecular dysfunctions that slowly evolve during recirculation after circulatory impairment (19, 20). Our observation of the absence of a therapeutic effect during the hypotensive period is in line with this interpretation and demonstrates that the hippocampus does not profit as much as other parts of the brain from the improved collateral blood supply.
The successful application of GM-CSF to improve the outcome of hemodynamic stroke opens up another approach for infarct prevention in patients with reduced hemodynamic reserve. This treatment, which aims to enhance blood flow to ischemic territories from outside the risk region, may supplement established surgical preventive treatments such as carotid endarterectomy or percutaneous transluminal balloon angioplasty. Our results strongly suggest that clinical studies should be initiated to test this promising approach.
Acknowledgments
We thank Mr. B. Huth (Max Planck Institute for Neurological Research) for the artwork. This work was supported by a grant from the Bundesministerium für Wissenschaft und Forschung (Kompetenznetzwerk Schlaganfall) and the Research Program of the Volkswagen Foundation.
Abbreviations: CBF, cerebral blood flow; GM-CSF, granulocyte-macrophage colony-stimulating factor; LDF, laser-Doppler flowmetry; 3-VO, three-vessel occlusion.
References
- 1.Busch, H. J., Buschmann, I. R., Mies, G., Bode, C. & Hossmann, K.-A. (2003) J. Cereb. Blood Flow Metab. 23, 621-628. [DOI] [PubMed] [Google Scholar]
- 2.Buschmann, I. R., Hoefer, I. E., van Royen, N., Katzer, E., Braun-Dulleaus, R., Heil, M., Kostin, S., Bode, C. & Schaper, W. (2001) Atherosclerosis 159, 343-356. [DOI] [PubMed] [Google Scholar]
- 3.Ito, W. D., Arras, M., Winkler, B., Scholz, D., Schaper, J. & Schaper, W. (1997) Circ. Res. 80, 829-837. [DOI] [PubMed] [Google Scholar]
- 4.Banai, S., Jaklitsch, M. T., Casscells, W., Shou, M., Shrivastav, S., Correa, R., Epstein, S. E. & Unger, E. F. (1991) Circ. Res. 69, 76-85. [DOI] [PubMed] [Google Scholar]
- 5.Lazarous, D. F., Unger, E. F., Epstein, S. E., Stine, A., Arevalo, J. L., Chew, E. Y. & Quyyumi, A. A. (2000) J. Am. Coll. Cardiol. 36, 1239-1244. [DOI] [PubMed] [Google Scholar]
- 6.Rissanen, T. T., Vajanto, I. & Yla-Herttuala, S. (2001) Eur. J. Clin. Invest. 31, 651-666. [DOI] [PubMed] [Google Scholar]
- 7.Unger, E. F., Banai, S., Shou, M., Jaklitsch, M., Hodge, E., Correa, R., Jaye, M. & Epstein, S. E. (1993) Cardiovasc. Res. 27, 785-791. [DOI] [PubMed] [Google Scholar]
- 8.Buschmann, I. R., Busch, H.-J., Mies, G. & Hossmann, K.-A. (2003) Circulation 108, 610-615. [DOI] [PubMed] [Google Scholar]
- 9.Pontén, U., Ratcheson, R. A., Salford, L. G. & Siesjo, B. K. (1973) J. Neurochem. 21, 1127-1138. [DOI] [PubMed] [Google Scholar]
- 10.Sakurada, O., Kennedy, C., Jehle, J., Brown, J. D., Carbin, G. L. & Sokoloff, L. (1978) Am. J. Physiol. 234, H59-H66. [DOI] [PubMed] [Google Scholar]
- 11.Kogure, K. & Alonso, O. F. (1978) Brain Res. 154, 273-284. [DOI] [PubMed] [Google Scholar]
- 12.Paschen, W. (1990) Prog. Histochem. Cytochem. 20, 1-122. [DOI] [PubMed] [Google Scholar]
- 13.Swanson, R. A., Morton, M. T., Tsao-Wu, G., Savalos, R. A., Davidson, C. & Sharp, F. R. (1990) J. Cereb. Blood Flow Metab. 10, 290-293. [DOI] [PubMed] [Google Scholar]
- 14.Seiler, C., Pohl, T., Wustmann, K., Hutter, D., Nicolet, P. A., Windecker, S., Eberli, F. R. & Meier, B. (2001) Circulation 104, 2012-2017. [DOI] [PubMed] [Google Scholar]
- 15.Bladin, C. F. & Chambers, B. R. (1994) Stroke (Dallas) 25, 2179-2182. [DOI] [PubMed] [Google Scholar]
- 16.Argentino, C., Toni, D., Rasura, M., Violi, F., Sacchetti, M. L., Allegretta, A., Balsano, F. & Fieschi, C. (1990) Stroke (Dallas) 21, 387-389. [DOI] [PubMed] [Google Scholar]
- 17.Eigenbrodt, M. L., Rose, K. M., Couper, D. J., Arnett, D. K., Smith, R. & Jones, D. (2000) Stroke (Dallas) 31, 2307-2313. [DOI] [PubMed] [Google Scholar]
- 18.Haapaniemi, H., Hillbom, M., Numminen, H., Juvela, S., Palomaki, H. & Kaste, M. (1992) Cerebrovasc. Dis. 2, 282-286. [Google Scholar]
- 19.Kogure, T. & Kogure, K. (1997) Clin. Neurosci. 4, 179-183. [PubMed] [Google Scholar]
- 20.Schreiber, S. S. & Baudry, M. (1995) Trends Neurosci. 18, 446-451. [DOI] [PubMed] [Google Scholar]