Abstract
The activation marker CD69 is expressed by skin γδ T cells. Here we demonstrate that CD69 controlled the aryl hydrocarbon receptor (AhR)-dependent interleukin 22 (IL-22) secretion in γδ T cells, which contributed to psoriasis development induced by IL-23. CD69 associated with the aromatic amino acid transporter complex LAT1-CD98 and regulated its surface expression, L-tryptophan (L-Trp) uptake and intracellular quantity of L-Trp-derived AhR activators. In vivo administration of L-Trp, an AhR inhibitor or IL-22 abrogated differences in skin inflammation between CD69-deficient and wild type mice. LAT1-mediated regulation of AhR activation and IL-22 secretion was also observed in circulating Vγ9 γδ T cells of psoriatic patients. Thus, CD69 is a key mediator of the pathogenesis of psoriasis by controlling LAT1-CD98-mediated metabolic cues.
Psoriasis is one of the most common chronic inflammatory skin diseases, affecting about 2% of the population worldwide 1. It is defined by a thickened epidermis caused by keratinocyte proliferation (acanthosis) and the massive skin infiltration of polimorfonuclear cells. Psoriatic lesions contain high amounts of the pro-inflammatory cytokines interleukin 17 (IL-17), IL-21, IL-22 and IL-23, leading to the classification of psoriasis as a disease mediated by the IL-17 producing helper T cells (TH17) 2. The importance of the IL-23 and IL-17 in psoriatic patients is demonstrated by the efficacy of treatment with monoclonal antibodies against IL-17 and IL-23R 3. Furthermore, intradermal administration of recombinant IL-23 in mice induces a psoriasiform dermatitis that mimics the human disease in histological and immunological aspects 4.
In addition to IL-17, the cytokine IL-22 also acts as a master regulator of psoriasis 5, 6, 7. Polymorphisms in the Il22 gene increased psoriasis susceptibility 8, and serum levels of IL-22 positively correlate with disease severity and negatively correlate with responsiveness to therapy 9. IL-22 signaling in keratinocytes induces the expression and phosphorylation of the transcription factor STAT3, which increases epidermal proliferation and de-differentiation 10. IL-22 expression is regulated by the ligand-dependent transcription factor AhR in TH17 cells and some populations of innate lymphocytes 11, 12. Currently described endogenous ligands for AhR also include naturally occurring dietary substances, such as L-Trp-derived metabolites 13. Upon exposure to light, L-Trp can be metabolized to several products, including the high affinity AhR agonist 6-formylindolo [3, 2-b] carbazole (FICZ). A light-independent, H2O2-dependent pathway for systemic generation of FICZ from L-Trp has also been described 14. Uptake of aromatic amino acids by activated lymphoid cells is predominantly conducted through the system L1 transporter, an heterodimer comprising a heavy chain, CD98 (also known as SLC3A2, 4F2) and a light chain, LAT1 (L-type amino acid transporter 1, also known as SLC7A5). Regulation of amino acid transport through the LAT1-CD98 heterodimer is linked to T cell activation and differentiation processes 15.
Although TH17 cells were previously considered an important source of IL-17 and IL-22 in the psoriatic skin, recent evidence indicates that these cytokines are mainly produced by a population of dermal γδ T cells already identified in both humans and mice 16, 17, 18, 19. Skin γδ T cells bear several markers of memory and effector T cells, including CD69 20. Lymphocytes from CD69-deficient mice show enhanced differentiation towards the TH17 lineage 21 and CD69-deficient mice exhibit increased severity in TH17-mediated inflammatory diseases, including collagen II-induced arthritis 22, allergic asthma and skin contact hypersensitivity 23, autoimmune myocarditis 24 and colitis 25. Whether CD69 exerts an immune-modulatory effect in psoriasis by controlling IL-17 and IL-22 responses in skin γδ T cells has remained unexplored until now.
In this study we show that CD69-deficient mice developed an attenuated skin inflammatory response to IL-23 administration, with decreased expression of IL-22 and STAT3 in the epidermis. We show that CD69 associated with the heterodimeric amino acid transporter LAT1-CD98 and regulated L-Trp uptake, which promoted AhR-induced IL-22 secretion in skin γδ T cells.
Results
CD69 is required for IL-23-induced psoriasiform inflammation
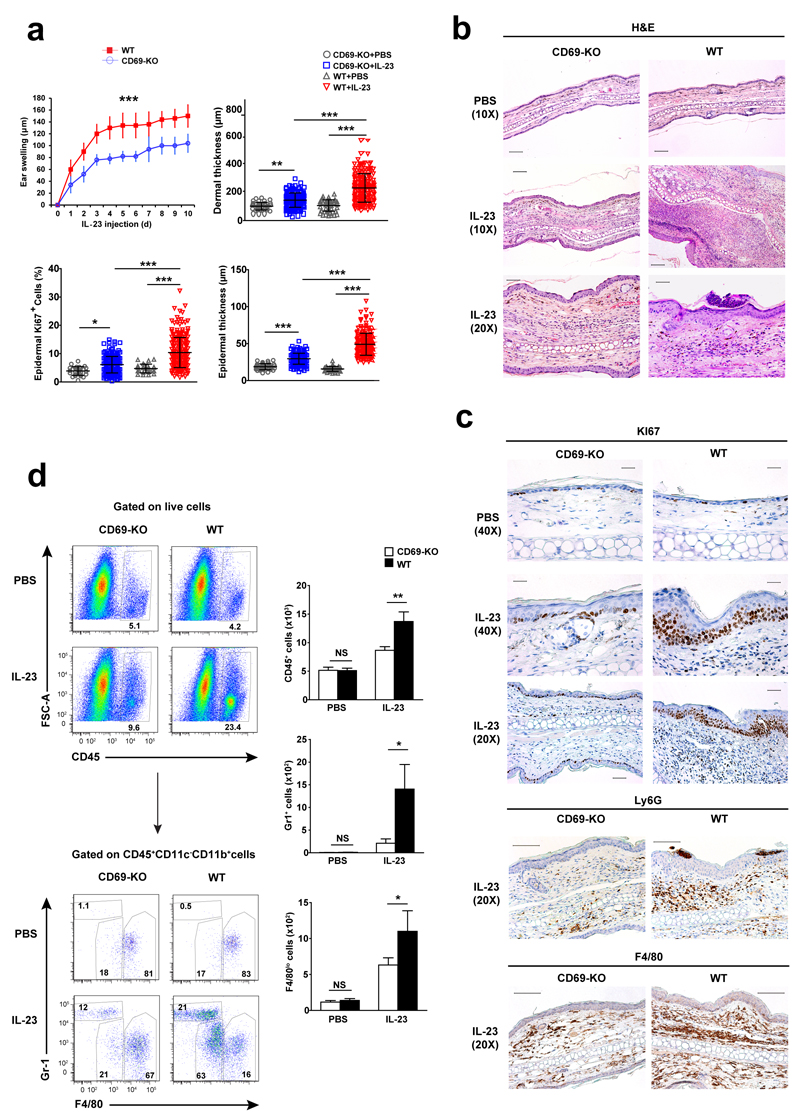
To assess the role of CD69 in psoriasis, consecutive intradermal injections of murine IL-23 protein were administered in the ears of wild-type and CD69-deficient mice. IL-23 induced more ear swelling, epidermal acanthosis, dermal inflammation and keratinocyte proliferation (Ki67+ nuclei) in the ears of wild-type than in CD69-deficient mice (Fig. 1a-c). Also, IL-23 significantly increased the total number of CD45+ cells in wild-type compared to CD69-deficient mice. These were mostly CD45+CD11c-CD11b+ myeloid (non-dendritic) cells, particularly Gr-1+F4/80-neutrophils, F4/80lo monocytes and F4/80hi macrophages (Fig. 1d). Immunohistochemistry also revealed increased numbers of Ly6G+ neutrophils and F4/80+ monocytes in wild-type compared to CD69-deficient mice after IL-23 treatment (Fig. 1c) These results indicate that the absence of CD69 prevents psoriasis development after intradermal injections of IL-23.
Figure 1. CD69-deficient mice develop a mild form of IL-23-induced psoriasis.
(a) Ear thickness assessment after IL-23 intradermal injection in CD69-deficient (CD69-KO) and wild-type (WT) mice. Dermal, epidermal and Ki67+ nuclei quantifications in histological sections of ears from CD69-KO and WT mice collected after 10 doses of IL-23. (b) Representative H&E sections. Scale bars indicate 100µm (10X), and 50µm (20X). (c) Representatives IHC of KI67, Ly6G and F4/80 in the skin of CD69-deficient and wild-type mice. Scale bars represent 25µm (40X) and 50µm (20X). (d) Dot plots of CD45+ population, and CD45+CD11b+CD11c- gated cells showing GR1+ granulocytes and F4/80+ monocytes and macrophages infiltrating the ears of CD69-deficient and wild-type mice upon 10 doses of IL-23. Numbers adjacent to outlined areas indicated percent. Bars indicated quantification of these populations in the ear skin per 104 live gated cells. Data are representative of three experiments (n=5 mice) and was analyzed using unpaired t test (p<0.0001) for ear swelling, one way ANOVA with newman-keuls multiple comparison post-test for histological assessments, and two way ANOVA with bonferroni multiple comparison test for flow cytometry analysis. NS, not significant; * P < 0.05, ** P < 0.01, *** P < 0.001.
CD69 deficiency reduces IL-22 and STAT3 expression
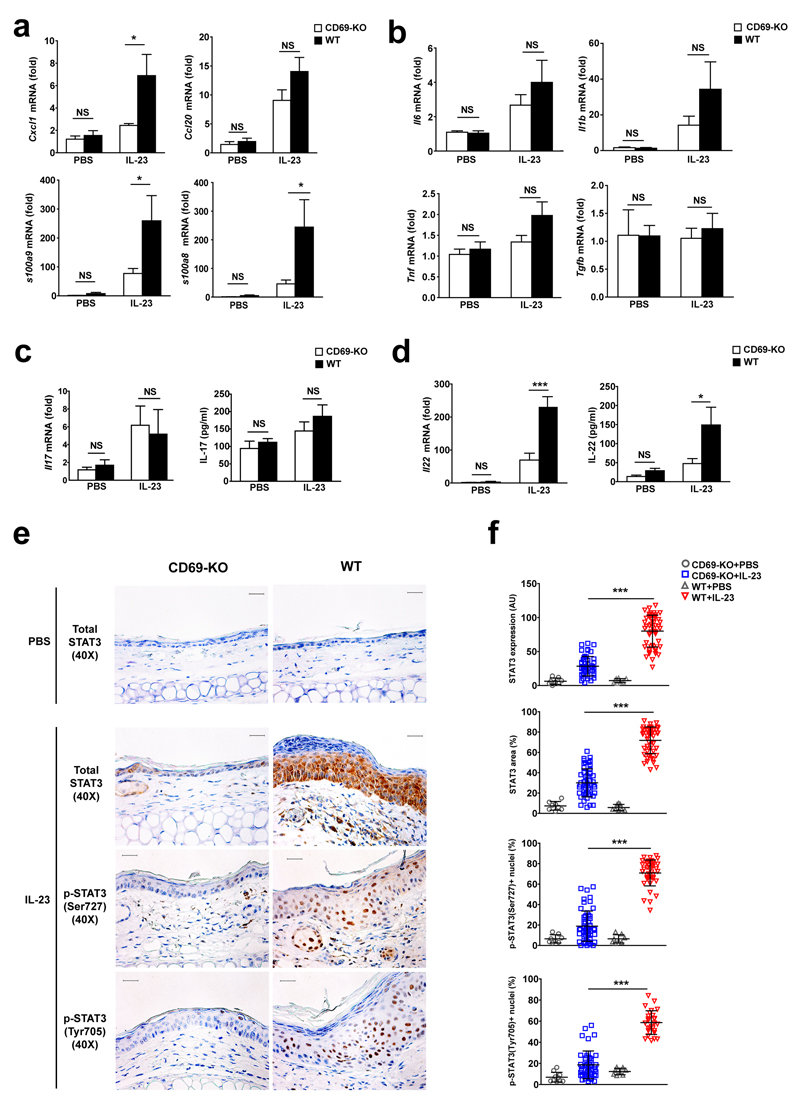
We next determined whether CD69 controls the expression of inflammatory mediators in the skin. IL-23 induced higher mRNA expression of Cxcl1, S100a8 and S100a9 in wild-type than in CD69-deficient mice, while both genotypes showed a similar increase in Ccl20 (Fig. 2a). IL-23 treatment increased the expression of Il6, Tnf, Il1b and IL-17 to a similar extent in both wild-type and CD69-deficient mice, while Tgfb expression in the skin was not altered (Fig. 2b-c). In contrast, IL-23 induced a very modest increase of IL-22 expression in the skin of CD69-deficient compared with wild-type mice (Fig. 2d). Neither genotype showed detectable expression of IL-22 or IL-17 in T cells from the ear draining lymph nodes of IL-23-injected mice after re-stimulation (data not shown). These results indicated that the weak cutaneous inflammatory response to IL-23 injection observed in the CD69-deficient mice might be related to an attenuated skin production of IL-22.
Figure 2. CD69-deficiency weakens IL-23-triggered IL-22 expression and STAT3 signaling in the epidermis.
(a) Quantitative PCR of chemoattractant factors and (b) cytokines in total mRNA obtained from ears of CD69-deficient (CD69-KO) and wild-type (WT) mice upon PBS or IL-23 treatment. (c) Quantification of IL-17A and (d) IL-22 mRNA and protein expression in mouse ear skin of IL-23 or PBS treated CD69-KO and WT mice. (e) Representative IHC staining of STAT3 and pSTAT3 (Ser727 and Tyr705) in mouse ears treated with PBS or IL-23. Scale bars indicated 25µm at 40X magnification. (f) Quantification of epidermal STAT3 expression expressed as mean of signal intensity (AU: arbitrary units) and percentage of total analyzed epidermal area; and the percentage of each pSTAT3-positive nuclei counted in the keratinocytes from PBS or IL-23-treated CD69-KO and WT mice. Data are representative of three experiments (n=5 mice per group) and data were analyzed with two-way ANOVA and bonferroni multiple comparison test. At least 5 histological images were analyzed by ear (n=5 mice) and data was analyzed using One Way ANOVA with newman-keuls multiple comparison test. NS, not significant; * P < 0.05 and *** P < 0.001.
The expression of IL-22R in skin is restricted to non-hematopoietic cells, e.g. keratinocytes and fibroblasts. In these cells, IL-22 binding to IL-22R activates STAT3 26. IL-23 ear injections caused a uniform increase of epidermal STAT3 expression and phosphorylation (Ser727 and Tyr705) in wild-type mice compared to a patched induction in CD69-deficient mice (Fig. 2e,f), indicating that CD69 controls the expression of IL-22 and STAT3.
CD69 enhances AhR and IL-22 expression
CD69 is a negative regulator of IL-17 secretion in TH17 cells 27, but its effect on IL-22 secretion is not known. This is a crucial question since IL-22 is produced by TH17 lymphocytes 13. Naïve CD4+ T cells from CD69-deficient mice polarized for 48h in vitro to TH17 cells secreted less IL-22 than wild-type cells (Supplementary Fig. 1a). Moreover, flow cytometry after intracellular staining showed that CD69-deficient TH17 cells had reduced expression of AhR compared to wild type TH17 cells (Supplementary Fig. 1b). We found that mRNA expression of AhR target genes Il22, Ahr, Ahrr, Cyp1a and Cyp1b was impaired in CD69-deficient TH17 cells compared to wild-type (Supplementary Fig. 1c).
In IL-23-driven psoriasis, IL-22 and IL-17 are secreted by dermal γδ T cells 16, 18. To characterize the resident T cell populations in the skin of both wild-type and CD69-deficient mice, we performed flow cytometry analysis of total skin cell suspensions and separated dermal and epidermal layers. These experiments revealed that CD3hi cells were dendritic epidermal γδ T cells, while 50-80 % of dermal CD3lo cells were γδ T cells (Supplementary Fig. 1d). Both CD3hi and CD3lo γδ T cell populations had high expression of CD69 in untreated skin (Supplementary Fig. 1e). Although CD69 expression is linked to the generation and migration of skin resident memory T cells 28, the percentage of skin resident epidermal (CD3hi) and dermal (CD3lo) γδ T cell populations was similar in wild-type and CD69-deficient mice (Supplementary Fig. 1f).
Simultaneous injection of intradermal IL-23 in the ears and systemic (intraperitoneal, i.p) administration of the secretion-inhibitor brefeldin A to wild-type mice promoted the accumulation of IL-22 in dermal CD3lo γδ T cells (Supplementary Figure 2a). Moreover, flow cytometry analysis of dermal γδ T cells sorted from wild-type mice and stimulated in vitro with IL-23 plus IL-1β revealed an acute peak of IL-22 (max. 24 h post-stimulation), and elevated AhR expression compared to non-stimulated cells. However, IL-17 secretion in sorted dermal γδ T cells was sustained up to 72 h post-stimulation (Supplementary Fig. 2b).
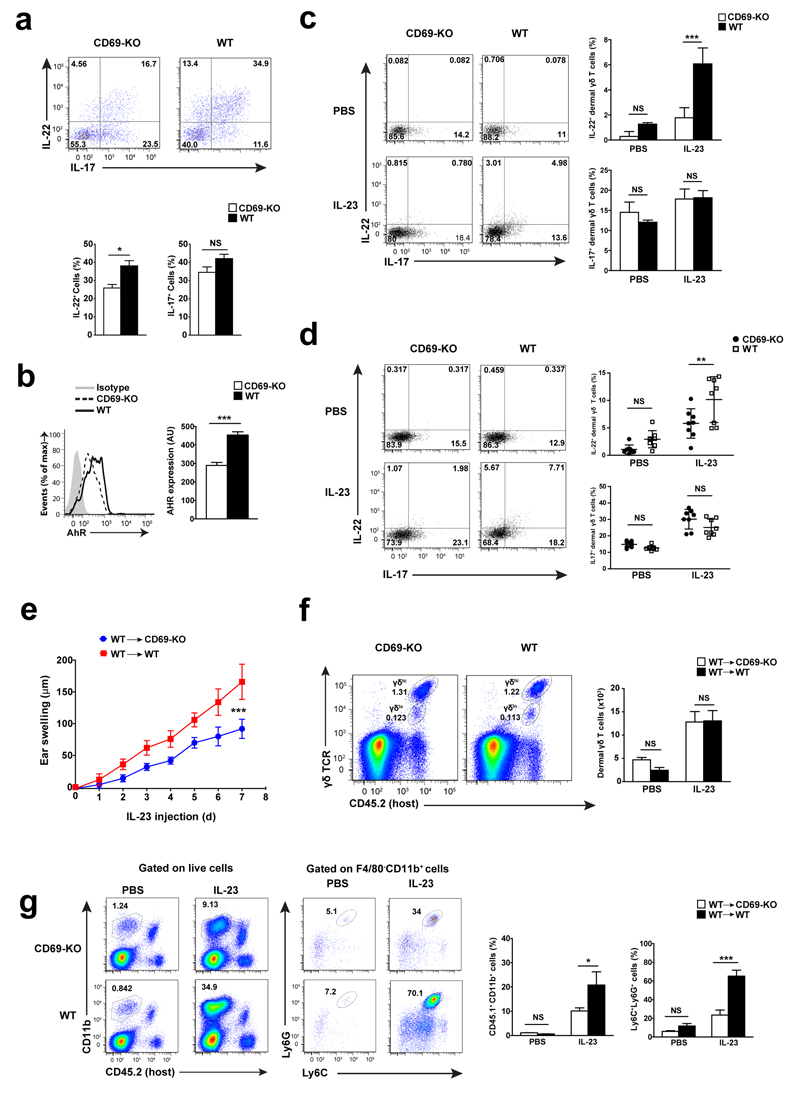
Sorted CD69-deficient dermal γδ T cells released lower amounts of IL-22 compared to wild-type dermal γδ T cells when stimulated with IL-23 and IL-1β in vitro, whereas IL-17 production was similar (Fig. 3a). Also, the expression of AhR induced by IL-23 and IL-1β in vitro stimulation was reduced in CD69-deficient dermal γδ T cells compared to wild-type cells (Fig. 3b)
Figure 3. CD69 regulates AHR and IL-22 expression in dermal resident γδ T cells.
(a) Flow cytometry analysis of dermal γδ T cells sorted from CD69-deficient (CD69-KO) and wild-type (WT) cells stimulated for 24 h in vitro with IL-23+IL-1β. (b) Representative histograms and bar charts of AhR expression in dermal γδ T cells after 24 h of in vitro stimulation with IL-23+IL-1β. At least three independent experiments of in vitro dermal γδ T cells stimulation were performed, with similar results (n=4). (c) Skin cell suspensions from the ears of mice receiving a single dose of IL-23 (12h) were stimulated in vitro with PMA and Ionomycin by 4h (n=8 mice per group). (d) Mice were injected with a single dose of IL-23 and Brefeldin A and sacrified after 12h (n=8 mice per group). Representative dot plots and quantification of IL-22 and IL-17-expressing cells (percentages) are shown. (e) Analysis of ear thickness during administration of 7 doses of IL-23 in CD69-KO and WT chimeric mice, represented as the mean ± SD; (n=5). (f) Representative dot plots of host resident dermal γδ T cells obtained from chimeric mice and quantification of total number of dermal γδ T cells detected per ears are represented by bars. (g) Percentage (%) of CD45.1+CD11b+ from live cells and CD45.1+CD11b+F480-Ly6G+Ly6C+ were indicated. Bars denote the mean ± SEM and data were analyzed with unpaired t-test or two-way ANOVA and bonferroni multiple comparison test. AU, arbitrary units. NS, not significant; * P < 0.05, ** P < 0.01, *** P < 0.001.
To parse the function of CD69 in IL-22 secretion by dermal γδ T cells in vivo, we prepared skin cell suspensions 12 h post-administration of a single dose of IL-23 in the ear of wild-type and CD69-deficient mice, followed by stimulation with PMA and Ionomycin. Flow cytometry analysis showed lower IL-22 expression in CD69-deficient dermal γδ T cells compared to wild-type cells (Fig. 3c). Simultaneous intradermal injection of IL-23 and intraperitoneal administration of brefeldin A resulted in a lower expression of IL-22 in dermal γδ T cells from CD69-deficient compared to wild-type mice (Fig. 3d).
We observed a similar expression of RORγt in dermal γδ T cells from CD69-deficient and wild-type mice (Supplementary Fig. 2c). Likewise, phosphorylation of STAT3 and STAT5 following IL-23 plus IL-1β stimulation was comparable in CD69-deficient and wild-type dermal γδ T cells (Supplementary Fig. 2c). Flow cytometry analysis of skin suspensions from a knock-in IL-23R-GFP-reporter mice (IL-23R-GFP+ KI) 29 showed that dermal γδ T cells (CD3lo) were the main population of IL-23R+(GFP+) in the skin in steady-state, while epidermal γδ T cells were mostly IL-23R- (GFP-) (Supplementary Fig. 2d). Staining of IL-23R showed that the number of IL-23R+ dermal γδ T cells and their molecular density of IL-23R were similar in CD69-deficient and wild-type mice (Supplementary Figure 2e).
CD69 is known to modulate effector T cells trafficking 30. To investigate whether CD69-mediated lymph node lymphocyte egress is involved in the IL-23 skin inflammation model, we transferred wild-type (CD45.1) whole bone marrow cells intravenously into-lethally irradiated CD69-deficient and wild-type host mice (CD45.2) (Fig. 3e). After two months, animals were administered intradermal IL-23. CD69-deficient host mice displayed lower skin inflammation than wild-type host mice (Fig. 3e). Flow cytometry analysis of skin cell suspensions showed a similar increase of host-derived (CD45.2+) dermal γδ T cells in CD69-deficient and wild-type host mice upon IL-23 treatment (Fig. 3f) We observed a significant increase of the number of infiltrating CD11b+ myeloid cells and Ly6G+Ly6C+ neutrophils of donor (CD45.1+) origin in the skin of wild-type compared to CD69-deficient mice (Fig. 3g). Because skin resident dermal γδ T cells are radioresistant and proliferate in situ 20, they persisted in the bone marrow chimeric hosts, accounting for the differences between the wild-type and CD69-deficient hosts. These observations rule out a possible role for CD69+ T lymphocytes migrating from the lymph nodes as a triggering mechanism.
IL-22 and AhR mediated skin inflammation downstream of CD69
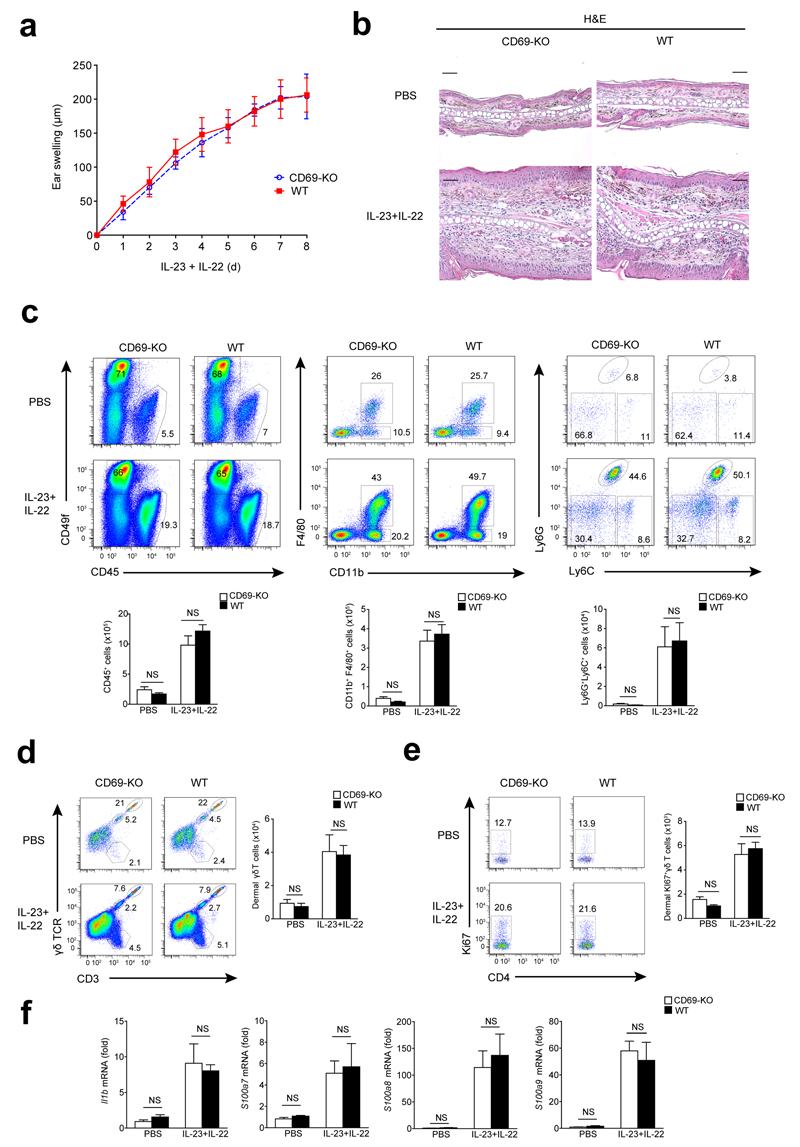
To address if the attenuated skin inflammation observed in CD69-deficient mice was due to reduced skin secretion of IL-22, we simultaneously injected IL-22 and IL-23 intradermally in wild-type and CD69-deficient mice. We observed a similar degree of ear swelling in both genotypes (Figure 4a), marked acanthosis, infiltration of myeloid cells in the dermis, proliferation rate of dermal γδ T cells, and expression of Il1b and Il22-related genes, e.g. S100a7/8/9 (Fig. 4b-f). Moreover, intradermal injection of IL-22 alone induced comparable ear swelling and expression of S100a8/9 in CD69-deficient and wild-type skin (Supplementary Fig. 3a). These observations suggest that the effect of IL-22 in the context of IL-23-induced skin inflammation is independent of CD69 expression.
Figure 4. IL-22 mediates skin inflammation downstream of CD69.
(a) Analysis of ear thickness during administration of 8 doses of IL-23 plus IL22 (500 ng of each) in CD69-deficient (CD69-KO) and wild-type (WT) mice. Data is the mean ± SD; (n=6 mice). This experiment was independently replicated with similar results. (b) Representative H&E-stained sections of PBS or IL-23+IL-22-treated ears of WT and CD69-KO. Scale bars indicated 50 µm (20X). (c) Representative dot plots indicating percentages (%) in the skin suspensions of CD45+ cells, keratinocytes (CD49f+), macrophages (CD45+CD11c-CD11b+F4/80+) and neutrophils (CD11b+F4/80-Ly6G+Ly6C+). Bar charts indicate total number of CD45+ cells, macrophages and neutrophils quantified per ears. (d) Representative dot plots indicating percentages in the skin suspensions of CD3+ populations: CD3+γδ- T cells, CD3+γδ+ dermal T cells, and CD3++γδ++ epidermal T cells. Bar chart indicates total number of dermal γδ T cell population detected per ear. (e) Ki67+ dermal γδ T cells percentages and total number in the skin is showed in the dot plots and bar chart, respectively. (f) Analysis of Il1b, S100a7-8-9 mRNA expression in the skin. Bars denote the mean ± SEM and data were analyzed with two-way ANOVA and Bonferroni multiple comparison test. NS, not significant.
A direct involvement of AhR in IL-22 secretion by dermal γδ T cells was addressed by simultaneous administration of three intradermal injections of IL-23 in the ears of AhR-deficient and wild-type mice, and brefeldin A (i.p.). Flow cytometry analysis of skin cell suspensions showed a reduced number of IL-22+ dermal γδ T cells in AhR-deficient mice compared to wild-type, while the number of IL-17+ γδ T cells were similarly increased in the skin of both genotypes (Supplementary Fig. 3b). In addition, sorted CD69-deficient and wild-type dermal γδ T cells were stimulated in vitro with IL-23+IL-1β in the presence of the AhR inhibitor CH-223191, which prevents IL-22 secretion in both genotypes (Supplementary Fig. 3c). To assess whether IL-22 secretion depended of CD69 in other γδ T subsets besides the skin, we used splenic CD27- γδ T cells, which secrete IL-17 and IL-22 upon IL-23 stimulation 31. These cells express CD69 upon IL-23 and/or IL-1β stimulation (Supplementary Fig 3d). Splenic CD27- γδ T cells from CD69-deficient mice expressed less IL-22 than wild-type cells, and this was abrogated by CH-223191 (Supplementary Fig. 3e). These observations indicate that AhR activation is required for CD69-regulation of IL-23-induced IL-22 expression.
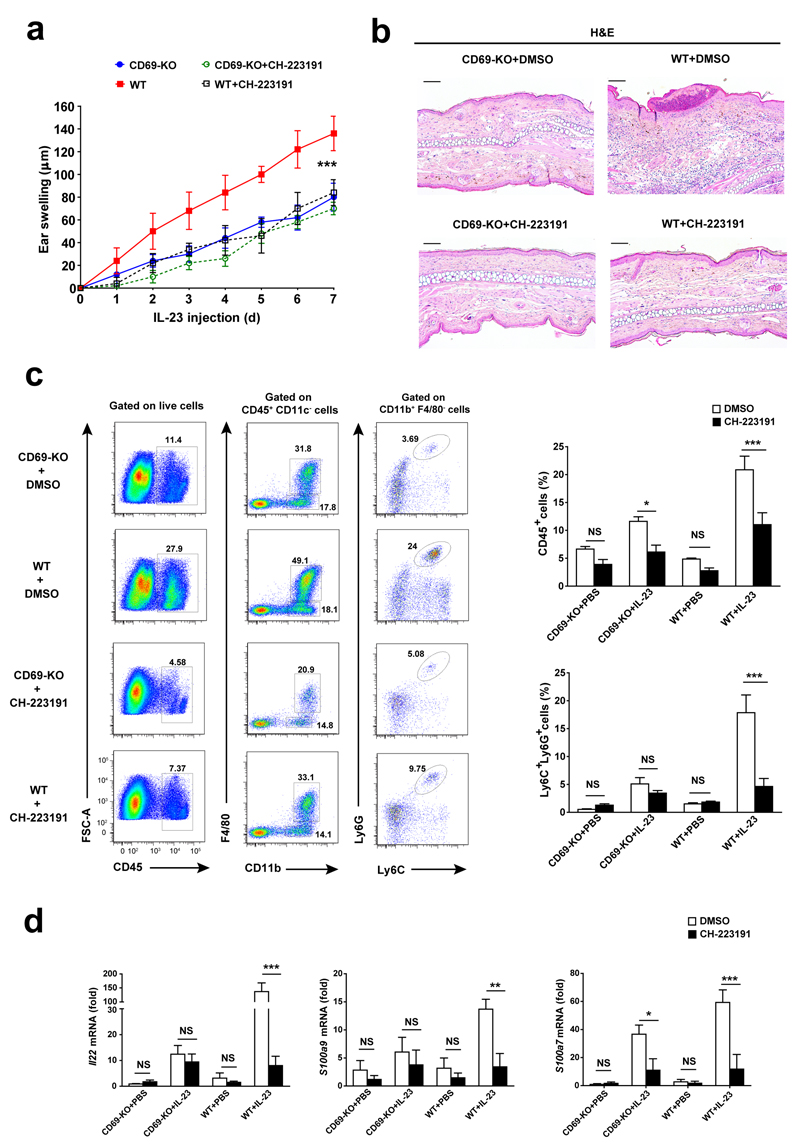
To address if AhR inhibition controlled IL-23-dependent skin inflammation, we injected IL-23 intradermally together with daily intraperitoneal administration of CH-223191 to wild-type and CD69-deficient mice. This treatment decreased inflammation in wild-type mice, but had a very modest effect in CD69-deficient mice (Fig. 5a, b). Systemic administration of CH-223191 also reduced the number of IL-23-driven Ly6G+Ly6C+ neutrophils in the wild-type skin to levels comparable to those in the CD69-deficient mice (Fig. 5c). Also, the amount of Il22 mRNA and its target genes S100a9/7 in whole skin were significantly reduced in wild-type mice receiving CH-223191+IL-23 compared to IL-23 alone, and similar to those detected in CD69-deficient mice treated with IL-23 (Fig. 5d). These results indicate that AhR and IL-22 contribute to skin inflammation controlled by CD69.
Figure 5. IL-23 skin inflammation is prevented by AhR inhibition.
(a) Analysis of ear thickness during administration of 7 doses of IL-23 in CD69-deficient (CD69-KO) and wild-type mice (WT) that received concomitantly intraperitoneal injections of CH-223191 or its vehicle (DMSO). Data is represented as the mean ± SD; (n=5 mice per group). (b) Representative H&E-stained sections of IL-23-treated ears of WT and CD69-KO mice treated with IL-23 and CH-223191 or DMSO. Scale bars indicated 100µm (10X). (c) Representative dot plots indicating CD45+ cells and the percentages of macrophages (CD45+CD11c-CD11b+F4/80+) and neutrophils (CD11b+F4/80-Ly6G+Ly6C+) from indicated gated cells. Right bar charts indicate the percentage of CD45+ cells from live-gated skin cells and the percentage of Ly6C+Ly6G+ from CD45+CD11c-CD11b+F4/80- subset. (d) Analysis of Il22, S100a9 and S100a7 mRNA expression in the skin. Bars denote the mean ± SEM and data were analyzed with two-way ANOVA and Bonferroni multiple comparison test. NS, not significant; * P < 0.05, ** P < 0.01, *** P < 0.001.
CD69 associates with LAT1-CD98 and controls amino acid uptake
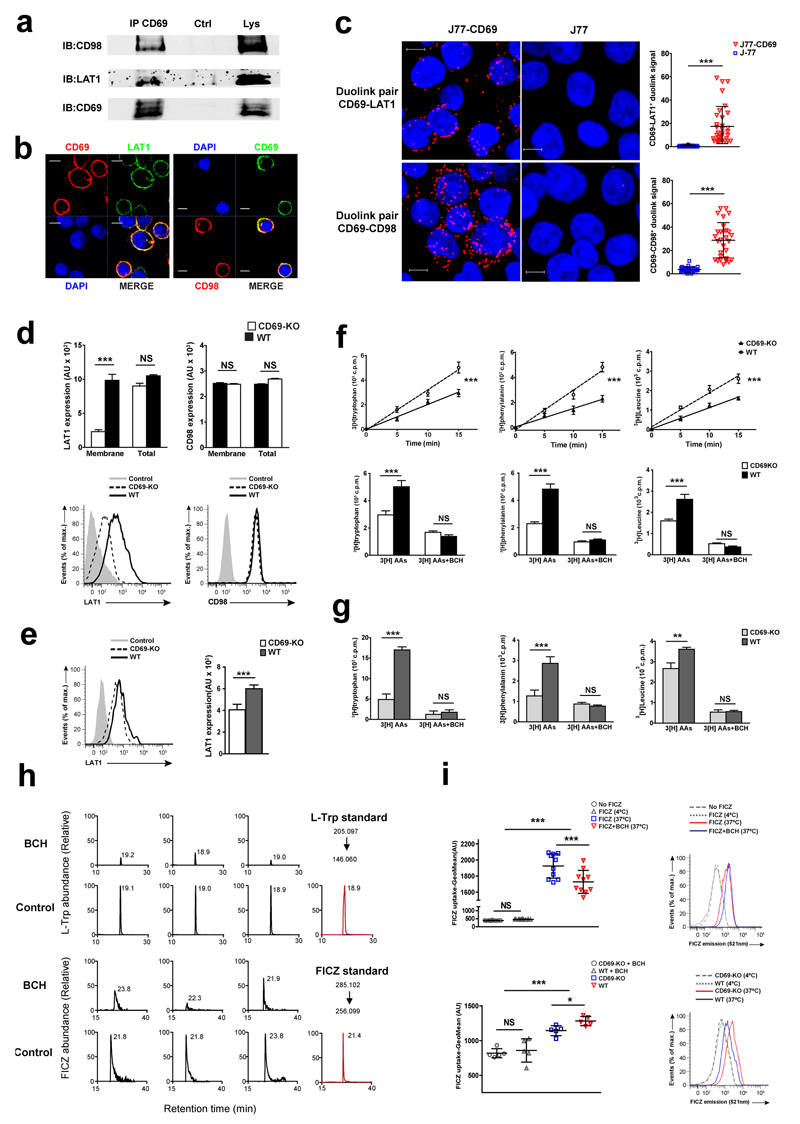
To address the mechanism by which CD69 regulated AhR-mediated IL-22 expression, we explored possible interactions of CD69 with regulators of AhR using mass spectrometry. We found that CD69 associates with several nutrient transporters, including CD98, LAT1, MOT1 and GTR1 (Table S1 and Fig. S4a). The LAT1-CD98 complex is a major intake receptor of aromatic amino acids, e.g. L-Trp, which is a source of ligands for AHR 14. Immuno-precipitation experiments showed that CD69 associates with both chains of the LAT1-CD98 transporter in human Jurkat T cells activated with PMA and ionomycin (Fig. 6a). Confocal imaging and a proximity ligation assay also indicated the association of CD69 with CD98 and LAT1 on the plasma membrane of activated Jurkat T cells (Fig. 6b,c).
Figure 6. CD69 associates with LAT1/CD98 and controls amino acid uptake.
(a) Immunoprecipitation of LAT1-CD98 with CD69 in activated J77 cells. (b) Immunofluorescence of LAT1-CD98 and CD69 in activated J77 cells. (c) In situ proximity ligation assay of CD69-LAT1 and CD69-CD98 protein interactions in non-activated J77 or stable-transfected J77-CD69 cells. Bars denote the mean ± SD of scatter plots and data was analyzed with unpaired t test. (d) Flow cytometry of LAT1 and CD98 membrane and total expression in activated CD4+ T cells and (e) sorted dermal γδ T cells. (f) Uptake of 3H-labeled amino acids by spleen/lymph-nodes γδ CD27+ T cells. Bar charts show uptake of each labeled amino acid after 15 min. (g) Uptake of labeled amino acids was assessed at 15 min in spleen/lymph-nodes-sorted γδ CD27-T cells. (h) L-Trp and FICZ were quantified by targeted mass spectrometry. These chromatograms correspond to three biological replicates and the numbers indicate retention times. MS2 extracted ion chromatograms of reference standards (in red) are illustrated to confirm retention times. (i) Flow cytometry of FICZ uptake after 16h (1µM) in wild-type activated CD4+ T cells (upper); and after 1h (100nM) by CD69-deficient (CD69-KO) and wild-type (WT) activated CD4+ T cells (lower panel). At least two experiments were performed (n=5 or 10). Data is the mean ± SEM and were analyzed with one or two way ANOVA and Newman Keuls or Bonferroni test. NS, not significant; * P < 0.05, ** P < 0.01, *** P < 0.001.
Activated CD4+ T cells from CD69-deficient mice had reduced expression of surface but not total LAT1 compared to wild-type cells, while plasma membrane and global expression of CD98 were similar (Fig. 6d). Membrane expression of LAT1 was also significantly higher in sorted wild-type dermal γδ T cells compared to CD69-deficient cells (Fig. 6e). Anti-CD3-stimulated T cells showed a similar increase in Slc7a5 (LAT1) and Slc3a2 (CD98) mRNA in wild-type and CD69-deficient CD4+ T cells (Supplementary Fig. 4b), suggesting that CD69 could be regulating LAT1 dynamics and/or its stability at the membrane. Moreover, no significant differences in the mRNA expression of other amino acid transporters was observed in activated CD4+ T cells from CD69-deficient and wild-type mice, except for an increase in Slc38a2 mRNA (encoding SNAT2 transporter), which is regulated by amino acid starvation 32, in CD69-deficient CD4+ T cells compared to wild-type (Supplementary Fig. 4c).
3H-labeled L-Trp, L-Phe and L-Leu uptake assays showed that CD69-deficient γδ T cells isolated from spleen and lymph nodes had a slower take up rate of L-Trp through LAT1 compared to wild-type cells (Fig. 6f, g). Likewise, reduced uptake of amino acids through LAT1 was detected in CD69-deficient CD4+ T cells compared to wild-type cells (Supplementary Fig. 5a). Incubation with an antibody that promotes CD69 internalization 23 also triggered LAT1 internalization in CD4+ T cells, but had no effect on surface expression of CD98 (Supplementary Fig. 5b). HEK-293 cells co-transfected with plasmids expressing CD69-GFP and LAT1-Cherry fusion proteins were incubated with labeled-anti-CD69 antibody, which showed co-internalization of CD69 and LAT1 (Supplementary Fig. 5c). Also, antibody-induced CD69 internalization impaired L-Trp and L-Phe uptake compared to control antibody-treated cells in CD4+ wild-type T cells, but had no effect on amino acid uptake in CD69-deficient cells (Supplementary Fig. 5d). These observations indicate that CD69 is associated with LAT1 and regulates its localization at the plasma membrane and amino acid transport.
Mass spectrometry analysis of Jurkat T cells cultured in L-Trp enriched medium with the LAT1 inhibitor showed reduced intracellular accumulation of FICZ, a metabolic and photoxidative derived product of L-Trp that activates AHR (Fig. 6h). Moreover, using the intrinsic fluorescence of FICZ, we observed enhanced intracellular transport of FICZ into T cells at 37°C but not 4ºC (Fig. 6i). FICZ uptake from the extracellular medium was decreased in CD69-deficient T cells compared to wild-type cells and differences were abrogated with LAT1 inhibitor Fig. 6i).
CD69-LAT1-CD98 regulate mTORC and AhR-mediated IL-22 expression
The LAT1-mediated transport of amino acids is required for activation of the mTORC pathway 33. CD69-deficient and wild-type naïve CD4+ T cells were polarized towards TH17 in vitro, and mTORC signaling was assessed by phosphorylation of mTORC1, S6 and 4E-BP1 kinases (24-96h). CD69 expression was dispensable for early activation of mTORC after 24h of TCR engagement (Supplementary Fig. 6A). However, the maintenance of mTORC signaling is influenced by amino acid uptake 33. TH17 cells from CD69-deficient mice showed impaired phosphorylation of S6 and 4E-BP1 kinases after 96h compared to wild-type cells (Supplementary Fig. 6b).
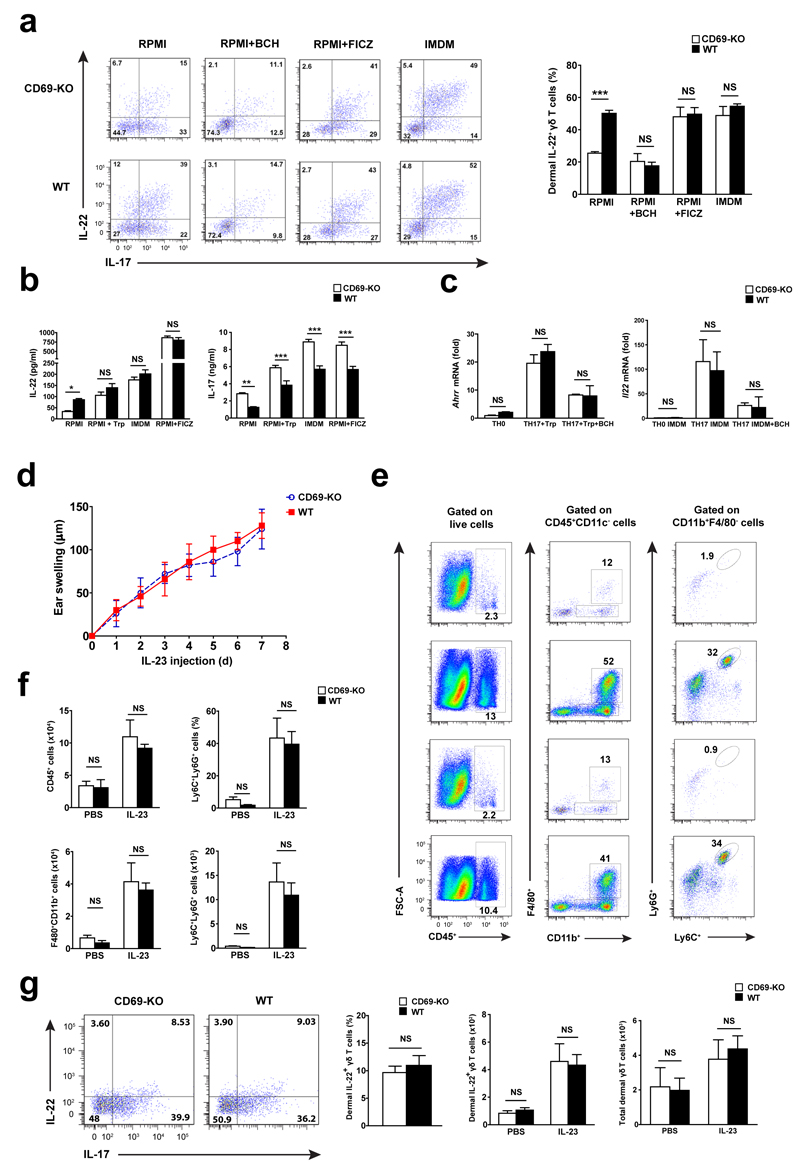
We next addressed the role of LAT1-CD98-mediated aromatic amino acid transport in AhR-dependent responses. Addition of the LAT1 inhibitor BCH abrogated IL-22 production in CD69-deficient and wild-type TH17 cells (Supplementary Fig. 6c). In contrast, addition of the AhR ligand FICZ induced IL-22 secretion in both genotypes. Culture of TH17 cells with L-Trp- enrich medium IMDM 13, restored IL-22 secretion in CD69–deficient TH17 compared to wild-type cells (Supplementary Fig. 6c). Also, IL-22 expression in wild-type dermal γδ T cells stimulated in vitro with IL-23 plus IL-1β was similar to that observed in CD69-deficient cells in the presence of BCH (Fig. 7a). Conversely, IMDM or addition of FICZ induced a similar elevation of IL-22 expression in CD69-deficient and wild-type dermal γδ T cells (Fig. 7a). Supplementation of RPMI medium with L-Trp also increased IL-22 release by CD69-deficient and wild-type TH17 cells (Fig. 7b), although differences in IL-17 secretion remained (Fig. 7b). Expression of the AhR-regulated genes Ahrr and Il22 was increased in CD69-deficient and wild-type in vitro polarized TH17 cells cultured in RPMI medium supplemented with L-Trp or IMDM, compared to cells activated with anti-CD3 and anti-CD28 antibodies (TH0 polarizing condition) (Fig. 7c). Addition of BCH decreased mRNA expression of Ahrr and Il22 in CD69-deficient and wild-type cells (Fig. 7c).
Figure 7. CD69 regulates AhR-induced IL-22 secretion in dermal γδ T cells in vitro and in vivo through control of L-Trp uptake.
(a) Dot plots of IL-22+ and IL-17+ cells from sorted dermal γδ T cells in vitro stimulated with IL-23+IL-1β in RPMI, supplemented with BCH, FICZ or in IMDM medium. Graphic bars (right) indicated the percentage of IL-22+ cells detected by flow cytometry. (b). IL-22 and IL-17 levels detected by ELISA in the supernatants of TH17 cells. (c) Ahrr and Il22 mRNA expression detected after 48h of in vitro TH0 and TH17 cells cultured cells. (d) Ear swelling of CD69-deficient (CD69-KO) and wild-type (WT) mice during administration of IL-23+L-Trp. Data is represented as the mean ± SEM; n=5 mice per group. (e) Dot plots of total CD45+, macrophages (CD11b+F4/80+) and neutrophils (CD11b+F4/80-Ly6G+Ly6C+) cells per ear in IL-23 or PBS treated ears from L-Trp-injected mice. (f) Graphic bars indicated the percentage of CD45+ cells from live-gates skin cells and the percentage of Ly6C+Ly6G+ from CD45+CD11b+F4/80- subset. Analysis of total number of infiltrating cells is also provided. (g) Dot plots and quantification of dermal γδ T cells secreting IL-22 and IL-17 from mice treated with IL-23 and L-Trp that received injection of Brefeldin A. At least two independent experiments were performed (n=5), with similar results. Bars are mean+/-SEM, and results were compared for each condition using two way ANOVA and bonferroni test. NS, not significant; * P < 0.05, ** P < 0.01, *** P < 0.001.
Psoriatic skin displays increased expression of tryptophan-degrading enzyme indoleamine 2, 3-dyoxigenase (IDO) and tryptophan 2,3-dioxygenase (TDO), which catalyze the first step in L-Trp catabolism by the kynurenine pathway, as well as L-kynureninase 34. Thus, we examined their expression in whole skin after IL-23 and IL-23+IL-22 intradermal injections. Ido and L-kynureninase mRNA were comparably induced in CD69-deficient and wild-type skin, while Tdo was not induced by IL-23 (Supplemental Fig. 6d).
To test whether uptake of L-Trp in vivo by dermal γδ T cells modulated psoriasis in CD69-deficient and wild-type mice, L-Trp was administered daily (i.p) simultaneously with IL-23 intradermal injection. L-Trp administration resulted in similar skin swelling (Fig. 7d) and comparable numbers of infiltrating CD45+CD11b+F4/80+ macrophages and CD45+CD11b+F4/80-Ly6G+Ly6C+ neutrophils in the skin of CD69-deficient and wild-type mice (Fig. 7e, f). Also, we observed similar numbers of IL-22+ and IL-17+ dermal γδ T cells in CD69-deficient and wild-type mice following L-Trp administration (Fig. 7g). Hence, these results indicate that CD69 regulates LAT1 surface expression, L-Trp uptake, FICZ intracellular increase, and subsequent AhR activation and IL-22 secretion in dermal γδ T cells (Supplemental Fig. 7).
CD69, LAT1 and IL-22 expression are upregulated in psoriatic patients
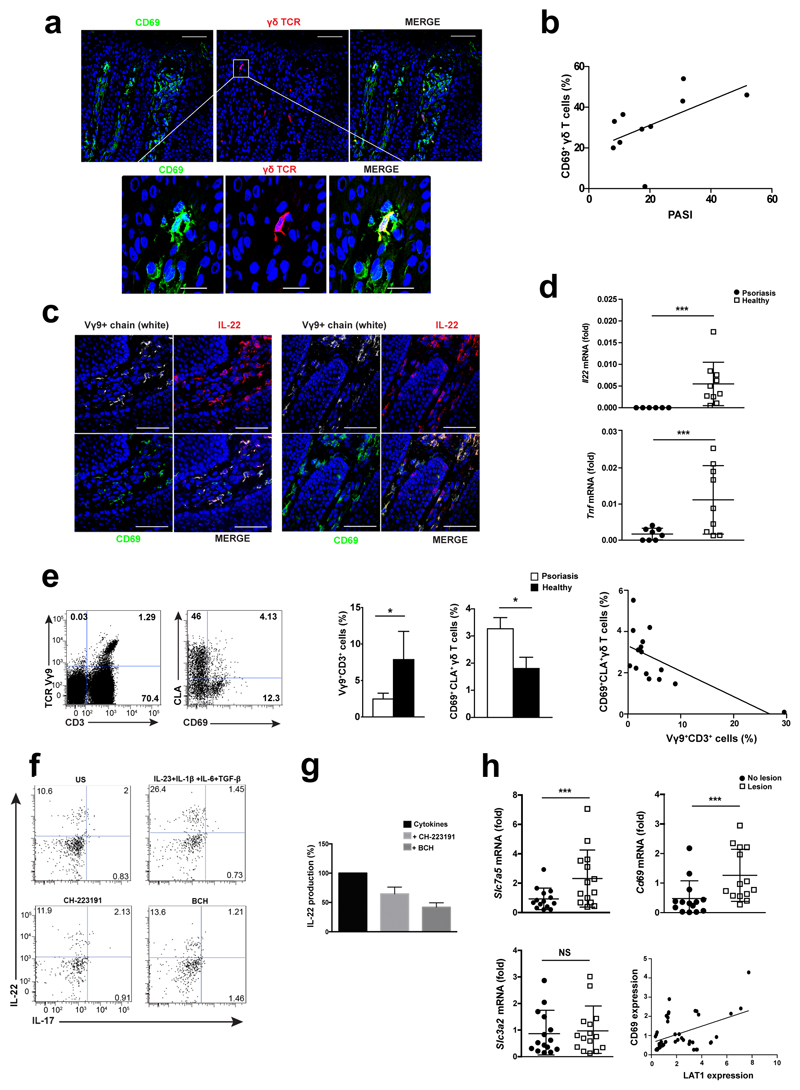
We next assessed if CD69+ γδ T cells were present in skin samples from 10 patients with moderate to severe psoriasis. CD69 expression was detected by immunofluorescence in Vγ9+ T cells in psoriatic skin lesions (Fig. 8a). The frequency of Vγ9+CD69+ T cells positively correlated with the psoriasis area severity index (PASI) (Fig. 8b). Immunofluorescence staining showed that IL-22-secreting Vγ9+ T cells in the dermal layer expressed CD69 (Fig. 8c). In agreement, a higher expression of Il22 and Tnf mRNA expression was found in psoriatic skin compared to healthy biopsies (Fig. 8d).
Figure 8. CD69 expression is detected in skin and circulating Vγ9+ T cells from psoriatic patients.
(a) Representative frozen section of human psoriatic lesion immunostained for CD69 (green), γδTCR (red) and nuclei (DAPI-blue). (b) Correlation between dermal Vγ9+CD69+ T cells (%) and the clinical Psoriasis Area Severity Index (PASI) of patients, Spearman test, r=0.64, p<0.05, (n=10). (c) Representative frozen sections from psoriatic lesions immunostained against Vγ9+ chain (white), IL-22 (red) and CD69 (green). Scale bar indicated 50µm and 25µm for zoom. (d) RT-PCR analysis of Il22 and Tnf mRNA in lesional skin (n=10) vs healthy subjects (n=9). (e) Flow cytometry of PBMCs from psoriatic (n=9) and healthy (n=6) subjects indicating CD3+Vγ9+ gated cells and expression of CLA and CD69. Bars showed percentage of CD3+Vγ9+ cells and analysis of CD69 expression in skin homing CLA+Vγ9 circulating T cells (n=14). (f-g) Peripheral γδ T cells from psoriatic patients were expanded and stimulated to release IL-22 in L-Trp supplemented medium. Addition of CH-223191 and BCH was assessed by flow cytometry and confirmed by ELISA (n=3). (h) Analysis of Slc7a5, Cd69 and Slc3a2 in the lesion and non-lesional area from psoriatic patients (n=14). Correlation analysis (r=0.34; p=0.02) between Cd69 and Slc7a5 is indicated. Each dot indicates one patient, and horizontal bars denote the mean ± SD of scatter plots. Graphic bars represent the mean ± SEM and data was analyzed with unpaired t-test. NS, not significant; * P < 0.05, *** P < 0.001.
An increased percentage of circulating CD69+CLA+Vγ9+ T cells was observed in psoriatic patients compared to healthy controls (Fig. 8e). To assess the ability of these cells to produce IL-22, circulating γδ T cells from psoriatic patients were stimulated in vitro with a cytokine cocktail (IL-23+IL-1β+IL-6+TGFβ). IL-22 expression in human Vγ9+ γδ T cells was decreased by addition of AhR and LAT1 inhibitors (Fig. 8f, g). Quantitative RT-PCR on whole skin biopsies of Slc7a5 and Cd69 mRNA expression showed their significantly increase in psoriatic lesions compared to unaffected regions, whereas Slc3a2 mRNA levels was not differentially expressed (Fig. 8h). These results show that LAT1-mediated regulation of AhR and IL-22 is observed in γδ T cells from psoriasis patients.
Discussion
The pathogenesis of psoriasis involves the cross-talk between skin-resident innate immune cells and keratinocytes, which is orchestrated by cytokines such as IL-22 and IL-17 1, 35. Here we report that CD69 associated with the transporter complex LAT1-CD98 and enhanced L-Trp uptake, a metabolic precursor of AhR ligands that promoted IL-22 secretion 13, 14. The transcriptional activity of STAT3 induced by IL-22 in keratinocytes upregulates the expression of pro-inflammatory molecules such as keratin17, S100A7, S100A8, S100A9, and several CXCL chemokines 6, 36. IL-22 also induces keratinocyte proliferation through the PI3K-Akt-mTORC signaling pathway 37. The deficient expression of IL-22 and activation of STAT3 in the skin of CD69-deficient mice provides a mechanistic link that explains the attenuated dermal inflammation and keratinocyte proliferation observed after IL-23 administration.
γδ TCR-deficient mice display attenuated psoriatic plaque formation in response to IL-23 16 and imiquimod 18. The role of CD69 as an enhancer of AhR activity and IL-22 release in γδ T cells in vitro and in vivo correlates well with clinical data that showed increased CD69 expression on IL-22+ Vγ9+ T cells from the skin and the bloodstream of psoriatic patients.
CD69 associated with the amino acid transporter complex LAT1-CD98 15, on the plasma membrane of activated T cells, controlling mTORC activation. Further studies are required to ascertain whether CD69 regulates other targets of mTORC, like autophagy route 38 and HIF-1α through AhR 39. Different regions of CD98 control amino acids uptake and β1-integrin-mediated cell proliferation 40. However, previous studies showed no differences in the proliferation rate of CD69-deficient and wild-type T lymphocytes 41, suggesting that the association of CD69 with LAT1-CD98 complex in activated immune cells modulates amino acids uptake specifically.
Cytoplasmic L-Trp behaves as a chromophore that is photoconverted into active AhR ligands, including FICZ 13. The recent characterization of a light-independent metabolic route for FICZ generation from the intracellular pool of L-Trp 14 suggests that the regulation of the precursor entrance may determine the amount of cellular FICZ and AhR activation. Our results demonstrate that LAT1-CD98 regulates the intracellular accumulation of FICZ.
AhR inhibition protects from inflammation induced by IL-23 without significant alterations of the keratinocyte layers. However, AhR depletion in keratinocytes disrupts epidermal homeostasis, enhancing psoriasis in response to imiquimod, although reduced expression of IL-22 was detected in the skin of full AhR-deficient mice compared to wild-type 42. Differences between the IL-23-induced and imiquimod-induced psoriasis models exist 2. TLR7-independent epidermal hyperproliferative responses induced by keratinocyte damage are involved in the induction of psoriasis in the imiquimod-model 18, 43. AhR controls terminal differentiation of epidermal cells and the expression of gene barrier function such as filaggrin 44 45. Hence, AhR in keratinocytes is required to skin homeostasis but its activation in inflammatory cells mediates inflammation.
CD69 did not affect IL-17 secretion by innate γδ T cells, in contrast to CD4+ TH17 T cells 27. Increased IL-17 secretion in CD69-deficient CD4+ TH17 cells compared to wild-type is due to increased phosphorylation of STAT3 and increased RORγt expression 27. This was not observed in dermal γδ T cells, which owes to the fact that transcription factors controlling CD4+ TH17 differentiation, e.g. STAT3, are not required for the development of fetal thymus-originated IL-17+(RORγt+) γδ T cells 46, 47.
Prevention of L-Trp uptake through LAT1 abrogated AhR-dependent IL-22 secretion in human peripheral γδ T cells from psoriatic patients, suggesting a possible role for dietary L-Trp uptake. Notably, in vivo administration of L-Trp increases the severity of inflammatory responses elicited by IL-23 independent of CD69 expression. Overall, these data indicate that L-Trp catabolism plays an important role in the pathophysiology of psoriasis, not only due to the regulation of kynurenine, but also by its effect on other metabolites that may contribute to skin inflammation.
In conclusion, this study establishes a biological role for CD69 in the control of amino acids uptake and the regulation of AhR activation and IL-22 expression in γδ and TH17 cells and indicates that CD69 contributes to psoriasis development.
Supplementary Material
Acknowledgments
We thank S. Bartlett for English editing and T. Hernandez and R. Brid Doohan for immunohistochemistry technical assistance. We also thanks to Dr. M. Navarro and Dr. D. Rotin for provide special reagents. This study was supported by grants from the Spanish Ministry of Economy and Competitiveness (SAF2011-25834 and SAF2014-55579-R to F.S-M, SAF2011-27330 to P.M.; and SAF2013-42850 to M.F.); grant INDISNET-S2011/BMD-2332 from Comunidad de Madrid to F.S-M; Red Cardiovascular RD 12-0042-0056 from Instituto Salud Carlos III (ISCIII), and ERC-2011-AdG 294340-GENTRIS to F.S-M. Additional grants to M.F. and F.S-M are BIOIMID (Proyecto de Excelencia Instituto Sanitario “La Princesa”) of Instituto de Salud “Carlos III”, and S-2010/BMD-2332 from Comunidad de Madrid and Ramón Areces foundation.
Footnotes
Author Contributions
D.C.V. performed mice experimentation, analyzed and interpreted data and wrote the manuscript; M.L.S. collaborated in mice experimentation, data interpretation and writing of the manuscript; H.F. performed psoriatic patients analysis, R.S and O.M.G. performed qPCR determinations; I.J.C., A.F. and J.V. performed proteomic and metabolic-mass spectrometry analyses; C.P. contributes with her expertise in radioactive assay; M.V. , M.F. and P.F.S. provided reagents and helped with the revision of the manuscript; E.D.T. provided psoriatic patient’s biopsies and its clinical diagnostic; P.M. helped to design research, provided reagents, collaborated in data interpretation and manuscript writing; and F.S.M. planned research, discussed results and collaborate to write the manuscript.
Competing Financial Interest
Authors declare no competing financial interest.
References
- 1.Di Meglio P, Villanova F, Nestle FO. Psoriasis. Cold Spring Harb Perspect Med. 2014;4(8):a015354. doi: 10.1101/cshperspect.a015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227–255. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366(13):1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 4.Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203(12):2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445(7128):648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 6.Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl) 2009;87(5):523–536. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- 7.Van Belle AB, de Heusch M, Lemaire MM, Hendrickx E, Warnier G, Dunussi-Joannopoulos K, et al. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol. 2012;188(1):462–469. doi: 10.4049/jimmunol.1102224. [DOI] [PubMed] [Google Scholar]
- 8.Prans E, Kingo K, Traks T, Silm H, Vasar E, Koks S. Copy number variations in IL22 gene are associated with Psoriasis vulgaris. Hum Immunol. 2013;74(6):792–795. doi: 10.1016/j.humimm.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Shimauchi T, Hirakawa S, Suzuki T, Yasuma A, Majima Y, Tatsuno K, et al. Serum interleukin-22 and vascular endothelial growth factor serve as sensitive biomarkers but not as predictors of therapeutic response to biologics in patients with psoriasis. J Dermatol. 2013;40(10):805–812. doi: 10.1111/1346-8138.12248. [DOI] [PubMed] [Google Scholar]
- 10.Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36(5):1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 11.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31(2):321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36(1):92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206(1):43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smirnova A, Wincent E, Vikstrom Bergander L, Alsberg T, Bergman J, Rannug A, et al. Evidence for New Light-Independent Pathways for Generation of the Endogenous Aryl Hydrocarbon Receptor Agonist FICZ. Chem Res Toxicol. 2016;29(1):75–86. doi: 10.1021/acs.chemrestox.5b00416. [DOI] [PubMed] [Google Scholar]
- 15.Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol. 2013;14(5):500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai Y, Shen X, Ding C, Qi C, Li K, Li X, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35(4):596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N, et al. Identification of a novel proinflammatory human skin-homing Vgamma9Vdelta2 T cell subset with a potential role in psoriasis. J Immunol. 2011;187(5):2783–2793. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, et al. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J Clin Invest. 2012;122(6):2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahlfors H, Morrison PJ, Duarte JH, Li Y, Biro J, Tolaini M, et al. IL-22 fate reporter reveals origin and control of IL-22 production in homeostasis and infection. J Immunol. 2014;193(9):4602–4613. doi: 10.4049/jimmunol.1401244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL, et al. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J Exp Med. 2011;208(3):505–518. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Amaro R, Cortes JR, Sanchez-Madrid F, Martin P. Is CD69 an effective brake to control inflammatory diseases? Trends Mol Med. 2013;19(10):625–632. doi: 10.1016/j.molmed.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sancho D, Gomez M, Viedma F, Esplugues E, Gordon-Alonso M, Garcia-Lopez MA, et al. CD69 downregulates autoimmune reactivity through active transforming growth factor-beta production in collagen-induced arthritis. J Clin Invest. 2003;112(6):872–882. doi: 10.1172/JCI19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin P, Gomez M, Lamana A, Matesanz Marin A, Cortes JR, Ramirez-Huesca M, et al. The leukocyte activation antigen CD69 limits allergic asthma and skin contact hypersensitivity. J Allergy Clin Immunol. 2010;126(2):355–365. doi: 10.1016/j.jaci.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Cruz-Adalia A, Jimenez-Borreguero LJ, Ramirez-Huesca M, Chico-Calero I, Barreiro O, Lopez-Conesa E, et al. CD69 limits the severity of cardiomyopathy after autoimmune myocarditis. Circulation. 2010;122(14):1396–1404. doi: 10.1161/CIRCULATIONAHA.110.952820. [DOI] [PubMed] [Google Scholar]
- 25.Radulovic K, Manta C, Rossini V, Holzmann K, Kestler HA, Wegenka UM, et al. CD69 regulates type I IFN-induced tolerogenic signals to mucosal CD4 T cells that attenuate their colitogenic potential. J Immunol. 2012;188(4):2001–2013. doi: 10.4049/jimmunol.1100765. [DOI] [PubMed] [Google Scholar]
- 26.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252(1):116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 27.Martin P, Gomez M, Lamana A, Cruz-Adalia A, Ramirez-Huesca M, Ursa MA, et al. CD69 association with Jak3/Stat5 proteins regulates Th17 cell differentiation. Mol Cell Biol. 2010;30(20):4877–4889. doi: 10.1128/MCB.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14(12):1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 29.Awasthi A, Riol-Blanco L, Jager A, Korn T, Pot C, Galileos G, et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182(10):5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 31.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10(4):427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashiwagi H, Yamazaki K, Takekuma Y, Ganapathy V, Sugawara M. Regulatory mechanisms of SNAT2, an amino acid transporter, in L6 rat skeletal muscle cells by insulin, osmotic shock and amino acid deprivation. Amino Acids. 2009;36(2):219–230. doi: 10.1007/s00726-008-0050-9. [DOI] [PubMed] [Google Scholar]
- 33.Taylor PM. Role of amino acid transporters in amino acid sensing. Am J Clin Nutr. 2014;99(1):223S–230S. doi: 10.3945/ajcn.113.070086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harden JL, Lewis SM, Lish SR, Suarez-Farinas M, Gareau D, Lentini T, et al. The tryptophan metabolism enzyme L-kynureninase is a novel inflammatory factor in psoriasis and other inflammatory diseases. J Allergy Clin Immunol. 2015;(15):01585–7. doi: 10.1016/j.jaci.2015.09.055. S0091-6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizzo HL, Kagami S, Phillips KG, Kurtz SE, Jacques SL, Blauvelt A. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J Immunol. 2011;186(3):1495–1502. doi: 10.4049/jimmunol.1001001. [DOI] [PubMed] [Google Scholar]
- 36.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174(6):3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 37.Mitra A, Raychaudhuri SK, Raychaudhuri SP. IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine. 2012;60(1):38–42. doi: 10.1016/j.cyto.2012.06.316. [DOI] [PubMed] [Google Scholar]
- 38.Finlay DK, Rosenzweig E, Sinclair LV, Feijoo-Carnero C, Hukelmann JL, Rolf J, et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med. 2012;209(13):2441–2453. doi: 10.1084/jem.20112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat Med. 2015;21(6):638–646. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fenczik CA, Zent R, Dellos M, Calderwood DA, Satriano J, Kelly C, et al. Distinct domains of CD98hc regulate integrins and amino acid transport. J Biol Chem. 2001;276(12):8746–8752. doi: 10.1074/jbc.M011239200. [DOI] [PubMed] [Google Scholar]
- 41.Lauzurica P, Sancho D, Torres M, Albella B, Marazuela M, Merino T, et al. Phenotypic and functional characteristics of hematopoietic cell lineages in CD69-deficient mice. Blood. 2000;95(7):2312–2320. [PubMed] [Google Scholar]
- 42.Di Meglio P, Duarte JH, Ahlfors H, Owens ND, Li Y, Villanova F, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40(6):989–1001. doi: 10.1016/j.immuni.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walter A, Schafer M, Cecconi V, Matter C, Urosevic-Maiwald M, Belloni B, et al. Aldara activates TLR7-independent immune defence. Nat Commun. 2013;4:1560. doi: 10.1038/ncomms2566. [DOI] [PubMed] [Google Scholar]
- 44.van den Bogaard EH, Podolsky MA, Smits JP, Cui X, John C, Gowda K, et al. Genetic and pharmacological analysis identifies a physiological role for the AHR in epidermal differentiation. J Invest Dermatol. 2015;135(5):1320–1328. doi: 10.1038/jid.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furue M, Tsuji G, Mitoma C, Nakahara T, Chiba T, Morino-Koga S, et al. Gene regulation of filaggrin and other skin barrier proteins via aryl hydrocarbon receptor. J Dermatol Sci. 2015;80(2):83–8. doi: 10.1016/j.jdermsci.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 46.Serre K, Silva-Santos B. Molecular Mechanisms of Differentiation of Murine Pro-Inflammatory gammadelta T Cell Subsets. Front Immunol. 2013;4:431. doi: 10.3389/fimmu.2013.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shibata K, Yamada H, Sato T, Dejima T, Nakamura M, Ikawa T, et al. Notch-Hes1 pathway is required for the development of IL-17-producing gammadelta T cells. Blood. 2011;118(3):586–593. doi: 10.1182/blood-2011-02-334995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.