Abstract
The analysis of immune responses in diverse malaria endemic regions provides more information to understand the host’s immune response to Plasmodium falciparum. Several plasmodial antigens have been reported as targets of human immunity. PfAMA1 is one of most studied vaccine candidates; PfRH5 and Pf113 are new promising vaccine candidates. The aim of this study was to evaluate humoral response against these three antigens among children of Lastourville (rural area) and Franceville (urban area). Malaria was diagnosed using rapid diagnosis tests. Plasma samples were tested against these antigens by enzyme-linked immunosorbent assay (ELISA). We found that malaria prevalence was five times higher in the rural area than in the urban area (p < 0.0001). The anti-PfAMA1 and PfRh5 response levels were significantly higher in Lastourville than in Franceville (p < 0.0001; p = 0.005). The anti-AMA1 response was higher than the anti-Pf113 response, which in turn was higher than the anti-PfRh5 response in both sites. Anti-PfAMA1 levels were significantly higher in infected children than those in uninfected children (p = 0.001) in Franceville. Anti-Pf113 and anti-PfRh5 antibody levels were lowest in children presenting severe malarial anemia. These three antigens are targets of immunity in Gabon. Further studies on the role of Pf113 in antimalarial protection against severe anemia are needed.
Keywords: P. falciparum, antibodies, vaccine candidates, Pf AMA1, Pf113, PfRh5, Gabon
Introduction
Plasmodium falciparum is responsible for most of the malaria-related deaths and accounts for more than 25% of children deaths in Africa [1]. The clinical manifestations of malaria are due to the repeated cycles of replication of the asexual parasite P. falciparum in the host’s red blood cells. Antigens presented by the asexual parasite stages in the bloodstream are critical in the development of protective immunity to the disease. In the course of malarial infection, red blood cells (RBC) are invaded by the merozoite during a very short moment through a complex multistep process. It begins with an initial attachment of the merozoite to the RBC surface via several protein– protein interactions, followed by an apical reorientation of the merozoite, a tight junction formation between the parasite and the host cell, and the final entry of the merozoite into the RBC [2–5]. Hence, any intervention that could block this multistep process could lead to the control of malaria parasite replication in RBC. The passive transfer of immunoglobulins from immune adults into P. falciparum-infected individuals has provided strong evidences that antibodies (Abs) play an important role in mediating protective immunity [6]. This indicates that the induction of appropriate antibody responses could be an important element in finding a way for an efficient vaccine strategy. Thus, the identification of P. falciparum antigens containing epitopes that are targets of naturally acquired immunity is important for the design of a vaccine. The Plasmodium parasite genome encodes over 5,000 proteins, a mere handful of which have been identified as candidate vaccine components [7]. More than 40 merozoite proteins involved in invasion have been identified, most of which have been shown to be targets of acquired immunity. Several of these are in early-stage clinical evaluation, making this an exciting time for the field. Among these antigens are PfAMA1, PfRh5, and Pf113.
The P. falciparum apical membrane antigen 1 (AMA1) is a membrane protein present in most apicomplexan parasites including all the Plasmodium species sequenced to date, Toxoplasma gondii, and Babesia divergens [8, 9]. PfAMA1 is a structurally conserved type I integral membrane protein (622 amino acids closely related to chimpanzee malaria, Plasmodium reichenowi) [10]. PfAMA1 is an important target and leading vaccine candidate which is presently being tested in clinical trials [11]. Antibodies to PfAMA1 are found in most people exposed to malaria, with the prevalence of antibody positivity increasing with age [12], and antibodies to PfAMA1 have been associated with reduced risk of clinical malaria in prospective studies [13, 14].
P. falciparum reticulocyte-binding protein homolog 5 (PfRH5) is a member of the family of PfRh invasion ligand recently identified among merozoite protein that is located in the rhoptries [15], secreted onto the merozoite surface prior to RBC invasion, and binds basigin, a RBC protein [16]. In contrast with other malarial antigens involved in invasion, PfRh5 exhibits a limited genetic diversity. In vitro studies have identified PfRH5 as the highest priority target in the blood-stage malaria vaccine field over the last decade [17].
The P. falciparum 113 antigen (Pf113) is thought to be located at the P. falciparum merozoite surface, a favorable location to interfere with the erythrocyte surface during RBC invasion [18]. The malarial adhesins and adhesin-like proteins predictor (MAAP) classifies Pf113 as an adhesin together with other well-characterized GPI-anchored RBC-binding proteins like MSP1, MSP2, and MSP10 [18]. Furthermore, it has been associated with protection from symptomatic malaria in Papua New Guinea (PNG) and Kenya [19]. Moreover, cumulative responses to combinations comprising 5 of the 10 top-ranked antigens, including Pf113, were associated with 100% protection against clinical episodes of malaria [20], suggesting that Pf113 is a promising candidate in the malaria vaccine development pipeline.
Gabon is an endemic area in which the malaria burden fluctuates according to the living area. After the implementation of artemisinin-based combination therapies (ACTs) in 2005, a decrease of malaria prevalence was observed, but for the past few years, a recrudescence appeared in the urban areas of Franceville and Libreville. However, prevalence did not change in rural areas [21], suggesting different profiles of epidemiology. It has been reported that ACT interventions induced a loss of acquired immunity [22] which could explain why differential epidemiology profiles exist between rural and urban areas.
Franceville is the third largest town in Gabon, with a good economic development. In 2011, a study estimated malaria prevalence among the febrile children at 17.9% [23], and a more recent study estimated the prevalence of malaria at 22.1% in febrile children [24].
The city of Lastourville had an estimated population of 8531 inhabitants in 2006. The city has a poor economic development, few industries are present. Lastourville is surrounded by forest and crossed by several rivers, among which the main river of Gabon: the river Ogooué. Malaria transmission is high year-round in Lastourville; a recent study estimated the prevalence of Plasmodium infections at 79.5% in children aged 6 to 168 months [24].
The main objective of the present study was to evaluate the naturally acquired antibody responses to three re-combinant proteins of P. falciparum (Pf113, PfAMA1, and PfRh5) among P. falciparum-infected malaria subjects in a rural area versus an urban area in Gabon.
Materials and methods
Study area and population
This study was conducted at the Amissa Bongo regional hospital of Franceville and at the medical center of Lastourville.
Franceville is an urban region of south-east Gabon (1° 37′ 15″ S, 13° 34′ 58″ E) of 56,002 inhabitants (estimated in 2010), and Lastourville is a rural region of eastern central Gabon (0° 49′ S, 12° 42′ E), the capital of the Mulundu department. The city of Lastourville had an estimated population of 8531 inhabitants in 2006. During 2015, children reporting to the pediatric ward aged 0 to 156 months were included in the study.
Blood samples
Venous blood (2.0–5.0 ml) was collected into ethylenediaminetetraacetic acid (EDTA)-containing vacutainer tubes. Of the 210 children included in this study, 104 were from Franceville and 106 from Lastourville. Blood smears were stained with Giemsa according to the Lambarené method for microscopic P. falciparum identification and quantification [25]. All slides were examined by two well-trained microscopists from the Centre International de Recherches Médicales de Franceville and two well-trained microscopists from the Centre Médical de Lastourville. Blood samples were centrifuged, and plasma samples were aliquoted and stored at –80 °C until use.
Antigens
The P. falciparum apical membrane antigen (PfAMA1), P. falciparum reticulocyte-binding homologue (PfRh5), and P. falciparum 113 (Pf113) were evaluated in this study. PfAMA1 and PfRh5 were provided by the Welcome Trust Institute of London. Recombinant P. falciparum proteins were expressed by transient transfection of HEK293 cells from the 3D7 strain using expression plasmids described in Crosnier et al. [26]. Recombinant PfRH5 was processed when expressed in HEK293 media supplemented with fetal calf serum; this processing was reduced by using HEK293 cells adapted to serum-free media and was prevented, when necessary, by the addition of 2 to 10 μg/ml aprotinin. Briefly, all plasmids were chemically synthesized using codons optimized for expression in human cells, potential N-linked glycosylation sequons were mutated, and a C-terminal rat Cd4d3+4 tag was appended. The monomeric “bait” proteins were enzymatically monobiotinylated by cotransfection with a plasmid encoding a secreted BirA enzyme as described in Bushell et al. [27]. Proteins were purified from supernatants using an ÄKTA Express or ÄKTA pure (GE Healthcare).
A recombinant form of Pf113 was produced in the course of this study. Briefly, a 616 amino acid domain of the Pf113 protein devoid of GPI anchor signal (D283-E899) was chosen to be expressed. The nucleotide sequence encoding for this Pf113 domain was amplified by PCR by using total FcB1 genomic DNA prepared as previously described, as template [28]. The PCR product was subcloned downstream of the trc promoter in plasmid pTrCHis2B, with in frame fusion to a C-terminal poly-Histidine (6xHis) tag for rapid purification affinity chro-matography on His GraviTrap column and detection with an anti-His antibody (Sigma-Aldrich). This Pf113 recombinant domain was expressed in Escherichia coli (E. coli) Rosetta 2 DE3, purified from supernatants on HisTrap HP columns using an ÄKTA pure (GE Healthcare). Purified recombinant Pf113 was migrated on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) and then transferred on nitrocellulose membranes for immunoblot staining, using anti-His tag antibodies (Sigma-Aldrich, St. Louis, USA).
Measurement of antibody levels
Enzyme-linked immunosorbent assay (ELISA) was performed to evaluate levels of malaria-specific antibodies from the patients’ plasma. Immunosorbent plates (Nunc-MaxiSorp®, Thermo Scientific) were coated with each antigen (5 ng/μl for PfAMA1, 20 ng/μl for PfRh5, or 15 ng/µl for Pf113), diluted in phosphate-buffered saline (PBS) in coating buffer overnight at 4 °C or 3 h at 37 °C, and then washed four times with PBS (pH 7.4) containing 0.05% Tween 20 (PBS-T). The plates were blocked with PBS containing 3% skim-milk for 1 h at 37 °C and washed four times with PBS-T. Plasma samples were diluted 1:100 in PBS containing 3% skim-milk, added in triplicate to microtiter plates (100 µl/well), and incubated at 37 °C for 2 h. The plates were then washed four times in PBS-T and incubated with peroxidase-conjugated mouse anti-human antibodies (Sigma-Aldrich, St. Louis, USA) diluted to 1:15,000 in PBS with 1% skim-milk for 1 h at room temperature. The plates were washed four times with a PBS-T washing solution. Tetramethylbenzidine substrate (TMB) solution (Thermo-Scientific, USA) was added to each well and incubated for 30 min. The reaction was stopped with 25 µl of 1 M sulfuric acid (H2SO4) per well. The plates were read at 450 nm with microplate reader (Sunrise™, Tecan). During the analyses, a pool of ten (10) sera from non-exposed donors, negative for P. falciparum infections, was used as negative controls and the washing solution PBST was used as a blank control. Microtiter plates were read using Sunrise™, Tecan at 450 nm. Test was considered positive if the sample optical density (OD) mean was >OD450 mean + 3 SD of negative controls (non-exposed donors). Antibody levels were expressed in arbitrary units (AU), where an AU of 0 is defined as the mean optical density (OD) value plus 3 standard deviations for ten malaria-naive European donors. Individuals with antigen specific AU < 0 or AU > 0 were defined as non-responders or responders to that antigen, respectively. Each sample was tested in triplicate.
Statistical analysis
To compare multiple groups of data, the non-parametric Kruskal-Wallis test was used. The Mann-Whitney U non-parametric test was used to test for differences in variables between two groups. The association of antibody levels with malaria protection was evaluated by logistic regression. Thus, the analysis was performed including age and antibodies showing an association or trend toward association with different groups in univariate analysis. p < 0.05 was considered as indicating a significant association. All statistical analyses were performed using SPSS version 17.0 for windows (SPSS Inc., Chicago, USA).
Ethic statements
The parents or legal guardians gave their written informed consent before each child’s enrollment in the study, which was approved by the Gabonese National Research Ethics Committee. This study was authorized by the Ministry of Health and has received the agreement of the National Ethics Committee on Research (authorization number: PROT N°0023/2013/SG/CNE).
Results
Clinical and biological characteristics of the study population
Table 1 summarizes the main characteristics of all children recruited for this study. A total of 210 children aged between 6 and 156 months were included: 71 children had P. falciparum infection (12 [11.5%] from Franceville and 59 [55.7%] from Lastourville), and 139 were free of malaria parasites (92 from Franceville and 47 from Lastourville).
Table 1.
Demographical and hematological parameters of included patients (mean ± SD)
| Lastourville children | Franceville children | |||||
|---|---|---|---|---|---|---|
| Infected (n = 59) | Uninfected (n = 47) | p | Infected (n = 12) | Uninfected (n = 92) | p | |
| Age (months) | 65.17 ± 5.3 | 48.36 ± 5.9 | 0.012 | 70.15 ± 10.56 | 46.71 ± 4.59 | 0.016 |
| Haemoglobin (g/dl) | 9.14 ± 0.25 | 10.5 ± 0.24 | <0.0001 | 11.58 ± 0.4 | 11.35 ± 0.3 | 0.9 |
| Red blood cells (106 cells/mm3) | 3.88 ± 0.12 | 4.6 ± 0.14 | <0.00001 | 3.97 ± 0.15 | 3.86 ± 0.12 | 0.6 |
| White blood cells (103 cells/mm3) | 8.95 ± 0.62 | 10.1 ± 1.22 | 0.38 | 6.75 ± 0.75 | 10.7 ± 0.86 | 0.036 |
| Platelets (105 cells/mm3) |
1.54 ± 0.14 | 3.17 ± 0.2 | <0.000001 | 2.04 ± 0.35 | 4.24 ± 0.77 | 0.015 |
| Parasitemia (parasites/μl) | 4529 ± 515 | 57727 ± 31103 | ||||
Age, leucocytes counts, hemoglobin concentrations, and parasite densities in uninfected and infected children. Plasmodium falciparum-exposed children negative for parasites in thick blood smears for P. falciparum were defined as uninfected children. SD = standard deviation
In the rural area, mean parasitemia was 4529 ± 515 parasites/μl. Infected children were older (65.17 ± 5.3 months) than uninfected children (48.3 ± 5.9 months, p = 0.012). Infected children had significant lower hemoglobin levels (9.14 ± 0.25 g/dl) than uninfected children (10.5 ± 0.24 g/dl), p < 0.0001. Red blood cell [(3.88 ± 0.12) · 106 cells/mm3] and platelet [(1.54 ± 0.14) · 105 cells/mm3] counts were also significantly lower in infected children than in uninfected children [(4.6 ± 0.14) • 106 cells/mm3 and (3.17 ± 0.2) · 105 cells/mm3, respectively, p < 0.00001]. There was no significant difference in the white blood cells counts between infected children [(8.95 ± 0.62) · 103 cells/mm3] and uninfected children [(10.1 ± 1.22) · 103 cells/mm3, p = 0.38].
However, in the urban region of Franceville, high levels of parasite density were found (mean of 57,727 ± 31,103 parasites/µl), infected children were also older (70.15 ± 10.56 months) than uninfected children (46.7 ± 4.6 months, p = 0.016). Infected children had significantly lower white blood cell [(6.75 ± 0.75) · 103 cells/mm3] and platelet [(2.04 ± 0.35) · 105 cells/mm3] counts than uninfected children [(10.7 ± 0.86) · 103 cells/mm3, (4.24 ± 0.77) · 105 cells/mm3, p = 0.036 and p = 0.015, respectively]. There was no difference regarding hemoglobin levels and red blood cell counts between the two clinical groups. The parasite densities found in Franceville (57,727 ± 31,103 parasites/µl) were stronger than in Lastourville (4529 ± 515 parasites/µl, p = < 0.00001, Table 1).
Anti-PfAMA1, Pf113, and PfRh5 IgG responses according to living area and age in children
Antibody responses against PfAMA1, Pf113, and PfRh5 were assessed in the plasma samples from Lastourville and Franceville children. When analyzing the data between the two areas, the Lastourville children had higher levels of both anti-PfAMA1 (0.67 ± 0.1 AU) and anti-PfRh5 (0.1 ± 0.02 AU) antibodies compared to the Franceville children (0.27 ± 0.06 AU for PfAMA1, 0.065 ± 0.02 AU for PfRh5; p < 0.0001 and p = 0.006, respectively; Fig. 1A and C). However, no such difference was seen for anti-Pf113 between the two living areas (p > 0.05, Fig. 1B). When comparing infected and uninfected children from Lastourville, there was no significant difference for the three antigens between these groups (Fig. 2A, B, and C). However, in Franceville, infected children had significant higher levels of anti-PfAMA1 antibodies (0.72 ± 0.3 AU) compared to uninfected Franceville children (0.21 ± 0.05 AU; p = 0.001, Fig. 2D). No such difference was seen for anti-Pf113 and anti-PfRh5 antibody levels between these groups in the Franceville population (Fig. 2E and F).
Fig. 1.
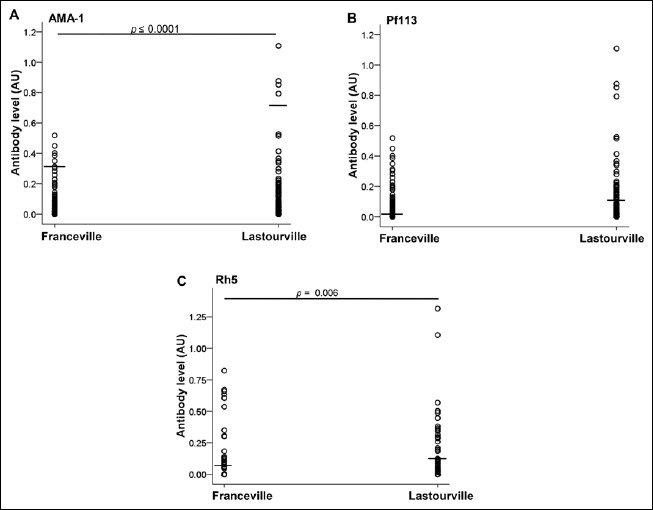
Malaria-specific PfAMA-1, Pf113, and PfRh5 antibodies in Franceville and Lastourville children. Blood plasma levels from 210 children were analyzed for malaria-specific Pf AMA-1 (A), Pf113 (B), and PfRh5 (C) antibodies. The children were subdivided according to the life area
Fig. 2.
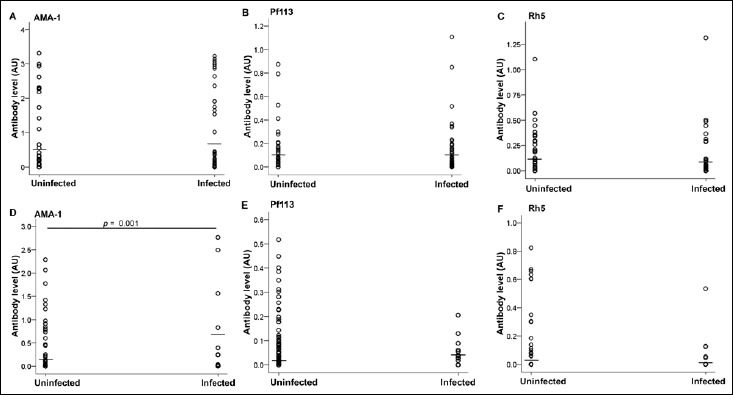
Distribution of malaria-specific PfAMA-1, Pf113, and PfRh5 antibodies in response to Plasmodium falciparum exposition in Franceville and Lastourville children. Plasma concentrations of anti-PfAMA-1 (A), anti-Pf113 (B), and anti-PfRh5 (C) in Lastourville children and anti-PfAMA-1 (D), anti-Pf113 (E), and anti-PfRh5 (F) in Franceville children were quantified in P. falciparum-uninfected and -infected children
When analyzing the data according to age categories, the Lastourville children aged 0 to 6 months exhibited significant higher titers of anti-PfAMA1 antibodies (1.47 ± 0.64 AU) as compared to the Franceville children of the same age group (0.17 ± 0.13 AU; p = 0.048, Table 2). The same differences were also found when comparing the children aged 61 to 108 months from the two localities for anti-PfAMA1 antibodies (0.88 ± 0.22 AU for Lastourville, 0.24 ± 0.12 AU for Franceville, p = 0.004) and for anti-PfRh5 antibodies (0.13 ± 0.049 AU for Lastourville, 0.01 ± 0.006 AU for Franceville; p = 0.006, Table 2). We used logistic regression to assess the associations between anti-PfAMA1, Pf113, PfRh5 specific antibody titers, age, and clinical malaria. Anti-PfAMA1 (p = 0.017) and age (p = 0.007) were associated with protection of malaria. An increase in anti-PfAMA1 levels was associated with a 0.1-fold (CI 0.02–0.16) decrease in the risk of malaria (data not showed).
Table 2.
Distribution of malaria-specific PfAMA-1, Pf113, and PfRh5 antibodies according to age in Franceville and Lastourville children
| ALMA-1 | Pf113 | Rh5 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Franceville | Lastourville | p | Franceville | Lastourville | p | Franceville | Lastourville | p | |
| 0–6 months |
0.17 ± 0.13 | 1.47 ± 0.64 | 0.048 | 0.12 ± 0.06 | 0.38 ± 0.21 | n.s. | 0.1 ± 0.1 | 0.45 ± 0.43 | n.s. |
| 7–24 months |
0.14 ± 0.006 | 0.37 ± 0.17 | n.s. | 0.072 ± 0.015 | 0.068 ± 0.023 | n.s. | 0.051 ± 0.023 | 0.061 ± 0.024 | n.s. |
| 25–60 months |
0.31 ± 0.14 | 0.56 ± 0.16 | n.s. | 0.042 ± 0.012 | 0.065 ± 0.015 | n.s. | 0.045 ± 0.026 | 0.1 ± 0.025 | n.s. |
| 61–108 months |
0.24 ± 0.12 | 0.88 ± 0.22 | 0.004 | 0.09 ± 0.029 | 0.19 ± 0.048 | n.s. | 0.01 ± 0.006 | 0.13 ± 0.049 | 0.006 |
| 109–156 months |
0.58 ± 0.26 | 1.04 ± 0.35 | n.s. | 0.056 ± 0.024 | 0.12 ± 0.09 | n.s. | 0.21 ± 0.08 | 0.058 ± 0.04 | n.s. |
n.s.: not significant
When comparing children from Lastourville, anti-PfAMA1 and anti-Pf113 responses were significantly different between age groups (p = 0.016 and 0.024, respectively, Fig. 3A and 3B). The highest responses against PfAMA1 (1.47 ± 0.6 AU) and Pf113 (0.38 ± 0.2 AU) were found in children aged under 6 months and then it decreased in children aged from 7 to 24 months (anti- PfAMA1 = 0.37 ± 0.17 AU and anti-Pf113 = 0.07 ± 0.02 AU; p = 0.03 and p = 0.013, respectively). When comparing different age groups together for the response to the same antigen, the children aged under 6 months had higher anti-Pf113 titers (0.38 ± 0.2 AU) compared to the children aged 25–60 months (0.065 ± 0.014 AU) and those aged 109–156 months (0.12 ± 0.09 AU), p = 0.03 (Fig. 3A). However, higher anti-Pf113 titers were observed among children aged to 61–108 months (0.19 ± 0.047 AU) as compared to children aged to 7–25 months (0.067 ± 0.02 AU, p = 0.03). The same difference was also seen when comparing the children aged to 61–108 months with the children aged to 25–60 months (p = 0.013). When the anti-PfAMA1 IgG response was compared, children exhibited an age-dependent increase of anti-PfAMA1 titers after 24 months of age (Fig. 3B). Significant differences were seen between age groups of 7–24 (0.37 ± 0.17 AU), 25–60 (0.56 ± 0.16 AU), 61–108 (0.88 ± 0.22 AU), and 109–156 (1.042 ± 0.35 AU) months, with p = 0.013 for 7–24 vs. 61–108 months, 0.002 for 7–24 vs. 109–156 months, and 0.04 for 25–60 vs. 109–156 months, respectively, p < 0.04. Anti-PfRh5 levels did not show a significant difference between all the age groups (p > 0.05; data not showed). We simultaneously tested all factors (anti-PfAMA1, Pf113, PfRh5, and age) for association with clinical malaria. The multivariate analysis detected no association between antibody levels and malaria protection. In Franceville, there were no statistically significant differences for any of the antigens tested between all the groups. However, the group aged 109 to 156 months exhibited significant higher anti-PfRh5 titers (0.22 ± 0.09 AU) than those of the 7 to 24 months age group (0.054 ± 0.03 AU; p = 0.02, Fig. 3C).
Fig. 3.
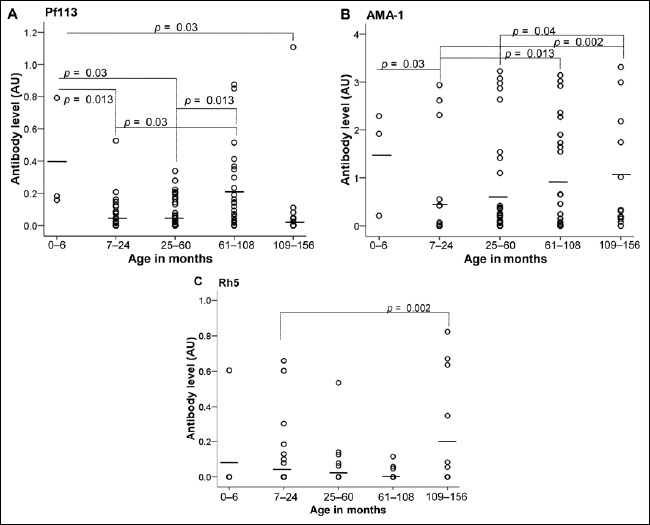
Levels of malaria-specific PfAMA-1, Pf113, and PfRh5 antibodies in Franceville and Lastourville children according to age. Plasma concentrations of anti-Pf113 (A) and anti-AMA-1 (B) in Lastourville children and anti-Rh5 (C) in Franceville children were quantified. P. falciparum-exposed infants negative for parasites in blood smears for P. falciparum were uninfected children
Anti-PfAMA1, Pf113, and PfRh5 antibody responses according to parasitemia and clinical groups in children
Infected children from Lastourville were investigated regarding P. falciparum density to determine the association between parasitemia and the production of antibodies. There was no significant difference between anti- PfAMA1 and anti-Pf113 responses, according to the parasite density. We also compared antibody levels according to parasite densities in Franceville. This analysis revealed no significant difference of antibody levels for the three P. falciparum antigens in all parasites density groups (Fig. 4).
Fig. 4.
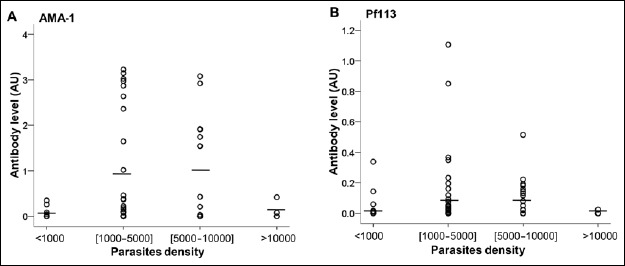
Distribution of antibodies (anti-PfAMA-1 and anti-Pf113) concentrations according to the parasitemia. Plasma concentrations of malaria-specific antibodies were quantified in P. falciparum-infected Lastourville children. P. falciparum-infected children were subdivided into four groups based upon the parasite density. The very low density group was characterized by parasite density <1000 parasites/μl of blood. The low density group was defined by parasite density included between 1000 and 5000 para-sites/μl. Medium and high density groups were defined by parasite density included between 5000 and 10000 parasites/μl and >10,000 parasites/μl of blood, respectively
We then analyzed antibody levels in children with unanemic malaria (n = 18), mild malarial anemia (n = 30), and severe malarial anemia (n = 5). Although the stratification into clinical groups did not show differences of antibody responses against the three tested P. falciparum malaria antigens in Lastourville, the comparison between the separate groups revealed that children with unanemic malaria (0.17 ± 0.07 AU) and mild malaria anemia (0.1 ± 0.02 AU) exhibited higher anti-Pf113 titers than those with severe malaria anemia (p = 0.03 and p = 0.004, respectively, Fig. 5). No significant difference in the antibody levels for anti-PfAMA1 and anti-PfRh5 was observed between the clinical groups. In Franceville, only children with un-anemic malaria were observed.
Fig. 5.
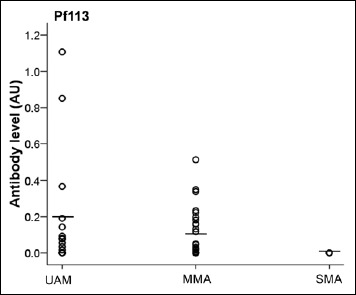
Malaria-specific antibody levels according to malaria anemia status. Plasma concentrations of anti-Pf113 antibodies were quantified in P. falciparum-infected Lastourville children. Children were subdivided into severe malaria anemia (SMA: Hb < 5.0 g/dl), mild malaria anemia (MMA: 5 ≤ Hb < 11 g/dl) and unanemial malaria (UAM: Hb ≥ 11 g/dl)
Discussion
The prevalence of malarial infection was significantly higher in the rural area (Lastourville) than in the urban area (Franceville), this result being probably due to a difference in urbanization level, equipment, housing, and access to treatment or education between both cities, consistent with previous data comparing prevalence of malaria in different areas of Gabon [29]. We observed that the mean age of infected children was significantly higher than the mean age of uninfected children. It has been demonstrated in Gabon that the mean age of children developing malaria has increased significantly and that children over 5 years old are now those who have the greatest risk to contract malaria [23, 29]. This could be the consequence of a better protection of children under five by the antimalarial programs in Gabon.
Although the etiology of anemia in tropical areas is multifactorial, P. falciparum malaria is commonly associated with anemia in children living in holoendemic malaria areas [30]. In this context, we found that hemoglobin levels were lower in infected Gabonese children, which is consistent with data from Kenya, where severe anemia was associated with parasitemia in 85% of the admissions [31]. The same result was obtained in a recent study from Nigeria [32]. Hemolysis may be due to destruction of the parasitized cells during schizogony and erythrophagocytosis in the spleen [33]. Another mechanism involved in malaria anemia is the transfer of parasite antigens such as RSP2 to uninfected red blood cells and the elimination of the erythroid lineage in the spleen and in some immune phagocytosis [34, 35].
In areas with intense P. falciparum transmission such as Gabon, clinical immunity to malaria and development of P. falciparum-specific antibodies are acquired gradually over the years, following repeated infections [36], but the acquisition of host immunity could vary according to the living areas. Data showed that children living in Lastourville, where the malaria prevalence was highest, had significant higher levels of anti-PfAMA1 and anti-PfRh5 antibodies compared to children living in Franceville; this is consistent with a study from Uganda [37]. This could be due to the frequency of contacts with the parasite leading to a better immunity boost [6]. It has been shown that naturally acquired immunoglobulin G (IgG) specific for PfRH5 and PfAMA1 increased with age and was boosted by P. falciparum transmission [19, 38]. Our findings suggest that antibody responses to PfAMA1 and PfRh5 appeared to best reflect the intensity of transmission and may be the best indicator for malaria surveillance, a result also supported by a previous study [37]. However, no such difference was seen for Pf113 in both sites, so the antibody response against Pf113 could be unrelated to the number of contacts with the P. falciparum parasite. An interpretation is that the acquisition of anti-Pf113 immunity would require fewer contacts with the parasite and would persist without any need for repeated stimulations.
Data showed antibody response classification depending on antigens. Anti-PfAMA1 response was higher than the anti-Pf113 response, which was itself higher than the anti-PfRh5 response, in terms of antibody levels. Such an observation is consistent with a recent study from Kenya and Mali, where it was observed that the IgG reactivity to PfRH5 was approximately 2 orders of magnitude lower than the IgG reactivity to PfAMA1 [17, 38]. This study shows new information regarding the Pf113 response which is established as an intermediary between the PfAMA1 and PfRh5 responses.
We confirm that the anti-PfAMA1 response was higher in infected children compared to those without P. falciparum infection; this is consistent with previous immuno-epidemiological studies [37, 38]. Anti-PfAMA1 response and age of children were also associated with protection against malaria. In previous studies, vaccination with PfAMA1 has been shown to elicit antibody responses that give good protection against homologous parasite challenges in a number of rodent and primate models [39–46].
The presence of higher levels of anti-PfAMA1 and anti-Pf113 antibodies in children under 6 months could be explained by IgG transfer across the maternal placenta and breastfeeding [47, 48].
From 7 to 24 months of age, anti-PfAMA1 levels increased with age in the area of Lastourville; this confirms the findings reported in the population of Nagongera in Uganda [37] and Kalifabougou in Mali [38].
No association between parasitemia and the production of antibodies could be established in both sites, even though the levels of antibodies were high. This supports the idea that the function of antibodies, and not just their presence, is important for protection against malaria as established by Reddy et al. [49].
The lowest level of anti-Pf113 antibody was found in children with severe malarial anemia contrary to the levels in children without anemia or mild malarial anemia, suggesting that anti-Pf113 antibodies could induce a protection against severe malarial anemia. We hypothesize that anti-Pf113 antibodies may not protect against Plasmodium parasite proliferation but rather against malarial anemia. Other studies reported that high levels of anti-MSP1 IgG1 antibodies are associated with protection against malaria attacks [50]. A more recent study from Ghana revealed that the risk of clinical malaria decreased with increasing antibody levels against GLURP R2, MSP3, AMA1, MSP1, and EBA175 [51]. However, the number of children with severe anemia was too low to draw conclusions; further studies are needed to confirm that anti-Pf113 antibodies are associated with protection against anemia.
Conclusion
This study provides the first evidences for naturally acquired immunity to PfAMA1, Pf113, and PfRh5 anti-malarial antigen vaccine candidates, in populations exposed to malaria transmission in Gabon. We established a classification of immunogenicity to these three antigens: PfAMA1 is the best, followed by Pf113, and the last being PfRh5. Antibody levels to PfAMA1 and PfRh5 appeared to be the best parameters reflecting transmission intensity and may be the best indicators for malaria surveillance. The anti-Pf113 and anti-PfRh5 responses could be associated with protection against anemia, but further studies are needed.
Acknowledgements
All the authors sincerely thank all the children and their parents for their participation in the study as well as the staff of the medical center, the hospital of Lastourville, and the hospital of Franceville. In addition, the English revision of the article by Heïdi Lançon is much appreciated.
Funding Statement
Funding sources: The study was funded by the Centre International de Recherches Médicales de Franceville (CIRMF, Gabon) and the Agence Universitaire de la Francophonie (AUF). This work was supported by a Wellcome Trust grant (number 098051).
References
- 1.WHO (2014): World Malaria Report. 2014, World Health Organization, Geneva, Switzerland [Google Scholar]
- 2.Cowman AF, Crabb BS: Invasion of red blood cells by malaria parasites. Cell 124, 755–766 (2006) [DOI] [PubMed] [Google Scholar]
- 3.Pasvol G, Wilson RJ: The interaction of malaria parasites with red blood cells. Br Med Bull 38, 133–140 (1982) [DOI] [PubMed] [Google Scholar]
- 4.Wright GJ, Rayner JC: Plasmodium falciparum erythrocyte invasion: combining function with immune evasion. PLoS Pathog 10, e1003943 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartholdson SJ, Crosnier C, Bustamante LY, Rayner JC, Wright GJ: Identifying novel Plasmodium falciparum erythrocyte invasion receptors using systematic extracellular protein interaction screens. Cell Microbiol 15, 1304–1312 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen S, Mc GI Carrington S: Gamma-globulin and acquired immunity to human malaria. Nature 192, 733–737 (1961) [DOI] [PubMed] [Google Scholar]
- 7.Doolan DL: Plasmodium immunomics. Int J Parasitol 41, 3–20 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donahue CG, Carruthers VB, Gilk SD, Ward GE: The Toxoplasma homolog of Plasmodium apical membrane antigen-1 (AMA-1) is a microneme protein secreted in response to elevated intracellular calcium levels. Mol Biochem Parasitol 111, 15–30 (2000) [DOI] [PubMed] [Google Scholar]
- 9.Montero E, Rodriguez M, Oksov Y, Lobo CA: Babesia divergens apical membrane antigen 1 and its interaction with the human red blood cell. Infect Immun 77, 4783–4793 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chesne-Seck ML, Pizarro JC, Vulliez-Le Normand B, Collins CR, Blackman MJ, Faber BW, Remarque EJ, Kocken CH, Thomas AW, Bentley GA: Structural comparison of apical membrane antigen 1 orthologues and paralogues in apicomplexan parasites. Mol Biochem Parasitol 144, 55–67 (2005) [DOI] [PubMed] [Google Scholar]
- 11.Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Ouattara A, Kone AK, Guindo AB, Traore K, Traore I, Kouriba B, Diallo DA, Diarra I, Daou M, Dolo A, Tolo Y, Sissoko MS, Niangaly A, Sissoko M, Takala-Harrison S, Lyke KE, Wu Y, Blackwelder WC, Godeaux O, Vekemans J, Dubois MC, Ballou WR, Cohen J, Thompson D, Dube T, Soisson L, Diggs CL, House B, Lanar DE, Dutta S, Heppner DG, Jr, Plowe CV: A field trial to assess a blood-stage malaria vaccine. N Engl J Med 365, 1004–1013 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chelimo K, Ofulla AV, Narum DL, Kazura JW, Lanar DE, John CC: Antibodies to Plasmodium falciparum antigens vary by age and antigen in children in a malaria-holoendemic area of Kenya. Pediatr Infect Dis J 24, 680–684 (2005) [DOI] [PubMed] [Google Scholar]
- 13.Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, Gilson PR, Murphy VJ, Anders RF, Mueller I, Beeson JG: Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun 77, 1165–1174 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osier FH, Fegan G, Polley SD, Murungi L, Verra F, Tetteh KK, Lowe B, Mwangi T, Bull PC, Thomas AW, Cavanagh DR, McBride JS, Lanar DE, Mackinnon MJ, Conway DJ, Marsh K: Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun 76, 2240–2248 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baum J, Chen L, Healer J, Lopaticki S, Boyle M, Triglia T, Ehlgen F, Ralph SA, Beeson JG, Cowman AF: Reticulocyte-binding protein homologue 5 – an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int J Parasitol 39, 371–380 (2009) [DOI] [PubMed] [Google Scholar]
- 16.Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, Uchikawa M, Mboup S, Ndir O, Kwiatkowski DP, Duraisingh MT, Rayner JC, Wright GJ: Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 480, 534–537 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas AD, Williams AR, Illingworth JJ, Kamuyu G, Biswas S, Goodman AL, Wyllie DH, Crosnier C, Miura K, Wright GJ, Long CA, Osier FH, Marsh K, Turner AV, Hill AV, Draper SJ: The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat Commun 2, 601 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obando-Martinez AZ, Curtidor H, Arevalo-Pinzon G, Vanegas M, Vizcaino C, Patarroyo MA, Patarroyo ME: Conserved high activity binding peptides are involved in adhesion of two detergent-resistant membrane-associated merozoite proteins to red blood cells during invasion. J Med Chem 53, 3907–3918 (2010) [DOI] [PubMed] [Google Scholar]
- 19.Richards JS, Arumugam TU, Reiling L, Healer J, Hodder AN, Fowkes FJ, Cross N, Langer C, Takeo S, Uboldi AD, Thompson JK, Gilson PR, Coppel RL, Siba PM, King CL, Torii M, Chitnis CE, Narum DL, Mueller I, Crabb BS, Cowman AF, Tsuboi T, Beeson JG: Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J Immunol 191, 795–809 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osier FH, Mackinnon MJ, Crosnier C, Fegan G, Kamuyu G, Wanaguru M, Ogada E, McDade B, Rayner JC, Wright GJ, Marsh K: New antigens for a multicomponent blood-stage malaria vaccine. Sci Transl Med 6, 247ra102 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assele V, Ndoh GE, Nkoghe D, Fandeur T: No evidence of decline in malaria burden from 2006 to 2013 in a rural Province of Gabon: implications for public health policy. BMC Public Health 15, 81 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogier C: Childhood malaria in endemic areas: epidemiology, acquired immunity and control strategies. Med Trop (Mars) 63, 449–464 (2003) [PubMed] [Google Scholar]
- 23.Lekana-Douki JB, Pontarollo J, Zatra R, Toure-Ndouo FS: Malaria in Gabon: results of a clinical and laboratory study at the Chinese–Gabonese Friendship Hospital of Franceville. Sante 21, 193–198 (2011) [DOI] [PubMed] [Google Scholar]
- 24.Maghendji-Nzondo S, Nzoughe H, Lemamy GJ, Kouna LC, Pegha-Moukandja I, Lekoulou F, Mbatchi B, Toure-Ndouo F, Lekana-Douki JB: Prevalence of malaria, prevention measures, and main clinical features in febrile children admitted to the Franceville Regional Hospital, Gabon. Parasite 23, 32 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Planche T, Krishna S, Kombila M, Engel K, Faucher JF, Ngou-Milama E, Kremsner PG: Comparison of methods for the rapid laboratory assessment of children with malaria. Am J Trop Med Hygiene 65, 599–602 (2001) [DOI] [PubMed] [Google Scholar]
- 26.Crosnier C, Wanaguru M, McDade B, Osier FH, Marsh K, Rayner JC, Wright GJ: A library of functional recombinant cell-surface and secreted P. falciparum merozoite proteins. Mol Cell Proteomics 12, 3976–3986 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bushell KM, Sollner C, Schuster-Boeckler B, Bateman A, Wright GJ: Large-scale screening for novel low-affinity extracellular protein interactions. Genome Res 18, 622–630 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinden RE: Plasmodium differentiation in the mosquito. Parassitologia 41, 139–148 (1999) [PubMed] [Google Scholar]
- 29.Mawili-Mboumba DP, Bouyou Akotet MK, Kendjo E, Nzamba J, Medang MO, Mbina JR, Kombila MMCORU team: Increase in malaria prevalence and age of at risk population in different areas of Gabon. Malar J 12, 3 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathanga DP, Campbell CH, Jr., Vanden Eng J, Wolkon A, Bronzan RN, Malenga GJ, Ali D, Desai M: Comparison of anaemia and parasitaemia as indicators of malaria control in household and EPI-health facility surveys in Malawi. Malar J 9, 107 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obonyo CO, Vulule J, Akhwale WS, Grobbee DE: In-hospital morbidity and mortality due to severe malarial anemia in western Kenya. Am J Trop Med Hygiene 77, 23–28 (2007) [PubMed] [Google Scholar]
- 32.Noland GS, Graves PM, Sallau A, Eigege A, Emukah E, Patterson AE, Ajiji J, Okorofor I, Oji OU, Umar M, Alphonsus K, Damen J, Ngondi J, Ozaki M, Cromwell E, Obiezu J, Eneiramo S, Okoro C, McClintic-Doyle R, Oresanya O, Miri E, Emerson PM, Richards FO, Jr.: Malaria prevalence, anemia and baseline intervention coverage prior to mass net distributions in Abia and Plateau States, Nigeria. BMC Infect Dis 14, 168 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips RE, Pasvol G: Anaemia of Plasmodium falcipar um malaria. Baillieres Clin Haematol 5, 315–330 (1992) [DOI] [PubMed] [Google Scholar]
- 34.Lekana Douki JB, Traore B, Costa FT, Fusai T, Pouvelle B, Sterkers Y, Scherf A, Gysin J: Sequestration of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A, a receptor for maternal malaria: monoclonal antibodies against the native parasite ligand reveal pan-reactive epitopes in placental isolates. Blood 100, 1478–1483 (2002) [DOI] [PubMed] [Google Scholar]
- 35.Layez C, Nogueira P, Combes V, Costa FT, Juhan-Vague I, da Silva LH, Gysin J: Plasmodium falciparum rhoptry protein RSP2 triggers destruction of the erythroid lineage. Blood 106, 3632–3638 (2005) [DOI] [PubMed] [Google Scholar]
- 36.Crompton PD, Kayala MA, Traore B, Kayentao K, Ongoiba A, Weiss GE, Molina DM, Burk CR, Waisberg M, Jasinskas A, Tan X, Doumbo S, Doumtabe D, Kone Y, Narum DL, Liang X, Doumbo OK, Miller LH, Doolan DL, Baldi P, Felgner PL, Pierce SK: A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci U S A 107, 6958–6963 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeka A, Nankabirwa J, Mpimbaza A, Kigozi R, Arinaitwe E, Drakeley C, Greenhouse B, Kamya MR, Dorsey G, Staedke SG: Factors associated with malaria parasitemia, anemia and serological responses in a spectrum of epide-miological settings in Uganda. PloS One 10, e0118901 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran TM, Ongoiba A, Coursen J, Crosnier C, Diouf A, Huang CY, Li S, Doumbo S, Doumtabe D, Kone Y, Bathily A, Dia S, Niangaly M, Dara C, Sangala J, Miller LH, Doumbo OK, Kayentao K, Long CA, Miura K, Wright GJ, Traore B, Crompton PD: Naturally acquired antibodies specific for Plasmodium falciparum reticulocyte-binding protein homologue 5 inhibit parasite growth and predict protection from malaria. J Infect Dis 209, 789–798 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amante FH, Crewther PE, Anders RF, Good MF: A cryptic T cell epitope on the apical membrane antigen 1 of Plasmodium chabaudi adami can prime for an anamnestic antibody response: implications for malaria vaccine design. J Immunol 159, 5535–5544 (1997) [PubMed] [Google Scholar]
- 40.Anders RF, Crewther PE, Edwards S, Margetts M, Matthew ML, Pollock B, Pye D: Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine 16, 240–247 (1998) [DOI] [PubMed] [Google Scholar]
- 41.Collins WE, Pye D, Crewther PE, Vandenberg KL, Galland GG, Sulzer AJ, Kemp DJ, Edwards SJ, Coppel RL, Sullivan JS, et al. : Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am J Trop Med Hygiene 51, 711–719 (1994) [DOI] [PubMed] [Google Scholar]
- 42.Crewther PE, Matthew ML, Flegg RH, Anders RF: Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect Immun 64, 3310–3317 (1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deans JA, Knight AM, Jean WC, Waters AP, Cohen S, Mitchell GH: Vaccination trials in rhesus monkeys with a minor, invariant, Plasmodium knowlesi 66 kD merozoite antigen. Parasite Immunol 10, 535–552 (1988) [DOI] [PubMed] [Google Scholar]
- 44.Narum DL, Ogun SA, Thomas AW, Holder AA: Immunization with parasite-derived apical membrane antigen 1 or passive immunization with a specific monoclonal antibody protects BALB/c mice against lethal Plasmodium yoelii yoelii YM blood-stage infection. Infect Immun 68, 2899–2906 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu H, Hodder AN, Yan H, Crewther PE, Anders RF, Good MF: CD4+ T cells acting independently of antibody contribute to protective immunity to Plasmodium chabaudi infection after apical membrane antigen 1 immunization. J Immunol 165, 389–396 (2000) [DOI] [PubMed] [Google Scholar]
- 46.Stowers AW, Kennedy MC, Keegan BP, Saul A, Long CA, Miller LH: Vaccination of monkeys with recombinant Plasmodium falciparum apical membrane antigen 1 confers protection against blood-stage malaria. Infect Immun 70, 6961–6967 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pitcher-Wilmott RW, Hindocha P, Wood CB: The placental transfer of IgG subclasses in human pregnancy. Clin Exp Immunol 41, 303–308 (1980) [PMC free article] [PubMed] [Google Scholar]
- 48.Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD: Placental transfer of immunoglobulin G subclasses. Clin Diagn Lab Immunol 1, 667–669 (1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reddy SB, Anders RF, Beeson JG, Farnert A, Kironde F, Berenzon SK, Wahlgren M, Linse S, Persson KE: High affinity antibodies to Plasmodium falciparum merozoite antigens are associated with protection from malaria. PloS One 7, e32242 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egan AF, Morris J, Barnish G, Allen S, Greenwood BM, Kaslow DC, Holder AA, Riley EM: Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J Infect Dis 173, 765–769 (1996) [DOI] [PubMed] [Google Scholar]
- 51.Dodoo D, Atuguba F, Bosomprah S, Ansah NA, Ansah P, Lamptey H, Egyir B, Oduro AR, Gyan B, Hodgson A, Koram KA: Antibody levels to multiple malaria vaccine candidate antigens in relation to clinical malaria episodes in children in the Kasena-Nankana district of Northern Ghana. Malar J 10, 108 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]


