Abstract
Introduction
Down syndrome (DS), caused by human trisomy 21 (Ts21), can be considered as a prototypical model for understanding the effects of chromosomal aneuploidies in other diseases. Human chromosome 21 (Hsa21) is syntenically conserved with three regions in the mouse genome.
Sources of data
A review of recent advances in genetic modeling and analysis of DS. Using Cre/loxP-mediated chromosome engineering, a substantial number of new mouse models of DS have recently been generated, which facilitates better understanding of disease mechanisms in DS.
Areas of agreement
Based on evolutionary conservation, Ts21 can be modeled by engineered triplication of Hsa21 syntenic regions in mice. The validity of the models is supported by the exhibition of DS-related phenotypes.
Areas of controversy
Although substantial progress has been made, it remains a challenge to unravel the relative importance of specific candidate genes and molecular mechanisms underlying the various clinical phenotypes.
Growing points
Further understanding of mechanisms based on data from mouse models, in parallel with human studies, may lead to novel therapies for clinical manifestations of Ts21 and insights to the roles of aneuploidies in other developmental disorders and cancers.
Keywords: Down syndrome, human trisomy 21, mouse models, chromosome engineering
Introduction
Human trisomy 21 (Ts21, Down syndrome, DS) is the most common chromosomal abnormality compatible with postnatal survival and occurs in one in ~691 and 1000 newborns in the USA1 and Europe,2 respectively. It is a leading genetic cause of congenital heart disease, acute megakaryoblastic leukemia and developmental cognitive deficits. It causes early onset Alzheimer-type neurodegeneration in nearly every individual with DS. The pregnancy termination rate after prenatal diagnosis of human Ts21 has not increased and the incidence rate of DS has not decreased in the last decade in countries like the USA.3 Among a constellation of DS phenotypes, some of them, such as developmental cognitive deficits and Alzheimer's disease (AD), impact both the affected individuals and their families, and are without effective treatments. After the discovery that individuals with DS carry an extra copy of human chromosome 21 (Hsa21),4,5 a subsequent major effort was to try to define subgenomic regions associated with various DS phenotypes by examining human segmental trisomies. In these experiments, data generated from individuals with segmental trisomy of Hsa21 were used to establish genotype–phenotype relationships.6–8 However, interpretation of these studies is not straightforward because some individuals carrying segmental Ts21 also have additional genomic abnormalities, such as unbalanced derivatives associated with non-Hsa21 genomic regions stemming from chromosomal translocations.6–8 Another inherent problem is that the endpoints of segmental trisomies are almost always unique among the cases. Therefore, for almost any segmental trisomy case, the sample size for a specific genotype is one, which is a major obstacle in distinguishing the contributions of trisomy, versus unique characteristics of a given subject, to a phenotype.9 For these reasons, research efforts have turned to well-controlled model organisms, particularly the mouse, to unravel the biology associated with DS.
Modeling DS at the early stage
Based on the findings that many Hsa21 gene orthologs mapped to mouse chromosome 16 (Mmu16), the first trisomic model of DS was mouse trisomy 16.10,11 However, this mutant, with the entire extra chromosome 16, is embryonic lethal and thus many important postnatal phenotypes of DS cannot be studied. Therefore, the discovery of postnatally viable Ts65Dn mice was considered as a major development in DS research.12 Ts65Dn mice carry an unbalanced derivative, Ts(1716)65Dn, of a balanced translocation, which was randomly induced by irradiation.12 The Ts(1716)65Dn chromosome consists of the entire genomic region distal to Mir155 on Mmu16 and a subcentromeric region on Mmu17, which is not syntenic to Hsa2113,14 (Supplementary Table S1). The second postnatally viable mouse model of DS is Ts1Cje, which carries an unbalanced derivative, Ts(1216)1Cje, of a balanced translocation, which was induced by gene-targeting in mouse ES cells.15 The Ts(1216)1Cje chromosome carries the entire genomic region distal to Sod1 on Mmu16 with Sod1 inactivated13,15 (Supplementary Table S1). Recent analyses showed a heterozygous deletion on Mmu12 in Ts1Cje mice, which is not syntenic to Hsa21.13,16 The phenotypes of Ts65Dn and Ts1Cje mice have been extensively characterized and, although these mice are not perfect molecular mimics of human DS, they do show many phenotypic features of the human syndrome17–21 (Supplementary Table S2).
Transchromosomal mouse models of DS
Another strategy to model DS is to generate mice carrying an actual Hsa21—thus, a ‘transchromosomal model’. Using microcell-mediated chromosome transfer, Hsa21 segments and an entire Hsa21 were introduced to mouse ES cells and mouse mutants were then generated using these cells.22–25 Among transchromosomal models, Tc1 mice carry more Hsa21 genetic materials than any other transchromosomal mouse models of DS. Probably because Hsa21 was irradiated before being transferred to mouse ES cells, the Hsa21 in Tc1 mice carries genetic alterations, including deletions, duplications and other rearrangements.26 The Tc1 mice have been extensively characterized and, like the models in the previous section, despite the presence of secondary molecular aberrations, they too show several phenotypic features similar to human DS25,27 (Supplementary Table S2).
Genetic modeling and dissection of DS using mouse mutants generated by Cre/loxP-mediated chromosome engineering
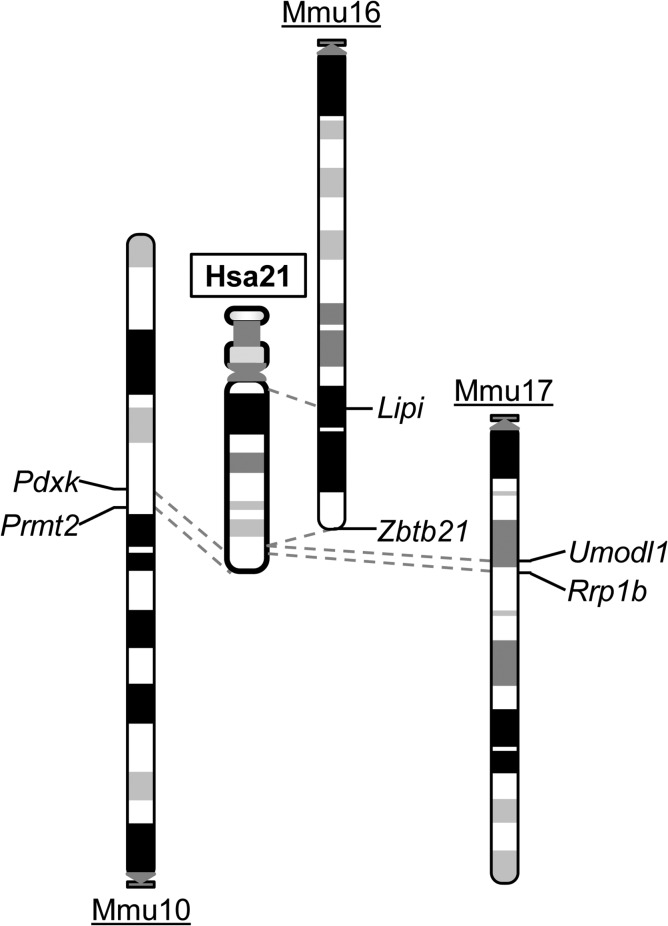
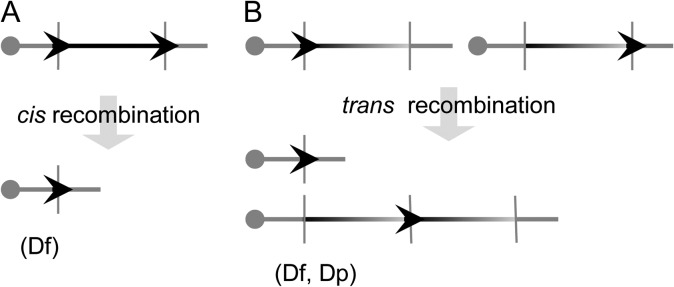
Ts65Dn and Ts1Cje mice are important viable trisomic mouse models. However, neither is a complete model. The comparison between the human and mouse genomes revealed that the regions of Hsa21 are syntenically conserved in three regions in the mouse genome located on Mmu10, Mmu16 and Mmu17 (Fig. 1) (www.ensembl.org). Only <65% of Hsa21 gene orthologs are triplicated in Ts65Dn and Ts1Cje mice. Because these models were discovered by serendipity, it will be difficult to generate additional models of DS trisomic for different Hsa21 syntenic regions by the procedures used for generating Ts65Dn and Ts1Cje mice. This difficult technical obstacle was finally overcome by the development of Cre/loxP-mediated chromosome engineering technology. This technology can be used to generate chromosomal duplications and deletions with predetermined endpoints via three steps.28 First, a loxP site is introduced into the first endpoint in the ES cell genome with a positive selection marker (Fig. 2). One of such positive selection markers is the neomycin resistance gene (neo). When G418, an antibiotic, is added to the culture medium, the cells expressing neo survive and are selected for. Next, a second loxP site is targeted to a second endpoint with an alternative positive selection marker such as the puromycin resistance gene. To induce recombination, a Cre expression vector is electroporated into double-targeted clones. If two targeted loxPs are located on the same chromosome homolog (in cis) and oriented in the same direction with respect to the centromere, recombination will result in a chromosomal deletion (deficiency, Df) (Fig. 2A). If two loxPs are located on two homologs (in trans) and oriented in the same direction, the recombination will result in a deletion (i.e. Df) and the reciprocal duplication (i.e. Dp) (Fig. 2B). The correct orientation of loxP on a chromosome can be achieved by choosing the desired orientation of the loxP in a targeting vector based on the genomic sequence. Clones carrying a desired rearrangement can be identified by analyzing the sib-selection result through positive selection drugs, and by analyzing recombination efficiencies,28 and the rearrangements can be verified by Southern analysis and fluorescence in situ hybridization. Chimeras are generated by injecting the ES cells confirmed to have the rearrangements of interest into mouse blastocysts, from which the progeny that carry the rearrangements are derived. Precise rearrangements of Hsa21 syntenic regions in mouse mutants can be verified by array-based comparative genome hybridization, and most recently by inferring DNA copy number data from NextGen sequencing.
Fig. 1.
Shared syntenies between Hsa21 and three regions in the mouse genome which are located on Mmu10, Mmu16 and Mmu17. The endpoints of the syntenic regions in mice are indicated.
Fig. 2.
The strategy to generate deletions and duplications in mouse ES cells using Cre/loxP-mediated chromosome engineering.28 To generate Dp and Df, loxP is inserted into two endpoints of an orthologous region of Hsa21 in the genome of mouse ES cells with two different positive selection markers, such as the neomycin and puromycin resistance genes. A Cre expression vector is then electroporated into the double-targeted cells to induce recombination. (A) If two loxP sites are located in cis and orientated in the same direction in relationship to the centromere, the recombination will result in a Df. (B) If two loxP sites are located in trans and orientated in the same direction in relationship to the centromere, the recombination will result in a Dp and a Df. The genotypes of engineered ES cells are confirmed by Southern blot analysis and fluorescence in situ hybridization. Afterwards, these cells are used to generate chimeras by injecting them into blastocysts. Germline transmission will lead to establishment of mouse mutants carrying a desired Dp or Df. Arrow head, loxP.
The rate-limiting factor in the aforementioned procedure is the time needed for cloning to construct targeting vectors. To improve efficiency, genomic libraries with pre-made targeting vectors were developed.29 The vectors from these libraries contain all the required genetic elements. Approximately 153 000 λ phage clones from these libraries were converted into the plasmid form. The end sequences of their genomic inserts were derived using sequencing primers located external to the cloning sites. Based on these sequences, the genome coordinates of the genomic inserts were determined. It is estimated that for every ~39 kb of the genome at least one targeting vector is available for endpoint targeting.30 The libraries have been designated the Mutagenic Insertion and Chromosome Engineering Resource (MICER). MICER substantially eliminates the need for constructing new targeting vectors for generating mutant mice carrying large genomic rearrangements. These vectors, which are available from ‘Source BioScience’, thus markedly accelerate progress in generating the desired mice. Besides engineering desired chromosomal rearrangements in ES cells and using these cells to generate mouse mutants, alternative strategies include first generating mouse mutants carrying a single targeted loxP at either desired endpoint. Afterwards, appropriate crossings of the mice carrying two targeted loxP sites with a transgenic cre mice, such as Sycp1-cre or Tg(Pgk1-cre)1Lni mice,31–33 will result in Cre/loxP-mediated trans-recombination in the compound mutants and lead to Dp and Df in the progeny.
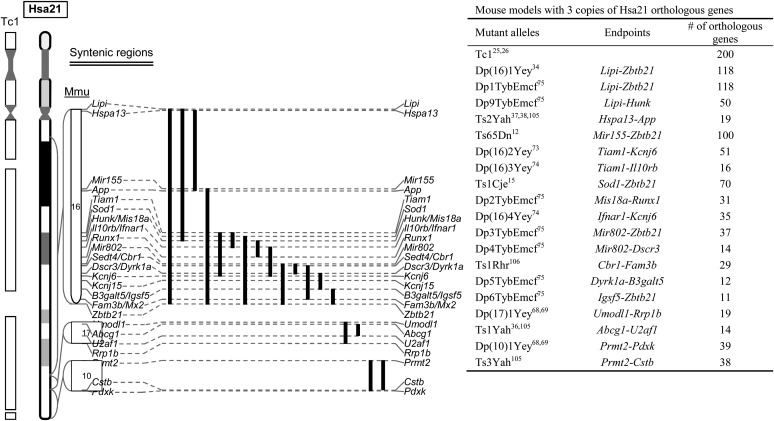
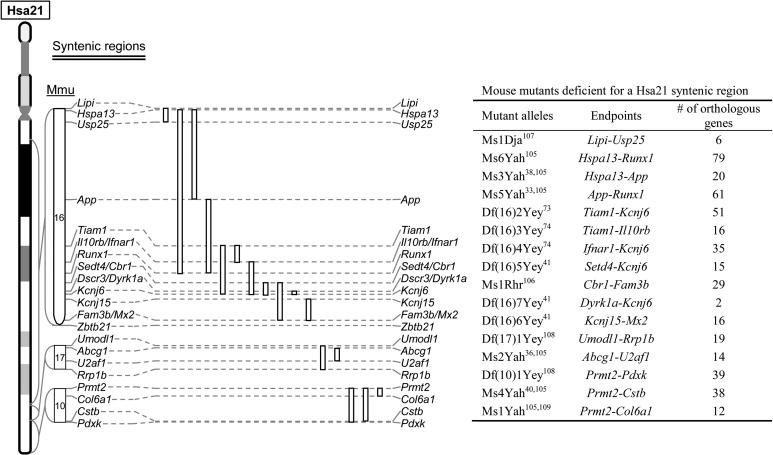
Using these procedures, several laboratories have generated a substantial number of chromosomal rearrangements in Hsa21 syntenic regions in mice (Figs 3 and 4). The duplication mutants have been used to determine if the triplication of the entire Hsa21 syntenic regions on Mmu10, Mmu16 and/or Mmu17 as well as a sub-segment within a Hsa21 syntenic region is sufficient to cause a DS-related phenotype.34–38 To determine if a given relatively small region is necessary for a phenotype, a subtractive strategy can be used by compounding a larger duplication with a deletion of the smaller sub-region.33,39–42 Using different combinations of Dp and Df mutants, the smallest genomic region can be identified for a specific DS phenotype. If this region contains 10 or more Hsa21 gene orthologs, it might be necessary to generate new Dp and/or Df mutants to further dissect the region. After a minimal critical genomic region is identified, a compound mutant could be generated to carry a duplication of the region and a null allele of the gene located within the region. The contribution of the gene to the phenotype could be established based on elimination or significant alleviation of a DS-related phenotype observed in a mouse mutant carrying the duplication alone. This type of ‘subtractive’ or ‘normalization’ strategy has been used by many labs to ascertain the contribution of individual Hsa21 genes orthologs.43–47 For this reason, mouse mutants carrying null alleles of Hsa21 gene orthologs are essential reagents for examining the contributions of these genes to DS phenotypes. Through the years, null alleles for many of these genes were generated by individual laboratories because of their importance as individual genes. The pace of null allele generation was drastically expedited after the launches of several systematic knockout (KO) projects, including ‘NIH Knockout Mouse Project’ and ‘The European Conditional Mouse Mutagenesis Program’.48–51 The current status of the KO mice for the Hsa21 gene orthologs in the public domain, including the status of targeting vector, targeted ES cells and mouse mutants, is presented in Supplementary Table S1.
Fig. 3.
Mouse mutants which carry a triplication of a Hsa21 syntenic region. A solid line represents the region triplicated for a Hsa21 syntenic region in a mouse model listed in the table.
Fig. 4.
Mouse mutants which carry a deletion of a Hsa21 syntenic region. An open line represents the region deleted for a Hsa21 syntenic region in a mouse model listed in the table.
Impact of Ts21 on DNA methylation patterns
Evidence has been continuously accumulating that the dosage increase of a Hsa21 gene or gene ortholog can contribute to a mutant phenotype associated with DS. Recent results suggest the possibility that epigenetic events may also be involved in such genotype–phenotype relationships. Gene-specific alterations in CpG methylation were first detected in blood leukocytes from adults with DS when compared to the samples from control individuals.52 The presence of such a DS-specific methylation profile was further supported by other studies on the samples isolated from Ts21 placentas, fibroblasts53–55 and more recently neural tissues.56 Interestingly, such a phenomenon was recapitulated in mouse models of DS,56 providing a system for further exploring the processes and the consequences of DS-associated methylation alterations. Moreover, the epigenetic responses to the presence of the extra genetic material are not restricted to altered CpG methylation patterns; changes in histone modifications have also been described.57 When considered as a well-defined and experimentally accessible model system, results from these studies of genetic–epigenetic interactions in human Ts21 and in the mouse lines with engineered genomic rearrangements will likely have important implications for understanding analogous genetic–epigenetic interactions in other developmental disorders associated with aneuploidies as well as in human cancers in which aneuploidies are often a hallmark.58
Phenotypic analysis of mouse models of DS
Developmental cognitive deficits are the most studied phenotype of DS because human Ts21 is a leading genetic cause of this phenotype.18,59–63 The average IQ of individuals with Ts21 is significantly lower when compared with individuals without Ts21,60,64 and while there are marked inter-individual variations, probably due to genetic background effects, some degree of intellectual disability is seen in all individuals with DS. Cognitive deficits include impairment in spatial memory and long-term memory as well as difficulties in acquiring new skills.61,65,66 Neuropsychological examinations have revealed that individuals with Ts21 exhibit hippocampal dysfunctions.61,67
Since developmental cognitive deficits of Ts65Dn, Ts1Cje and Tc1 mice have been extensively reviewed, here we will focus on this phenotype analyzed in newly engineered triplication mouse models. To determine if the triplication of a specific Hsa21 syntenic region, including an entire Hsa21 sytenic region on a mouse chromosome, is necessary and/or sufficient to cause developmental cognitive deficits, cognitively relevant phenotypes of duplication mutants and/or compound mutants were characterized, which include T-maze test, Morris water maze tests and fear conditioning tests as well as analysis of synaptic plasticity using extracellular recording of hippocampal slices. In parallel with Ts65Dn mice, abnormal cognitively relevant phenotypes were observed in Dp(16)1Yey/+ mice68 and any compound mutants carrying Dp(16)1Yey and a duplication(s) of any other Hsa21 syntenic regions,69,70 which include Dp(10)1Yey/+;Dp(16)1Yey/+;Dp(17)1Yey/+ mice carrying duplications of the entireties of all the three syntenic regions located on Mmu10, Mmu16 and Mmu17. Although Hsa21 syntenic region on Mmu10 contains 39 Hsa21 gene orthologs, a duplication of this syntenic region alone has not been shown to cause an abnormal cognitively relevant phenotype.68 Interestingly in view of the small chromosomal sub-region involved, a duplication of the Hsa21 syntenic region on Mmu17 was able to consistently cause abnormal hippocampal long-term potentiation.36,68 Many deletion mutants have been used to determine if a Hsa21 syntenic region is necessary for a phenotype. Such an approach has been used to show that the so-called DS critical region is necessary for cognitive deficits in young adult mice.39,41 The extension of such a subtractive strategy has also led to show that the triplications of some Hsa21 gene orthologs are necessary for cognitively relevant phenotypes, including Dyrk1a.41,71 The data from this type of analysis also suggest potential interactions between different triplicated Hsa21 gene orthologs.36,72
Besides developmental cognitive deficits, other phenotypes of DS that have also been analyzed in new models include heart defects,33,73–75 craniofacial abnormalities,76 leukemia77 and middle ear infection.37
Another key phenotype in DS is AD, which is early onset with AD-type neurodegeneration detected by age 40 for all the individuals carrying Ts21.78 The neuropathological findings of AD in DS are very similar to AD without Ts21 in the pattern of emergence of specific pathological markers, which include neuritic plaques and neurofibrillary tangles.79–81 Neuron loss is present in the locus coeruleus and basal forebrain.82–84 The evidence from individuals carrying segmental Ts21 suggests the triplication of the amyloid beta precursor protein gene (APP) is necessary for Alzheimer-type neurodegeneration.85–89 This is also consistent with a more recent report, in which a study of 30 people partially trisomic for Hsa21 provided evidence that an increased dose of APP is necessary for AD in DS.8 Interestingly, mosaic Ts21 or segmental Ts21 has recently been detected in sporadic AD cases.90–94 Detections of mosaic wild-type APP triplication in brains of patients with sporadic AD suggest the possible causative relationship between APP dosage increase and neurodegeneration.95 Therefore, AD pathogenesis in DS may provide insights into AD pathogenesis in other populations, including sporadic AD.96,97
Mouse models of DS have demonstrated important parallels with AD in DS. In Ts65Dn mice, age-related neurodegeneration impacts neurons of the locus coeruleus and cholinergic neurons in the basal forebrain medial septum.44,98 Significantly, increased App dose was shown to be necessary for degeneration of both neuronal populations in Ts65Dn mice.44,98 Interestingly, both the temporal and spatial patterns of neurodegeneration are also consistent with those in AD with or without Ts21; degeneration of locus coeruleus neurons predates basal forebrain cholinergic neurons.99 Specifically, in Ts65Dn mice, locus coeruleus showed progressive age-related changes in volume and cell number at 3–6 months of age, with changes in basal forebrain cholinergic neurons at 9–12 months.44,100–103 Another important AD neuropathology is enlargement of early endosomes. The implication is associated with the fact that APP processing occurs in endosomes.104 One of the consequences of the abnormalities associated with endosomes is impaired retrograde trafficking of neurotrophins in endosomes of axons, which has been implicated as the cause for degeneration of basal forebrain cholinergic neurons.44 Evidence has shown that the triplication of the App ortholog is required for mutant mice to exhibit enlargement of endosomes and impaired neurotrophin transport.43,44 Together, these findings suggest that neuronal degeneration relevant to AD in DS is recapitulated in mutant mice.
Future prospects
Fueled by sequencing the human and mouse genomes and development of chromosome engineering technology, the mouse has continued to serve as a rewarding organism for genetic modeling and dissection of DS. With the development of new genome manipulating tools, such as CRISPR/Cas9, the pace of mouse-based genetic studies of DS is anticipated to be further accelerated. With null alleles of Hsa21 gene orthologs in combination with new mouse mutants carrying a duplication or deletion of Hsa21 syntenic regions, we expect to define the contributions of Hsa21 genes to various DS phenotypes, including at the behavioral, physiological, cellular and epigenetic levels, which will lay the groundwork to unravel the true mechanisms underlying these phenotypes. All these efforts are a prelude to building a sufficient knowledge basis for rational designing of therapeutic interventions to enhance the quality of life for individuals with DS.
Supplementary Material
Supplementary material
Funding
The work at the authors’ laboratories is supported in part by grants from the National Institutes of Health (R01NS66072, R01HL91519, P01HD35897, R21GM114645 and P30CA16056) and the Children's Guild Foundation.
Conflict of interest
The authors have no potential conflicts of interest.
References
- 1.Parker SE, Mai CT, Canfield MA, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol 2010;88:1008–16. [DOI] [PubMed] [Google Scholar]
- 2.Khoshnood B, Greenlees R, Loane M, et al. Paper 2: EUROCAT public health indicators for congenital anomalies in Europe. Birth Defects Res A Clin Mol Teratol 2011;91:S16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Natoli JL, Ackerman DL, McDermott S et al. Prenatal diagnosis of Down syndrome: a systematic review of termination rates (1995–2011). Prenat Diagn 2012;32:142–53. [DOI] [PubMed] [Google Scholar]
- 4.LeJeune J, Gautier M, Turpin R. Etudes des chromosomes somatiques de neuf enfants mongoliens. Comptes Renus de l’ Academic les Sciences 1959;248:1721–2. [PubMed] [Google Scholar]
- 5.LeJeune J, Turpin R, Gautier M. Le mongolism: premier éxample d'aberration autosomique humaine. Ann Genet 1959;1:41–9. [Google Scholar]
- 6.Korenberg JR, Chen XN, Schipper R et al. Down syndrome phenotypes: the consequences of chromosomal imbalance. Proc Natl Acad Sci USA 1994;91:4997–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinet PM, Theophile D, Rahmani Z et al. Mapping of the Down syndrome phenotype on chromosome 21 at the molecular level. Biomed Pharmacother 1994;48:247–52. [DOI] [PubMed] [Google Scholar]
- 8.Korbel JO, Tirosh-Wagner T, Urban AE et al. The genetic architecture of Down syndrome phenotypes revealed by high-resolution analysis of human segmental trisomies. Proc Natl Acad Sci USA 2009;106:12031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noble J. Natural history of Down's syndrome: a brief review for those involved in antenatal screening. J Med Screen 1998;5:172–7. [DOI] [PubMed] [Google Scholar]
- 10.Epstein CJ, Cox DR, Epstein LB. Mouse trisomy 16: an animal model of human trisomy 21 (Down syndrome). Ann NY Acad Sci 1985;450:157–68. [DOI] [PubMed] [Google Scholar]
- 11.Holtzman DM, Li YW, DeArmond SJ, et al. Mouse model of neurodegeneration: atrophy of basal forebrain cholinergic neurons in trisomy 16 transplants. Proc Natl Acad Sci USA 1992;89:1383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davisson MT, Schmidt C, Akeson EC. Segmental trisomy of murine chromosome 16: a new model system for studying Down syndrome. Prog Clin Biol Res 1990;360:263–80. [PubMed] [Google Scholar]
- 13.Duchon A, Raveau M, Chevalier C, et al. Identification of the translocation breakpoints in the Ts65Dn and Ts1Cje mouse lines: relevance for modeling Down syndrome. Mamm Genome 2011;22:674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reinholdt LG, Ding Y, Gilbert GJ, et al. Molecular characterization of the translocation breakpoints in the Down syndrome mouse model Ts65Dn. Mamm Genome 2011;22:685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sago H, Carlson EJ, Smith DJ, et al. Ts1Cje, a partial trisomy 16 mouse model for Down syndrome, exhibits learning and behavioral abnormalities. Proc Natl Acad Sci USA 1998;95:6256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laffaire J, Rivals I, Dauphinot L, et al. Gene expression signature of cerebellar hypoplasia in a mouse model of Down syndrome during postnatal development. BMC Genomics 2009;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeves RH, Irving NG, Moran TH, et al. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet 1995;11:177–84. [DOI] [PubMed] [Google Scholar]
- 18.Antonarakis SE, Lyle R, Dermitzakis ET, et al. Chromosome 21 and down syndrome: from genomics to pathophysiology. Nat Rev Genet 2004;5:725–38. [DOI] [PubMed] [Google Scholar]
- 19.Liu C, Belichenko PV, Zhang L. et al. Mouse models for Down syndrome-associated developmental cognitive disabilities. Dev Neurosci 2011;33:404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rueda N, Florez J, Martinez-Cue C. Mouse models of Down syndrome as a tool to unravel the causes of mental disabilities. Neural Plast 2012;2012:584071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruparelia A, Pearn ML, Mobley WC. Aging and intellectual disability: insights from mouse models of Down syndrome. Dev Disabil Res Rev 2013;18:43–50. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez D, Mee PJ, Martin JE, et al. Transchromosomal mouse embryonic stem cell lines and chimeric mice that contain freely segregating segments of human chromosome 21. Hum Mol Genet 1999;8:923–33. [DOI] [PubMed] [Google Scholar]
- 23.Inoue T, Shinohara T, Takehara S, et al. Specific impairment of cardiogenesis in mouse ES cells containing a human chromosome 21. Biochem Biophys Res Commun 2000;273:219–24. [DOI] [PubMed] [Google Scholar]
- 24.Shinohara T, Tomizuka K, Miyabara S, et al. Mice containing a human chromosome 21 model behavioral impairment and cardiac anomalies of Down's syndrome. Hum Mol Genet 2001;10:1163–75. [DOI] [PubMed] [Google Scholar]
- 25.O'Doherty A, Ruf S, Mulligan C, et al. An aneuploid mouse strain carrying human chromosome 21 with Down syndrome phenotypes. Science 2005;309:2033–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gribble SM, Wiseman FK, Clayton S, et al. Massively parallel sequencing reveals the complex structure of an irradiated human chromosome on a mouse background in the Tc1 model of Down syndrome. PLoS One 2013;8:e60482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morice E, Andreae LC, Cooke SF, et al. Preservation of long-term memory and synaptic plasticity despite short-term impairments in the Tc1 mouse model of Down syndrome. Learn Mem 2008;15:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Y, Bradley A. Engineering chromosomal rearrangements in mice. Nat Rev Genet 2001;2:780–90. [DOI] [PubMed] [Google Scholar]
- 29.Zheng B, Mills AA, Bradley A. A system for rapid generation of coat color-tagged knockouts and defined chromosomal rearrangements in mice. Nucleic Acids Res 1999;27:2354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams DJ, Biggs PJ, Cox T, et al. Mutagenic insertion and chromosome engineering resource (MICER). Nat Genet 2004;36:867–71. [DOI] [PubMed] [Google Scholar]
- 31.Herault Y, Rassoulzadegan M, Cuzin F, et al. Engineering chromosomes in mice through targeted meiotic recombination (TAMERE). Nat Genet 1998;20:381–4. [DOI] [PubMed] [Google Scholar]
- 32.Lallemand Y, Luria V, Haffner-Krausz R, et al. Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res 1998;7:105–12. [DOI] [PubMed] [Google Scholar]
- 33.Raveau M, Lignon JM, Nalesso V, et al. The App-Runx1 region is critical for birth defects and electrocardiographic dysfunctions observed in a Down syndrome mouse model. PLoS Genet 2012;8:e1002724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Yu T, Morishima M, et al. Duplication of the entire 22.9-Mb human chromosome 21 syntenic region on mouse chromosome 16 causes cardiovascular and gastrointestinal abnormalities. Hum Mol Genet 2007;16:1359–66. [DOI] [PubMed] [Google Scholar]
- 35.Belichenko NP, Belichenko PV, Kleschevnikov AM, et al. The ‘Down syndrome critical region’ is sufficient in the mouse model to confer behavioral, neurophysiological, and synaptic phenotypes characteristic of Down syndrome. J Neurosci 2009;29:5938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira PL, Magnol L, Sahun I, et al. A new mouse model for the trisomy of the Abcg1-U2af1 region reveals the complexity of the combinatorial genetic code of down syndrome. Hum Mol Genet 2009;18:4756–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhutta MF, Cheeseman MT, Herault Y, et al. Surveying the Down syndrome mouse model resource identifies critical regions responsible for chronic otitis media. Mamm Genome 2013;24:439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brault V, Duchon A, Romestaing C, et al. Opposite phenotypes of muscle strength and locomotor function in mouse models of partial trisomy and monosomy 21 for the proximal Hspa13-App region. PLoS Genet 2015;11:e1005062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson LE, Roper RJ, Sengstaken CL, et al. Trisomy for the Down syndrome ‘critical region’ is necessary but not sufficient for brain phenotypes of trisomic mice. Hum Mol Genet 2007;16:774–82. [DOI] [PubMed] [Google Scholar]
- 40.Duchon A, Pothion S, Brault V, et al. The telomeric part of the human chromosome 21 from Cstb to Prmt2 is not necessary for the locomotor and short-term memory deficits observed in the Tc1 mouse model of Down syndrome. Behav Brain Res 2010;217:271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang X, Liu C, Yu T, et al. Genetic dissection of the Down syndrome critical region. Hum Mol Genet 2015;24:6540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marechal D, Lopes Pereira P, Duchon A, et al. Dosage of the Abcg1-U2af1 region modifies locomotor and cognitive deficits observed in the Tc1 mouse model of Down syndrome. PLoS One 2015;10:e0115302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cataldo AM, Petanceska S, Peterhoff CM, et al. App gene dosage modulates endosomal abnormalities of Alzheimer's disease in a segmental trisomy 16 mouse model of down syndrome. J Neurosci 2003;23:6788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salehi A, Delcroix JD, Belichenko PV, et al. Increased App expression in a mouse model of Down's syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron 2006;51:29–42. [DOI] [PubMed] [Google Scholar]
- 45.Sussan TE, Yang A, Li F, et al. Trisomy represses Apc(Min)-mediated tumours in mouse models of Down's syndrome. Nature 2008;451:73–5. [DOI] [PubMed] [Google Scholar]
- 46.Baek KH, Zaslavsky A, Lynch RC, et al. Down's syndrome suppression of tumour growth and the role of the calcineurin inhibitor DSCR1. Nature 2009;459:1126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chakrabarti L, Best TK, Cramer NP, et al. Olig1 and Olig2 triplication causes developmental brain defects in Down syndrome. Nat Neurosci 2010;13:927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Austin CP, Battey JF, Bradley A, et al. The knockout mouse project. Nat Genet 2004;36:921–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skarnes WC, Rosen B, West AP, et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature 2011;474:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bradley A, Anastassiadis K, Ayadi A, et al. The mammalian gene function resource: the International Knockout Mouse Consortium. Mamm Genome 2012;23:580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White JK, Gerdin AK, Karp NA, et al. Genome-wide generation and systematic phenotyping of knockout mice reveals new roles for many genes. Cell 2013;154:452–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kerkel K, Schupf N, Hatta K, et al. Altered DNA methylation in leukocytes with trisomy 21. PLoS Genet 2010;6:e1001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin S, Lee YK, Lim YC, et al. Global DNA hypermethylation in down syndrome placenta. PLoS Genet 2013;9:e1003515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bacalini MG, Gentilini D, Boattini A, et al. Identification of a DNA methylation signature in blood cells from persons with Down Syndrome. Aging (Albany NY) 2015;7:82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sailani MR, Santoni FA, Letourneau A, et al. DNA-methylation patterns in Trisomy 21 using cells from monozygotic twins. PLoS One 2015;10:e0135555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mendioroz M, Do C, Jiang X, et al. Trans effects of chromosome aneuploidies on DNA methylation patterns in human Down syndrome and mouse models. Genome Biol 2015;16:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Letourneau A, Santoni FA, Bonilla X, et al. Domains of genome-wide gene expression dysregulation in Down's syndrome. Nature 2014;508:345–50. [DOI] [PubMed] [Google Scholar]
- 58.Davoli T, Xu AW, Mengwasser KE, et al. Cumulative haploinsufficiency and triplosensitivity drive aneuploidy patterns and shape the cancer genome. Cell 2013;155:948–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Epstein CJ. The Consequences of Chromosome Imbalance: Principles, Mechanism and Models. New York: Cambridge University Press, 1986;486. [Google Scholar]
- 60.Pulsifer MB. The neuropsychology of mental retardation. J Int Neuropsychol Soc 1996;2:159–76. [DOI] [PubMed] [Google Scholar]
- 61.Pennington BF, Moon J, Edgin J, et al. The neuropsychology of Down syndrome: evidence for hippocampal dysfunction. Child Dev 2003;74:75–93. [DOI] [PubMed] [Google Scholar]
- 62.Roizen NJ, Patterson D. Down's syndrome. Lancet 2003;361:1281–9. [DOI] [PubMed] [Google Scholar]
- 63.Antonarakis SE, Epstein CJ. The challenge of Down syndrome. Trends Mol Med 2006;12:473–9. [DOI] [PubMed] [Google Scholar]
- 64.Chapman RS, Hesketh LJ. Behavioral phenotype of individuals with Down syndrome. Ment Retard Dev Disabil Res Rev 2000;6:84–95. [DOI] [PubMed] [Google Scholar]
- 65.Haxby JV. Neuropsychological evaluation of adults with Down's syndrome: patterns of selective impairment in non-demented old adults. J Ment Defic Res 1989;33:193–210. [DOI] [PubMed] [Google Scholar]
- 66.Nadel L. Down syndrome in cognitive neuroscience perspective In: Tager-Flusberg H. (ed). Neurodevelopmental Disorders. Cambridge, MA: MIT Press, 1999;197–221. [Google Scholar]
- 67.Uecker A, Mangan PA, Obrzut JE, et al. Down syndrome in neurobiological perspective: an emphasis on spatial cognition. J Clin Child Psychol 1993;22:266–76. [Google Scholar]
- 68.Yu T, Liu C, Belichenko P, et al. Effects of individual segmental trisomies of human chromosome 21 syntenic regions on hippocampal long-term potentiation and cognitive behaviors in mice. Brain Res 2010;1366:162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu T, Li Z, Jia Z, et al. A mouse model of Down syndrome trisomic for all human chromosome 21 syntenic regions. Hum Mol Genet 2010;19:2780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L, Meng K, Jiang X, et al. Human chromosome 21 orthologous region on mouse chromosome 17 is a major determinant of Down syndrome-related developmental cognitive deficits. Hum Mol Genet 2014;23:578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia-Cerro S, Martinez P, Vidal V, et al. Overexpression of Dyrk1A is implicated in several cognitive, electrophysiological and neuromorphological alterations found in a mouse model of Down syndrome. PLoS One 2014;9:e106572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xing L, Salas M, Zhang H, et al. Creation and characterization of BAC-transgenic mice with physiological overexpression of epitope-tagged RCAN1 (DSCR1). Mamm Genome 2013;24:30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu C, Morishima M, Yu T, et al. Genetic analysis of Down syndrome-associated heart defects in mice. Hum Genet 2011;130:623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu C, Morishima M, Jiang X, et al. Engineered chromosome-based genetic mapping establishes a 3.7 Mb critical genomic region for Down syndrome-associated heart defects in mice. Hum Genet 2014;133:743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lana-Elola E, Watson-Scales S, Slender A, et al. Genetic dissection of Down syndrome-associated congenital heart defects using a new mouse mapping panel. Elife 2016;5:e11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Starbuck JM, Dutka T, Ratliff TS, et al. Overlapping trisomies for human chromosome 21 orthologs produce similar effects on skull and brain morphology of Dp(16)1Yey and Ts65Dn mice. Am J Med Genet A 2014;164A:1981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lane AA, Chapuy B, Lin CY, et al. Triplication of a 21q22 region contributes to B cell transformation through HMGN1 overexpression and loss of histone H3 Lys27 trimethylation. Nat Genet 2014;46:618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hyman BT, West HL, Rebeck GW, et al. Quantitative analysis of senile plaques in Alzheimer disease: observation of log-normal size distribution and molecular epidemiology of differences associated with apolipoprotein E genotype and trisomy 21 (Down syndrome). Proc Natl Acad Sci USA 1995;92:3586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Struwe F. Histopathology Untersuchungen uber Entstenhung und Wesen der senile Plaques Z. ges. Z Gesamte Neuro Psy 1929;122:291–307. [Google Scholar]
- 80.Bertrand I, Koffas D. Cas d'idiotie mongolienne adulte avec nombrueses plaues seniles et concretions calcarires pallidales. Rev Neurol 1946;78:338–43. [Google Scholar]
- 81.Malamud N. Neuropathology of organic brain syndromes associated with aging In: Gaitz CM. (ed). Aging and the Brain: Advances in Behavioural Biology. New York: Plenum Press, ; 1972,63–87. [Google Scholar]
- 82.Mann DM, Royston MC, Ravindra CR. Some morphometric observations on the brains of patients with Down's syndrome: their relationship to age and dementia. J Neurol Sci 1990;99:153–64. [DOI] [PubMed] [Google Scholar]
- 83.Hof PR, Bouras C, Perl DP, et al. Age-related distribution of neuropathologic changes in the cerebral cortex of patients with Down's syndrome. Quantitative regional analysis and comparison with Alzheimer's disease. Arch Neurol 1995;52:379–91. [DOI] [PubMed] [Google Scholar]
- 84.Wilcock DM. Neuroinflammation in the aging down syndrome brain; lessons from Alzheimer's disease. Curr Gerontol Geriatr Res 2012;2012:170276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prasher VP, Farrer MJ, Kessling AM, et al. Molecular mapping of Alzheimer-type dementia in Down's syndrome. Ann Neurol 1998;43:380–3. [DOI] [PubMed] [Google Scholar]
- 86.Cabrejo L, Guyant-Marechal L, Laquerriere A, et al. Phenotype associated with APP duplication in five families. Brain 2006;129:2966–76. [DOI] [PubMed] [Google Scholar]
- 87.Rovelet-Lecrux A, Hannequin D, Raux G, et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet 2006;38:24–6. [DOI] [PubMed] [Google Scholar]
- 88.Sleegers K, Brouwers N, Gijselinck I, et al. APP duplication is sufficient to cause early onset Alzheimer's dementia with cerebral amyloid angiopathy. Brain 2006;129:2977–83. [DOI] [PubMed] [Google Scholar]
- 89.Kasuga K, Shimohata T, Nishimura A, et al. Identification of independent APP locus duplication in Japanese patients with early-onset Alzheimer disease. J Neurol Neurosurg Psychiatry 2009;80:1050–2. [DOI] [PubMed] [Google Scholar]
- 90.Kingsbury MA, Yung YC, Peterson SE, et al. Aneuploidy in the normal and diseased brain. Cell Mol Life Sci 2006;63:2626–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ringman JM, Rao PN, Lu PH, et al. Mosaicism for trisomy 21 in a patient with young-onset dementia: a case report and brief literature review. Arch Neurol 2008;65:412–5. [DOI] [PubMed] [Google Scholar]
- 92.Iourov IY, Vorsanova SG, Liehr T, et al. Aneuploidy in the normal, Alzheimer's disease and ataxia-telangiectasia brain: differential expression and pathological meaning. Neurobiol Dis 2009;34:212–20. [DOI] [PubMed] [Google Scholar]
- 93.Hulten MA, Jonasson J, Nordgren A, et al. Germinal and somatic trisomy 21 mosaicism: how common is it, what are the implications for individual carriers and how does it come about. Curr Genomics 2010;11:409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Potter H, Granic A, Caneus J. Role of trisomy 21 mosaicism in sporadic and familial Alzheimer's disease. Curr Alzheimer Res 2016;13:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bushman DM, Kaeser GE, Siddoway B, et al. Genomic mosaicism with increased amyloid precursor protein (APP) gene copy number in single neurons from sporadic Alzheimer's disease brains. Elife 2015;4:e05116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hartley D, Blumenthal T, Carrillo M, et al. Down syndrome and Alzheimer's disease: common pathways, common goals. Alzheimers Dement 2015;11:700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Head E, Lott IT, Wilcock DM, et al. Aging in Down syndrome and the development of Alzheimer's disease neuropathology. Curr Alzheimer Res 2016;13:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salehi A, Faizi M, Colas D, et al. Restoration of norepinephrine-modulated contextual memory in a mouse model of Down syndrome. Sci Transl Med 2009;1:7–17. [DOI] [PubMed] [Google Scholar]
- 99.Braak H, Del Tredici K. Alzheimer's pathogenesis: is there neuron-to-neuron propagation. Acta Neuropathol 2011;121:589–95. [DOI] [PubMed] [Google Scholar]
- 100.Cooper JD, Salehi A, Delcroix JD, et al. Failed retrograde transport of NGF in a mouse model of Down's syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion. Proc Natl Acad Sci USA 2001;98:10439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Contestabile A, Fila T, Bartesaghi R, et al. Choline acetyltransferase activity at different ages in brain of Ts65Dn mice, an animal model for Down's syndrome and related neurodegenerative diseases. J Neurochem 2006;97:515–26. [DOI] [PubMed] [Google Scholar]
- 102.Lockrow J, Boger H, Gerhardt G, et al. A noradrenergic lesion exacerbates neurodegeneration in a Down syndrome mouse model. J Alzheimers Dis 2011;23:471–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fortress AM, Hamlett ED, Vazey EM, et al. Designer receptors enhance memory in a mouse model of Down syndrome. J Neurosci 2015;35:1343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jiang Y, Mullaney KA, Peterhoff CM, et al. Alzheimer's-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition. Proc Natl Acad Sci USA 2010;107:1630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Herault Y, Lopes PP, Magnol L, et al. Tackling the complexity of the genotype-phenotype relationship in the Down syndrome with the mouse aneuploidy zoo: a resource of new models to study aneuploidies involving human chromosome 21. In: The American Society of Human Genetics 59th Annual Meeting, 2009, Honolulu HI.
- 106.Olson LE, Richtsmeier JT, Leszl J, et al. A chromosome 21 critical region does not cause specific Down syndrome phenotypes. Science 2004;306:687–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Migdalska AM, van der Weyden L, Ismail O, et al. Modeling partial monosomy for human chromosome 21q11.2-q21.1 reveals haploinsufficient genes influencing behavior and fat deposition. PLoS One 2012;7:e29681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu T, Clapcote SJ, Li Z, et al. Deficiencies in the region syntenic to human 21q22.3 cause cognitive deficits in mice. Mamm Genome 2010;21:258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Besson V, Brault V, Duchon A, et al. Modeling the monosomy for the telomeric part of human chromosome 21 reveals haploinsufficient genes modulating the inflammatory and airway responses. Hum Mol Genet 2007;16:2040–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.