Abstract
Background
African Americans and patients with chronic kidney disease (CKD) are at high risk for clinical heart failure (HF). In this study, we aimed to determine the association of markers of kidney disease with subclinical HF (by echocardiogram) and risk of clinical HF among a large, well-characterized community-based cohort of African American patients. We also examined whether the association of markers of kidney disease with HF was attenuated with adjustment for echocardiographic measures.
Methods
We studied participants in the Jackson Heart Study, a large community-based cohort of African Americans. Estimated glomerular filtration rate (eGFR) and urine albumin:creatinine ratio (ACR) were measured at baseline. We tested the association of eGFR and urine ACR with left ventricular mass (LVM), left ventricular ejection fraction (LVEF) and physician-adjudicated incident HF.
Results
Among the 3332 participants in the study, 166 (5%) had eGFR <60 mL/min/1.73 m2 and 405 (12%) had urine ACR ≥30 mg/g. In models adjusted for demographics, comorbidity and the alternative measure of kidney disease, lower eGFR and higher urine ACR were associated with higher LVM {β-coefficient 1.54 [95% confidence interval (CI) 0.78–2.31] per 10 mL/min/1.73 m2 decrease in eGFR and 2.87 (95% CI 1.85–3.88) per doubling of urine ACR}. There was no association of eGFR and urine ACR with LVEF [β-coefficient −0.12 (95% CI −0.28–0.04) and −0.11 (95% CI −0.35–0.12), respectively]. There was no association of eGFR with the risk of incident HF [HR 1.02 (95% CI 0.91–1.14) per 10 mL/min/1.73 m2 decrease], while there was a significant association of urine ACR [HR 2.22 (95% CI 1.29–3.84) per doubling of urine ACR]. This association was only modestly attenuated with adjustment for LVM [HR 1.95 (95% CI 1.09–3.49)].
Conclusions
Among a community-based cohort of African Americans, lower eGFR and higher ACR were associated with higher LVM. Furthermore, higher urine ACR was associated with incident HF, which was not entirely explained by the presence of left ventricular disease.
Keywords: African Americans, chronic kidney disease, echocardiogram, heart failure, left ventricular hypertrophy
INTRODUCTION
Chronic kidney disease (CKD), defined as estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 or elevated urine albumin:creatinine ratio (ACR), affects 14% of the US population (http://www.usrds.org/adr.htm). The burden of CKD and ESRD is disproportionately high in African Americans compared with other racial/ethnic groups [1, 2]. African Americans have a faster decline in eGFR compared with whites [3–7].
Heart failure (HF) is the leading cause of cardiovascular morbidity and mortality among patients with CKD. Patients with CKD have 3-fold greater risk of HF compared with those without CKD [8], with higher rates of complications such as hospitalizations and death [9–15]. Prior studies have also reported strong associations between CKD and early changes in left ventricular structure and function, specifically elevated left ventricular mass (LVM) and decreased left ventricular ejection fraction (LVEF), even in patients without known clinical HF [16–21]. The risk of HF is particularly high among African Americans [22, 23]. Previous studies of HF risk in CKD populations have generally had underrepresentation of African Americans or have studied primarily research populations, which may not be generalizable to African Americans with CKD in general. In a previous study of adults in the Health, Aging and Body Composition Study, the association of eGFR with incident HF was stronger in African Americans compared with whites [22]. However, this study included only older adults and did not examine other important markers of kidney damage (urine albuminuria).
Thus, in this study, we determined the association of markers of kidney disease with the risk of subclinical measures of HF (as determined by echocardiography) and the risk of incident HF among participants in the Jackson Heart Study (JHS) without clinical HF at baseline. We also examined whether the association of kidney disease with HF was attenuated with adjustment for echocardiographic measures.
MATERIALS AND METHODS
Study population
The JHS is a community-based cohort study of African Americans designed to evaluate risk factors for cardiovascular disease (CVD) [24, 25]. A total of 5301 participants ages 21–94 years were recruited from the tri-county region (Hinds, Madison and Rankin) of metropolitan Jackson, MS, USA [26]. Participants were recruited during calendar years 2000–04 and underwent a second and third in-person exam in 2005–08 and 2009–10, respectively. For this study, participants were excluded if they were missing any of our four echocardiographic measures from baseline (n = 1915) or serum creatinine (n = 54) at baseline, which left a final analytic sample of 3332. Participants who were excluded were more likely to be older and have lower income and higher comorbidity burden compared with those included in the analysis (Supplementary data, Table S1). Institutional review board approval was obtained from all participating institutions.
eGFR and urine ACR
eGFR and ACR were our primary exposures. eGFR was calculated from serum concentration of creatinine measured at baseline using the Chronic Kidney Disease Epidemiology Collaboration equation [27]. CKD was defined as eGFR <60 mL/min/1.73 m2. Creatinine was measured using the Jaffe method and calibrated to measurements traceable to isotope dilution mass spectrometry (IDMS) [28]. The urine ACR was obtained from either 24-h urine collections or spot urine samples at Exam 1. Urinary albumin was measured by two different methods at Exam 1: a random spot morning urine collection (n = 1589) or a timed 24-h urine collection (n = 570) [29]. A subset of participants (n = 223) collected urine samples using both methods. The urine albumin (nephelometric immunoassay, Dade Behring) to creatinine (enzymatic Jaffe method) ratio (mg/g) was calculated for both sample types and was found to be highly correlated (r = 0.965) [30]. For missing ACRs, multiple imputation using chained equations with sex and age as the main predictor variables (but including all the covariates in Model 2: age, sex, education, BMI, tobacco use, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, lipid-lowering medications, history of coronary heart disease (CHD), history of stroke, history of diabetes, hemoglobin and eGFR) was conducted (n = 1271).
Echocardiogram measures
Echocardiograms were performed at Examination 1 by certified ultrasonography technicians (Sonos 4500 echocardiograph; Hewlett Packard, Andover, MA, USA) and following American Society of Echocardiography recommendations [29, 31]. The 2D and M-mode examination was similar to typical clinical echocardiography with parasternal, apical and subcostal windows, and assessment of all four cardiac chambers. A single observer who was blinded to participants' clinical data read all measurements. The calculation of left ventricular (LV) mass was based on the standard formula: LV mass (g) = 0.8 × 1.04 [(LV end diastolic diameter + IVST + PWT)3 − (LV end diastolic diameter)3] + 0.6, where IVST is the interventricular septal thickness and PWT is the posterior wall thickness. LV mass was divided by height2.7 to calculate the LV mass index. Left ventricular systolic function was described in terms of the LV ejection fraction (LVEF). The LVEF was derived semiquantitatively by the primary cardiologist using a modified Quinones technique and visual assessment of the LV apex. The modified Quinones formula is as follows: LVEF = (left ventricular internal diameter in systole [LVIDS]2 − LVIDD2)/LVIDD2 × 100% [32].
Incident HF
Incident HF was ascertained from Exam 2 to the end of follow-up (through 2010). Participants who died prior to Exam 2 (n = 223) or had prevalent HF (by self-report) at Exam 2 (n = 36) were excluded. To capture incident cardiovascular events and death, trained interviewers conducted annual telephone follow-up interviews to ascertain any significant health event since the last JHS contact, including diagnostic tests, hospitalizations or death. Information on cohort hospitalizations and deaths was transmitted to the medical record abstraction unit, which reviews death certificates and hospital records to identify cardiovascular events in the cohort. Interviews with the next of kin and completed questionnaires by physicians and medical examiners or coroners were used to obtain information on deaths in the cohort. The first computer-generated diagnosis during the JHS period with follow-up review and adjudication by trained medical personnel completed the final, disease-specific event classification of hospitalized and fatal cardiovascular events [33]. Ascertainment for outcomes of interest began at Exam 2. Participants were censored at death, loss to follow-up or end of the study period.
Covariates
Baseline covariates were included in the analysis. Demographic characteristics (age, sex and race), education and income were determined by self-report. Hypertension was defined by the use of blood pressure medications or blood pressure >140/90 mmHg. Diabetes was defined as fasting glucose >126 mg/dL or use of oral hypoglycemic medications or insulin. Information on tobacco use was collected from self-reports (never, former or current). Physical examination measures (systolic and diastolic blood pressure, body mass index in kg/m2) and laboratory values (total cholesterol, LDL cholesterol, HDL cholesterol) were obtained at each study visit. Medication use was determined by the evaluation of participant pill bottles and recorded by study personnel.
Statistical methods
We compared baseline characteristics of the study population across categories of eGFR at baseline. We reported means of our echocardiographic variables of interest across categories of eGFR and urine ACR. Urine ACR was log-transformed given the skewed distribution.
We used cubic splines to assess the function form of the unadjusted association of each kidney measure with each echocardiogram measure. In cross-sectional analyses, we used linear regression to examine the association of eGFR (per 10 mL/min/1.73 m2 decrease and <60 versus ≥60 mL/min/1.73 m2) and urine ACR (per doubling as a continuous variable and in >30 g/mg versus ≤30 g/mg) with LVM, left ventricular mass index (LVMI) and LVEF (all continuous). We adjusted for (i) age, sex and education; (ii) age, sex, education and other possible confounders, including BMI, tobacco use, HDL, LDL, lipid-lowering medications, history of CHD, history of stroke, history of diabetes, hemoglobin, eGFR and urine ACR and (iii) possible mediators, including systolic blood pressure, diastolic blood pressure and anti-hypertensive medications. We also tested for interaction by age and gender.
In longitudinal analyses, we calculated rates and cumulative incidence of HF per year across categories of baseline eGFR and urine ACR. We confirmed that the Cox proportional hazards assumption was not violated. We then utilized Cox proportional hazards model to examine the association of baseline eGFR and urine ACR with the risk of incident HF. We adjusted for age, sex, systolic blood pressure and the alternative measure of kidney disease in these models. In a series of ‘mediation’ analyses, we adjusted (individually) for LVM, LVMI and LVEF. The rationale for these models was to determine if the associations of markers of kidney disease with incident HF were attenuated with adjustment of subclinical HF at baseline.
All analyses were conducted in STATA version 13 (StataCorp, College Station, TX, USA) and P-values <0.05 were considered statistically significant.
RESULTS
Characteristics of the study population
Among the 3332 participants in the study, 166 (5%) had eGFR <60 mL/min/1.73 m2 at baseline. Overall, participants with lower eGFR were older, more likely to be male, had lower income and lower educational attainment. Participants with lower eGFR and higher urine ACR. Also had higher systolic blood pressure, were more likely to be taking blood pressure medications and were more likely to have a history of coronary heart disease or stroke at baseline (Tables 1 and 2).
Table 1.
Baseline characteristics of participants in the Jackson Heart Study by level of eGFR (N = 3332)
| eGFR (mL/min/1.73 m2) |
|||
|---|---|---|---|
| ≥60 | <60 | Total | |
| n | 3166 | 166 | 3332 |
| Age, years, mean (SD) | 52 (12) | 66 (10) | 53 (13) |
| Male | 1174 (37) | 52 (31) | 1226 (37) |
| Income | |||
| Poor | 360 (14) | 41 (31) | 401 (14) |
| Lower-middle | 597 (22) | 32 (24) | 629 (22) |
| Upper-middle | 814 (31) | 44 (33) | 858 (31) |
| Affluent | 902 (34) | 17 (13) | 919 (33) |
| Education | |||
| <High school | 481 (15) | 60 (36) | 541 (16) |
| High school graduate | 558 (18) | 35 (21) | 593 (18) |
| ≥GED | 730 (23) | 30 (18) | 760 (23) |
| College degree | 1384 (44) | 41 (25) | 1425 (43) |
| Body mass index, kg/m2, mean (SD) | 31.4 (6.9) | 31.2 (6.7) | 31.3 (6.9) |
| Systolic blood pressure, mmHg, mean (SD) | 125 (18) | 132 (21) | 125 (18) |
| Diastolic blood pressure, mmHg, mean (SD) | 79 (10) | 76 (12) | 79 (10) |
| Smoking | |||
| Never | 2208 (70) | 105 (64) | 2313 (70) |
| Former | 521 (17) | 45 (27) | 566 (17) |
| Current | 408 (13) | 14 (9) | 422 (13) |
| Diabetes mellitus | 549 (17) | 77 (46) | 626 (19) |
| Hypertension | 1725 (55) | 155 (93) | 1880 (56) |
| Use of hypertension medications | 1362 (55) | 147 (92) | 1509 (57) |
| Use of RAAS inhibitors | 650 (21) | 91 (55) | 741 (22) |
| Use of β-blockers | 266 (11) | 39 (24) | 305 (12) |
| Prevalent coronary heart disease | 153 (5) | 41 (25) | 194 (6) |
| Prevalent stroke | 98 (3) | 20 (12) | 118 (4) |
| Total cholesterol, mg/dL, mean (SD) | 198 (39) | 213 (47) | 198 (40) |
| LDL, mg/dL, mean (SD) | 126 (36) | 137 (43) | 126 (36) |
| HDL, mg/dL, mean (SD | 51 (14) | 52 (15) | 52 (14) |
| Triglycerides, mg/dL, median (IQR) | 87 (62–121) | 108 (77–148) | 87 (63–122) |
| Statins | 287 (12) | 38 (24) | 325 (12) |
| HbA1c, %, mean (SD) | 5.8 (1.2) | 6.5 (1.5) | 5.9 (1.2) |
Values presented as n (%) unless otherwise noted.
GED, General Educational Development test; HbA1c, hemoglobin A1c; RAAS, renin-angiotensin-aldosterone system.
Table 2.
Baseline characteristics of participants in the Jackson Heart Study by level of ACR (N = 3332)
| ACR |
||
|---|---|---|
| <30 mg/g | ≥30 mg/g | |
| n | 2915 | 417 |
| Age, years, mean (SD) | 52 (13) | 57 (13) |
| Male | 1083 (37) | 143 (34) |
| Income | ||
| Poor | 335 (14) | 66 (18) |
| Lower-middle | 510 (21) | 119 (33) |
| Upper-middle | 758 (31) | 100 (28) |
| Affluent | 846 (35) | 73 (20) |
| Education | ||
| <High school | 433 (15) | 109 (26) |
| High school graduate | 510 (18) | 85 (20) |
| ≥GED | 671 (23) | 93 (22) |
| College degree | 1301 (45) | 130 (31) |
| Body mass index, kg/m2, mean (SD) | 31.1 (6.8) | 33.2 (7.6) |
| Systolic blood pressure, mmHg, mean (SD) | 124 (17) | 133 (20) |
| Diastolic blood pressure mmHg, mean (SD) | 79 (10) | 80 (11) |
| Smoking | ||
| Never | 2047 (70) | 287 (69) |
| Former | 495 (17) | 75 (18) |
| Current | 375 (13) | 55 (13) |
| Diabetes mellitus | 451 (16) | 175 (42) |
| Hypertension | 1553 (53) | 327 (78) |
| Use of hypertension medications | 1229 (54) | 280 (76) |
| Use of RAAS inhibitors | 583 (20) | 158 (38) |
| Use of β-blockers | 244 (11) | 61 (16) |
| Prevalent coronary heart disease | 140 (5) | 54 (13) |
| Prevalent stroke | 84 (3) | 34 (8) |
| Total cholesterol, mg/dL, mean (SD) | 197 (39) | 204 (42) |
| LDL, mg/dL, mean (SD) | 126 (36) | 130 (38) |
| HDL, mg/dL, mean (SD) | 52 (14) | 51 (15) |
| Triglycerides, mg/dL, median (IQR) | 86 (61–120) | 97 (71–145) |
| Statins | 308 (11) | 68 (16) |
| HbA1c, %, mean (SD) | 5.8 (1.1) | 6.6 (1.7) |
Values presented as n (%) unless otherwise noted.
GED, General Educational Development test; HbA1c, hemoglobin A1c; RAAS, renin-angiotensin-aldosterone system.
Association of markers of kidney disease with subclinical HF as determined by echocardiogram measures
In unadjusted models, every 10 mL/min/1.73 m2 decrease in eGFR, eGFR <60 mL/min/1.73 m2 and higher urine ACR were associated with higher LVM (Table 3). These associations remained statistically significant with adjustment for demographics, cardiovascular risk factors, eGFR (when urine ACR was predictor) and urine ACR (when eGFR was the predictor). Every 10 mL/min/1.73 m2 decrease in eGFR was associated with 1.54 g (95% CI 0.78–2.31) higher LVM (Table 3). This association remained statistically significant even with adjustment for blood pressure and blood pressure medications, possible mediators of this association. In multivariable models, every doubling of urine ACR was associated with 2.87 g (95% CI 1.85–3.88) higher LVM. This association was only partially attenuated with adjustment for blood pressure and blood pressure medications. In multivariable models, there was no association of eGFR or urine ACR with LVEF. Interactions by age and gender were not statistically significant (all P > 0.05).
Table 3.
Cross-sectional association of markers of kidney disease with echocardiographic measures among participants in the Jackson Heart Study
| LVM, β (95% CI) | LVEF, β (95% CI) | |
|---|---|---|
| eGFR (per 10 mL/min/1.73 m2 decrease) | ||
| Unadjusted | 3.89 (3.17, 4.61) | 0.03 (−0.10, 0.17) |
| Model 1 | 2.08 (1.67, 2.48) | −0.15 (−0.23, −0.08) |
| Model 2 | 1.54 (0.78, 2.31) | −0.12 (−0.28, 0.04) |
| Model 3 (mediation) | 1.26 (0.52, 1.99) | −0.13 (−0.28, 0.03) |
| eGFR <60 mL/min/1.73 m2 (ref ≥60 mL/min/1.73 m2) | ||
| Unadjusted | 25.86 (19.22, 32.51) | −0.72 (−1.94, 0.49) |
| Model 1 | 18.93 (15.70, 22.16) | −1.51 (−2.13, −0.88) |
| Model 2 | 10.62 (4.34, 16.91) | −1.07 (−2.38, 0.23) |
| Model 3 (mediation) | 11.08 (5.03, 17.13) | −1.09 (−2.40, 0.22) |
| ACR ≥30 g/mg (ref <30 g/mg) | ||
| Unadjusted | 22.31 (16.86, 27.77) | −0.46 (−1.60, 0.68) |
| Model 1 | 18.74 (13.71, 23.76) | −0.64 (−1.71, 0.43) |
| Model 2 | 10.98 (6.20, 15.76) | −0.51 (−1.69, 0.66) |
| Model 3 (mediation) | 7.58 (2.65, 12.51) | −0.57 (−1.77, 0.62) |
| ACR (per doubling) | ||
| Unadjusted | 4.89 (3.78, 6.00) | −0.28 (−0.21, 0.15) |
| Model 1 | 4.39 (3.36, 5.42) | −0.14 (−0.35, 0.08) |
| Model 2 | 2.87 (1.85, 3.88) | −0.11 (−0.35, 0.12) |
| Model 3 (mediation) | 1.95 (0.96, 2.95) | −0.13 (−0.36, 0.10) |
Values presented as mean (95% CI) difference in LVM and LVEF.
Model 1: Adjusted for age, sex and education.
Model 2: Model 1 + BMI, tobacco use, LDL cholesterol, HDL cholesterol, lipid-lowering medications, history of CHD, history of stroke, history of diabetes, hemoglobin and eGFR/ACR (adjusting for the alternative kidney disease marker).
Model 3: Model 2 + systolic blood pressure, diastolic blood pressure and hypertension medications. ref, reference.
Association of markers of kidney disease with incident HF
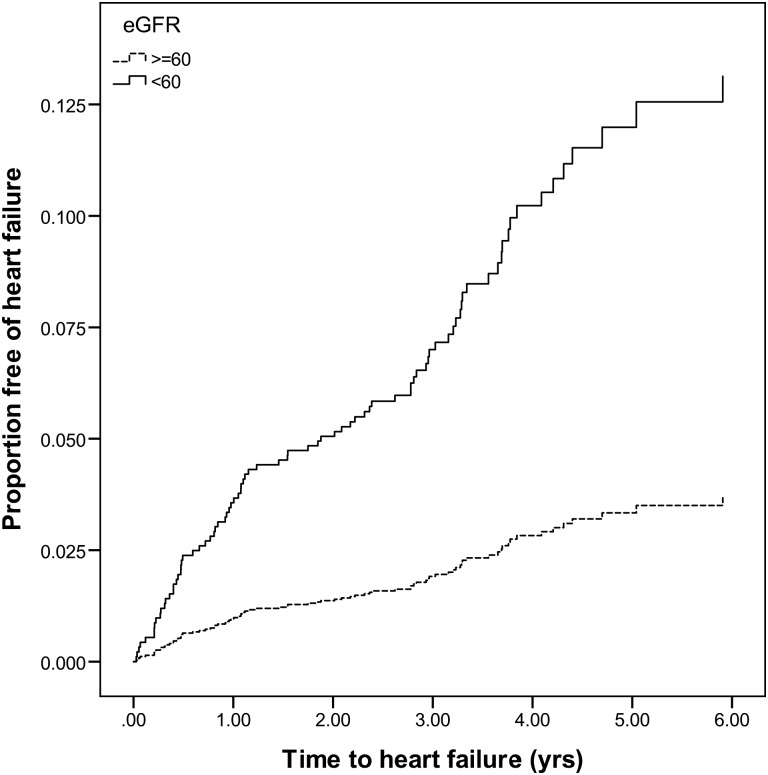
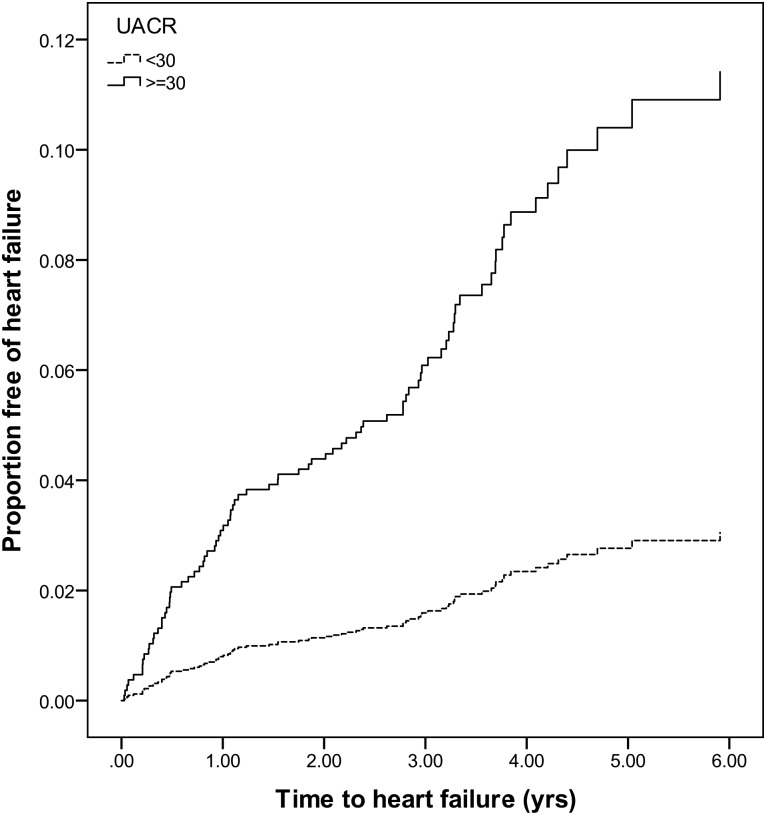
Over a median of 3.36 [interquartile range (IQR) 2.73–4.47] years of follow-up, there were 84 cases of incident HF. The cumulative incidence of HF was higher in those with eGFR <60 mL/min/1.73 m2 and urine ACR >30 mg/g (Figures 1 and 2). In unadjusted models, every 10 mL/min/1.73 m2 decrease in eGFR was associated with a 28% higher risk of incident HF. This association was attenuated with adjustment for demographics, systolic blood pressure and urine ACR. Further adjustment for LVM and LVEF did not attenuate the point estimate. Similarly, eGFR <60 mL/min/1.73 m2 was associated with a >3-fold greater risk of incident HF. This association was attenuated with multivariable adjustment (Table 4). Every doubling of urine ACR was associated with a 26% greater risk of incident HF. This association was attenuated but remained statistically significant with adjustment for demographics, systolic blood pressure and eGFR. Urine ACR ≥30 g/mg was associated with a >3-fold greater risk of incident HF in adjusted models. This association remained robust with multivariable adjustment. While further adjustment for LVM and LVEF somewhat attenuated the association between urine ACR ≥30 g/mg, it remained statistically significant (Table 4).
FIGURE 1:
Cumulative incidence of HF by eGFR category.
FIGURE 2:
Cumulative incidence of HF by urine ACR category.
Table 4.
Association of markers of kidney disease with risk of incident HF among participants in the Jackson Heart Study
| n | With HF, n | Rate/year | Unadjusted, HR (95% CI) | Model 1,a HR (95% CI) | M1a + LVM, HR (95% CI) | M1a + LVMI, HR (95% CI) | M1a + LVEF, HR (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| eGFR (per 10 mL/min/1.73 m2 decrease) | 3073 | 84 | 0.75 | 1.28 (1.17–1.39) | 1.02 (0.91–1.14) | 0.99 (0.89–1.11) | 1.01 (0.91–1.12) | 1.00 (0.89–1.12) |
| eGFR | ||||||||
| ≥60 | 2939 | 71 | 0.66 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| <60 | 134 | 13 | 2.51 | 3.78 (2.09–6.84) | 1.37 (0.71–2.66) | 1.09 (0.55–2.15) | 1.19 (0.61–2.32) | 1.24 (0.63–2.43) |
| ACR (per doubling) | 3073 | 84 | 0.75 | 1.26 (1.15–1.39) | 1.18 (1.06–1.32) | 1.12 (1.01–1.26) | 1.13 (1.01–1.26) | 1.18 (1.06–1.32) |
| ACR | ||||||||
| <30 | 2745 | 61 | 0.60 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| ≥30 | 328 | 23 | 2.01 | 3.16 (1.86–5.35) | 2.22 (1.29–3.84) | 1.95 (1.09–3.49) | 1.98 (1.12–3.50) | 2.28 (1.24–4.20) |
ref, reference.
aAdjusted for age, sex, systolic blood pressure, ACR (for eGFR) or eGFR (for ACR).
DISCUSSION
This study of African American participants from a community-based cohort found that lower eGFR and higher urine ACR were significantly associated with higher LVM and LVMI. Furthermore, urine ACR ≥30 mg/g was associated with a >2-fold higher risk of incident HF in multivariable models. This association was only partially attenuated when we adjusted for baseline echocardiographic measures. These data further support the link between kidney disease and HF risk in African Americans. While structural heart disease likely contributes to this risk of HF, our findings suggest that it may not fully explain the entire risk for HF among African Americans with kidney disease.
Our study noted an association between lower eGFR and higher urine ACR with higher LVM and LVMI. Consistent with our findings, prior studies have also noted an association of CKD with higher LVM [16–18, 21, 34]. We also found an association with even mild reductions in eGFR with higher LVM among African Americans. This is similar to the findings in a cross-sectional study of 4971 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) study, where a 1.6 greater odds for LVH was noted among participants with eGFRs between 60 and −75 mL/min/1.73 m2 [35]. This association was consistent across race/ethnic categories, including African Americans. Interestingly, we found a strong association of urine ACR with higher LVM, independent of eGFR level. A study of young hypertensive African American men also reported an association between urine ACR with elevated LVMI [36]. In previous studies of Caucasian participants, higher urine ACR (even <30 mg/g) was associated with LVH [37, 38]. Our study extends the findings of prior reports that have shown an important link between kidney disease and subclinical HF to a large, community-based cohort of African Americans with a wide range of eGFR.
We did not find an association of lower eGFR or higher urine ACR with LVEF. This is consistent with a previous study that did not show an association between CKD and LVEF in a large CKD population [34]. In contrast, a community-based cohort of Caucasian participants found an association between eGFR and LVEF [39]. In a study of patients with HF with preserved ejection fraction, higher urine ACR was associated with greater LVEF [40]. Our study differed from these previous studies in that we included a large, relatively healthy cohort of African American participants, many of them younger in age and largely with preserved kidney function, which may explain the difference in findings. It is plausible that at this range of kidney disease, development of LVH may precede reductions in LVEF.
There was a significant association of higher urine ACR with the risk of incident HF in our study. However, the association of lower eGFR with incident HF was attenuated with multivariable adjustment. It is possible that we did not have adequate power to detect an association between eGFR and incident HF, as our results differ from prior studies. A previous study of young African American participants noted that CKD was one of the strongest predictors of incident HF [23]. Among older participants, the association of eGFR with the risk of incident HF was stronger in blacks compared with whites [22]. Similar to our findings, among clinical trial participants at high risk for incident HF, urine ACR was identified as a novel predictor of HF [41]. Another analysis noted that any level of measurable urine ACR was associated with a greater risk of CVD (including HF) [42]. These studies and ours highlight the importance of comprehensive measurement of kidney disease, which includes testing of urine ACR, in high-risk patient populations. Even in the absence of reduced eGFR, high urine albumin excretion may have important cardiovascular pathological consequences, including endothelial damage and inflammation, which may contribute to the risk of HF. This hypothesis is supported in part by clinical trials, which have shown that renin–angiotensin–aldosterone system inhibitors decrease the risk of subclinical and clinical HF across many populations [43–46].
Adjusting for echocardiographic variables from baseline only partially attenuated the association between higher urine ACR and incident HF. While structural heart disease is an important risk factor for the development of clinical HF, the pathogenesis of HF is likely very complex and multifactorial, particularly in the setting of kidney disease. Kidney disease is associated with retention of uremic toxins, impaired sodium handling, deranged mineral metabolism, inflammation and other processes, which may contribute to altered biological pathways that lead to HF. For example, alterations in FGF-23 and parathyroid hormone, which are common in the setting of kidney disease, have been linked to the development of HF [47–50]. Further understanding of the mechanisms linking kidney disease with HF may lead to effective targeted primary and secondary therapies.
Our study had several strengths. We studied a large, well-characterized community-based African American population. We had standardized echocardiogram data available that were centrally quantified. HF events were ascertained by central physician adjudication. We recognize a few limitations as well. Determination of prevalent HF (by self-report) and HF adjudication did not begin until Exam 2. Only one serum creatinine value was available and a large proportion of participants were missing urine ACR measures. Although we used multiple imputation to address the issue of missing data, we do recognize that missing data were likely accrued in a nonrandom fashion. Exposures and covariates were modeled as a single measure (rather than time-varying) due to limited availability of repeat measures of some covariates. There were relatively few participants with CKD (either by reduced eGFR or high urine ACR) at baseline, and the number of HF events was low. We had only baseline measures of our echocardiographic measures of interest. The variables diastolic dysfunction and left atrial diameter were not quantified from JHS echocardiograms. We were unable to make comparisons with other patient populations such as white Americans. The study was conducted among a community-based African American population in the South, and thus results may not be generalizable to all African American populations.
In conclusion, among participants in a community-based cohort of African Americans, lower eGFR and higher urine ACR were significantly associated with higher LVM. Additionally, there was an independent association of higher urine ACR with the risk of incident HF, which may identify urine ACR as an important modifiable risk factor in this high-risk patient population. Further studies are needed to understand the mechanisms linking early signs of kidney damage with the risk of HF in high-risk patient populations.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflicts of interest to disclose. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health or the U.S. Department of Health and Human Services. The US Department of Veterans Affairs does not endorse any of the statements or opinions advocated by this article.
(See related article by Tamez. African Americans with left ventricular hypertrophy and chronic kidney disease: what should we do? Nephrol Dial Transplant 2016; 31: 1969–1970)
Supplementary Material
ACKNOWLEDGEMENTS
We thank all participants of the Jackson Heart Study for their time, effort and dedication toward this study. This study was supported by the following funding sources: R01DK102134 (B.Y.) and K23DK088865 (N.B.). The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C and HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. B.Y. and I.H.d.B. are also supported in part by funding from the Veterans Affairs Puget Sound Health Care System.
REFERENCES
- 1.Babayev R, Whaley-Connell A, Kshirsagar A et al. Association of race and body mass index with ESRD and mortality in CKD stages 3–4: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2013; 61: 404–412 [DOI] [PubMed] [Google Scholar]
- 2.Grams ME, Chow EK, Segev DL et al. Lifetime incidence of CKD stages 3–5 in the United States. Am J Kidney Dis 2013; 62: 245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derose SF, Rutkowski MP, Crooks PW et al. Racial differences in estimated GFR decline, ESRD, and mortality in an integrated health system. Am J Kidney Dis 2013; 62: 236–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peralta CA, Vittinghoff E, Bansal N et al. Trajectories of kidney function decline in young black and white adults with preserved GFR: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Kidney Dis 2013; 62: 261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muntner P, Newsome B, Kramer H et al. Racial differences in the incidence of chronic kidney disease. Clin J Am Soc Nephrol 2012; 7: 101–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer H, Palmas W, Kestenbaum B et al. Chronic kidney disease prevalence estimates among racial/ethnic groups: the Multi-Ethnic Study of Atherosclerosis. Clin J Am Soc Nephrol 2008; 3: 1391–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu CC, Kao WH, Coresh J et al. Apolipoprotein E and progression of chronic kidney disease. JAMA 2005; 293: 2892–2899 [DOI] [PubMed] [Google Scholar]
- 8.Kottgen A, Russell SD, Loehr LR et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol 2007; 18: 1307–1315 [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg I, Moss AJ, McNitt S et al. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol 2006; 98: 485–490 [DOI] [PubMed] [Google Scholar]
- 10.Givertz MM, Postmus D, Hillege HL et al. Renal function trajectories and clinical outcomes in acute heart failure. Circ Heart Fail 2014; 7: 59–67 [DOI] [PubMed] [Google Scholar]
- 11.Komukai K, Ogawa T, Yagi H et al. Decreased renal function as an independent predictor of re-hospitalization for congestive heart failure. Circ J 2008; 72: 1152–1157 [DOI] [PubMed] [Google Scholar]
- 12.Carrasco-Sanchez FJ, Galisteo-Almeda L, Paez-Rubio I et al. Prognostic value of cystatin C on admission in heart failure with preserved ejection fraction. J Card Fail 2011; 17: 31–38 [DOI] [PubMed] [Google Scholar]
- 13.Shirakabe A, Hata N, Kobayashi N et al. Long-term prognostic impact after acute kidney injury in patients with acute heart failure. Int Heart J 2012; 53: 313–319 [DOI] [PubMed] [Google Scholar]
- 14.Hillege HL, Nitsch D, Pfeffer MA et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006; 113: 671–678 [DOI] [PubMed] [Google Scholar]
- 15.Hillege HL, Girbes AR, de Kam PJ et al. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 2000; 102: 203–210 [DOI] [PubMed] [Google Scholar]
- 16.Eckardt KU, Scherhag A, Macdougall IC et al. Left ventricular geometry predicts cardiovascular outcomes associated with anemia correction in CKD. J Am Soc Nephrol 2009; 20: 2651–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin A, Thompson CR, Ethier J et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 1999; 34: 125–134 [DOI] [PubMed] [Google Scholar]
- 18.Paoletti E, Bellino D, Cassottana P et al. Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis 2005; 46: 320–327 [DOI] [PubMed] [Google Scholar]
- 19.Foley RN, Curtis BM, Randell EW et al. Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clin J Am Soc Nephrol 2010; 5: 805–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foley RN, Parfrey PS, Kent GM et al. Serial change in echocardiographic parameters and cardiac failure in end-stage renal disease. J Am Soc Nephrol 2000; 11: 912–916 [DOI] [PubMed] [Google Scholar]
- 21.Levin A, Singer J, Thompson CR et al. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis 1996; 27: 347–354 [DOI] [PubMed] [Google Scholar]
- 22.Bibbins-Domingo K, Chertow GM, Fried LF et al. Renal function and heart failure risk in older black and white individuals: the Health, Aging, and Body Composition Study. Arch Intern Med 2006; 166: 1396–1402 [DOI] [PubMed] [Google Scholar]
- 23.Bibbins-Domingo K, Pletcher MJ, Lin F et al. Racial differences in incident heart failure among young adults. N Engl J Med 2009; 360: 1179–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor HA., Jr The Jackson Heart Study: an overview. Ethn Dis 2005; 15(4 Suppl 6): S6-1-3. [PubMed] [Google Scholar]
- 25.Taylor HA., Jr The Jackson Heart Study of the future. Ethn Dis 2012; 22(3 Suppl 1): S1-49–1-54 [PubMed] [Google Scholar]
- 26.Fuqua SR, Wyatt SB, Andrew ME et al. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis 2005; 15(4 Suppl 6): S6-18–6-29 [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Young BA, Fulop T et al. Effects of serum creatinine calibration on estimated renal function in African Americans: the Jackson Heart Study. Am J Med Sci 2015; 349: 379–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpenter MA, Crow R, Steffes M et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci 2004; 328: 131–144 [DOI] [PubMed] [Google Scholar]
- 30.Young BA, Katz R, Boulware LE et al. Risk factors for rapid kidney function decline among African-Americans: the Jackson Heart Study. Am J Kidney Dis 2016; doi:10.1053/j.ajkd.2016.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox ER, Taylor J, Taylor H et al. Left ventricular geometric patterns in the Jackson cohort of the Atherosclerotic Risk in Communities (ARIC) Study: clinical correlates and influences on systolic and diastolic dysfunction. Am Heart J 2007; 153: 238–244 [DOI] [PubMed] [Google Scholar]
- 32.Choudhary G, Jankowich M, Wu WC. Elevated pulmonary artery systolic pressure predicts heart failure admissions in African Americans: Jackson Heart Study. Circ Heart Fail 2014; 7: 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keku E, Rosamond W, Taylor HA Jr. et al. Cardiovascular disease event classification in the Jackson Heart Study: methods and procedures. Ethn Dis 2005; 15(4 Suppl 6): S6-62–S6-70 [PubMed] [Google Scholar]
- 34.Park M, Hsu CY, Li Y et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 2012; 23: 1725–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moran A, Katz R, Jenny NS et al. Left ventricular hypertrophy in mild and moderate reduction in kidney function determined using cardiac magnetic resonance imaging and cystatin C: the multi-ethnic study of atherosclerosis (MESA). Am J Kidney Dis 2008; 52: 839–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Post WS, Blumenthal RS, Weiss JL et al. Spot urinary albumin–creatinine ratio predicts left ventricular hypertrophy in young hypertensive African-American men. Am J Hypertens 2000; 13: 1168–1172 [DOI] [PubMed] [Google Scholar]
- 37.Lieb W, Mayer B, Stritzke J et al. Association of low-grade urinary albumin excretion with left ventricular hypertrophy in the general population: the MONICA/KORA Augsburg Echocardiographic Substudy. Nephrol Dial Transplant 2006; 21: 2780–2787 [DOI] [PubMed] [Google Scholar]
- 38.Wachtell K, Palmieri V, Olsen MH et al. Urine albumin/creatinine ratio and echocardiographic left ventricular structure and function in hypertensive patients with electrocardiographic left ventricular hypertrophy: the LIFE Study. Losartan Intervention for Endpoint Reduction. Am Heart J 2002; 143: 319–326 [DOI] [PubMed] [Google Scholar]
- 39.Nerpin E, Ingelsson E, Riserus U et al. The association between glomerular filtration rate and left ventricular function in two independent community-based cohorts of elderly. Nephrol Dial Transplant 2014; 29: 2069–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katz DH, Burns JA, Aguilar FG et al. Albuminuria is independently associated with cardiac remodeling, abnormal right and left ventricular function, and worse outcomes in heart failure with preserved ejection fraction. JACC Heart Fail 2014; 2: 586–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong YW, Thomas L, Sun JL et al. Predictors of incident heart failure hospitalizations among patients with impaired glucose tolerance: insight from the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research study. Circ Heart Fail 2013; 6: 203–210 [DOI] [PubMed] [Google Scholar]
- 42.Ruggenenti P, Porrini E, Motterlini N et al. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol 2012; 23: 1717–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verdecchia P, Sleight P, Mancia G et al. Effects of telmisartan, ramipril, and their combination on left ventricular hypertrophy in individuals at high vascular risk in the ongoing Telmisartan Alone and in Combination with Ramipril Global End Point Trial and the Telmisartan Randomized Assessment Study in ACE intolerant subjects with cardiovascular disease. Circulation 2009; 120: 1380–1389 [DOI] [PubMed] [Google Scholar]
- 44.Pitt B, Reichek N, Willenbrock R et al. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation 2003; 108: 1831–1838 [DOI] [PubMed] [Google Scholar]
- 45.Yusuf S, Teo KK, Pogue J et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358: 1547–1559 [DOI] [PubMed] [Google Scholar]
- 46.Wing LM, Reid CM, Ryan P et al. A comparison of outcomes with angiotensin-converting-enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med 2003; 348: 583–592 [DOI] [PubMed] [Google Scholar]
- 47.Faul C, Amaral AP, Oskouei B et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gutierrez OM, Januzzi JL, Isakova T et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009; 119: 2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kestenbaum B, Katz R, de Boer I et al. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol 2011; 58: 1433–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bansal N, Zelnick L, Robinson-Cohen C et al. Serum parathyroid hormone and 25-hydroxyvitamin D concentrations and risk of incident heart failure: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2014; 3: e001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.