Abstract
Background
Chronic kidney disease (CKD) is common, but the frequency of albuminuria testing and referral to nephrology care has been difficult to measure. We here characterize CKD prevalence and recognition in a complete healthcare utilization cohort of the Stockholm region, in Sweden.
Methods
We included all adult individuals (n = 1 128 058) with at least one outpatient measurement of IDMS-calibrated serum creatinine during 2006–11. Estimated glomerular filtration rate (eGFR) was calculated via the CKD-EPI equation and CKD was solely defined as eGFR <60 mL/min/1.73 m2. We also assessed the performance of diagnostic testing (albuminuria), nephrology consultations, and utilization of ICD-10 diagnoses.
Results
A total of 68 894 individuals had CKD, with a crude CKD prevalence of 6.11% [95% confidence interval (CI): 6.07–6.16%] and a prevalence standardized to the European population of 5.38% (5.33–5.42%). CKD was more prevalent among the elderly (28% prevalence >75 years old), women (6.85 versus 5.24% in men), and individuals with diabetes (17%), hypertension (17%) or cardiovascular disease (31%). The frequency of albuminuria monitoring was low, with 38% of diabetics and 27% of CKD individuals undergoing albuminuria testing over 2 years. Twenty-three per cent of the 16 383 individuals satisfying selected KDIGO criteria for nephrology referral visited a nephrologist. Twelve per cent of CKD patients carried an ICD-10 diagnostic code of CKD.
Conclusions
An estimated 6% of the adult Stockholm population accessing healthcare has CKD, but the frequency of albuminuria testing, nephrology consultations and registration of CKD diagnoses was suboptimal despite universal care. Improving provider awareness and treatment of CKD could have a significant public health impact.
Keywords: chronic renal failure, creatinine, nephrology, public health, referral
INTRODUCTION
Chronic kidney disease (CKD) is associated with increased risk of cardiovascular events, death and high healthcare costs [1]. Although there is increasing recognition of CKD and its associated morbidity [2, 3], little is known regarding the diagnosis, treatment and referral of CKD in real-world settings. Recently, a large disparity in CKD prevalence was reported among selected European countries with population samples from the late 1990s and early 2000s [4]. Differences in study design, biomarker assays, estimating equations and population representativeness may influence these estimates [5]. However, treatment practices may also affect CKD prevalence. There is a need for reliable estimates of CKD prevalence connected to existing practice patterns to inform CKD management and prevention planning locally and globally.
CKD remains a neglected non-communicable disease [6] frequently under-recognized by both patient and provider [7–9]. Health systems and policies supporting the care of people with CKD vary across countries [10], and there have been few attempts to quantify the extent of CKD monitoring and nephrologist referral [11]. Current guidelines recognize albuminuria as central to CKD staging and provide criteria for referral to a nephrologist [12–14]. It is important to determine the potential gap between these guideline recommendations and current CKD care practice. Settings with universal health coverage and aggregated data for the entire population provide an ideal situation to compare current care to guideline recommendations [15, 16]. We here evaluated the prevalence of CKD in a healthcare utilization cohort of the region of Stockholm, Sweden, and assessed the frequency of albuminuria surveillance, referral to nephrologist and utilization of appropriate diagnostic codes.
MATERIALS AND METHODS
Patient selection
The region of Stockholm is the largest in Sweden, accounting for 20–25% of the Swedish population. Sweden's healthcare system is universally accessible and publicly financed [17]. The Stockholm CREAtinine Measurements (SCREAM) project is a healthcare utilization cohort from the region of Stockholm [18]. All Swedish citizens residing or accessing healthcare in Stockholm with a valid personal identification number [19] and a measurement of serum creatinine during 2006–11 were included. These records were linked with regional and national administrative databases including sociodemographic data, migration, diagnoses, healthcare consumption, validated end-stage renal disease (ESRD) outcomes, vital status and pharmacy-dispensed prescription drugs. SCREAM includes all healthcare utilization in the region. Given the commonness of creatinine testing in healthcare, our previous analysis showed a coverage of ∼66% of the average census adult population [18]. Serum creatinine testing showed increased coverage across older age strata: 54% coverage for age range 18–44 years, 78% for 45–64 years and 92% coverage for ≥65 years [18]. The regional institutional review board and the Swedish National Board of Welfare approved the study for use of de-identified data.
Assessment of CKD prevalence
The cohort-entry point was the first available creatinine measured in connection with an outpatient consultation. We used the CKD-EPI creatinine-based equation to estimate glomerular filtration rate (eGFR) [20]. We defined CKD solely as eGFR <60 mL/min/1.73 m2 (eGFR groups G3 or above), using when available the average of two eGFR assessments 3–12 months apart [21]. Because albuminuria was tested much less frequently than creatinine, we did not consider the current definition of CKD combining these two metrics as our primary analysis, which would infer in indication bias. Ethnicity is not registered in Sweden by law, and all citizens were assumed Caucasian [22]. eGFR groups were defined as follows: G1–2 = eGFR ≥60 mL/min/1.73 m2; G3a = eGFR ≥45 and <60 mL/min/1.73 m2; G3b = eGFR ≥30 and <45 mL/min/1.73 m2; G4 = eGFR ≥15 and <30 mL/min/1.73 m2; eGFR non-dialysis = eGFR <15 mL/min/1.73 m2; and finally, ESRD was defined as undergoing dialysis or kidney transplantation, ascertained via linkage with the Swedish Renal Registry [23].
Biochemical assessments and study covariates
Laboratory tests were performed as part of healthcare encounters. Three laboratory companies provide services to Stockholm healthcare, with minimal between and within laboratory variation as shown by quality and harmonization control programmes (www.equalis.se). All serum creatinine measurements were standardized to isotope dilution mass spectrometry standards. Serum creatinine <25 μmol/L and >1500 μmol/L were considered implausible and discarded. For albuminuria measurements, we accepted both albumin-to-creatinine ratio (ACR) and dipstick proteinuria (DPR) tests. We defined albuminuria based on the test closest to the index creatinine (±6 months). All albuminuria assessments were considered for estimation of surveillance. However, for identification of patients with macroalbuminuria we excluded in-hospital albuminuria measurements.
Age was defined at the first available creatinine assessment. Comorbidities were defined according to the Charlson score [24] and based on ICD-10 diagnoses. Diabetes mellitus was enriched with the use of anti-diabetic drugs (ATC codes: A10) ±3 months from inclusion; hypertension was defined by ICD-10 codes or use antihypertensive medication (ATC codes: C03, C07, C08, C09) ±3 months from inclusion. Previous history of cardiovascular disease (CVD) was defined by the presence of myocardial infarction, congestive heart failure, peripheral vascular disease or cerebrovascular disease [24]. Information on ICD-10 diagnoses was obtained from the Regional Healthcare Utilization Database, VAL [25]. Information on dispensed prescriptions was obtained from the Swedish Prescribed Drug Registry [26].
Assessment of albuminuria monitoring, nephrology care and CKD recognition
To assess the degree of albuminuria monitoring, we identified the presence of at least one testing for albuminuria ±12 months from inclusion date. We assessed the degree of referral overall and among three specific patient populations recommended by guidelines [13]: individuals with eGFR <30 mL/min/1.73 m2, individuals with severe albuminuria (ACR >30 mg/mmol) irrespective of eGFR and individuals with both eGFR <60 mL/min/1.73 m2 and hypertension refractory to treatment (concurrent treatment with 4+ antihypertensive medications). Nephrology referral was defined as attendance to an outpatient nephrology consultation, inclusion in the Swedish Renal Registry (SRR) or current use of drugs prescribed by a nephrologist. Referral was estimated at study inclusion (up to 12 months before for nephrology consultations, up to 6 months before for drug purchases and with no time restriction for inclusion in SRR) and during follow-up (12 months). Physician diagnosis of CKD was defined by the presence of relevant ICD-10 codes in any position during the preceding 3 years and during follow-up.
Deaths within 1 year
We calculated the proportion of deaths within 12 months from inclusion. Information on death was obtained via linkage with the Swedish population Registry, which records vital status for all Swedish citizens with no loss to follow-up.
Statistical analyses
Values are presented as median with interquartile interval (IQI) or count with percentage. Age-gender standardization of prevalence was performed using the direct method, with the adult population of Sweden as of 31 December 2011 (www.scb.se) and the EU27 adult population as of 1 January 2012 (ec.europa.eu/eurostat) as standard populations. Confidence intervals (CIs) were obtained with the exact method for the crude and stratified prevalence, and with the Gamma distribution for the standardized prevalences. We also analysed CKD prevalence using alternative assumptions: (i) using only one eGFR, (ii) using strictly the average of two measurements 3–12 months apart, (iii) assuming all non-healthcare users of the region as not having CKD (census population obtained from www.scb.se), (iv) including albuminuria in the definition when available and (v) using strictly individuals with concurrent eGFR and albuminuria assessments. Multivariable risk ratios were estimated using Poisson regression models with no offset specified [27]. All analyses were performed using R (https://www.r-project.org) and Stata/MP (StataCorp).
RESULTS
Participant selection and baseline characteristics
A total of 1 334 190 individuals underwent at least one creatinine measurement in the region of Stockholm during 2006–11. Supplementary data, Figure S1 shows the selection flow-chart for this study, which resulted in 1 128 058 eligible individuals for analysis.
Median age was 50.5 years (IQI 36.2–64.4), 46% were men, median eGFR was 95.9 mL/min/1.73 m2 (82.2–109.0). A total of 62.9% of individuals had no comorbidities, 29.7% had mild, 5.5% had moderate and the remaining 1.9% had severe comorbidity burden. In particular, 28.4% of individuals had hypertension, 6.6% had diabetes and 7.0% had history of CVD. A concurrent albuminuria test was only available in 13% of the population (Supplementary data, Table S1).
Prevalence of CKD
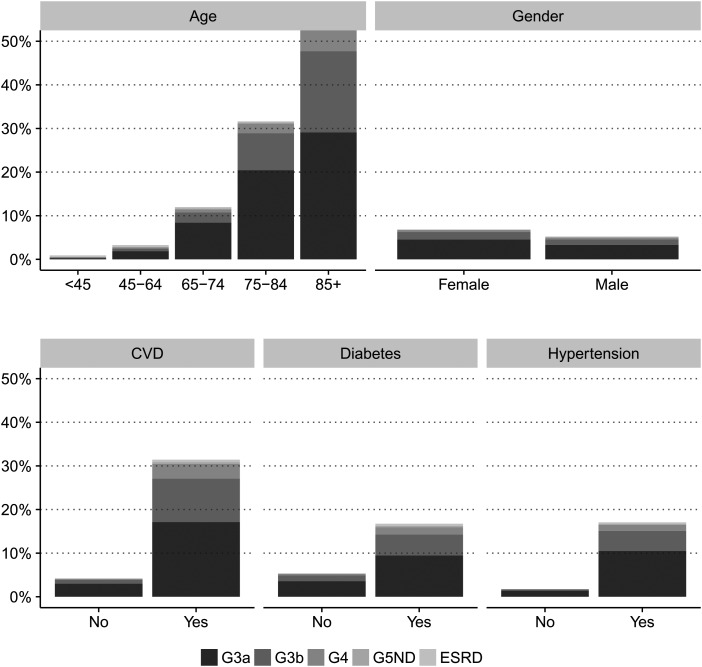
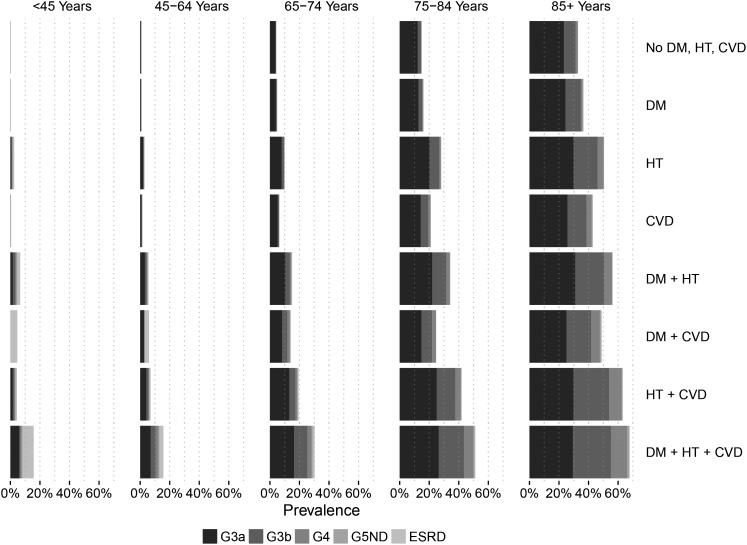
Prevalence of eGFR (G) stages is shown in Table 1. We identified 68 964 individuals with CKD, corresponding to a crude CKD prevalence of 6.11% (95% CI 6.07–6.16%). After age-sex standardization to the adult Swedish [5.62% (5.57–5.66%)] or the EU27 population [5.38% (5.33–5.42%)], CKD prevalence was slightly reduced. The CKD burden was markedly higher in older age strata, and relatively higher among women compared with men. The prevalence of CKD was considerably higher in individuals with diabetes mellitus, hypertension or CVD, overall, within each CKD strata and in combination (Figures 1 and 2). In multivariable regression, older age strata, hypertension, diabetes and CVD were the factors most strongly associated with a higher relative risk of CKD (Supplementary data, Table S2). A total of 42 644 (61.8%) individuals with CKD had the qualifying eGFR based on two measurements (Supplementary data, Table S3). The CKD prevalence estimated based on one single eGFR was similar to our main analysis: 6.20% (6.16–6.25%, Supplementary data, Table S4). In individuals with more frequent healthcare utilization and two eGFR 3–12 months apart, CKD prevalence reached 11.30% (11.20–11.39%, Supplementary data, Table S5). Assuming that all non-healthcare users in the region have no CKD yielded a conservative prevalence of 4.40% (4.37–4.44%, Supplementary data, Table S6). Including albuminuria in the definition of CKD conveyed a sicker population selection with CKD prevalence ranging between 7.01% (6.96–7.05%) and 16.67% (16.48–16.86%, Supplementary data, Table S7).
Table 1.
Participants' stratification according to eGFR strata, and analysis of CKD prevalence (eGFR <60 mL/min/1.73 m2 defines CKD)
| eGFR category |
|||||||
|---|---|---|---|---|---|---|---|
| G1–2 | G3a | G3b | G4 | G5ND | ESRD | G3+ | |
| eGFR range, mL/min/1.73 m2 | ≥60 | 45–59 | 30–44 | 15–29 | <15 | – | <60 |
| Cases | 1 059 094 | 44 802 | 17 120 | 4883 | 763 | 1396 | 68 964 |
| Crude prevalence | 93.89% (93.84–93.93)% | 3.97% (3.94–4.01)% | 1.52% (1.50–1.54)% | 0.43% (0.42–0.45)% | 0.07% (0.06–0.07)% | 0.12% (0.12–0.13)% | 6.11% (6.07–6.16)% |
| Standardized prevalence | |||||||
| To the Swedish population | 94.38% (94.20–94.57)% | 3.66% (3.62–3.69)% | 1.38% (1.36–1.40)% | 0.40% (0.39–0.41)% | 0.06% (0.06–0.07)% | 0.12% (0.12–0.13)% | 5.62% (5.57–5.66)% |
| To the EU27 population | 94.62% (94.44–94.81)% | 3.53% (3.49–3.56)% | 1.30% (1.28–1.32)% | 0.37% (0.36–0.38)% | 0.06% (0.06–0.07)% | 0.12% (0.11–0.13)% | 5.38% (5.33–5.42)% |
| Stratified prevalence | |||||||
| Age strata | |||||||
| 18–44 years | 456 304 | 594 | 204 | 111 | 61 | 336 | 1306 |
| 99.71% (99.70–99.73)% | 0.13% (0.12–0.14)% | 0.04% (0.04–0.05)% | 0.02% (0.02–0.03)% | 0.01% (0.01–0.02)% | 0.07% (0.07–0.08)% | 0.29% (0.27–0.30)% | |
| 45–64 years | 391 653 | 5127 | 1096 | 438 | 157 | 654 | 7472 |
| 98.13% (98.09–98.17)% | 1.28% (1.25–1.32)% | 0.27% (0.26–0.29)% | 0.11% (0.10– 0.12)% | 0.04% (0.03–0.05)% | 0.16% (0.15–0.18)% | 1.87% (1.83–1.91)% | |
| 65–74 years | 123 598 | 9451 | 2143 | 587 | 150 | 246 | 12 577 |
| 90.76% (90.61–90.92)% | 6.94% (6.81–7.08)% | 1.57% (1.51–1.64)% | 0.43% (0.40–0.47)% | 0.11% (0.09–0.13)% | 0.18% (0.16–0.20)% | 9.24% (9.08–9.39)% | |
| 75–84 years | 66 829 | 17 717 | 6472 | 1599 | 212 | 137 | 26 137 |
| 71.89% (71.60–72.17)% | 19.06% (18.81–19.31)% | 6.96% (6.80–7.13)% | 1.72% (1.64–1.81)% | 0.23% (0.20–0.26)% | 0.15% (0.12–0.17)% | 28.11% (27.83–28.40)% | |
| 85+ years | 20 710 | 11 913 | 7205 | 2148 | 183 | 23 | 21 472 |
| 49.10% (48.62–49.58)% | 28.24% (27.81–28.67)% | 17.08% (16.72–17.44)% | 5.09% (4.88–5.31)% | 0.43% (0.37–0.50)% | 0.05% (0.03–0.08)% | 50.90% (50.42–51.38)% | |
| Sex strata | |||||||
| Men | 489 822 | 17 108 | 6554 | 2096 | 440 | 878 | 27 076 |
| 94.76% (94.70–94.82)% | 3.31% (3.26–3.36)% | 1.27% (1.24–1.30)% | 0.41% (0.39–0.42)% | 0.09% (0.08–0.09)% | 0.17% (0.16–0.18)% | 5.24% (5.18–5.30)% | |
| Women | 569 272 | 27 694 | 10 566 | 2787 | 323 | 518 | 41 888 |
| 93.15% (93.08–93.21)% | 4.53% (4.48–4.58)% | 1.73% (1.70–1.76)% | 0.46% (0.44–0.47)% | 0.05% (0.05–0.06)% | 0.08% (0.08–0.09)% | 6.85% (6.79–6.92)% | |
| Diabetes mellitus | |||||||
| Present | 61 506 | 7005 | 3532 | 1227 | 225 | 378 | 12 367 |
| 83.26% (82.99–83.53)% | 9.48% (9.27–9.70)% | 4.78% (4.63–4.94)% | 1.66% (1.57–1.76)% | 0.30% (0.27–0.35)% | 0.51% (0.46–0.57)% | 16.74% (16.47–17.01)% | |
| Absent | 997 588 | 37 797 | 13 588 | 3656 | 538 | 1018 | 56 597 |
| 94.63% (94.59–94.67)% | 3.59% (3.55–3.62)% | 1.29% (1.27–1.31)% | 0.35% (0.34–0.36)% | 0.05% (0.05–0.06)% | 0.10% (0.09–0.10)% | 5.37% (5.33–5.41)% | |
| Hypertension | |||||||
| Present | 265 666 | 33 582 | 14 801 | 4374 | 685 | 1224 | 54 666 |
| 82.93% (82.82–83.05)% | 10.48% (10.38–10.58)% | 4.62% (4.55–4.69)% | 1.37% (1.33–1.41)% | 0.21% (0.20–0.23)% | 0.38% (0.36–0.40)% | 17.07% (16.95–17.18)% | |
| Absent | 793 428 | 11 220 | 2319 | 509 | 78 | 172 | 14 298 |
| 98.23% (98.20–98.26)% | 1.39% (1.36–1.41)% | 0.29% (0.28–0.30)% | 0.06% (0.06–0.07)% | 0.01% (0.01–0.01)% | 0.02% (0.02–0.02)% | 1.77% (1.74–1.80)% | |
| Cardiovascular disease | |||||||
| Present | 53 796 | 13 436 | 7792 | 2654 | 336 | 437 | 24,65 |
| 68.57% (68.25–68.90)% | 17.13% (16.86–17.39)% | 9.93% (9.72–10.14)% | 3.38% (3.26–3.51)% | 0.43% (0.38–0.48)% | 0.56% (0.51–0.61)% | 31.42% (31.10–31.75)% | |
| Absent | 1 005 298 | 31 366 | 9328 | 2229 | 427 | 959 | 44 309 |
| 95.78% (95.74–95.82)% | 2.99% (2.96–3.02)% | 0.89% (0.87–0.91)% | 0.21% (0.20–0.22)% | 0.04% (0.04–0.04)% | 0.09% (0.09–0.10)% | 4.22% (4.18–4.26)% | |
FIGURE 1:
Prevalence of CKD in the region of Stockholm, stratified by age, gender and comorbidities.
FIGURE 2:
Prevalence of CKD, stratified by comorbid combinations and age categories. DM, diabetes mellitus; HT, hypertension.
Albuminuria monitoring
The frequency of albuminuria monitoring in the community was low, with 38.3% of all diabetics and 27.3% of CKD individuals undergoing albuminuria testing in the 2-year period (Table 2). Albuminuria monitoring was also low in non-diabetic individuals with hypertension (20.1%) or CVD (17.6%). Assessment of dipstick proteinuria was more common than ACR (24 versus 8% overall, Supplementary data, Table S8). In multivariable regression (Supplementary data, Table S9), albuminuria testing was more often performed in older age categories, in participants with lower eGFR, and comorbidities such as diabetes and rheumatoid disease. Individuals with dementia, on the other hand, had a lower probability of being tested for albuminuria. The same pattern was observed when stratifying by the presence of diabetes.
Table 2.
Albuminuria monitoring, referral to nephrology care and physician diagnosis of CKD
| eGFR category |
||||||||
|---|---|---|---|---|---|---|---|---|
| G1–2 | G3a | G3b | G4 | G5ND | ESRD | G3+ | Overall | |
| Albuminuria monitoring | ||||||||
| Overall (n = 1 128 058) | 162 020 (15.30%) | 11 119 (24.82%) | 4709 (27.51%) | 1771 (36.27%) | 417 (54.65%) | 787 (56.38%) | 18 803 (27.26%) | 180 823 (16.03%) |
| In diabetic patients (n = 73 873) | 23 179 (37.69%) | 2796 (39.91%) | 1428 (40.43%) | 581 (47.35%) | 132 (58.67%) | 180 (47.62%) | 5117 (41.38%) | 28 296 (38.30%) |
| In non-diabetic, hypertensive patients (n = 269 886) | 43 237 (19.08%) | 6156 (22.62%) | 2837 (24.74%) | 1079 (33.72%) | 260 (55.56%) | 540 (61.78%) | 10 872 (25.15%) | 54 109 (20.05%) |
| In non-diabetic, CVD patients (n = 62 643) | 7128 (16.21%) | 2037 (19.46%) | 1183 (20.20%) | 500 (26.33%) | 88 (42.51%) | 81 (33.20%) | 3889 (20.82%) | 11 017 (17.59%) |
| Referral to nephrology care | ||||||||
| Overall (n = 68 964) | NA | 1601 (3.57%) | 1525 (8.91%) | 1489 (30.49%) | 567 (74.31%) | 1396 (100.00%) | 6578 (9.54%) | NA |
| KDIGO criteria | ||||||||
| Individuals with eGFR <30 mL/min (n = 5646) | NA | NA | NA | 1489 (30.49%) | 567 (74.31%) | NA | NA | 2056 (36.42%) |
| Individuals with albuminuria >30 mg/mmol (n = 3720) | 504 (21.88%) | 122 (26.35%) | 174 (44.16%) | 247 (78.66%) | 149 (94.90%) | 88 (100.00%) | NA | 1284 (34.52%) |
| Individuals with eGFR <60 mL/min and refractory hypertension (n = 16 383) | NA | 282 (6.08%) | 399 (14.02%) | 474 (42.82%) | 205 (89.13%) | 273 (100.00%) | NA | 1633 (17.96%) |
| Any KDIGO criteria (n = 16 383) | 504 (21.88%) | 368 (7.37%) | 503 (16.16%) | 1489 (30.49%) | 567 (74.31%) | 325 (100.00%) | NA | 3756 (22.93%) |
| Physician diagnosis of CKD | ||||||||
| Overall (n = 68 964) | NA | 1343 (3.00%) | 2439 (14.25%) | 2313 (47.37%) | 650 (85.19%) | 1384 (99.14%) | 8129 (11.79%) | 10 231 (0.91%) |
| In diabetic patients (n = 73 873) | NA | 351 (5.01%) | 717 (20.30%) | 722 (58.84%) | 203 (90.22%) | 374 (98.94%) | 2367 (19.14%) | 2667 (3.61%) |
| In hypertensive patients (n = 320 332) | NA | 1144 (3.41%) | 2212 (14.94%) | 2143 (48.99%) | 609 (88.91%) | 1215 (99.26%) | 7323 (13.40%) | 8621 (2.69%) |
| In CVD patients (n = 78 451) | NA | 594 (4.42%) | 1323 (16.98%) | 1262 (47.55%) | 283 (84.23%) | 436 (99.77%) | 3898 (15.81%) | 4244 (5.41%) |
NA, not applicable or not assessed.
Referral to nephrology care
Less than 10% of identified CKD cases were seen by a nephrologist (Table 2). When we addressed selected patient populations in which referral is recommended by KDIGO, 36% of individuals with eGFR stages G4 and G5 not yet on dialysis, 35% of individuals with severe albuminuria and 8% of CKD individuals with hypertension refractory to treatment were referred to nephrology care. Pooling individuals satisfying any of these three criteria (n = 16 383), only 23% were referred. In multivariable regression, the likelihood of referral was higher across participants with lower eGFR and in individuals with hypertension. On the other hand, older patients, women and individuals with diabetes or CVD were less likely to be referred (Supplementary data, Table S10).
Physician diagnosis of CKD
Only 12% of the patients in CKD stage G3+ carried an ICD-10-CM diagnosis of CKD (Table 2). Although CKD diagnosis was more common among individuals with comorbid conditions (diabetes, CVD and hypertension), it did not surpass 22% in any of these populations. In multivariable regression, older patients, women and earlier CKD stages were less likely to be diagnosed. On the other hand, patients with hypertension, diabetes and CVD history were more likely to carry a diagnosis (Supplementary data, Table S11).
Deaths within 1 year
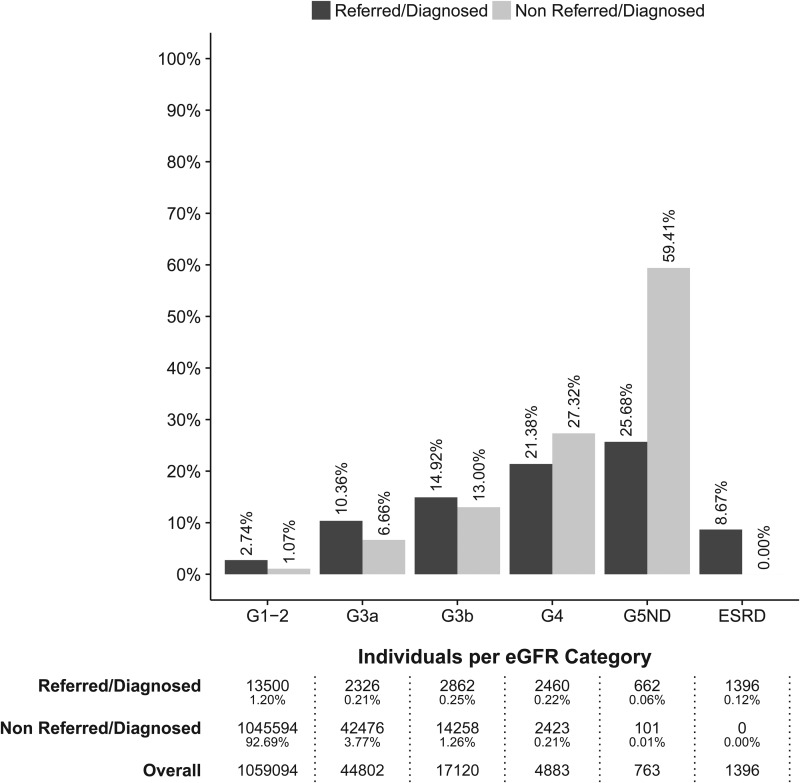
Collectively, 23 206 individuals were referred to nephrology care or carried a physician diagnosis. Of those, 9706 were individuals with G3+ (14% of all identified CKD patients) (Table 2, Supplementary data, Figure S2). A total of 18 491 (2%) individuals died within 12 months. Of those, 6891 deaths occurred among CKD cases (10% of all CKD cases) (Supplementary data, Table S12). In eGFR categories G1–2 to G3b, the proportion of deaths was higher among referred/diagnosed individuals than among non-referred/diagnosed (Figure 3). In eGFR categories G4 and G5 non-dialysis, however, the opposite was true and death occurred more often in non-referred/diagnosed compared with referred/diagnosed individuals (21 versus 27% and 26 versus 60%, G4 and G5 non-dialysis, respectively).
FIGURE 3:
Proportions of deaths within 12 months, by eGFR group (G3+ defines CKD) and presence of nephrology referral/physician diagnosis. Also shown are the number and proportion of individuals in each category.
DISCUSSION
We provide a comprehensive picture of the full CKD spectrum in the largest metropolitan area of Sweden. We confirm that CKD (defined solely by eGFR) is common, present in ∼6% of the population accessing healthcare and in the most conservative scenario (assuming all non-healthcare users as not having CKD), present in 4.4%. We also show that albuminuria measurement is conducted in a minority of individuals and that disease recognition and referral to specialists are suboptimal, even in high-risk groups. Overall, there is a considerable gap between the CKD diagnosis, staging and referral patterns and recent clinical practice guidelines [12], identifying areas for improvement also in this setting with government funded healthcare.
Our CKD prevalence estimates are in line with those reported from other developed countries [28, 29]. The large sample size allows us to estimate severe CKD and informative subgroups (cross-classification by diabetes, hypertension, CVD and albuminuria testing) with unprecedented precision. Direct comparison estimates across studies is hampered by differences in study design, data coverage, methodology and reporting. For example, probability samples, such as NHANES or ARIC, require participants to volunteer, inevitably excluding sicker individuals [30]. On the other hand, healthcare utilization cohorts, such as this one, may overestimate CKD estimates since non-users of the health system are excluded. However, the high frequency of creatinine testing in healthcare favours our approach, which captures all cases and provides a large sample size to study severe disease, subgroups of interest and disease management. The lowest (54%) population coverage in SCREAM, found for the age range 18–44 years [18], is not far from the response rate of many of the existing sampling surveys [4]. Similar to previous reports, CKD in our study was more common among the elderly, among women and among those with comorbid diabetes, hypertension or CVD [2, 4, 31]. Old age was the strongest risk factor for prevalent CKD, followed by CVD and diabetes, in agreement with US trends [2]. Given the overlap in disease, strong CVD and diabetes prevention efforts can also target CKD.
Debate exists as to whether general screening programmes are cost-effective [32, 33]. Our study and others [34] show that screening is already taking place on a large scale in healthcare. The problem may lie in the integration of this existing information into the medical decision process. Despite frequent creatinine measurements being available in most individuals, the recognition of CKD in our region was worryingly low, even in advanced CKD stages. This under-utilization of ICD diagnoses of CKD is in agreement with current literature [7, 9, 35, 36], and overall emphasizes the importance of estimating CKD burden on the basis of laboratory values. The strong interaction between albuminuria and eGFR in predicting the risk of ESRD and mortality is well established [37, 38], and screening and monitoring for albuminuria is recommended in high-risk individuals [14, 21]. We show a suboptimal proportion of the population being tested for albuminuria. In the interpretation of these findings we must acknowledge the possibility of on-site dipstick albuminuria testing by primary healthcare centres not captured in our underestimates. Nevertheless, a recent USRDS study describes probabilities of albuminuria testing close to ours [39].
Our analysis identified a large population of individuals in whom referral was indicated but who did not receive a nephrology consultation. Because the data collection period of SCREAM precedes the publication of the latest KDIGO guidelines [12], we are not evaluating their implementation in our practice, but merely quantifying the magnitude of the population in need of nephrology care. If Swedish practitioners were to strictly follow KDIGO recommendations, our region would experience an unfeasible >300% increase in the nephrology consultation rates. It has been suggested that a shift towards a primary care model with an integrated programme among different healthcare professionals would serve best in the identification and treatment of early CKD [40, 41]. In current practice, this is the predominant model in Sweden, whereby the management of CKD patients is initially performed by general practitioners and sometimes guided at distance by a nephrologist. To date, the success of these models has not been evaluated in our country, but it has been found to be moderate in other settings [11]. In our study, women were 43% more likely to be diagnosed with CKD, but 27% less likely to consult a nephrologist. It is possible that physicians still base their decision of referral on serum creatinine rather than on eGFR. Automated reporting of eGFR may facilitate physician awareness and has shown to lead to a sustained increase in referrals [42, 43]. From 2016, the Stockholm region will implement automatic eGFR reporting, which may influence CKD recognition and care. Another interesting finding in our study relates to the observed difference in mortality rates between referred and non-referred patients with advanced CKD; presumably, non-referred patients with advanced CKD are too sick or fragile for a referral to be useful. Alternatively, these patients may have been identified too late. The high mortality rate among non-referred patients with CKD 5ND is particularly striking. This is an important area that has been poorly studied, and vindicates the suitability of representative healthcare extractions such as this study, given that this subgroup is captured in neither CKD referral cohorts nor population-based studies [44, 45].
This is the largest European study to assess the burden of CKD. An important strength is the inclusion of all serum creatinine measurements performed in our region, IDMS-standardization of these measures and the large coverage of the population. Another strength is our ability to assess CKD based on two measurements in a large population subset (62% of CKD cases), which increases our accuracy. Finally, due to the universal and publicly funded healthcare of Sweden and to the possibility of linking to administrative and healthcare utilization records, we were able to assess nephrology consultation patterns, and the level of CKD recognition and monitoring with precision. However, the study also has limitations, starting with the focus on healthcare-utilizing individuals, which under-represents the healthy young population. Given the low frequency of albuminuria monitoring, we were unable to classify CKD with these combined metrics. When we did so in our sensitivity analyses, the indication/selection bias became more severe and increased CKD prevalence by almost 3-fold. We acknowledge that referral is affected both by the recognition of CKD in primary/specialist care and by the culture of referral acceptance at the nephrology department. Sweden has a decentralized care system; the nephrologists may deny referrals and the patient can be sent back without a physical consultation, generally accompanied by standard treatment recommendations. We also acknowledge the lack of information on body mass index, diabetes duration or blood pressure. Finally, our results reflect CKD prevalence and practice in Stockholm during 2006–11, and may not necessarily extrapolate to other regions or to other periods.
Among 1.1 million adults from the region of Stockholm accessing healthcare and being tested for creatinine, 6% were found to have eGFR <60 mL/min/1.73 m2. Based on conservative projections, we conclude that between 4.5 and 6% of the population census of the region have CKD on the basis of eGFR. Overall, the frequency of albuminuria monitoring, consultation by nephrology care and registration of diagnosis of renal disease was low, even among subgroups with more severe disease or indications for referral. From a public health perspective, there is a clear need to better quantify the prevalence and distribution of CKD. However, this is also important for healthcare systems, as moderate CKD stages are potentially good targets for preventative therapy and more advanced ones require timely identification and care, as well as planning of renal replacement therapy.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
ACKNOWLEDGEMENTS
SCREAM has obtained financial support from Stockholm County. We also acknowledge grant support from the Swedish Heart and Lung Foundation, The Swedish Research Council, Dalarna University, Martin Rind's and Westman's foundations. J.C. and M.E.G. acknowledge support from the US National Kidney Foundation (NKF) and the NIDDK (R01DK100446-01; K08DK092287). Funding sources had no role in the conception, design, conduct or analysis of this study, or in the decision to submit the manuscript for publication.
REFERENCES
- 1.Matsushita K, van der Velde M, Astor BC et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038–2047 [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Wang F, Wang L et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012; 379: 815–822 [DOI] [PubMed] [Google Scholar]
- 4.Bruck K, Stel VS, Gambaro G et al. CKD prevalence varies across the European general population. J Am Soc Nephrol 2016, 27: 2135–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCullough K, Sharma P, Ali T et al. Measuring the population burden of chronic kidney disease: a systematic literature review of the estimated prevalence of impaired kidney function. Nephrol Dial Transplant 2012; 27: 1812–1821 [DOI] [PubMed] [Google Scholar]
- 6.Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385: 117–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Wen XJ, Pavkov ME et al. Awareness of kidney disease among US adults: findings from the 2011 behavioral risk factor surveillance system. Am J Nephrol 2014; 39: 306–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couser WG, Remuzzi G, Mendis S et al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 2011; 80: 1258–1270 [DOI] [PubMed] [Google Scholar]
- 9.Ravera M, Noberasco G, Weiss U et al. CKD awareness and blood pressure control in the primary care hypertensive population. Am J Kidney Dis 2011; 57: 71–77 [DOI] [PubMed] [Google Scholar]
- 10.Bello AK, Levin A, Manns BJ et al. Effective CKD care in European countries: challenges and opportunities for health policy. Am J Kidney Dis 2015; 65: 15–25 [DOI] [PubMed] [Google Scholar]
- 11.Allen AS, Forman JP, Orav EJ et al. Primary care management of chronic kidney disease. J Gen Intern Med 2011; 26: 386–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapter 3: Management of progression and complications of CKD. Kidney Int Suppl 2013; 3: 73–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapter 5: Referral to specialists and models of care. Kidney Int Suppl 2013; 3: 112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Clinical Guideline Centre (UK). Chronic Kidney Disease (Partial Update): Early Identification and Management of Chronic Kidney Disease in Adults in Primary and Secondary Care. Investigating chronic kidney disease http://www-ncbi-nlm-nih-gov.proxy.kib.ki.se/books/NBK328146/; National Institute for Health and Care Excellence (UK), 2014. (19 March 2016, date last accessed) [PubMed] [Google Scholar]
- 15.Vart P, Gansevoort RT, Joosten MM et al. Socioeconomic disparities in chronic kidney disease: a systematic review and meta-analysis. Am J Prev Med 2015; 48: 580–592 [DOI] [PubMed] [Google Scholar]
- 16.Bello A, Hemmelgarn B, Manns B et al. Use of administrative databases for health-care planning in CKD. Nephrol Dial Transplant 2012; 27 (Suppl 3): iii12–iii18 [DOI] [PubMed] [Google Scholar]
- 17.Anell A, Glenngard AH, Merkur S. Sweden health system review. Health Syst Transit 2012; 14: 1–159 [PubMed] [Google Scholar]
- 18.Runesson B, Gasparini A, Qureshi AR et al. The Stockholm CREAtinine Measurements (SCREAM) project: protocol overview and regional representativeness. Clin Kidney J 2016; 9: 119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009; 24: 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Group KDIGOKCW. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 22.Wandell PE. Population groups in dietary transition. Food Nutr Res 2013; 57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swedish Renal Registry. Swedish Renal Registry: Annual report. www.snronline.se (20 June 2016, date last accessed)
- 24.Quan H, Sundararajan V, Halfon P et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1073–1077 [DOI] [PubMed] [Google Scholar]
- 25.Zarrinkoub R, Wettermark B, Wändell P et al. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail 2013; 15: 995–1002 [DOI] [PubMed] [Google Scholar]
- 26.Wettermark B, Zoega H, Furu K et al. The Nordic prescription databases as a resource for pharmacoepidemiological research—a literature review. Pharmacoepidemiol Drug Saf 2013; 22: 691–699 [DOI] [PubMed] [Google Scholar]
- 27.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol 2004; 160: 301–305 [DOI] [PubMed] [Google Scholar]
- 28.De Nicola L, Zoccali C. Chronic kidney disease prevalence in the general population: heterogeneity and concerns. Nephrol Dial Transplant 2016; 31: 331–335 [DOI] [PubMed] [Google Scholar]
- 29.Matsushita K, Mahmoodi BK, Woodward M et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 2012; 307: 1941–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebholz CM, Coresh J, Ballew SH et al. Kidney failure and ESRD in the Atherosclerosis Risk in Communities (ARIC) study: comparing ascertainment of treated and untreated kidney failure in a cohort study. Am J Kidney Dis 2015; 66: 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stack AG, Casserly LF, Cronin CJ et al. Prevalence and variation of Chronic Kidney Disease in the Irish health system: initial findings from the National Kidney Disease Surveillance Programme. BMC Nephrol 2014; 15: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fink HA, Ishani A, Taylor BC et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the U.S. Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2012; 156: 570–581 [DOI] [PubMed] [Google Scholar]
- 33.Komenda P, Ferguson TW, Macdonald K et al. Cost-effectiveness of primary screening for CKD: a systematic review. Am J Kidney Dis 2014; 63: 789–797 [DOI] [PubMed] [Google Scholar]
- 34.So BH, Methven S, Hair MD et al. Socio-economic status influences chronic kidney disease prevalence in primary care: a community-based cross-sectional analysis. Nephrol Dial Transplant 2015; 30: 1010–1017 [DOI] [PubMed] [Google Scholar]
- 35.Ronksley PE, Tonelli M, Quan H et al. Validating a case definition for chronic kidney disease using administrative data. Nephrol Dial Transplant 2012; 27: 1826–1831 [DOI] [PubMed] [Google Scholar]
- 36.Robertson LM, Denadai L, Black C et al. Is routine hospital episode data sufficient for identifying individuals with chronic kidney disease? A comparison study with laboratory data. Health Informatics J 2016; 22: 383–396 [DOI] [PubMed] [Google Scholar]
- 37.Tangri N, Grams ME, Levey AS et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA 2016; 315: 164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with mortality and end-stage renal disease: a collaborative meta-analysis of kidney disease cohorts. Kidney Int 2011; 79: 1331–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.US Renal Data System. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 40.Shahinian VB, Saran R. The role of primary care in the management of the chronic kidney disease population. Adv Chronic Kidney Dis 2010; 17: 246–253 [DOI] [PubMed] [Google Scholar]
- 41.Becker BN. Filling the gap in CKD: the health care workforce and faculty development. Am J Kidney Dis 2011; 57: 198–201 [DOI] [PubMed] [Google Scholar]
- 42.Wang V, Hammill BG, Maciejewski ML et al. Impact of automated reporting of estimated glomerular filtration rate in the veterans health administration. Med Care 2015; 53: 177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips LA, Phillips BM, Meran S et al. The long-term impact of eGFR reporting on referral patterns. Eur J Intern Med 2014; 25: 97–101 [DOI] [PubMed] [Google Scholar]
- 44.O'Hare AM, Choi AI, Bertenthal D et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 2007; 18: 2758–2765 [DOI] [PubMed] [Google Scholar]
- 45.De Nicola L, Chiodini P, Zoccali C et al. Prognosis of CKD patients receiving outpatient nephrology care in Italy. Clin J Am Soc Nephrol 2011; 6: 2421–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.