Abstract
Although the modulation of social behaviors by most major neurochemical systems has been explored, there are still standouts, including the study of vasoactive intestinal polypeptide (VIP). VIP is a modulator of circadian, reproductive, and seasonal rhythms and is well known for its role in reproductive behavior, as it is the main vertebrate prolactin-releasing hormone. Originally isolated as a gut peptide, VIP and its cognate receptors are present in virtually every brain area that is important for social behavior, including all nodes of the core “social behavior network” (SBN). Furthermore, VIP cells show increased transcriptional activity throughout the SBN in response to social stimuli. Using a combination of comparative and mechanistic approaches in socially diverse species of estrildid finches and emberizid sparrows, we have identified neural “hotspots” in the SBN that relate to avian affiliative behavior, as well as neural “hotspots” that may represent critical nodes underlying a trade-off between aggression and parental care. Specifically, we have found that: (1) VIP fiber densities and VIP receptor binding in specific brain sites, such as the lateral septum, medial extended amygdala, arcopallium, and medial nidopallium, correlate with species and/or seasonal differences in flocking behavior, and (2) VIP cells and fibers within the anterior hypothalamus—caudocentral septal circuit relate positively to aggression and negatively to parental care while VIP elements in the mediobasal hypothalamus relate negatively to aggression and positively to parental care. Thus, while a given behavior or social context likely activates VIP circuitry throughout the SBN and beyond, key brain sites emerge as potential “hotspots” for the modulation of affiliation, aggression, and parental care.
Introduction
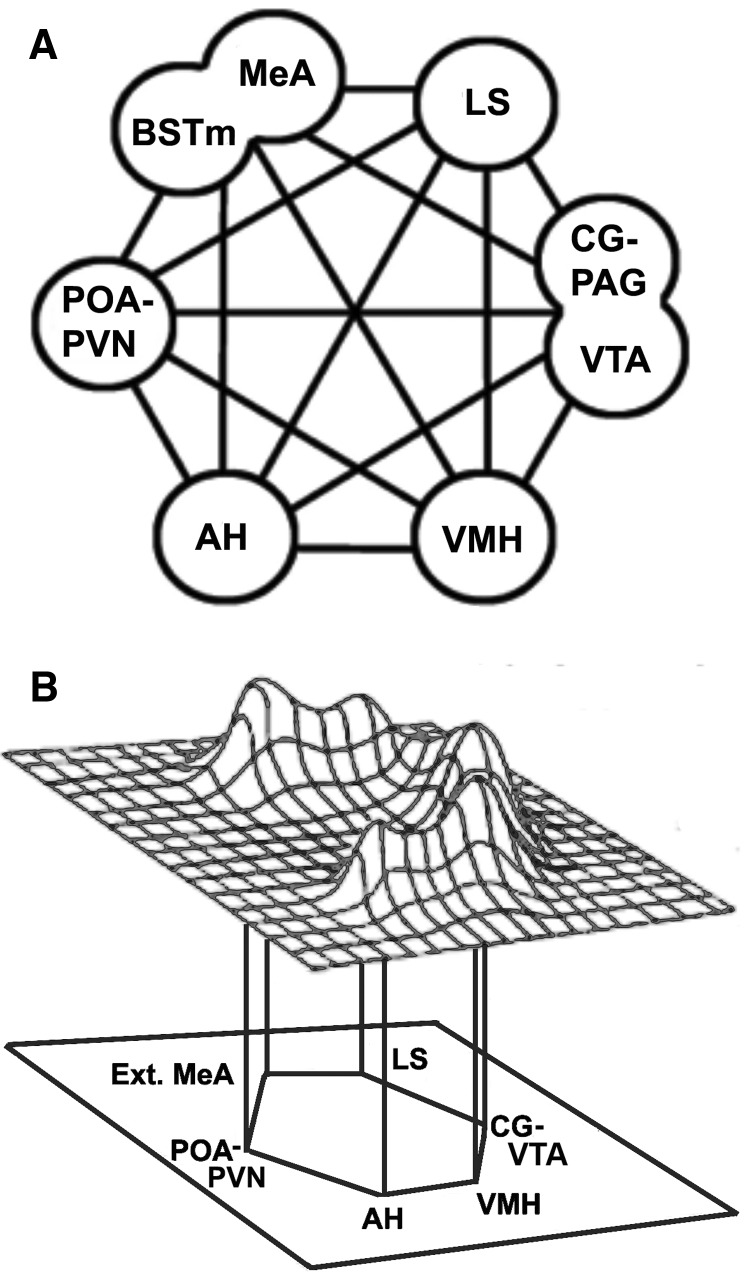
The “social behavioral network” (SBN) originally described by Newman (1999) has been uncovered in the basal forebrain and midbrain of reptiles, birds, and mammals (Crews 2003; Goodson 2005; O'Connell and Hofmann 2011; Goodson and Kingsbury 2013). This conserved network functions as the core circuitry for the regulation of vertebrate social behaviors, such as courtship, reproduction, parental care, aggression, social affiliation, social recognition, social communication, anxiety-like behavior, and response to stressors (Newman 1999; Goodson 2005; Goodson and Kingsbury 2013). The central nodes of this network represent highly interconnected brain regions and each node is involved in the modulation of multiple social behaviors (Fig. 1A). These nodes include the preoptic area (POA), anterior hypothalamus (AH), ventromedial hypothalamus (VMH), medial extended amygdala (medial amygdala, MeA, and medial bed nucleus of the stria terminalis, BSTm), midbrain central gray (CG; includes intercollicular nucleus, Kingsbury et al. 2011), lateral septum (LS), as well as the ventral tegmental area (VTA) and paraventricular nucleus of the hypothalamus (PVN) as more recent additions (Maney et al. 2008; Goodson and Kingsbury 2013)(Fig. 1A). O’Connell and Hofmann (2011) have proposed an expanded model of the SBN called the social decision making (SDM) network, which includes the mesolimbic dopaminergic reward circuitry that is central for the evaluation of stimulus salience and appetitive/avoidance behavior. Thus, together with the SBN, the SDM adds additional mesolimbic dopaminergic areas such as the striatum, nucleus accumbens (NAcc), ventral pallidum (VP), basolateral amygdala (blAMY), hippocampus, and prefrontal cortex (PFC; in mammalian models) (O'Connell and Hofmann 2011).
Fig. 1.
The “social behavior network” based on Newman (1999). (A) The core components of the social behavior network (SBN) include the medial extended amygdala (medial amygdala, MeA, and the medial bed nucleus of the stria terminalis, BSTm), medial preoptic area (POA) and paraventricular nucleus (PVN), anterior hypothalamus (AH), ventromedial hypothalamus (VMH), lateral septum (LS), central gray (CG) or periaqueductal gray (PAG), and the ventral tegmental area (VTA). (B) Schematic representation of immediate early gene data for the behavioral context of aggression, which is characterized by a distinct pattern of activation across the nodes of the SBN. Taken from Goodson (2005). Of note is that another behavior, such as affiliation, would elicit a different pattern of activation. Furthermore, each node will likely have distinct cell populations (e.g., VIP cells) with different response profiles for a given social context.
Within the SBN, Newman (1999) proposed that behavior is not processed in a segregated linear fashion. Rather, she suggested that many nodes within the network respond to a social stimulus, and it is the unique pattern of activation throughout the network that is associated with a particular social context and behavioral response (Fig. 1B). Indeed, when we examine cell responses across the network following a given social stimulus or context, we observe network-wide activation (Goodson 2005; Goodson et al. 2005, 2015; Kingsbury et al. 2015). Similar to the core structure, the subnuclear organization within the nodes is likewise strongly conserved (Risold and Swanson 1997; Goodson et al. 2004, 2009a; Kingsbury et al. 2011). Despite this remarkable conservation, we continue to identify features that likely contribute to individual and species differences in behavior, such as variation in the number and distribution of neuropeptide cells, fibers, and receptors within the SBN, as well as differential activation of neuropeptide cells across the SBN following a given social stimulus.
Vasoactive intestinal polypeptide
While the roles of many neuropeptides and neuromodulators within the SBN have been explored, the role of vasoactive intestinal polypeptide (VIP) remains relatively unknown. VIP is a brain neuropeptide that is produced and released by many hypothalamic and extrahypothalamic cell groups (Table 1) and is best known as the major regulator of prolactin (PRL) secretion from the pituitary in vertebrates (Kato et al. 1978; Lam 1991; EL Halawani 1997; Maney et al. 1999). PRL modulates a number of reproductive behaviors in a variety of species, such as nesting building in rabbits (Gonzalez-Mariscal et al. 1996; Gonzalez-Mariscal 2001), parental feeding in ring doves (Buntin et al. 1991), lactation in mammals (Grattan and Bridges 2009), parental egg fanning in fish (de Ruiter et al. 1986), egg incubation in turkeys and bantam hens (Sharp et al. 1989; el Halawani et al. 1996), and chick-rearing in native Thai hens (Chaiyachet et al. 2013). Several studies suggest that it is the VIP cells within the mediobasal hypothalamus, such as those in the inferior nucleus (IH), infundibular nucleus (INF), and median eminence (ME), which induce the PRL release that is associated with parental care (Cloues et al., 1990; Youngren et al., 1996; Chaiseha et al., 1998; Chaiyachet et al., 2013). These mediobasal VIP cells, together with those in the lateral septal organ (LSO), are also important for detecting photoperiodic cues that seasonally stimulate the reproductive axis and may facilitate migration (Silver et al. 1988; Saldanha et al. 1994; Li and Kuenzel 2008; Rastogi et al. 2013; Kuenzel et al. 2015). However, in addition to cells within the mediobasal hypothalamus, VIP cells, fibers, and receptors are present in all core SBN nodes (Sims et al. 1980; Kuenzel and Blahser 1994; Kuenzel et al. 1997; Joo et al. 2004; Goodson et al. 2006) and dopaminergic SDM sites (Kuenzel et al. 1997; Goodson et al. 2006, 2012b) (Table 1). Given this network-wide expression of VIP cells and receptors, it is likely that VIP signaling modulates a variety of social behaviors. Until recently, however, the role of VIP in the behaviors affiliated with these networks remained largely unexplored. Based on comparisons of VIP circuitry in socially diverse species of estrildid finches and emberizid sparrows, and manipulations of endogenous VIP signaling in vivo, we describe VIP neural “hotspots” in the SBN that relate to avian affiliative behavior, as well as neural “hotspots” that may represent critical nodes underlying a trade-off between aggression and parental care. These neural “hotspots,” as we define them, are SBN nodes where VIP signaling appears to be particularly important for the regulation of a given social behavior, based on findings across multiple studies and/or through the use of multiple techniques to assess VIP function (Table 1).
Table 1.
The presence of VIP elements in the vertebrate SBN and mesolimbic reward system and proposed neural hotspots for the regulation of affiliation, aggression, and parental care by VIP
| Area | VIP cells | VIP fibers | VIP receptors |
|---|---|---|---|
| SBN | |||
| POA-hypothalamus | |||
| POA | +++ | +++ | + |
| AH | ++ + (Ag/Pa, c) | ++ + (Ag/Pb) | + |
| PVN | + | +++ | ++ |
| VMH | ++ + (Ag/Pc) | ++ (Ag/Pb) | + |
| IH | +++ | ++ | |
| INF | ++ + (Ag/Pa) | ++ | + |
| ME | ++ + (Ag/Pa) | ++ | |
| Extended amygdala | |||
| MeA, anterior | +++ | ++ | ++ (A) |
| MeA, posterior | +++ | ++ | ++ (A) |
| BSTm | + | ++ (A) | + (A) |
| BSTl | +++ | ++ | |
| Septum | |||
| LSr | − | ++ | ++ (A) |
| LSc.d (pallial) | − | +++ | +++ |
| LSc, (subpallial), | − | +++ | ++ (A) |
| nPC | − | ++ | |
| CcS | − | ++ + (Ag/Pb) | + |
| LSO, medial | + | + | |
| Midbrain | |||
| CG | +++ | ++ | |
| ICo, medial | +++ | +++ | |
| ICo, lateral | + | ||
| VTA, rostral* | +++ | +++ | |
| VTA, caudal* | +++ | ++ | |
| Mesolimbic reward system | |||
| Str | +++ | + | ++ |
| NAcc | +++ | +++ | |
| VP | ++ | ||
| HIP | ++ | ++ | ++ |
| Other telencephalic | |||
| Medial nidopallium | − | + (A) | ++ + (A) |
| Rostral arcopallium, dorsal | + | ++ (A) | |
| Rostral arcopallium, lateral | + | ++ (A) |
Source: Data was generated from Goodson et al. (2006, 2012b), Kingsbury et al. (2015), and Wilson et al. (2016).
Notes: (−) Areas where no VIP elements were observed. An absence of − or + indicate brain areas not measured. (A): Neural hotspot for the regulation of affiliative behavior by VIP. For (A), a positive relationship between VIP elements and social affiliation was observed.
(Ag/P): Neural hotspot for the regulation of a trade-off between aggression and parental care by VIP.
Superscripts indicate the different studies where the trade-off was observed:
c Kingsbury et al. (2015); findings for VIP/Fos co-labeled cells are presented rather than VIP cell number.
Neural hotspots for VIP’s modulation of gregariousness
LS and BSTm
Both comparative and manipulative studies suggest that VIP elements in select SBN nodes play an important role in avian grouping behavior and social affiliation. In both birds and other vertebrates, VIP effects are mediated by the VPAC receptors, VPAC1 and VPAC2, which bind both VIP and pituitary adenylate cyclase activating peptide (PACAP) (Vaudry et al. 2000), although PACAP also acts through a single PACAP receptor, PAC1, which binds PACAP with high affinity and VIP with very poor affinity (Vaudry et al. 2000; Zawilska et al. 2003). In a cross-species comparison, we examined VIP receptor densities in five estrildid finches that differ selectively in their species-typical group size but are similar in other major aspects of behavior and physiology (i.e., all are socially monogamous, long-term pair bonders, bi-parental, semi-opportunistic breeders, and inhabit semi-arid and/or grassland scrub habitat) (Goodson et al. 2006). These species included two highly territorial asocial finches, the violet-eared waxbill (Uraeginthus granatina) and melba finch (Pytilia melba), that independently evolved territoriality and have a species-typical group size of two (male–female pair); one moderately gregarious finch species, the Angolan blue waxbill (Uraeginthus angolensis), that has an average group size of 8–40 individuals; and two highly gregarious finch species, the zebra finch (Taeniopygia guttata) and spice finch (Lonchura punctulata), that independently evolved coloniality and have a species-typical group size of 100 or more (Skead 1975; Goodwin 1982; Zann 1996). We found significantly higher densities of VIP binding sites within LS nuclei (i.e., rostral LS, LSr; ventral and ventraolateral nuclei of the caudal LS, LSc.v, LSc.vl) in gregarious finches relative to territorial finches (Fig. 2A–C). A similar pattern was observed for the BSTm (Fig. 2C). Thus, VIP receptor sites within two key nodes of the SBN appear to have evolved in relation to sociality, with enhanced VIP binding sites in more gregarious species (Goodson et al. 2006).
Fig. 2.
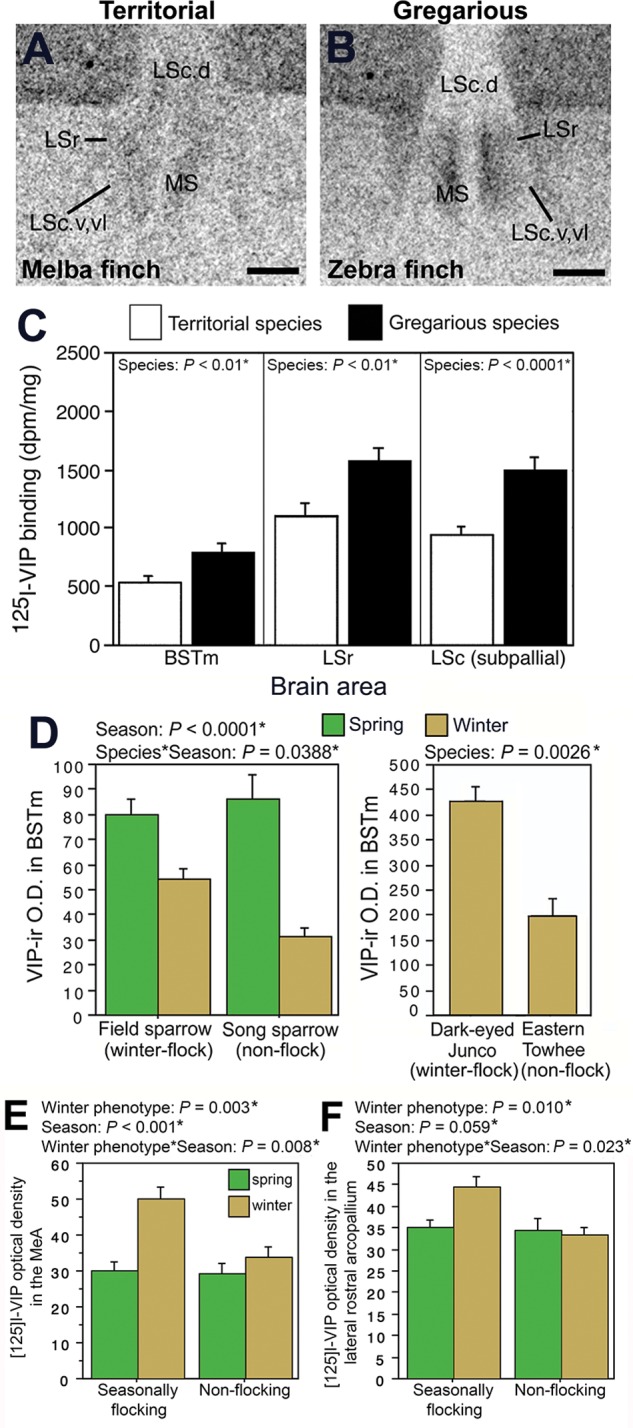
VIP elements within specific brain sites are enhanced in flocking species relative to non-flocking species. A–B. Photomicrographs of 125I-VIP binding in the lateral septum of a territorial melba finch (A) and a gregarious zebra finch (B). Relative to territorial birds, gregarious birds have greater 125I-VIP binding in the rostral lateral septum (LSr) and subpallial caudal lateral septum (LSc.v and LSc.vl). Taken from Goodson et al. (2006). (C) Gregarious species have significantly greater 125I-VIP binding in the medial bed nucleus of the stria terminalis (BSTm), LSr, and LSc, as compared to territorial species (adapted from Table 1; Goodson et al. 2006). (D) VIP immunoreactive (-ir) fiber density in the BSTm is greater in winter flocking emberizid sparrows (field sparrows and dark-eyed juncos) compared to winter non-flocking sparrows (song sparrows and eastern towhees). Taken from Goodson et al. (2012b). E–F. Seasonally flocking sparrows show a winter increase in 125I-VIP binding density in the medial amygdala (MeA) (E) and lateral rostral arcopallium (F) as compared to the non-flocking sparrows. Taken from Wilson et al. (2016), courtesy of S. Karger AG, Basel.
Given that many species exhibit temporal variation in group size, we were interested in exploring whether seasonal variation in grouping behavior is associated with similar VIP mechanisms. For instance, does VIP circuitry in a species that switches to a gregarious phenotype resemble that of a year-round gregarious species? For this cross-species comparison, we examined VIP fiber densities in four species of emberizid sparrows: two species that switch between a territorial phenotype in the breeding season and a flocking phenotype in the winter (field sparrow, Spizella pusilla, and dark-eyed junco, Junco hyemalis); a sparrow that is territorial year-round (song sparrow, Melospiza melodia); and a sparrow that switches from breeding territorially to loosely distributing, but not flocking, in the winter (eastern towhee, Pipilo erythrophthalmus)(Goodson et al. 2012b). We found that VIP innervation of the BSTm is greater in the two sparrow species that flock during the winter compared to the two species that do not winter flock (Fig. 2D). Thus, VIP elements in the BSTm seem to be important for both species-typical grouping and seasonal grouping: BSTm VIP receptor density is associated with a species-typical flocking phenotype and BSTm VIP fiber density is associated with a winter flocking phenotype.
MeA and arcopallium
We conducted an additional comparison in our four sparrow species to determine if VIP receptor densities in sparrows that seasonally switch to a gregarious winter phenotype resemble receptor density patterns in year-round gregarious finches (Wilson et al. 2016). We predicted that increased VIP binding would be observed in the LS and BSTm of winter flocking sparrows (field sparrows and dark-eyed juncos), as compared to non-winter flockers (song sparrows and eastern towhees), as these SBN nodes are characterized by greater VIP receptor density in year-round flocking finches as compared to year-round asocial finches. While we did not observe a positive association between winter flocking and VIP binding density in the LS or BSTm, we did find that winter flocking is associated with a specific winter increase in VIP binding in the MeA (Fig. 2E) and rostral arcopallium, both lateral (Fig. 2F) and dorsal arcopallial divisions (Wilson et al. 2016). The arcopallium, formerly termed the archistriatum, has both amygdaloid and somatic properties (Zeier and Karten 1971; Reiner et al. 2004). We refer to the rostral arcopallium as the arcopallial region dorsal to MeA (nucleus taeniae) and immediately dorsal and lateral to the robust nucleus of the arcopallium (RA) (see Fig. 2F from Wilson et al. 2016). The MeA, together with the BSTm, are components of the medial extended amygdala, and the arcopallium, whose homology still remains to be clarified (Reiner et al. 2004), has been hypothesized to encompass parts of pallial amygdala (Jarvis 2009; Hanics et al. 2016), which is part of the mammalian SDM (O'Connell and Hofmann 2011).
Medial nidopallium
Outside the core nodes of the SBN, VIP circuitry in the medial nidopallium is also important for avian affiliative behavior. The medial nidopallium, formerly termed the neostriatum (Reiner et al. 2004), is part of the medial pallium, an area that is proposed to be a prefrontal cortex homolog (Reiner et al. 2004; Husband and Shimizu 2011; Kingsbury et al. 2013). Given the links described above between flocking behavior and increased VIP binding sites, we hypothesized that activation of VPAC receptors in gregarious zebra finches (T. guttata) promotes preferences for larger groups. To test this hypothesis, we infused a selective VPAC receptor antagonist (neurotensin6-11-mouseVIP7-28; Nowak et al. 2003) or saline control into the medial nidopallium of both male and female zebra finches and examined whether each subject chose to affiliate with 2 or 10 unfamiliar same-sex conspecifics (Kingsbury et al. 2013). The medial nidopallium of zebra finches is characterized by an incredibly high density of VIP binding sites in both male and female zebra finches (Fig. 3A, white arrow; Goodson et al. 2006) and VPAC receptor antagonism at this brain site reduces gregariousness (i.e., preference for the larger group) in both sexes (Fig. 3B), but has no effect on social contact or anxiety-like behaviors (Kingsbury et al. 2013). We have also compared VIP fiber density in the medial nidopallium of a seasonally flocking sparrow (field sparrow) and a year-round territorial sparrow (song sparrow). Whereas both sparrows have similar VIP fiber innervation of the medial nidopallium in the spring breeding season, within the non-breeding season, the winter flocking sparrow has greater VIP innervation compared to the non-flocking sparrow (Fig. 3C). Together, these results suggest that gregariousness is modulated via VPAC activation in the medial nidopallium.
Fig. 3.
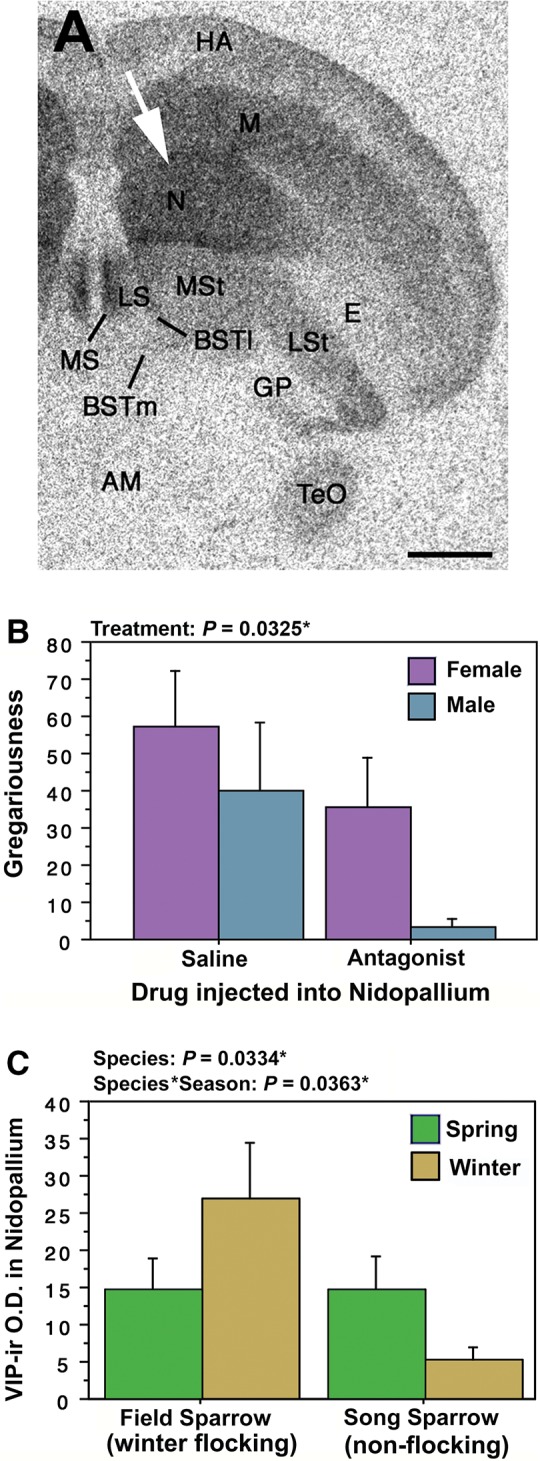
VIP in the medial nidopallium modulates gregariousness. (A) 125I-VIP binding in a cross section through a zebra finch brain. The medial nidopallium has an extremely high density of VIP binding sites (white arrowhead). Taken from Goodson et al. (2006). (B) VPAC antagonism in the medial nidopallium significantly decreases gregariousness (i.e., preference for a larger group of same-sex conspecifics) in both male and female zebra finches. Taken from Kingsbury et al. (2013). (C) VIP immunoreactive (-ir) fiber density in the medial nidopallium is greater in winter flocking male field sparrows compared to winter non-flocking male song sparrows.
VIP elements as modulators of affiliative behavior at key brain sites
While many nodes within the SBN and SDM respond to a social stimulus, it is the unique pattern of activation throughout the network that is associated with a particular behavioral response (Goodson 2005; Goodson et al. 2005; O'Connell and Hofmann 2011). Furthermore, the magnitude of cell activity within each individual node depends on the particular social behavior being assessed, the behavioral phenotype of the animal, and the neurochemical phenotype of the cells being examined (Goodson 2005). Based on our cross-species comparisons, we propose that variation in VIP fiber and receptor density in the LS, BSTm, MeA, rostral arcopallium, and medial nidopallium contribute to species differences in grouping behavior by modulating VIP signaling throughout the network.
It is perhaps not surprising that several SBN nodes, such as the LS, BSTm, and MeA, emerge as neural hotspots for the regulation of avian affiliative behavior. Peptide circuits of the BSTm and LS are known to be important for grouping behavior and social affiliation in both birds and mammals, although this has been mostly studied with regard to the nonapeptides, oxytocin and vasopressin (Kelly and Goodson 2014). The LS is also part of a brain circuit linking contextual stimuli with reward processing via projections to the VTA (Luo et al. 2011), an area that is part of the mesolimbic dopamine circuitry and implicated in pair bonding in male prairie voles (Curtis and Wang 2005). With regards to the medial extended amygdala, it is also not unexpected that we observe a positive association between VIP elements in the BSTm and MeA with affiliative behavior since both regions (1) are highly interconnected (Newman 1999; Goodson 2005), (2) are involved in the processing of stimuli with positive and negative valence (Nishijo et al. 1988; Knapska et al. 2006; Paton et al. 2006), and (3) mediate social approach and avoidance (Newman 1999; Sheehan et al. 2001; Goodson and Wang 2006).
Multiple lines of evidence, including our work, suggest that the arcopallium may be a hotspot of social modulation. We find that facultative flocking is associated with enhanced VIP binding in the rostral arcopallium (Wilson et al. 2016), an area whose homology is not yet clear but which is believed to be sensory somatic and/or pallial amygdalar in nature (Reiner et al. 2004; Jarvis 2009). The pallial amygdala includes the blAMY (Martinez-Garcia et al. 2002), a structure that integrates multi-model sensory information for emotional regulation (LeDoux 2000) and is involved in the modulation of goal-directed behaviors (O'Connell and Hofmann 2011). Interestingly, similar to VIP binding sites, oxytocin binding sites are also enhanced in the lateral rostral arcopallium in winter flocking species compared to non-flocking species (Wilson et al. 2016). Like VIP (Kingsbury et al. 2013), oxytocin modulates grouping and affiliative behavior (Young and Wang 2004; Goodson et al. 2009b; Goodson and Kingsbury 2011; Young et al. 2011). In pigeons, the dorsal arcopallium is rich in dopaminergic fibers (Reiner et al. 2004). Within golden-collared manakins, the arcopallium, together with the MeA, express an extremely high density of androgen receptors (Fusani et al. 2014), and thus the arcopallium has been implicated in the premotor control of courtship displays (Fusani et al. 2014). In Japanese quail, cells within the arcopallium are activated in response to appetitive and consummatory sexual behavior, which may relate to activation in both limbic and sensorimotor systems that coordinate copulatory behavior (Ball and Balthazar 2001). Finally, VIP cells in the acropallium are implicated in the vocal control of song (Ball et al. 1988). We speculate that neuropeptide signaling in the arcopallium could facilitate social approach that is a component of affiliative behavior, either for courtship/copulation or flocking (Wilson et al. 2016).
Despite the high concentration of VIP receptors in the medial nidopallium of gregarious zebra finches (Goodson et al. 2006), we were initially surprised to discover that endogenous VIP signaling within this area promotes gregariousness (Kingsbury et al. 2013). However, the medial nidopallium, which is part of the medial pallium, may be homologous to the mammalian PFC based on connectional and functional data. For instance, both the medial pallium of pigeons and the mammalian PFC (1) are part of a “limbic loop” that includes the mediodorsal thalamus, VP, and NAcc, (2) have extensive connections to the NAcc, and (3) have a direct connection from a homologous thalamic nucleus (the anterior dorsomedial nucleus in birds and the mediodorsal nucleus in mammals) (Metzger et al. 1996; Veenman et al. 1997; Montagnese et al. 2003; Husband and Shimizu 2011). Furthermore, both the mammalian PFC and NAcc are components of the mesolimbic dopaminergic reward circuitry, mediate the reinforcing effects of drugs and natural stimuli, and are important for affiliation and social attachment (McBride et al. 1999; Young et al. 2001). In birds, cells within the medial pallium respond to both appetitive and consummatory sexual behavior (Ball and Balthazar 2001). Thus, the avian medial nidopallium may regulate social approach to rewarding stimuli.
While the arcopallium and medial nidopallium are not core components of the SBN, these structures may be part of the mesolimbic reward circuitry, and hence, the SDM proposed by O’Connell and Hofmann. For instance, the arcopallium is hypothesized to encompass parts of pallial blAMY (Reiner et al. 2004; Jarvis 2009), a structure that is part of the mesolimbic dopaminergic system (O'Connell and Hofmann 2011). The medial nidopallium is part of the medial pallium, a proposed homologue for mammalian PFC (Husband and Shimizu 2011), and within mammals, the PFC is included as part of the mesolimbic reward circuitry (McBride et al. 1999).
In summary, we hypothesize that the modulation of affiliation by VIP is mediated by increases in VIP fiber density within the BSTm and/or medial nidopallium, increases in VIP receptors within the MeA, BSTm, LS, medial nidopallium and/or rostral arcopallium, or some combination thereof. As regards to whether VPAC receptors are activated by locally produced VIP or VIP produced in more distal cell bodies, it is possible that VPAC receptors in the extended medial amygdala are activated by locally produced VIP, as well as VIP from distant sites, due to the presence of both VIP cells and fibers in the MeA and BSTm. We speculate that activation of VPAC receptors in the LS, medial nidopallium, and arcopallium is likely due to VIP produced at distal cell bodies due to a lack of VIP cells observed with these regions.
Neural hotspots for VIP’s modulation of parental care and aggression
Mediobasal hypothalamus
The mediobasal hypothalamus includes the VMH, IH, INF, and median eminence and previous research suggests that VIP cells within these regions (1) mediate the PRL release that is associated with reproductive and parental behaviors (Cloues et al. 1990; Youngren et al. 1996; Chaiseha et al. 1998; Chaiyachet et al. 2013) and (2) detect the photoperiodic changes necessary for the seasonal stimulation of gonadal development (Saldanha et al. 1994; Li and Kuenzel 2008; Kuenzel et al. 2015). VIP-immunization in domestic fowl blocks VIP-induced increases in plasma PRL levels, reducing nesting activity in turkeys (el Halawani et al. 1996) and increasing nest desertion in incubating bantam hens (Sharp et al. 1989).
AH and caudal septum
Previous work in territorial violet-eared waxbills demonstrates that VIP signaling in the AH and caudal septum mediates aggression. Septal infusions of VIP targeting the caudal septum in male violet-eared waxbills significantly increase aggressive behaviors (i.e., chases and threats) and decrease the latency to aggress in a modified resident-intruder paradigm (Goodson 1998). In a detailed study of the chemoarchitectonic divisions of the avian septum, VIP fibers were found to be concentrated in the subpallial LS and caudocentral septum (CcS)(Goodson et al. 2004). The septum receives projections from the AH (Atoji and Wild 2004), a brain area well known for the mediation of aggression and agonistic behavior (Nelson and Trainor 2007). Because the dorsal AH (AHd) contains a discrete population of VIP cells, we hypothesized that VIP in the AHd modulates aggression. Indeed, knockdown of VIP peptide using antisense oligonucleotides significantly decreases resident-intruder aggression in violet-eared waxbills and nest defense aggression in zebra finches compared to control animals (Goodson et al. 2012a).
VIP mediated trade-off between aggression and parental care
A common avian life history trade-off involves effort directed toward mating (i.e., mate competition and resident-intruder aggression) versus effort directed toward parental care. This trade-off can be modulated by testosterone, with higher levels generally increasing mating effort at the expense of parental care (McGlothlin et al. 2007). Based on data from three independent studies, we propose that two different populations of VIP neurons may represent critical neural nodes underlying a trade-off between male aggression and male parental care. Within the context of the SBN, we hypothesize that aggression is facilitated by a simultaneous increase in VIP signaling within the AH/CcS and decrease within the mediobasal hypothalamic areas. Similarly, we hypothesize that parental care is facilitated by a concurrent enhancement of VIP signaling in the mediobasal hypothalamus and suppression within the AH/CcS.
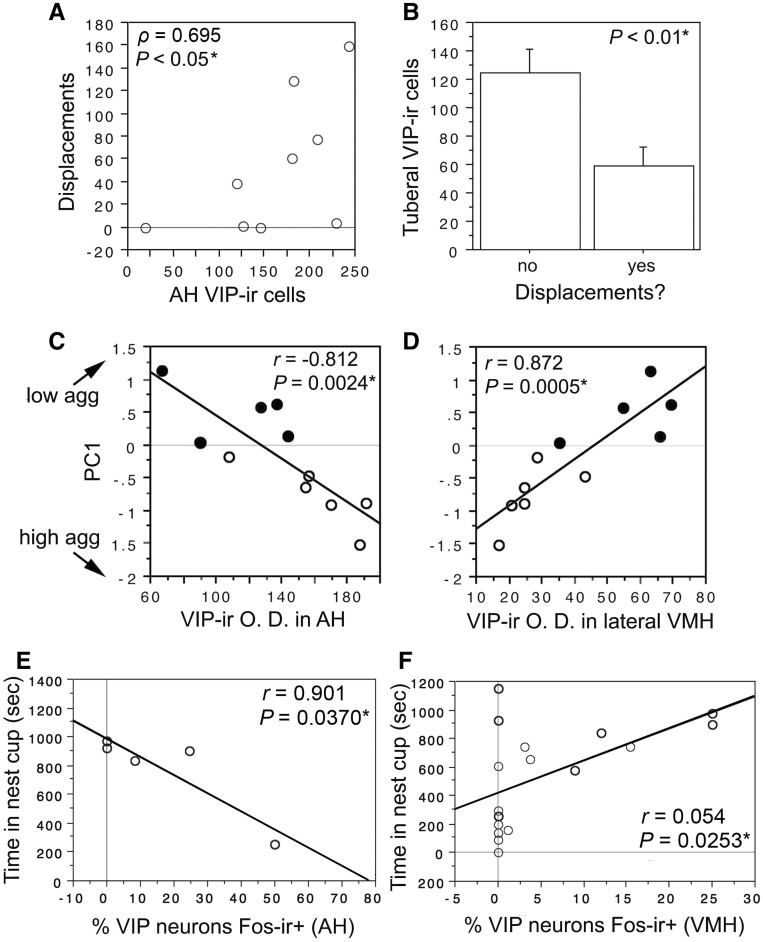
In support of this hypothesis, we find that VIP cell number in the AH and tuberal hypothalamus (INF and ME; parts of the mediobasal hypothalamus) differentially relates to aggression in male violet-eared waxbills (Fig. 4A–B). For instance, the number of VIP-immunoreactive (-ir) cells in the AH correlates positively with aggression (Fig. 4A) and violet-eared waxbills that did not displace intruders had significantly more VIP-ir tuberal cells, as compared to those who actively displaced intruders (Fig. 4B). More support for this hypothesis comes from our sparrow dataset where we investigated whether individual and species differences in VIP neurochemistry can predict aggressive behavior, given that field sparrows are less aggressive than song sparrows during the breeding system (Goodson et al. 2012b). A principle component analysis was conducted using aggressive behaviors measured during the spring breeding season for field and song sparrows. This analysis yielded a single component, PC1, which strongly loaded all four aggressive variables with positive scores indicating low aggression and negative scores indicating high aggression. A significant negative correlation with PC1, and hence, positive correlation with aggression was observed for VIP-ir fiber density in the AH (Fig. 4C) and CcS (Goodson et al. 2012b). In contrast, a significant positive correlation with PC1, and hence, negative correlation with aggression was observed for VIP-ir fiber density in VMH (Fig. 4D). Note that VIP-ir fiber density predicts both individual differences in aggression as well as species differences (Fig. 4C–D). Finally, a study examining VIP cell number and VIP cell activation in nesting zebra finches lends further support for this hypothesis (Kingsbury et al. 2015). Whereas time spent in a nest cup correlates negatively with the percent of VIP/Fos double-labeled cells in the AH (Fig. 4E), time spent in the nest cup correlates positively with the percent of VIP/Fos double-labeled cells in the VMH (Fig. 4F). Thus, studies from male territorial violet-eared waxbills, field sparrows, song sparrows, and gregarious zebra finches support the hypothesis that VIP elements within the AH and mediobasal hypothalamus mediate a trade-off between aggressive behavior and reproduction. We do not know as much about VIP’s proposed trade-off for reproduction versus parental care in females, although what we do know is partially consistent with what we have found for males. Similar to results from males, knockdown of AHd VIP production in female violet-eared waxbills drastically reduces aggression, indicating that the AHd VIP cell group regulates aggression in both sexes. Furthermore, time spent in a nest cup is negatively correlated with VIP cell activation in the AHd of both male and female zebra finches (Kingsbury et al. 2015). However, whereas nest cup time is positively correlated with VIP cell activation in the VMH for male zebra finches, no such relationship is found for females (Kingsbury et al. 2015). In addition, while we present additional evidence to support our hypothesis for VIP’s modulation of a trade-off between reproduction and parental care in Fig. 4A–D, we were only able to examine males within these studies due to the greater difficulty in catching females in the field. Thus, it remains to be determined whether the regulation of a trade-off between aggression and parental care by VIP exists for females.
Fig. 4.
VIP elements in the anterior hypothalamus (AH) and mediobasal hypothalamus (tuberal hypothalamus and ventromedial hypothalamus, VMH) suggest that VIP signaling mediates a trade-off between aggression and parental care. A–B. VIP cell numbers in the AH and tuberal hypothalamus of male violet-eared waxbills differentially relate to aggression. (A) VIP-ir cells in the AH of controls infused with scrambled oligonucleotides correlate positively with aggressive displacements (Spearman rank correlation). (B) For controls infused with scrambled oligonucleotides, subjects that did not exhibit displacements have significantly more VIP-ir cells in the tuberal hypothalamus compared to controls that did exhibit aggressive displacements (n = 2 and 7, respectively, unpaired t-test). Taken from Goodson et al. (2012a). C–D. VIP fiber density in the AH and VMH of male sparrows differentially relates to aggression. Aggression (measured as PC1 from a principal component analysis) in field sparrows (closed circles) and song sparrows (open circles) correlates positively with the optical density (O.D.) of VIP-ir fibers in the AH (C) and negatively with the O. D. of VIP-ir fibers in the lateral VMH (D). Note that more negative PC1 values stand for higher aggression (arrows on Y axis). Taken from Goodson et al. (2012b). E–F. VIP cell activation in the AH and VMH of zebra finches differentially relates to nesting behavior. (E) VIP-Fos colocalization in the AH correlates negatively with the amount of time spent in the nest cup by nesting males. F. VIP-Fos colocalization in the VMH correlates positively with time in nest for males (F). Data points from nesting males are shown in heavy circles; data points from males with nest cups only (no nest material) are shown in lighter circles. Taken from Kingsbury et al. (2015).
Within the context of the SBN, we hypothesize that the trade-off between aggression versus parental care is mediated by an opposing change in VIP cell number, fiber density, and/or VIP cell activation within the mediobasal hypothalamus (VMH, INF, ME) and the AH/CcS (Table 1).
Conclusions
The comparative and mechanistic studies presented here within socially diverse species of estrildid finches and emberizid sparrows highlight a role for VIP signaling in avian grouping, aggression, and parental care. We propose that enhancement of VIP receptors and/or VIP fiber innervation in neural “hotspots,” such as the LS, BSTm, medial nidopallium, and arcopallium, promote flocking in species characterized by stable year-round group size, as well as in species that switch between gregarious and territorial phenotypes. Based on correlations of VIP cell number, VIP fiber density, and VIP cell activity with aggressive and reproductive behaviors, we also propose that VIP elements in the AH/CcS and mediobasal hypothalamus may represent critical nodes that underlie a trade-off between male aggression and male reproductive behavior. Thus, while VIP circuitry throughout multiple nodes of the SBN and SDM is likely activated in response to a given social context, key brain sites emerge as potential “hotspots” for a given social behavior.
Outside of birds, we know surprisingly little with regards to the role of VIP in affiliation, aggression, and parental care. Most of the work in mammals has focused on the role of VIP in the modulation of circadian and reproductive rhythms (reviewed in Kingsbury 2015). Furthermore, while our findings in birds are complemented by studies in mice, which demonstrate that a reduction of VIP signaling in utero leads to deficits in social approach and sociability in adolescent and adult offspring (Hill et al. 2007; Stack et al. 2008), the brain site of VIP action for social affiliation within mammals is unknown. Thus, a future direction could be to examine the role of VIP signaling with the SBN of different vertebrate taxa with regards to the social behaviors studied here.
VIP can stimulate the release of other brain neuropeptides, such as oxytocin and vasopressin (Bardrum et al. 1987 , 1988), which are also important for the social behaviors described here. However, a major gap in our knowledge is how these brain neuropeptides might act together to modulate behavior, especially since each of these peptides is found widely distributed throughout the SBN and SDM. Thus, another future direction is to examine how these neuropeptide systems collectively act with the SBN and SDM to coordinate various social behaviors.
Funding
National Institutes of Health grant R01 MH092331 supports work in the laboratory of the author.
References
- Atoji Y, Wild JM. 2004. Fiber connections of the hippocampal formation and septum and subdivisions of the hippocampal formation in the pigeon as revealed by tract tracing and kainic acid lesions. J Comp Neurol 475:426–61. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazar J. 2001. Ethological concepts revisited: immediate early gene induction in response to sexual stimuli in birds. Brain Behav Evol 57:252–70. [DOI] [PubMed] [Google Scholar]
- Ball GF, Faris PL, Hartman BK, Wingfield JC. 1988. Immunohistochemical localization of neuropeptides in the vocal control regions of two songbird species. J Comp Neurol 268:171–80. [DOI] [PubMed] [Google Scholar]
- Bardrum B, Ottesen B, Fahrenkrug J, Fuchs AR. 1988. Release of oxytocin and vasopressin by intracerebroventricular vasoactive intestinal polypeptide. Endocrinology 123:2249–54. [DOI] [PubMed] [Google Scholar]
- Bardrum B, Ottesen B, Fuchs AR. 1987. Preferential release of oxytocin in response to vasoactive intestinal polypeptide in rats. Life Sci 40:169–73. [DOI] [PubMed] [Google Scholar]
- Buntin JD, Becker GM, Ruzycki E. 1991. Facilitation of parental behavior in ring doves by systemic or intracranial injections of prolactin. Horm Behav 25:424–44. [DOI] [PubMed] [Google Scholar]
- Chaiseha Y, Tong Z, Youngren OM, El Halawani ME. 1998. Transcriptional changes in hypothalamic vasoactive intestinal peptide during a photo-induced reproductive cycle in the turkey. J Mol Endocrinol 21:267–75. [DOI] [PubMed] [Google Scholar]
- Chaiyachet OA, Chokchaloemwong D, Prakobsaeng N, Sartsoongnoen N, Kosonsiriluk S, Rozenboim I, el Halawani ME, Porter TE, Chaiseha Y. 2013. Neuroendocrine regulation of rearing behavior in the native Thai hen. Acta Histochem 115:209–18. [DOI] [PubMed] [Google Scholar]
- Cloues R, Ramos C, Silver R. 1990. Vasoactive intestinal polypeptide-like immunoreactivity during reproduction in doves: influence of experience and number of offspring. Horm Behav 24:215–31. [DOI] [PubMed] [Google Scholar]
- Crews D. 2003. The development of phenotypic plasticity: where biology and psychology meet. Develop Psychobiol 43:1–10. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Wang Z. 2005. Ventral tegmental area involvement in pair bonding in male prairie voles. Physiol Behav 86:338–46. [DOI] [PubMed] [Google Scholar]
- de Ruiter AJ, Wendelaar Bonga SE, Slijkhuis H, Baggerman B. 1986. The effect of prolactin on fanning behavior in the male three-spined stickleback, Gasterosteus aculeatus L. Gen Comp Endocr 64:273–83. [DOI] [PubMed] [Google Scholar]
- El Halawani ME. 1997. Vasoactive intestinal peptide as the avian prolactin-releasing factor In: Harvey S, Etches RJ, editors. Perspectives in avian endocrinology. Bristol: The Society of Endocrinology: p. 403–16. [Google Scholar]
- EL Halawani ME, Pitts GR, Sun S, Silsby JL, Sivanandan V. 1996. Active immunization against vasoactive intestinal peptide prevents photo-induced prolactin secretion in turkeys. Gen Comp Endocr 104:76–83. [DOI] [PubMed] [Google Scholar]
- Fusani L, Donaldson Z, London SE, Fuxjager MJ, Schlinger BA. 2014. Expression of androgen receptor in the brain of a sub-oscine bird with an elaborate courtship display. Neurosci Lett 578:61–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal G. 2001. Neuroendocrinology of maternal behavior in the rabbit. Horm Behav 40:125–32. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal G, Melo AI, Jimenez P, Beyer C, Rosenblatt JS. 1996. Estradiol, progesterone, and prolactin regulate maternal nest-building in rabbits. J Neuroendocrinol 8:901–7. [DOI] [PubMed] [Google Scholar]
- Goodson JL. 1998. Vasotocin and vasoactive intestinal polypeptide modulate aggression in a territorial songbird, the violet-eared waxbill (Estrildidae: Uraeginthus granatina). Gen Comp Endocr 111:233–44. [DOI] [PubMed] [Google Scholar]
- Goodson JL. 2005. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav 48:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L. 2004. Chemoarchitectonic subdivisions of the songbird septum and a comparative overview of septum chemical anatomy in jawed vertebrates. J Comp Neurol 473:293–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L, Allen CD. 2005. Neuro-evolutionary patterning of sociality. Proc R Soc B 272:227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Wang Y. 2006. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm Behav 50:223–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D, Kelly AM, Rinaldi J, Klatt JD. 2009a. Midbrain dopamine neurons reflect affiliation phenotypes in finches and are tightly coupled to courtship. Proc Natl Acad Sci USA 106:8737–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kelly AM, Kingsbury MA, Thompson RR. 2012a. An aggression-specific cell type in the anterior hypothalamus of finches. Proc Natl Acad Sci USA 109:13847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kingsbury MA. 2011. Nonapeptides and the evolution of social group sizes in birds. Front Neuroanat 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kingsbury MA. 2013. What's in a name? Considerations of homologies and nomenclature for vertebrate social behavior networks. Horm Behav 64:103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Kingsbury MA. 2015. Oxytocin mechanisms of stress response and aggression in a territorial finch. Physiol Behav 141:154–63. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. 2009b. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science 325:862–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Wang Y. 2006. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc Natl Acad Sci USA 103:17013–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Wilson LC, Schrock SE. 2012b. To flock or fight: neurochemical signatures of divergent life histories in sparrows. Proc Natl Acad Sci USA 109:10685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin D. 1982. Estrildid finches of the world. Ithaca (NY: ): Cornell University Press. [Google Scholar]
- Grattan DR, Bridges RS. 2009. Prolactin actions in the brain In: Pfaff DW, editor. Hormones, brain and behavior. Oxford: (UK): Elsevier; p. 2471–503. [Google Scholar]
- Hanics J, Teleki G, Alpar A, Szekely AD, Csillag A. 2016. Multiple amygdaloid divisions of arcopallium send convergent projections to the nucleus accumbens and neighboring subpallial amygdala regions in the domestic chicken: a selective pathway tracing and reconstruction study. Brain Struct Funct published online (doi: 10.1007/s00429-016-1219-8). [DOI] [PMC free article] [PubMed]
- Hill JM, Cuasay K, Abebe DT. 2007. Vasoactive intestinal peptide antagonist treatment during mouse embryogenesis impairs social behavior and cognitive function of adult male offspring. Exp Neurol 206:101–13. [DOI] [PubMed] [Google Scholar]
- Husband SA, Shimizu T. 2011. Calcium-binding protein distributions and fiber connections of the nucleus accumbens in the pigeon (Columba livia). J Comp Neurol 519:1371–94. [DOI] [PubMed] [Google Scholar]
- Jarvis E. 2009. Evolution of the pallium in birds and reptiles In: Binder M, Hirokawa N, Windhorst U, editors. Encyclopedia of neuroscience. Berlin: Springer; p. 1390–400. [Google Scholar]
- Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI. 2004. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol 476:388–413. [DOI] [PubMed] [Google Scholar]
- Kato Y, Iwasaki Y, Iwasaki J, Abe H, Yanaihara N, Imura H. 1978. Prolactin release by vasoactive intestinal polypeptide in rats. Endocrinology 103:554–8. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL. 2014. Social functions of individual vasopressin-oxytocin cell groups in vertebrates: what do we really know? Front Neuroendocrinol 35:512–29. [DOI] [PubMed] [Google Scholar]
- Kingsbury MA. 2015. New perspectives on vasoactive intestinal polypeptide as a widespread modulator of social behavior. Curr Opin Behav Sci 6:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, Jan N, Klatt JD, Goodson JL. 2015. Nesting behavior is associated with VIP expression and VIP-Fos colocalization in a network-wide manner. Horm Behav 69:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, Kelly AM, Schrock SE, Goodson JL. 2011. Mammal-like organization of the avian midbrain central gray and a reappraisal of the intercollicular nucleus. PLoS One 6:e20720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, Miller KM, Goodson JL. 2013. VPAC receptor signaling modulates grouping behavior and social responses to contextual novelty in a gregarious finch: A role for a putative prefrontal cortex homologue. Horm Behav 64:511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Walasek G, Nikolaev E, Neuhausser-Wespy F, Lipp HP, Kaczmarek L, Werka T. 2006. Differential involvement of the central amygdala in appetitive versus aversive learning. Learn Mem 13:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzel WJ, Blahser S. 1994. Vasoactive intestinal polypeptide (VIP)-containing neurons: distribution throughout the brain of the chick (Gallus domesticus) with focus upon the lateral septal organ. Cell Tissue Res 275:91–107. [DOI] [PubMed] [Google Scholar]
- Kuenzel WJ, Kang SW, Zhou ZJ. 2015. Exploring avian deep-brain photoreceptors and their role in activating the neuroendocrine regulation of gonadal development. Poult Sci 94:786–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzel WJ, McCune SK, Talbot RT, Sharp PJ, Hill JM. 1997. Sites of gene expression for vasoactive intestinal polypeptide throughout the brain of the chick (Gallus domesticus). J Comp Neurol 381:101–18. [DOI] [PubMed] [Google Scholar]
- Lam KS. 1991. Vasoactive intestinal peptide in the hypothalamus and pituitary. Neuroendocrinology 53:45–51. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. 2000. Emotion circuits in the brain. Annu Rev Neurosci 23:155–84. [DOI] [PubMed] [Google Scholar]
- Li H, Kuenzel WJ. 2008. A possible neural cascade involving the photoneuroendocrine system (PNES) responsible for regulating gonadal development in an avian species, Gallus gallus. Brain Res Bull 76:586–96. [DOI] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. 2011. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science 333:353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. 2008. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol 511:173–86. [DOI] [PubMed] [Google Scholar]
- Maney DL, Schoech SJ, Sharp PJ, Wingfield JC. 1999. Effects of vasoactive intestinal peptide on plasma prolactin in passerines. Gen Comp Endocr 113:323–30. [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia F, Martinez-Marcos A, Lanuza E. 2002. The pallial amygdala of amniote vertebrates: evolution of the concept, evolution of the structure. Brain Res Bull 57:463–9. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. 1999. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res 101:129–52. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Jawor JM, Ketterson ED. 2007. Natural variation in a testosterone-mediated trade-off between mating effort and parental effort. The Amer Nat 170:864–75. [DOI] [PubMed] [Google Scholar]
- Metzger M, Jiang S, Wang J, Braun K. 1996. Organization of the dopaminergic innervation of forebrain areas relevant to learning: a combined immunohistochemical/retrograde tracing study in the domestic chick. J Comp Neurol 376:1–27. [DOI] [PubMed] [Google Scholar]
- Montagnese CM, Mezey SE, Csillag A. 2003. Efferent connections of the dorsomedial thalamic nuclei of the domestic chick (Gallus domesticus). J Comp Neurol 459:301–26. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. 2007. Neural mechanisms of aggression. Nat Rev Neurosci 8:536–46. [DOI] [PubMed] [Google Scholar]
- Newman SW. 1999. The medial extended amygdala in male reproductive behavior: a node in the mammalian social behavior network. Ann N Y Acad Sci 877:242–57. [DOI] [PubMed] [Google Scholar]
- Nishijo H, Ono T, Nishino H. 1988. Single neuron responses in amygdala of alert monkey during complex sensory stimulation with affective significance. J Neurosci 8:3570–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JZ, Sedkowska P, Zawilska JB, Gozes I, Brenneman DE. 2003. Antagonism of VIP-stimulated cyclic AMP formation in chick brain. J Mol Neurosci 20:163–72. [DOI] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA. 2011. The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. J Comp Neurol 519:3599–639. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. 2006. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature 439:865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi A, Kumari Y, Rani S, Kumar V. 2013. Neural correlates of migration: activation of hypothalamic clock(s) in and out of migratory state in the blackheaded bunting (Emberiza melanocephala). PLoS One 8:e70065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, et al. 2004. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol 473: 377–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. 1997. Chemoarchitecture of the rat lateral septal nucleus. Brain Res Rev 24:91–113. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Leak RK, Silver R. 1994. Detection and transduction of daylength in birds. Psychoneuroendocrinology 19:641–56. [DOI] [PubMed] [Google Scholar]
- Sharp PJ, Sterling RJ, Talbot RT, Huskisson NS. 1989. The role of hypothalamic vasoactive intestinal polypeptide in the maintenance of prolactin secretion in incubating bantam hens: observations using passive immunization, radioimmunoassay and immunohistochemistry. J Endocrinol 122:5–13. [DOI] [PubMed] [Google Scholar]
- Sheehan T, Paul M, Amaral E, Numan MJ, Numan M. 2001. Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience 106:341–56. [DOI] [PubMed] [Google Scholar]
- Silver R, Witkovsky P, Horvath P, Alones V, Barnstable CJ, Lehman MN. 1988. Coexpression of opsin- and VIP-like-immunoreactivity in CSF-contacting neurons of the avian brain. Cell Tissue Res 253:189–98. [DOI] [PubMed] [Google Scholar]
- Sims KB, Hoffman DL, Said SI, Zimmerman EA. 1980. Vasoactive intestinal polypeptide (VIP) in mouse and rat brain: an immunocytochemical study. Brain Res 186: 165–83. [DOI] [PubMed] [Google Scholar]
- Skead DM. 1975. Ecological studies of four Estrildines in the central Transvaal: Ostrich, Suppl. 11: 1–55.
- Stack CM, Lim MA, Cuasay K, Stone MA, Seibert KM, Spivak-Pohis I, Crawley JN, Waschek JA, Hill JM. 2008. Deficits in social behavior and reversal learning are more prevalent in male offspring of VIP deficient female mice. Exp Neurol 211:67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. 2000. Pituitary adenylate cyclase-activating polypeptide and its receptors: From structure to functions. Pharmacol Rev 52:269–324. [PubMed] [Google Scholar]
- Veenman CL, Medina L, Reiner A. 1997. Avian homologues of mammalian intralaminar, mediodorsal and midline thalamic nuclei: immunohistochemical and hodological evidence. Brain Behav Evol 49:78–98. [DOI] [PubMed] [Google Scholar]
- Wilson LC, Goodson JL, Kingsbury MA. 2016. Seasonal variation in group size is related to seasonal variation in neuropeptide receptor density. Brain Behav Evol published online (doi: 10.1159/000448372). [DOI] [PubMed]
- Young KA, Gobrogge KL, Liu Y, Wang Z. 2011. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front Neuroendocrinol 32:53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. 2001. Cellular mechanisms of social attachment. Horm Behav 40:133–8. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. 2004. The neurobiology of pair bonding. Nature neuroscience 7:1048–54. [DOI] [PubMed] [Google Scholar]
- Youngren O, Chaiseha Y, Phillips R, El Halawani ME. 1996. Vasoactive intestinal peptide concentrations in turkey hypophysial portal blood differ across the reproductive cycle. Gen Comp Endocrinol 103:323–30. [DOI] [PubMed] [Google Scholar]
- Zann RA. 1996. The zebra finch: a synthesis of field and laboratory studies. Oxford: Oxford University Press. [Google Scholar]
- Zawilska JB, Niewiadomski P, Nowak JZ. 2003. PAC1 receptors in chick cerebral cortex: characterization by binding of pituitary adenylate cyclase-activating polypeptide, [125I]-PACAP27. Neurosci Lett 338:155–65. [DOI] [PubMed] [Google Scholar]
- Zeier H, Karten HJ. 1971. The archistriatum of the pigeon: organization of afferent and efferent connections. Brain Res 31:313–26. [DOI] [PubMed] [Google Scholar]