In Strategic Timing of Antiretroviral Treatment, immediate combination antiretroviral therapy (cART) reduced risk of infection-related cancer by 74% and infection-unrelated cancer by 51%. The benefit of immediate cART doesn't appear to be solely attributable to human immunodeficiency virus RNA suppression and may also be mediated by other mechanisms.
Keywords: HIV, antiretroviral therapy, cancer, Kaposi sarcoma, non-Hodgkin lymphoma
Abstract
Background. In the Strategic Timing of Antiretroviral Treatment (START) study, immediate combination antiretroviral therapy (cART) initiation reduced cancer risk by 64%. We hypothesized that risk reduction was higher for infection-related cancer and determined by differences in CD4 cell counts and human immunodeficiency virus (HIV) RNA between the study arms.
Methods. Incident malignancies in START were categorized into infection-related and infection-unrelated cancer. We used Cox models to assess factors associated with both cancer categories. We used sequential adjustment for baseline covariates, cancer risk factors, and HIV-specific variables to investigate potential mediators of cancer risk reduction with immediate cART.
Results. There were 14 cancers among persons randomized to immediate cART (6 infection-related and 8 infection-unrelated) and 39 cancers in the deferred arm (23 infection-related and 16 infection-unrelated); hazard ratios of immediate vs deferred cART initiation were 0.26 (95% confidence interval [CI], .11–.64) for infection-related and 0.49 (95% CI, .21–1.15) for infection-unrelated cancer. Independent predictors of infection-related cancer were older age, higher body mass index, low- to middle-income region, HIV RNA, and baseline CD8 cell count. Older age and baseline CD8 cell count were independent predictors of infection-unrelated cancer. Adjustment for latest HIV RNA level had little impact on the protective effect of immediate cART on infection-related cancer. Adjustment for latest HIV RNA level, but not for CD4 cell count or cancer risk factors, attenuated the effect of immediate cART on infection-unrelated cancer.
Conclusions. Immediate cART initiation significantly reduces risk of cancer. Although limited by small sample size, this benefit does not appear to be solely attributable to HIV RNA suppression and may be also mediated by other mechanisms.
Individuals infected with human immunodeficiency virus (HIV) have an increased risk of cancer compared with the general population. Many reasons have been postulated for this including immunodeficiency [1–4], higher prevalence of traditional cancer risk factors [5], and coinfection by oncogenic viruses, direct pro-oncogenic effects of HIV [6], long-term combination antiretroviral therapy (cART) toxicity [7, 8], and activated inflammation and coagulation [9, 10]. Because of this inherent increased risk and given the fact that HIV-infected persons are now living much longer [11], cancer has emerged as a major cause of morbidity and death in the cART era [12].
HIV infection [13] and other immunodeficiency disorders [14, 15] are associated with a higher risk of infection-related cancer. According to recent estimates, up to 40% of cancer cases among HIV-infected persons in the United States are infection related [16], an attributable fraction 10 times as high as in the general population. HIV-infected persons also have an increased risk of infection-unrelated cancer [17]. Reduced control of oncogenic viruses and impaired immune surveillance of malignant cells may facilitate carcinogenesis during HIV infection [18].
The Strategic Timing of Antiretroviral Treatment (START) trial randomized HIV-infected adults with a CD4 count >500 cells/µL to immediate cART initiation or cART deferral until CD4 counts dropped below 350 cells/µL. Immediate cART reduced risk of cancer by 64% [19]; this was a surprising finding as START recruited young participants with early HIV infection and a median CD4 count of 651 cells/µL at study entry. Here, we set out to determine factors associated with cancer development among START participants and to assess mediators of the benefit of immediate cART in reducing cancer risk. Our a priori hypothesis was that the reduction in cancer risk was higher for infection-related vs infection-unrelated cancer and was mainly determined by differences in CD4 counts and HIV RNA levels between the study arms.
METHODS
The methods and results of the START study have been described in detail elsewhere [19]. In short, START randomized 4685 HIV-infected participants from 35 countries to start cART immediately or to defer cART initiation until CD4 counts dropped below 350 cells/µL or AIDS-defining illnesses developed. After a mean follow-up of 3.0 years, the START data and safety monitoring board determined that predefined stopping rules were met for the primary endpoint of a composite of serious AIDS-related and non-AIDS-related events, and recommended that all participants be offered cART. In the immediate cART arm, average CD4 counts during follow-up were 194 cells/µL higher than in the deferred cART arm [19]. The START trial was conducted in compliance with the Declaration of Helsinki Guidelines, registered on clinical trial databases (ClinicalTrials.gov number: NCT00867048), and reviewed by an independent data and safety monitoring board. The institutional review board at each study site approved the original study protocol, and written informed consent was obtained from all participants.
An endpoint review committee independently validated all reported cancer events. All cancer cases were considered definitive events with the exception of 1 case of leukemia that was classified as probable. We categorized incident malignancies in START into infection-related and infection-unrelated cancer categories after individual review of case report forms and source documentation. Infection-related cancer was defined as cancer driven by the following infectious agents: human herpesvirus 8 (HHV-8) (Kaposi sarcoma), Epstein-Barr virus (EBV) (non-Hodgkin lymphoma, Hodgkin lymphoma), and human papillomavirus (anal cancer, cervical cancer) [20]. All other malignancies were classified as infection-unrelated cancer, including 1 case of liver cancer in a participant with no evidence of hepatitis B or C coinfection and 1 case of gastric cancer in a participant without previous history of Helicobacter pylori infection. No cases of pharyngeal lymphoepithelioma or Merkel cell carcinoma were observed.
Statistical Analyses
Participants were followed from study entry until first cancer event, death, loss to follow-up, or 25 May 2015 (date of database closure), whichever occurred first. We calculated annual rates of any type of cancer in each study arm and examined the trends within study group using unadjusted Poisson regression models. Kaplan–Meier curves giving the cumulative percentage of participants with infection-related and infection-unrelated cancer were constructed for each study arm. We tested for the difference in treatment group hazard ratios (HRs) for infection-related cancer vs infection-unrelated cancer by fitting a Cox regression model that simultaneously estimated both treatment group HRs and stratified by type of event.
Multivariable Cox models identified factors independently associated with risk of infection-related and infection-unrelated cancer. The statistical power was insufficient to investigate individual cancer types. Epidemiologically important and biologically plausible baseline variables were investigated: demographics (age, sex, race, and region), medical history (current smoking, alcohol abuse, body mass index [BMI], hepatitis B or C coinfection, and prior cancer), and HIV-specific variables (CD4 count, CD8 count, CD4:CD8 ratio, and HIV RNA level). Variables associated with cancer risk in univariate analyses were subsequently mutually adjusted for.
To explore potential mediators of the reduced cancer risk from immediate cART, Cox proportional hazards models with an indicator for treatment group were adjusted sequentially for demographics, traditional cancer risk factors, and baseline and time-updated HIV-specific variables. The following models were used: (A) univariable with a single treatment group indicator; (B) adjusted for baseline covariates: age, sex, race, geographical region, smoking, BMI, hepatitis B/C, CD4 count, and log10 HIV RNA; (C) adjusted for latest CD4 count, modeled as a continuous variable; (D) adjusted for latest HIV RNA, modeled as ≤200 copies/mL vs >200 copies/mL; (E) adjusted for latest CD4 count and latest HIV RNA (≤200 vs >200 copies/mL); (F) as in (B) with further adjustment for latest CD4 count and latest HIV RNA (≤200 vs >200 copies/mL); (G) adjusted for latest log10 HIV RNA; and (H) adjusted for latest CD4:CD8 ratio.
Secondary Analyses
We repeated analyses excluding premalignant or low-grade lesions not requiring chemotherapy (1 participant had microinvasive squamous cell carcinoma and anal intraepithelial neoplasia grade III, which was classified as anal cancer in the main analyses), localized skin squamous carcinoma (n = 2), and Kaposi sarcoma restricted to the skin without internal organ involvement (n = 7). This was done because these lesions have low morbidity or can be reversed or cured by cART initiation alone [21, 22].
RESULTS
There were 14 malignancies among persons randomized to the immediate cART arm (6 infection-related and 8 infection-unrelated) and 39 malignancies in the deferred arm (23 infection-related and 16 infection-unrelated). Types of infection-related and infection-unrelated cancer stratified by treatment group are listed in Table 1. Baseline characteristics of participants with no cancer and with infection-related and infection-unrelated cancer are shown in Table 2.
Table 1.
Types of Malignancies by Treatment Group—Strategic Timing of Antiretroviral Treatment (START) Trial
| Malignancy | Immediate cART | Deferred cART |
|---|---|---|
| Infection-related cancer | ||
| Human herpesvirus 8: Kaposi sarcoma | 1 | 11 |
| Epstein-Barr virus | ||
| Non-Hodgkin lymphoma | 2 | 9 |
| Hodgkin lymphoma | 1 | 1 |
| Human papillomavirus | ||
| Anal cancer | 1 | 2 |
| Cervical cancer | 1 | 0 |
| Total | 6 | 23 |
| Infection-unrelated cancer | ||
| Prostate | 2 | 3 |
| Lung | 2 | 2 |
| Testicular | 0 | 2 |
| Multiple myeloma | 1 | 0 |
| Fibrosarcoma | 1 | 0 |
| Breast | 0 | 1 |
| Bladder | 1 | 0 |
| Ureteral | 0 | 1 |
| Malignant melanoma | 0 | 1 |
| Myeloid leukemia | 0 | 1 |
| Thyroid | 0 | 1 |
| Leiomyosarcoma | 0 | 1 |
| Squamous cell carcinoma of the skin | 1 | 0 |
| Squamous cell carcinoma of the head/neck | 0 | 1 |
| Gastric adenocarcinoma | 0 | 1 |
| Liver | 0 | 1 |
| Total | 8 | 16 |
Abbreviation: cART, combination antiretroviral therapy.
Table 2.
Baseline Characteristics by Cancer Event Status—Strategic Timing of Antiretroviral Treatment (START) Trial
| Characteristic | No Cancer | Infection-Related Cancer | Infection-Unrelated Cancer |
|---|---|---|---|
| No. of participants | 4632 | 29 | 24 |
| Demographics | |||
| Age, y, median (IQR) | 36 (29–44) | 44 (31–48) | 51 (36–59) |
| Sex, % female | 26.9 | 10.3 | 25.0 |
| Race, % | |||
| Asian | 8.4 | 0.0 | 4.2 |
| Black | 30.3 | 13.8 | 16.7 |
| Latino/Hispanic | 13.7 | 10.3 | 4.2 |
| White | 44.2 | 75.9 | 75.0 |
| Other | 3.5 | 0.0 | 0.0 |
| Region, % | |||
| High income | 45.8 | 51.7 | 79.2 |
| Low to middle income | 54.2 | 48.3 | 20.8 |
| HIV history, % | |||
| Likely mode of HIV infection | |||
| Sexual contact with person of same sex | 55.1 | 79.3 | 54.2 |
| Sexual contact with person of opposite sex | 38.3 | 20.7 | 37.5 |
| Injection drug use | 1.4 | 0.0 | 4.2 |
| Blood products/other/unknown | 5.3 | 0.0 | 4.2 |
| Time participant known to be HIV infected, y, median (IQR) | 1.0 (0.4–3.0) | 0.7 (0.3–3.4) | 2.3 (0.6–4.4) |
| Laboratory results | |||
| CD4 counta, cells/µL, median (IQR) | 651 (584–764) | 671 (587–770) | 638 (562–759) |
| CD4:CD8 ratio, median (IQR) | 0.66 (0.48–0.90) | 0.57 (0.42–0.73) | 0.56 (0.38–0.75) |
| CD8 count, cells/µL, median (IQR) | 1040 (774–1400) | 1152 (986–1892) | 1150 (821–1499) |
| HIV RNA, log10 copies/mL, median (IQR) | 4.1 (3.5–4.6) | 4.6 (4.0–5.0) | 4.2 (3.9–4.5) |
| Highest HIV RNAb, log10copies/mL, median (IQR) | 4.3 (3.7–4.8) | 4.8 (4.1–5.3) | 4.5 (4.2–5.0) |
| Medical history, % | |||
| Hepatitis B | 2.8 | 0.0 | 0.0 |
| Hepatitis C | 3.7 | 0.0 | 12.5 |
| Prior non-AIDS cancer | 0.4 | 0.0 | 4.2 |
| Current smoker | 31.8 | 44.8 | 41.7 |
| Alcoholism or other substance dependence | 3.3 | 0.0 | 4.2 |
| Body mass index, kg/m2, median (IQR) | 24.5 (22.1–27.9) | 25.9 (22.8–29.6) | 25.4 (22.3–27.2) |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
a Average of screening and baseline values.
b Documented in participant record.
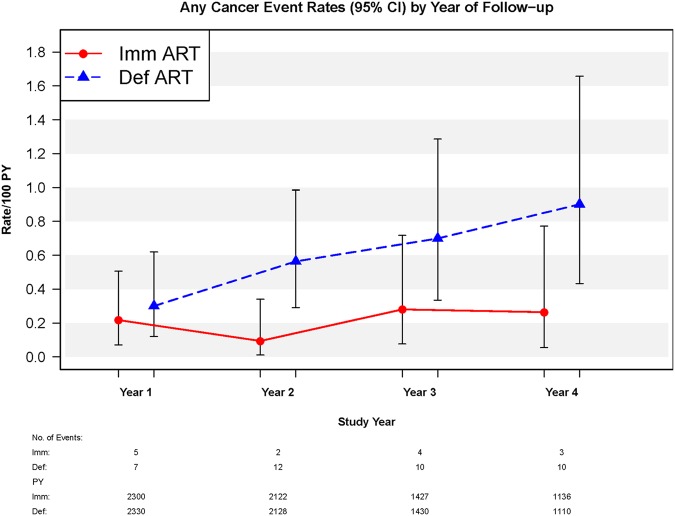
On the one hand, in the immediate cART arm, annual incidences of any type of cancer remained stable throughout the entirety of follow-up (P value for trend = .790). On the other hand, in the deferred cART arm, annual incidences of any type of cancer increased significantly during follow-up (P = .026) (Figure 1). The HR of immediate vs deferred cART initiation was 0.28 (95% confidence interval [CI], .13–.58; P < .001) for any type of cancer after the exclusion of cancer events occurring in the first year of follow-up. Among participants with any type of cancer, the latest median CD4 count at time of cancer diagnosis was 861 (interquartile range [IQR], 680–1082) cells/µL in the immediate cART arm and 586 (IQR, 452–664) cells/µL in the deferred cART arm. In the deferred arm, 13 malignancies (8 infection-related and 5 infection-unrelated) were diagnosed after cART initiation. The median time from cART initiation to cancer diagnosis was 134 (IQR, 39–1225) days in the deferred arm.
Figure 1.
Annual rates of any type of cancer by year of follow-up, stratified by Strategic Timing of Antiretroviral Treatment arm. P value for trend was .026 in the deferred (Def) combination antiretroviral therapy (cART) arm and .790 in the immediate (Imm) cART arm. Abbreviations: ART, antiretroviral therapy; CI, confidence interval; PY, person-years.
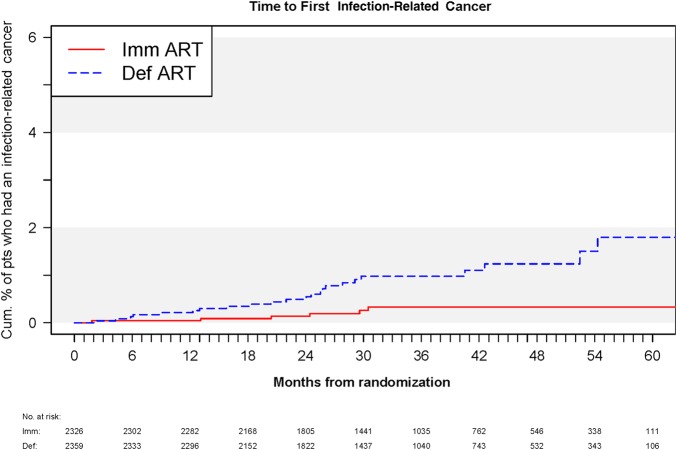
Immediate cART significantly reduced the risk of infection-related cancer by 74% (HR of immediate vs deferred cART initiation, 0.26 [95% CI, .11–.64]; P = .003). Kaplan–Meier curves overlapped during the first year; afterward, the lower risk of infection-related cancer in the immediate cART arm became evident and remained so for the rest of follow-up (Figure 2). The lower risk of infection-related cancer in the immediate cART arm was driven mainly by a reduction of cases of Kaposi sarcoma and non-Hodgkin lymphoma (Table 1).
Figure 2.
Kaplan–Meier curves giving the cumulative percentage of participants who developed infection-related cancer in each Strategic Timing of Antiretroviral Treatment arm. Abbreviations: ART, antiretroviral therapy; Def, deferred; Imm, immediate; pts, patients.
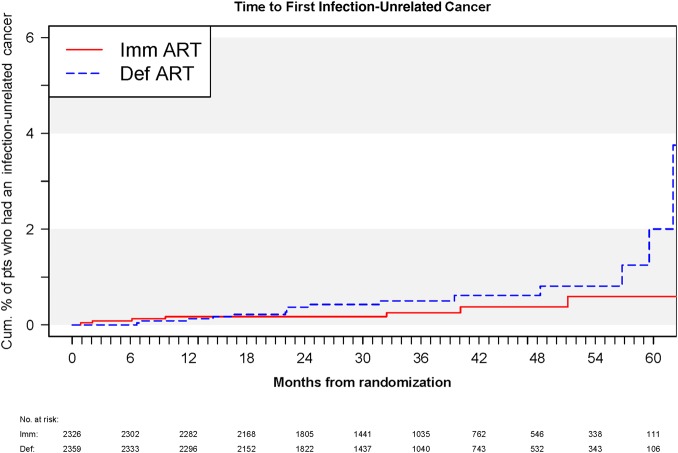
Immediate cART lowered the risk of infection-unrelated cancer by 51% (95% CI, 21%–115%; P = .103). This risk reduction did not reach statistical significance (Figure 3). This seemed to suggest that immediate cART reduced the risk for infection-related cancer more than infection-unrelated cancer. However, when we tested the difference in HRs for infection-related vs infection-unrelated cancer, we found no statistical significance (P = .27 for comparing the treatment group HRs for infection-related vs infection-unrelated cancers).
Figure 3.
Kaplan–Meier curves giving the cumulative percentage of participants who developed infection-unrelated cancer in each Strategic Timing of Antiretroviral Treatment arm. Abbreviations: ART, antiretroviral therapy; Def, deferred; Imm, immediate; pts, patients.
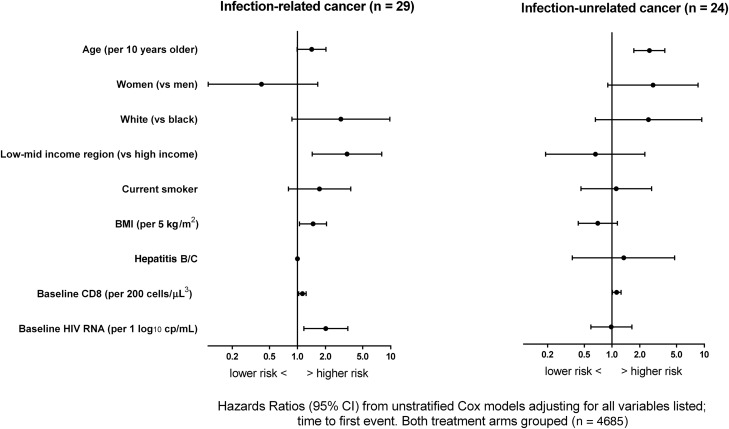
In adjusted analyses with both treatment groups combined, independent baseline predictors of infection-related cancer were older age (HR, 1.42 [95% CI, .99–2.02] per 10 years older), higher BMI (HR, 1.47 [95% CI, 1.05–2.05] per 5 kg/m2), low- to middle-income region (HR, 3.40 [95% CI, 1.44–8.02] vs high-income region), baseline HIV RNA (HR, 2.01 [95% CI, 1.17–3.47] per 1 log10 higher) and baseline CD8 count (HR, 1.13 [95% CI, 1.03–1.24] per 200 cells/µL higher) (Figure 4). Older age (HR, 2.54 [95% CI, 1.72–3.73] per 10 years older) and baseline CD8 count (HR, 1.12 [95% CI, 1.01–1.25] per 200 cells/µL higher) were the only independent predictors of infection-unrelated cancer (Figure 4).
Figure 4.
Factors independently associated with infection-related and infection-unrelated cancer. Hepatitis B/C could not be estimated for infection-related cancer. Abbreviations: BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus.
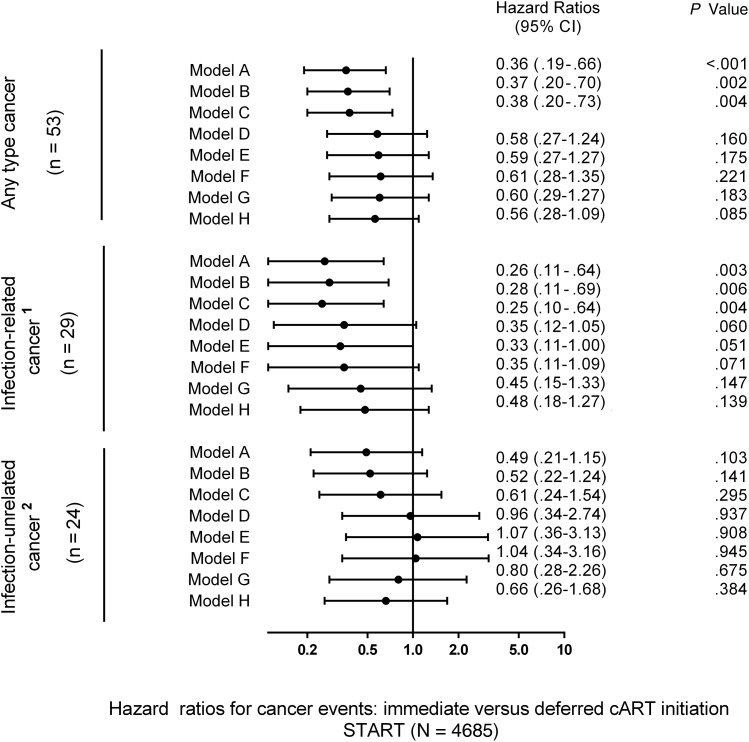
Figure 5 shows the impact of sequential adjustment for baseline and time-updated variables on the protective effect of immediate cART initiation on any-type cancer risk. Adjustment for demographics, traditional cancer risk factors, and CD4 counts (models B and C) had no appreciable effect on the estimates of overall effect. Adjustment for latest HIV RNA level (models D–G) and CD4:CD8 ratio (model H) attenuated the protective effect of immediate cART on any type of cancer by partially attenuating the overall effect and enlarging 95% CIs. The effect of immediate cART was no longer significant in models D–H, suggesting that the effect of cART is partially mediated through effects on these parameters.
Figure 5.
Immediate vs deferred combination antiretroviral therapy (cART) initiation and the risk of any type, infection-related and infection-unrelated cancer in the Strategic Timing of Antiretroviral Treatment (START) study. 1Infection-related cancer: human herpesvirus 8 (Kaposi sarcoma), Epstein-Barr virus (non-Hodgkin lymphoma, Hodgkin lymphoma), human papillomavirus (anal cancer, cervical cancer); 2Infection-unrelated cancer: prostate cancer, lung cancer, testis cancer, plasma cell myeloma, fibrosarcoma, breast cancer, bladder cancer, ureteric cancer, malignant melanoma, myeloid leukemia, thyroid cancer, leiomyosarcoma, squamous cell carcinoma of the head and neck, squamous cell cancer, gastric adenocarcinoma, liver cancer. Models: (A) Univariable, estimated in a Cox proportional hazards model with a single treatment indicator. (B) Adjusted for baseline covariates: age, sex, race, geographical region, smoking, body mass index, hepatitis B/C, baseline CD4 cell count, and baseline log10 human immunodeficiency virus (HIV) RNA. (C) Adjusted for latest CD4 cell count, modeled as a continuous variable. (D) Adjusted for latest HIV RNA, modeled as ≤200 copies/mL vs >200 copies/mL. (E) Adjusted for latest CD4 cell count and latest HIV RNA. (F) As in (B) with further adjustment for latest CD4 cell count and latest HIV RNA. (G) Adjusted for latest log10 HIV RNA. (H) Adjusted for latest CD4:CD8 ratio. Abbreviation: CI, confidence interval.
As for infection-related cancer, results were broadly consistent across the models, with HRs varying from 0.26 to 0.48 (Figure 5). Adjustment for latest HIV RNA levels (models D–G), in particular when modeled as a continuous variable (model G), and CD4:CD8 ratio (model H) partially attenuated the effect of immediate cART on the risk of infection-related cancer.
With respect to infection-unrelated cancer, the measurable effect of sequential adjustments on the protective effect of immediate cART appears to be stronger (models D–F). For instance, HRs (immediate cART/deferred cART) for infection-unrelated cancer increased from 0.49 (model A) to 1.07 (model E) after adjustment. However, the overall number of cancer events was too small, and no definitive statement can be made about the degree of attenuation of the protective effect of immediate cART after adjustment.
Secondary analyses excluding premalignant lesions and localized cancer did not change these conclusions.
DISCUSSION
In a large, randomized HIV trial from around the globe, we report that immediate cART initiation significantly reduced the risk of infection-related cancer. This effect was mainly driven by a reduction in cases of Kaposi sarcoma and non-Hodgkin lymphoma. These were unexpected results because START enrolled participants with early HIV infection and follow-up time was shorter than initially planned. Contrary to what we first hypothesized, adjustment for CD4 counts had no impact on the protective effect of immediate cART on any-type and infection-related cancer, and adjustment for HIV RNA levels only partially attenuated this association. Taken together, these findings suggest that the benefit of immediate cART in reducing cancer goes beyond HIV RNA suppression and immunostatus as assessed by CD4 counts and seems likely to be also mediated by other mechanisms impacting coinfections with pro-oncogenic virus and immune surveillance.
With the changing epidemiology of cancer during HIV infection, the traditional classification into AIDS-defining and non-AIDS-defining cancer, implemented in the early 1990s for surveillance purposes [23], became outdated [18]. The classification of cancer into infection-related and infection-unrelated seems to us to be more appropriate because it considers a wider range of cancer types whose incidence is increased during immunosuppression and establishes a framework to better understand the interplay between viral coinfections, HIV, and carcinogenesis. However, as pointed out by others [24], this classification is not perfect because, even when considering a given cancer type such as non-Hodgkin lymphoma, an infectious origin can only be ascertained in a subset of cases. This classification can only be definitive after a thorough search for pathogens in tissue samples using in situ hybridization.
Consistent with previous reports [25], we observed that infection-related and infection-unrelated cancer may have different predictors. Low- to middle-income region and baseline HIV RNA were associated with risk of infection-related cancer, which reflects a higher burden of these malignancies in resource-constrained settings and suggests a stronger link between HIV RNA viremia and malignancies caused by pro-oncogenic viruses [26, 27]. Older age and higher CD8 count were independent predictors of both infection-related and infection-unrelated cancer. Indeed, a recent report linked elevations in CD8 cells after cART initiation to viral coinfections and non-AIDS death [28].
Because cART improves immune function, lowers HIV viral load, and reduces inflammation [29], earlier cART initiation had previously been proposed as an approach to reduce cancer risk among HIV-infected persons [2–4]. Furthermore, cohort studies had long identified a relationship between immunodeficiency and uncontrolled HIV RNA replication with risk of non-Hodgkin lymphoma and Kaposi sarcoma [1, 2]. However, this benefit was estimated in previous studies when cART was initiated at much lower CD4 counts. In START, cART was initiated at higher CD4 counts before the development of overt immunosuppression; still, the benefit of immediate cART in reducing cancer risk seems to be larger than previously estimated by observational studies [30].
The benefit of immediate cART initiation in reducing infection-related cancer was determined by a marked decrease in cases of non-Hodgkin lymphoma and Kaposi sarcoma. The mechanisms by which EBV and HHV-8 may foster carcinogenesis are complex and not fully understood [31, 32]. In both cases, common viral infections cause rare malignancies; this highlights the potential contribution of multifactorial nonviral factors to the development of infection-related cancer.
EBV establishes long-term latent infections in B cells and may act just as an initial promoter of cellular transformation from which subsequent progression to cancer is independent [31, 32]. The dominant EBV latency patterns observed in non-Hodgkin lymphoma tissue differ between pre- [33] and post-cART studies [34]. It was hypothesized that cART increases immune surveillance of proteins expressed by cells latently infected by EBV, resulting in a shift to a less oncogenic latency pattern [34].
As for HHV-8 infection, immunosuppression is clearly a major contributor to progression to Kaposi sarcoma, whereas recent evidence points out the importance of complex immune modulation mechanisms [35]. Questions remain as to how cART interferes with such mechanisms. In a randomized trial involving participants with tuberculous pericarditis predominantly coinfected with HIV, glucocorticoids and immunotherapy with nonpathogenic mycobacteria acted synergistically to increase risk of Kaposi sarcoma [35].
CD8 T-cell dysfunction in the setting of untreated HIV infection may also be an important contributor. Depleted response from EBV- and HHV-8-specific CD8 cytotoxic cells may facilitate carcinogenesis among immunocompromised hosts [36]. The extent to which the normalization of the CD4:CD8 ratio following cART initiation reflects a recovery of CD8 cell response is unclear [37], but this may explain why adjustment by CD4:CD8 partially attenuated the benefit of immediate cART in reducing cancer risk.
Our study had important limitations. First, this is a post hoc analysis of a trial whose sample size was not calculated to assess reductions in cancer risk. Second, START had a follow-up shorter than initially planned. As a result, we cannot exclude the possibility that immediate cART could have significantly reduced the risk of infection-unrelated cancer if a longer follow-up with a larger number of events had accrued. It is unclear whether the nonsignificant reduction in infection-unrelated cancer simply reflected limited power to detect differences or was instead a true observation. The first possibility is favored by the lack of significant difference in the HRs for infection-related vs infection-unrelated cancer (P = .27). From our studies, activated inflammation is associated with an increased risk of both infection-related and infection-unrelated cancers [9]. Therefore, reduction of inflammation could have been a common mechanism by which cART may have reduced the risk of both types of cancer [38]. Third, we did not perform in situ hybridization analyses in cancer tissues; therefore, a definitive infectious origin could not be ascertained in cancer events classified as infection related. Fourth, the number of cancer events was relatively small; this forced us to group heterogeneous malignancies into 2 categories and hampered our ability to quantify variation in risk of individual cancer types. We also had a hampered ability to fully explore potential mediators of the benefit of immediate cART and take our results as preliminary rather than definitive. A longer follow-up is needed in START to better understand the treatment differences and determine with accuracy which are the predictors for infection-related and infection-unrelated cancer. Nevertheless, few other randomized experiments are available to investigate the impact of cART on cancer risk. The Strategies for Management of Antiretroviral Therapy (SMART) study compared continuous cART use to CD4-guided structured cART interruptions [39]. Because SMART participants who interrupted cART also had higher risk of cancer, in particular Kaposi sarcoma and non-Hodgkin lymphoma [40], an individual participant data analysis combining START and SMART may be useful for a better estimation of the impact of cART on the risk of specific cancer types.
CONCLUSIONS
Immediate cART initiation significantly reduces risk of cancer during HIV infection. Adjustment for latest HIV RNA level appeared to attenuate HRs of immediate vs deferred cART for infection-unrelated cancers more than infection-related cancers. The benefit of immediate cART does not appear to be solely attributable to HIV RNA suppression and may also be mediated by other mechanisms, such as a curb on oncogenic virus coinfection and reduction of inflammation. Further research is needed to identify mediators of the benefit of immediate cART initiation in reducing cancer risk.
Notes
Acknowledgments. We thank the Strategic Timing of Antiretroviral Treatment (START) investigators and participants. For the complete member list of the INSIGHT START Study Group, see the supplementary material in [19].
Author contributions. A. H. B., J. N., A. G. B., T. W., and R. M. conceived the study. J. N. performed the statistical analyses. A. H. B. drafted the manuscript. All authors contributed to data interpretation, critically revised the manuscript, and approved the final version.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of this manuscript.
Financial support. The START study was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health Clinical Center (grant numbers UM1-AI068641 and UM1-AI120197), National Cancer Institute, National Heart, Lung, and Blood Institute, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, National Institute of Arthritis and Musculoskeletal and Skin Diseases, Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (France), National Health and Medical Research Council (Australia), National Research Foundation (Denmark), Bundesministerium für Bildung und Forschung (Germany), European AIDS Treatment Network, Medical Research Council (United Kingdom), National Institute for Health Research, National Health Service (United Kingdom), and University of Minnesota. Antiretroviral drugs were donated to the central drug repository by AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline (GSK)/ViiV Healthcare, Janssen Scientific Affairs, and Merck. The present study was also supported by the Research Council at Rigshospitalet and by the Danish National Research Foundation (grant number DNRF126). A. H. B. is supported by Lundbeckfonden (grant number R219-2016-762).
Potential conflicts of interest. K. H. conducts research sponsored by GSK/ViiV Healthcare, Merck, Janssen, and Gilead. M. K. J. has received research grant support from Gilead Sciences, AbbVie, Janssen, GSK/ViiV Healthcare, Merck, and Bristol-Myers Squibb. T. W. has received grant funding (paid to Weill Cornell Medicine) from Gilead Sciences, GSK/ViiV Healthcare, and Bristol-Myers Squibb. T. W. has also served as an ad hoc consultant to GSK/ViiV Healthcare. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Clifford GM, Polesel J, Rickenbach M et al. . Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst 2005; 97:425–32. [DOI] [PubMed] [Google Scholar]
- 2.Guiguet M, Boué F, Cadranel J et al. . Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol 2009; 10:1152–9. [DOI] [PubMed] [Google Scholar]
- 3.Reekie J, Kosa C, Engsig F et al. . Relationship between current level of immunodeficiency and non-acquired immunodeficiency syndrome-defining malignancies. Cancer 2010; 116:5306–15. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg MJ, Chao C, Leyden WA et al. . HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev 2011; 20:2551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helleberg M, Gerstoft J, Afzal S et al. . Risk of cancer among HIV-infected individuals compared to the background population: impact of smoking and HIV. AIDS 2014; 28:1499–508. [DOI] [PubMed] [Google Scholar]
- 6.Harrod R, Nacsa J, Van Lint C et al. . Human immunodeficiency virus type-1 Tat/co-activator acetyltransferase interactions inhibit p53Lys-320 acetylation and p53-responsive transcription. J Biol Chem 2003; 278:12310–8. [DOI] [PubMed] [Google Scholar]
- 7.Worm SW, Bower M, Reiss P et al. . Non-AIDS defining cancers in the D:A:D Study—time trends and predictors of survival: a cohort study. BMC Infect Dis 2013; 13:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruyand M, Ryom L, Shepherd L et al. . Cancer risk and use of protease inhibitor or nonnucleoside reverse transcriptase inhibitor-based combination antiretroviral therapy: the D:A:D study. J Acquir Immune Defic Syndr 2015; 68:568–77. [DOI] [PubMed] [Google Scholar]
- 9.Borges ÁH, Silverberg MJ, Wentworth D et al. . Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS 2013; 27:1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borges ÁH, Lundgren JD, Ridolfo A et al. . Thrombocytopenia is associated with an increased risk of cancer during treated HIV disease. AIDS 2014; 28:2565–71. [DOI] [PubMed] [Google Scholar]
- 11.Shiels MS, Pfeiffer RM, Gail MH et al. . Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011; 103:753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith CJ, Ryom L, Weber R et al. . Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–8. [DOI] [PubMed] [Google Scholar]
- 13.Silverberg MJ, Chao C, Leyden WA et al. . HIV infection and the risk of cancers with and without a known infectious cause. AIDS 2009; 23:2337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007; 370:59–67. [DOI] [PubMed] [Google Scholar]
- 15.Vajdic CM, Mao L, van Leeuwen MT, Kirkpatrick P, Grulich AE, Riminton S. Are antibody deficiency disorders associated with a narrower range of cancers than other forms of immunodeficiency? Blood 2010; 116:1228–34. [DOI] [PubMed] [Google Scholar]
- 16.de Martel C, Shiels MS, Franceschi S et al. . Cancers attributable to infections among adults with HIV in the United States. AIDS 2015; 29:2173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr 2009; 52:611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borges AH, Dubrow R, Silverberg MJ. Factors contributing to risk for cancer among HIV-infected individuals, and evidence that earlier combination antiretroviral therapy will alter this risk. Curr Opin HIV AIDS 2014; 9:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.INSIGHT START Study Group; Lundgren JD, Babiker AG et al. . Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouvard V, Baan R, Straif K et al. . A review of human carcinogens—part B: biological agents. Lancet Oncol 2009; 10:321–2. [DOI] [PubMed] [Google Scholar]
- 21.Bower M, Dalla Pria A, Coyle C et al. . Prospective stage-stratified approach to AIDS-related Kaposi sarcoma. J Clin Oncol 2014; 32:409–14. [DOI] [PubMed] [Google Scholar]
- 22.de Pokomandy A, Rouleau D, Ghattas G et al. . HAART and progression to high-grade anal intraepithelial neoplasia in men who have sex with men and are infected with HIV. Clin Infect Dis 2011; 52:1174–81. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992; 41:1–19. [PubMed] [Google Scholar]
- 24.Gopal S, Achenbach CJ, Yanik EL et al. . Moving forward in HIV-associated cancer. J Clin Oncol 2014; 32:876–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shepherd L, Borges Á, Ledergerber B et al. . Infection-related and -unrelated malignancies, HIV and the aging population. HIV Med 2016; 17:590–600. [DOI] [PubMed] [Google Scholar]
- 26.Kowalkowski MA, Day RS, Du XL, Chan W, Chiao EY. Cumulative HIV viremia and non-AIDS-defining malignancies among a sample of HIV-infected male veterans. J Acquir Immune Defic Syndr 2014; 67:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riedel DJ, Rositch AF, Redfield RR. Patterns of HIV viremia and viral suppression before diagnosis of non-AIDS-defining cancers in HIV-infected individuals. Infect Agent Cancer 2015; 10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helleberg M, Kronborg G, Ullum H, Ryder LP, Obel N, Gerstoft J. Course and clinical significance of CD8+ T-cell counts in a large cohort of HIV-infected individuals. J Infect Dis 2015; 211:1726–34. [DOI] [PubMed] [Google Scholar]
- 29.Baker JV, Neuhaus J, Duprez D et al. . Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr 2011; 56:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lodi S, Phillips A, Logan R et al. . Comparative effectiveness of immediate antiretroviral therapy versus CD4-based initiation in HIV-positive individuals in high-income countries: observational cohort study. Lancet HIV 2015; 2:e335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer 2010; 10:878–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White MK, Pagano JS, Khalili K. Viruses and human cancers: a long road of discovery of molecular paradigms. Clin Microbiol Rev 2014; 27:463–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kersten MJ, Van Gorp J, Pals ST, Boon F, Van Oers MH. Expression of Epstein-Barr virus latent genes and adhesion molecules in AIDS-related non-Hodgkin's lymphomas: correlation with histology and CD4-cell number. Leuk Lymphoma 1998; 30:515–24. [DOI] [PubMed] [Google Scholar]
- 34.Arvey A, Ojesina AI, Pedamallu CS et al. . The tumor virus landscape of AIDS-related lymphomas. Blood 2015; 125:e14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayosi BM, Ntsekhe M, Bosch J et al. . Prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis. N Engl J Med 2014; 371:1121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pietersma F, Piriou E, van Baarle D. Immune surveillance of EBV-infected B cells and the development of non-Hodgkin lymphomas in immunocompromised patients. Leuk Lymphoma 2008; 49:1028–41. [DOI] [PubMed] [Google Scholar]
- 37.Serrano-Villar S, Sainz T, Lee SA et al. . HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014; 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis 2011; 70:104–8. [DOI] [PubMed] [Google Scholar]
- 39.El-Sadr WM, Lundgren JD, Neaton JD et al. . CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355:2283–96. [DOI] [PubMed] [Google Scholar]
- 40.Silverberg MJ, Neuhaus J, Bower M et al. . Risk of cancers during interrupted antiretroviral therapy in the SMART study. AIDS 2007; 21:1957–63. [DOI] [PubMed] [Google Scholar]