Due to drift of the A/H3N2 influenza virus, vaccine effectiveness (VE) against the predominant A/H3N2 virus was insignificant at 6% but was 55% against B/Yamagata. VE did not differ between live attenuated influenza vaccine and inactivated influenza vaccine among young children. Quadrivalent vaccine use increased.
Keywords: influenza vaccine, vaccine effectiveness
Abstract
Background. Circulating A/H3N2 influenza viruses drifted significantly after strain selection for the 2014–2015 vaccines. Also in 2014–2015, the Advisory Committee on Immunization Practices recommended preferential use of live attenuated influenza vaccine (LAIV) over inactivated influenza vaccine (IIV) among children aged 2–8 years.
Methods. Vaccine effectiveness (VE) across age groups and vaccine types was examined among outpatients with acute respiratory illness at 5 US sites using a test-negative design, that compared the odds of vaccination among reverse transcription polymerase chain reaction–confirmed influenza positives and negatives.
Results. Of 9311 enrollees with complete data, 7078 (76%) were influenza negative, 1840 (19.8%) were positive for influenza A (A/H3N2, n = 1817), and 395 (4.2%) were positive for influenza B (B/Yamagata, n = 340). The overall adjusted VE was 19% (95% confidence interval [CI], 10% to 27%) and was statistically significant in all age strata except those aged 18–64 years. The adjusted VE of 6% (95%CI, −5% to 17%) against A/H3N2-associated illness was not statistically significant, unlike VE for influenza B/Yamagata, which was 55% (95%CI, 43% to 65%). Among those aged 2–8 years, VE against A/H3N2 was 15% (95%CI, −16% to 38%) for IIV and −3% (CI, −50% to 29%) for LAIV; VE against B/Yamagata was 40% (95%CI, −20% to 70%) for IIV and 74% (95%CI, 25% to 91%) for LAIV.
Conclusions. The 2014–2015 influenza vaccines offered little protection against the predominant influenza A/H3N2 virus but were effective against influenza B. Preferential use of LAIV among young children was not supported.
(See the Editorial Commentary by Omer and Yildirim on pages 1574–6.)
Rapid changes in influenza vaccine science have occurred recently. The potential impact of these changes on vaccine effectiveness (VE) has not been thoroughly examined. For example, quadrivalent influenza vaccine (containing 2 A strains and 2 B lineages) is gaining market share compared with the older, trivalent vaccine (containing 2 A strains and 1 B lineage), but the effect of this trend on VE is unknown. Second, a metaanalysis of live attenuated influenza vaccine (LAIV) suggested superior VE compared with inactivated influenza vaccine (IIV) among children [1]. For the 2014–2015 influenza season only, the Advisory Committee on Immunization Practices (ACIP) released recommendations stating a preference for LAIV over IIV for children aged 2–8 years [2]. In the 2013–2014 season, LAIV VE against A/H1N1pdm2009 [3] (A/H1N1) was poor. Furthermore, the 2014–2015 season was characterized by the emergence of an A/H3N2 drifted virus that was associated with low VE in an early season estimate [4, 5], and with an earlier-than-normal peak in influenza cases occurring in late December [6]. Thus, the unpredictability of influenza epidemiology continues to necessitate effectiveness studies of available influenza vaccines in a range of population subgroups.
In a related study, the US Flu VE Network reported the VE of genetic subtypes of antigenically matched and drifted A/H3N2 influenza viruses circulating in 2014–2015 [7] but did not provide VE estimates for influenza overall by age groups or vaccine type. The purposes of this study were to provide influenza VE estimates against medically attended acute respiratory illness (ARI) for influenza A and B; estimate VE in children aged 2–8 years by vaccine type; identify trends in trivalent vs quadrivalent vaccine use; and compare VE of the most widely available influenza vaccines, specifically standard-dose trivalent IIV vs high-dose trivalent IIV and trivalent IIV vs quadrivalent IIV.
METHODS
Participants
Since the 2011–2012 influenza season, the US Flu VE Network in Michigan, Pennsylvania, Texas, Washington, and Wisconsin has enrolled participants seeking outpatient medical care for an ARI with cough. Details of the sites, enrollment, and laboratory methods have been previously published [8, 9]. Briefly, after confirmation of local influenza circulation in the 2014–2015 season, eligible patients with ARI onset ≤7 days prior who presented with cough were enrolled. Eligibility criteria included date of birth before 1 March 2014, not taking influenza antiviral medication in the previous 7 days, and not previously enrolled within 14 days. After obtaining informed consent from patients or parents/guardians for their children, participants were interviewed to collect demographic data, general and current health status, symptom and illness severity information, and 2014–2015 influenza vaccination status. Presence of 1 or more high-risk medical conditions in the previous 12 months, as defined by the International Classification of Diseases, 9th Edition, Clinical Modification [10], was extracted from electronic medical records (EMRs).
Vaccination Status
Current season vaccination status was self-reported at enrollment and documented by review of EMRs, employee health records, and state or local immunization registries, collectively termed electronic immunization records (EIRs). All participants with EIR-documented 2014–2015 influenza vaccination ≥14 days prior to illness onset were considered vaccinated. Participants in Michigan, Pennsylvania, and Texas were additionally considered vaccinated if they were able to report a plausible place and time of vaccine receipt from an off-site provider without vaccination records (plausible self-report), and participants in Washington who reported any off-site vaccination ≥14 days before illness onset were considered vaccinated [3]. Those vaccinated 0–13 days prior to illness onset were excluded. All other participants were considered unvaccinated. Information on prior seasons' vaccine receipt was derived from EIRs.
2014–2015 Influenza Vaccine Formulation and Type
Recommended reference viruses for 2014–2015 northern hemisphere vaccines were A/California/7/2009(H1N1), A/Texas/50/2012(H3N2) (A/Victoria/361/2011-like), and B/Massachusetts/2/2012 (B/Yamagata lineage); quadrivalent vaccines also contained B/Brisbane/60/2008 (B/Victoria lineage) [11]. The type of influenza vaccine received, including trivalent (IIV3) or quadrivalent inactivated (IIV4) and quadrivalent live-attenuated (LAIV) vaccines, and high dose or standard dose, was derived from lot number, name of vaccine, manufacturer, and route of administration from EIR. For those with only self-reported vaccination, influenza vaccine type was recorded from self or parental report at enrollment as either “shot” or “nasal mist.”
Laboratory Methods
Participants who were aged ≥2 years provided nasal and throat swabs (children aged <2 years provided nasal swabs only) for confirmation of influenza using real-time reverse transcription polymerase chain reaction (RT-PCR) assays in US Flu VE Network laboratories, as previously described [8, 9]. The influenza outbreak period was unique to each site and was defined as the time between the week of illness onset for the first RT-PCR–positive case and the week of illness onset for the last influenza-positive case collected.
Statistical Analyses
A test-negative design was used to estimate VE by comparing influenza vaccination status among influenza RT-PCR–positive (case) and influenza-negative (control) participants [12, 13]. Enrollees with inconclusive RT-PCR results, controls with illness onset dates outside each site's influenza outbreak period, and children aged <9 years who were considered partially vaccinated per 2014–2015 ACIP criteria [2] were excluded from analyses.
The primary analysis estimated VE against RT-PCR–confirmed influenza-associated ARI in participants aged ≥6 months during the influenza A and B outbreak period. Logistic regression models were used to calculate odds ratios (ORs) to compare influenza-positive and influenza-negative participants; VE was estimated as 100% × (1 − OR). For comparison with previous season estimates [9], we calculated adjusted ORs in models that include network site, participant age (by category for overall VE or by year for age group–specific estimates), presence of any high-risk condition (vs none), and calendar time in 2-week intervals. Additional potential confounders, including sex, race/Hispanic ethnicity, self- or parent-rated general health status, and days from illness onset to specimen collection, did not change the adjusted VE by ≥5%, which was the predetermined threshold for inclusion [9]. Primary VE analyses used vaccination status from EIR and plausible self-report. Separate sensitivity analyses were performed that included only self-reported doses; excluded participants with a plausible self-report of vaccination that was not confirmed by EIR; and included, but considered unvaccinated, participants with a plausible self-report of vaccination that was not confirmed by EIR.
All reported tests were 2 sided, and a P value < .05 was considered statistically significant. VE estimates were considered statistically significant if 95% confidence intervals (CIs) excluded 0. The Hosmer-Lemeshow test was used to assess goodness of fit. Statistical analyses were conducted using SAS for Windows (version 9.3, Cary, North Carolina).
RESULTS
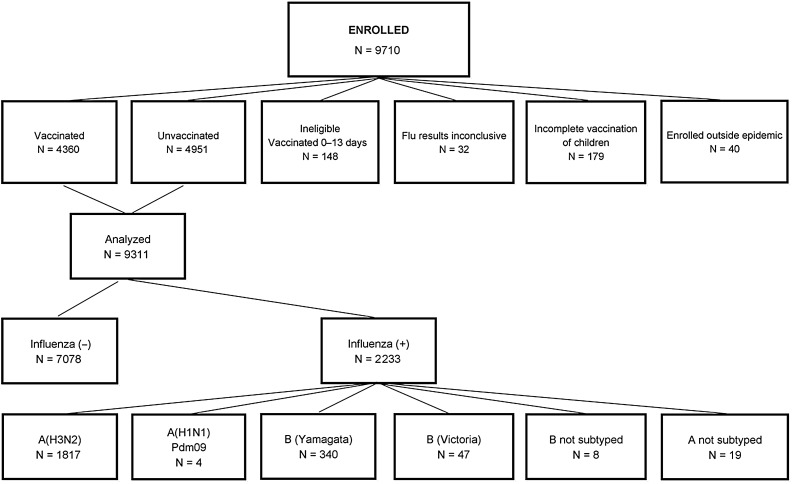
At US Flu VE Network sites, 9710 participants aged ≥6 months with ARI were enrolled from 10 November 2014 through 10 April 2015 (Figure 1). Of these, 399 were excluded from all analyses due to inconclusive or unrepeatable influenza results (n = 32), incomplete vaccination of children (n = 179), vaccination 0–13 days before onset (n = 148), and enrollment outside of the combined-site epidemic period (n = 40). Of the 9311 analyzed, 7078 (76%) were negative for influenza; of the remainder, 1840 were positive for influenza A and 395 were positive for influenza B, including 2 participants coinfected with A/H3N2 and B viruses. Nineteen influenza A samples could not be subtyped. Of the remainder, more than 99% (n = 1817) were A/H3N2 and fewer than 1% (n = 4) were A/H1N1. Eighty-eight percent (n = 340) of influenza B infections were B/Yamagata, 12% (n = 47) were B/Victoria, and 8 had no lineage identified. Antigenic characterization of B virus specimens indicated that 86% (24/28) of B/Victoria lineage viruses and 100% (35/35) of B/Yamagata lineage viruses were similar to the respective 2014–2015 vaccine strains; whereas, more than 80% of A/H3N2 viruses belonged to a drifted genetic group [7].
Figure 1.
Distribution of enrollees by eligibility and vaccination status, analysis, influenza status and influenza type. Two enrollees were positive for both A/H3N2 and B.
The epidemic curve shows a large peak of cases in December, with a smaller secondary peak in March (Supplementary Figure 1). Influenza A/H3N2 was the dominant virus, causing disease in November 2014 through February 2015, but B/Yamagata became the dominant virus in March 2015.
Detection of influenza virus infections varied by site, age, race, presence of asthma or diabetes diagnosis ICD code in year prior to enrollment, interval from ARI onset to enrollment, general health status, exposure to cigarette smoke, and current health assessment (P < .05; Table 1). The relative proportions of influenza A and B varied across sites, and those with influenza more often had diabetes, reported fewer days between ARI onset and enrollment, reported less exposure to cigarette smoke, had a lower score for current health assessment (ie, felt worse), and were less frequently vaccinated.
Table 1.
Descriptive Characteristics of Participants With Medically Attended Acute Respiratory Illness by Influenza Case Status
| Characteristic | Influenza-Negative Controls | Influenza-Positive Cases | A/H3N2 Positive Cases | Influenza B Positive Cases | P Valuea |
|---|---|---|---|---|---|
| N = 7078 | N = 2233 | N = 1817 | N = 395 | ||
| N (Column %) | N (Column %) | N (Column %) | N (Column %) | ||
| Site | <.001 | ||||
| Michigan | 1152 (16.3) | 331 (14.8) | 307 (16.9) | 18 (4.6) | |
| Pennsylvania | 1024 (14.5) | 469 (21.0) | 424 (23.3) | 42 (10.6) | |
| Texas | 1318 (18.6) | 374 (16.8) | 280 (15.4) | 89 (22.5) | |
| Washington | 2272 (32.1) | 500 (22.4) | 416 (22.9) | 82 (20.8) | |
| Wisconsin | 1312 (18.5) | 559 (25.0) | 390 (21.5) | 164 (41.5) | |
| Age, y | <.001 | ||||
| 6 mo–8 | 1946 (27.5) | 473 (21.2) | 396 (21.8) | 72 (18.2) | |
| 9–17 | 950 (13.4) | 392 (17.6) | 306 (16.8) | 80 (20.3) | |
| 18–49 | 2206 (31.2) | 642 (28.8) | 531 (29.2) | 106 (26.8) | |
| 50–64 | 1118 (15.8) | 378 (16.9) | 281 (15.5) | 94 (23.8) | |
| ≥65 | 858 (12.1) | 348 (15.6) | 303 (16.7) | 43 (10.9) | |
| Male | 2969 (42.0) | 954 (42.7) | 767 (42.2) | 179 (45.3) | .52 |
| Race/ethnicityb | .001 | ||||
| White, non-Hispanic | 5182 (73.4) | 1706 (76.6) | 1397 (77.1) | 300 (76.1) | |
| Black, non-Hispanic | 522 (7.4) | 166 (7.5) | 133 (7.3) | 29 (7.4) | |
| Hispanic | 685 (9.7) | 155 (7.0) | 119 (6.6) | 32 (8.1) | |
| Other, non-Hispanic | 673 (9.5) | 199 (8.9) | 162 (9.0) | 33 (8.4) | |
| High-risk condition in last 12 mo (International Classification of Diseases, 9th Edition, Clinical Modification) | |||||
| Any high-risk condition | 2636 (37.2) | 812 (36.4) | 671 (36.9) | 135 (34.2) | .45 |
| Asthma/pulmonary | 1525 (21.6) | 423 (18.9) | 349 (19.2) | 71 (18.0) | .008 |
| Cardiovascular | 773 (10.9) | 247 (11.1) | 204 (11.2) | 41 (10.4) | .85 |
| Diabetes | 504 (7.1) | 194 (8.7) | 164 (9.0) | 26 (6.6) | .01 |
| Morbid obesity (body mass index ≥ 40 kg/m2) | 527 (7.5) | 161 (7.2) | 125 (6.9) | 35 (8.9) | .71 |
| Other | 881 (12.5) | 291 (13.0) | 234 (12.9) | 55 (13.9) | .47 |
| Interval from onset to enrollment | <.001 | ||||
| 0–2 d | 1904 (26.9) | 964 (43.2) | 839 (46.2) | 118 (29.9) | |
| 3–4 d | 2771 (39.2) | 836 (37.4) | 638 (35.1) | 191 (48.4) | |
| 5–7 d | 2403 (34.0) | 433 (19.4) | 340 (18.7) | 86 (21.8) | |
| Reported general health statusc | .006 | ||||
| Excellent/very good | 4991 (70.6) | 1671 (73.9) | 1342 (74.2) | 291 (73.7) | |
| Good | 1639 (23.2) | 459 (20.3) | 368 (20.3) | 80 (20.3) | |
| Fair/poor | 442 (6.3) | 123 (5.4) | 99 (5.5) | 24 (6.1) | |
| Self/household exposure to smoked | 1248 (17.7) | 295 (13.2) | 229 (12.6) | 63 (16.0) | <.001 |
| Number of children aged <12 y in householde | .17 | ||||
| 0 | 4244 (60.0) | 1346 (60.5) | 1101 (60.9) | 233 (59.0) | |
| 1 | 1672 (23.6) | 489 (22.0) | 398 (22.0) | 86 (21.8) | |
| ≥2 | 1156 (16.4) | 390 (17.5) | 310 (17.1) | 76 (19.2) | |
| Reported current health assessment, median (interquartile range)f | |||||
| Scale 1 (worst)–100 (best) | 60 (45–75) | 50 (40–70) | 50 (40–70) | 50 (40–70) | <.001 |
| Vaccination status 2014–2015g | |||||
| Any inactivated vaccine | 3438 (48.6) | 962 (43.1) | 823 (45.3) | 130 (32.9) | |
| Vaccinated with IIV3 | 1397 | 405 | 335h | 66h | |
| Vaccinated with IIV4 | 1628 | 413 | 360h | 48h | |
| Vaccinated with LAIV4 | 394 (5.6) | 123 (5.5) | 108 (5.9) | 10 (2.5) | |
| Vaccinated with other/unknown type | 34 (0.5) | 13 (0.6) | 8 (0.4) | 5 (1.3) | |
| Unvaccinated | 3212 (45.4) | 1135 (50.8) | 878 (48.3) | 250 (63.3) | |
The interquartile range represents the 25th–75th percentile.
Abbreviations: IIV3/4, inactivated influenza vaccine, trivalent/quadrivalent; LAIV4, live attenuated influenza vaccine.
a The χ2 statistic was used to assess the differences between the number of persons with influenza-negative and influenza-positive test results with respect to the distributions of site, age group, sex, race/ethnicity, presence of any high-risk condition, vaccination status, interval from illness onset to enrollment, general health status, smoke exposure in self/household, and number of children aged <12 years in the household. The Wilcoxon Mann–Whitney test was used to assess differences with respect to the distribution of the current health assessment. P < .05 is statistically significant and bolded.
b Data on race/ethnicity were missing for 23 enrollees.
c Data on general health status were missing for 14 enrollees.
d Data on exposure to smoke were missing for 14 enrollees.
e Data on children aged <12 years in the household were missing for 14 enrollees.
f Data on current health assessment were missing for 17 enrollees.
g Vaccination status (14 days or more prior to onset) determined by electronic immunization record (includes electronic medical records, employee health records, and state immunization registry).
h Some influenza cases were caused by A/H1N1 influenza, were unsubtyped, or were coinfections.
Vaccine effectiveness was determined overall (A and B) by influenza A subtype and influenza B lineage and by age groups; estimates are shown in Table 2. For influenza A and B combined, the overall adjusted VE was 19% (CI, 10% to 27%) against all medically attended influenza and was statistically significant in all age groups except those aged 18–49 years. The Hosmer–Lemeshow test showed that the goodness of fit was acceptable and similar for adjusted models.
Table 2.
Percentage Vaccinated Among Influenza-Positive Cases and Test-Negative Controls and Unadjusted and Adjusted Vaccine Effectiveness Estimates by Age Group and Influenza Type/Subtype
| Influenza Type/Age Group | Influenza Positive |
Influenza Negative |
VE |
VE | |||
|---|---|---|---|---|---|---|---|
| No. Vaccinated/ Total | % | No. Vaccinated/ Total | % | Unadjusted % (95% CI) | Adjusteda % (95% CI) | Fully Adjustedb % (95% CI) | |
| Influenza A and B | |||||||
| Overall | 1098/2233 | 49.2 | 3866/7078 | 54.6 | 20 (12 to 27) | 19 (10 to 27) | 22 (13 to 30) |
| 6 mo–8 y | 186/473 | 39.3 | 1013/1946 | 52.1 | 40 (27 to 51) | 25 (6 to 40) | 26 (7 to 41) |
| 9–17 y | 137/392 | 35.0 | 391/950 | 41.2 | 23 (2 to 40) | 25 (2 to 42) | 26 (3 to 44) |
| 18–49 y | 272/642 | 42.4 | 996/2206 | 45.2 | 11 (−7 to 25) | 7 (−12 to 33) | 9 (−11 to 26) |
| 50–64 y | 229/378 | 60.6 | 739/1118 | 66.1 | 21 (0 to 38) | 20 (−3 to 38) | 25 (2 to 42) |
| ≥65 y | 274/348 | 78.7 | 727/858 | 84.7 | 33 (8 to 51) | 32 (3 to 52) | 33 (3 to 54) |
| Influenza A/H3N2 | |||||||
| Overall | 939/1817 | 51.7 | 3866/7078 | 54.6 | 11 (2 to 20) | 6 (−5 to 17) | 11 (−1 to 21) |
| 6 mo–8 y | 160/396 | 40.4 | 1013/1946 | 52.1 | 38 (22 to 50) | 20 (−3 to 37) | 23 (1 to 40) |
| 9–17 y | 119/306 | 38.9 | 391/950 | 41.2 | 9 (−18 to 30) | 7 (−26 to 31) | 7 (−26 to 32) |
| 18–49 y | 236/531 | 44.4 | 996/2206 | 45.2 | 3 (−18 to 20) | −6 (−31 to 24) | −3 (−28 to 18) |
| 50–64 y | 176/281 | 62.6 | 739/1118 | 66.1 | 14 (−13 to 34) | 12 (−19 to 34) | 18 (−13 to 40) |
| ≥65 y | 248/303 | 81.9 | 727/858 | 84.7 | 19 (−15 to 42) | 12 (−29 to 40) | 15 (−28 to 43) |
| Influenza B/Yamagata | |||||||
| Overall | 128/340 | 37.7 | 3866/7078 | 54.6 | 50 (37 to 60) | 55 (43 to 65) | 54 (41 to 64) |
| 6 mo–8 y | 18/60 | 30.0 | 1013/1946 | 52.1 | 60 (31 to 77) | 54 (17 to 74) | 50 (9 to 72) |
| 9–17 y | 9/60 | 15.0 | 391/950 | 41.2 | 75 (48 to 88) | 77 (51 to 89) | 77 (50 to 89) |
| 18–49 y | 26/90 | 28.9 | 996/2206 | 45.2 | 51 (21 to 69) | 55 (27 to 73) | 53 (22 to 71) |
| 50–64 y | 52/90 | 57.8 | 739/1118 | 66.1 | 30 (−9 to 55) | 24 (−20 to 52) | 24 (−22 to 52) |
| ≥65 y | 23/40 | 57.5 | 727/858 | 84.7 | 76 (53 to 87) | 74 (45 to 87) | 74 (43 to 88) |
Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
a Adjusted for site, age (spline for all ages, years for age groups), calendar time, high risk.
b Fully adjusted model includes site, age (spline for all ages, years for age groups), race/Hispanic ethnicity, interval from onset to enrollment, sex, general self-rated health status, calendar time (2-week intervals), any high-risk International Classification of Diseases, 9th Edition, Clinical Modification code in the year prior to enrollment.
VE against A/H3N2 was 6% (95% CI, −5% to 17%), and estimates were similar across age groups (Table 2), consistent with a mismatch between the vaccine and circulating viruses. Overall adjusted VE for influenza B/Yamagata was 55% (CI, 43% to 65%) and was similarly significant in all age strata except those aged 50–64 years. Inclusion of the small number of influenza B/Victoria cases did not change the adjusted VE for any B virus (54%; CI, 43% to 64%). No effect modification due to prior year vaccination status was observed (Table 3).
Table 3.
Unadjusted and Adjusted Vaccine Effectiveness Stratified by Combinations of Prior (2013–2014) and Current (2014–2015) Influenza Vaccination Status Among Patients Aged ≥9 Years
| Vaccination Status | Influenza-Positive Cases |
Influenza-Negative Controls |
Unadjusted | Adjusteda | ||
|---|---|---|---|---|---|---|
| No. Cases/ Row Total | (%) | No. Controls/ Row Total | (%) | VE, % (95% CI) | VE, % (95% CI) | |
| Influenza A/H3N2b | ||||||
| Vaccinated current 2014–2015 only | 175/910 | (19.2) | 735/910 | (80.8) | 15 (−4 to 30) | 8 (−14 to 26) |
| Vaccinated current 2014–2015 and prior 2013–2014 | 576/2564 | (22.5) | 1988/2564 | (77.5) | −4 (−19 to 9) | −2 (−20 to 13) |
| Vaccinated prior 2013–2014 only | 133/589 | (22.6) | 456/589 | (77.4) | −5 (−30 to 16) | 3 (−23 to 23) |
| Not vaccinated either 2013–2014 or 2014–2015 | 461/2115 | (21.8) | 1654/2115 | (78.2) | REF | REF |
| Influenza B/Yamagatac | ||||||
| Vaccinated current 2014–2015 only | 23/758 | (3.0) | 735/758 | (97.0) | 61 (39 to 75) | 59 (34 to 74) |
| Vaccinated current 2014–2015 and prior 2013–2014 | 85/2073 | (4.1) | 1988/2073 | (95.9) | 47 (30 to 60) | 56 (39 to 68) |
| Vaccinated prior 2013–2014 only | 21/477 | (4.4) | 456/477 | (95.6) | 43 (8 to 34) | 43 (7 to 65) |
| Not vaccinated either 2013–2014 or 2014–2015 | 133/1787 | (7.4) | 1654/1787 | (92.6) | REF | REF |
Abbreviations: CI, confidence interval; REF, reference; VE, vaccine effectiveness.
a Adjusted for site, age (spline), any high-risk International Classification of Diseases, 9th Edition, Clinical Modification code in the year prior to enrollment, and calendar time.
b The P value for interaction of prior (2013–2014) and current (2014–2015) season vaccination was .40.
c The P value for interaction of prior (2013–2014) and current (2014–2015) season vaccination was .07.
Distribution of Influenza Vaccine Type
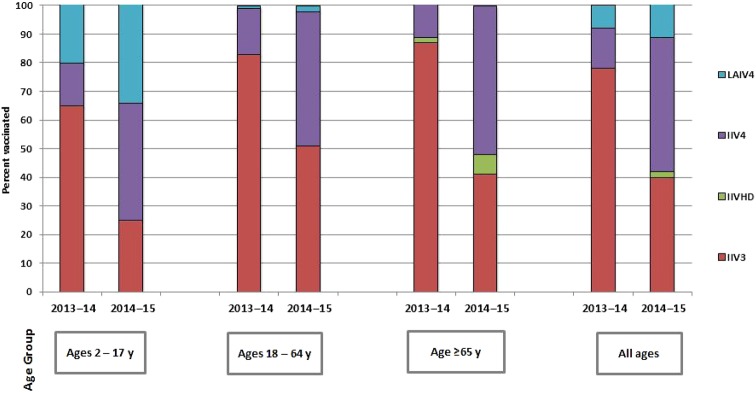
Of the 4360 vaccinated participants with known vaccine type, 1733 (39.7%) received standard-dose IIV3, 69 (1.6%) received high-dose IIV3, 2041 (46.8%) received standard-dose IIV4, and 517 (11.9%) received LAIV4 (Table 4). This distribution represents a statistically significant change from the 2013–2014 season in the US Flu VE Network in which 78% received standard-dose IIV3, 0.3% received high-dose IIV3, 14% received IIV4, and 8% received LAIV4 (P < .001) [3]. Figure 2 shows the differences in the type of influenza vaccine given across 3 age groups—2–17 years, 18–64 years, and ≥65 years—in the 2013–2014 and 2014–2015 influenza seasons. Generally, the use of IIV3 decreased, commensurate with increases in IIV4 and LAIV4.
Table 4.
Descriptive Characteristics of Participants With Medically Attended Acute Respiratory Illness by Influenza Vaccination Status
| Characteristic | All Vaccinateda | Standard-Dose IIV3b | IIV4 | High-Dose IIV3 | LAIV4 | P Valuec |
|---|---|---|---|---|---|---|
| All, no. (%)d | 4360 | 1733 (39.7) | 2041 (46.8) | 69 (1.6) | 517 (11.9) | |
| Site | <.001 | |||||
| Michigan | 720 | 52 (7.2) | 550 (76.4) | 44 (6.1) | 74 (10.3) | |
| Pennsylvania | 540 | 136 (25.2) | 322 (59.6) | 8 (1.5) | 74 (13.7) | |
| Texas | 603 | 80 (13.3) | 446 (74.0) | 14 (2.3) | 63 (10.4) | |
| Washington | 1568 | 697 (44.5) | 682 (43.5) | 0 (0) | 189 (12.1) | |
| Wisconsin | 929 | 769 (82.7) | 41 (4.4) | 3 (0.3) | 117 (12.6) | |
| Age | <.001 | |||||
| 6 mo–8 y | 1191 | 340 (28.5) | 545 (45.8) | 0 (0) | 306 (25.7) | |
| 6 mo–23 mo | 309 | 128 (41.3) | 181 (58.4) | 0 (0) | 0 (0) | |
| 2–8 y | 882 | 212 (24.0) | 364 (41.2) | 0 (0) | 306 (34.7) | |
| 9–17 y | 506 | 130 (25.7) | 203 (40.1) | 0 (0) | 173 (34.2) | |
| 18–49 y | 956 | 469 (49.1) | 449 (47.0) | 1 (0.1) | 37 (3.9) | |
| 50–64 y | 791 | 418 (52.8) | 372 (47.0) | 1 (0.1) | 0 (0) | |
| ≥65 y | 916 | 376 (41.0) | 472 (51.5) | 67 (7.3) | 1 (0.1) | |
| Male | 1792 | 675 (37.7) | 840 (46.9) | 25 (1.4) | 252 (14.1) | <.001 |
| Race/ethnicitye | <.001 | |||||
| White, non-Hispanic | 3354 | 1451 (43.3) | 1472 (43.9) | 57 (1.7) | 374 (11.2) | |
| Black, non-Hispanic | 231 | 57 (24.7) | 150 (64.9) | 4 (1.7) | 20 (8.7) | |
| Hispanic | 314 | 57 (18.2) | 192 (61.1) | 6 (1.9) | 59 (18.8) | |
| Other, non-Hispanic | 426 | 135 (31.7) | 226 (53.1) | 2 (0.5) | 63 (14.8) | |
| High-risk condition | <.001 | |||||
| Any high-risk condition | 2029 | 838 (41.3) | 1070 (52.7) | 50 (2.5) | 71 (3.5) | <.001 |
| Asthma/pulmonary | 1088 | 439 (40.3) | 582 (53.5) | 14 (1.3) | 53 (4.9) | <.001 |
| Cardiovascular | 712 | 303 (42.6) | 374 (52.5) | 26 (3.7) | 9 (1.3) | <.001 |
| Diabetes | 480 | 203 (42.3) | 253 (52.7) | 22 (4.6) | 2 (0.4) | <.001 |
| Morbid obesityf | 353 | 165 (46.7) | 180 (51.0) | 3 (0.8) | 5 (1.4) | <.001 |
| Other | 824 | 375 (45.5) | 407 (49.4) | 26 (3.2) | 16 (1.9) | <.001 |
| Interval from onset to enrollment | .001 | |||||
| 0–2 d | 1294 | 486 (37.6) | 605 (46.8) | 18 (1.4) | 185 (14.3) | |
| 3–4 d | 1646 | 631 (38.3) | 790 (48.0) | 28 (1.7) | 197 (12.0) | |
| 5–7 d | 1420 | 616 (43.4) | 646 (45.5) | 23 (1.6) | 135 (9.5) | |
| Reported general health statusg | <.001 | |||||
| Excellent/very good | 3008 | 1121 (37.3) | 1390 (46.2) | 35 (1.2) | 462 (15.4) | |
| Good | 1026 | 466 (45.4) | 491 (47.9) | 24 (2.3) | 45 (4.4) | |
| Fair/poor | 320 | 145 (45.3) | 156 (48.8) | 10 (3.1) | 9 (2.8) | |
| Self/household exposure to smokeh | 519 | 242 (46.6) | 207 (39.9) | 2 (0.4) | 68 (13.1) | <.001 |
| Number of children aged <12 y in householdi | <.001 | |||||
| 0 | 2766 | 1199 (43.3) | 1320 (47.7) | 66 (2.4) | 181 (6.5) | |
| 1 | 965 | 311 (32.2) | 444 (46.0) | 3 (0.3) | 207 (21.5) | |
| ≥2 | 628 | 222 (35.4) | 277 (44.1) | 0 (0) | 129 (20.5) | |
| Reported current health assessment, median (interquartile range)j | <.001 | |||||
| Scale 1 (worst) – 100 (best) | 60 (45–75) | 55 (40–70) | 60 (45–75) | 60 (45–70) | 65 (50–75) | |
| Reverse transcription polymerase chain reaction–confirmed Influenza status | .19 | |||||
| Positive | 941 | 388 (41.2) | 413 (43.9) | 17 (1.8) | 123 (13.1) | |
| Negative | 3419 | 1345 (39.3) | 1628 (47.6) | 52 (1.5) | 394 (11.5) |
The interquartile range represents the 25th–75th percentile.
Abbreviations: IIV, inactivated influenza vaccine; IIV3, trivalent inactivated influenza vaccine; IIV4, quadrivalent inactivated influenza vaccine; LAIV, live attenuated influenza vaccine.
a Defined as having ≥1 dose of any influenza vaccine ≥14 days before illness onset. Excludes 604 participants who received a vaccine of undetermined type.
b Among those who received standard-dose IIV3, 9 were cell culture IIV3 and 1 was intradermal.
c The χ2 test statistic was used to assess differences by vaccine type received, with respect to the distributions of site, age group, sex, race/ethnicity, presence of high-risk conditions, interval from illness onset to enrollment, general health status, self/household smoke exposure, number of children <12 in the household, and reverse transcription polymerase chain reaction–confirmed influenza positivity. The Kruskal–Wallis test was used to assess differences with respect to the distribution of the current health assessment. P < .05 is statistically significant and bolded.
d Data are presented as no. (row %).
e Data on race/ethnicity were missing for 5 participants.
f Morbid obesity was defined as a body mass index ≥40 kg/m2 at enrollment or at least 1 International Classification of Diseases, 9th Edition, Clinical Modification code for morbid obesity in the year prior to enrollment.
g Data on general health status were missing for 6 participants.
h Data on exposure to smoke were missing for 5 participants.
i Data on children aged <12 years in the household were missing for 1 participant.
j Data on current health assessment were missing for 6 participants.
Figure 2.
Distribution of vaccine type among enrollees in 2013–2014 and 2014–2015. Abbreviations: HD, high does; IIV, inactivated influenza vaccine; IIV3, trivalent inactivated influenza vaccine; IIV4, quadrivalent inactivated influenza vaccine; LAIV, live attenuated influenza vaccine.
Some differences in distribution of vaccine types across demographic groups were to be expected, given their recommendations for specific age groups. Vaccine type varied significantly across age groups, with the number of children in the household, with the presence of high-risk conditions, with the level of self-rated health, and by sex, race/ethnicity, and exposure to cigarette smoke (Table 4). Although there was no significant difference in the number of influenza cases across the vaccine types, some differences in illness measures were observed, such as self-rated health, days between ARI onset and enrollment, and current health assessment. Self-reported health at enrollment varied by vaccine type, with the lowest ratings among standard-dose IIV3 recipients.
Influenza Vaccine Effectiveness by Vaccine Type
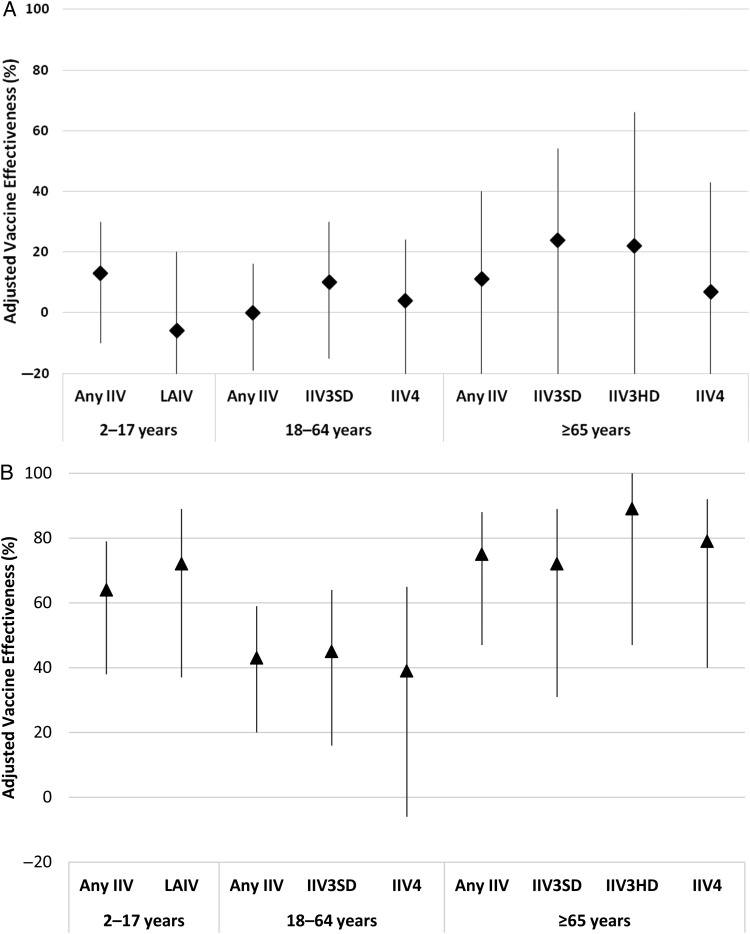
VE estimates by vaccine type were conducted using 1817 A/H3N2-positive, 340 B/Yamagata-positive, and 7078 influenza test–negative participants and were specific to the age groups for which the various vaccine types are recommended. Adjusted VE against A/H3N2 did not vary significantly by vaccine type, with all CIs including zero (Figure 3A). For B/Yamagata, which is contained in both trivalent and quadrivalent vaccines, point estimates for adjusted VE within each age group were similar and CIs overlapped across vaccine types (Figure 3B). However, the VE against B/Yamagata was not statistically significant for IIV4 in those aged 18–64 years. Of note, no cases of B/Yamagata occurred among high-dose IIV3 recipients (VE = 89%; CI, 47% to 100% by exact logistic regression). Overall adjusted VE for influenza B remained significant at 54% (CI, 43% to 64%), with no differences between VE for IIV3 and IIV4 in both 2–17 and 18–64 year-old age groups (data not shown).
Figure 3.
A, Adjusted vaccine effectiveness against influenza A/H3N2 by age group and vaccine type. Confidence intervals (CIs) for those aged 2–17 years: any inactivated influenza vaccine (IIV) (−9, 30); live attenuated influenza vaccine (LAIV) (−40, 20); for those aged 18–64 years: any IIV (−19, 16); standard-dose trivalent IIV (IIV3SD) (−15, 30); quadrivalent IIV (IIV4) (−23, 24); for those aged ≥65 years: any IIV (−30, 40); standard-dose trivalent IIV (IIV3SD) (−25, 54); high-dose trivalent IIV (IIV3HD) (−82, 66); IIV4 (−52, 43). B, Adjusted vaccine effectiveness against influenza B/Yamagata by age group and vaccine type. There were no cases of high-dose trivalent IIV. CIs for those aged 2–17 years: any IIV (41, 80); LAIV (43, 90); for those aged 18–64 years: any IIV (59, 20); IIV3SD (16, 64); IIV4 (−6, 65); for those aged ≥65 years: any IIV (47, 88); IIV3SD (31, 89); IIV3HD (47, 100); IIV4 (40, 92).
VE against A/H3N2 was 13% (CI, −9% to 30%) for any IIV and −5% (CI, −40% to 21%) for LAIV among those aged 2–17 years. VE against B/Yamagata was 66% (CI, 41% to 80%) for any IIV and 76% (CI, 43% to 90%) for LAIV in the same age group. Among those aged 2–8 years, VE against A/H3N2 was 15% (CI, −16% to 38%) for IIV and −3% (CI, −50% to 29%) for LAIV; VE against B/Yamagata was 40% (CI, −20% to 70%) for IIV and 74% (CI, 25% to 91%) for LAIV.
Sensitivity analyses were conducted to test the effect of different vaccination-reporting methods on VE. Adjusted VE against A/H3N2 was 3% (CI, −11% to 16%) for self-reported vaccination only (Supplementary Table 1) vs 12% (CI, 1% to 22%) for EIR-documented vaccination only, vs 10% (CI, −2% to 20%) for plausible self-reported vaccination excluded. Against B/Yamagata, the adjusted VE was 46% (CI, 25% to 62%) for self-reported vaccination only vs 53% (CI, 39% to 63%) for EIR-documented vaccination only, vs 55% (CI, 42% to 65%) for plausible self-reported vaccination excluded.
DISCUSSION
For the past 4 years, the US Flu VE Network has provided effectiveness estimates for seasonal influenza vaccine [3, 4, 8, 9, 11, 14, 15] for the purposes of monitoring and guiding influenza vaccine policy. These 4 influenza seasons have differed from each other in terms of timing and severity of the epidemic, from the relatively mild 2011–2012 season, with peak incidence in March, to moderately severe seasons from 2012–2013 through 2014–2015, with peak incidence in late December to early January [6]. The predominant influenza A virus and VE have also varied by season, from 39% against A/H3N2 during 2011–2012 [8, 9] to 51% against A/H1N1 in 2013–2014 [3]. VE estimates for influenza B viruses have simultaneously varied from 51% to 66% [8, 9]. In 2014–2015, the vaccine was ineffective at 6% VE against the predominant A/H3N2 strain due to significant drift [7]. Later in March, B/Yamagata became dominant and the VE was significant at 55% (CI, 43% to 65%). These results highlight the importance of vaccinating throughout the influenza season because the vaccine may protect against a second wave of influenza.
The changing landscape of available influenza vaccines also suggests the need for VE estimates by vaccine type. Across US Flu VE Network sites, small increases in LAIV and high-dose IIV3 use were reported in addition to a large increase in IIV4, with a correspondingly large decease in IIV3. In 2014–2015, the B/Yamagata lineage predominated over B/Victoria, and B/Yamagata was included in both the trivalent and quadrivalent vaccine types. Consequently, overall IIV4 VE was similar to that of IIV3, consistent with previous US Flu VE Network findings [3, 8, 9]. Given the uncertainty of predicting which B lineage will circulate in any given year, a quadrivalent vaccine obviates the possibility of a mismatch between vaccine and circulating B lineages.
Although previous studies [1, 16] have suggested that LAIV has greater VE than IIV in young children, several recent studies have not supported those findings. LAIV had low VE in 2013–2014 [3, 17, 18], possibly due to the heat instability of the A/H1N1 [19, 20] construct, which was changed for the 2015–2016 vaccine. A review of LAIV VE over several past years of the US Flu VE Network also failed to find superiority of LAIV [21]. While LAIV has been reported to have higher VE than IIV during drift seasons [22], LAIV and IIV VE did not differ statistically among children aged 2–8 years in the 2014–2015 season. Thus, the cumulative US Flu VE Network data did not suggest superior effectiveness of LAIV over IIV in young children following the clinical trials. With the current ACIP recommendation not to use LAIV during the 2016–2017 season (from the opposite of an LAIV preference in 2014–2015) [2, 11], there may be little opportunity to evaluate LAIV effectiveness in the coming season in the United States. However, offering choice of vaccine type (eg, either LAIV or IIV) has been shown to increase vaccine uptake [23], resulting in protection for a larger number of people.
Strengths and Limitations
The strengths of this study include the fact that the US Flu VE Network is a multistate effort that offers a substantial sample size. Furthermore, the study used the test-negative design with an established, highly specific RT-PCR diagnostic test. While it can be difficult to confirm adult influenza vaccinations that are administered at occupational health programs and pharmacies, we used a previously published method to determine plausible self-report of vaccination [3]. Self-reported vaccination status by adults has been shown to be reliable [24], and sensitivity analyses with only EIR-documented vaccinations did not materially change VE estimates, confirming the validity of this method. Residual unmeasured confounding cannot be ruled out in an observational study, even with the use of sophisticated regression analyses. Finally, small numbers were enrolled with some new influenza vaccine types (eg, high-dose IIV3).
CONCLUSIONS
Although the mismatch between the circulating A/H3N2 influenza strains and the 2014–2015 influenza vaccine strains resulted in low VE against the A strains, VE was high against influenza B. In this year with mostly a B/Yamagata lineage, quadrivalent vaccine was not shown to be more effective than trivalent vaccine and LAIV was not more effective than IIV among young children.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. University of Pittsburgh: G.K. Balasubramani, PhD; Donald S. Burke, MD; Rina Chabra, MD; Edward Garafolo, MD; Philip Iozzi, MD; Barbara Kevish, MD; Donald B. Middleton, MD; Christopher Olbrich, MD; Evelyn C. Reis, MD; Sandra Sauereisen, MD; Leonard Urbanski, MD; and Stephen Wisniewski, PhD.
Marshfield Clinic Research Foundation: Hannatu Amaza, Braiden Andersen, Elizabeth Armagost, Yvonne Cerne, Deanna Cole, Kathleen Cushman, Shelia Drowatzky, Keith Gilge, William Gillaspie, Gregg Greenwald, Krista Herkert, Deborah Hilgemann, Tara Johnson, Bryan Joosse, Sarah Kopitzke, Alex Krenzke, Kelly Mathews, Madalyn Minervini, Vicki Moon, Suellyn Murray, Rebecca Pilsner, Deeann Polacek, Zoe Retzlaff, Carla Rottscheit, Jacklyn Salzwedel, Kirsten Schultz, Teresa Schultz, Adam Smith, Sandra Strey, Laurel Verhagen, Kristja Vittallo, Jane Wesely, Kelly Wirkus, Patrick Stockwell, Jennifer Anderson, Brooke Olson, and Klevi Hoxha.
Baylor Scott and White: Madhava Beeram, MD; Michael Reis, MD; Alejandro Arroliga, MD; Donald Wesson, MD; Archana Nagrani, MBBS; Patricia Sleeth, Virginia Gandy, Teresa Ponder, Mary Kylberg, Hope Gonzales, Martha Zayed, Deborah Furze, Jeremy Ray, Jessica Rotoscykj, and Glen Cryer.
US Flu VE Investigators. University of Pittsburgh: Arlene Bullota, Heather Eng, Samantha Ford, Jennifer Gray, Krissy K. Moehling, Jonathan M. Raviotta, Edmund M. Ricci, Chuck Rinaldo, Sean Saul, Terri Sax, and Michael Susick.
Group Health Research Institute: Erika Kiniry, Joyce Benoit, and C. Hallie Phillips.
University of Michigan: Suzanne E. Ohmit, Emily T. Martin, Ryan E. Malosh, E.J. McSpadden, Caroline K. Cheng, Rachel Truscon, Emileigh Johnson, Lois E. Lamerato, and Heather R. Lipkovich.
Marshfield Clinic Research Foundation: Jennifer P. King, Jennifer K. Meece, Lynn Ivacic, and Phil Bertz.
Baylor Scott and White: Jessica Pruszyunski, Lydia Clipper, Anne Robertson, Pedro A. Piedra, and Vasanthi Avadhanula.
Centers for Disease Control and Prevention: Sarah Spencer, LaShondra Berman, Angie Foust, Wendy Sessions, and Swathi N. Thaker.
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC) or the National Institutes of Health (NIH).
Financial support. This work was supported by the CDC through cooperative agreements with the University of Michigan (U01 IP000474), Group Health Research Institute (U01 IP000466), Marshfield Clinic Research Foundation (U01 IP000471), University of Pittsburgh (U01 IP000467), and Baylor Scott and White Health (U01 IP000473) and by the NIH (grants UL1 RR024153 and UL1TR000005 to the University of Pittsburgh).
Potential conflicts of interest. R. K. Z. has received research funding from Sanofi Pasteur, Inc., Pfizer, Inc., and Merck & Co., Inc. M. P. N. has received research funding from Pfizer, Inc. and Merck & Co., Inc. E. A. B. and H. Q. M. have received research funding from MedImmune, LLC. M. G. has an institutional research contract with MedImmune/AstraZeneca. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the U.S. Flu VE Investigators, G.K. Balasubramani, Donald S. Burke, Rina Chabra, Edward Garafolo, Philip Iozzi, Barbara Kevish, Donald B. Middleton, Christopher Olbrich, Evelyn C. Reis, Sandra Sauereisen, Leonard Urbanski, Stephen Wisniewski, Hannatu Amaza, Braiden Andersen, Elizabeth Armagost, Yvonne Cerne, Deanna Cole, Kathleen Cushman, Shelia Drowatzky, Keith Gilge, William Gillaspie, Gregg Greenwald, Krista Herkert, Deborah Hilgemann, Tara Johnson, Bryan Joosse, Sarah Kopitzke, Alex Krenzke, Kelly Mathews, Madalyn Minervini, Vicki Moon, Suellyn Murray, Rebecca Pilsner, Deeann Polacek, Zoe Retzlaff, Carla Rottscheit, Jacklyn Salzwedel, Kirsten Schultz, Teresa Schultz, Adam Smith, Sandra Strey, Laurel Verhagen, Kristja Vittallo, Jane Wesely, Kelly Wirkus, Patrick Stockwell, Jennifer Anderson, Brooke Olson, Klevi Hoxha, Madhava Beeram, Michael Reis, Alejandro Arroliga, Donald Wesson, Jessica Pruszynski, Anne Robertson, Archana Nagrani, Patricia Sleeth, Virginia Gandy, Teresa Ponder, Mary Kylberg, Hope Gonzales, Martha Zayed, Deborah Furze, Jeremy Ray, Jessica Rotoscykj, Glen Cryer, Arlene Bullota, Heather Eng, Samantha Ford, Jennifer Gray, Krissy K. Moehling, Jonathan M. Raviotta, Edmund M. Ricci, Chuck Rinaldo, Sean Saul, Terri Sax, Michael Susick, Erika Kiniry, Joyce Benoit, C. Hallie Phillips, Suzanne E. Ohmit, Emily T. Martin, Ryan E. Malosh, E.J. McSpadden, Caroline K. Cheng, Rachel Truscon, Emileigh Johnson, Lois E. Lamerato, Heather R. Lipkovich, Jennifer P. King, Jennifer K. Meece, Lynn Ivacic, Phil Bertz, Jessica Pruszyunski, Lydia Clipper, Anne Robertson, Pedro A. Piedra, Vasanthi Avadhanula, Sarah Spencer, LaShondra Berman, Angie Foust, Wendy Sessions, and Swathi N. Thaker
References
- 1.Centers for Disease Control and Prevention. Characteristics associated with seasonal influenza vaccination of preschool children—Oregon, 2006–2008. Morb Mortal Wkly Rep (MMWR) 2011; 60:981–4. [PubMed] [Google Scholar]
- 2.Grohskopf LA, Olsen SJ, Sokolow LZ et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014–15 influenza season. Morb Mortal Wkly Rep (MMWR) 2014; 63:691–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Gaglani M, Pruszynski J, Murthy K et al. Influenza vaccine effectiveness against 2009 pandemic influenza A(H1N1) virus differed by vaccine type during 2013–2014 in the United States. J Infect Dis 2016; 213:1546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flannery B, Clippard J, Zimmerman RK et al. Early estimates of seasonal influenza vaccine effectiveness—United States, January 2015. Morb Mortal Wkly Rep (MMWR) 2015; 64:10–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Skowronski DM, Chambers C, Sabaiduc S et al. A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–15 season. Clin Infect Dis 2016; 63:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. National and Regional Level Outpatient Illness and Viral Surveillance. Available at: http://gis.cdc.gov/grasp/fluview/fluportaldashboard.html Accessed 17 February 2016.
- 7.Flannery B, Zimmerman RK, Gubareva LV et al. Enhanced genetic characterization of influenza A(H3N2) viruses and vaccine effectiveness by genetic group, 2014–2015. J Infect Dis 2016; 214:1010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohmit SE, Thompson MG, Petrie JG et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLean HQ, Thompson MG, Sundaram ME et al. Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type. J Infect Dis 2015; 211:1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Classification of Diseases, Clinical Modification. Available at: http://www.cdc.gov/nchs/icd/icd9cm.htm Accessed 17 February 2016.
- 11.Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 influenza season. Morb Mortal Wkly Rep (MMWR) 2015; 64:818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foppa IM, Haber M, Ferdinands JM, Shay DK. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine 2013; 31:3104–9. [DOI] [PubMed] [Google Scholar]
- 13.Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 31:2165–8. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. Morb Mortal Wkly Rep (MMWR) 2013; 62:119–23. [PMC free article] [PubMed] [Google Scholar]
- 15.Flannery B, Thaker SN, Clippard J et al. Interim estimates of 2013–14 seasonal influenza vaccine effectiveness—United States, February 2014. Morb Mortal Wkly Rep (MMWR) 2014; 63:137–42. [PMC free article] [PubMed] [Google Scholar]
- 16.McElligott JT, Roberts JR, O'Brien ES et al. Improving immunization rates at 18 months of age: implications for individual practices. Public Health Rep 2011; 126(suppl 2):33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cost AA, Hiser MJ, Hu Z et al. Brief report: mid-season influenza vaccine effectiveness estimates for the 2013–2014 influenza season. MSMR 2014; 21:15–7. [PubMed] [Google Scholar]
- 18.Caspard H, Gaglani M, Clipper L et al. Effectiveness of live attenuated influenza vaccine and inactivated influenza vaccine in children 2–17 years of age in 2013–2014 in the United States. Vaccine 2016; 34:77–82. [DOI] [PubMed] [Google Scholar]
- 19.Cotter CR, Jin H, Chen Z. A single amino acid in the stalk region of the H1N1pdm influenza virus HA protein affects viral fusion, stability and infectivity. PLoS Pathog 2014; 10:e1003831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Donnell CD, Vogel L, Matsuoka Y, Jin H, Subbarao K. The matrix gene segment destabilizes the acid and thermal stability of the hemagglutinin of pandemic live attenuated influenza virus vaccines. J Virol 2014; 88:12374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung JR, Flannery B, Thompson MG et al. Seasonal effectiveness of live attenuated and inactivated influenza vaccine. Pediatrics 2016; 137:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ambrose CS, Wu XH, Knuf M, Wutzler P. The efficacy of intranasal live attenuated influenza vaccine in children 2 through 17 years of age: a meta-analysis of 8 randomized controlled studies. Vaccine 2012; 30:886–92. [DOI] [PubMed] [Google Scholar]
- 23.Nowalk MP, Lin CJ, Toback SL et al. Improving influenza vaccination rates in the workplace: a randomized trial. Am J Prev Med 2010; 38:237–46. [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman RK, Raymund M, Janosky JE, Nowalk MP, Fine MJ. Sensitivity and specificity of patient self-report of influenza and pneumococcal polysaccharide vaccinations among elderly outpatients in diverse patient care strata. Vaccine 2003; 21:1486–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.