Abstract
Cytomegalovirus (CMV) infection is a significant complication in hematopoietic cell transplantation (HCT) recipients. Four antiviral drugs are used for preventing or treating CMV: ganciclovir, valganciclovir, foscarnet, and cidofovir. With prolonged and repeated use of these drugs, CMV can become resistant to standard therapy, resulting in increased morbidity and mortality, especially in HCT recipients. Antiviral drug resistance should be suspected when CMV viremia (DNAemia or antigenemia) fails to improve or continue to increase after 2 weeks of appropriately dosed and delivered antiviral therapy. CMV resistance is diagnosed by detecting specific genetic mutations. UL97 mutations confer resistance to ganciclovir and valganciclovir, and a UL54 mutation confers multidrug resistance. Risk factors for resistance include prolonged or previous anti-CMV drug exposure or inadequate dosing, absorption, or bioavailability. Host risk factors include type of HCT and degree of immunosuppression. Depending on the genotyping results, multiple strategies can be adopted to treat resistant CMV infections, albeit no randomized clinical trials exist so far, after reducing immunosuppression (if possible): ganciclovir dose escalation, ganciclovir and foscarnet combination, and adjunct therapy such as CMV-specific cytotoxic T-lymphocyte infusions. Novel therapies such as maribavir, brincidofovir, and letermovir should be further studied for treatment of resistant CMV.
Introduction
Cytomegalovirus (CMV) infection is ubiquitous and largely asymptomatic in individuals with a competent immune system.1,2 However, it is a clinically significant complication in cancer patients with impaired cellular immunity, particularly hematopoietic stem cell transplantation (HCT) recipients.3-5 Despite the decrease in the prevalence of early CMV disease to 3% to 6% with widespread antiviral use, an optimal prevention strategy has not been identified. Early CMV reactivation remains associated with lower overall survival and higher nonrelapse mortality.6 In an earlier study, the risk of late CMV disease was as high as 18%, with a mortality rate of 46%.7 However, with polymerase chain reaction (PCR)–guided preemptive therapy or prophylaxis with oral valganciclovir, the incidence of late CMV disease and all-cause mortality after HCT could be as low as 5% and 17%, respectively.8 In addition to directly causing end-organ disease, CMV infection may be associated with “indirect effects” manifesting as graft failure,9 graft-versus-host disease (GVHD),10 accelerated atherosclerosis,11 and secondary bacterial and fungal infections.12
Two strategies are adopted for prevention of CMV infection and/or disease in HCT recipients: universal prophylaxis and the preferred preemptive therapy.13-15 Four effective antiviral drugs are used for the prevention or treatment of CMV infection: ganciclovir, the ganciclovir prodrug valganciclovir, foscarnet, and cidofovir.3,13,16 Despite reducing the risk of CMV infection after HCT,8,17-24 universal prophylaxis remains unpopular owing to the increased risk of treatment-related toxic effects (mainly myelosuppression with subsequent bacterial and fungal infections).17,20,25 Prophylactic use of foscarnet remains dubious; uncontrolled studies have reported breakthrough CMV infections, renal toxicities, and electrolyte imbalances.26-28
Before ganciclovir was approved for use as anti-CMV therapy, ganciclovir-resistant CMV strains were generated in the laboratory29 and subsequently detected in clinical settings, leading to treatment failures.30 During prolonged and repeated use of anti-CMV therapies, as doses are adjusted to prevent or reverse subsequent toxic effects, CMV can develop resistance to standard therapy. With the widespread use of anti-CMV drugs and the availability and access to genotypic antiviral resistance testing in HCT recipients, CMV resistance to one or more standard antivirals has been increasingly recognized, along with cross-resistance that may be conferred by various mutations. Although CMV resistance remains uncommon in HCT recipients from matched related or unrelated donors in particular (range, 0% to 7.9%),8,31-33 in high-risk patients, the incidence of CMV resistance has been reported to be as high as 14.5%.34 Nevertheless, to date, there are no systematic evaluations of different outcomes including morality when comparing CMV infections with wild-type vs resistant strains.
How I treat
To illustrate how we approach resistant or refractory CMV infection in HCT recipients in our practice, we present 2 cases. It is important to note that none of the recommendations are based on randomized controlled clinical trials, and the proposed algorithms are, in some important aspects, based on our expert opinion (the way we approach such clinical scenarios at the University of Texas MD Anderson Cancer Center) and the opinions of others in the field. The algorithms are intended as general guidance for management of this specific type of an infection, and data from randomized clinical trials are crucial.
Case 1
A 55-year-old, CMV-seropositive (R+) man with acute myelogenous leukemia underwent matched unrelated HCT from a CMV-seronegative (D−) donor who was a complete HLA match. The patient had received conditioning with busulfan, fludarabine, and antithymocyte gammaglobulin (ATG). Standard prophylactic therapy was started, and he engrafted at day 12 after HCT (D+12). On D+33, serum CMV PCR analysis was positive for 570 IU/mL, without evidence of CMV disease, and the patient was started on valganciclovir induction therapy at a dose of 450 mg twice daily, adjusted for creatinine clearance (CrCl) of 59 mL/min. Five days into his antiviral therapy, serum CMV increased to 1276 IU/mL but then decreased to <137 IU/mL at day 12 of therapy. The patient subsequently developed nausea and vomiting, and upper endoscopy showed acute gastrointestinal GVHD, for which he received high-dose corticosteroids. The patient was switched to intravenous ganciclovir at a dose of 2.5 mg/kg every 12 hours. After 3 weeks of induction therapy, the ganciclovir was decreased to a maintenance dose of 1.25 mg/kg daily and then switched to valganciclovir. The patient then developed recurrent gastrointestinal GVHD, for which he received further corticosteroids, and at D+78, his serum CMV had increased to 10 320 IU/mL while receiving valganciclovir for secondary prophylaxis. The ganciclovir induction was restarted at a dose of 2.5 mg/kg every 12 hours, maintaining CrCl at 48 mL/min. Repeat upper and lower endoscopy showed CMV disease with CMV enterocolitis, which was confirmed by histopathologic analysis. After 2 weeks of treatment with ganciclovir, the patient’s serum CMV decreased to <137 IU/mL and, while on ganciclovir, peaked again, at D+98, at 22 829 IU/mL; absolute lymphocyte count was 470 cells/mm3. At that point, empiric treatment with foscarnet was started, and CMV genotypic analysis showed only the C592G UL97 mutation. Within 2 weeks of starting treatment with foscarnet, the patient’s serum CMV was <137 IU/mL, and he was asymptomatic.
Case 2
A 27-year-old, CMV R+ woman with T-cell acute lymphoblastic leukemia underwent double cord blood HCT following conditioning with busulfan, clofarabine, ATG, and total body irradiation. Standard prophylactic therapy was started and she engrafted on D+18. On D+30, her course was complicated by human herpesviurs-6 reactivation. She was treated for 2 weeks with foscarnet. While receiving this therapy, she developed acute kidney injury and severe electrolytes imbalance, which resolved after completion of therapy. On D+60, PCR analysis showed that serum CMV was detectable at 255 IU/mL, for which she again started treatment with foscarnet at a dose of 90 mg/kg every 12 hours for 21 days. Initially, serum CMV increased to 2,370 IU/mL but then decreased to <137 IU/mL after 11 days of antiviral therapy. While receiving foscarnet, she had recurrent acute kidney injury requiring dose adjustment for decreased CrCl. Following induction therapy, the patient completed 2 weeks of maintenance therapy with foscarnet and then received valganciclovir as secondary prophylaxis thereafter. At D+112, she developed recurrent CMV reactivation with serum CMV of 377 IU/mL. She again started treatment with foscarnet; however, within 7 days of antiviral treatment, serum CMV peaked at 38 440 IU/mL. Ganciclovir at a dose of 5 mg/kg every 12 hours was added, and genotypic analysis showed evidence of a V715M UL54 mutation; foscarnet was discontinued. After 21 days of treatment with ganciclovir, serum CMV decreased to <137 IU/mL. At D+169, she developed nausea and vomiting, and upper endoscopy revealed gastrointestinal GVHD, for which she received high-dose corticosteroids. CMV reactivated again at 475 IU/mL, and treatment with ganciclovir was restarted; however, after 2 weeks of antiviral therapy at optimal dosing, serum CMV remained high at 12 552 IU/mL, without evidence of CMV end-organ disease. Absolute lymphocyte count was 170 cells/mm3. Genotypic analysis showed V715M UL54 and a new A594V UL97 mutation. Both foscarnet and ganciclovir were stopped, and the patient received cidofovir, leflunomide, and 2 CMV-specific CTL infusions. Two weeks into the new regimen, serum CMV decreased to 907 IU/mL. Two additional CMV-specific CTL infusions and a total of 4 weeks of intravenous cidofovir at a dose of 5 mg/kg once per week were given, while the patient maintained treatment with leflunomide at serum levels ≥50 μg/mL. At D+253, serum CMV was <137 IU/mL, and she continued leflunomide monotherapy with intermittent CMV “blips” ranging from 137 to 250 IU/mL without overt CMV disease.
Diagnosis of resistant or refractory CMV infection
Definitions
Antiviral drug resistance can manifest as true virological resistance secondary to one or a combination of clinically significant mutations or as “clinical resistance” in the absence of these mutations. The distinction between these two entities is important, as clinical resistance is mainly secondary to host or viral factors rather than genotypic mutations, and altering antiviral therapy without addressing the host factors could be detrimental to the patients in many instances.
Antiviral drug resistance should be suspected when CMV viremia (DNAemia or antigenemia) fails to improve (ie, >1 log10 increase in CMV DNA levels in blood or serum) after 2 weeks of appropriately dosed and delivered antiviral therapy. Antiviral drug resistance should also be suspected when CMV end-organ disease occurs during prolonged antiviral therapy (>6 weeks of antiviral drug exposure, including 2 weeks of full-dose therapy) in the presence of risk factors. In drug-naive patients, owing to underlying immunosuppression and corticosteroid use,35-37 a modest quantitative increase in viral loads (or antigenemia) during the first 2 weeks of anti-CMV therapy may occur; this is not an indication of drug resistance and may not necessitate a change of therapy.36 In adult drug-naive patients, it is unusual for resistance to develop during the first 6 weeks of therapy,38,39 despite some reports in pediatric HCT recipients40,41 or patients with primary congenital immunodeficiency who underwent T-cell–depleted HCT,42,43perhaps related to high CMV viral loads. Furthermore, in some instances, certain CMV diseases are not always associated with measurable viral loads in blood, and a low threshold for suspicion is warranted when a lack of improvement is observed or progression occurs, because these findings could be the only indications of drug resistance. Refractory CMV infection is defined similarly to antiviral-drug–resistant CMV infection, except that known genetic mutations conferring resistance are not detected (clinical resistance).
Laboratory assays
There are 2 types of assays to diagnose antiviral drug resistance: the classic phenotypic plaque reduction assay (PRA) and the more common and practical genotypic analysis. The PRA was the first such assay to be developed and was considered the “gold standard.”44,45 Despite efforts for standardization,44 interassay and interlaboratory variability remain a problem. In addition, PRA is time-consuming (it takes up to 4 to 6 weeks to complete) and labor-intensive. Nowadays, phenotypic methods are rarely done, because they are not practical for clinical use. Recombinant mutation phenotypic methods are now used only to confirm new genotypic drug resistance. The latter can also be confirmed using marker transfer of the mutated gene in a wild-type virus.
Genotypic assays have the advantages of rapid diagnosis of drug resistance (turnaround time varies from hours to few days), objective identification of mutations, and ability to differentiate drug resistance from clinical resistance secondary to host factors. In addition, genotypic analysis can be performed directly on clinical isolates in blood, fluids, or tissue specimens with or without cell cultures.46 Multiple genotypic techniques have been reported,47,48 but DNA sequencing is currently the standard for detection of drug resistance, and the analysis is more reliable if the CMV viral load is at least 1000 IU/mL. However, interpretation of the results remains a challenge, because random and irrelevant mutations associated with natural polymorphism may be detected. Moreover, false negatives can occur, because mutant strains comprising <20% to 30% of the viral population are not reported.49 This limitation might be overcome with the recently developed deep sequencing techniques that detect smaller subpopulations50; however, this technology is not readily available and is currently expensive.
Antiviral activity and genetic mechanisms of CMV drug resistance
CMV genome structure and key proteins
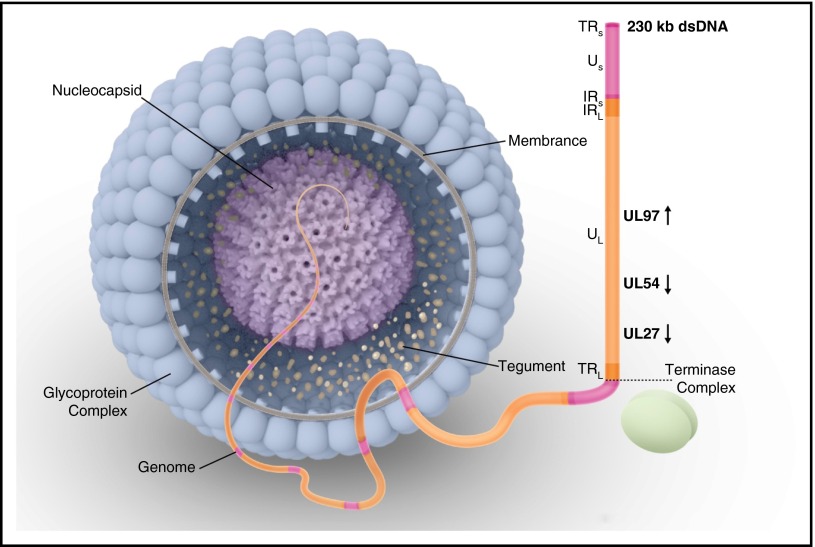
CMV is the largest member of the Betaherpesvirinae subfamily and the most complex human herpesvirus in this subfamily. The CMV genome is a linear, double-stranded DNA molecule comprising a total of 236 kilobase pairs51 containing nonoverlapping open reading frames (ORFs) that encode 164 proteins (Figure 1).52,53 The DNA genome is formed by 2 covalently linked genome segments, each consisting of a central unique region (Figure 1).54 These unique regions are flanked by inverted repeats either at the ends or internally at the intersection of long and short segments (Figure 1).
Figure 1.
Human CMV virion structure and structural components. The genome is packaged in an icosahedral capsid surrounded by a lipid envelope. Viral phosphoproteins (eg, pp65 antigen) are found in the tegument, a space between the nucleocapsid and the envelope. The DNA genome is formed by 2 covalently linked genome segments (L, long; S, short), each consisting of a central unique region (UL, unique long; US, unique short). These unique regions are flanked by inverted repeats either at the ends (IR, inverted repeat; TR, terminal repeat) or internally at the intersection of the long and short segments (IRL, internal repeat long; IRS, internal repeat short). Vertical arrows show the direction of the open reading frame for each gene of interest for drug resistance (UL97, UL54, and UL27). During CMV replication, a single long DNA chain is synthesized. This chain contains multiple repeated gene sequences known as concatemers. Each concatemer is cleaved into multiple gene sequences known as monomers, forming the genetic material for each virion. This process of replication, cleavage, and packaging is performed by a terminase complex. dsDNA, double-stranded DNA.
The CMV genome encodes for its own DNA polymerase, which has conserved functional regions.55 All currently approved anti-CMV drugs target and inhibit this DNA polymerase by incorporating a deoxyguanosine triphosphate into the replicating viral DNA. ORFs and their corresponding proteins are named by the unique segment and the numerical order in which they occur. ORF UL54 (pol) encodes the highly conserved CMV DNA polymerase56 (Pol or pUL54). The highly conserved regions are the 3′-5′ exonuclease domains (I-III) and the polymerization domains I to VII (Figure 2). pUL97, a protein kinase, is the product of the UL97 ORF.57,58 pUL97 phosphorylates some CMV viral and cellular proteins58 and controls the phosphorylation of ganciclovir in CMV-infected cells. During CMV replication, a single long DNA chain is synthesized and then cleaved into multiple gene sequences that form the genetic material for each virion (Figure 1).59 This process of replication, cleavage, and packaging is performed by a terminase complex.60 pUL56, which is encoded by UL56 ORF, is 1 of 2 proteins forming the CMV terminase complex.
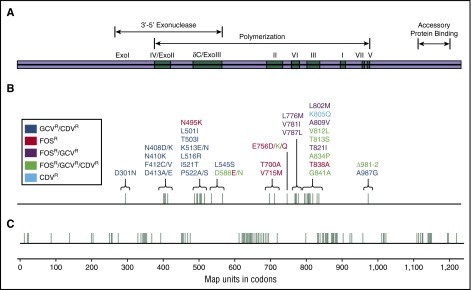
Figure 2.
Map of the CMV DNA polymerase gene (UL54 or pol). Most of the UL54 mutations occur within the cluster of conserved regions of homology (Exo I through III and polymerization domains I through VII). Mutations conferring ganciclovir (GCV) and cidofovir (CDV) cross-resistance are most commonly found within the exonuclease domains and polymerization region V. Mutations conferring resistance to foscarnet (FOS) are located in the δC/Exo III domains and polymerization II and III domains. FOS, GCV, and valganciclovir cross-resistance mutations are located in polymerization regions VI and III. Mutations conferring resistance to all 4 drugs (FOS, GCV, valganciclovir, and CDV) are located in Exo III domains and polymerization regions III, VII, and V, and those conferring resistance to only CDV are in polymerization region III. (A) Functional regions of the DNA polymerase. (B) UL54 mutations with corresponding antiviral resistance profiles. (C) Codons corresponding to functional DNA polymerase regions and UL54 mutations. Reprinted from Lurain and Chou69 with permission.
Ganciclovir, valganciclovir, and UL97
Ganciclovir, a nucleoside analog, is currently the drug of choice to treat CMV infections.61-63 Ganciclovir is administered intravenously and undergoes activation to ganciclovir-triphosphate upon phosphorylation by pUL97 and cellular kinases in CMV-infected cells (Figure 3).64,65 Ganciclovir-triphosphate is a potent inhibitor of CMV polymerase enzyme; it competitively inhibits the incorporation of deoxyguanosine triphosphate, which grows viral DNA, and slows viral DNA replication by incorporating at the end of the elongating viral DNA.66 Valganciclovir, the valine ester of ganciclovir, undergoes hydrolysis upon administration by a human intestinal mucosal valine esterase, and it then enters systemic circulation as ganciclovir. It has ∼60% bioavailability, which is 10-fold higher than oral ganciclovir,67 with the same mechanism of action and toxicity profile.68
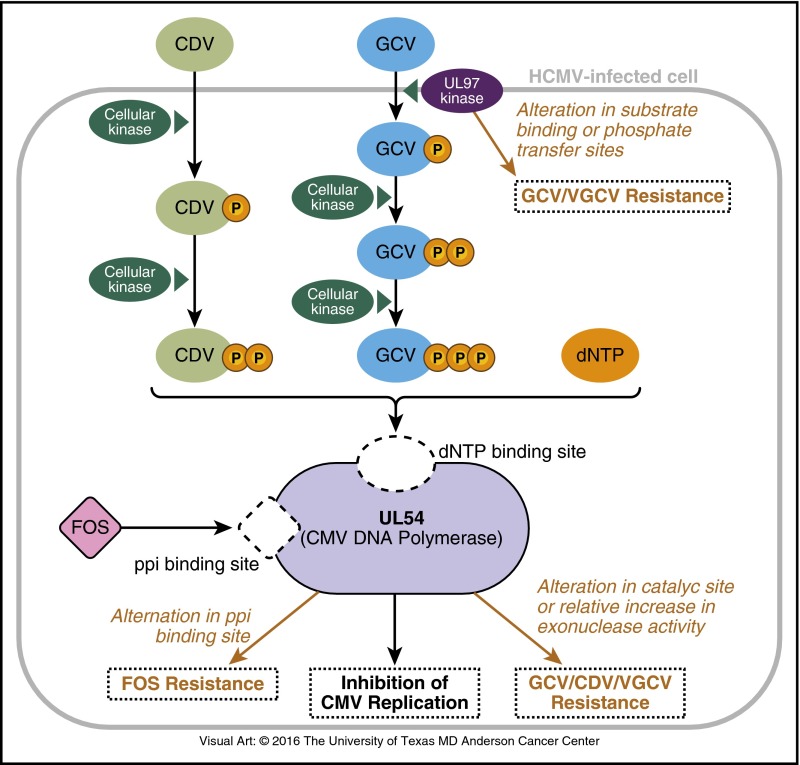
Figure 3.
Mechanism of action of antiviral drugs for CMV. In the CMV-infected cell, GCV and valganciclovir (VGCV) undergo phosphorylation by UL97 kinase (pUL97) and cellular kinases. CDV phosphorylation is independent of pUL97; cellular kinases add an additional phosphate. GCV, VGCV, and CDV compete with deoxynucleotide triphosphate (dNTP) for the binding site on pUL54 (CMV DNA polymerase [pol]). FOS does not require phosphorylation. Once inside the CMV-infected cell, FOS directly inhibits CMV DNA replication by binding to the pyrophosphate (ppi) site of pUL54. Alterations in the substrate binding or phosphate transfer sites of pUL97 confer UL97 resistance to GCV and VGCV. Alterations in the catalytic site or relative increases in the exonuclease activity of pUL54 confer UL54 resistance to GCV, VGCV, and CDV. Alterations of the ppi binding site of pUL54 confer UL54 resistance to FOS.
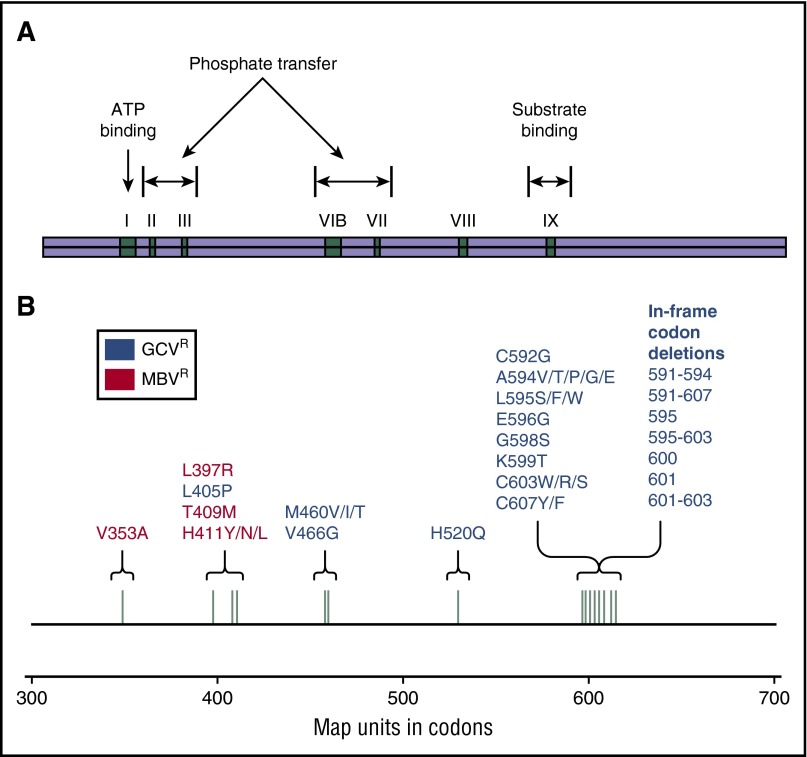
CMV resistance to ganciclovir can result from either modification of pUL97 or alteration of pUL54 (discussed in the next section) (Figure 4). Multiple UL97 mutations with amino acid substitutions have been described,69,70 and the 7 most common “canonical” mutations, constituting >80% of the UL97 resistance mutations, confer a 5- to 15-fold increase in IC50 (Table 1). Other deletion mutations conferring varying levels of resistance have been reported in the codon range 590 to 607. With mutations in this region, pUL97 fails to recognize ganciclovir as a substrate. Mutations at codons 460 and 520 are more critical to overall kinase activity by modifying adenosine triphosphate (ATP) binding.69 Rare in vitro mutations have been described, but their clinical relevance is yet to be determined.71,72
Figure 4.
Map of the cytomegalovirus UL97 gene. (A) Functional regions of the UL97 kinase (pUL97). (B) Codons corresponding to functional pUL97 regions and UL97 mutations with corresponding antiviral resistance profiles. Reprinted from Lurain and Chou69 with permission.
Table 1.
Clinically relevant, confirmed CMV resistance substitution mutations occurring in UL97, identified by recombinant phenotyping
| Codon number | Wild-type | Mutant | GCV ratio* | References |
|---|---|---|---|---|
| Canonical mutations | ||||
| 460 | M | I | 5 | 47,72,80,142,152-163 |
| 460 | M | V | 8.3 | 43,47,80,82,83,142,154-156,159-161,164-169 |
| 520 | H | Q | 10 | 47,72,83,142,154,155,159,161,164,165,169-171 |
| 592 | C | G | 2.9 | 72,80,82,83,142,153,154,159-161,163,165,166,172,173 |
| 594 | A | V | 8.3 | 43,72,80,82,83,152-156,158-161,164-166,171,173 |
| 595 | L | S | 9.2 | 43,72,80,82,83,153-155,158-166 |
| 603 | C | W | 8 | 72,80,83,142,153-155,159-161,163,164,171,172,174,175 |
| Other clinically relevant substitution mutations | ||||
| 595 | L | F | 15.7 | 72,156,161,175 |
| 595 | L | W | 5.1 | 72,154,155,159-161,175 |
GCV ratio = IC50 of mutant/IC50 of wild-type (IC50 = 50% inhibitory concentration, the concentration at which there is a 50% reduction in the number of plaques on a phenotypic plaque reduction assay; GCV IC50 values ≤6 μM indicate sensitivity, and values >6 μM indicate resistance44).
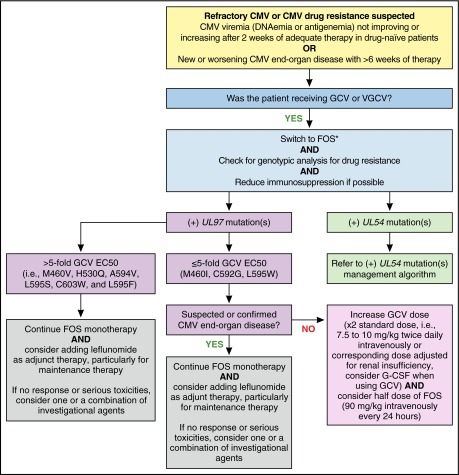
Because C592G UL97 mutation confers low-level resistance to ganciclovir (Table 1), when managing CMV resistance for case 1, we could have increased the ganciclovir dose up to double the standard induction dose, with successful outcomes in some reported cases (Figure 5).73,74 However, in our patient with CMV disease, switching empirically to foscarnet was thought to be more appropriate. For patients with UL97 mutations conferring a 5- to 10-fold or more increase in ganciclovir resistance, we recommend an immediate switch to foscarnet and avoiding high-dose ganciclovir monotherapy. Although our patient had renal dysfunction, an empirical switch to foscarnet was proper because of his prior exposure to ganciclovir and the high likelihood of emergence of UL97 before a UL54 mutation.
Figure 5.
MD Anderson Cancer Center proposed algorithm for management of refractory or resistant CMV infection with UL97 mutation(s). *While awaiting genotypic analysis results, maintaining GCV or VGCV and refraining from switching to FOS in low-risk patients (ie, HLA-identical HCT recipients without GVHD and/or without risk factors for CMV resistance) may be considered. EC50, concentration of a drug that gives half-maximal response; G-CSF, granulocyte colony-stimulating factor. Professional illustration by Patrick Lane, ScEYEnce Studios.
Cross-resistance: foscarnet, cidofovir, ganciclovir, valganciclovir, and UL54
Foscarnet is an organic pyrophosphate analog that reversibly inhibits pUL54 by blocking the pyrophosphate binding site75 without incorporation into the elongating viral DNA (Figure 3).76 High intracellular foscarnet levels are required to inhibit UL54.77 Cidofovir is a nucleotide analog of cytosine with potent activity against CMV. After phosphorylation by cellular kinases,78 cidofovir triphosphate inhibits pUL54, and similar to ganciclovir, cidofovir incorporates into the viral DNA, disrupting further elongation (Figure 3).79
All approved CMV antivirals inhibit pUL54 as a final target. UL54 mutations induce various patterns of cross-resistance among all these drugs. UL54 mutations distributed over large areas (covering the codon range 300-1000) have been reported, and unlike with UL97 mutations, there is no short list of canonical mutations (Table 1).69,71 Most UL54 mutations occur within the cluster of conserved regions of homology (exonuclease domains I through III and polymerization domains I through VII). Mutations conferring resistance to one or more of these CMV antivirals are found within different exonuclease and polymerization domains (Figure 2). Ganciclovir and valganciclovir are affected by both UL97 and UL54 mutations.80 In clinical settings, UL97 mutations are much more likely (>90%) to emerge first.80 In the setting of prolonged ganciclovir exposure, UL54 resistance mutations would more commonly confer additional resistance to preexisting UL97 mutations.81-84 Foscarnet and cidofovir activity is independent of pUL97 and is therefore unaffected by these mutations.
For case 1, another option would have been cidofovir, because its phosphorylation is independent of UL97. However, experience with cidofovir in HCT recipients remains limited.85 In addition, transient efficacy has been reported and concerns about low thresholds for resistance and significant kidney injury have been raised. Furthermore, if the patient had a concomitant UL54 mutation, cidofovir should be avoided because almost all UL54 mutations that confer ganciclovir resistance confer cidofovir cross-resistance. In these instances, we may use an empirical ganciclovir-foscarnet combination86-90 or an investigational agent if possible. As for case 2, because V715M UL54 confers resistance only to foscarnet (Figure 2), ganciclovir was used. Perhaps, the availability of a more rapid genotypic testing and results would have made the switch to an appropriate antiviral agent timelier in both cases. Whether it would have an impact on morbidity or mortality needs to be determined in future studies.
Risk factors and clinical outcomes of resistant or refractory CMV
CMV drug resistance appears to be an emerging problem in patients with specific underlying risk factors (Table 2). It has been shown that prolonged drug exposure (>3 months, before or after HCT) and incomplete viral replication with subtherapeutic drug activity play a major role in the development of resistance.35,91-93 Furthermore, subtherapeutic antiviral levels could lead to the emergence of resistance in HCT recipients,94 and therapeutic drug monitoring, a relatively common practice in pediatric patients,95 has been suggested. Since the serum concentrations of valganciclovir are quite difficult to predict, therapeutic drug monitoring could be helpful in some instances. Previous drug exposure for CMV prophylaxis was found to be another important risk factor,91,93 although preemptive therapy seems to be protective against drug resistance.31 Other risk factors include T-cell depletion,42,96 high peak CMV viral loads,34,71 and recurrent CMV infections.33 On the one hand, intermittent low-level viral replication with profound immunosuppression may induce resistance; on the other hand, in some instances, there is evidence of slow response to preemptive antiviral therapy, with persistent low-level viremia without evidence of resistance.97 The type of HCT has also emerged as a risk factor; haploidentical, allogeneic unrelated, and cord blood HCT increase the risk.3,34,41 With these types of HCT, the delay in immune reconstitution and impaired crosstalk or interactions between donor and recipient T cells may result in ongoing viral replication.34
Table 2.
Risk factors for CMV resistance in HCT recipients
| Host factors |
| Prolonged antiviral CMV drug exposure (>3 mo) |
| Previous antiviral CMV drug exposure |
| Recurrent CMV infection |
| Inadequate antiviral CMV drug absorption and bioavailability |
| Inadequate antiviral CMV oral prodrug conversion |
| Variation in antiviral CMV drug clearance |
| Subtherapeutic antiviral CMV drug level |
| Poor compliance |
| T-cell depletion |
| Haploidentical, allogeneic, and cord blood HCT |
| Delayed immune reconstitution |
| CMV-seropositive recipient |
| Treatment with antithymocyte antibodies |
| Active GVHD |
| Young age |
| Congenital immunodeficiency syndromes |
| Viral factors |
| CMV viral load rise while receiving treatment (after >2 wk with adequate dosing) |
| Failure of CMV viral load to fall despite appropriate treatment |
| Rise in CMV viral load after decline while receiving appropriate therapy |
| Intermittent low-level CMV viremia |
| High CMV viral loads |
Case 1 had a constellation of risk factors for developing CMV drug resistance: allogeneic HCT, CMV R+/D−, active GVHD treated with high-dose corticosteroids, prolonged anti-CMV drug exposure (>6 weeks), rebound in CMV viremia after clearance, and CMV end-organ disease. The patient also had renal insufficiency, which complicated the picture because the patient was receiving a renally adjusted dose of valganciclovir with an unclear absorption pattern. Case 2 also had risk factors predisposing her to resistant CMV: cord blood HCT, prolonged anti-CMV drug exposure (>6 weeks), GVHD and corticosteroid immunosuppression, CMV R+, and a conditioning regimen containing ATG.
Outcomes of drug-resistant CMV infections have ranged from complete recovery to fatal disease. Clinical features are nonspecific and mostly similar to CMV disease; however, life-threatening infections have been described in the setting of a paucity of effective antiviral therapy. Interestingly, previously uncommon central nervous system CMV disease and retinitis have been found to be associated with ganciclovir-resistant CMV strains.97-99
Novel and future therapies
Maribavir
Maribavir, a benzimidazole antiviral agent, competes with ATP for binding to pUL97.100-102 This mechanism of action is novel, because it does not require intracellular phosphorylation and is independent of pUL54. Similar to deletions in UL97, maribavir severely inhibits CMV growth.103-105 After successful phase 1 and 2 trials,106,107 maribavir, at a potentially suboptimal selected dosage, failed to show efficacy in the prevention of CMV disease in a phase 3 trial.108 However, a case series of ganciclovir-resistant CMV infection showed that maribavir had potential activity.109 Still, maribavir may not completely inhibit CMV replication, resulting in persistent low-level viremia.109,110 A phase 2 randomized study assessing maribavir for resistant CMV infections has been completed and on intent-to-treat analysis, 68% of the patients had undetectable plasma CMV viral loads within 6 weeks; however, 68% developed serious treatment related adverse events within 25 weeks and 25% had recurrent CMV infection within 36 weeks (www.clinicaltrials.gov #NCT01611974). Although various in vitro mutations have been shown to confer maribavir resistance by modifying the pUL97 ATP binding site,100,102,111 CMV isolates that are resistant to maribavir remain susceptible to ganciclovir (and vice versa). Only 2 of these in vitro mutations have confirmed clinical significance: T409M and H411Y.112 However, since maribavir inhibits UL97, it impairs phosphorylation of ganciclovir; in theory, these 2 drugs should not be co-administered.113 This potential antagonistic effect seems not to be a concern when combining maribavir and foscarnet.
Brincidofovir
Brincidofovir is the oral lipid conjugate of cidofovir. It is more potent and less nephrotoxic and achieves higher serum concentrations than cidofovir.114,115 After crossing the intestinal wall into the bloodstream, brincidofovir reaches the target cells, where the lipid moiety is cleaved and the cidofovir component is phosphorylated by cellular kinases; inhibition of pUL54 then ensues. However, brincidofovir (administered weekly) is associated with increased gastrointestinal toxicities (mainly diarrhea) compared with cidofovir.116 Unfortunately, in a phase 3 trial, brincidofovir failed to prevent clinically significant CMV infection through week 24 after HCT.117 Resistance to brincidofovir is expected to be similar to cidofovir after mutations in UL54, although in vitro novel resistance mutations have been identified as well.118 Brincidofovir can be used for ganciclovir-resistant CMV infection, unless resistance is acquired through a UL54 mutation (Figure 2).119,120
Letermovir
Letermovir has a novel mechanism of action: it inhibits CMV DNA synthesis at a late step by targeting the pUL56 subunit of the terminase enzyme complex.121-123 Letermovir is the most potent in vitro anti-CMV agent to date, with a low nanomolar IC50121 and no tolerability or safety issues reported in either phase 1 or phase 2 studies.123 With its distinct mode of action, letermovir can overcome any mutation conferred through exposure to any of the other anti-CMV drugs, as was shown in a lung transplantation recipient in whom letermovir controlled a multidrug-resistant strain of CMV.124 However, in vitro letermovir resistance mutations in the codon range 231 to 369 of UL56 have already been identified, suggesting a low genetic barrier to resistance.125 The clinical implications of these in vitro resistance mutations have yet to be determined. Because letermovir does not prevent viral DNA synthesis, CMV DNAemia may remain detectable early on in patients receiving treatment with letermovir without evidence of drug resistance.126 Surrogate monitoring techniques for response to treatment with letermovir may become necessary in the future,127 and current treatment algorithms would need to be revised.
CMV-specific CTLs
Cellular adoptive immunotherapy has proven efficacious for the treatment of CMV infection after transplantation by restoring CMV-specific T-cell responses.128-132 More recently, cellular adoptive immunotherapy has been used to treat CMV infections that are unresponsive to antiviral therapy, with promising results.133 However, use of cellular adoptive immunotherapy has been limited by logistical difficulties: availability, cost, time to generate adequate cells for infusion, limited use in patients receiving high-dose corticosteroids or with significant GVHD, and durability of immunity. When treating resistant CMV infections, we recommend cytotoxic T-lymphocyte (CTL) infusions when available as adjunct therapy, particularly if multidrug resistance is identified. Multiple infusions maybe needed, especially if the initial response is suboptimal or rebound of CMV viremia occurs. Interestingly, in a case report of a patient with a multidrug-resistant CMV infection, adoptive transfer of CMV-specific T cells was preceded with retransplantation from a CMV-seropositive donor.134 However, major adverse events, such as graft failure and transplantation-associated microangiopathy, have been reported in a very small number of patients undergoing donor-derived CTL infusions.132
Other therapies
Several drugs with anti-CMV activity have been repurposed for the treatment of resistant CMV infections; however, no controlled clinical trials are available to confirm the efficacy of these treatments. Leflunomide, approved for the treatment of rheumatoid arthritis, inhibits protein kinase activity and may interfere with CMV virion assembly.135,136 Because leflunomide showed in vitro activity against wild-type and ganciclovir-resistant CMV,135 it has been used to treat multidrug-resistant or refractory CMV infections, with variable results.137-140 We recommend leflunomide as adjunct therapy in addition to other anti-CMV drugs or other strategies. Higher doses and monitoring of teriflunomide (active metabolite) levels in the serum may be necessary to keep drug levels above 40 μg/mL. In some instances, low-level CMV viremia without overt CMV disease persists in clinically stable HCT recipients who are receiving only leflunomide.141 Moreover, we closely monitor patients for leflunomide side effects, which may include elevated liver enzymes, impaired bone marrow function, and potential severe life-threatening toxicities.
Artesunate, an antimalarial agent, has shown in vitro activity against ganciclovir-resistant CMV.142 Artesunate interferes with the host cell kinase signaling system required for CMV replication.143 The clinical efficacy of artesunate remains questionable, because only a few cases were reported, with variable success.144,145 Artesunate is generally well-tolerated; however, transient neurologic abnormalities and neutropenia have been observed in patients treated for malaria.146 We suggest using artesunate as an adjunct therapy and only when everything else has failed.
The mammalian target of rapamycin drugs sirolimus and everolimus may reduce the incidence of CMV infections in HCT recipients.147 The mechanism of action of these drugs against CMV is still unclear; however, it is thought that these drugs have an indirect effect by inhibiting host cell proliferation and signaling pathways.148,149 Whether switching to a mammalian target of rapamycin drugs inhibitor could be beneficial for management of resistant CMV infection still need to be determined in future trials.
CSJ148, a combination of 2 newly discovered monoclonal antibodies against CMV, binds and inhibits the CMV viral glycoprotein B (gB) and gH/gL/UL128/UL130/UL131, a pentameric complex essential for CMV infectivity.150 CSJ148 showed 100- to 1000-fold more potency than CMV hyperimmunoglobulin at inhibiting CMV replication. In the first-in-human study, CSJ148 was safe and well tolerated by healthy volunteers.151 Interestingly, no resistance was seen when CMV was cultured in the presence of the compound for >400 days. Currently, CSJ148 is being studied for its efficacy in preventing CMV replication in HCT recipients (www.clinicaltrials.gov #NCT02268526).
The use of CMV intravenous immunoglobulins as adjunct therapy for CMV end-organ disease, particularly for CMV pneumonitis, in HCT recipients remains at best controversial. To date, available data on the utility of adding CMV intravenous immunoglobulins for the treatment of resistant CMV infection in this patient population are lacking.
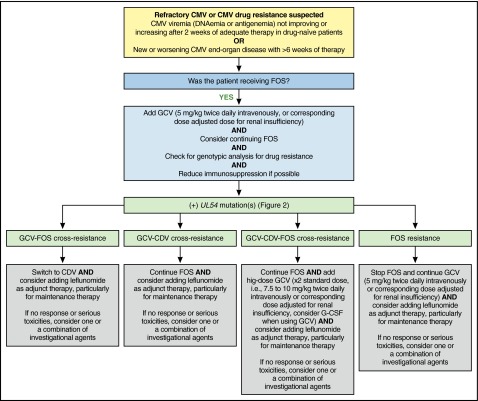
When UL97 was detected for case 2, the addition of cidofovir along with adjunct therapy with leflunomide and CMV-specific CTLs controlled CMV viremia, with persistent low-level “blips” and no overt CMV disease. Investigational therapies such as maribavir or brincidofovir could have been used in this case (Figure 6).
Figure 6.
MD Anderson Cancer Center proposed algorithm for management of refractory or resistant CMV infection with UL54 mutation(s). Professional illustration by Patrick Lane, ScEYEnce Studios.
Conclusions
CMV resistance to the available antiviral agents remains a major threat to HCT recipients. While treating CMV reactivation, the clinician should be vigilant to the risk factors for drug resistance and perform genotypic analysis when indicated. Multiple approaches, based mainly on expert opinion, exist for the treatment of resistant CMV infections; however, because of major side effects and limited drug options with currently approved antiviral agents, there is an urgent need for randomized trials to identify novel therapies with fewer toxicities, greater potency, and lack of cross-resistance with current therapies.
Acknowledgments
The authors thank Erica Goodoff in MD Anderson’s Department of Scientific Publications for editing the manuscript.
The study was supported in part by National Cancer Institute, National Institutes of Health award P30CA016672 and used the Cancer Center Support Grant resources.
Authorship
Contribution: F.E.C., D.P.S., and R.F.C. designed the study, reviewed the articles, wrote the manuscript, and gave final approval for the manuscript.
Conflict-of-interest disclosure: R.F.C. has received research funding from Merck, Chimerix, Novartis, and Oxford Immunotec and is also a consultant for Oxford Immunotec, Merck, Astellas, and Chimerix. The remaining authors declare no competing financial interests.
Correspondence: Roy F. Chemaly, Department of Infectious Diseases, Infection Control, and Employee Health, Unit 402, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030-4009; e-mail: rfchemaly@mdanderson.org; and Firas El Chaer, Department of Infectious Diseases, Infection Control, and Employee Health, Unit 402, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030-4009; e-mail: elchaer.firas@gmail.com.
References
- 1.Gershon AAGE, Nankervis GA. Viral Infections of Humans. New York: Plenum Press; 1997. [Google Scholar]
- 2.Zhang LJ, Hanff P, Rutherford C, Churchill WH, Crumpacker CS. Detection of human cytomegalovirus DNA, RNA, and antibody in normal donor blood. J Infect Dis. 1995;171(4):1002-1006. [DOI] [PubMed] [Google Scholar]
- 3.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113(23):5711-5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34(8):1094-1097. [DOI] [PubMed] [Google Scholar]
- 5.Zaia JA. Viral infections associated with bone marrow transplantation. Hematol Oncol Clin North Am. 1990;4(3):603-623. [PubMed] [Google Scholar]
- 6.Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9(9):543-558. [DOI] [PubMed] [Google Scholar]
- 8.Boeckh M, Nichols WG, Chemaly RF, et al. Valganciclovir for the prevention of complications of late cytomegalovirus infection after allogeneic hematopoietic cell transplantation: a randomized trial. Ann Intern Med. 2015;162(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reusser P, Fisher LD, Buckner CD, Thomas ED, Meyers JD. Cytomegalovirus infection after autologous bone marrow transplantation: occurrence of cytomegalovirus disease and effect on engraftment. Blood. 1990;75(9):1888-1894. [PubMed] [Google Scholar]
- 10.Cantoni N, Hirsch HH, Khanna N, et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16(9):1309-1314. [DOI] [PubMed] [Google Scholar]
- 11.Koskinen P, Lemstrøm K, Mattila S, Häyry P, Nieminen MS. Cytomegalovirus infection associated accelerated heart allograft arteriosclerosis may impair the late function of the graft. Clin Transplant. 1996;10(6 Pt 1):487-493. [PubMed] [Google Scholar]
- 12.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis. 2002;185(3):273-282. [DOI] [PubMed] [Google Scholar]
- 13.Tomblyn M, Chiller T, Einsele H, et al. ; Center for International Blood and Marrow Research; National Marrow Donor program; European Blood and MarrowTransplant Group; American Society of Blood and Marrow Transplantation; Canadian Blood and Marrow Transplant Group; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America; Association of Medical Microbiology and Infectious Disease Canada; Centers for Disease Control and Prevention. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green ML, Leisenring W, Stachel D, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1687-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollack M, Heugel J, Xie H, et al. An international comparison of current strategies to prevent herpesvirus and fungal infections in hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2011;17(5):664-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ariza-Heredia EJ, Nesher L, Chemaly RF. Cytomegalovirus diseases after hematopoietic stem cell transplantation: a mini-review. Cancer Lett. 2014;342(1):1-8. [DOI] [PubMed] [Google Scholar]
- 17.Goodrich JM, Bowden RA, Fisher L, Keller C, Schoch G, Meyers JD. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann Intern Med. 1993;118(3):173-178. [DOI] [PubMed] [Google Scholar]
- 18.Winston DJ, Yeager AM, Chandrasekar PH, Snydman DR, Petersen FB, Territo MC; Valacyclovir Cytomegalovirus Study Group. Randomized comparison of oral valacyclovir and intravenous ganciclovir for prevention of cytomegalovirus disease after allogeneic bone marrow transplantation. Clin Infect Dis. 2003;36(6):749-758. [DOI] [PubMed] [Google Scholar]
- 19.Winston DJ, Ho WG, Bartoni K, et al. Ganciclovir prophylaxis of cytomegalovirus infection and disease in allogeneic bone marrow transplant recipients. Results of a placebo-controlled, double-blind trial. Ann Intern Med. 1993;118(3):179-184. [DOI] [PubMed] [Google Scholar]
- 20.Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88(10):4063-4071. [PubMed] [Google Scholar]
- 21.Prentice HG, Gluckman E, Powles RL, et al. ; European Acyclovir for CMV Prophylaxis Study Group. Impact of long-term acyclovir on cytomegalovirus infection and survival after allogeneic bone marrow transplantation. Lancet. 1994;343(8900):749-753. [DOI] [PubMed] [Google Scholar]
- 22.Meyers JD, Reed EC, Shepp DH, et al. Acyclovir for prevention of cytomegalovirus infection and disease after allogeneic marrow transplantation. N Engl J Med. 1988;318(2):70-75. [DOI] [PubMed] [Google Scholar]
- 23.Prentice HG, Gluckman E, Powles RL, et al. ; The European Acyclovir for CMV Prophylaxis Study Group. Long-term survival in allogeneic bone marrow transplant recipients following acyclovir prophylaxis for CMV infection. Bone Marrow Transplant. 1997;19(2):129-133. [DOI] [PubMed] [Google Scholar]
- 24.Ljungman P, de La Camara R, Milpied N, et al. ; Valacyclovir International Bone Marrow Transplant Study Group. Randomized study of valacyclovir as prophylaxis against cytomegalovirus reactivation in recipients of allogeneic bone marrow transplants. Blood. 2002;99(8):3050-3056. [DOI] [PubMed] [Google Scholar]
- 25.Salzberger B, Bowden RA, Hackman RC, Davis C, Boeckh M. Neutropenia in allogeneic marrow transplant recipients receiving ganciclovir for prevention of cytomegalovirus disease: risk factors and outcome. Blood. 1997;90(6):2502-2508. [PubMed] [Google Scholar]
- 26.Bacigalupo A, Tedone E, Van Lint MT, et al. CMV prophylaxis with foscarnet in allogeneic bone marrow transplant recipients at high risk of developing CMV infections. Bone Marrow Transplant. 1994;13(6):783-788. [PubMed] [Google Scholar]
- 27.Bregante S, Bertilson S, Tedone E, et al. Foscarnet prophylaxis of cytomegalovirus infections in patients undergoing allogeneic bone marrow transplantation (BMT): a dose-finding study. Bone Marrow Transplant. 2000;26(1):23-29. [DOI] [PubMed] [Google Scholar]
- 28.Reusser P, Gambertoglio JG, Lilleby K, Meyers JD. Phase I-II trial of foscarnet for prevention of cytomegalovirus infection in autologous and allogeneic marrow transplant recipients. J Infect Dis. 1992;166(3):473-479. [DOI] [PubMed] [Google Scholar]
- 29.Biron KK, Fyfe JA, Stanat SC, et al. A human cytomegalovirus mutant resistant to the nucleoside analog 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine (BW B759U) induces reduced levels of BW B759U triphosphate. Proc Natl Acad Sci USA. 1986;83(22):8769-8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erice A, Chou S, Biron KK, Stanat SC, Balfour HH Jr, Jordan MC. Progressive disease due to ganciclovir-resistant cytomegalovirus in immunocompromised patients. N Engl J Med. 1989;320(5):289-293. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert C, Roy J, Belanger R, et al. Lack of emergence of cytomegalovirus UL97 mutations conferring ganciclovir (GCV) resistance following preemptive GCV therapy in allogeneic stem cell transplant recipients. Antimicrob Agents Chemother. 2001;45(12):3669-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hantz S, Garnier-Geoffroy F, Mazeron MC, et al. ; French CMV Resistance Survey Study Group. Drug-resistant cytomegalovirus in transplant recipients: a French cohort study. J Antimicrob Chemother. 2010;65(12):2628-2640. [DOI] [PubMed] [Google Scholar]
- 33.Allice T, Busca A, Locatelli F, Falda M, Pittaluga F, Ghisetti V. Valganciclovir as pre-emptive therapy for cytomegalovirus infection post-allogenic stem cell transplantation: implications for the emergence of drug-resistant cytomegalovirus. J Antimicrob Chemother. 2009;63(3):600-608. [DOI] [PubMed] [Google Scholar]
- 34.Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis. 2014;209(4):557-561. [DOI] [PubMed] [Google Scholar]
- 35.Nichols WG, Corey L, Gooley T, et al. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood. 2001;97(4):867-874. [DOI] [PubMed] [Google Scholar]
- 36.Gerna G, Lilleri D, Zecca M, et al. Rising antigenemia levels may be misleading in pre-emptive therapy of human cytomegalovirus infection in allogeneic hematopoietic stem cell transplant recipients. Haematologica. 2005;90(4):526-533. [PubMed] [Google Scholar]
- 37.Park SY, Lee SO, Choi SH, et al. Paradoxical rising cytomegalovirus antigenemia during preemptive ganciclovir therapy in hematopoietic stem cell transplant recipients: incidence, risk factors, and clinical outcomes. J Clin Microbiol. 2011;49(12):4179-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drew WL, Miner RC, Busch DF, et al. Prevalence of resistance in patients receiving ganciclovir for serious cytomegalovirus infection. J Infect Dis. 1991;163(4):716-719. [DOI] [PubMed] [Google Scholar]
- 39.Jabs DA, Enger C, Forman M, Dunn JP; The Cytomegalovirus Retinitis and Viral Resistance Study Group. Incidence of foscarnet resistance and cidofovir resistance in patients treated for cytomegalovirus retinitis. Antimicrob Agents Chemother. 1998;42(9):2240-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eckle T, Lang P, Prix L, et al. Rapid development of ganciclovir-resistant cytomegalovirus infection in children after allogeneic stem cell transplantation in the early phase of immune cell recovery. Bone Marrow Transplant. 2002;30(7):433-439. [DOI] [PubMed] [Google Scholar]
- 41.Choi SH, Hwang JY, Park KS, et al. The impact of drug-resistant cytomegalovirus in pediatric allogeneic hematopoietic cell transplant recipients: a prospective monitoring of UL97 and UL54 gene mutations. Transpl Infect Dis. 2014;16(6):919-929. [DOI] [PubMed] [Google Scholar]
- 42.Wolf DG, Yaniv I, Honigman A, Kassis I, Schonfeld T, Ashkenazi S. Early emergence of ganciclovir-resistant human cytomegalovirus strains in children with primary combined immunodeficiency. J Infect Dis. 1998;178(2):535-538. [DOI] [PubMed] [Google Scholar]
- 43.Wolf DG, Yaniv I, Ashkenazi S, Honigman A. Emergence of multiple human cytomegalovirus ganciclovir-resistant mutants with deletions and substitutions within the UL97 gene in a patient with severe combined immunodeficiency. Antimicrob Agents Chemother. 2001;45(2):593-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landry ML, Stanat S, Biron K, et al. A standardized plaque reduction assay for determination of drug susceptibilities of cytomegalovirus clinical isolates. Antimicrob Agents Chemother. 2000;44(3):688-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wentworth BB, French L. Plaque assay of cytomegalovirus strains of human origin. Proc Soc Exp Biol Med. 1970;135(2):253-258. [DOI] [PubMed] [Google Scholar]
- 46.Hall Sedlak R, Castor J, Butler-Wu SM, et al. Rapid detection of human cytomegalovirus UL97 and UL54 mutations directly from patient samples. J Clin Microbiol. 2013;51(7):2354-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Göhring K, Mikeler E, Jahn G, Hamprecht K. Rapid simultaneous detection by real-time PCR of cytomegalovirus UL97 mutations in codons 460 and 520 conferring ganciclovir resistance. J Clin Microbiol. 2006;44(12):4541-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Göhring K, Mikeler E, Jahn G, Rohde F, Hamprecht K. Rapid semiquantitative real-time PCR for the detection of human cytomegalovirus UL97 mutations conferring ganciclovir resistance. Antivir Ther. 2008;13(3):461-466. [PubMed] [Google Scholar]
- 49.Chou S, Erice A, Jordan MC, et al. Analysis of the UL97 phosphotransferase coding sequence in clinical cytomegalovirus isolates and identification of mutations conferring ganciclovir resistance. J Infect Dis. 1995;171(3):576-583. [DOI] [PubMed] [Google Scholar]
- 50.Chou S, Ercolani RJ, Sahoo MK, Lefterova MI, Strasfeld LM, Pinsky BA. Improved detection of emerging drug-resistant mutant cytomegalovirus subpopulations by deep sequencing. Antimicrob Agents Chemother. 2014;58(8):4697-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chee MS, Bankier AT, Beck S, et al. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125-169. [DOI] [PubMed] [Google Scholar]
- 52.Davison AJ, Dolan A, Akter P, et al. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J Gen Virol. 2003;84(Pt 1):17-28. [DOI] [PubMed] [Google Scholar]
- 53.Dolan A, Cunningham C, Hector RD, et al. Genetic content of wild-type human cytomegalovirus. J Gen Virol. 2004;85(Pt 5):1301-1312. [DOI] [PubMed] [Google Scholar]
- 54.Murphy E, Shenk T. Human cytomegalovirus genome. Curr Top Microbiol Immunol. 2008;325:1-19. [DOI] [PubMed] [Google Scholar]
- 55.Teo IA, Griffin BE, Jones MD. Characterization of the DNA polymerase gene of human herpesvirus 6. J Virol. 1991;65(9):4670-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chou S, Lurain NS, Weinberg A, Cai GY, Sharma PL, Crumpacker CS; Adult AIDS Clinical Trials Group CMV Laboratories. Interstrain variation in the human cytomegalovirus DNA polymerase sequence and its effect on genotypic diagnosis of antiviral drug resistance. Antimicrob Agents Chemother. 1999;43(6):1500-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chee MS, Lawrence GL, Barrell BG. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J Gen Virol. 1989;70(Pt 5):1151-1160. [DOI] [PubMed] [Google Scholar]
- 58.He Z, He YS, Kim Y, et al. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J Virol. 1997;71(1):405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griffiths PD, Emery VC. Taming the transplantation troll by targeting terminase. N Engl J Med. 2014;370(19):1844-1846. [DOI] [PubMed] [Google Scholar]
- 60.Bogner E. Human cytomegalovirus terminase as a target for antiviral chemotherapy. Rev Med Virol. 2002;12(2):115-127. [DOI] [PubMed] [Google Scholar]
- 61.Martin JC, Dvorak CA, Smee DF, Matthews TR, Verheyden JP. 9-[(1,3-Dihydroxy-2-propoxy)methyl]guanine: a new potent and selective antiherpes agent. J Med Chem. 1983;26(5):759-761. [DOI] [PubMed] [Google Scholar]
- 62.Ashton WT, Karkas JD, Field AK, Tolman RL. Activation by thymidine kinase and potent antiherpetic activity of 2′-nor-2′-deoxyguanosine (2'NDG). Biochem Biophys Res Commun. 1982;108(4):1716-1721. [DOI] [PubMed] [Google Scholar]
- 63.Crumpacker CS. Ganciclovir. N Engl J Med. 1996;335(10):721-729. [DOI] [PubMed] [Google Scholar]
- 64.Littler E, Stuart AD, Chee MS. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 1992;358(6382):160-162. [DOI] [PubMed] [Google Scholar]
- 65.Sullivan V, Talarico CL, Stanat SC, Davis M, Coen DM, Biron KK. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 1992;359(6390):85. [DOI] [PubMed] [Google Scholar]
- 66.Cheng YC, Grill SP, Dutschman GE, Nakayama K, Bastow KF. Metabolism of 9-(1,3-dihydroxy-2-propoxymethyl)guanine, a new anti-herpes virus compound, in herpes simplex virus-infected cells. J Biol Chem. 1983;258(20):12460-12464. [PubMed] [Google Scholar]
- 67.Jung D, Dorr A. Single-dose pharmacokinetics of valganciclovir in HIV- and CMV-seropositive subjects. J Clin Pharmacol. 1999;39(8):800-804. [DOI] [PubMed] [Google Scholar]
- 68.Martin DF, Sierra-Madero J, Walmsley S, et al. ; Valganciclovir Study Group. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N Engl J Med. 2002;346(15):1119-1126. [DOI] [PubMed] [Google Scholar]
- 69.Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev. 2010;23(4):689-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kleiboeker S, Nutt J, Schindel B, Dannehl J, Hester J. Cytomegalovirus antiviral resistance: characterization of results from clinical specimens. Transpl Infect Dis. 2014;16(4):561-567. [DOI] [PubMed] [Google Scholar]
- 71.Chou S. Approach to drug-resistant cytomegalovirus in transplant recipients. Curr Opin Infect Dis. 2015;28(4):293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baldanti F, Lurain N, Gerna G. Clinical and biologic aspects of human cytomegalovirus resistance to antiviral drugs. Hum Immunol. 2004;65(5):403-409. [DOI] [PubMed] [Google Scholar]
- 73.West P, Schmiedeskamp M, Neeley H, Oberholzer J, Benedetti E, Kaplan B. Use of high-dose ganciclovir for a resistant cytomegalovirus infection due to UL97 mutation. Transpl Infect Dis. 2008;10(2):129-132. [DOI] [PubMed] [Google Scholar]
- 74.Gracia-Ahufinger I, Gutiérrez-Aroca J, Cordero E, et al. Use of high-dose ganciclovir for the treatment of cytomegalovirus replication in solid organ transplant patients with ganciclovir resistance-inducing mutations. Transplantation. 2013;95(8):1015-1020. [DOI] [PubMed] [Google Scholar]
- 75.Derse D, Bastow KF, Cheng Y. Characterization of the DNA polymerases induced by a group of herpes simplex virus type I variants selected for growth in the presence of phosphonoformic acid. J Biol Chem. 1982;257(17):10251-10260. [PubMed] [Google Scholar]
- 76.Eriksson B, Oberg B, Wahren B. Pyrophosphate analogues as inhibitors of DNA polymerases of cytomegalovirus, herpes simplex virus and cellular origin. Biochim Biophys Acta. 1982;696(2):115-123. [DOI] [PubMed] [Google Scholar]
- 77.Crumpacker CS. Mechanism of action of foscarnet against viral polymerases. Am J Med. 1992;92(2A):3S-7S. [DOI] [PubMed] [Google Scholar]
- 78.Lea AP, Bryson HM. Cidofovir. Drugs. 1996;52(2):225-230, discussion 231. [DOI] [PubMed] [Google Scholar]
- 79.De Clercq E. Clinical potential of the acyclic nucleoside phosphonates cidofovir, adefovir, and tenofovir in treatment of DNA virus and retrovirus infections. Clin Microbiol Rev. 2003;16(4):569-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith IL, Cherrington JM, Jiles RE, Fuller MD, Freeman WR, Spector SA. High-level resistance of cytomegalovirus to ganciclovir is associated with alterations in both the UL97 and DNA polymerase genes. J Infect Dis. 1997;176(1):69-77. [DOI] [PubMed] [Google Scholar]
- 81.Boivin G, Goyette N, Gilbert C, Covington E. Analysis of cytomegalovirus DNA polymerase (UL54) mutations in solid organ transplant patients receiving valganciclovir or ganciclovir prophylaxis. J Med Virol. 2005;77(3):425-429. [DOI] [PubMed] [Google Scholar]
- 82.Chou S, Van Wechel LC, Lichy HM, Marousek GI. Phenotyping of cytomegalovirus drug resistance mutations by using recombinant viruses incorporating a reporter gene. Antimicrob Agents Chemother. 2005;49(7):2710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Erice A, Gil-Roda C, Pérez JL, et al. Antiviral susceptibilities and analysis of UL97 and DNA polymerase sequences of clinical cytomegalovirus isolates from immunocompromised patients. J Infect Dis. 1997;175(5):1087-1092. [DOI] [PubMed] [Google Scholar]
- 84.Gilbert C, Azzi A, Goyette N, Lin SX, Boivin G. Recombinant phenotyping of cytomegalovirus UL54 mutations that emerged during cell passages in the presence of either ganciclovir or foscarnet. Antimicrob Agents Chemother. 2011;55(9):4019-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ljungman P, Deliliers GL, Platzbecker U, et al. ; The Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Cidofovir for cytomegalovirus infection and disease in allogeneic stem cell transplant recipients. Blood. 2001;97(2):388-392. [DOI] [PubMed] [Google Scholar]
- 86.Mylonakis E, Kallas WM, Fishman JA. Combination antiviral therapy for ganciclovir-resistant cytomegalovirus infection in solid-organ transplant recipients. Clin Infect Dis. 2002;34(10):1337-1341. [DOI] [PubMed] [Google Scholar]
- 87.The Studies of Ocular Complications of AIDS Research Group in Collaboration with the AIDS Clinical Trials Group. Combination foscarnet and ganciclovir therapy vs monotherapy for the treatment of relapsed cytomegalovirus retinitis in patients with AIDS. The Cytomegalovirus Retreatment Trial. Arch Ophthalmol. 1996;114(1):23-33. [DOI] [PubMed] [Google Scholar]
- 88.Combination therapy improves CMV treatment. Aids Alert. 1996;11(3):32-33. [PubMed] [Google Scholar]
- 89.Bacigalupo A, Bregante S, Tedone E, et al. Combined foscarnet -ganciclovir treatment for cytomegalovirus infections after allogeneic hemopoietic stem cell transplantation (Hsct). Bone Marrow Transplant. 1996;18(Suppl 2):110-114. [PubMed] [Google Scholar]
- 90.Sastry SM, Epps CH III, Walton RC, Rana SN, Sanders RJ. Combined ganciclovir and foscarnet in pediatric cytomegalovirus retinitis. J Natl Med Assoc. 1996;88(10):661-662. [PMC free article] [PubMed] [Google Scholar]
- 91.Erice A, Borrell N, Li W, Miller WJ, Balfour HH Jr. Ganciclovir susceptibilities and analysis of UL97 region in cytomegalovirus (CMV) isolates from bone marrow recipients with CMV disease after antiviral prophylaxis. J Infect Dis. 1998;178(2):531-534. [DOI] [PubMed] [Google Scholar]
- 92.Drobyski WR, Knox KK, Carrigan DR, Ash RC. Foscarnet therapy of ganciclovir-resistant cytomegalovirus in marrow transplantation. Transplantation. 1991;52(1):155-157. [PubMed] [Google Scholar]
- 93.Slavin MA, Bindra RR, Gleaves CA, Pettinger MB, Bowden RA. Ganciclovir sensitivity of cytomegalovirus at diagnosis and during treatment of cytomegalovirus pneumonia in marrow transplant recipients. Antimicrob Agents Chemother. 1993;37(6):1360-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fletcher C, Sawchuk R, Chinnock B, de Miranda P, Balfour HH Jr. Human pharmacokinetics of the antiviral drug DHPG. Clin Pharmacol Ther. 1986;40(3):281-286. [DOI] [PubMed] [Google Scholar]
- 95.Villeneuve D, Brothers A, Harvey E, et al. Valganciclovir dosing using area under the curve calculations in pediatric solid organ transplant recipients. Pediatr Transplant. 2013;17(1):80-85. [DOI] [PubMed] [Google Scholar]
- 96.Eckle T, Prix L, Jahn G, et al. Drug-resistant human cytomegalovirus infection in children after allogeneic stem cell transplantation may have different clinical outcomes. Blood. 2000;96(9):3286-3289. [PubMed] [Google Scholar]
- 97.van der Beek MT, Marijt EW, Vossen AC, et al. Failure of pre-emptive treatment of cytomegalovirus infections and antiviral resistance in stem cell transplant recipients. Antivir Ther. 2012;17(1):45-51. [DOI] [PubMed] [Google Scholar]
- 98.Hamprecht K, Eckle T, Prix L, Faul C, Einsele H, Jahn G. Ganciclovir-resistant cytomegalovirus disease after allogeneic stem cell transplantation: pitfalls of phenotypic diagnosis by in vitro selection of an UL97 mutant strain. J Infect Dis. 2003;187(1):139-143. [DOI] [PubMed] [Google Scholar]
- 99.Wolf DG, Lurain NS, Zuckerman T, et al. Emergence of late cytomegalovirus central nervous system disease in hematopoietic stem cell transplant recipients. Blood. 2003;101(2):463-465. [DOI] [PubMed] [Google Scholar]
- 100.Biron KK, Harvey RJ, Chamberlain SC, et al. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob Agents Chemother. 2002;46(8):2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Townsend LB, Devivar RV, Turk SR, Nassiri MR, Drach JC. Design, synthesis, and antiviral activity of certain 2,5,6-trihalo-1-(beta-D-ribofuranosyl)benzimidazoles. J Med Chem. 1995;38(20):4098-4105. [DOI] [PubMed] [Google Scholar]
- 102.Chou S, Marousek GI. Accelerated evolution of maribavir resistance in a cytomegalovirus exonuclease domain II mutant. J Virol. 2008;82(1):246-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wolf DG, Courcelle CT, Prichard MN, Mocarski ES. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc Natl Acad Sci USA. 2001;98(4):1895-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Komazin G, Ptak RG, Emmer BT, Townsend LB, Drach JC. Resistance of human cytomegalovirus to the benzimidazole L-ribonucleoside maribavir maps to UL27. J Virol. 2003;77(21):11499-11506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Prichard MN, Britt WJ, Daily SL, Hartline CB, Kern ER. Human cytomegalovirus UL97 Kinase is required for the normal intranuclear distribution of pp65 and virion morphogenesis. J Virol. 2005;79(24):15494-15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang LH, Peck RW, Yin Y, Allanson J, Wiggs R, Wire MB. Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti-human cytomegalovirus agent, in healthy and human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 2003;47(4):1334-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Winston DJ, Young JA, Pullarkat V, et al. Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem cell transplant recipients: a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood. 2008;111(11):5403-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marty FM, Ljungman P, Papanicolaou GA, et al. ; Maribavir 1263-300 Clinical Study Group. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis. 2011;11(4):284-292. [DOI] [PubMed] [Google Scholar]
- 109.Avery RK, Marty FM, Strasfeld L, et al. Oral maribavir for treatment of refractory or resistant cytomegalovirus infections in transplant recipients. Transpl Infect Dis. 2010;12(6):489-496. [DOI] [PubMed] [Google Scholar]
- 110.Prichard MN. Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir. Rev Med Virol. 2009;19(4):215-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chou S, Wechel LC, Marousek GI. Cytomegalovirus UL97 kinase mutations that confer maribavir resistance. J Infect Dis. 2007;196(1):91-94. [DOI] [PubMed] [Google Scholar]
- 112.Strasfeld L, Lee I, Tatarowicz W, Villano S, Chou S. Virologic characterization of multidrug-resistant cytomegalovirus infection in 2 transplant recipients treated with maribavir. J Infect Dis. 2010;202(1):104-108. [DOI] [PubMed] [Google Scholar]
- 113.Chou S, Marousek GI. Maribavir antagonizes the antiviral action of ganciclovir on human cytomegalovirus. Antimicrob Agents Chemother. 2006;50(10):3470-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hostetler KY. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: current state of the art. Antiviral Res. 2009;82(2):A84-A98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Painter W, Robertson A, Trost LC, Godkin S, Lampert B, Painter G. First pharmacokinetic and safety study in humans of the novel lipid antiviral conjugate CMX001, a broad-spectrum oral drug active against double-stranded DNA viruses. Antimicrob Agents Chemother. 2012;56(5):2726-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marty FM, Winston DJ, Rowley SD, et al. ; CMX001-201 Clinical Study Group. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med. 2013;369(13):1227-1236. [DOI] [PubMed] [Google Scholar]
- 117.Chimerix. Chimerix announces top-line results from phase 3 SUPPRESS trial of brincidofovir. Available at: http://ir.chimerix.com/releasedetail.cfm?releaseid=948172. Accessed 1 April, 2016.
- 118.James SH, Price NB, Hartline CB, Lanier ER, Prichard MN. Selection and recombinant phenotyping of a novel CMX001 and cidofovir resistance mutation in human cytomegalovirus. Antimicrob Agents Chemother. 2013;57(7):3321-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Papanicolau GKJ, Westervelt P, Gea-Banacloche J, et al. Experience with CMX001, a novel antiviral drug, for cytomegalovirus infections in stem cell transplant patients. Biol Blood Marrow Transplant. 2011;17(2):S273-S274. [Google Scholar]
- 120.El-Haddad D, El Chaer F, Vanichanan J, et al. Brincidofovir (CMX-001) for refractory and resistant CMV and HSV infections in immunocompromised cancer patients: A single-center experience. Antiviral Res. 2016;134:58-62. [DOI] [PubMed] [Google Scholar]
- 121.Lischka P, Hewlett G, Wunberg T, et al. In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246. Antimicrob Agents Chemother. 2010;54(3):1290-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goldner T, Hewlett G, Ettischer N, Ruebsamen-Schaeff H, Zimmermann H, Lischka P. The novel anticytomegalovirus compound AIC246 (Letermovir) inhibits human cytomegalovirus replication through a specific antiviral mechanism that involves the viral terminase. J Virol. 2011;85(20):10884-10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chemaly RF, Ullmann AJ, Stoelben S, et al. ; AIC246 Study Team. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med. 2014;370(19):1781-1789. [DOI] [PubMed] [Google Scholar]
- 124.Kaul DR, Stoelben S, Cober E, et al. First report of successful treatment of multidrug-resistant cytomegalovirus disease with the novel anti-CMV compound AIC246. Am J Transplant. 2011;11(5):1079-1084. [DOI] [PubMed] [Google Scholar]
- 125.Goldner T, Hempel C, Ruebsamen-Schaeff H, Zimmermann H, Lischka P. Geno- and phenotypic characterization of human cytomegalovirus mutants selected in vitro after letermovir (AIC246) exposure. Antimicrob Agents Chemother. 2014;58(1):610-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stoelben S, Arns W, Renders L, et al. Preemptive treatment of Cytomegalovirus infection in kidney transplant recipients with letermovir: results of a Phase 2a study. Transpl Int. 2014;27(1):77-86. [DOI] [PubMed] [Google Scholar]
- 127.Hebart H, Lengerke C, Ljungman P, et al. Prospective comparison of PCR-based vs late mRNA-based preemptive antiviral therapy for HCMV infection in patients after allo-SCT. Bone Marrow Transplant. 2011;46(3):408-415. [DOI] [PubMed] [Google Scholar]
- 128.Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362(9393):1375-1377. [DOI] [PubMed] [Google Scholar]
- 129.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202(3):379-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257(5067):238-241. [DOI] [PubMed] [Google Scholar]
- 131.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333(16):1038-1044. [DOI] [PubMed] [Google Scholar]
- 132.Blyth E, Clancy L, Simms R, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013;121(18):3745-3758. [DOI] [PubMed] [Google Scholar]
- 133.Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99(11):3916-3922. [DOI] [PubMed] [Google Scholar]
- 134.Stuehler C, Stüssi G, Halter J, et al. Combination therapy for multidrug-resistant cytomegalovirus disease. Transpl Infect Dis. 2015;17(5):751-755. [DOI] [PubMed] [Google Scholar]
- 135.Waldman WJ, Knight DA, Blinder L, et al. Inhibition of cytomegalovirus in vitro and in vivo by the experimental immunosuppressive agent leflunomide. Intervirology. 1999;42(5-6):412-418. [DOI] [PubMed] [Google Scholar]
- 136.Waldman WJ, Knight DA, Lurain NS, et al. Novel mechanism of inhibition of cytomegalovirus by the experimental immunosuppressive agent leflunomide. Transplantation. 1999;68(6):814-825. [DOI] [PubMed] [Google Scholar]
- 137.Avery RK, Bolwell BJ, Yen-Lieberman B, et al. Use of leflunomide in an allogeneic bone marrow transplant recipient with refractory cytomegalovirus infection. Bone Marrow Transplant. 2004;34(12):1071-1075. [DOI] [PubMed] [Google Scholar]
- 138.Avery RK, Mossad SB, Poggio E, et al. Utility of leflunomide in the treatment of complex cytomegalovirus syndromes. Transplantation. 2010;90(4):419-426. [DOI] [PubMed] [Google Scholar]
- 139.Ehlert K, Groll AH, Kuehn J, Vormoor J. Treatment of refractory CMV-infection following hematopoietic stem cell transplantation with the combination of foscarnet and leflunomide. Klin Padiatr. 2006;218(3):180-184. [DOI] [PubMed] [Google Scholar]
- 140.Battiwalla M, Paplham P, Almyroudis NG, et al. Leflunomide failure to control recurrent cytomegalovirus infection in the setting of renal failure after allogeneic stem cell transplantation. Transpl Infect Dis. 2007;9(1):28-32. [DOI] [PubMed] [Google Scholar]
- 141.El Chaer F, Mori N, Shah D, et al. Adjuvant and salvage therapy with leflunomide for recalcitrant cytomegalovirus infections in hematopoietic cell transplantation recipients: A case series. Antiviral Res. 2016;135:91-96. [DOI] [PubMed] [Google Scholar]
- 142.Schreiber A, Härter G, Schubert A, Bunjes D, Mertens T, Michel D. Antiviral treatment of cytomegalovirus infection and resistant strains. Expert Opin Pharmacother. 2009;10(2):191-209. [DOI] [PubMed] [Google Scholar]
- 143.Efferth T, Romero MR, Wolf DG, Stamminger T, Marin JJ, Marschall M. The antiviral activities of artemisinin and artesunate. Clin Infect Dis. 2008;47(6):804-811. [DOI] [PubMed] [Google Scholar]
- 144.Germi R, Mariette C, Alain S, et al. Success and failure of artesunate treatment in five transplant recipients with disease caused by drug-resistant cytomegalovirus. Antiviral Res. 2014;101:57-61. [DOI] [PubMed] [Google Scholar]
- 145.Shapira MY, Resnick IB, Chou S, et al. Artesunate as a potent antiviral agent in a patient with late drug-resistant cytomegalovirus infection after hematopoietic stem cell transplantation. Clin Infect Dis. 2008;46(9):1455-1457. [DOI] [PubMed] [Google Scholar]
- 146.Bethell D, Se Y, Lon C, et al. Dose-dependent risk of neutropenia after 7-day courses of artesunate monotherapy in Cambodian patients with acute Plasmodium falciparum malaria. Clin Infect Dis. 2010;51(12):e105-e114. [DOI] [PubMed] [Google Scholar]
- 147.Marty FM, Bryar J, Browne SK, et al. Sirolimus-based graft-versus-host disease prophylaxis protects against cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation: a cohort analysis. Blood. 2007;110(2):490-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Johnson RA, Wang X, Ma XL, Huong SM, Huang ES. Human cytomegalovirus up-regulates the phosphatidylinositol 3-kinase (PI3-K) pathway: inhibition of PI3-K activity inhibits viral replication and virus-induced signaling. J Virol. 2001;75(13):6022-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kudchodkar SB, Yu Y, Maguire TG, Alwine JC. Human cytomegalovirus infection induces rapamycin-insensitive phosphorylation of downstream effectors of mTOR kinase. J Virol. 2004;78(20):11030-11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Macagno A, Bernasconi NL, Vanzetta F, et al. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol. 2010;84(2):1005-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dole K, Segal FP, Feire A, et al. A first-in-human study to assess the safety and pharmacokinetics of monoclonal antibodies against human cytomegalovirus in healthy volunteers. Antimicrob Agents Chemother. 2016;60(5):2881-2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Abraham B, Lastere S, Reynes J, Bibollet-Ruche F, Vidal N, Segondy M. Ganciclovir resistance and UL97 gene mutations in cytomegalovirus blood isolates from patients with AIDS treated with ganciclovir. J Clin Virol. 1999;13(3):141-148. [DOI] [PubMed] [Google Scholar]
- 153.Boutolleau D, Deback C, Bressollette-Bodin C, et al. Genetic analysis and putative role in resistance to antivirals of the human cytomegalovirus DNA polymerase UL44 processivity factor. Antivir Ther. 2009;14(6):847-852. [DOI] [PubMed] [Google Scholar]
- 154.Chou S, Waldemer RH, Senters AE, et al. Cytomegalovirus UL97 phosphotransferase mutations that affect susceptibility to ganciclovir. J Infect Dis. 2002;185(2):162-169. [DOI] [PubMed] [Google Scholar]
- 155.Lurain NS, Bhorade SM, Pursell KJ, et al. Analysis and characterization of antiviral drug-resistant cytomegalovirus isolates from solid organ transplant recipients. J Infect Dis. 2002;186(6):760-768. [DOI] [PubMed] [Google Scholar]
- 156.Wolf DG, Smith IL, Lee DJ, Freeman WR, Flores-Aguilar M, Spector SA. Mutations in human cytomegalovirus UL97 gene confer clinical resistance to ganciclovir and can be detected directly in patient plasma. J Clin Invest. 1995;95(1):257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lurain NS, Spafford LE, Thompson KD. Mutation in the UL97 open reading frame of human cytomegalovirus strains resistant to ganciclovir. J Virol. 1994;68(7):4427-4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Baldanti F, Underwood MR, Stanat SC, et al. Single amino acid changes in the DNA polymerase confer foscarnet resistance and slow-growth phenotype, while mutations in the UL97-encoded phosphotransferase confer ganciclovir resistance in three double-resistant human cytomegalovirus strains recovered from patients with AIDS. J Virol. 1996;70(3):1390-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lurain NS, Weinberg A, Crumpacker CS, Chou S; Adult AIDS Clinical Trials Group-CMV Laboratories. Sequencing of cytomegalovirus UL97 gene for genotypic antiviral resistance testing. Antimicrob Agents Chemother. 2001;45(10):2775-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Castor J, Cook L, Corey L, Jerome KR. Rapid detection directly from patient serum samples of human cytomegalovirus UL97 mutations conferring ganciclovir resistance. J Clin Microbiol. 2007;45(8):2681-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Erice A. Resistance of human cytomegalovirus to antiviral drugs. Clin Microbiol Rev. 1999;12(2):286-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gilbert C, Boivin G. New reporter cell line to evaluate the sequential emergence of multiple human cytomegalovirus mutations during in vitro drug exposure. Antimicrob Agents Chemother. 2005;49(12):4860-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mousavi-Jazi M, Schloss L, Drew WL, et al. Variations in the cytomegalovirus DNA polymerase and phosphotransferase genes in relation to foscarnet and ganciclovir sensitivity. J Clin Virol. 2001;23(1-2):1-15. [DOI] [PubMed] [Google Scholar]
- 164.Boivin G, Gilbert C, Gaudreau A, Greenfield I, Sudlow R, Roberts NA. Rate of emergence of cytomegalovirus (CMV) mutations in leukocytes of patients with acquired immunodeficiency syndrome who are receiving valganciclovir as induction and maintenance therapy for CMV retinitis. J Infect Dis. 2001;184(12):1598-1602. [DOI] [PubMed] [Google Scholar]
- 165.Jabs DA, Martin BK, Forman MS, et al. ; Cytomegalovirus Retinitis and Viral Resistance Study Group. Longitudinal observations on mutations conferring ganciclovir resistance in patients with acquired immunodeficiency syndrome and cytomegalovirus retinitis: The Cytomegalovirus and Viral Resistance Study Group Report Number 8. Am J Ophthalmol. 2001;132(5):700-710. [DOI] [PubMed] [Google Scholar]
- 166.Limaye AP, Corey L, Koelle DM, Davis CL, Boeckh M. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet. 2000;356(9230):645-649. [DOI] [PubMed] [Google Scholar]
- 167.Marfori JE, Exner MM, Marousek GI, Chou S, Drew WL. Development of new cytomegalovirus UL97 and DNA polymerase mutations conferring drug resistance after valganciclovir therapy in allogeneic stem cell recipients. J Clin Virol. 2007;38(2):120-125. [DOI] [PubMed] [Google Scholar]
- 168.Mousavi-Jazi M, Schloss L, Wahren B, Brytting M. Point mutations induced by foscarnet (PFA) in the human cytomegalovirus DNA polymerase. J Clin Virol. 2003;26(3):301-306. [DOI] [PubMed] [Google Scholar]
- 169.Kuo IC, Imai Y, Shum C, Martin DF, Kuppermann BD, Margolis TP. Genotypic analysis of cytomegalovirus retinitis poorly responsive to intravenous ganciclovir but responsive to the ganciclovir implant. Am J Ophthalmol. 2003;135(1):20-25. [DOI] [PubMed] [Google Scholar]
- 170.Hanson MN, Preheim LC, Chou S, Talarico CL, Biron KK, Erice A. Novel mutation in the UL97 gene of a clinical cytomegalovirus strain conferring resistance to ganciclovir. Antimicrob Agents Chemother. 1995;39(5):1204-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Limaye AP. Ganciclovir-resistant cytomegalovirus in organ transplant recipients. Clin Infect Dis. 2002;35(7):866-872. [DOI] [PubMed] [Google Scholar]
- 172.Cherrington JM, Fuller MD, Lamy PD, et al. In vitro antiviral susceptibilities of isolates from cytomegalovirus retinitis patients receiving first- or second-line cidofovir therapy: relationship to clinical outcome. J Infect Dis. 1998;178(6):1821-1825. [DOI] [PubMed] [Google Scholar]
- 173.Springer KL, Chou S, Li S, et al. How evolution of mutations conferring drug resistance affects viral dynamics and clinical outcomes of cytomegalovirus-infected hematopoietic cell transplant recipients. J Clin Microbiol. 2005;43(1):208-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chou S. Recombinant phenotyping of cytomegalovirus UL97 kinase sequence variants for ganciclovir resistance. Antimicrob Agents Chemother. 2010;54(6):2371-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gilbert C, Bestman-Smith J, Boivin G. Resistance of herpesviruses to antiviral drugs: clinical impacts and molecular mechanisms. Drug Resist Updat. 2002;5(2):88-114. [DOI] [PubMed] [Google Scholar]