Abstract
Background:
Menopausal hormone therapy (MHT) has been proven to have beneficial effects on several components of metabolic syndrome. However, the effects vary according to different regimens, dosages, and duration of MHT. The aim of the study was to evaluate the effect of standard-dose 0.625 mg conjugated equine estrogen (CEE) and half-dose 0.3 mg CEE daily with different progestogens in a continuous sequential regimen on postmenopausal metabolic parameters in generally healthy postmenopausal women.
Methods:
A prospective, open-label, randomized controlled clinical trial was conducted between February 2014 and December 2015. Totally 123 Chinese postmenopausal women with climacteric symptoms were included in this study and were randomly assigned to three groups: Group A received CEE 0.3 mg/micronized progesterone (MP) 100 mg daily; Group B received CEE 0.625 mg/MP 100 mg daily; and Group C received CEE 0.625 mg/dydrogesterone 10 mg daily. Drugs were given in a continuous sequential pattern. The duration of treatment was 12 months. Clinical, anthropometrical, and metabolic variables were measured. Data were analyzed according to intention-to-treat analysis, using Student's t-test and analysis of variance.
Results:
A total of 107 participants completed the 12-month follow-up and were included in the data analysis. At 12 months of treatment, high-density lipoprotein cholesterol and apolipoprotein A significantly increased, and low-density lipoprotein cholesterol, fasting glucose, and glycosylated hemoglobin significantly decreased in Groups B and C, compared with baseline (all P < 0.05). Among the three groups, only Group C showed significantly increased triglycerides compared with baseline (1.61 ± 0.80 mmol/L vs. 1.21 ± 0.52 mmol/L, P = 0.026). Each group showed a neutral effect on total cholesterol, lipoprotein A, apolipoprotein B, and fasting insulin levels. No cardiovascular and venous thromboembolic events occurred in the three groups.
Conclusions:
Among Chinese postmenopausal women, half-dose CEE was not sufficient to induce a favorable lipid and carbohydrate profile compared with standard-dose CEE. Adding natural MP may counterbalance the TG-increasing effect of CEE.
Trial Registration:
ClinicalTrials.gov, NCT01698164; https://clinicaltrials.gov/ct2/show/NCT01698164?term=NCT01698164&rank=1.
Keywords: Chinese Postmenopausal Women, Conjugated Equine Estrogen, Dydrogesterone, Menopausal Hormone Therapy, Micronized Progesterone
Introduction
Menopause is characterized by a decline of endogenous estrogen that is associated with vasomotor symptoms, vaginal dryness, osteoporosis, and disturbed metabolism. Postmenopausal changes in lipid and glucose metabolism may increase the risk of developing metabolic syndrome (MetS).[1] The concept of MetS was first described in 1988 by Reaven[2] as a constellation of risk factors including visceral adiposity, atherogenic dyslipidemia, elevated blood pressure (BP), and insulin resistance (IR) that increase the risk of developing cardiovascular disease (CVD). Menopause is associated with all components of MetS. As the average life expectancy has increased during the past decades, women will spend an increasing duration in menopause. MetS will likely increase dramatically in women around the world.[3]
Menopausal hormone therapy (MHT) was originally prescribed primarily to treat vasomotor symptoms, but had been increasingly viewed as a way to forestall many chronic diseases of aging, including MetS and osteoporosis.[4] Regarding MetS, orally administered conjugated equine estrogens (CEEs) or estradiol alone has been shown to lower low-density lipoprotein cholesterol (LDL-C) and total cholesterol (TC) and to increase high-density lipoprotein cholesterol (HDL-C); however, CEE and estradiol were also associated with increases in triglycerides (TG).[5] The addition of progestogens may, however, affect some of the beneficial effects of estrogen, which may be dependent on the dosage and structure of the progestogens. Heterogeneity exists in the lipid profile response to MHT, which may be due to the differences in MHT composition, doses, dosing regimen, route of administration, compliance, etc.
Several large randomized controlled trials have explored the effect of MHT on the components of MetS.[6,7,8,9,10] The durations of the studies were long with a span of 2–5.2 years. They consistently found beneficial effects of MHT on metabolic homeostasis. However, the estrogens used in these studies were all standard-dose CEE or estradiol, and the progestogens were medroxyprogesterone acetate (MPA) or norethisterone acetate, which suppressed the favorable effects of estrogen than natural progesterone. In our study, standard- and half-dose CEEs were chosen to compare their effect on the components of MetS. In addition, we chose natural micronized progesterone (MP) and dydrogesterone, which lack androgenic effect and had less unfavorable effect on metabolic homeostasis, to explore the beneficial effect of CEE on the components of MetS.
Methods
Study design
This was a prospective, open-label, randomized controlled clinical trial conducted at Peking Union Medical College Hospital (PUMCH) in China, between February 2014 and December 2015. Participants were all apparently healthy postmenopausal women with intact uteri seeking treatment for menopausal symptoms. They were aged between 40 and 60 years and experienced spontaneous amenorrhea for ≥6 months and ≤5 years with serum follicle-stimulating hormone levels >40 mU/ml and serum estradiol <30 pg/ml. They were required to have an indication for MHT, which was evaluated by the investigator. No contraindication for MHT was also included in the inclusion criteria. Exclusion criteria were any of the following conditions: a uterine myoma with a diameter >3 cm; endometriosis with obvious symptoms and signs; uncontrolled diabetes and severe hypertension; thromboembolic disease history or inclination for thromboembolic diseases; epilepsy; asthma; hyperprolactinemia; a history of breast cancer among the first-degree relatives; and presence of clinically significant disease. Women who abused alcohol or drugs within 3 months of enrollment and those who smoked above 20 cigarettes per day were also excluded from the study. Other exclusion criteria were use of estrogen/progestin products within 3 months; a endometrial thickness of ≥5 mm even after progestin withdrawal; and allergic to any ingredient of the drug. The study was approved by the Ethics Committee of PUMCH (No. S-648, dated February 20, 2014) and conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guideline E6: Good Clinical Practice. All participants provided written informed consent before the study entry.
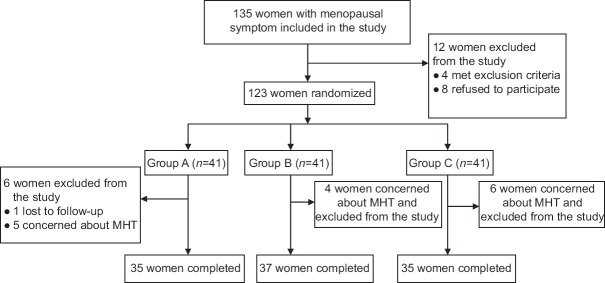
During consultation, all participants underwent a thorough clinical evaluation, including physical, breast, gynecological examinations, laboratory evaluations, transvaginal ultrasonography, mammography, and dual-energy X-ray absorptiometry (DEXA). All participants were given a number according to their order of inclusion in the study. Randomization process was conducted using IBM SPSS Statistics version 20 (IBM, Armonk, NY, USA) of the study protocol. The women were randomly assigned to three groups: Group A (n = 41) received 0.3 mg CEE (Xinjiang Xinziyuan Pharmaceutical Co., Ltd., China) and 100 mg MP (Zhejiang Xianju Pharmaceutical Co., Ltd., China) daily; Group B (n = 41) received 0.625 mg CEE and 100 mg MP daily; and Group C (n = 41) received 0.625 mg CEE and 10 mg dydrogesterone (Solvay Pharmaceuticals, Inc., France) daily. All drugs were given orally. The CEE was given once daily for consecutive 28 days with progestogen added once daily at the 17th day of CEE use for 12 days. The following cycle would begin without discontinuation of drugs. All drugs were required to be taken before sleep at night to avoid side effects such as dizziness brought by taking progesterone. The participants who received supplementation were instructed to return with capsule containers at each visit to record the medication that was not used and to establish compliance. Duration of the treatment was 12 cycles. The assessments were performed at 0 (baseline) and 12 months. Figure 1 shows flowchart of this study.
Figure 1.
Flow diagram of this study. Group A: Received CEE 0.3 mg + MP 100 mg daily; Group B: Received CEE 0.625 mg + MP 100 mg daily; Group C: Received CEE 0.625 mg + dydrogesterone 10 mg daily. MHT: Menopausal hormone therapy; CEE: Conjugated equine estrogen; MP: Micronized progesterone.
Assessments
The anthropometric data included weight, height, body mass index (BMI = weight/height2), and waist and hip circumference. Waist circumference (WC) was measured at the midpoint between the lowest rib and the top of the iliac crest. The hip circumference was measured at the maximum circumference over the buttocks. These measurements for the anthropometric data and BPs were performed by a single evaluator. DEXA instrument (Lunar Prodigy, GE®, USA) was used to measure body composition, and the examination was performed by a single examiner. Blood samples were collected from each participant after 12 h of fasting at baseline and at 12 months. After centrifugation to remove the clot, sera underwent biochemical analysis immediately. Lipid parameters and fasting glucose were processed by an automated analyzer, AU5800® (Beckman Coulter, USA). Insulin was quantified using the ADVIA Centaur XP® (Siemens®, Germany), which uses chemiluminescent immunoassay. To evaluate IR, we used a method that was based on statistical measurement of two plasma components: insulin and fasting glucose. Homeostasis model assessment-IR (HOMA-IR) was calculated using the following formula: insulin (mU/ml) × fasting glucose (mg/L)/405. IR was defined as HOMA-IR >2.7.[11]
Statistical analysis
Data were analyzed according to intention-to-treat analysis, including all participants in each group. The quantitative variables were shown as mean ± standard deviation (SD), and qualitative variables were presented as frequencies and percentages. In case of quantitative variables, Student's t-test was used. In case of quantitative variables where data were not normally distributed, Kruskal-Wallis test and Wilcoxon Mann-Whitney test were used. Analysis of variance was used to explore the effect of drugs across various groups in case of continuous variables. A P < 0.05 was considered statistically significant. Analyses were performed using the IBM SPSS statistics version 20 (IBM, Armonk, NY, USA).
Results
Baseline characteristics of all participants
Of all women screened, 123 women were randomized. During 12 months of follow-up, no one was withdrawn due to intolerable side effects, but 15 women were withdrawn due to concerns about MHT and one due to lost to follow-up. Finally, 107 participants completed the 12-month follow-up and were included in the data analysis. They were treated with CEE 0.3 mg/MP 100 mg (n = 35, Group A), CEE 0.625 mg/MP 100 mg (n = 37, Group B), or CEE 0.625 mg/dydrogesterone 10 mg (n = 35, Group C). The overall rate of discontinuation among the three groups was similar (14.6%, 9.8%, and 14.6%, respectively). A comparison of baseline characteristics of postmenopausal women among the three groups is presented in Table 1. No significant difference was found among and between groups for all variables (all P > 0.05).
Table 1.
Baseline characteristics of postmenopausal women among the three groups in this study
| Characteristics | Group A (n = 35) | Group B (n = 37) | Group C (n = 35) | F | P |
|---|---|---|---|---|---|
| Age (years) | 53.7 ± 4.2 | 53.1 ± 3.1 | 53.4 ± 4.5 | 0.277 | 0.827 |
| Age at menopause (years) | 49.4 ± 3.7 | 49.3 ± 2.8 | 49.4 ± 4.0 | 0.095 | 0.980 |
| Length of menopause (years) | 4.4 ± 1.5 | 3.9 ± 1.3 | 4.0 ± 1.3 | 0.941 | 0.394 |
| Systolic BP (mmHg) | 117.2 ± 13.4 | 111.3 ± 9.6 | 110.1 ± 11.3 | 2.549 | 0.085 |
| Diastolic BP (mmHg) | 73.6 ± 8.6 | 69.1 ± 7.8 | 69.3 ± 7.9 | 2.604 | 0.081 |
| BMI (kg/m2) | 23.6 ± 3.1 | 22.8 ± 3.0 | 23.6 ± 2.3 | 0.721 | 0.484 |
| WC (cm) | 78.9 ± 8.4 | 75.9 ± 7.1 | 77.5 ± 7.5 | 0.941 | 0.395 |
| Waist-hip ratio | 0.77 ± 0.05 | 0.75 ± 0.06 | 0.79 ± 0.05 | 0.935 | 0.390 |
| TFM (g) | 17,441 ± 1779 | 18,215 ± 2238 | 17,608 ± 1641 | 0.337 | 0.715 |
| TC (mmol/L) | 5.22 ± 0.77 | 5.26 ± 0.63 | 5.30 ± 1.36 | 0.975 | 0.377 |
| Triglycerides (mmol/L) | 1.43 ± 0.73 | 1.20 ± 0.73 | 1.21 ± 0.52 | 0.652 | 0.570 |
| HDL-C (mmol/L) | 1.52 ± 0.28 | 1.48 ± 0.32 | 1.42 ± 0.35 | 0.785 | 0.468 |
| LDL-C (mmol/L) | 3.24 ± 0.80 | 3.33 ± 0.69 | 3.49 ± 1.20 | 0.562 | 0.572 |
| Apolipoprotein A (g/L) | 1.51 ± 0.12 | 1.49 ± 0.14 | 1.44 ± 0.17 | 2.347 | 0.115 |
| Apolipoprotein B (g/L) | 0.98 ± 0.22 | 1.00 ± 0.15 | 0.98 ± 0.17 | 0.574 | 0.566 |
| Fasting glucose (mmol/L) | 5.10 ± 0.37 | 5.65 ± 0.90 | 5.44 ± 0.67 | 2.824 | 0.078 |
| HbA1c (%) | 5.56 ± 0.32 | 5.69 ± 0.68 | 5.61 ± 0.40 | 0.244 | 0.812 |
| Insulin (mU/ml) | 7.96 ± 4.41 | 8.49 ± 3.05 | 9.49 ± 4.63 | 1.012 | 0.320 |
| HOMA-IR | 1.97 ± 0.93 | 2.17 ± 0.98 | 2.37 ± 1.35 | 0.560 | 0.573 |
Data are given as mean ± SD. Group A: Received CEE 0.3 mg + MP 100 mg daily; Group B: Received CEE 0.625 mg + MP 100 mg daily; Group C: Received CEE 0.625 mg + dydrogesterone 10 mg daily. CEE: Conjugated equine estrogen; MP: Micronized progesterone; HOMA-IR: Homeostasis model assessment-insulin resistance; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol; HbA1c: Glycosylated hemoglobin; SD: Standard deviation; WC: Waist circumference; BP: Blood pressure; TC: Total cholesterol; TFM: Total fat mass; BMI: Body mass index.
Lipid and carbohydrate parameters
The comparison of lipid and carbohydrate parameters between baseline and 12 months after treatment is presented in Table 2. The Groups B and C (receiving standard-dose CEE) showed a significant increase in HDL-C level at 12 months after treatment (P = 0.033 in Group B; P = 0.006 in Group C) while HDL-C level in Group A (the half-dose CEE) did not significantly changed (P = 0.519), compared with baseline. The mean increases of HDL-C levels in Groups B and C were similar (P = 0.679) and were significantly more than that in Group A (P = 0.012 and P = 0.024, respectively). The LDL-C levels were decreased at 12 months after treatment in Groups B and C by 9.6% and 12.9%, respectively, compared with baseline (P = 0.039 in Group B; P = 0.029 in Group C), without significant difference between the two groups (P = 0.524). In Group A, no significant change was observed in LDL-C level compared with baseline (P = 0.426). The change of LDL-C level in Group B and Group C was significantly higher than that in Group A (P = 0.026 and P = 0.014, respectively). TC levels in each group were all decreased but without significant difference compared with baseline (all P > 0.05).
Table 2.
Lipid and carbohydrate parameters at baseline and 12 months after treatment in the three groups
| Parameters | Baseline | 12 months after treatment | Absolute change | Statistical values | P |
|---|---|---|---|---|---|
| TC (mmol/L) | |||||
| Group A | 5.22 ± 0.77 | 5.19 ± 1.05 | −0.03 (−2.5) | −0.112* | 0.906 |
| Group B | 5.26 ± 0.63 | 5.22 ± 0.89 | −0.04 (−2.2) | −0.194* | 0.848 |
| Group C | 5.30 ± 1.36 | 5.28 ± 0.77 | −0.02 (−3.0) | −0.060* | 0.953 |
| Triglycerides (mmol/L) | |||||
| Group A | 1.43 ± 0.73 | 1.40 ± 0.98 | −0.03 (−2.1) | −0.112* | 0.912 |
| Group B | 1.20 ± 0.73 | 1.56 ± 0.97 | 0.36 (30.0) | 1.529* | 0.140 |
| Group C | 1.21 ± 0.52 | 1.61 ± 0.80 | 0.40 (33.1) | 2.387* | 0.026 |
| HDL-C (mmol/L) | |||||
| Group A | 1.52 ± 0.28 | 1.56 ± 0.24 | 0.04 (2.6) | 0.654* | 0.519 |
| Group B | 1.48 ± 0.32 | 1.66 ± 0.32 | 0.18 (12.2) | 2.263* | 0.033 |
| Group C | 1.42 ± 0.35 | 1.65 ± 0.35 | 0.23 (16.2) | 3.0278 | 0.006 |
| LDL-C (mmol/L) | |||||
| Group A | 3.24 ± 0.80 | 3.08 ± 0.70 | −0.16 (−4.9) | −0.811* | 0.426 |
| Group B | 3.33 ± 0.69 | 3.01 ± 0.75 | −0.32 (−9.6) | −2.215* | 0.039 |
| Group C | 3.49 ± 1.20 | 3.04 ± 0.77 | −0.45 (−12.9) | −2.308* | 0.029 |
| Apolipoprotein A (g/L) | |||||
| Group A | 1.51 ± 0.12 | 1.52 ± 0.17 | 0.01 (0.7) | 0.440* | 0.664 |
| Group B | 1.49 ± 0.14 | 1.63 ± 0.20 | 0.14 (9.4) | 3.030* | 0.006 |
| Group C | 1.44 ± 0.17 | 1.63 ± 0.18 | 0.19 (13.2) | 5.529* | <0.001 |
| Apolipoprotein B (g/L) | |||||
| Group A | 0.98 ± 0.22 | 1.11 ± 0.41 | 0.13 (13.3) | 2.583* | 0.162 |
| Group B | 1.00 ± 0.15 | 0.97 ± 0.20 | −0.03 (−3.0) | −0.404* | 0.690 |
| Group C | 0.98 ± 0.17 | 0.98 ± 0.18 | 0 (0.0) | 0.008* | 0.994 |
| Fasting glucose (mmol/L) | |||||
| Group A | 5.10 ± 0.37 | 5.36 ± 0.67 | 0.26 (5.1) | 1.698* | 0.103 |
| Group B | 5.65 ± 0.90 | 4.97 ± 0.60 | −0.68 (−12.0) | −3.323* | 0.003 |
| Group C | 5.44 ± 0.67 | 4.91 ± 0.85 | −0.53 (−9.7) | −3.027* | 0.006 |
| HbA1c (%) | |||||
| Group A | 5.56 ± 0.32 | 5.61 ± 0.39 | 0.05 (0.9) | 0.467* | 0.645 |
| Group B | 5.69 ± 0.68 | 5.35 ± 0.40 | −0.34 (−5.1) | −2.220* | 0.037 |
| Group C | 5.61 ± 0.40 | 5.35 ± 0.43 | −0.26 (−4.6) | −2.731* | 0.012 |
| Insulin (mU/ml) | |||||
| Group A | 7.96 ± 4.41 | 8.97 ± 4.24 | 1.01 (12.7) | 0.862* | 0.399 |
| Group B | 8.49 ± 3.05 | 7.37 ± 4.21 | −1.12 (−13.2) | −1.109* | 0.279 |
| Group C | 9.49 ± 4.63 | 8.60 ± 4.10 | −0.89 (−9.4) | −0.773* | 0.448 |
| HOME-IR | |||||
| Group A | 1.97 ± 0.93 | 2.23 ± 1.06 | 0.26 (13.2) | 0.918† | 0.378 |
| Group B | 2.17 ± 0.98 | 1.66 ± 1.06 | −0.51 (−23.5) | −1.988† | 0.072 |
| Group C | 2.37 ± 1.35 | 1.96 ± 1.16 | −0.41 (17.3) | −1.244† | 0.226 |
Data are given as mean ± SD, or different value (%). *Student’s t-test; †Kruskal-Wallis test and Wilcoxon Mann-Whitney test. Group A (n = 35): Received CEE 0.3 mg + MP 100 mg daily; Group B (n = 37): Received CEE 0.625 mg + MP 100 mg daily; Group C (n = 35): Received CEE 0.625 mg + dydrogesterone 10 mg daily. CEE: Conjugated equine estrogen; MP: Micronized progesterone; HOMA-IR: Homeostasis model assessment-insulin resistance; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol; HbA1c: Glycosylated hemoglobin; SD: Standard deviation; TC: Total cholesterol.
Triglyceride level in Group C increased significantly by 33.1% at 12-month treatment compared with baseline (P = 0.026), and the significant difference was found in triglyceride level at 12 months after treatment between Groups A and C (P < 0.001); although triglyceride level in Group B also increased by 30.0% at 12 months after treatment compared with baseline, but the difference was not significant (P = 0.140). Moreover, the elevations in Groups B and C were also comparable (P = 0.342).
The levels of apolipoprotein A at 12 months after treatment in Groups B and C significantly increased by 9.4% and 13.2% compared with baseline and were significantly more than that of Group A (P = 0.005 and P = 0.002, respectively). Apolipoprotein B levels of the three groups did not change significantly at 12 months after treatment compared with baseline and were comparable among the three groups (all P > 0.05).
The levels of fasting glucose decreased significantly in both Group B (by 12.0%, P = 0.003) and Group C (by 9.7%, P = 0.006) at 12 months after treatment compared with baseline, and no significant difference was found between these two groups (P = 0.573). In Group A, the level of fasting glucose increased slightly compared with baseline, but without a significant difference (P = 0.103). Compared with baseline, levels of glycosylated hemoglobin (HbA1c) decreased significantly in Groups B and C (P = 0.037 and P = 0.012, respectively) and increased slightly in Group A (P = 0.645) at 12 months after treatment. The levels of fasting insulin in three groups did not reach significance at 12 months after treatment compared with baseline and were comparable among the three groups (all P > 0.05), as was also the case in HOMA-IR.
Body composition and blood pressure
After 12-month treatment, no significant increases in BMI were found in any of the three groups compared with baseline, so as to WC and total fat mass. Waist-hip ratio decreased significantly in Group C at 12 months after treatment compared with baseline (P = 0.004), but there were no significant differences in waist-hip ratio at 12 months between Group C and other two groups (all P > 0.05). There were no significant differences in systolic BP among the three groups. A significant decrease in diastolic BP compared with baseline was found in Group A [P = 0.007; Table 3].
Table 3.
Body composition parameters and BP at baseline and 12 months after treatment in the three groups
| Parameters | Baseline | 12 months after treatment | Absolute change | Statistical values | P |
|---|---|---|---|---|---|
| BMI (kg/m2) | |||||
| Group A | 23.6 ± 3.1 | 23.8 ± 3.2 | 0.2 (0.8) | 0.167 | 0.866 |
| Group B | 22.8 ± 3.0 | 22.8 ± 2.8 | 0 (0.0) | 0.316 | 0.758 |
| Group C | 23.6 ± 2.3 | 23.5 ± 2.3 | 0.1 (0.4) | 0.098 | 0.923 |
| WC (cm) | |||||
| Group A | 78.9 ± 8.4 | 81.4 ± 6.3 | 2.5 (3.2) | 0.997 | 0.319 |
| Group B | 75.9 ± 7.1 | 79.6 ± 8.2 | 3.7 (4.9) | 0.605 | 0.579 |
| Group C | 77.5 ± 7.5 | 80.1 ± 7.1 | 2.6 (3.4) | 0.167 | 0.878 |
| Waist-hip ratio | |||||
| Group A | 0.77 ± 0.05 | 0.75 ± 0.05 | −0.02 (−2.6) | −1.307 | 0.194 |
| Group B | 0.75 ± 0.06 | 0.72 ± 0.05 | −0.03 (−4.0) | −1.978 | 0.067 |
| Group C | 0.79 ± 0.05 | 0.76 ± 0.04 | −0.03 (−3.8) | −3.212 | 0.004 |
| TFM (g) | |||||
| Group A | 17,441 ± 1779 | 18,209 ± 1885 | 768 (4.4) | 1.529 | 0.140 |
| Group B | 18,215 ± 2238 | 17,167 ± 1883 | −1048 (−5.8) | −1.817 | 0.092 |
| Group C | 17,608 ± 1641 | 18,270 ± 2115 | 662 (3.8) | 1.244 | 0.223 |
| Systolic BP (mmHg) | |||||
| Group A | 117.2 ± 13.4 | 119.0 ± 10.4 | 1.8 (1.5) | 0.928 | 0.366 |
| Group B | 111.3 ± 9.6 | 111.7 ± 12.4 | 0.4 (0.4) | 0.135 | 0.894 |
| Group C | 110.1 ± 11.3 | 109.3 ± 12.4 | −0.8 (0.7) | 0.322 | 0.750 |
| Diastolic BP (mmHg) | |||||
| Group A | 73.6 ± 8.6 | 67.2 ± 6.5 | −6.4 (8.7) | −2.926 | 0.007 |
| Group B | 69.1 ± 7.8 | 70.7 ± 8.3 | 1.6 (2.3) | 0.645 | 0.525 |
| Group C | 69.3 ± 7.9 | 66.1 ± 7.6 | −3.2 (4.5) | −1.536 | 0.133 |
Data are given as mean ± SD, or different value (%). Group A (n = 35): Received CEE 0.3 mg + MP 100 mg daily; Group B (n = 37): Received CEE 0.625 mg + MP 100 mg daily; Group C (n = 35): Received CEE 0.625 mg + dydrogesterone 10 mg daily. CEE: Conjugated equine estrogen; MP: Micronized progesterone; BMI: Body mass index; WC: Waist circumference; BP: Blood pressure; TFM: Total fat mass; SD: Standard deviation.
Discussion
Our study demonstrated that the effect of standard-dose CEE supplementation was better than that of half-dose CEE on the parameters of MetS components among generally healthy postmenopausal women in China. Adding natural MP will not increase triglyceride levels compared with adding dydrogesterone after 12-month continuous sequential MHT. The CEE 0.625 mg/MP 100 mg and CEE 0.625 mg/dydrogesterone 10 mg resulted in improvements of HDL-C, LDL-C, apolipoprotein A, fasting glucose, and HbA1c levels, compared with CEE 0.3 mg/MP 100 mg at 12 months of treatment. However, CEE 0.625 mg/dydrogesterone 10 mg increased the triglyceride levels compared with the other two groups. All the three groups had neutral effects on TC, apolipoprotein B, and fasting insulin levels.
The alterations of lipid profile during perimenopause and after menopause are toward a more atherogenic direction, with increased levels of LDL-C and TG and decreased levels of HDL-C although the mechanisms behind these changes are unknown. All large randomized controlled trials for MHT using CEE, including postmenopausal estrogen/progestin interventions (PEPI), Heart and Estrogen/Progestin Replacement Study (HERS), and Women's Health Initiative (WHI) trials, confirmed that MHT produced a reduction in LDL-C, an increase in HDL-C, and an increase in TG compared with placebo.[10,12,13] In the PEPI trial, the greatest increase in HDL-C was seen with oral administration of CEE alone and the HDL-C increase was attenuated in women treated with a combination of CEE and progestin (least with micronized progestin and most with MPA).[10] CEE 0.625 mg/d plus cyclic MP 200 mg/d 12 d/months for 3 years increased HDL-C by 0.11 mmol/L. The HERS trial (CEE 0.625 mg/MPA 2.5 mg) found a mean LDL-C decreased by 11%, mean HDL-C increased by 6%, and mean TG levels increased by 7%. Our study found that a 12-month continuous sequential oral intake of 0.625 mg CEE combined with 100 mg MP or 10 mg dydrogesterone daily could increase HDL-C by 12.2–16.2% and decrease LDL-C by 9.6–12.9%. Half-dosage CEE seemed not to make any improvement in lipid profile. Compared with other major trials, the better improvement of LDL-C and HDL-C in our trial attributable to the progestin part was MP and dydrogesterone or racial differences. Another interesting issue found in our trial was that adding MP to standard-dosage CEE seemed not to improve triglyceride levels (P = 0.140) although this might due to small sample size of our trial.
Our findings were consistent with the results of large randomized controlled trials and observational studies of MHT on apparently healthy postmenopausal women, which found decreased fasting glucose levels[10,14,15,16] and hemoglobin A1C levels,[17,18,19] but this only referred to standard-dosage CEE, not half-dosage CEE. In the PEPI trial, 875 younger postmenopausal women (45–64 years of age) were followed up for 3 years and a statistically significant decrease of 2–3% in fasting glucose level (P < 0.03) was found.[10] Our study found no significant change in fasting glucose in women taking 0.3 mg CEE daily continuously with sequential natural MP 100 mg daily for 12 days, but found a significant decrease in fasting glucose level for those who take 0.625 mg CEE daily (12% when MP added, P = 0.003; 9.7% when dydrogesterone added, P = 0.006). No significant difference was found in fasting glucose level after 12 months of treatment between Group B and Group C (P = 0.573). This suggested that the treatment benefit on fasting glucose level might be attributable to the estrogen component, but did not change when progestin was added.
Several studies have reported favorable effects of MHT on BMI, abdominal fat, and waist-hip ratio. Our study demonstrated that 12-month continuous sequential supplement of CEE 0.3 mg/MP 100 mg, CEE 0.625 mg/MP 100 mg, or CEE 0.625 mg/dydrogesterone 10 mg did not alter BMI, WC, and total fat mass (TFM). However, CEE 0.625 mg/dydrogesterone 10 mg could reduce waist-hip ratio slightly but significantly by 3.8% (P = 0.004). The WHI trial (CEE 0.625 mg/MPA 2.5 mg) showed a statistically significant decrease in BMI (−0.19 ± 0.04, P < 0.01) and WC (0.77 ± 0.10 cm, P < 0.01) during the 1st year of treatment.[20] The HERS (HERS; CE 0.625 mg/MPA 2.5 mg) also found that women receiving MHT experienced slight but significant weight loss (−0.5 kg), decreased BMI (−0.2 kg/m2), and decreased abdominal obesity (waist hip ratio: −0.01; WC: −0.8 cm) during follow-up, compared with placebo.[8] All women in the PEPI trial (CEE 0.625 mg/d + MPA 2.5 mg or MP 200 mg) gained weight, but the mean increase from baseline was smaller among women assigned to unopposed CEE at 3 years of follow-up.[10] The half-dose CEE group reduced diastolic pressure significantly by 8.7%. This might be due to the reduced protective effect of half-dose CEE compared with standard-dose CEE on elasticity of the vascular wall. And, this was supported by a pile of basic researches.[21,22,23,24]
MHT affects many metabolic processes that have a potential influence on CVD risk. The randomized controlled trials provided evidence that MHT products may differ substantially with regard to their impact on the components of MetS parameters, which depend on the estrogen and progestin used, as well as regimen, dosage, and routes of administration. The favorable effects of MHT on the components of MetS parameters may be in a CEE dose-dependent manner, which explained why standard-dosage CEE was effective in improving serum lipid and sugar parameters while half-dosage CEE was not in our trial. Adding MP or dydrogesterone has similar effects on biochemical markers of MetS, but adding 100 mg MP daily sequentially for 10 days may not improve TG level. Larger sample size is needed to further prove this phenomenon. Our study will follow the participants for the extended 12 months to further discover the 24-month effects of the three MHT regimens on MetS parameters.
In conclusion, among Chinese postmenopausal women, half-dose CEE was not sufficient to induce a favorable lipid and carbohydrate profile compared with standard-dose CEE. Adding natural MP may counterbalance the TG-increasing effect of CEE.
Financial support and sponsorship
This study was supported by a grant from National “Twelfth Five-Year” Plan for Science and Technology Support (No. 2014BAI10B10-1).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
References
- 1.Spencer CP, Godsland IF, Stevenson JC. Is there a menopausal metabolic syndrome? Gynecol Endocrinol. 1997;11:341–55. doi: 10.3109/09513599709152559. doi: 10.3109/09513599709152559. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Lovre D, Lindsey SH, Mauvais-Jarvis F. Effect of menopausal hormone therapy on components of the metabolic syndrome. Ther Adv Cardiovasc Dis. 2016 doi: 10.1177/1753944716649358. pii: 1753944716649358. [Epub ahead of print] doi: 10.1177/1753944716649358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manson JE. Current recommendations: What is the clinician to do? Fertil Steril. 2014;101:916–21. doi: 10.1016/j.fertnstert.2014.02.043. doi: 10.1016/j.fertnstert.2014.02.043. [DOI] [PubMed] [Google Scholar]
- 5.Godsland IF. Effects of postmenopausal hormone replacement therapy on lipid, lipoprotein, and apolipoprotein (a) concentrations: Analysis of studies published from 1974-2000. Fertil Steril. 2001;75:898–915. doi: 10.1016/s0015-0282(01)01699-5. doi: 10.1016/S0015-0282(01)01699-5. [DOI] [PubMed] [Google Scholar]
- 6.Lobo RA, Pinkerton JV, Gass ML, Dorin MH, Ronkin S, Pickar JH, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril. 2009;92:1025–38. doi: 10.1016/j.fertnstert.2009.03.113. doi: 10.1016/j.fertnstert.2009.03.113. [DOI] [PubMed] [Google Scholar]
- 7.Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: Results from the Women's Health Initiative Hormone Trial. Diabetologia. 2004;47:1175–87. doi: 10.1007/s00125-004-1448-x. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- 8.Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, et al. Glycemic effects of postmenopausal hormone therapy: The Heart and Estrogen/Progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- 9.Jensen LB, Vestergaard P, Hermann AP, Gram J, Eiken P, Abrahamsen B, et al. Hormone replacement therapy dissociates fat mass and bone mass, and tends to reduce weight gain in early postmenopausal women: A randomized controlled 5-year clinical trial of the Danish Osteoporosis Prevention Study. J Bone Miner Res. 2003;18:333–42. doi: 10.1359/jbmr.2003.18.2.333. doi: 10.1359/jbmr.2003.18.2.333. [DOI] [PubMed] [Google Scholar]
- 10.Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA. 1995;273:199–208. doi: 10.1001/jama.1995.03520270033028. [PubMed] [Google Scholar]
- 11.Geloneze B, Repetto EM, Geloneze SR, Tambascia MA, Ermetice MN. The threshold value for insulin resistance (HOMA-IR) in an admixtured population IR in the Brazilian metabolic syndrome study. Diabetes Res Clin Pract. 2006;72:219–20. doi: 10.1016/j.diabres.2005.10.017. doi: 10.1016/j.diabres.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 13.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women's health initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 14.Lobo RA, Pickar JH, Wild RA, Walsh B, Hirvonen E. Metabolic impact of adding medroxyprogesterone acetate to conjugated estrogen therapy in postmenopausal women. The Menopause Study Group. Obstet Gynecol. 1994;84:987–95. [PubMed] [Google Scholar]
- 15.Espeland MA, Hogan PE, Fineberg SE, Howard G, Schrott H, Waclawiw MA, et al. Effect of postmenopausal hormone therapy on glucose and insulin concentrations. PEPI Investigators. Postmenopausal Estrogen/Progestin Interventions. Diabetes Care. 1998;21:1589–95. doi: 10.2337/diacare.21.10.1589. doi: 10.2337/diacare.21.10.1589. [DOI] [PubMed] [Google Scholar]
- 16.Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–53. doi: 10.7326/0003-4819-135-11-200112040-00005. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 17.Sargeant LA, Wareham NJ, Khaw KT. Hormone replacement therapy and glucose tolerance in EPIC-Norfolk: A population-based study. Diabetes Metab Res Rev. 2000;16:20–5. doi: 10.1002/(sici)1520-7560(200001/02)16:1<20::aid-dmrr76>3.0.co;2-a. doi: 10.1002/(SICI)1520-7560(200001/02)16: 1<20::AID-DMRR76>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Howard BV, Cowan LD, Yeh J, Schaefer CF, Wild RA, et al. The effect of estrogen use on levels of glucose and insulin and the risk of type 2 diabetes in american Indian postmenopausal women: The strong heart study. Diabetes Care. 2002;25:500–4. doi: 10.2337/diacare.25.3.500. doi: 10.2337/diacare.25.3.500. [DOI] [PubMed] [Google Scholar]
- 19.Nabulsi AA, Folsom AR, White A, Patsch W, Heiss G, Wu KK, et al. Association of hormone-replacement therapy with various cardiovascular risk factors in postmenopausal women. The Atherosclerosis Risk in Communities Study Investigators. N Engl J Med. 1993;328:1069–75. doi: 10.1056/NEJM199304153281501. doi: 10.1056/NEJM199304153281501. [DOI] [PubMed] [Google Scholar]
- 20.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 21.Kong D, Zhan Y, Liu Z, Ding T, Li M, Yu H, et al. SIRT1-mediated ERβ suppression in the endothelium contributes to vascular aging. Aging Cell. 2016 doi: 10.1111/acel.12515. [Epub ahead of print] doi: 10.1111/acel.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Gou Y, Zhang H, Zuo H, Zhang H, Liu Z, et al. Estradiol improves cardiovascular function through up-regulation of SOD2 on vascular wall. Redox Biol. 2014;3:88–99. doi: 10.1016/j.redox.2014.11.001. doi: 10.1016/j.redox.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matrai M, Hetthéssy JR, Nadasy GL, Szekacs B, Mericli M, Acs N, et al. Estrogen therapy may counterbalance eutrophic remodeling of coronary arteries and increase bradykinin relaxation in a rat model of menopausal hypertension. Menopause. 2016;23:778–83. doi: 10.1097/GME.0000000000000654. doi: 10.1097/GME.0000000000000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarhouni K, Guihot AL, Vessieres E, Procaccio V, Grimaud L, Abraham P, et al. Estrogens are needed for the improvement in endothelium-mediated dilation induced by a chronic increase in blood flow in rat mesenteric arteries. Vascul Pharmacol. 2016;80:35–42. doi: 10.1016/j.vph.2015.10.004. doi: 10.1016/j.vph.2015.10.004. [DOI] [PubMed] [Google Scholar]