Abstract
Background:
Percutaneous coronary intervention (PCI) causes endothelial damage, resulting in an inflammatory response with elevation of markers such as high-sensitive C-reactive protein (hs-CRP) and vascular cell adhesion molecule-1 (VCAM-1), which are associated with restenosis after PCI. Evidence suggests that microRNA-126 (miR-126) plays an important role in vascular inflammation, but its correlation with PCI-mediated inflammation has not been investigated. In this study, we investigated the effect of PCI on circulating miR-126 and inflammation markers such as hs-CRP and VCAM-1.
Methods:
We enrolled 130 patients with coronary artery disease (CAD) in the Second Hospital of Jilin University from October 2015 to December 2015. Among them, 82 patients with CAD, defined as at least one major epicardial vessel with >70% stenosis who planned to undergo PCI, were divided into acute coronary syndrome (ACS) group (46 patients) and stable angina (SA) group (36 patients). Forty-eight patients confirmed by coronary angiography without PCI were used as controls. The plasmas of all patients were collected prior to PCI and at 30 min, 24 h, and 72 h after PCI. The plasma VCAM-1 and hs-CRP were detected by enzyme-linked immunosorbent assay, and the miR-126 was evaluated by quantitative reverse transcription-polymerase chain reaction.
Results:
Plasma concentrations of hs-CRP and VCAM-1 in patients with either ACS (n = 46) or SA (n = 36) were significantly higher than in controls (n = 48) (P < 0.01) prior to PCI, and increased further at 24 h and 72 h after PCI, compared with prior PCI. Moreover, VCAM-1 was positively correlated with balloon time and pressure. In contrast, the plasma concentration of miR-126 was significantly lower in patients with CAD than in controls, and further decreased with time post-PCI. A negative correlation was observed between miR-126 and hs-CRP and VCAM-1 at 72 h after PCI.
Conclusion:
There was a negative correlation of miR-126 with the PCI-induced markers of inflammation such as hs-CRP and VCAM-1.
Keywords: High-sensitivity C-reactive Protein, MicroRNA126, Percutaneous Coronary Intervention, Vascular Cell Adhesion Molecule-1
Introduction
Coronary artery disease (CAD) is a complex cardiovascular disease with a high mobility.[1] Percutaneous coronary intervention (PCI) is the most effective treatment for patients with CAD, but restenosis after PCI is one of the major limitations.[2] Chronic inflammation has been found to be related to the progression of CAD, and PCI is associated with vessel wall injuries resulting in the activation of an inflammatory process.[3] In addition, many studies have reported that inflammatory status prior to PCI and inflammatory response to PCI are also implicated in restenosis.[3] An inflammatory response, with elevation of markers such as high-sensitivity C-reactive protein (hs-CRP) and vascular cell adhesion molecule-1 (VCAM-1), has been observed in patients with acute coronary syndromes (ACSs) who are undergoing PCI, and the inflammation caused by PCI has been shown to be associated with restenosis.[4] However, the underlying mechanisms remain poorly understood.
MicroRNAs (miRNAs), a class of single-stranded noncoding RNA molecules approximately 22 nucleotides in length, are posttranscriptional modulators of gene expression in various physiological and pathological conditions including inflammation and cardiovascular disease.[5] Several lines of evidence highlight the role of miRNA-126 in the regulation of inflammation and vascular pathology.[6] miR-126 has been reported to inhibit vascular inflammation by targeting VCAM-1-mediated leukocyte infiltration.[7] In addition to inflammation, miR-126 also regulates vascular integrity and angiogenesis. miR-126 can promote the vascular endothelial cell repair and angiogenesis through upregulating VEGF expression, whereas deletion of miR-126 in mice or zebrafish causes vascular leakage.[8,9] However, the specific roles of miR-126 in inflammation following PCI have not yet been investigated.
In this study, we investigated the status of serum miR-126 and its correlation with the inflammatory markers such as hs-CRP and VCAM-1 in patients with CAD following PCI.
Methods
Patients
We enrolled 130 patients with CAD in the Second Hospital of Jilin University from October 2015 to December 2015. Among them, 82 patients with CAD, defined as at least one major epicardial vessel with >70% stenosis who planned to undergo PCI with stenting, were selected. The 82 CAD patients with PCI were divided into ACS group (46 patients) and stable angina (SA) group (36 patients). In addition, 48 patients admitted to the hospital because of chest pain, confirmed by coronary angiography who did not proceed to coronary stenting, were used as controls. Baseline venous blood was collected at the beginning of the angiography procedure. In the present study, the basic clinical characteristics of patients were quantified by testing the levels of total cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, and hs-CRP. Written consent was obtained from all patients, and the study protocol was approved by the Ethics Committee of Jilin University.
Percutaneous coronary intervention procedure
PCI was performed in the standard fashion and consisted of balloon angioplasty and stenting. In brief, patients receiving stents were treated with a combination of aspirin and clopidogrel for a week. A bolus of 10,000 U heparin was administered intravenously before the procedure. This was followed by an intravenous injection during the procedure to maintain an activated clotting time of 250 s. Balloon predilatation and stent implantation were performed according to the standard techniques. The use of drug-eluting stents was left to the physician's discretion. Procedural success was defined as optimal position of the stent, residual stenosis 30%, forward blood flow of thrombolysis in myocardial infarction Class 3, and no serious complications.
Plasma sampling
Repeat venous blood samples were collected 5 min prior to stent deployment and 30 min, 24 h, and 72 h after stent deployment. Patients who underwent diagnostic angiography but did not proceed to coronary stenting were included as controls and underwent serial blood sampling at the same time points. Blood samples were prepared immediately in the laboratory according to the following protocol: pyrogen-free blood collection tubes containing ethylenediaminetetraacetic acid and plasma were immediately immersed in melting ice and centrifuged within 20 min at 2500 ×g for 20 min to obtain platelet-poor plasma after coagulation. Plasma aliquots were stored at −80°C for subsequent analysis.
Plasma vascular cell adhesion molecule-1 and high-sensitivity C-reactive protein levels
Plasmas of all the 130 patients were collected at different time points following PCI. We used the human VCMA-1 and hs-CRP enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN, USA) to measure the plasma levels of VCMA-1, as per manufacturer's instruction. Absorbance was measured at 450 nm (primary wave length) (MRX, Dynatech Laboratories Inc., Chantilly, VA, USA). Concentrations of human VCMA-1 were determined by plotting the absorbance of each sample against a standard curve of recombinant human VCAM-1.
RNA isolation and real-time quantitative reverse transcription-polymerase chain reaction
For measuring microRNAs, miRNA was extracted from plasma samples using the miRcute miRNA isolation kit (Tiangen, Beijing City, China). The samples were performed both poly-(A) tailing and reverse transcription with the miScript reverse transcription kit (Tiangen, Beijing City, China). miR-126 was quantified by using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) assay according to the protocol of the manufacturer (Tiangen, Beijing City, China). Each reaction was performed in a total volume of 20 μl containing 2 μl RT products, 1 μl 20× microRNA-126 assay primer, 10 μl 2× Universal PCR Master Mix, and nuclease-free H2O to adjust the volume. The PCR reaction was performed as follows: 95°C for 5 min, followed by 40 cycles of 95°C for 15 s and 60°C for 30 s. qRT-PCR was performed using the 7500 FAST Real-time PCR System (Applied Biosystems, Naerum, Denmark). The RT forward primers (Sangon, Shanghai, China) used were (1) miR-126 forward: 5′-TCGTACCGTGAGTAATAATGC-3′ (2) U6 forward: 5′-GCTTCGGCAGCACATATACTAAAAT-3′. Analysis of relative gene expression levels was performed using the following formula: 2–ΔCT with ΔCT = CT (target gene) – CT (control).
Statistical analysis
Data were shown as mean ± standard deviation (SD) and median for the general characteristics of the patients. All statistical analyses were performed using the software SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Differences among groups were assessed using the one-way analysis of variance (ANOVA) test followed by post hoc test. Correlations of miR-126 with VCAM-1 or hs-CRP were analyzed with Spearman's correlation. A value of P < 0.05 was considered statistically significant.
Results
Basic clinical characteristics of patients
Patients with CAD were divided into ACS and SA groups [Table 1]. There were 46 patients (mean age: 57.2 ± 7.2 years, 23 males) in the ACS group, 36 patients (mean age: 56.7 ± 8.0, 22 males) in the SA group, and 48 patients (mean age: 54.2 ± 7.2, 29 males) in the control group. Patients showed no differences in diabetes, body mass index, blood urea nitrogen, creatinine, total cholesterol, LDL cholesterol, triglycerides, history of hypertension, diabetes, or smoking (P > 0.05 for all).
Table 1.
Basic clinical characteristics of patients
| Characteristics | ACS (n = 46) | SA (n = 36) | Control (n = 48) |
|---|---|---|---|
| Age, years | 57.2 ± 7.2 | 56.7 ± 8.0 | 54.2 ± 7.4 |
| Sex (n) | |||
| Male/female | 23/13 | 22/14 | 29/19 |
| BMI (kg/m2) | 25.10 ± 3.82 | 24.84 ± 3.61 | 24.72 ± 3.72 |
| BUN (mmol/L) | 5.98 ± 1.46 | 5.77 ± 1.51 | 5.45 ± 1.23 |
| Creatinine (µmol/L) | 80.3 ± 12.9 | 82.5 ± 11.7 | 81.7 ± 12.2 |
| LDL cholesterol (mmol/L) | 3.44 ± 1.25 | 3.31 ± 1.28 | 3.24 ± 1.31 |
| Triglyceride (mmol/L) | 1.94 ± 0.76 | 1.89 ± 0.69 | 1.82 ± 0.57 |
| Total cholesterol (mmol/L) | 4.61 ± 1.31 | 4.54 ± 1.37 | 4.42 ± 1.33 |
| Hypertension, n (%) | 13 (28.26) | 10 (27.78) | 11 (22.92) |
| Diabetes, n (%) | 12 (26.08) | 9 (25.00) | 5 (10.42) |
| Smoking, n (%) | 20 (43.47) | 23 (63.88) | 15 (31.25) |
All data were expressed as mean ± SD or n, n (%). Differences among groups were assessed using the one-way analysis of variance test. ACS: Acute coronary syndrome group; SA: Stable angina group; BMI: Body mass index; BUN: Blood urea nitrogen; LDL: Low-density lipoprotein.
Plasma high-sensitivity C-reactive protein, vascular cell adhesion molecule-1, and microRNA-126 in patients with coronary artery disease before and after percutaneous coronary intervention
To test the effect of PCI on inflammation and its correlation with miR-126, we tested the plasma levels of hs-CRP, VCAM-1, and miR-126, before and after PCI. Our results confirmed that baseline hs-CRP and VCAM-1 were significantly higher in the ACS and SA groups than in control group [Tables 2 and 3]. In addition, the baseline hs-CRP and VCAM-1 levels were significantly higher in the ACS group than in the SA group [Tables 2 and 3]. In contrast, the baseline miR-126 was significantly lower in the ACS and SA groups than in control group. Following PCI, the plasma levels of hs-CRP and VCAM-1 increased with time in the ACS and SA groups. Moreover, plasma hs-CRP and VCAM-1 levels were significantly higher in the ACS and SA groups at 24 h or 72 h after PCI than in controls. Similarly, plasma levels of miR-126 decreased with time post-PCI in the ACS and SA groups. Plasma miR-126 levels were significantly higher in the ACS and SA groups at 72 h after PCI [Table 4].
Table 2.
Plasma sVCAM-1 levels (μg/L) of different groups before and after PCI
| Groups | Before PCI | 30 min after PCI | 24 h after PCI | 72 h after PCI |
|---|---|---|---|---|
| ACS (n = 46) | 695.06 ± 145.64*,† | 726.00 ± 135.52 | 898.57 ± 146.45‡,§ | 1184.05 ± 142.54‡,§,|| |
| SAP (n = 36) | 376.11 ± 69.63* | 419.00 ± 82.83 | 634.25 ± 141.14‡,§ | 816.45 ± 138.04‡,§,|| |
| Control (n = 48) | 206.24 ± 36.38 | 220.172 ± 35.61 | 226.22 ± 43.19 | 237.03 ± 44.47 |
All data were expressed as mean ± SD. Differences among groups were assessed using the one-way analysis of variance test. *P<0.05, the levels of sVCAM-1 in ACS or SAP group versus that in control group prior to PCI; †P<0.05, the level of sVCAM-1 in ACS versus that in SAP group prior to PCI; ‡P<0.05, the levels of sVCAM-1 at different time points after PCI versus that prior to PCI; §P<0.05, the levels of sVCAM-1 at different time points after PCI versus that 30 min after PCI; ||P<0.05, the levels of sVCAM-1 at different time points after PCI versus that 24 h after PCI. ACS: Acute coronary syndrome group; SAP: Stable angina pectoris group; sVCAM: Soluble vascular cell adhesion molecule; PCI: Percutaneous coronary intervention; SD: Standard deviation.
Table 3.
Plasma hs-CRP levels (μg/L) of different groups before and after PCI
| Groups | Before PCI | 30 min after PCI | 24 h after PCI | 72 h after PCI |
|---|---|---|---|---|
| ACS (n = 46) | 15.63 ± 2.42*,† | 15.43 ± 2.62 | 19.88 ± 4.30‡,§ | 27.53 ± 3.50‡,§,|| |
| SAP (n = 36) | 7.81 ± 1.57* | 7.65 ± 1.91 | 10.37 ± 3.10‡,§ | 16.14 ± 1.53‡,§,|| |
| Control (n = 48) | 1.26 ± 0.35 | 1.30 ± 0.34 | 1.35 ± 0.34 | 1.35 ± 0.35 |
All data were expressed as mean ± SD. Differences among groups were assessed using the one-way analysis of variance test. *P<0.05, the levels of hs-CRP in ACS or SAP group versus that in control group prior to PCI; †P<0.05, the levels of hs-CRP in ACS versus that in SAP group prior to PCI; ‡P<0.05, the levels of hs-CRP at different time points after PCI versus that prior to PCI; §P<0.05, the levels of hs-CRP at different time points after PCI versus that 30 min after PCI; ||P<0.05, the levels of hs-CRP at different time points after PCI versus that 24 h after PCI. ACS: Acute coronary syndrome group; SAP: Stable angina pectoris group; VCAM: Vascular cell adhesion molecule; PCI: Percutaneous coronary intervention; SD: Standard deviation; hs-CRP: High-sensitivity C-reactive protein.
Table 4.
Plasma miR-126 level in different groups before and after PCI (fold change)
| Groups | Before PCI | 30 min after PCI | 24 h after PCI | 72 h after PCI |
|---|---|---|---|---|
| ACS (n = 46) | 0.52 ± 0.14* | 0.52 ± 0.12 | 0.49 ± 0.12 | 0.40 ± 0.12† |
| SAP (n = 36) | 0.54 ± 0.14* | 0.52 ± 0.14 | 0.50 ± 0.13 | 0.41 ± 0.13† |
| Control (n = 48) | 1.00 ± 0.21 | 1.07 ± 0.22 | 1.05 ± 0.11 | 1.06 ± 0.17 |
All data were expressed as mean ± SD. Differences among groups were assessed using the one-way ANOVA test. *P<0.05, the levels of miR-126 in ACS or SAP group versus that in control group prior to PCI; †P<0.05, the level of miR-126 at 72 h after PCI versus that prior to PCI in the same group. ACS: Acute coronary syndrome group; SAP: Stable angina pectoris group; VCAM: Vascular cell adhesion molecule; PCI: Percutaneous coronary intervention; SD: Standard deviation; miR-126: MicroRNA-126.
Correlation of high-sensitivity C-reactive protein, vascular cell adhesion molecule-1, and balloon time and pressure
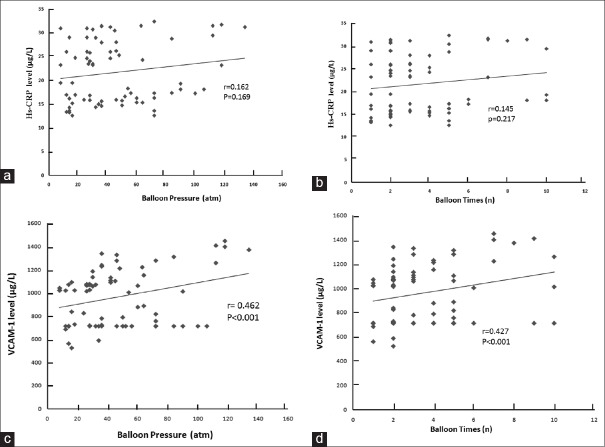
PCI has been reported to cause vascular damage resulting in inflammation.[3] The details of balloon time and pressure in the different groups are shown in Table 5. Our data also showed that PCI could increase the plasma levels of hs-CRP and VCAM-1 and reach the highest at 72 h after PCI [Figure 1]. We further tested the effect of balloon time and pressure on the plasma levels of hs-CRP and VCAM-1 at 72 h after PCI. The results revealed a positive correlation between VCAM-1 and balloon time or pressure (r = 0.462 and r = 0.427, respectively, P < 0.05). However, no significant correlation was observed between hs-CRP and balloon time or pressure.
Table 5.
Details of balloon times and pressure
| Items | ACS (n = 46) | SAP (n = 36) |
|---|---|---|
| Diameter of balloon (mm) | 2.67 ± 0.99 | 2.41 ± 0.61 |
| Times of balloon, n | 3.31 ± 2.34 | 3.66 ± 2.65 |
| Pressure of balloon (atm) | 49.15 ± 13.45 | 51.46 ± 14.40 |
All data were expressed as mean ± SD. Differences among groups were assessed using the one-way analysis of variance test. 1atm = 101,325 Pa. ACS: Acute coronary syndrome group; SAP: Stable angina pectoris group; SD: Standard deviation.
Figure 1.
The correlation between balloon pressure and time with hs-CRP or VCAM-1. (a) The correlation between balloon pressure and hs-CRP. (b) The correlation between balloon time and hs-CRP. (c) The correlation between balloon pressure and VCAM-1. (d) The correlation between balloon time and VCAM-1. A significant positive relationship was observed between balloon pressure and time and VCAM-1. Spearman's correlation = 0.462 and 0.427, respectively, P < 0.001. 1 atm=101,325 Pa. hs-CRP: High-sensitivity C-reactive protein; VCAM-1: Vascular cell adhesion molecule-1.
Correlation of microRNA-126 levels with high-sensitivity C-reactive protein and vascular cell adhesion molecule-1
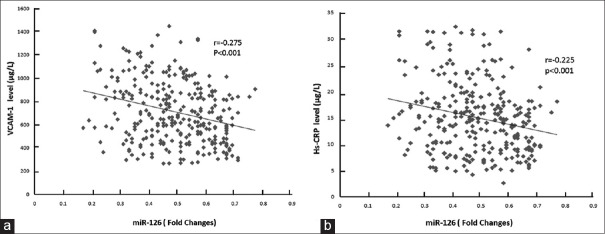
To test the anti-inflammatory activity of miR-126, we analyzed the relationship between miRNA-126, hs-CRP, and VCAM-1 levels. As the levels of plasma miR-126 only displayed a significant difference at 72 h after PCI, we analyzed the correlation at 72 h after PCI. As shown in Figure 2, miR-126 showed a strong negative correlation with hs-CRP and VCAM-1 in patients with CAD following PCI, (r = −0.225 and r = −0.275, respectively, P < 0.01).
Figure 2.
The correlation between miR-126 and hs-CRP or VCAM-1. (a) Circulating levels of miR-126 were decreased in CAD patients who showed higher levels of hs-CRP (n = 130). A significant negative relationship of miR-126 with hs-CRP was observed. Spearman's correlation = −0.225, significance P < 0.001. (b) Circulating levels of miR-126 were decreased in CAD patients who showed higher levels of VCAM-1 (n = 130). A significant negative relationship of miR-126 with VCAM-1 was observed. Coefficient of correlation = −0.275, P < 0.001. hs-CRP: High-sensitivity C-reactive protein; VCAM-1: Vascular cell adhesion molecule-1; CAD: Coronary artery disease; miR-126: MicroRNA-126.
Discussion
In this study, the inflammation markers such as hs-CRP and VCAM-1 were found to be elevated in patients with CAD prior to PCI, which has been reported to be associated with an increased risk of CAD. hs-CRP is an acute-phase reactant and a marker of inflammation that is released 6 h after a coronary event.[10] Elevation of hs-CRP has been reported in ACSs and associated with cardiac events.[11] VCAM-1, an adhesion molecule, has been detected both in atherosclerotic plaques and in endothelial cells. VCAM-1-mediated leukocyte adherence to endothelial cells may contribute to the development of atherosclerosis.[12]
PCI has been reported to cause vascular disease resulting in inflammation.[3] A positive association has been reported between increasing CRP levels and endothelial dysfunction. In addition, elevation of VCAM-1 has been reported after balloon injury to the rabbit iliac artery.[13] In this study, we also found that plasma hs-CRP and VCAM-1 levels were upregulated after PCI. Furthermore, a positive correlation between VCAM-1 and balloon time and pressure was observed in patients following PCI, demonstrating that PCI can cause vascular damage and result in inflammation.
Inflammation plays a central role in the development of restenosis.[14] Several clinical trials have shown that restenosis rates are higher in patients who have elevated systemic markers of inflammation. High levels of CRP and VCAM-1 predict greater rates of restenosis.[15,16] A significant correlation between the vascular occlusion score and baseline hs-CRP levels has been reported.[17] Elevated serum levels of inflammatory markers (CRP, interleukin-6) 6 months after PCI were associated with a risk of in-stent restenosis.[18] VCAM-1 participates in neointimal formation, as it facilitates monocyte infiltration into injured arteries and enhances smooth muscle cell proliferation. Inhibition of VCAM-1 reduces neointimal formation after surgical mechanical injury of the rat carotid artery.[13]
By suppressing VCAM-1 expression, miR-126 could decrease leukocyte interactions with endothelial cells and attenuate vascular inflammation after injury.[19] Overexpression of miR-126 could lead to repression in tumor necrosis factor-α-induced protein expression of VCAM-1 and leukocyte adhesion.[20] Other studies confirmed that VCAM-1 was the target gene of miR-126, knockdown of miR-126 antisense oligonucleotide could lead to upregulating the expression of VCAM-1. miR-126 is one of the noncoding small single-strand RNAs, which inhibit gene expression in posttranscription.[21] In addition, a decrease in plasma miR-126 and high levels of hs-CRP were found in patients with CAD. Notably, miR-126 would be decreased in the presence of CRP and in vascular injury. It has been reported that plasma concentrations of miR-126 were downregulated in CAD patients. Plasma levels of CRP negatively affected the expression levels of endothelial-specific miR-126, while mild inflammatory conditions, as well as ischemia, positively influenced plasma concentrations of miR-126.[18] Consistent with these results, we observed a negative correlation between miR-126 and VCAM-1 or hs-CRP in patients with CAD following PCI, demonstrating that miR-126 could affect the inflammation caused by PCI.
Possible limitations of the present study are the single-center focus and the small number of patients. In addition, patients who underwent primary PCI were treated with a baseline combination of aspirin and statins. Statins exert anti-inflammatory activity and could inhibit the expression of hs-CRP and VCAM-1.[22] Aspirin was found to affect the expression of microRNAs, including miR-126.[23] Other factors such as white blood cell count, renal and liver function might influence on the results of the study, but we did not evaluate those factors. In addition, it was reported that inflammation reached its peak at 48 h after PCI.[24] We just picked up 30 min, 24 h, and 72 h as the study points to test the inflammatory response after PCI. We should check 48 h and some longer time points.
In summary, our data suggest that PCI could elevate the inflammation markers such as hs-CRP and VCAM-1. Plasma VCAM-1 levels were associated with balloon time and pressure. In addition, we observed a negative correlation between miR-126 and hs-CRP and VCAM-1 in patients with CAD following PCI. We will further investigate the underlying mechanism and enroll more patients in future.
Financial support and sponsorship
This study was supported by grants from the National Natural Science Foundation of China (No. 81470024), the Development and Reform Commission of Jilin Province (No. 2013C023), and the Doctoral Fund of Ministry of Education of China (No. 2013061120051).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
References
- 1.Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: A scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572–84. doi: 10.1161/CIRCULATIONAHA.107.185214. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 2.Douglas JS, Jr, Holmes DR, Jr, Kereiakes DJ, Grines CL, Block E, Ghazzal ZM, et al. Coronary stent restenosis in patients treated with cilostazol. Circulation. 2005;112:2826–32. doi: 10.1161/CIRCULATIONAHA.104.530097. doi: 10.1161/CIRCULATIONAHA.104.530097. [DOI] [PubMed] [Google Scholar]
- 3.Toutouzas K, Colombo A, Stefanadis C. Inflammation and restenosis after percutaneous coronary interventions. Eur Heart J. 2004;25:1679–87. doi: 10.1016/j.ehj.2004.06.011. doi: 10.1016/j.ehj.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Zakynthinos E, Pappa N. Inflammatory biomarkers in coronary artery disease. J Cardiol. 2009;53:317–33. doi: 10.1016/j.jjcc.2008.12.007. doi: 10.1016/j.jjcc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–8. doi: 10.1093/cvr/cvn156. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 6.van Solingen C, Bijkerk R, de Boer HC, Rabelink TJ, van Zonneveld AJ. The Role of microRNA-126 in Vascular Homeostasis. Curr Vasc Pharmacol. 2015;13:341–51. doi: 10.2174/15701611113119990017. doi: 10.2174/15701611113119990017. [DOI] [PubMed] [Google Scholar]
- 7.Hu J, Zeng L, Huang J, Wang G, Lu H. miR-126 promotes angiogenesis and attenuates inflammation after contusion spinal cord injury in rats. Brain Res. 2015;1608:191–202. doi: 10.1016/j.brainres.2015.02.036. doi: 10.1016/j.brainres.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–71. doi: 10.1016/j.devcel.2008.07.002. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–84. doi: 10.1016/j.devcel.2008.07.008. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrivastava AK, Singh HV, Raizada A, Singh SK. C-reactive protein, inflammation and coronary heart disease. Egypt Heart J. 2015;67:89–97. doi: 10.1016/j.ehj.2014.11.005. [Google Scholar]
- 11.Raposeiras Roubín S, Barreiro Pardal C, Roubín-Camiña F, Ocaranza Sanchez R, Alvarez Castro E, Paradela Dobarro B, et al. High-sensitivity C-reactive protein predicts adverse outcomes after non-ST-segment elevation acute coronary syndrome regardless of GRACE risk score, but not after ST-segment elevation myocardial infarction. Rev Port Cardiol. 2013;32:117–22. doi: 10.1016/j.repc.2012.05.026. doi: 10.1016/j.repc.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–51. doi: 10.1161/01.atv.18.5.842. doi: 10.1161/01.ATV.18.5.842. [DOI] [PubMed] [Google Scholar]
- 13.Krejcy K, Schwarzacher S, Ferber W, Plesch C, Cybulsky MI, Weidinger FF. Expression of VCAM-1 in rabbit iliac arteries is associated with vasodilator dysfunction of regenerated endothelium following balloon injury. Atherosclerosis. 1996;122:59–67. doi: 10.1016/0021-9150(95)05747-1. doi: 10.1016/0021-9150(95)05747-1. [DOI] [PubMed] [Google Scholar]
- 14.Drachman DE, Simon DI. Inflammation as a mechanism and therapeutic target for in-stent restenosis. Curr Atheroscler Rep. 2005;7:44–9. doi: 10.1007/s11883-005-0074-5. doi: 10.1007/s11883-005-0074-5. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez Garcia B, Ruiz C, Chacon P, Sabin JA, Matas M. High-sensitivity C-reactive protein in high-grade carotid stenosis: Risk marker for unstable carotid plaque. J Vasc Surg. 2003;38:1018–24. doi: 10.1016/s0741-5214(03)00709-2. doi: 10.1016/S0741. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu N, Suzuki H, Wakabayashi K, Iso Y, Shibata M, Yorozuya M, et al. Expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in the pig coronary artery injury model: Comparison of plain old balloon angioplasty and stent implantation. J Cardiol. 2004;43:131–9. doi: 10.1378/chest.125.4.1213. [PubMed] [Google Scholar]
- 17.Arroyo-Espliguero R, Avanzas P, Cosín-Sales J, Aldama G, Pizzi C, Kaski JC. C-reactive protein elevation and disease activity in patients with coronary artery disease. Eur Heart J. 2004;25:401–8. doi: 10.1016/j.ehj.2003.12.017. doi: 10.1016/j.ehj.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Azar RR, Badaoui G, Sarkis A, Kassab R, Salamé E, Klaymé S, et al. Effects of tirofiban and statins on high-sensitivity C-reactive protein, interleukin-6, and soluble CD40 ligand following percutaneous coronary interventions in patients with stable coronary artery disease. Am J Cardiol. 2005;95:236–40. doi: 10.1016/j.amjcard.2004.08.093. doi: 10.1016/j.amjcard.2004.08.093. [DOI] [PubMed] [Google Scholar]
- 19.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008;105:1516–21. doi: 10.1073/pnas.0707493105. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen LJ, Lim SH, Yeh YT, Lien SC, Chiu JJ. Roles of microRNAs in atherosclerosis and restenosis. J Biomed Sci. 2012;19:79. doi: 10.1186/1423-0127-19-79. doi: 10.1186/1423-0127-19-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Q, Luan T, Fu S, Yang J, Jiang C, Xia F. Effects of pitavastatin on the expression of VCAM-1 and its target gene miR-126 in cultured human umbilical vein endothelial cells. Cardiovasc Ther. 2014;32:193–7. doi: 10.1111/1755-5922.12081. doi: 10.1111/1755-5922.12081. [DOI] [PubMed] [Google Scholar]
- 22.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, et al. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–11. doi: 10.1161/CIRCULATIONAHA.106.646380. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 23.de Boer HC, van Solingen C, Prins J, Duijs JM, Huisman MV, Rabelink TJ, et al. Aspirin treatment hampers the use of plasma microRNA-126 as a biomarker for the progression of vascular disease. Eur Heart J. 2013;34:3451–7. doi: 10.1093/eurheartj/eht007. doi: 10.1093/eurheartj/eht007. [DOI] [PubMed] [Google Scholar]
- 24.Masoud K, Fereshtah T, Seyedhossein H, Nasrin G, Mahdi R, Vahid K, et al. Circulating miR-126 and miR-499 reflect progression of cardiovascular disease;correlations with uric acid and ejection fraction. Heart Int. 2016;11:e1–9. doi: 10.5301/heartint.5000226. doi: 10.5301/heartint.5000226. [DOI] [PMC free article] [PubMed] [Google Scholar]