Abstract
Background:
Acne inversa (AI), also called hidradenitis suppurativa, is a chronic, inflammatory, recurrent skin disease of the hair follicle. Familial AI shows autosomal-dominant inheritance caused by mutations in the γ-secretase genes. This study was aimed to identify the specific mutations in the γ-secretase genes in two Chinese families with AI.
Methods:
In this study, two Chinese families with AI were investigated. All the affected individuals in the two families mainly manifested with multiple comedones, pitted scars, and a few inflammatory nodules on their face, neck, trunk, axilla, buttocks, upper arms, and thighs. Reticulate pigmentation in the flexures areas resembled Dowling-Degos disease clinically and pathologically. In addition, one of the affected individuals developed anal canal squamous cell carcinoma. Molecular mutation analysis of γ-secretase genes including PSENEN, PSEN1, and NCSTN was performed by polymerase chain reaction and direct DNA sequencing.
Results:
Two novel mutations of PSENEN gene were identified, including a heterozygous missense mutation c.194T>G (p.L65R) and a splice site mutation c.167-2A>G.
Conclusions:
The identification of the two mutations could expand the spectrum of mutations in the γ-secretase genes underlying AI and provide valuable information for further study of genotype-phenotype correlations.
Keywords: Acne Inversa, Comedones, Dowling-Degos Disease, Hidradenitis Suppurativa, PSENEN, Squamous Cell Carcinoma
Introduction
Acne inversa (AI), also known as hidradenitis suppurativa (HS), is a chronic disease of follicular occlusion, characterized by recurrent inflamed nodules, cysts, deep abscesses, draining sinuses, subsequent scarring, and chronic seepage, involving the apocrine gland-bearing areas of the body, most commonly the axillae, inguinal, and anogenital regions.[1,2] AI usually occurs after puberty with a mean disease incidence of 6.0/100,000 person-years and an average prevalence of approximately 1% of the general population in Europe.[3]
The pathogenesis of AI is complex and far from clarified. About one-third of patients with AI have a positive family history with an autosomal-dominant pattern of inheritance and high penetrance and genetic heterogeneity. The γ-secretase complex, comprising four essential protein subunits including presenilin, presenilin enhancer 2, nicastrin, and anterior pharynx defective 1, is a transmembrane protease that controls a number of important cellular functions through substrate cleavage.[4] Loss-of-function mutations in γ-secretase complex have recently been identified in familial AI.[1] Familial AI-1 (ACNINV1; OMIM 142690) is caused by mutations in the NCSTN gene (or APH2, encoding nicastrin) on chromosome 1q22-q23; familial AI-2 (ACNINV2; OMIM 613736) is caused by haploinsufficiency of the PSENEN gene (or PEN2, encoding presenilin enhancer 2) on chromosome 19q13.12; familial AI-3 (ACNINV3; OMIM 613737) is caused by haploinsufficiency of the PSEN1 gene (encoding presenilin) on chromosome 14q24.3, making this disorder be an allelic disorder of early-onset familial Alzheimer's disease.
In this study, we report a heterozygous missense mutation and a splice site mutation in the PSENEN gene in two Chinese AI families with clinical manifestation of familial multiple comedones and Dowling-Degos disease (DDD).
Methods
Participants
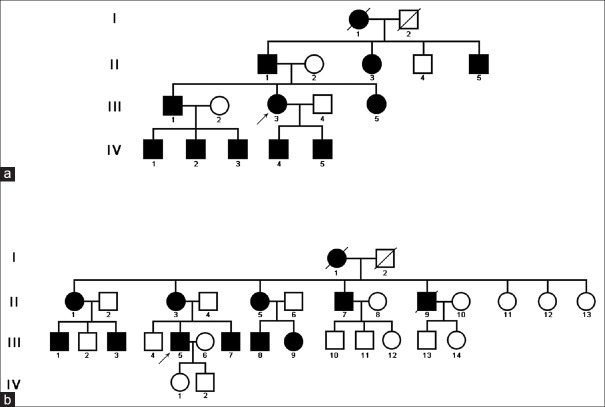
Two Chinese families were included in the present study. A four-generation pedigree was drawn for the two families as shown in Figure 1. All the affected individuals shared extensive comedones and pitted scars on the face, neck, and trunk, and reticulate pigmentation in the flexures areas. The onset age of all the affected individuals is about 15 to 18 years. No difference in severity of the skin lesions between male and female was noticed. Informed consent was obtained from the patients and their family members, and this study adhered to the tenets of the Declaration of Helsinki.
Figure 1.
Pedigrees of the two acne inversa families in the study. (a) Pedigree of family 1. (b) Pedigree of family 2.
DNA sample preparation
Peripheral blood samples were obtained, and DNA was isolated from these blood samples using a Blood DNA Kit (Tiangen Biotech Co., Ltd., China) according to the manufacturer's instructions. In addition, genomic DNA from fifty normal healthy Chinese individuals was extracted as control. DNA integrity and quantity were verified by agarose gel electrophoresis.
Mutation analysis of the PSENEN gene
After genomic DNA was extracted, the encoding exons of the PSENEN, PSEN1, and NCSTN and their flanking intron regions were amplified by polymerase chain reaction (PCR). Twenty-six pairs of primers used for amplification were designed with the DNAMAN (version 6.0) program (Lynnon BioSoft, USA). The primer sequences are listed in Table 1. All PCR reactions were prepared to a final volume of 50 µl with the following conditions: 94°C for 5 min, followed by 35 cycles of 94°C for 30 s, 57°C–59°C for 30 s, 72°C for 45 s, and then final extension at 72°C for 10 min. The amplified PCR products were purified and directly sequenced on an ABI 3700 automated DNA sequencer (Applied Biosystems, USA). Sequence comparisons and analysis were performed with Chromas software (version 2.33, Technelysium Pty Ltd., Australia).
Table 1.
Twenty-six pairs of PCR primers used in this study
| Gene | Number of primer | Forward primer (5’→3’) | Reverse primer (5’→3’) |
|---|---|---|---|
| NCSTN | Primer-1 | AAACACGAACTTCCGGTCTC | GAACTTCTGTCGTGGGAACG |
| Primer-2 | CCTTTGAGGCACATAGCTGG | TGCCTAGCTTGACAGACGG | |
| Primer-3 | AGAGGTGCCTGTATTCACCC | TTCCTTCTGAGGACCACCC | |
| Primer-4 | AGAGCAGATCATTGTCCAGACTC | AGGAGAGGGAAGGGATGAGC | |
| Primer-5 | CCCCTTCCTTTGCCTTAC | CACTGAAACCTCTGCCTC | |
| Primer-6 | ACTGAGTCTGCAACCCTTTG | CAGAGCTCCTTCATGGTGTC | |
| Primer-7 | TGGATAGTGGCAGAGAAGCC | CCTCCTTTCTTGCTCCAGTC | |
| Primer-8 | AAATTGGGAAGCCTCAAATG | CACATCCAGAAGCTCTGTGC | |
| Primer-9–10 | GATAGTCACAGCTGGGAGGTG | ACACCCAATCCAATCCTGTC | |
| Primer-11 | ATTCAGAGAGCCTTGGTCCC | GGACCTGAAGTTCTGGAGGG | |
| Primer-12–13 | ACCACCTCACCCTCACTCC | ATAAGATGCAGATGAGGCCC | |
| Primer-14–15 | CTTGCCCTAGTGTCCCAAAC | CTGCATCTCTCCACCTCTCC | |
| Primer-16 | GGAGAGGTGGAGAGATGCAG | GGGACAGATTTGCAGTAGGC | |
| Primer-17 | TAGGCTGGAGAGATGTTGCC | GGAGCGGAGGAAGGGAG | |
| PSENEN | Primer-2 | AGCTCTTTAATCCAGCCAGC | GTTCGCAGGTCCTTCATCTC |
| Primer-3–4 | ATTCCTGGATCCCAAAGAGG | GTCAGCAGAGAACGTGGGAC | |
| PSEN1 | Primer-3 | CCTTTGCGGTCCTTAGACAG | GTGTCCTCCAGCAATCAGC |
| Primer-4 | CAGAGAGAATGGAGCAAGCC | ATCACAGAGGATGGGCTCTC | |
| Primer-5 | ATGGAGCCAGTGTCTGCTTC | AGCTGCTTGTGAACTCCTGG | |
| Primer-6 | ACCAATATCTAGGTAAAGCCATTC | TTATAAGCAAGGAGCAACAGAAG | |
| Primer-7 | AATAGCACAGTTGATATAGGTTATGG | TGAAGAGTTATGGGATGTACACG | |
| Primer-8 | CAGTTCACCTGCCATTTATTTC | AGATCTGCAGGAGTTCCAGG | |
| Primer-9 | GCAGCATTAGGAAGACTGGC | CCTTTCCATGCTGGTATTCTG | |
| Primer-10 | AGAGGCTTGGTGGGATTACC | CCAGGTACAGTGGCTCACG | |
| Primer-11 | AACAGCAGCATCTACAGTTAAGAC | GCTGGAATATTTAACCCACCTG | |
| Primer-12 | TTCCAGATTGAATGAACGTCTG | AGTGCAAGGTGGTCAGGAAG |
PCR: Polymerase chain reaction.
Results
Clinical examination
The proband of family 1 is a 48-year-old Chinese female (III-3 in Figure 1), who was born from a nonconsanguineous family. She was referred to our department because of extensive asymptomatic comedones, pitted scars, multiple inflammatory papules, and nodules since the age of 15 years. The lesions first appeared on the back of her neck as 2–5 mm comedones and small inflammatory papules, which subsequently developed into pitted scars. The lesions then spread to her face, upper trunk, axilla, buttocks, upper arms, and thighs. Physical examination revealed numerous 1–5 mm monomorphic pitted scars, brown to black papules with a firm central keratotic plugging, and reticulate pigmentation in the flexures areas [Figure 2]. Several nodulocystic lesions in various sizes were also noted. Her general health was otherwise normal. Most of the affected individuals in her family shared the similar clinical manifestations. However, one of her brothers (III-1 in Figure 1) showed more abscesses, cysts, scars, and unfortunately, he developed a well-differentiated squamous cell carcinoma (SCC) on his anal canal at the age of 38 years. Two years after a tumorectomy, he developed lymph nodes and bone metastasis. All the family members had normal teeth, nails, stature, and intelligence.
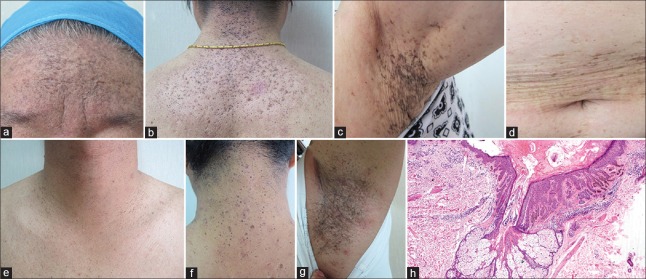
Figure 2.
Clinical and pathological features of the acne inversa families. (a and b) The proband (III-3) of family 1 showed extensive comedones, pitted scars, and hyperpigmentation on her face, back of the neck, and upper back with a few inflammatory papules. (c and d) II-3 of family 1 showed reticulate hyperpigmentation with pitted scar in the axilla and over the trunk. (e-g) The proband of family 2 showed extensive comedones with pitted scars over his neck and upper trunk, and reticulate hyperpigmentation in the axilla. (h) Skin biopsy from the proband (III-3) of family 1 showed dilated hair follicle with follicular plugging and perifollicular acanthosis with downward elongations of rete ridges in a reticulated pattern, which resembled Dowling-Degos disease (H and E, ×40).
In family 2, the affected individuals had been suffering from extensive asymptomatic comedones, pitted scars, and reticulate pigmentation, which were similar to family 1 [Figure 2e-2g]. However, inflammatory nodules, cysts, and abscesses were more severe on the buttocks and groin of the affected individuals in family 2. No malignant alteration of the skin lesions was identified in any of the affected family members.
Histopathologic examination of a skin biopsy from the comedonal and pigmented lesions on back of the neck of the proband in family 1 revealed a dilated hair follicle with follicular plugging and acanthosis with downward elongations of rete ridges in a reticulated or fenestrated pattern and a few horn cysts [Figure 2h]. The pathologic changes were similar to previous reported familial comedo and DDD cases.[5,6]
Mutation analysis
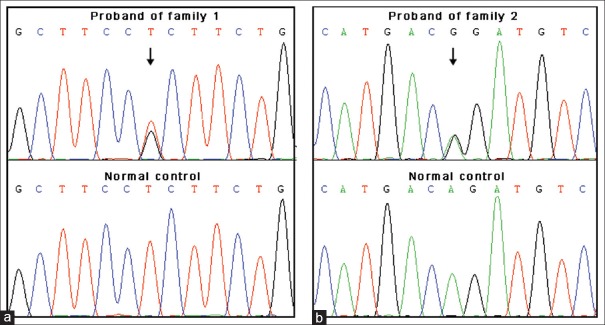
After direct sequencing of PCR products in the two patients, a heterozygous missense mutation c.194T>G (p.L65R) in the PSENEN gene was identified in Case 1, and a splice site mutation c.167-2A>G in the PSENEN gene was identified in Case 2 [Figure 3]. No mutation in PSEN1 and NCSTN genes was identified. Both of the two mutations were verified in the family members and absent in fifty ethnically matched normal controls (100 chromosomes).
Figure 3.
Identification of mutations in the PSENEN gene. (a) In the proband of family 1, a heterozygous missense mutation c.194T>G (p.L65R) was identified while it was absent in normal controls. (b) In the proband of family 2, a heterozygous splice site mutation c.167-2A>G was found.
Discussion
For the diagnosis of AI, three criteria should be fulfilled.[3,7] First, typical lesions such as nodules (inflamed or noninflamed), sinus tracts (inflamed or noninflamed), abscesses, and scars (atrophic, mesh-like, red, hypertrophic, or linear) must be present. Second, these elements mainly occur in more than one of the predilection sites for AI: axillae, groin, perineal region, buttocks, and infra- and inter-mammary folds. Third, there must be a clear history of chronicity and recurrence (more than two recurrences over a period of 6 months). Our cases met the criteria well, but the main manifestations were multiple comedones and DDD, which are not common.
In 2013, Canoui-Poitrine et al.[8] identified three AI phenotype subgroups: axillary-mammary type, follicular type, and gluteal type. However, several other possible clinical subtypes were also proposed recently, such as regular type, frictional furuncle type, scarring folliculitis type, conglobata type, syndromic type, and ectopic type.[7] In addition, several cases mainly manifested as asymptomatic comedones and pitted scars without notable inflammation had been reported namely as familial comedones,[5,6] which were similar to our cases. Rerknimitr et al.[9] then reported more families and extended the familial comedones spectrum to include multiple severe inflammatory lesions and scars together with generalized comedones. Interestingly, using whole-genome linkage analysis and whole-exome sequencing in this family, a heterozygous one-base pair insertion, 84_85insT (p.L28FfsX93) in PSENEN gene was identified,[10] which shared the same causative gene in AI and the present cases. According to the overlapping clinical and genetic findings, we suggest that AI and familial comedones should be regarded as a spectrum disorder with different clinical variations. However, several inflammatory nodules, cysts, and abscesses had also been identified in the affected family members of the present study, and the diagnosis of AI was favored. Nevertheless, we proposed a disseminated comedone and pitted scar subtype as a complement of AI clinical spectrum.
A number of diseases, such as Crohn's diseases, synovitis-acne-pustulosis-hyperostosis-osteitis syndrome, pyoderma gangrenosum, and tumors (SCC, keratoacanthoma, and adenocarcinoma) have been described to coexist with AI. Several reports have illustrated a rare association between AI and DDD.[11,12,13] DDD is an autosomal dominant genodermatosis characterized by reticular pigmented anomaly mainly affecting flexures. KRT5, POFUT1, and POGLUT1 have been identified to be the causal genes of DDD.[14] The histopathological change of DDD is very characteristic, showing dilated follicular, fingerlike projections called rete ridges, with thinning of the suprapapillary plates, resulting in an “antler-like” pattern and increased pigmentation of the basal layer.[15] The histological change of the family 1 in the present study resembled DDD or suggested a coexistence of AI and DDD. AI has been reported to be associated with DDD, and it is possible that the defect of follicular proliferation may account for the coexistence of these conditions.[12]
The patient III-1 of family 2 developed SCC as well as AI and DDD. Two of the previous reports showed SCC with HS and DD: one in the groin[11] and the other in the perianal region.[16] Another report described multiple keratoacanthomas with AI and DDD.[17] However, it is unclear whether DDD itself could increase the risk of SCC independently of AI.
Genetic background, follicular occlusion, bacteria, inflammation, obesity, smoking, and mechanical stress have been implied to be involved in the pathogenesis of AI. However, it is still far from clarified.[18] The γ-secretase is an intramembranous protease complex capable of cleaving in excess of thirty Type 1 transmembrane proteins including amyloid precursor protein, Notch receptors, N-cadherin, and E-cadherin.[4,19] In the skin, Notch is expressed in developing or differentiating epidermis and hair follicles. Disruption of a Notch signaling pathway causes epidermal and follicular hyperkeratosis and epidermal cyst formation.
According to the reported mutations summarized recently by Ratnamala et al.,[20] the study on familial comedones by Panmontha et al.,[21] and the results of the present study, 30 families with 29 different mutations of the γ-secretase genes have been reported to date in AI, including 22 mutations in NCSTN, 6 mutations in PSENEN, and 1 mutation in PSEN1. Interestingly, all the four missense mutations reported in AI previously (p.V75I, p.D185N, p.P211R, and p.Q216P) are located within the ectodomain of NCSTN.[19] To the best of our knowledge, no missense mutation located in the transmembrane domain of any γ-secretase genes has been reported before. It is noteworthy that the missense mutation c.194T>G (p.65 L>R) reported in the present study located in the second transmembrane domain of PSENEN. As far as we know, it is the first reported missense mutation located in the transmembrane domain of any γ-secretase genes. This implies that the second transmembrane domain of PEN2 might be critical for functional γ-secretase activity. The splice site mutation c.167-2A>G in the PSENEN gene might destroy the second transmembrane domain, the evolutionarily conserved DYLSF domain and the hydrophilic C terminus of PEN2. The DYLSF domain is necessary for the binding of PEN2 to other components in the presenilin complex and the C terminus is critical for functional γ-secretase activity.[21]
There is no current cure for AI. Lots of treatments, such as topical and systemic antibiotics, intralesional corticosteroids, systemic using of corticosteroids, retinoids, dapsone, cyclosporine A, hormones as well as biologics such as adalimumab and laser therapy, might be useful.[3]
In conclusion, we reported two AI families manifested as familial multiple comedones and DDD, and one of the affected individuals developed anal canal SCC. Then, we identified two novel mutations of PSENEN gene involved in AI, including a heterozygous missense mutation c.194T>G (p.L65R) and a splice site mutation c.167-2A>G. The identification of the two mutations could expand the spectrum of mutations in the γ-secretase genes underlying AI and provide valuable information for further study of genotype-phenotype correlations.
Financial support and sponsorship
This work was supported by grants from the National Natural Science Foundation of China (No. 81201221) and Beijing Municipal Natural Science Foundation (No. 7154247).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We are grateful to the patients and members of the two families for their participation in this study.
Footnotes
Edited by: Qiang Shi
References
- 1.Wang B, Yang W, Wen W, Sun J, Su B, Liu B, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi: 10.1126/science.1196284. doi: 10.1126/science.1196284. [DOI] [PubMed] [Google Scholar]
- 2.Pink AE, Simpson MA, Brice GW, Smith CH, Desai N, Mortimer PS, et al. PSENEN and NCSTN mutations in familial hidradenitis suppurativa (Acne Inversa) J Invest Dermatol. 2011;131:1568–70. doi: 10.1038/jid.2011.42. doi: 10.1038/jid.2011.42. [DOI] [PubMed] [Google Scholar]
- 3.Zouboulis CC, Desai N, Emtestam L, Hunger RE, Ioannides D, Juhász I, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619–44. doi: 10.1111/jdv.12966. doi: 10.1111/jdv.12966. [DOI] [PubMed] [Google Scholar]
- 4.Zhao G, Liu Z, Ilagan MX, Kopan R. Gamma-secretase composed of PS1/Pen2/Aph1a can cleave notch and amyloid precursor protein in the absence of nicastrin. J Neurosci. 2010;30:1648–56. doi: 10.1523/JNEUROSCI.3826-09.2010. doi: 10.1523/JNEUROSCI.3826-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng MJ, Chen WC, Happle R, Song ZQ. Familial disseminated comedones without dyskeratosis: Report of an affected family and review of the literature. Dermatology. 2014;228:303–6. doi: 10.1159/000360818. doi: 10.1159/000360818. [DOI] [PubMed] [Google Scholar]
- 6.Cantú JM, Gómez-Bustamente MO, González-Mendoza A, Sánchez-Corona J. Familial comedones. Evidence for autosomal dominant inheritance. Arch Dermatol. 1978;114:1807–9. [PubMed] [Google Scholar]
- 7.van der Zee HH, Jemec GB. New insights into the diagnosis of hidradenitis suppurativa: Clinical presentations and phenotypes. J Am Acad Dermatol. 2015;73(5 Suppl 1):S23–6. doi: 10.1016/j.jaad.2015.07.047. doi: 10.1016/j.jaad.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 8.Canoui-Poitrine F, Le Thuaut A, Revuz JE, Viallette C, Gabison G, Poli F, et al. Identification of three hidradenitis suppurativa phenotypes: Latent class analysis of a cross-sectional study. J Invest Dermatol. 2013;133:1506–11. doi: 10.1038/jid.2012.472. doi: 10.1038/jid.2012.472. [DOI] [PubMed] [Google Scholar]
- 9.Rerknimitr P, Korkij W, Wititsuwannakul J, Panmontha W, Suphapeetiporn K, Shotelersuk V. Expanding phenotypic spectrum of familial comedones. Dermatology. 2014;228:215–9. doi: 10.1159/000358170. doi: 10.1159/000358170. [DOI] [PubMed] [Google Scholar]
- 10.Dereure O. Familial multiple comedones and PEN-2 mutation. Ann Dermatol Venereol. 2016;143:171–2. doi: 10.1016/j.annder.2015.12.005. doi: 10.1016/j.annder.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Weber LA, Kantor GR, Bergfeld WF. Reticulate pigmented anomaly of the flexures (Dowling-Degos disease): A case report associated with hidradenitis suppurativa and squamous cell carcinoma. Cutis. 1990;45:446–50. [PubMed] [Google Scholar]
- 12.Choudhary SV, Jain D, Agrawal P, Singh A. Dowling-Degos disease and hidradenitis suppurativa: Co occurrence or association? Indian Dermatol Online J. 2013;4:191–4. doi: 10.4103/2229-5178.115514. doi: 10.4103/2229-5178.115514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arjona-Aguilera C, Linares-Barrios M, Albarrán-Planelles C, Jiménez-Gallo D. Dowling-Degos disease associated with hidradenitis suppurativa: A case report. Actas Dermosifiliogr. 2015;106:337–8. doi: 10.1016/j.ad.2014.09.010. doi: 10.1016/j.ad.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Basmanav FB, Oprisoreanu AM, Pasternack SM, Thiele H, Fritz G, Wenzel J, et al. Mutations in POGLUT1, encoding protein O-glucosyltransferase 1, cause autosomal-dominant Dowling-Degos disease. Am J Hum Genet. 2014;94:135–43. doi: 10.1016/j.ajhg.2013.12.003. doi: 10.1016/j.ajhg.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loo WJ, Rytina E, Todd PM. Hidradenitis suppurativa, Dowling-Degos and multiple epidermal cysts: A new follicular occlusion triad. Clin Exp Dermatol. 2004;29:622–4. doi: 10.1111/j.1365-2230.2004.01631.x. doi: 10.1111/j.1365-2230.2004.01631.x. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Hunt MJ, Commens CA. Hidradenitis suppurativa, Dowling Degos disease and perianal squamous cell carcinoma. Australas J Dermatol. 1997;38:209–11. doi: 10.1111/j.1440-0960.1997.tb01700.x. doi: 10.1111/j.1440-0960.1997.tb01700.x. [DOI] [PubMed] [Google Scholar]
- 17.Fenske NA, Groover CE, Lober CW, Espinoza CG. Dowling-Degos disease, hidradenitis suppurativa, and multiple keratoacanthomas. A disorder that may be caused by a single underlying defect in pilosebaceous epithelial proliferation. J Am Acad Dermatol. 1991;24(5 Pt 2):888–92. [PubMed] [Google Scholar]
- 18.Prens E, Deckers I. Pathophysiology of hidradenitis suppurativa: An update. J Am Acad Dermatol. 2015;73(5 Suppl 1):S8–11. doi: 10.1016/j.jaad.2015.07.045. doi: 10.1016/j.jaad.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 19.Pink AE, Simpson MA, Desai N, Trembath RC, Barker JN. g-Secretase mutations in hidradenitis suppurativa: New insights into disease pathogenesis. J Invest Dermatol. 2013;133:601–7. doi: 10.1038/jid.2012.372. doi: 10.1038/jid.2012.372. [DOI] [PubMed] [Google Scholar]
- 20.Ratnamala U, Jhala D, Jain NK, Saiyed NM, Raveendrababu M, Rao MV, et al. Expanding the spectrum of γ-secretase gene mutation-associated phenotypes: Twonovel mutations segregating with familial hidradenitis suppurativa (acne inversa) and acne conglobata. Exp Dermatol. 2016;25:314–6. doi: 10.1111/exd.12911. doi: 10.1111/exd.12911. [DOI] [PubMed] [Google Scholar]
- 21.Panmontha W, Rerknimitr P, Yeetong P, Srichomthong C, Suphapeetiporn K, Shotelersuk V. A frameshift mutation in PEN-2 causes familial comedones syndrome. Dermatology. 2015;231:77–81. doi: 10.1159/000382122. doi: 10.1159/000382122. [DOI] [PubMed] [Google Scholar]