Abstract
Background:
Patients with diabetes mellitus (DM) have a higher risk of thromboembolic events; however, the optimal duration of dual antiplatelet therapy (DAPT) remains unclear. The goal of this study was to assess the efficacy and safety of various DAPT durations in patients with DM undergoing drug-eluting stent implantation.
Methods:
We conducted a literature search for randomized controlled trials (RCTs). We searched databases including EMBASE, PubMed, Cochrane Library, and Scopus up to June 2016. Investigators extracted data independently, including outcomes, characteristics, and study quality. A random-effect model was used to pool odds ratios (ORs) with 95% confidence intervals (CIs) of the clinical outcomes.
Results:
Six RCTs totaling 6040 patients with DM were included in the study. Shorter-duration DAPT resulted in an increased rate of stent thrombosis (ST) (OR, 1.83, 95% CI: 1.03–3.26, P = 0.04), but did not increase the risk of myocardial infarction (OR, 1.33, 95% CI: 0.71–2.47, P = 0.37), stroke (OR, 0.96, 95% CI: 0.52–1.77, P = 0.90), target vessel revascularization (OR, 1.19, 95% CI: 0.46–3.07, P = 0.71), all-cause death (OR: 0.72, 95% CI: 0.48–1.09, P = 0.12), or cardiac death (OR, 0.82, 95% CI: 0.49–1.36, P = 0.44) significantly. Shorter-duration DAPT was associated with a decreased risk of major bleeding (OR, 0.60, 95% CI: 0.38–0.94, P = 0.02).
Conclusion:
In patients with DM, longer-duration DAPT had a lower risk of ST, but was associated with an increased bleeding risk.
Keywords: Diabetes Mellitus, Dual Antiplatelet Therapy, Meta-analysis, Stent Thrombosis
Introduction
Patients with diabetes mellitus (DM) have a higher risk of thromboembolic events, and DM is a strong and independent predictor of stent thrombosis (ST) after drug-eluting stent (DES) implantation.[1,2] Current clinical practice guidelines recommend 6–12-month dual antiplatelet therapy (DAPT) following DES implantation;[3,4] however, the optimal DAPT duration in patients with DM has not yet been defined. Recently, large-scale trials, such as the SECURITY and DAPT trials, published conflicting results of the DM subgroup;[5,6] therefore, we performed the meta-analysis to assess the optimal DAPT duration in this special population.
Methods
Search strategy and eligibility criteria
We conducted a systematic search to compare shorter- and longer-duration DAPT in patients with DM after DES implantation from databases including PubMed, Cochrane Library, EMBASE, and Scopus for randomized controlled trials (RCTs) up to June 2016. We also searched ClinicalTrials.gov databases and checked references of reviews to locate other eligible studies. Our search also included conference abstracts and did not restrict language or publication dates. Search terms included “dual antiplatelet”, “stent”, and “diabetes mellitus”. The specific search strategies for each database are listed in Supplementary Table 1.
Supplementary Table 1.
Study search strategy
| Database | Search strategy | Records |
|---|---|---|
| Scopus | TITLE-ABS-KEY (antiplatelet OR clopidogrel OR ticagrelor OR prasugrel) AND TITLE-ABS-KEY (stent OR stents) AND diabetes | 4156 |
| PubMed | (((antiplatelet [Title/Abstract] OR clopidogrel [Title/Abstract] OR ticagrelor [Title/Abstract] OR prasugrel[Title/Abstract])) AND stent [Title/Abstract]) AND diabetes | 472 |
| Cochrane | There are 158 results from 907144 records for your search on ‘antiplatelet OR clopidogrel OR ticagrelor OR prasugrel in title, abstract, keywords and stent in title, abstract, keywords and diabetes in trials’ | 188 |
| Embase | antiplatelet:ab, ti OR clopidogrel:ab, ti OR ticagrelor:ab, ti OR prasugrel:ab, ti AND stent:ab, ti AND diabetes | 1545 |
The eligibility criteria included: (1) RCTs; (2) patients with DM who received DAPT after coronary DES implantation; (3) shorter-duration DAPT with standard therapy (12-month DAPT) or standard therapy with prolonged DAPT; (4) any of the following endpoints: ST, myocardial infarction (MI), stroke, target vessel revascularization (TVR), all-cause death, cardiac death, and major bleeding; and (5) at least 12-month follow-up. Exclusion criteria included: bare-metal stent implantation, biodegradable polymer-based DES, and discontinuation or interruption (but not duration) of DAPT.
Study selection, data abstraction, and quality assessment
Two reviewers reviewed titles and abstracts independently to exclude irrelevant records and then obtained full-text records of potentially suitable articles. The third reviewer identified discrepancies. After full agreement on the included studies, two reviewers first extracted the data independently and assessed the risk of each study and then cross-examined these results. The quality of the RCTs was assessed with the Cochrane collaboration's risk-of-bias tool.[7]
Outcome variables
Major bleeding was defined differently across RCTs according to major thrombolysis in myocardial infarction bleeding in the EXCELLENT, REAL/ZEST-LATE, and RESET trials, while Bleeding Academic Research Consortium Type 3 or 5 was used in the SECURITY and DAPT trials.
Statistical analysis
All statistical analyses were conducted by Review Manager 5.3 (The Cochrane Collaboration, Copenhagen, Denmark). A random-effect model was used to pool odds ratios (ORs). Heterogeneity across studies was checked by Chi-square test and I2 statistic, with P < 0.10 or I2 > 50% representing a significant heterogeneity. All reported P values were two-sided, with P < 0.05 considered statistically significant. We did not test for publication bias because of the limited number of studies. Sensitivity analyses were performed by excluding sequentially one study at a time to test the robustness of the results.
Results
Study selection and quality assessment
The process of study selection is listed in Figure 1. Finally, six RCTs totaling 6040 patients with DM were included in the analysis.[5,6,8,9,10,11] The main features of the included RCTs are reported in Table 1. The baseline clinical, angiographic, and procedural characteristics of patients are listed in Supplementary Table 2. The quality of the RCTs is shown in Supplementary Table 3. In the subgroup of shorter versus 12-month DAPT, the definition of short- and long-duration DAPT was 3–6 months for short duration and 12 months for long duration. In the subgroup of 12-month versus longer DAPT, the definition of short- and long-duration DAPT was 12 months for the shorter duration and 24–30 months for the longer duration.[12,13] Two RCTs compared 3-month versus 12-month DAPT (the OPTIMIZE and RESET trials); two compared 6-month versus 12-month DAPT (the EXCELLENT and SECURITY trial), and the others compared 12-month versus >12-month DAPT (the DAPT study and REAL/ZEST-LATE trial). Second-generation DESs were used in two RCTs, and the others mixed the first- and second-generation DESs together in analyses. Overall, 63.9% of the patients received second-generation DESs; 18.8% received prasugrel, and the rest were treated with clopidogrel. Three RCTs provided outcomes at 12 months after DES implantation, two at 24 months, and one at 33 months. Three trials were carried out in Korea, one in Brazil, and two internationally.
Figure 1.
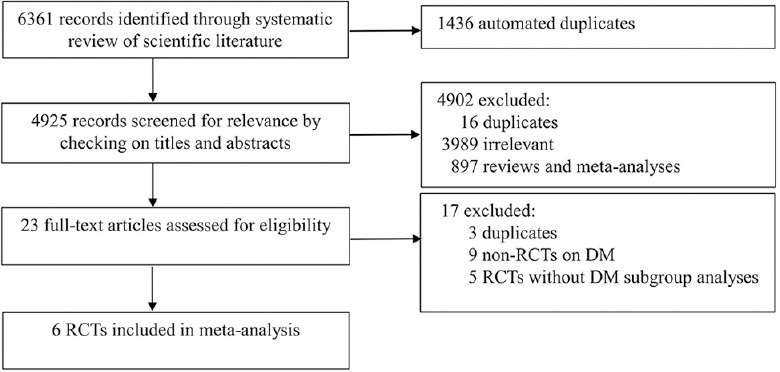
Search flow diagram of the studies included in the meta-analysis. DM: Diabetes mellitus; RCT: Randomized controlled trial.
Table 1.
Characteristics of the included RCTs
| Study, year | Setting | Definition of shorter and longer durations | Total number | Type of DES | P2Y12 inhibitor | Follow-up (months) |
|---|---|---|---|---|---|---|
| Shorter versus 12-month DAPT | ||||||
| SECURITY, 2016 | International | Shorter: 6 months | 429 | 2G | Clopidogrel | 24 |
| Longer: 12 months | Prasugrel | |||||
| OPTIMIZE, 2014 | Brazil | Shorter: 3 months | 1103 | 2G | Clopidogrel | 12 |
| Longer: 12 months | ||||||
| RESET, 2012 | Korea | Shorter: 3 months | 292 | 1G | Clopidogrel | 12 |
| Longer: 12 months | 2G | |||||
| EXCELLENT, 2012 | Korea | Shorter: 6 months | 550 | 1G | Clopidogrel | 12 |
| Longer: 12 months | 2G | |||||
| 12-month versus prolonged DAPT | ||||||
| REAL/ZEST-LATE, 2010 | Korea | Shorter: 12 months | 704 | 1G | Clopidogrel | 24 |
| Longer: 24 months | 2G | |||||
| DAPT, 2014 | 11 countries | Shorter: 12 months | 3391 | 1G | Clopidogrel | 33 |
| Longer: 30 months | 2G | Prasugrel |
1G: First-generation; 2G: Second-generation; DES: Drug-eluting stent; RCTs: Randomized controlled trials; DAPT: Dual antiplatelet therapy.
Supplementary Table 2.
Baseline clinical, angiographic, and procedural characteristics of patients enrolled among trials included in the meta–analysis
| Characteristics | REAL/ZEST–LATE‡ | RESET‡ | OPTIMIZE‡ | DAPT | EXCELLENT‡ | SECURITY | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shorter | Longer | Shorter | Longer | Shorter | Longer | Shorter | Longer | Shorter | Longer | Shorter | Longer | |
| Age (years) | 61.9 | 62.0 | 62.4 | 62.4 | 61.3 | 61.9 | 62.8 | 62.5 | 63.0 | 62.4 | 65.5 | 66.7 |
| Female (%) | 30.6 | 30.0 | 35.1 | 37.1 | 36.5 | 36.9 | 29.2 | 31.0 | 34.9 | 36.1 | 28.2 | 26.0 |
| DM (%) | 27.1‡ | 25.1‡ | 30.1‡ | 28.8 | 35.4‡ | 35.3‡ | 100.0 | 100.0 | 37.7‡ | 38.6‡ | 100 | 100 |
| Type 1 (%) | – | – | – | – | 10.2 | 10.4 | 28.1 | 29.3 | – | – | 21.4 | 19.7 |
| Type 2 (%) | – | – | – | – | 25.2 | 24.9 | 71.9 | 70.9 | – | – | 78.6 | 80.3 |
| Hypertension (%) | 56.9 | 57.1 | 62.6 | 61.4 | 86.4 | 88.2 | 88.6 | 87.2 | 72.7 | 73.8 | 82.5 | 80.3 |
| Dyslipidemia (%) | 43.5 | 43.2 | 58.2 | 59.9 | 63.2 | 63.7 | – | – | 75.2 | 76.3 | 69.4 | 70.9 |
| Current smoking (%) | 32.1 | 29.8 | 25.0 | 22.8 | 18.6 | 17.3 | 21.4 | 20.1 | 27.4 | 25.8 | 18.9 | 20.2 |
| Prior MI (%) | 3.3 | 3.8 | 1.8 | 1.6 | 34.6 | 34.8 | 24.8 | 23.2 | 6.5 | 3.7 | 23.8 | 17.1 |
| Prior PCI (%) | 11.8 | 13.0 | 3.7 | 3.0 | 20.9 | 19.1 | 34.1 | 35.6 | 9.3 | 8.6 | 22.8 | 17.0 |
| Prior CABG (%) | – | – | 0.2 | 0.6 | 7.1 | 8.2 | 16.4 | 15.2 | 1.5 | 1.0 | 5.8 | 7.2 |
| Prior stroke (%) | 3.3 | 4.3 | 0.0 | 0.0 | 2.5 | 2.5 | 4.2 | 5.3 | 6.5 | 6.7 | – | – |
| Peripheral arterial disease (%) | – | – | 0.0 | 0.0 | 2.8 | 3.0 | 8.6 | 8.6 | – | – | – | – |
| Heart failure (%) | – | – | 10.0 | 11.8 | 4.3 | 4.2 | 8.2 | 7.5 | 0.6 | 0.7 | – | – |
| Renal insufficiency (%) | – | – | –§ | –§ | 7.4 | 5.8 | 7.2 | 6.2 | 0.8 | 1.2 | –§ | –§ |
| LVEF (%) | 59.7 | 59.2 | 64.3 | 63.9 | – | – | – | – | 61.0 | 61.4 | 55.8 | 55.7 |
| ACS (%) | 21.2 | 22.1 | 14.6 | 13.8 | 31.6* | 32.3* | 21.4 | 22.3 | 51.1† | 52.0† | 35.9 | 32.3 |
| Therapy at discharge | ||||||||||||
| Aspirin + clopidogrel | 99.9 | 99.7 | 100.0 | 100.0 | 100.0 | 100.0 | 64.6 | 68.3 | 100.0 | 100.0 | 99.0 | 98.7 |
| Aspirin + prasugrel | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 35.4 | 31.7 | 0.0 | 0.0 | 0.5 | 0.0 |
| Multivessel disease | 48.1 | 48.0 | 42.2 | 42.9 | – | – | – | – | 51.9 | 52.0 | 54.3 | 47.8 |
| Bifurcation | 12.5 | 12.1 | 0.0 | 0.0 | 14.7 | 14.9 | – | – | 10.2 | 11.4 | 10.7 | 12.1 |
| Stents implanted | ||||||||||||
| Per patient | 1.6 | 1.8 | 1.3 | 1.5 | 1.6 | 1.6 | 11.5 | 1.4 | 1.6 | 1.6 | 1.6 | 1.6 |
| Per lesion | 1.2 | 1.3 | 1.0 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.1 | 1.2 |
| Stent length per lesion (mm) | 30.9 | 31.8 | 22.7 | 22.9 | 20.4 | 20.4 | 19.2 | 18.1 | 27.8 | 28.3 | 19.2 | 19.3 |
| Treated vessel | ||||||||||||
| Left main coronary | 2.4 | 2.9 | 0.0 | 0.0 | 1.2 | 1.5 | 0.8 | 0.9 | 0.0 | 0.0 | 0.0 | 0.0 |
| Left anterior descending | 49.9 | 48.7 | 52.7 | 53.6 | 47.9 | 46.6 | 37.4 | 38.2 | 49.6 | 50.4 | 39.0 | 41.0 |
| Left circumflex | 18.1 | 19.9 | 21.3 | 19.2 | 23.4 | 24.3 | 24.3 | 24.1 | 22.0 | 21.7 | 19.0 | 18.0 |
| Right coronary | 29.6 | 28.5 | 67.6 | 69.2 | 27.6 | 27.7 | 33.0 | 33.2 | 28.3 | 27.9 | 32.0 | 28.0 |
| Stent type | ||||||||||||
| First-generation | 80.2 | 80.9 | 0.0 | 28.5 | 0.0 | 0 | 36.2 | 35.5 | 74.8 | 74.8 | 0.0 | 0.0 |
| Second-generation | 18.8 | 18.7 | 100.0 | 71.5 | 100.0 | 100.0 | 53.3 | 54.0 | 25.2 | 25.2 | 100.0 | 100.0 |
*Low–risk ACS (UA or MI <30 days); †Low–risk ACS (MI <72 h was excluded); ‡The characteristics of patients were extracted from the overall population but not only diabetic patients; §Patients with serum creatinine >2.0 mg/dl were not included in the study. ACS: Acute coronary syndrome; CABG: Coronary artery bypass grafting; DES: Drug–eluting stent; LVEF: Left ventricular ejection fraction; MI: Myocardial infarction; PCI: Percutaneous coronary intervention; –: Could not be calculated; DAPT: Dual antiplatelet therapy; DM: Diabetes mellitus; UA: Unstable angina.
Supplementary Table 3.
Assessments of risk bias
| Study, year | Randomization sequence generation | Allocation concealment | Blinding of participants, personnel | Blinding of outcomes assessment | Incomplete outcome data | Selective reporting | Other sources of bias |
|---|---|---|---|---|---|---|---|
| REAL/ZEST-LATE, 2010 | Low risk: A preestablished, computer-generated randomization scheme | Unclear risk | High risk: Open-label study | Low risk | Low risk: 17 lost to follow-up (0.6%), but with an intention-to-treat analysis | Low risk | Low risk |
| RESET, 2012 | Low risk: Web-based response system | Unclear risk | High risk: Open-label study | Low risk | Low risk: 31 lost to follow-up (1.5%), but with an intention-to-treat analysis | Low risk | Low risk |
| OPTIMIZE, 2013 | Low risk: A dedicated web-based system and stratified by the presence of DM and institution | Unclear risk | High risk: Open-label study | Low risk | Low risk: 76 lost to follow-up (2.4%), but with an intention-to-treat analysis | Low risk | Low risk |
| DAPT, 2014 | Low risk: A central interactive voice response system | Unclear risk | High risk: Open-label study | Low risk | Low risk: 571 lost to follow-up (5.7%), but with an intention-to-treat analysis | Low risk | Low risk |
| EXCELLENT, 2012 | Low risk: A web-based online randomization system | Unclear risk | High risk: Open-label study | Low risk | Low risk: 15 lost to follow-up (1%), but with an intention-to-treat analysis | Low risk | Low risk |
| SECURITY, 2016 | Low risk: By electronic case report, and balanced within center by blocks of 4 | Unclear risk | High risk: Open-label study | Low risk | Low risk: 263 loss to follow-up (19%), but none excluded from the analysis | Low risk | Low risk |
DAPT: Dual antiplatelet therapy; DM: Diabetes mellitus.
Primary endpoints
The definitions of the primary endpoints in each record are listed in Supplementary Table 4. There was no significant difference for the primary endpoints between shorter- and longer-duration DAPT [OR, 1.04, 95% confidence interval [CI]: 0.65–1.65, P = 0.88; I2 = 60%; Supplementary Figure 1 (567.6KB, tif) ]. The results were consistent between abbreviated-term and prolonged-term DAPT studies (P for interaction = 0.42).
Supplementary Table 4.
The definition of primary endpoint of RCTs
| Study, year | The definition of primary endpoint |
|---|---|
| EXCELLENT, 2012 | TVF: A composite of cardiac death, MI, or TVR |
| OPTIMIZE, 2013 | NACCE: Composite of all-cause death, MI, stroke, or major bleeding |
| RESET, 2012 | NACE: A composite of cardiac death, MI, ST, TVR, or bleeding |
| SECURITY, 2016 | A composite of cardiac death, MI, stroke, definite or probable ST, or BARC Type 3 or 5 bleeding |
| DAPT, 2014 | MACCE: Death, MI, stroke |
| REAL/ZEST-LATE, 2010 | MI or cardiac death |
BARC: Bleeding Academic Research Consortium; MACCE: Major adverse cardiac and cerebrovascular event; MI: Myocardial infarction; NACE: Net adverse cardiac event; NACCE: Net adverse cardiac and cerebrovascular event; ST: Stent thrombosis; TVF: Target vessel failure; TVR: Target vessel revascularization; DAPT: Dual antiplatelet therapy; RCTs: Randomized controlled trials.
Primary endpoints for shorter versus longer DAPT duration in patients with DM. DAPT: Dual antiplatelet therapy; DM: Diabetes mellitus.
Definite/probable stent thrombosis
Definite or probable ST occurred in 34 patients (1.06%) with shorter-duration DAPT and 18 patients (0.55%) with longer-duration DAPT. Compared with longer-duration DAPT, shorter-duration DAPT had an increased risk of ST [OR, 1.83, 95% CI: 1.03–3.26, P = 0.04; I2 = 0%; Figure 2a]. The results were consistent between abbreviated-term and prolonged-term DAPT studies (P for interaction = 0.76).
Figure 2.
Risk estimates of definite or probable ST (a), MI (b), stroke (c), and TVR (d) with shorter and longer DAPT durations in patients with DM. DAPT: Dual antiplatelet therapy; ST: Stent thrombosis; MI: Myocardial infarction; TVR: Target vessel revascularization; DM: Diabetes mellitus.
Myocardial infarction
MI occurred in 96 patients (3.63%) with shorter-duration DAPT and 73 patients (2.68%) with longer-duration DAPT. No significant difference was found between shorter- and longer-duration DAPT [OR, 1.33, 95% CI: 0.71–2.47, P = 0.37; I2 = 32%; Figure 2b]. The results were consistent between abbreviated-term and prolonged-term DAPT studies (P for interaction = 0.66).
Stroke
Stroke occurred in 21 patients (0.84%) with shorter-duration DAPT and 23 patients (0.89%) with longer-duration DAPT. No significant difference was observed between shorter- and longer-duration DAPT [OR, 0.96, 95% CI: 0.52–1.77, P = 0.90; I2 = 0%; Figure 2c]. The results were consistent between abbreviated-term and prolonged-term DAPT studies (P for interaction = 0.63).
Target vessel revascularization
TVR occurred in 25 patients (2.53%) with shorter-duration DAPT and 20 patients (2.03%) with longer-duration DAPT. No significant difference was observed between shorter- and longer-duration DAPT [OR, 1.19, 95% CI: 0.46–3.07, P = 0.71; I2 = 46%; Figure 2d]. The results were consistent between abbreviated-term and prolonged-term DAPT studies (P for interaction = 0.05).
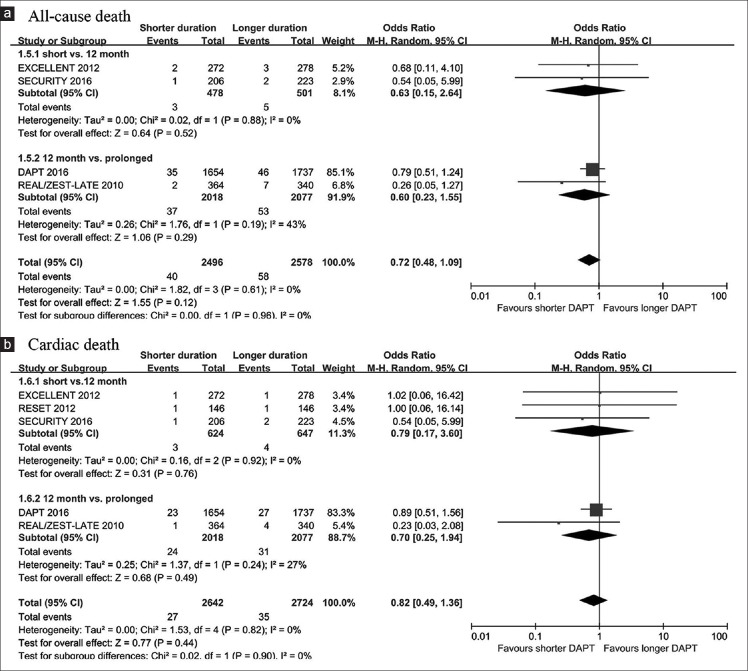
All-cause death and cardiac death
All-cause death occurred in 40 patients (1.60%) with shorter-duration treatment and 58 patients (2.25%) with longer-duration treatment. No significant difference was found between shorter- and longer-duration DAPT [OR, 0.72, 95% CI: 0.48–1.09, P = 0.12; I2 = 0%; Figure 3a]. The results were consistent between abbreviated-term and prolonged-term DAPT studies (P for interaction = 0.96). Similar results were observed for cardiac death [OR, 0.82, 95% CI: 0.49–1.36, P = 0.44; I2 = 0%; P for interaction = 0.90; Figure 3b].
Figure 3.
Risk estimates of all-cause death (a) and cardiac death (b) with shorter and longer DAPT duration in patients with DM. DAPT: Dual antiplatelet therapy; DM: Diabetes mellitus.
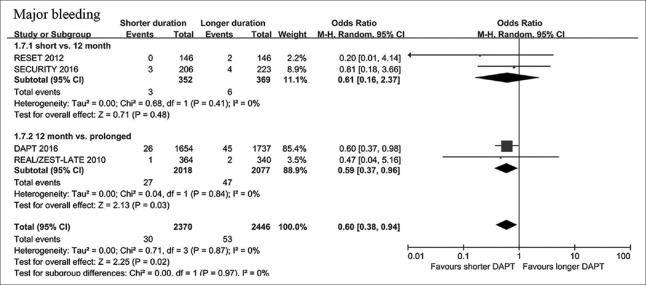
Major bleeding
Major bleeding occurred in 30 patients (1.27%) with shorter-duration DAPT and 53 patients (2.17%) with longer-duration DAPT. Shorter-duration DAPT was associated with a decreased risk of major bleeding [OR, 0.60, 95% CI: 0.38–0.94, P = 0.02; I2 = 0%; Figure 4]. The results were consistent between abbreviated-term and prolonged-term DAPT studies (P for interaction = 0.97).
Figure 4.
Risk estimates of major bleeding with shorter and longer DAPT duration in patients with DM. DAPT: Dual antiplatelet therapy; DM: Diabetes mellitus.
Discussion
In this meta-analysis involving 6040 patients with DM, we found that longer-duration DAPT was associated with a lower risk of ST, but with an increased risk of bleeding.
The optimal DAPT duration following DESs implantation has been assessed by many previous meta-analyses.[12,13] DM took more than 25% of patients undergoing percutaneous coronary intervention,[14] and it is an independent predictor of thrombotic events after coronary stenting.[1,2,15] Endothelial dysfunction, increased monocyte activation, altered smooth muscle cell migration in DM—which were caused by the effects of hyperglycemia—insulin resistance, and altered free fatty acids may account for the increased risk of thromboembolic events.[16] In this way, patients with DM may benefit from longer-duration DAPT. We found that longer-duration DAPT decreased the risk of ST significantly. As the patients involved in our meta-analysis were at a low risk of cardiac events (26.7% patients were presented with low-risk acute coronary syndrome, 20.7% with prior MI, 4.5% with heart failure, 6.1% with renal insufficiency, and 4.3% with prior stroke), our findings might underestimate the benefit of longer-duration DAPT for DM. The observational studies also indicate that patients with DM who received prolonged DAPT had a lower risk of death or MI.[17,18]
Different types of DESs used in patients with DM may make a difference. New-generation DESs might attenuate the benefit of longer-duration DAPT. Two RCTs included in our analyses indicated that patients with DM who received second-generation DESs did not experience any additional benefit from prolonged DAPT.[5,11] The CREATE study also found that in patients with DM who received biodegradable polymer-based DESs, longer DAPT (>6 months) was not beneficial in reducing major adverse cardiovascular events (4.1% vs. 4.9%, P = 0.76).[19]
Registries and clinical trials have found consistently that patients with DM are at higher risk of bleeding compared to patients without DM.[20,21,22,23] We also found that longer-duration DAPT carried an increased risk of bleeding. As patients with DM had activated platelets and increased atherothrombosis and had poor response to clopidogrel,[24,25,26] newer antiplatelet agents were recommended. Recent trials have indicated that patients with DM who received prasugrel or ticagrelor had a greater reduction in ischemic events, without increasing bleeding risk compared to clopidogrel.[27,28]
The DAPT and TL-PAS trials found longer duration of the more intensive DAPT (prasugrel and aspirin), significantly reduced MI, and ST risk with an increased bleeding rate,[29,30] but the subgroup analyses of patients with DM were lacking. Overall, although patients with DM are a high-risk group, with a stronger indication for more intensive DAPT, the optimal duration for DM remains unknown. More trials are needed to determine whether shorter-duration DAPT is enough for patients with DM who receive more intensive DAPT.
Our meta-analysis had several potential limitations. First, the included subgroup analyses were a preset group of large-scale RCTs, and the data regarding DM were not sufficiently reported. For these reasons, we could not explore the impact of the types or the severity or duration of DM on the optimal DAPT duration. Those requiring insulin therapy had high frequencies of chronic kidney disease, heart failure, and previous MI, and were at an increased risk of adverse cardiovascular events compared to noninsulin-dependent DM.[31,32,33,34] Such a high-risk subgroup might require longer-duration DAPT, and further studies are needed for insulin-dependent DM. Second, as literature is scant concerning DAPT durations for DM, the current meta-analysis was hindered in determining the impact of second-generation DESs or new-generation P2Y12 inhibitors on the optimal DAPT duration. Future studies on this topic are needed. Third, as the included patients were at a low risk of thromboembolic events, our finding could not be generalized to high-risk populations.
In conclusion, the current meta-analysis demonstrates that for patients with DM, longer-duration DAPT has a lower risk of ST after DES implantation, but with an increased risk of bleeding. Our results need to be confirmed in RCTs designed specifically for patients with DM.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank Dr. Yan-Ling Liu (the Frederick National Laboratory for Cancer Research) and Dr. Yi-Hua Wu (the Department of Epidemiology and Health Statistics, Zhejiang University School of Public Health) for their comments to improve the manuscript.
Footnotes
Edited by: Yuan-Yuan Ji
References
- 1.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–30. doi: 10.1001/jama.293.17.2126. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 2.Zhao YL, Xu B, Généreux P, Yang YJ, Xu L, Qiao SB, et al. Impact of diabetes status on long-term (6 years) outcomes after percutaneous coronary intervention of left main disease: Result from a real world experience of 1,528 consecutive patients. J Am Coll Cardiol. 2014;63:S13. doi: 10.1016/j.jacc.2014.02.056. [Google Scholar]
- 3.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2013;82:E266–355. doi: 10.1002/ccd.23390. doi: 10.1002/ccd.23390. [DOI] [PubMed] [Google Scholar]
- 4.Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, et al. Authors/Task Force members. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;35:2541–619. doi: 10.1093/eurheartj/ehu278. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 5.Tarantini G, Nai Fovino L, Tellaroli P, Chieffo A, Barioli A, Menozzi A, et al. Optimal duration of dual antiplatelet therapy after second-generation drug-eluting stent implantation in patients with diabetes: The SECURITY (second-generation drug-eluting stent implantation followed by six- versus twelve-month dual antiplatelet therapy)-diabetes substudy. Int J Cardiol. 2016;207:168–76. doi: 10.1016/j.ijcard.2016.01.068. doi: 10.1016/j.ijcard.2016.01.068. [DOI] [PubMed] [Google Scholar]
- 6.Meredith IT, Tanguay JF, Kereiakes DJ, Cutlip DE, Yeh RW, Garratt KN, et al. Diabetes mellitus and prevention of late myocardial infarction after coronary stenting in the randomized dual antiplatelet therapy study. Circulation. 2016;133:1772–82. doi: 10.1161/CIRCULATIONAHA.115.016783. doi: 10.1161/CIRCULATIONAHA.115.016783. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song H, Park GM, Lee CH, Park JS, Ahn JM, Oh JH, et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents in diabetic patients: A subgroup analysis of the randomized, clinical trial. J Am Coll Cardiol. 2010;56:B27. [Google Scholar]
- 9.Kim BK, Hong MK, Shin DH, Nam CM, Kim JS, Ko YG, et al. A new strategy for discontinuation of dual antiplatelet therapy: The RESET Trial (REal safety and efficacy of 3-month dual antiplatelet therapy following endeavor zotarolimus-eluting stent implantation) J Am Coll Cardiol. 2012;60:1340–8. doi: 10.1016/j.jacc.2012.06.043. doi: 10.1016/j.jacc.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 10.Gwon HC, Hahn JY, Park KW, Song YB, Chae IH, Lim DS, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: The efficacy of xience/promus versus cypher to reduce late loss after stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012;125:505–13. doi: 10.1161/CIRCULATIONAHA.111.059022. doi: 10.1161/CIRCULATIONAHA.111.059022. [DOI] [PubMed] [Google Scholar]
- 11.Feres F, Costa R, Bhatt D, De Paula J, Botelho R, Marin-Neto JA, et al. Impact of short- versus long-term DAPT in patients with diabetes mellitus undergoing percutaneous intervention with endeavor zotarolimus-eluting stents –A subanalysis of the large, prospective, randomized, multicenter optimize trial. J Am Coll Cardiol. 2014;63:A1859. [Google Scholar]
- 12.Navarese EP, Andreotti F, Schulze V, Kolodziejczak M, Bufon A, Brouwer M, et al. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: Meta-analysis of randomised controlled trials. BMJ. 2015;350:h1618. doi: 10.1136/bmj.h1618. doi: 10.1136/bmj.h1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassese S, Byrne RA, Ndrepepa G, Schunkert H, Fusaro M, Kastrati A. Prolonged dual antiplatelet therapy after drug-eluting stenting: Meta-analysis of randomized trials. Clin Res Cardiol. 2015;104:887–901. doi: 10.1007/s00392-015-0860-1. doi: 10.1007/s00392-015-0860-1. [DOI] [PubMed] [Google Scholar]
- 14.Smith SC, Jr, Faxon D, Cascio W, Schaff H, Gardner T, Jacobs A, et al. Prevention conference VI: Diabetes and cardiovascular disease: Writing group VI: Revascularization in diabetic patients. Circulation. 2002;105:e165–9. doi: 10.1161/01.cir.0000013957.30622.05. doi: 10.1161/01.CIR.0000013957.30622.05. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Qiao B, Han YL, Li Y, Xu K, Zhang QY, et al. Gender difference on five-year outcomes of EXCEL biodegradable polymer-coated sirolimus-eluting stents implantation: Results from the CREATE study. Chin Med J. 2013;126:1039–45. [PubMed] [Google Scholar]
- 16.Armstrong EJ, Rutledge JC, Rogers JH. Coronary artery revascularization in patients with diabetes mellitus. Circulation. 2013;128:1675–85. doi: 10.1161/CIRCULATIONAHA.113.002114. doi: 10.1161/circulationaha.113.002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faxon DP, Lawler E, Young M, Gaziano M, Kinlay S. Prolonged clopidogrel use after bare metal and drug-eluting stent placement: The Veterans Administration drug-eluting stent study. Circ Cardiovasc Interv. 2012;5:372–80. doi: 10.1161/CIRCINTERVENTIONS.111.967257. doi: 10.1161/circinterventions.111.967257. [DOI] [PubMed] [Google Scholar]
- 18.Thukkani AK, Agrawal K, Prince L, Smoot KJ, Dufour AB, Cho K, et al. Long-term outcomes in patients with diabetes mellitus related to prolonging clopidogrel more than 12 months after coronary stenting. J Am Coll Cardiol. 2015;66:1091–101. doi: 10.1016/j.jacc.2015.06.1339. doi: 10.1016/j.jacc.2015.06.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Han Y, Zhang L, Jing Q, Wang X, Yan G, et al. Clinical efficacy and safety of biodegradable polymer-based sirolimus-eluting stents in patients with diabetes mellitus insight from the 4-year results of the create study. Catheter Cardiovasc Interv. 2013;81:1127–33. doi: 10.1002/ccd.24649. doi: 10.1002/ccd.24649. [DOI] [PubMed] [Google Scholar]
- 20.De Berardis G, Lucisano G, D'Ettorre A, Pellegrini F, Lepore V, Tognoni G, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286–94. doi: 10.1001/jama.2012.5034. doi: 10.1001/jama.2012.5034. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Li Y, Jing QM, Wang XZ, Ma YY, Wang G, et al. Dual antiplatelet therapy over 6 months increases the risk of bleeding after biodegradable polymer-coated sirolimus eluting stents implantation: Insights from the CREATE study. J Interv Cardiol. 2014;27:119–26. doi: 10.1111/joic.12104. doi: 10.1111/joic.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolsky E, Stone GW, Kirtane AJ, Dangas GD, Lansky AJ, McLaurin B, et al. Gastrointestinal bleeding in patients with acute coronary syndromes: Incidence, predictors, and clinical implications: Analysis from the ACUITY (acute catheterization and urgent intervention triage strategy) trial. J Am Coll Cardiol. 2009;54:1293–302. doi: 10.1016/j.jacc.2009.07.019. doi: 10.1016/j.jacc.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al. Antithrombotic Trialists'(ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60. doi: 10.1016/S0140-6736(09)60503-1. doi: 10.1016/s0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavender MA, Steg PG, Smith SC, Jr, Eagle K, Ohman EM, Goto S, et al. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: Outcomes at 4 years from the reduction of atherothrombosis for continued health (REACH) registry. Circulation. 2015;132:923–31. doi: 10.1161/CIRCULATIONAHA.114.014796. doi: 10.1161/circulationaha.114.014796. [DOI] [PubMed] [Google Scholar]
- 25.Wu ZK, Wang JJ, Wang T, Zhu SS, Chen XL, Liu C, et al. Clopidogrel resistance response in patients with coronary artery disease and metabolic syndrome: The role of hyperglycemia and obesity. J Geriatr Cardiol. 2015;12:378–82. doi: 10.11909/j.issn.1671-5411.2015.04.009. doi: 10.11909/j.issn.1671-5411.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Li HY, Qiao R, Yu HY, Zeng H, Gao W, et al. Predictive value of antiplatelet resistance on early stent thrombosis in patients with acute coronary syndrome. Chin Med J. 2013;126:626–33. [PubMed] [Google Scholar]
- 27.Wiviott SD, Braunwald E, Angiolillo DJ, Meisel S, Dalby AJ, Verheugt FW, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38. Circulation. 2008;118:1626–36. doi: 10.1161/CIRCULATIONAHA.108.791061. doi: 10.1161/circulationaha.108.791061. [DOI] [PubMed] [Google Scholar]
- 28.James S, Angiolillo DJ, Cornel JH, Erlinge D, Husted S, Kontny F, et al. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: A substudy from the PLATelet inhibition and patient outcomes (PLATO) trial. Eur Heart J. 2010;31:3006–16. doi: 10.1093/eurheartj/ehq325. doi: 10.1093/eurheartj/ehq325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155–66. doi: 10.1056/NEJMoa1409312. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garratt KN, Weaver WD, Jenkins RG, Pow TK, Mauri L, Kereiakes DJ, et al. Prasugrel plus aspirin beyond 12 months is associated with improved outcomes after TAXUS Liberté paclitaxel-eluting coronary stent placement. Circulation. 2015;131:62–73. doi: 10.1161/CIRCULATIONAHA.114.013570. doi: 10.1161/circulationaha.114.013570. [DOI] [PubMed] [Google Scholar]
- 31.Kuchulakanti PK, Chu WW, Torguson R, Clavijo L, Wolfram R, Mishra S, et al. Sirolimus-eluting stents versus paclitaxel-eluting stents in the treatment of coronary artery disease in patients with diabetes mellitus. Am J Cardiol. 2006;98:187–92. doi: 10.1016/j.amjcard.2006.01.074. doi: 10.1016/j.amjcard.2006.01.074. [DOI] [PubMed] [Google Scholar]
- 32.Stone GW, Kedhi E, Kereiakes DJ, Parise H, Fahy M, Serruys PW, et al. Differential clinical responses to everolimus-eluting and paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation. 2011;124:893–900. doi: 10.1161/CIRCULATIONAHA.111.031070. doi: 10.1161/circulationaha.111.031070. [DOI] [PubMed] [Google Scholar]
- 33.Jain AK, Lotan C, Meredith IT, Feres F, Zambahari R, Sinha N, et al. Twelve-month outcomes in patients with diabetes implanted with a zotarolimus-eluting stent: Results from the E-five registry. Heart. 2010;96:848–53. doi: 10.1136/hrt.2009.184150. doi: 10.1136/hrt.2009.184150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rha SW, Choi CU, Na JO, Lim HE, Kim JW, Kim EJ, et al. Comparison of 12-month clinical outcomes in diabetic and nondiabetic patients with chronic total occlusion lesions: A multicenter study. Coron Artery Dis. 2015;26:699–705. doi: 10.1097/MCA.0000000000000304. doi: 10.1097/MCA.0000000000000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary endpoints for shorter versus longer DAPT duration in patients with DM. DAPT: Dual antiplatelet therapy; DM: Diabetes mellitus.