In the present study, we provide a detailed analysis of the catalytic properties of the bifunctional thiosulfate dehydrogenases/tetrathionate reductases (TsdA) of the human food-borne pathogen Campylobacter jejuni. Structural differences in the immediate environment of Haem 2 were shown to influence the reaction directionality.
Keywords: axial haem ligation, cytochrome c, reaction directionality, tetrathionate reductase, thiosulfate
Abstract
Bifunctional dihaem cytochrome c thiosulfate dehydrogenases/tetrathionate reductases (TsdA) exhibit different catalytic properties depending on the source organism. In the human food-borne intestinal pathogen Campylobacter jejuni, TsdA functions as a tetrathionate reductase enabling respiration with tetrathionate as an alternative electron acceptor. In the present study, evidence is provided that Cys138 and Met255 serve as the sixth ligands of Haem 1 and Haem 2 respectively, in the oxidized CjTsdA wt protein. Replacement of Cys138 resulted in a virtually inactive enzyme, confirming Haem 1 as the active site haem. Significantly, TsdA variants carrying amino acid exchanges in the vicinity of the electron-transferring Haem 2 (Met255, Asn254 and Lys252) exhibited markedly altered catalytic properties of the enzyme, showing these residues play a key role in the physiological function of TsdA. The growth phenotypes and tetrathionate reductase activities of a series of ΔtsdA/*tsdA complementation strains constructed in the original host C. jejuni 81116, showed that in vivo, the TsdA variants exhibited the same catalytic properties as the pure, recombinantly produced enzymes. However, variants that catalysed tetrathionate reduction more effectively than the wild-type enzyme did not allow better growth.
INTRODUCTION
Although tetrathionate (−O3S–S–S–SO3−) has long been known to be used by some bacteria as an electron acceptor under anaerobic conditions, recent evidence suggests that the biochemical and environmental significance of tetrathionate respiration has been hugely underestimated [1,2]. In a single step requiring input of two electrons, tetrathionate is reduced to two molecules of thiosulfate (−S–SO3−). The midpoint reduction potential of the tetrathionate/thiosulfate couple was only very recently determined by experimental means and found to be +198 mV compared with standard hydrogen electrode (SHE) [1], a value considerably more positive than the calculation-based value of +24 mV cited in many bacterial bioenergetics studies [3]. As a consequence, more free energy is available to be harnessed during the respiratory reduction of tetrathionate than was previously recognized. Furthermore, the high relevance of tetrathionate as an in vivo electron acceptor for bacterial pathogenesis is emphasized by the finding that the human intestinal pathogen Salmonella typhimurium induces host-driven formation of tetrathionate from thiosulfate by reactive oxygen species produced during inflammation [4]. The tetrathionate thus formed is used as terminal electron acceptor providing S. typhimurium with a growth advantage over the majority of the commensal flora lacking the capacity for tetrathionate reduction. Nevertheless, the extent to which this capacity is exploited by other enteric pathogens has yet to be fully appreciated. For example, although it has long been known that Citrobacter and Proteus species are also able to perform tetrathionate respiration [5], the mechanism and in vivo significance of this have not been studied.
In terms of the burden of disease, the microaerophilic food-borne pathogen Campylobacter jejuni is responsible for the majority of cases of bacterial gastroenteritis in the Western world, often being far more prevalent than any other enteric bacterium including Salmonella [6]. Normally commensal in the intestinal tract of chickens, in humans C. jejuni causes acute bloody diarrhoea and in some cases the sequelae include neuromuscular paralysis and even death [7,8]. Previously, it was found that some strains of C. jejuni are capable of tetrathionate respiration [2] and it was suggested that this plays an important role in growth in the oxygen-limited human intestinal mucosa; the ability to respire tetrathionate is thus relevant for understanding the pathogenicity of the organism [2]. The finding that C. jejuni strains are capable of tetrathionate reduction and that this can stimulate growth under oxygen-limited conditions added a further dimension to the complex, branched electron transport pathways employed in this organism. Other alternatives to oxygen as the terminal respiratory electron acceptor include fumarate, nitrite, trimethylamine N-oxide and dimethyl sulfoxide [9–11].
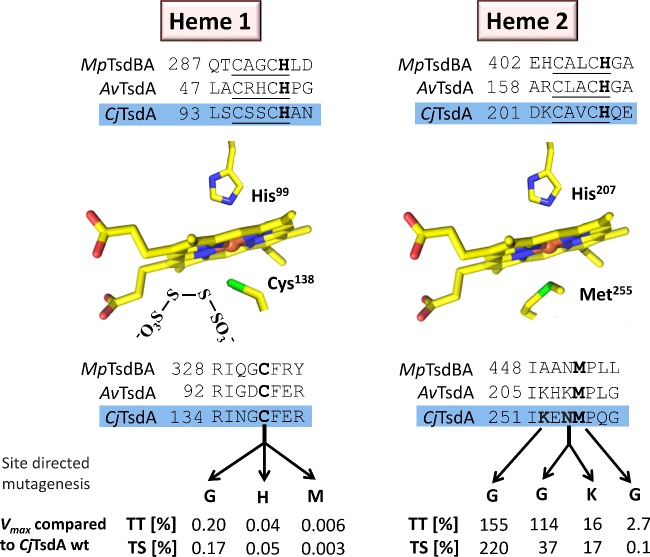
Surprisingly, the enzyme catalysing tetrathionate reduction in C. jejuni was found to be distinct from other known tetrathionate reductases, i.e. the iron–sulfur molybdoenzyme TtrABC found in S. typhimurium [12,13] and the octahaem Otr enzyme from Shewanella oneidensis [14]. Instead, the Campylobacter enzyme belongs to a novel, widely-distributed class of bifunctional thiosulfate dehydrogenase/tetrathionate reductases (TsdA) residing in the bacterial periplasm. TsdA enzymes represent a distinct type of dihaem c-type cytochrome [2,15,16]. An axial histidine/cysteine ligation of the central iron atom has been established for the active site haem (Haem 1) of the enzyme from Allochromatium vinosum, AvTsdA [15,16] (Figure 1). This type of ligation is rare among prokaryotes and appears to be of special importance in sulfur-based energy metabolism. In AvTsdA, Haem 2 exhibits axial His/Lys and His/Met co-ordination in the oxidized and reduced state respectively [16].
Figure 1. Schematic overview of Haem 1 and Haem 2 environments in Tsd(B)A proteins.
Relevant sequence alignments are shown for TsdA from C. jejuni (CjTsdA), A. vinosum (AvTsdA) and the TsdBA fusion protein from M. purpuratum (MpTsdBA). Amino acid numbers are given for the recombinant proteins without signal peptides. In case of CjTsdA, the sequence of the N-terminal Strep-tag is included. In the central left panel, a tetrathionate molecule is shown in vicinity of the active site Haem 1 iron-ligating cysteine, based on the AvTsdA crystal structure [16]. Amino acid numbers in the central panels refer to CjTsdA. In the lower part of the figure, changes in the environments of Haem 1 and Haem 2 are indicated that were introduced by site-directed mutagenesis. The effects of these changes on maximal reaction velocity are listed as percent of Vmax for the wild-type enzyme in the tetrathionate-reducing (TT) and the thiosulfate-oxidizing (TS) direction.
Although all TsdA enzymes characterized to date catalyse both the reduction of tetrathionate and the oxidation of two molecules of thiosulfate to tetrathionate at measurable rates, the enzymes from different bacteria still exhibit very different catalytic properties in vitro and have been shown to possess differences in reaction directionality using biochemical assays with redox dyes and also electrochemical protein film voltammetry [1,2,15,16]. The extent to which tetrathionate production or thiosulfate oxidation is favoured, is intrinsic to each TsdA. The A. vinosum enzyme catalyses tetrathionate reduction at a very low rate that is only measurable in colorimetric solution assays [15,16]. No evidence for reductive catalysis by AvTsdA could be found via protein film cyclic voltammetry whereas CjTsdA appeared clearly biased towards tetrathionate reduction relative to thiosulfate oxidation in these experiments [1]. On graphite electrodes, the enzyme from the anoxygenic phototrophic sulfur bacterium Marichromatium purpuratum, MpTsdBA, displays higher catalytic rates for thiosulfate oxidation than tetrathionate reduction revealing this enzyme's bias to oxidative catalysis [1]. The enzymatic features measured in vitro are in line with the different physiological functions of the TsdA enzymes which can enable either the use of tetrathionate as a terminal electron acceptor as in C. jejuni or the use of thiosulfate as an electron donor for respiration or photosynthesis as is the case in A. vinosum or M. purpuratum.
Very many if not all sequenced C. jejuni strains harbour a second tsdA-related gene. The corresponding protein CccC from C. jejuni strain NCTC 11168 (formerly Cj0037; C8j_0040 in strain 81116) has recently been identified as an efficient electron donor to the cb-oxidase [17]. These proteins contain a methionine in place of the probable Haem 1 ligating cysteine in TsdA. In addition, variation is apparent in the vicinity of the probable Haem 2-ligating methionine. Although all bona fide C. jejuni tsdA sequences encode an asparagine one residue upstream of the methionine, an alanine is present in the C8j_0040-related sequences. In a previous experimental approach towards the in vivo role of TsdA and related proteins in C. jejuni 81116, gene inactivation and complementation showed that the protein corresponding to typical TsdA is absolutely essential for growth on tetrathionate under oxygen limitation. A tsdA null mutant still had low tetrathionate reductase and thiosulfate dehydrogenase activities and slowly reduced tetrathionate probably due to low activity of CccC (Cj8_0040) as tetrathionate reductase, albeit this did not support growth [2].
There are still significant gaps in our knowledge about the physiological roles and biochemical properties of the proteins of the TsdA family. The enzyme from the sulfur oxidizer A. vinosum is the only one for which variants have so far been studied structurally and by UV–Vis spectroscopy. Kinetic characterization was initiated and showed that the replacement of either one of the Haem 2 distal axial ligands lysine or methionine did not render the enzyme catalytically inactive in vitro [15,16]. Initial characterization by UV–Vis absorption spectroscopy indicated that TsdA from C. jejuni 81116 has similar properties as the protein from A. vinosum [2]. However, experimental evidence has so far not been available providing conclusive information about axial haem iron ligation in the C. jejuni enzyme. It has therefore not been possible to assess whether structural differences in the immediate environment of one or both haems may be determinants underlying the enzyme's adaptation to catalysis in the tetrathionate-reducing direction [1]. Detailed kinetic data for a TsdA enzyme adapted to tetrathionate reduction is neither available for a wild-type enzyme nor have any variants been produced or analysed. In vivo data for performance of enzyme variants in the original host organism are so far not available for any TsdA. In the present study we address these areas, initially by characterization of pure recombinant CjTsdA variants, which have allowed us to assess whether the nature of the haem-ligating residues alters the catalytic properties of the enzyme and whether both or only one catalytic direction(s) are affected. In addition, we have determined by in vivo complementation of the C. jejuni 81116 tsdA null mutant with a set of variant TsdAs, the growth capabilities and tetrathionate reduction rates as well as specific tetrathionate reductase and thiosulfate dehydrogenase activities in crude extracts of the resulting strains.
MATERIALS AND METHODS
Bacterial strains, plasmids and growth conditions
Table 1 lists the bacterial strains and plasmids used for the present study. Escherichia coli BL21 (DE3) was used for recombinant protein production and was grown in LB medium. E. coli DH5α was used for molecular cloning. Wild-type and mutant strains of C. jejuni 81116 (NCTC 11828) were used for in vivo studies.
Table 1. Strains and plasmids used in the present study.
| Strains and plasmids | Description | References or sources |
|---|---|---|
| Strains | ||
| E. coli DH5α | F− φ80d lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1hsdR17 (rk− mk+) supE44 λ−thi-1 gyrA relA1 | [37] |
| E. coli BL21 (DE3) | F− ompT hsdSB (rB−mB−) gal dcm met(DE3) | Novagen |
| C. jejuni 81116 (NCTC 11828) | Wild-type | [38] |
| C. jejuni 81116 ΔtsdA | Kanr; insertion of kanamycin resistance cassette in place of c8j_0815 | [2] |
| C. jejuni 81116 ΔtsdA/*tsdA wt | Kanr, Cmr; ΔtsdA mutant complented with c8j_0815 in cj0046 pseudogene locus behind fdxA promotor | The present study |
| C. jejuni 81116 ΔtsdA/*tsdA CG | Kanr, Cmr; ΔtsdA mutant complented with tsdA C138G in cj0046 pseudogene locus behind fdxA promotor | The present study |
| C. jejuni 81116 ΔtsdA/*tsdA MG | Kanr, Cmr; ΔtsdA mutant complented with tsdA M255G in cj0046 pseudogene locus behind fdxA promotor | The present study |
| C. jejuni 81116 ΔtsdA/*tsdA NG | Kanr, Cmr; ΔtsdA mutant complented with tsdA N254G in cj0046 pseudogene locus behind fdxA promotor | The present study |
| C. jejuni 81116 ΔtsdA/*tsdA KG | Kanr, Cmr; ΔtsdA mutant complented with tsdA K252G in cj0046 pseudogene locus behind fdxA promotor | The present study |
| Plasmids | ||
| pEC86 | Cmr, product from pEC66 and pACYC184 with E. coli ccmABCDEFGH genes | [20] |
| pC46-fdxA | C. jejuni complementation vector for integration at the cj0046c pseudogene locus, expressing cloned genes from the fdxA promoter | [39] |
| pET-N-Strep-TsdACj | Apr; N-terminal Strep-tag, f1 origin, T7 Promoter, NcoI-HindIII fragment of PCR amplified tsdA in digested pETStrepDsrJ | [1] |
| pET-N-Strep-TsdACj-C138G | Apr; C138G mutation introduced into pET-N-Strep-TsdACj | The present study |
| pET-N-Strep-TsdACj-C138H | Apr; C138H mutation introduced into pET-N-Strep-TsdACj | The present study |
| pET-N-Strep-TsdACj-C138M | Apr; C138M mutation introduced into pET-N-Strep-TsdACj | The present study |
| pET-N-Strep-TsdACj-M255G | Apr; M255G mutation introduced into pET-N-Strep-TsdACj | The present study |
| pET-N-Strep-TsdACj-N254G | Apr; N254G mutation introduced into pET-N-Strep-TsdACj | The present study |
| pET-N-Strep-TsdACj-N254K | Apr; N254K mutation introduced into pET-N-Strep-TsdACj | The present study |
| pET-N-Strep-TsdACj-K252G | Apr; K252G mutation introduced into pET-N-Strep-TsdACj | The present study |
| pET-N-Strep-TsdACj-KMG | Apr; K252G mutation introduced into pET-N-Strep-TsdACj-M255G | The present study |
| pET-N-Strep-TsdACj-NMG | Apr; N254G mutation introduced into pET-N-Strep-TsdACj-M255G | The present study |
| pET-N-Strep-TsdACj-KNMG | Apr; K252G andN254G mutation introduced into pET-N-Strep-TsdACj-M255G | The present study |
| pC46-fdxA-0815HIS | Cmr; c8j_0815 cloned into pC46-fdxA to produce C-terminal His-tagged TsdA in C. jejuni | The present study |
| pC46-fdxA-TsdACj-C138G | Cmr, C138G mutation introduced into pC46-fdxA-0815 | The present study |
| pC46-fdxA-TsdACj-M255G | Cmr, M255G mutation introduced into pC46-fdxA-0815 | The present study |
| pC46-fdxA-TsdACj-N254G | Cmr, N254G mutation introduced into pC46-fdxA-0815 | The present study |
| pC46-fdxA-TsdACj-K252G | Cmr, K252G mutation introduced into pC46-fdxA-0815 | The present study |
Recombinant DNA techniques
All general molecular genetics techniques were described earlier [18]. Restriction enzymes, T4 ligase and Pfu or Phusion DNA polymerase were obtained from Thermo Scientific or NEB and used according to the manufacturer's instructions. Oligonucleotides for cloning were obtained from Eurofins MWG.
Construction of expression plasmids and site-directed mutagenesis
The C. jejuni tsdA gene coding for the mature protein without the signal peptide was amplified and cloned as described earlier [1]. Point mutations were introduced into tsdA by overlap extension [19] using standard PCR with Pfu DNA polymerase and pET-N-Strep-TsdACj [1] as the template (Table 1). For the TsdA–C138G exchange, two fragments were amplified with the following primers: for the first fragment pET-N-TsdACj_fw and tsdACj-C-Gly_rev, for the second fragment tsdACj-C-Gly_fw and pET-N-TsdACj_rev (Table 2). Both fragments were used as templates for amplification of the complete tsdA gene carrying the desired point mutation. In this step, pET-N-TsdACj_fw and pET-N-TsdACj_rev served as primers (Table 2). The resulting fragment was restricted with BamHI and HindIII and cloned into pET-N-Strep-TsdACj resulting in plasmid pET-N-Strep-TsdACj-C138G. The following plasmids were generated applying the same general strategy: pET-N-Strep-TsdACj-C138G, pET-N-Strep-TsdACj-C138H, pET-N-Strep-TsdACj-C138M, pET-N-Strep-TsdACj-M255G, pET-N-Strep-TsdACj-N254G pET-N-Strep-TsdACj-N254K and pET-N-Strep-TsdACj-K252G (Table 1). For construction of pET-N-Strep-TsdACj-KMG, pET-N-Strep-TsdACj-NMG and pET-N-Strep-TsdACj-KNMG pET-N-Strep-TsdACj-M255G was used as template in PCR.
Table 2. Primer used in the present study.
| Primer | Sequence 5′–3′ | References |
|---|---|---|
| pET-N-TsdACj_fw | CCGGATCCTTAGATCCAAATTTG | [1] |
| pET-N-TsdACj_rev | CCGAAGCTTCTACTTCTTGATCA | [1] |
| 0815-com-F/ATG | GGAGCGTCTCACATGAATAAATTTT CTATAGTTTTAACTTTG | The present study |
| 0815-com-R5XHis | ACGTCTCACATGTTAATGATGATGATGTTTTTTGATCATATTTGTA | The present study |
| tsdACj-C-Gly_fw | GATCAATGGCGGTTTTGAACGCT | The present study |
| tsdACj-C-Gly_rev | AGCGTTCAAAACCGCCATTGATC | The present study |
| tsdACj-C-His_fw | GATCAATGGCCATTTTGAACGCT | The present study |
| tsdACj-C-His_rev | AGCGTTCAAAATGGCCATTGATC | The present study |
| tsdACj-C-Met_fw | GATCAATGGCATGTTTGAACGCT | The present study |
| tsdACj-C-Met_rev | AGCGTTCAAACATGCCATTGATC | The present study |
| tsdACj-M-Gly_fw | TAAAGAAAATGGTCCTCAAGGTG | The present study |
| tsdACj-M-Gly_rev | CACCTTGAGGACCATTTTCTTTA | The present study |
| tsdACj-N-Gly_fw | TATTAAAGAAGGTATGCCTCAAGG | The present study |
| tsdACj-N-Gly_rev | CCTTGAGGCATACCTTCTTTAATA | The present study |
| tsdACj-N-Lys_fw | TATTAAAGAAAAGATGCCTCAAGG | The present study |
| tsdACj-N-Lys_rev | CCTTGAGGCATCTTTTCTTTAATA | The present study |
| tsdACj-K-Gly_fw | CTTCTTATATTGGCGAAAATATGC | The present study |
| tsdACj-K-Gly_rev | GCATATTTTCGCCAATATAAGAAG | The present study |
| tsdACj-NM-Gly_fw | TATTAAAGAAGGTGGTCCTCAAG | The present study |
| tsdACj-NM-Gly_rev | CTTGAGGACCACCTTCTTTAATA | The present study |
| tsdACj-KM-Gly_fw | TTCTTATATTGGCGAAAATGGTCC | The present study |
| tsdACj-KM-Gly_rev | GGACCATTTTCGCCAATATAAGAA | The present study |
| tsdACj-KNM-Gly_fw | TTATATTGGAGAAGGTGGTCCTC | The present study |
| tsdACj-KNM-Gly_fw | GAGGACCACCTTCTCCAATATAA | The present study |
| pC46_TsdA_Mfe_fw | GCCCAATTGAAAATCTAAGTAAAAT | The present study |
| pC46_TsdA_Cla_rev | TGAATCGATAAGGGAATATAGTATT | The present study |
| p46-Scr-F | CCACTCCTGAAGATGGAACAC | The present study |
| p46-Scr-R | ATCATTACAAGAGCTGAAAACCATAC | The present study |
| Cat-Scr-F | AAAAGTTATACCCAACTCTTTTATATGGAG | The present study |
| Cj46-Scr-R | GTGCAAGTTTTAGATCAGTTTATGGA | The present study |
Overproduction, purification and preparation of recombinant TsdA wild-type and variant proteins
Single colonies of E. coli BL21(DE3) containing pET-N-Strep-TsdACj or one of the tsdA variant expression plasmids in addition to plasmid pEC86 [20] were inoculated in 800 ml batches of LB medium containing 100 μg·ml−1 ampicillin and 25 μg·ml−1 chloramphenicol and incubated at 37°C and 180 rpm. Cells were harvested by centrifugation after 15 to 17 h, resuspended in 100 mM Tris/HCl buffer (pH 8) containing 150 mM NaCl and lysed by sonication. After removal of insoluble cell material by centrifugation (10000 g for 25 min at 4°C), TsdA or its variants were purified by Strep-Tactin affinity chromatography with a 5 ml StrepTrap HP column (GE Healthcare) according to manufacturer's instructions. Afterwards, a gel filtration chromatography was performed on a HiLoad 16/60 Superdex 75 pg column (GE Healthcare) using an ÄKTApurifier system (GE Healthcare). The column was equilibrated with 20 mM BisTris/HCl buffer, pH 6.5. The purified protein was concentrated with Amicon Ultra-15 30K centrifugal filter units (Merck Millipore) and desalted with a 5 ml HiTrap Desalting column (GE Healthcare). The protein was stored in 50 mM BisTris buffer, pH 6.5 at −70°C. The concentration of purified protein was determined with the BCA Kit from Pierce. For assessment of purity, SDS/PAGE was performed. Haem staining in acrylamide gels followed an established procedure [21]. Haem content was determined by the pyridine haemochrome method [22].
Construction of C. jejuni ΔtsdA/*tsdA complementation strains
The pC46-fdxA-0815HIS complementation vector integrates a copy of the tsdA gene at the phenotypically silent cj0046c pseudogene locus in C. jejuni, with expression driven by the moderately strong C. jejuni fdxA promoter. It was constructed by cloning the amplicon resulting from PCR of genomic DNA of C. jejuni strain 81116 with primers 0815-com-F/ATG and 0815-com-R5XHis (Table 2) into the BsmBI site of pC46-fdxA (8). The produced TsdA protein has a C-terminal 5 residue His-tag added, that was used for detection in immunoblots (see below). For the TsdA–C138G exchange, two fragments were amplified from pC46-fdxA-0815 with the following primers: for the first fragment pC46_TsdA_Mfe_fw and tsdACj-C-Gly_fw, for the second fragment tsdACj-C-Gly_rev and pC46_TsdA_Cla_rev (Table 2). Both fragments were used as templates for amplification of the complete tsdA gene carrying the desired point mutation. In this step, pC46_TsdA_Mfe_fw and pC46_TsdA_Cla_rev served as primers (Table 2). The resulting fragment was restricted with MfeI and ClaI and cloned into pC46-fdxA resulting in plasmid pC46-fdxA-TsdACj-C138G. The following plasmids were generated applying the same general strategy: pC46-fdxA-TsdACj-M255G, pC46-fdxA-TsdACj-N254G and pC46-fdxA-TsdACj-K255G (Table 1). Plasmids were transformed into the C. jejuni 81116 ΔtsdA mutant strain [2] by electroporation and transformants were selected on Columbia blood agar plates supplemented with chloramphenicol (20 μg·ml−1 final concentration). Complementations were confirmed by PCR of genomic DNA with primers pC46F-Scr-F + pC46-Scr-R and Cat-Scr-F + Cj46-Scr-R (Table 2).
Culture conditions for C. jejuni growth
Campylobacter jejuni strain 81116 was routinely cultured at 42°C under microaerobic conditions [10% (v/v) O2, 5% (v/v) CO2 and 85% (v/v) N2] in a MACS growth cabinet (Don Whitley Scientific) on Columbia agar containing 5% (v/v) lysed horse blood and 10 μg·ml−1 each of amphotericin B and vancomycin. To select C. jejuni mutants, kanamycin or chloramphenicol was added at a final concentration of 50 or 20 μg·ml−1 respectively. Liquid cultures of C. jejuni were routinely grown in Mueller-Hinton broth (Oxoid) supplemented with 20 mM L-serine (MH-S) under standard microaerobic conditions (gas concentrations as above), with 50–100 ml of medium contained in 250 ml conical flasks with continuous orbital shaking at 180 rpm. Except precultures, all cultures contained 50 μM ammonium iron (II) sulfate to induce the fdxA promoter in front of tsdA in the complementation strains. To study tetrathionate-dependent growth, oxygen-limited cultures were grown in 250 ml of brain heart infusion medium with 20 mM L-serine (BHI-S, brain heart infusion medium supplemented with serine) contained in a 250 ml conical flask with no shaking. In this case, 20 mM sodium formate and 15 mM sodium tetrathionate (final concentrations) were added from filter-sterilized stock solutions. Cultures were maintained in the MACS-VA500 incubator and growth was monitored by measuring absorbance at 600 nm. Values for each growth curve were measured with two independent cultures.
SDS/PAGE and immunoblotting with C. jejuni crude cell extract
C. jejuni cells were disrupted by bead beating (Bead Ruptor 12, Omni International) at maximal intensity for 30 s and the crude cell extracts were used for SDS/PAGE. For Western blot analysis, proteins were electroblotted on to nitrocellulose membranes (Amersham Protran 0.45 μm NC, GE Healthcare) for 35 min at 15 V using the Transblot SD semi-dry transfer apparatus (Bio-Rad Laboratories). His-tagged protein was detected with His-tag monoclonal antibody (#70796-3, Novagen) and Goat Anti-Mouse IgG horseradish peroxidase (HRP) Conjugate (#71045-3, Novagen) via the associated peroxidase activity using either chloronaphthol or the SignalFire ECL Reagent kit (Cell Signaling Technology).
Measurement of tetrathionate reduction and thiosulfate respiration rates
Rates of tetrathionate reduction by cell suspensions were measured by adding washed cells to 14 ml 25 mM phosphate buffer (pH 7.4) containing 20 mM sodium formate. Before addition of cells, the buffer was sparged with N2 for 10 min. The buffer-cell suspension was incubated at 42°C for 15 min to allow all of the dissolved oxygen to be consumed. Then, sodium tetrathionate was added to 2 mM final concentration and 0.5 ml samples were taken every 2 or 5 min. The cells were immediately removed from these samples by brief centrifugation (12000 × g, 2 min) and the thiosulfate concentration in the supernatants was measured by the method of Urban [23]. Tetrathionate formation rates were calculated by dividing thiosulfate oxidation rates (μmol·min−1·ml−1) by two and dividing the result by the protein content. For determination of the protein content, cell suspensions were first incubated at 100°C for 20 min. To remove residual thiosulfate and tetrathionate, which interfere with the BCA assay, an acetone precipitation was performed as the next step: 200 μl of cold (−20°C) acetone was added to 50 μl of the pre-boiled cell suspension, followed by mixing and incubation at -20°C for 1 h. BCA standards were treated accordingly. After centrifugation for 15 min at 15000 × g, the supernatant was discarded. The protein pellets were dried by incubation for 25 min at room temperature and subsequently resuspended in 50 μl of H2O. Protein concentration was determined with the BCA Kit from Pierce.
UV–Vis spectroscopy with TsdA in solution
UV–Vis spectra were recorded with an Analytik Jena Specord 210.
Assay of tetrathionate reductase activity with reduced methyl viologen
Spectrophotometric measurements of tetrathionate reductase activity were performed with recombinant C. jejuni TsdA at 42°C for tetrathionate concentrations up to 0.5 mM and used reduced methyl viologen as electron donor were performed along similar lines as described before [2]. However, in our previous work recombinant enzyme was added to reaction mixtures before these were made anoxic by sparging with argon and addition of 2% titanium (III) citrate oxygen scavenger solution. The assays were started by addition of tetrathionate. Here, enzyme was added to completely oxygen-free reaction mixtures containing tetrathionate and pre-reduced methyl viologen. This improved method yielded 20-fold higher specific tetrathionate reductase activities and thus enabled more precise analysis of kinetic parameters. In addition, the range from 0.01 to 0.5 mM tetrathionate was studied at higher resolution. The assay was carried out in a 1 ml quartz glass cuvette closed with a rubber stopper and purged with N2 for 10 min. All buffers and solutions were made anoxic by sparging with N2. The final assay mixture contained 100 mM ammonium acetate buffer, pH 5, 0.3 mM methyl viologen reduced with 1–3 μl of 2% titanium (III) citrate oxygen scavenger solution [24] and different final concentrations of tetrathionate. Enzyme solutions were injected after a relatively stable absorbance at 585 nm was achieved (below 2.0). The rate before addition of enzyme was subtracted from the rate after enzyme addition. Control experiments ensured that reduced methyl viologen was provided at a saturating concentration during all measurements with CjTsdA wt and its derivatives. The specific activity for tetrathionate reductase was calculated using molar absorption coefficient for methyl viologen of 11.8 mM−1·cm−1 at 585 nm. For enzyme activity measurements with crude cell extracts, C. jejuni 81116 (wt), ΔtsdA mutant (ΔtsdA) and the different complementation strains (WT, CG, MG, NG, KG) were first disrupted by bead beating (Bead Ruptor 12, Omni International) at maximal intensity for 30 s. Tetrathionate reduction was measured with 0.1 mM tetrathionate. Protein determination was performed with BCA-Kit from Pierce. Activity is expressed as μmol tetrathionate reduced min−1·mg protein−1 on the basis of a 1:2 molar ratio of tetrathionate reduced to methyl viologen oxidized.
The activity of TsdA and its variants is inhibited as substrate concentration increases. The Ki values given in Table 4 were derived from fitting complete data sets to the general equation for substrate inhibition (eqn 1) [25] using Graph Pad Prism (version 6; Graph Pad).
| 1 |
Table 4. Thiosulfate oxidation catalysed by CjTsdA wt and derivatives.
Enzyme assays were performed at 42°C in 50 mM BisTris buffer (pH 6.5) with a saturating concentration of 80 μM horse heart cytochrome c as electron acceptor and 0.05–8 mM thiosulfate. The units for Vmax are μmol·min−1·mg·protein−1. v compared with [S] plots were fitted to the Hill equation. Ligand changes affected either Haem 1 or Haem 2 as indicated; n, Hill coefficient.
| TsdA | Vmax (units/mg) | S0.5 (μM) | n | |
|---|---|---|---|---|
| WT | 1265±70 | 280±45 | 0.87±0.07 | |
| C138G | 2.1±0.3 | 527±273 | 0.71±0.12 | |
| Haem 1 | C138H | 0.6±0.1 | 343±205 | 0.77±0.32 |
| C138M | 0.04±0.01 | 660±532 | 0.52±0.13 | |
| M255G | 0.7±0.0 | 162±15 | 1.07±0.11 | |
| N254G | 469±29 | 194±34 | 0.94±0.10 | |
| N254K | 211±16 | 157±34 | 0.95±0.18 | |
| Haem 2 | K252G | 2782±275 | 330±79 | 1.24±0.31 |
| NMG | 0.6±0.1 | 72±27 | 0.61±0.13 | |
| KMG | 0.8±0.1 | 67±41 | 0.60±0.18 | |
| KNMG | 0.1±0.0 | 101±29 | 0.78±0.16 |
Kinetic constants other than Ki can be derived from eqn (1). In that case, Vmax is the maximum enzyme velocity if the substrate did not inhibit enzyme activity.
Assay of thiosulfate dehydrogenase activity with horse heart cytochrome c
In the article by Liu et al. [2], ferricyanide was used as the artificial electron acceptor during measurements of thiosulfate dehydrogenase activity at pH 4.0. However, a preliminary set of experiments already showed a much lower S0.5 value for thiosulfate and a near neutral pH optimum of the reaction when horse heart cytochrome c was used as electron acceptor [2]. Therefore, all measurements of thiosulfate dehydrogenase activity were performed with horse heart cytochrome c (80 μM; C7752, Sigma–Aldrich) as the electron acceptor at 42°C in 50 mM BisTris buffer, pH 6.5. Thiosulfate was varied in the range of 0.05–8 mM. Reactions were started by addition of the enzyme and followed by the increase in absorbance at 550 nm. As the reduction of horse heart cytochrome c was measured, a molar absorption coefficient at 550 nm of 21.1 mM−1·cm−1 [26] was used. The presence of oxygen did not affect these measurements. This was ensured by control reactions with CjTsdA wt and derivatives under anoxic conditions.
In the case of enzymes that use two molecules of the same substrate (here thiosulfate) primary v compared with [S] plots provide the best way to examine the data [27]. Data were fitted to the empirical Hill eqn (2) using Graph Pad Prism (version 6; Graph Pad).
| 2 |
The Hill equation resembles the classical Henri–Michaelis–Menten equation; however, the n term allows accounting for non-hyperbolic shapes. A substrate concentration [S]0.5 can be reported that yields half maximal velocity and is characteristic of the process. The constant K, which is not equivalent to Km, characterizes enzyme–substrate interaction. The relationship between K and [S]0.5 is K=[S]0.5n.
For enzyme activity measurements with crude cell extracts, C. jejuni 81116 cells (wild-type), ΔtsdA mutant (ΔtsdA) and the different complementation strains (WT, CG, MG, NG, KG) were first disrupted by bead beating (Bead Ruptor 12, Omni International) at maximal intensity for 30 s. Thiosulfate oxidation was measured with 2 mM thiosulfate. Protein determination was performed with BCA Kit from Pierce. Activity is expressed as μmol tetrathionate produced per min and mg protein on the basis of one molecule tetrathionate formed per two molecules horse heart cytochrome c reduced.
Protein film cyclic voltammetry of CjTsdA wt, N254G and K252G variant
Experiments were performed with a graphite working electrode as described in [1]. Cyclic voltammograms were measured under identical conditions with the freshly polished electrode placed directly into the desired solution, or, coated with enzyme prior to placement in the desired solution. The catalytic currents (icat) due to the activity of the enzyme were defined by subtraction of the response of the bare electrode from that of the enzyme coated electrode. The dependence of icat on potential was independent of whether the scan was to more negative, or more positive, potentials.
Statistical analysis
Average values and standard deviations for Vmax values shown in Tables 3 and 4 as well as for all values shown in Table 5 were calculated from the appropriate data sets using Microsoft Excel 2010. Standard deviations for all further kinetic parameters shown in Tables 3 and 4 were calculated with Graph Pad Prism (version 6; Graph Pad).
Table 3. Tetrathionate reduction catalysed by CjTsdA wt and derivatives.
Enzyme activity was measured under anoxic conditions at 42°C in 100 mM ammonium acetate buffer (pH 5) with a saturating concentration of 0.3 mM methyl viologen previously reduced with titanium (III) citrate. Tetrathionate was varied between 0.01 to 0.7 mM. The units for Vmax are μmol·min−1·mg·protein−1. Data sets were fitted to the general equation for substrate inhibition [25]. Ligand changes affected either Haem 1 or Haem 2 as indicated.
| TsdA | Vmax measured (units/mg) | Vmax (units/mg) | Km (μM) | Ki (μM) | |
|---|---|---|---|---|---|
| WT | 1316±38 | 2328±273 | 74±14 | 420±100 | |
| C138G | 2.63±0.08 | 3.7±0.5 | 34±9 | 810±440 | |
| Haem 1 | C138H | 0.49±0.01 | 0.5±0.1 | 24±10 | 1150±890 |
| C138M | 0.08±0.01 | – | – | – | |
| M255G | 35±1 | 88±13 | 317±61 | 570±160 | |
| N254G | 1502±136 | 3284±457 | 77±15 | 220±70 | |
| N254K | 216±1 | 2214±918 | 360±162 | 20±10 | |
| Haem 2 | K252G | 2045±112 | 3823±528 | 69±15 | 390±130 |
| NMG | 178±3 | 412±127 | 113±51 | 250±130 | |
| KMG | 281±1 | 652±251 | 151±84 | 440±300 | |
| KNMG | 95±3 | 129±16 | 55±14 | 1620±970 |
Table 5. Growth rate and doubling time of different C. jejuni cultures.
Growth parameters were calculated from A600 values taken in the exponential phase of the 81116 wt (81116), ΔtsdA mutant (ΔtsdA) and the different ΔtsdA/*tsdA complementation strains (*tsdA WT, *tsdA C138G, *tsdA M255G, *tsdA N254G, *tsdA K252G). Cells were grown under oxygen-limited conditions at 42°C in almost completely filled 500 ml shake flasks containing BHI-S medium plus 20 mM sodium formate and 15 mM tetrathionate.
| Strain | 81116 | ΔtsdA | *tsdA WT | *tsdA C138G | *tsdA M255G | *tsdA N254G | *tsdA K252G |
|---|---|---|---|---|---|---|---|
| μ | 0.12±0.01 | 0.01±0.00 | 0.24±0.01 | 0.01±0.00 | 0.05±0.00 | 0.25±0.02 | 0.19±0.02 |
| td (h) | 5.6±0.4 | 57.8±14.9 | 2.9±0.2 | 52.3±11.1 | 13.4±1.1 | 2.8±0.3 | 3.6±0.3 |
RESULTS
Production and characterization of wild-type and variant CjTsdA enzymes
CjTsdA wt and variant proteins (see ‘Materials and Methods’ section for details of construction) were produced recombinantly in E. coli BL21 (DE3) containing pEC86 to promote cytochrome c synthesis, purified to homogeneity by Strep-tag affinity chromatography and gel filtration. All CjTsdA proteins behaved as homodimers upon analytical gel permeation chromatography (70–76 kDa) and showed the expected size of 37 kDa (exactly: 37103 Da not including the signal peptide, with two covalently bound haems) upon SDS/PAGE (Supplementary Figure S1). Quantification of CjTsdA by the pyridine haemochrome method established that full haem loading had occurred in all cases (1.8–2.2 mol haem per mol protein). Amino acid numbering applies to recombinant protein without signal peptide but including the N-terminal Strep-tag.
Haem ligation in wild-type CjTsdA
On the basis of amino acid sequence comparisons [2,15], the iron atom of CjTsdA Haem 1 is predicted to be in a six-coordinated low spin state. Strictly conserved histidine (His99) and cysteine (Cys138) residues are likely to serve as axial haem iron ligands (Figure 1). Accordingly, a replacement of CjTsdA Cys138 by a glycine, an amino acid not capable of haem iron co-ordination, led to a low-intensity spectral feature at 622 nm in the oxidized state indicative of the presence of high-spin haem either with water as a sixth ligand or with five-coordinate iron (Figure 2) [28,29] thus confirming Cys138 as the sixth haem ligand of Haem 1.
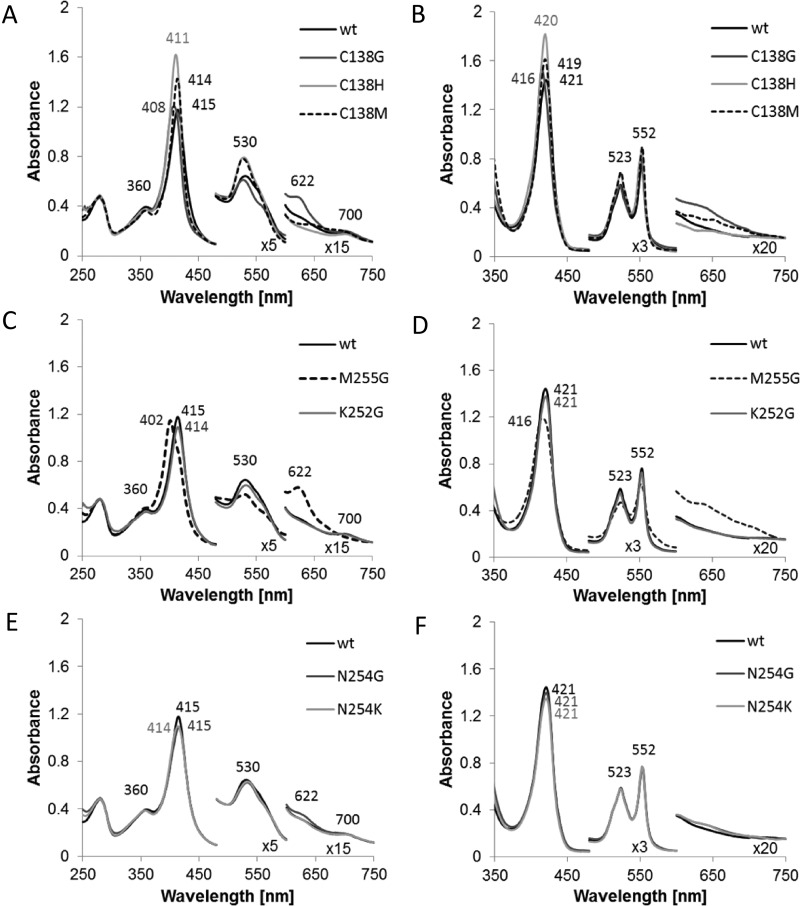
Figure 2. UV–Vis spectra of CjTsdA wt and variants.
UV–Vis spectra of CjTsdA wt are compared with spectra of variants with affected Haem 1 ligation (A and B) and with spectra of variants with affected Haem 2 ligation (C–F). As some of the proteins are partly reduced in the ‘as isolated’ state, up to 30 μM ferricyanide was added to record the oxidized spectrum (A, C and E). For full reduction of the proteins, 5–16 mM Na-dithionite was added (B, D and F). Thirty millimolars BisTris buffer (pH 6.5) were used and spectra were normalized to 280 nm and 750 nm. For CjTsdA M255G and CjTsdA C138G, a high-spin feature appears at 622 nm in the oxidized state. The oxidized spectrum of TsdA wt and all variants except of M255G exhibits a 700 nm peak indicating methionine as haem iron ligand. Protein concentration: 8 μM.
In AvTsdA, Haem 2 exhibits axial His/Lys co-ordination in the oxidized state. A ligand switch from the iron-coordinating Lys208 to Met209 is observed upon reduction of the enzyme [16]. Although the methionine residue is strictly conserved in all TsdA sequences (residue 255 in CjTsdA), variation is apparent at the position of the lysine, which is located one residue closer to the N-terminus (position 254 in CjTsdA). In CjTsdA, an asparagine (Asn254) is found instead of the lysine in the A. vinosum protein (Figure 1). UV–Vis spectroscopy and amino acid replacement clearly revealed that Met255 acts as the sixth axial ligand of Haem 2 iron in CjTsdA, at least in the oxidized state. First, a peak at 700 nm, which is characteristic for methionine as haem iron ligand [28], is clearly apparent in the oxidized spectra of CjTsdA wt (Figure 2A) as well as in CjTsdA variants with N254G, K252G and N254K exchanges (Figures 2C and 2E) but no longer present in the spectrum of a variant with a M255G replacement (Figure 2C). Second, the exchange of Met255 to the ligation-incompetent glycine leads to appearance of a high-spin haem feature at 622 nm [28,29] in the oxidized spectrum (Figure 2C).
Oxidative and reductive assays–experimental approaches and data analysis procedures
Solution assays with redox dyes yielded the first kinetic parameters for CjTsdA [2]. In the present study, we expanded this work to TsdA variants carrying amino acid replacements in the immediate environment of Haem 1 or Haem 2 (Figures 3 and 4, Tables 3 and 4). In addition, parameters for the wild-type enzyme were reassessed under optimized conditions (cf. Materials and Methods). Before describing specific results, some justification of the choice of assays and methods of data analysis are warranted.
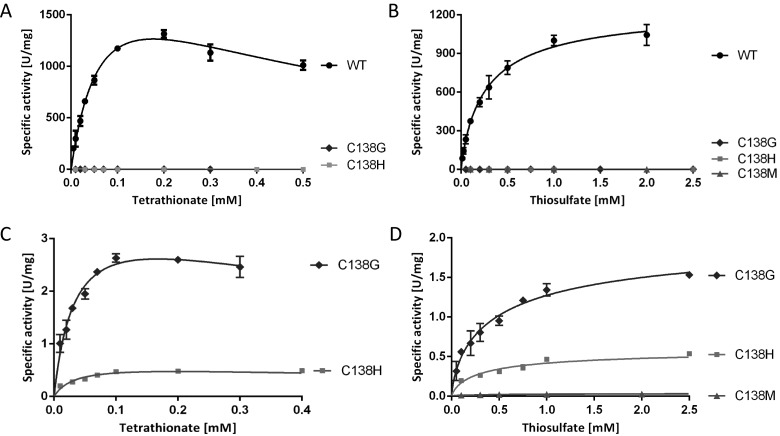
Figure 3. Enzyme activity assays with TsdA wt and Haem 1 ligation affected variants.
TsdA wt was compared with TsdA C138G, C138H and C138M concerning tetrathionate reduction (A) and thiosulfate oxidation (B). Tetrathionate reduction (C) and thiosulfate oxidation (D) of the TsdA C138 variants are shown in detail in the panels below. Tetrathionate reduction (A and C) was measured under anoxic conditions at 42°C in 100 mM ammonium acetate buffer (pH 5) with 0.3 mM methyl viologen previously reduced with titanium (III) citrate. Control measurements showed that methyl viologen was provided at a saturating concentration in all cases. Tetrathionate reduction activity of TsdA C138M was so low that exact kinetic parameters could not be derived with confidence. Different tetrathionate concentrations (0.01–0.7 mM) were used. Thiosulfate oxidation (B and D) was measured at 42°C in 50 mM BisTris buffer (pH 6.5) with 80 μM horse heart cytochrome c as electron acceptor and 0.05–8 mM thiosulfate. Control measurements showed that horse heart cytochrome c was provided at a saturating concentration in all cases.
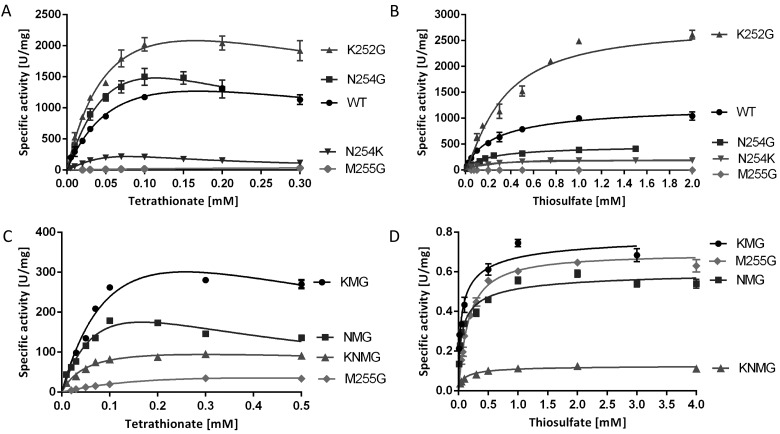
Figure 4. Enzyme activity assays with TsdA wt and Haem 2 ligation affected variants.
Tetrathionate reduction (A and C) was measured under anoxic conditions at 42°C in 100 mM ammonium acetate buffer (pH 5) with 0.3 mM methyl viologen previously reduced with titanium (III) citrate. Different tetrathionate concentrations (0.01–0.7 mM) were used. Thiosulfate oxidation (B and D) was measured at 42°C in 50 mM BisTris buffer (pH 6.5) with 80 μM horse heart cytochrome c as electron acceptor and 0.05–8 mM thiosulfate.
In vivo, one or more of a set of four monohaems cytochrome c’s probably serve as the periplasmic electron carrier mediating electron flow between the cytochrome bc1 complex and the periplasmic TsdA enzyme [2]. This is substantiated by the finding that neither the dihaem cytochrome c TsdB, which functions as natural electron acceptor for TsdA in a number of other organisms [15,30] nor the endogenous Campylobacter dihaem cytochrome c C8j_0040 (Cj0037 or CccC in the reference strain NCTC 11168 [17] is reduced by CjTsdA in vitro [2]. As a consequence, all measurements of thiosulfate dehydrogenase activity reported in the present study were performed at pH 6.5 in the presence of 80 μM horse heart cytochrome c. In the direction of thiosulfate oxidation, data sets need to be fitted to the Hill equation [27]. Conventional Michaelis–Menten kinetics does not apply because the enzyme catalyses the reaction of two molecules of the same substrate (here thiosulfate). The Hill equation does not yield Km but a substrate concentration [S]0.5 can be reported that yields half maximal velocity and is characteristic of the process. The wt enzyme yielded a Vmax of 1265±70 U·mg−1 and [S]0.5 of 280±45 μM.
The assay for tetrathionate reduction used reduced methyl viologen as electron donor. This enzyme activity is inhibited by tetrathionate as a substrate when its concentration is increased (Figure 3). Therefore, data sets have to be fitted to the general equation for substrate inhibition [25], yielding Ki. Kinetic constants other than Ki can also be derived and are tabulated in Table 3. However, in this case Vmax represents the maximum enzyme velocity and Km represents the Michaelis–Menten constant only if the substrate did not inhibit enzyme activity. As such, the calculated Vmax does not yield very useful information for the comparison of different enzyme variants. To circumvent this problem, the highest specific activities actually measured in vitro (Vmax measured) for CjTsdA and its derivatives are also given in Table 3. The wt enzyme yielded a Vmax measured of 1316±38 U·mg−1. Half maximal apparent reaction velocity was reached at 30 μM tetrathionate, whereas the calculated Km (disregarding substrate inhibition) was 74±14 μM.
In view of the above considerations, values for the specificity constant Kcat/Km cannot be derived for CjTsdA in a meaningful way for either of the two reaction directions. Furthermore, general use of Kcat/Km as a comparative index for the catalytic effectiveness of enzyme variants has been seriously questioned [31].
Identification of Haem 1 as the active site of CjTsdA
In addition to glycine, the Haem 1 iron-ligating Cys138 of CjTsdA was replaced by histidine and methionine (Figure 1). Electronic absorption spectra strongly indicated that histidine and methionine acted as haem ligands in the CjTsdA C138H and CjTsdA C138M variants respectively. The Soret peaks increased in intensity in the oxidized as well as in the reduced state (Figures 2A and 2B) consistent with replacement of Cys138 with the stronger field ligands histidine or methionine [32] and in contrast with the C138G variant a high-spin haem feature was not observed. Exchange of Cys138 by glycine, histidine or methionine led to drastic decrease in enzyme activity in all three cases. Tetrathionate reduction and thiosulfate oxidation were equally affected (Figure 3, Tables 3 and 4).
Effect of Haem 2 environment on catalytic properties
As Met255 was clearly identified as an important distal ligand of Haem 2 iron, this residue was changed to glycine. Furthermore, Asn254 and Lys252 were also replaced by glycine (Figure 1). These amino acids are in close vicinity to Haem 2 iron and have theoretical competence for temporary haem ligation. Exchange of Met255 had a dramatic effect on catalysis, with Vmax in the thiosulfate-oxidizing direction reduced by a factor of 1900 as compared with the wild-type protein (Figure 4, Table 4). Thiosulfate oxidation was much more impaired than tetrathionate reduction (Tables 3 and 4, Figure 1). The CjTsdA K252G variant exhibited a higher Vmax than the wild-type enzyme in both catalytic directions with a significantly stronger improvement in the oxidative direction. In contrast, the CjTsdA N254G variant exhibited virtually unaltered catalytic parameters in the tetrathionate-reducing direction, but a much lower activity in the oxidative direction than the wild-type enzyme (Tables 3 and 4). Thus, the N254G variant appears more adapted to catalysing the reductive direction.
In an attempt to create a haem environment in CjTsdA that resembles the situation in AvTsdA, Asn254 was replaced by lysine. This variant exhibited especially pronounced substrate inhibition by tetrathionate with a Ki of 0.02 mM as compared with 0.42 mM for the wild-type enzyme (Table 3, Figure 4). As a consequence, the calculated Vmax exceeded the highest measurable specific activity by a factor of ten. Vmax measured for this variant was 6-fold lower than that for CjTsdA wt. Catalysis in the thiosulfate-oxidizing direction was affected in a comparable manner.
When Asn254 or Lys252 or both residues were changed in addition to Met255, resulting in the CjTsdA KNG, NMG or KNMG derivatives, highest measured Vmax increased in the tetrathionate-reducing direction in all three cases when compared with the M255G variant (Table 3, Figure 4C). In contrast, in the thiosulfate-oxidizing direction the activity of the variants carrying double and triple replacements was impaired in a similar manner as observed for the M255G single mutant (Table 4, Figure 4D). This indicates that a lack of Asn254 and/or Lys252 has a positive effect on tetrathionate reduction. The triple mutant exhibited the lowest specific activity.
Taken together, the above results indicate that the Haem 2 environment has an impact on the catalytic directionality of CjTsdA. Recently, a catalytic bias towards tetrathionate reduction was demonstrated for CjTsdA by protein film voltammetry in solutions containing equal concentrations of thiosulfate and tetrathionate [1]. In these experiments, the enzyme was adsorbed in an electrocatalytically active form on graphite electrodes such that the electrode serves both an electron-accepting and an electron-donating function depending on the potential. Thus, the same reaction is assayed in the forward and the reverse direction and any differences in rate that may be introduced by using different redox dyes for the two different directions are eliminated. As a consequence, we compared protein film voltammetry of CjTsdA WT with that of the two most active variants with an altered Haem 2 environment, TsdA N254G and K252G, to provide an independent measure of the relative catalytic directionality of these proteins. Cyclic voltammetry was performed in solutions containing tetrathionate and thiosulfate at equal concentration for values between 0.025 mM and 0.15 mM (Figure 5). In each case, the negative catalytic currents (icatred) that quantify tetrathionate reduction reached greater magnitudes than the positive catalytic currents (icatox) that quantify thiosulfate oxidation. To assess catalytic bias, values for icatred and icatox were measured at 0.1 and 0.3 V compared with SHE respectively, where there is equal thermodynamic driving force (0.1 V) for each reaction. For a given substrate concentration, icatox (0.3 V)/icatred (0.1 V) followed the order K252G > WT > N254G (Figure 5, inset), which confirms the catalytic directionality deduced from the spectrophotometric assays (Tables 3 and 4). Because the voltammetric experiments were performed in equimolar concentrations of tetrathionate and thiosulfate, the catalytic rate for each reaction is defined by factors that include not just the Km and Vmax values for the relevant substrate but also the impact of substrate and production inhibition on each reaction [1]. As a consequence, comparison of the magnitude of icatox (0.3 V)/icatred (0.1V) with a simple counterpart deduced from the spectrophotometric analyses is not warranted.
Figure 5. Baseline subtracted protein film cyclic voltammetry of CjTsdA wild-type (black), N254G (blue) and K252G variant (red) in 0.05 mM tetrathionate and 0.075 mM thiosulfate.
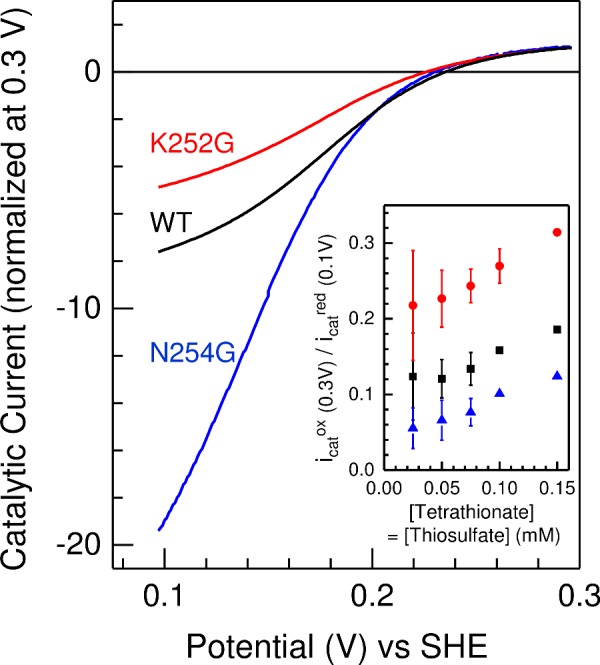
Inset: The dependence of icatox/icatred on substrate concentration for equimolar substrate concentrations; wild-type (black), N254G (blue) and K252G variant (red), see text for details. Values are the average of two independent measurements, errors show maximum and minimum values, except for N254G at 0.1 and 0.15 mM where single values are presented. Scan rate 10 mV·s−1, electrode rotation 500 rpm in 100 mM ammonium acetate, 50 mM NaCl, pH 5 at 42°C.
Impact of the immediate haem environments on the in vivo catalytic properties of CjTsdA
The in vitro studies on recombinant CjTsdA and its variants were complemented by analysing the extent to which substitutions of the Haem 1 ligand Cys138 and the immediate Haem 2 environment (M255G, N254G and K252G) affect the activity of CjTsdA in vivo (i.e with the correct natural physiological electron donor/acceptor) and whether the changes can be correlated to the observations in vitro. Selected CjTsdA variants studied above were produced in the original host C. jejuni strain 81116 in order to study the influence of alterations in CjTsdA axial haem ligation on growth and tetrathionate reduction. To investigate the role of Haem 1 ligation, we employed the C138G variant. However, as our major focus was on the effect of changing the environment of Haem 2 on in vivo activities, we chose the M255G, N254G and K252G variants for particular attention. The previously constructed tsdA deletion mutant [2] was complemented by integration of either the wild-type or the tsdA genes encoding these variant proteins at the cj0046 pseudogene locus, using a vector in which gene expression was driven by the strong fdxA (ferredoxin) promoter from C. jejuni. Strains with complemented tsdA genes produced C-terminally His-tagged CjTsdA enabling Western Blot analysis, thus allowing a comparison of the amounts of wild-type and TsdA variants in vivo.
SDS/PAGE analysis of crude cell extracts of C. jejuni 81116, the ΔtsdA mutant and the ΔtsdA/*tsdA complementation strains did not reveal a specific stained protein band correlating in size with TsdA (37103 Da; without signal peptide, with haems) (Figure 6A). Under the conditions applied, TsdA thus does not appear to be of major abundance in C. jejuni. Haem staining (Figure 6B) as well as Western blot analysis (Figures 6C and 6D) clearly revealed that the *tsdA wt, *tsdA N254G and *tsdA K252G complementation strains produced the different TsdA variants in comparable amounts. TsdA M255G was produced in a lower amount and TsdA C138G was hardly detectable. Either these variants are inefficiently produced or are degraded in C. jejuni. C. jejuni 81116 as well as the ΔtsdA mutant do not produce His-tagged protein and were used as negative controls in Western Blot analysis. The haem stained gel shows that less TsdA is formed in C. jejuni 81116 than in the ΔtsdA/*tsdA wt complementation strain, which suggests that the fdxA promoter in front of tsdA in the complementation strains is stronger than the natural tsdA promoter. For the ΔtsdA mutant, a protein was detected after haem staining, which is slightly larger than TsdA (Figure 6B). This band is likely to be C8j_0040, the dihaem cytochrome c equivalent to CccC in C. jejuni NCTC 11168 [17]. C8j_0040 has detectable sequence homology to TsdA, but does not exhibit clear enzyme activity [2]. The uppermost visible band in the haem stained gels can be unequivocally identified as the 71 kDa protein MccA, a multi-haem sulfite reductase, which is the largest c-type cytochrome encoded in the strain 81116 genome [17].
Figure 6. SDS/PAGE of crude cell extracts of C. jejuni 81116 wt, ΔtsdA mutant and complementation strains.
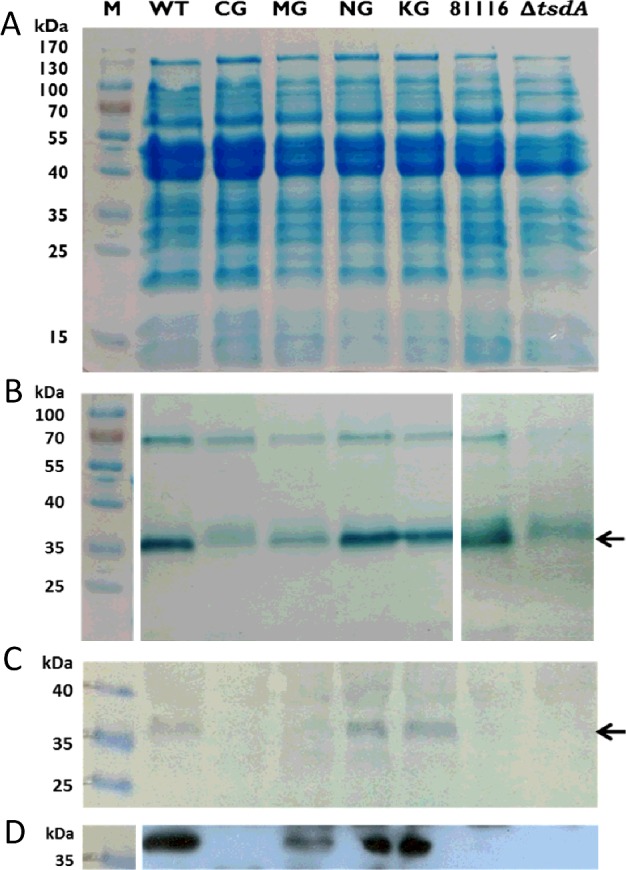
C. jejuni cells of 81116 wt (81116), ΔtsdA mutant (ΔtsdA) and the different complementation strains (*tsdA WT, *tsdA C138G, *tsdA M255G, *tsdA N254G, *tsdA K252G) were disrupted by bead beating and the crude cell extracts were used for SDS/PAGE. Forty micrograms of protein per lane were loaded on the gels for Coomassie staining (A) and Western blots (C and D), and 27 μg of protein per lane was used for haem staining (B). For the Western blots, antibody against His-tag was used and the second antibody was a HRP conjugate. The detection was based on the reaction of HRP with chloronaphthol (C) or the interaction between HRP and ECL reagent (D). The 37 kDa protein CjTsdA is marked with an arrow. The approximately 70 kDa haem stained band in panel (B) is the MccA multi-haem sulfite reductase (see text).
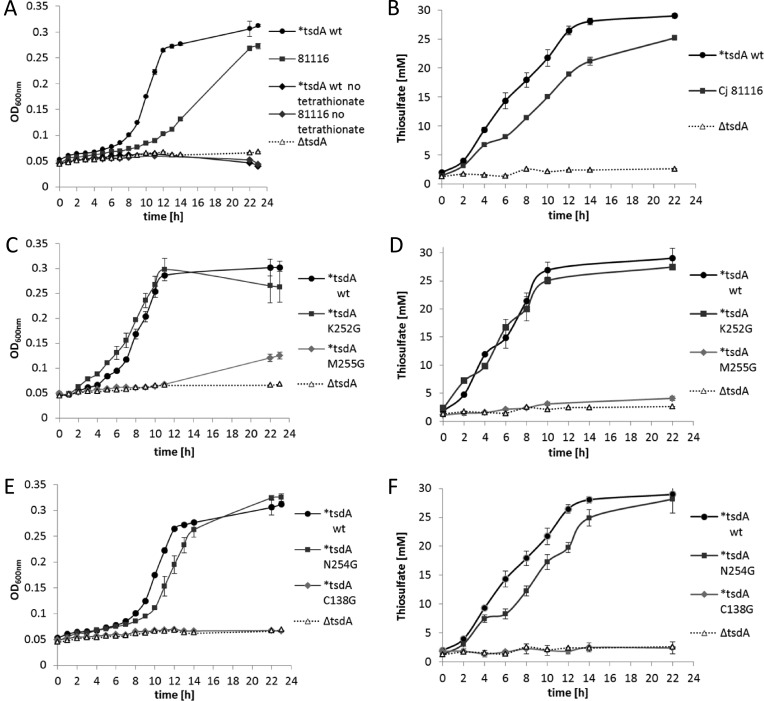
Growth experiments were performed with C. jejuni 81116, the ΔtsdA mutant and the different ΔtsdA/*tsdA complementation strains to analyse the impact of changes in TsdA haem ligation on the growth of C. jejuni with tetrathionate as electron acceptor. The cells were grown under oxygen-limited conditions at 42°C in almost completely filled 500 ml shake flasks containing BHI-S medium plus 20 mM sodium formate and 15 mM tetrathionate. As a control experiment, C. jejuni 81116 and the ΔtsdA/*tsdA wt complementation strain were grown with and without tetrathionate (Figure 7A). As the strains did not grow without added tetrathionate, but did grow with it, we ensured that the growth of the different C. jejuni strains used in this experiment is solely supported by tetrathionate as electron acceptor and not by residual oxygen in the medium. The ΔtsdA mutant did not grow with tetrathionate (Figure 7A) confirming that TsdA is indispensable for tetrathionate respiration in C. jejuni. The ΔtsdA/*tsdA wt complementation strain grew faster than the wild-type strain and exhibited a 2-fold lower doubling time (Table 5) indicating again that the fdxA promoter in front of tsdA in the complementation strains is stronger than the natural tsdA promoter. In accordance, the ΔtsdA/*tsdA wt complementation strain converted 15 mM tetrathionate much faster into 30 mM thiosulfate than the C. jejuni 81116 strain (Figure 7B).
Figure 7. Anaerobic growth of C. jejuni 81116 wt, ΔtsdA mutant and complementation strains.
C. jejuni cells of 81116 wt (81116), ΔtsdA mutant (ΔtsdA) and the different ΔtsdA/*tsdA complementation strains (*tsdA WT, *tsdA C138G, *tsdA M255G, *tsdA N254G, *tsdA K252G) were grown under oxygen-limited conditions at 42°C in almost completely filled 500 ml shake flasks containing BHI-S medium plus 20 mM sodium formate and 15 mM tetrathionate. In (A, C and E), the absorbance at 600 nm of the wild-type 81116 strain (closed black circles) is compared with that of an 81116 tsdA mutant (open triangles) and the different ΔtsdA/*tsdA complementation strains. In the absence of added tetrathionate [dark grey and black diamonds in (A)], no growth of either strain occurred. In (B, D and F), the conversion of tetrathionate to thiosulfate in each of the culture supernatants at each time point corresponding to the growth curves in (A, C and E) is shown.
As shown in Figure 6, only the *tsdA wt, *tsdA N254G and *tsdA K252G complementation strains produced the respective TsdA variants in similar amounts and are thus comparable between each other. The ΔtsdA/*tsdA NG and *tsdA KG complementation strains all show growth behaviour (Figures 7C and 7E) and doubling times (Table 5) similar to the ΔtsdA/*tsdA wt complementation strain. Moreover, all these strains converted 15 mM tetrathionate completely into thiosulfate (Figures 7D and 7F). Obviously, growth is not limited by this step of the respiratory chain. The ΔtsdA/*tsdA MG complementation strain showed only very little growth after several hours of incubation (Figure 7C, Table 5) and only small amounts of thiosulfate were produced (Figure 7D). This strain did not produce high amounts of TsdA M255G (Figure 6). The ΔtsdA/*tsdA C138G complementation strain showed a similar growth behaviour as the ΔtsdA mutant (Figure 7E, Table 5) and thiosulfate was not formed (Figure 7F).
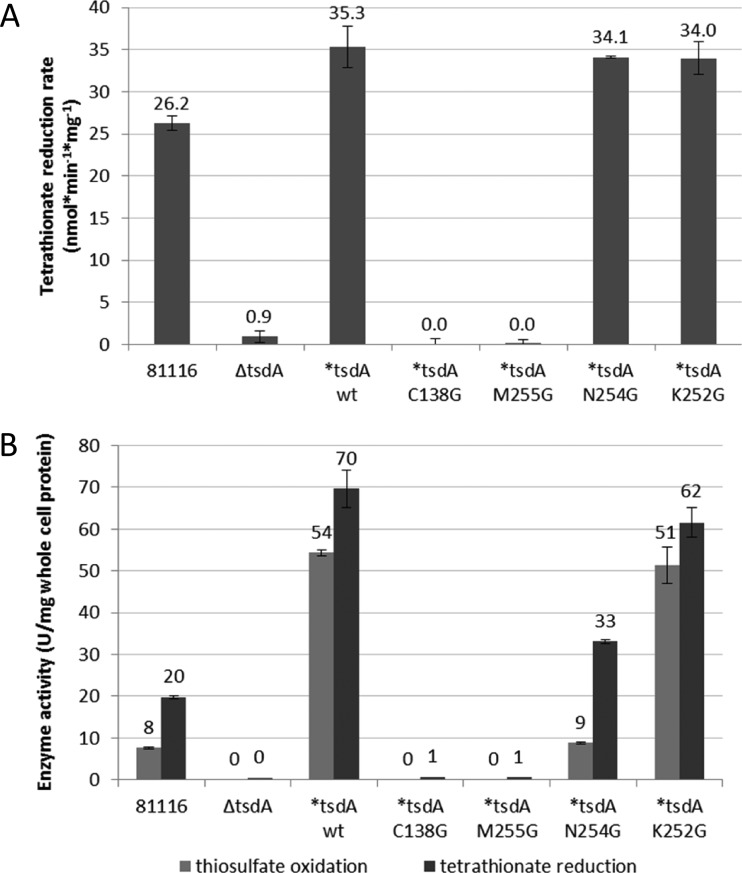
In order to evaluate the rate of electron transfer to TsdA in vivo, the formate-dependent tetrathionate reduction rate over a short time period was determined with resting cells of C. jejuni 81116, the ΔtsdA mutant and the different ΔtsdA/*tsdA complementation strains (Figure 8A). The ΔtsdA/*tsdA wt, the *tsdA K252G and the *tsdA N254G complementation strains did not show large differences in the tetrathionate reduction rate. The C. jejuni 81116 strain exhibits a lower tetrathionate reduction rate than the ΔtsdA/*tsdA wt complementation strain, because this strain does not produce as much TsdA as the complementation strains (Figure 8). The ΔtsdA/*tsdA M255G and the *tsdA C138G complementation strains as well as the ΔtsdA mutant do not reduce tetrathionate, as previously discussed.
Figure 8. Determination of tetrathionate reduction rate with whole cells and enzyme activity assays with crude cell extract.
For determination of tetrathionate reduction rate with whole cells (A) C. jejuni cells of 81116 wt (81116), ΔtsdA mutant (ΔtsdA) and the different complementation strains (*tsdA WT, *tsdA C138G, *tsdA M255G, *tsdA N254G, *tsdA K252G) were incubated at 42°C in 14 ml phosphate buffer (pH 7.4) containing 20 mM sodium formate and 2 mM tetrathionate. The thiosulfate concentrations in the supernatants of samples taken at 2 or 5 min intervals were determined directly after the experiment. For enzyme activity assays with crude cell extract (B) C. jejuni cells of 81116 wt (81116), ΔtsdA mutant (ΔtsdA) and the different complementation strains (*tsdA WT, *tsdA C138G, *tsdA M255G, *tsdA N254G, *tsdA K252G) were disrupted by bead beating. Tetrathionate reduction was measured anaerobically at 42°C in 100 mM ammonium acetate buffer (pH 5) with 0.3 mM methyl viologen previously reduced with titanium (III) citrate and 0.1 mM tetrathionate. Thiosulfate oxidation was performed at 42°C in 50 mM BisTris buffer (pH 6.5) with 80 μM horse heart cytochrome c as electron acceptor and 2 mM thiosulfate.
In a last set of in vivo experiments, we used crude cell extracts of the same strains to measure specific enzyme activities (Figure 8B). C. jejuni strain 81116 showed much higher tetrathionate reduction than thiosulfate oxidation activity, demonstrating that wild-type CjTsdA works best as a tetrathionate reductase and agreeing with earlier results on the pure enzyme [2]. The activities of the ΔtsdA/*tsdA wt complementation strain are higher than those of C. jejuni 81116 in both directions, as more TsdA is produced in this strain. The ΔtsdA mutant, the ΔtsdA/*tsdA M255G and the *tsdA C138G complementation strains did not show enzyme activity (Figure 8B), explaining the inability of the respective strains to reduce or to grow on tetrathionate. The N254G and K252G complementation strains showed tetrathionate reductase activity of same order of magnitude as for WT (consistent with expression levels and growth phenotypes).
DISCUSSION
In the present study, we assessed how the nature of the haem environments alters the catalytic properties of the dihaem cytochrome c tetrathionate reductase/thiosulfate dehydrogenase TsdA in vivo and in vitro and chose the enzyme from C. jejuni as an example. First, we demonstrated without ambiguity that Haem 1 in CjTsdA is axially ligated by a cysteine (Figure 1). Haem 1 constitutes the active site of CjTsdA, as enzyme activity is almost completely abolished in both directions when the Haem 1 ligating Cys138 is replaced. Only very recently it has been unambiguously demonstrated that catalysis of thiosulfate oxidation by TsdA and very probably also that by the other c-type cytochrome catalysing an oxidative reaction affecting thiosulfate, namely SoxXA, involves formation of a covalent adduct between the sulfane sulfur atom of thiosulfate and the Sγ of the active site cysteine [30]. The finding that the CjTsdA C138G, C138M and C138H variants still exhibit some residual activity shows that the covalent reaction intermediate is not absolutely required for catalysis. In CjTsdA, a change of Cys138 to iron-ligation competent histidine or methionine impaired catalysis much more than a replacement by glycine (Figure 1, Table 1), which cannot ligate the haem iron and yields a significant enzyme population containing five-coordinated high-spin haem or high-spin haem with water as a sixth ligand (Figure 2A). This shows that the temporary presence of five-coordinated high-spin haem, which cannot be generated in the C138H and C138M variants, is an important though not absolutely essential prerequisite for the reaction to proceed. Thus, the concept is strengthened that movement of the Haem 1 ligating cysteine out of the haem iron co-ordination sphere and covalent attachment of thiosulfate to the cysteine's Sγ in this state is a central part of the TsdA reaction cycle [16,33]. The finding that a cysteine S-thiosulfonate intermediate is formed during thiosulfate oxidation catalysed by TsdA in combination with the reaction mechanism proposed by Grabarczyk et al. [34] for AvTsdA catalysis led to the conclusion that in case of thiosulfate oxidation formation of the cysteine S-thiosulfonate releases two electrons that reduce the iron atoms of the two haems in TsdA to the Fe(II) state. After haem reoxidation by an external electron acceptor a thiol–disulfide exchange reaction is likely to proceed via an attack of the sulfane atom of a second thiosulfate molecule on the thiosulfonate group. In the tetrathionate-reducing direction, the central sulfur–sulfur bond of the tetrathionate molecule first would be cleaved by attack of the active site cysteine's Sγ atom thus releasing the first thiosulfate molecule and creating the cysteine S-thiosulfonate. From this intermediate, the second thiosulfate would be reductively released with two electrons delivered by an external electron donor via the two TsdA haem groups.
TsdA Haem 2 acts as an electron relay centre and wires the active site to the enzyme's redox partner [16]. An important difference between the tetrathionate-reduction adapted CjTsdA and the thiosulfate-oxidizing enzyme from A. vinosum, is indeed apparent at this haem. At AvTsdA Haem 2, a ligand change occurs from lysine to methionine upon reduction [16]. In contrast, we found for CjTsdA that the equivalent methionine residue is ligating Haem 2 iron in the oxidized state. The replacement of Met255 by glycine led to a strong impairment in both catalytic directions, however the effect on tetrathionate formation was much more severe. The simple attempt to create a haem environment in CjTsdA resembling the situation in AvTsdA by exchanging Asn254 for lysine did not have the expected effect. As evidenced by UV–Vis spectroscopy, the exchange did not lead to a replacement of Met255 as the haem iron ligand by the newly introduced lysine in the oxidized state (Figure 2E). Furthermore, the replacement negatively affected Vmax in both the tetrathionate-forming and the tetrathionate-reducing direction and did not cause adaptation of TsdA to catalysing thiosulfate oxidation as seen for AvTsdA.
Unexpectedly, the largest changes in CjTsdA reaction directionality and catalytic efficiency were observed upon replacing Asn254 by glycine instead of lysine and exchanging Lys252 to glycine (Figures 1 and 5) demonstrating the importance of those amino acid residues for functionality of the enzyme. The K252G exchange improved CjTsdA, resulting in higher Vmax in both catalytic directions. However, the reaction directionality of this variant also appeared to shift towards thiosulfate oxidation in solution assays and this was substantiated by protein film electrochemistry. In contrast, the CjTsdA N254G variant exhibited lower specific activity in the oxidative direction than the wild-type enzyme and proved more adapted to catalysing the tetrathionate-reducing direction in electrochemical experiments. These differences might be explicable by altered redox potentials of Haem 2, but more subtle changes in intramolecular electron transfer or reorganization energy might also apply [35,36].
A surprising level of complexity became apparent through the finding that replacement of Asn254 by glycine or lysine leads to a much stronger substrate inhibition by tetrathionate than observed for wild-type CjTsdA. This demonstrates that alterations at Haem 2 have an effect on the active site haem and its catalytic properties, indicating interaction between the two haem groups of TsdA. This is corroborated by the finding that the Km and S0.5 values vary between the CjTsdA derivatives with altered Haem 2 ligands (Tables 3 and 4). The same has been observed for AvTsdA [16].
Adaptation of a given TsdA to catalyse one or the other reaction direction, i.e. tetrathionate reduction or thiosulfate oxidation should have implications on its function in metabolic networks in vivo, i.e. how effectively tetrathionate reduction can be used as an alternative respiratory process. In the present study, we assessed this question by complementing a C. jejuni 81116 mutant strain devoid of the tsdA gene with TsdA variants exhibiting different specific activity and catalytic adaptation. The C138G variant lacking the active site cysteine was produced in very small amounts (Figure 6) and its specific activity was very low. It was therefore not surprising that this complementation strain was unable to grow with tetrathionate as electron acceptor. The same holds true for the M255G variant, which did not support significant growth with tetrathionate as respiratory electron acceptor. The catalytic properties of TsdA measured in crude cell extracts of the ΔtsdA/*tsdA wt complementation strain appear similar to those of pure recombinant TsdA wt (Tables 3 and 4, Figure 8B). Crude extracts of the ΔtsdA/*tsdA K252G complementation strain showed activities very similar to the ΔtsdA/*tsdA wt complementation strain. For the ΔtsdA/*tsdA N254G complementation strain, enzyme activities per mg cell protein were not as high as for the ΔtsdA/*tsdA wt complementation strain, but activity in the thiosulfate oxidizing direction was much more negatively affected than tetrathionate reduction. Growth characteristics for the strains complemented with the wild-type enzyme and the CjTsdA N254G and K252G variants did not differ significantly. This was surprising for the *tsdA K252G complementation strain because the TsdA K252G variant clearly exhibited higher specific activity than the wild-type enzyme in vitro and was present in similar amounts (Figure 6). We conclude that tetrathionate reduction by TsdA is not the limiting step in the respiratory chain of C. jejuni under the conditions applied.
In conclusion, we identified the haem iron ligands of CjTsdA and showed that Haem 2 iron ligation is different to that observed for AvTsdA in the oxidized state. The Haem 1 axial ligand cysteine and the Haem 2 iron-ligating methionine are indispensable for efficient enzyme function in vitro and in vivo. Structural differences in the immediate environment of Haem 2 were shown to contribute to defining the reaction directionality. Ongoing studies in our laboratories aim to gain further understanding of the factors that define the catalytic bias of TsdA enzymes.
Acknowledgments
We thank Yang-Wei Liu for construction of the pC46-fdxA-0815HIS vector and Nitanshu Garg and Michael White for assistance with the experiments performed in Sheffield. This article is dedicated to Christiane Dahl's esteemed academic teacher Hans G. Trüper who passed away on March 9, 2016.
Abbreviations
- BHI-S
brain heart infusion medium supplemented with serine
- HRP
horseradish peroxidase
- SHE
standard hydrogen electrode
- TsdA
thiosulfate dehydrogenases/tetrathionate reductases
- wt
wild-type
AUTHOR CONTRIBUTION
Julia Kurth performed all experiments. Julia Kurth, Christiane Dahl, Julea Butt and David Kelly designed and analysed experiments. Christiane Dahl conceived and co-ordinated the study. David Kelly supervised and co-ordinated the in vivo experiments shown in Figures 6 and 7 and Table 5. Julea Butt supervised and co-ordinated the electrochemical experiments shown in Figure 5. Julia Kurth and Christiane Dahl wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
FUNDING
This work was supported by the Deutsche Forschungsgemeinschaft [grant number DA 351/7-2]; the Aventis Foundation and Stiftung Stipendien-Fonds des Verbandes der Chemischen Industrie [grant number 700051 (to J.M.K.)]; and the UK Biotechnology and Biological Sciences Research Council [grant numbers BB/L022176/1 and BB/K009885/1].
References
- 1.Kurth J., Dahl C., Butt J.N. Catalytic protein film electrochemistry provides a direct measure of the tetrathionate/thiosulfate reduction potential. J. Am. Chem. Soc. 2015;137:13232–13235. doi: 10.1021/jacs.5b08291. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y.W., Denkmann K., Kosciow K., Dahl C., Kelly D.J. Tetrathionate stimulated growth of Campylobacter jejuni identifies TsdA as a new type of bi-functional tetrathionate reductase that is widely distributed in bacteria. Mol. Microbiol. 2013;88:173–188. doi: 10.1111/mmi.12176. [DOI] [PubMed] [Google Scholar]
- 3.Thauer R.K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winter S.E., Thiennimitr P., Winter M.G., Butler B.P., Huseby D.L., Crawford R.W., Russell J.M., Bevins C.L., Adams L.G., Tsolis R.M., et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oltman L.F., Claasen V.P., Kastelein P., Reijinders W.N.M., Stouthammer A.H. The influence of tungstate on the formation and activities of four reductases of Proteus mirabilis: identification of two new molybdoenzymes, chlorate reductase and tetrathionate reductase. FEBS Lett. 1979;106:43–46. doi: 10.1016/0014-5793(79)80691-2. [DOI] [PubMed] [Google Scholar]
- 6.Havelaar A.H., Ivarsson S., Lofdahl M., Nauta M.J. Estimating the true incidence of campylobacteriosis and salmonellosis in the European Union, 2009. Epidemiol. Infect. 2013;141:293–302. doi: 10.1017/S0950268812000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaser M.J., Engberg J. Clinical aspects of Campylobacter jejuni and Campylobacter coli infections. In: Nachamkin I., Szymanski G., Blaser M.J., editors. Campylobacter. Washington, DC: ASM Press; 2008. pp. 99–121. [Google Scholar]
- 8.Jacobs B.C., van Belkum A., Endtz H.P. Gullain-Barré syndrome and Campylobacter infection. In: Nachamkin I., Szymanski G., Blaser M.J., editors. Campylobacter. Washington, DC: ASM Press; 2008. pp. 245–261. [Google Scholar]
- 9.Sellars M.J., Hall S.J., Kelly D.J. Growth of Campylobacter jejuni supported by respiration of fumarate, nitrate, nitrite, trimethylamine-N-oxide, or dimethyl sulfoxide requires oxygen. J. Bacteriol. 2002;184:4187–4196. doi: 10.1128/JB.184.15.4187-4196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weingarten R.A., Taveirne M.E., Olson J.W. The dual functioning fumarate reductase is the sole succinate:quinone reductase in Campylobacter jejuni and is required for full host colonization. J. Bacteriol. 2009;191:5293–5300. doi: 10.1128/JB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pittman M.S., Elvers K.T., Lee L., Jones M.A., Poole R.K., Park S.F., Kelly D.J. Growth of Campylobacter jejuni on nitrate and nitrite: electron transport to NapA and NrfA via NrfH and distinct roles for NrfA and the globin Cgb in protection against nitrosative stress. Mol. Microbiol. 2007;63:575–590. doi: 10.1111/j.1365-2958.2006.05532.x. [DOI] [PubMed] [Google Scholar]
- 12.Hinojosa-Leon M., Dubourdieu M., Sanchez-Crispin J.A., Chippaux M. Tetrathionate reductase from Salmonella typhimurium: a molybdenum containing enzyme. Biochem. Biophys. Res. Commun. 1986;136:577–581. doi: 10.1016/0006-291X(86)90479-1. [DOI] [PubMed] [Google Scholar]
- 13.Hensel M., Hinsley A.P., Nikolaus T., Sawers G., Berks B.C. The genetic basis of tetrathionate respiration in Salmonella typhimurium. Mol. Microbiol. 1999;32:275–287. doi: 10.1046/j.1365-2958.1999.01345.x. [DOI] [PubMed] [Google Scholar]
- 14.Mowat C.G., Rothery E., Miles C.S., McIver L., Doherty M.K., Drewette K., Taylor P., Walkinshaw M.D., Chapman S.K., Reid G.A. Octaheme tetrathionate reductase is a respiratory enzyme with novel heme ligation. Nat. Struct. Mol. Biol. 2004;11:1023–1024. doi: 10.1038/nsmb827. [DOI] [PubMed] [Google Scholar]
- 15.Denkmann K., Grein F., Zigann R., Siemen A., Bergmann J., van Helmont S., Nicolai A., Pereira I.A.C., Dahl C. Thiosulfate dehydrogenase: a wide-spread unusual acidophilic c-type cytochrome. Environ. Microbiol. 2012;14:2673–2688. doi: 10.1111/j.1462-2920.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 16.Brito J.A., Denkmann K., Pereira I.A.C., Archer M., Dahl C. Thiosulfate dehydrogenase (TsdA) from Allochromatium vinosum: structural and functional insights into thiosulfate oxidation. J. Biol. Chem. 2015;290:9222–9238. doi: 10.1074/jbc.M114.623397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y.W., Kelly D.J. Cytochrome c biogenesis in Campylobacter jejuni requires cytochrome c6 (CccA; Cj1153) to maintain apocytochrome cysteine thiols in a reduced state for haem attachment. Mol. Microbiol. 2015;96:1298–1317. doi: 10.1111/mmi.13008. [DOI] [PubMed] [Google Scholar]
- 18.Dahl C., Schulte A., Stockdreher Y., Hong C., Grimm F., Sander J., Kim R., Kim S.H., Shin D.H. Structural and molecular genetic insight into a wide-spread bacterial sulfur oxidation pathway. J. Mol. Biol. 2008;384:1287–1300. doi: 10.1016/j.jmb.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Horton R.M. PCR mediated recombination and mutagenesis: SOEing together tailor-made genes. Mol. Biotechnol. 1995;3:93–99. doi: 10.1007/BF02789105. [DOI] [PubMed] [Google Scholar]
- 20.Arslan E., Schulz H., Zufferey R., Kunzler P., Thöny-Meyer L. Overproduction of Bradyrhizobium japonicum c-type cytochrome subunits of the cbb3 oxidase in Escherichia coli. Biochem. Biophys. Res. Commun. 1998;251:744–747. doi: 10.1006/bbrc.1998.9549. [DOI] [PubMed] [Google Scholar]
- 21.Thomas P.E., Ryan D., Levin W. Improved staining procedure for detection of peroxidase-activity of cytochrome P-450 on sodium dodecyl-sulfate polyacrylamide gels. Anal. Biochem. 1976;75:168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- 22.Berry E.A., Trumpower B.L. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal. Biochem. 1987;161:1–15. doi: 10.1016/0003-2697(87)90643-9. [DOI] [PubMed] [Google Scholar]
- 23.Urban P.J. Colorimetry of sulfur anions. I. An improved colorimetric method for the determination of thiosulfate. Z. Analyt. Chem. 1961;179:415–422. doi: 10.1007/BF00465088. [DOI] [Google Scholar]
- 24.Zehnder A.J.B., Wuhrmann K. Titanium(III) citrate as a nontoxic oxidation-reduction buffering system for culture of obligate anaerobes. Science. 1976;194:1165–1166. doi: 10.1126/science.793008. [DOI] [PubMed] [Google Scholar]
- 25.Cleland W.W. Substrate inhibition. Methods Enzymol. 1979;63:500–513. doi: 10.1016/0076-6879(79)63022-7. [DOI] [PubMed] [Google Scholar]
- 26.van Gelder B., Slater E.C. The extinction coefficient of cytochrome c. Biochim. Biophys. Acta. 1962;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]
- 27.Segel I.H. Enzyme Kinetics: Behaviour and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems. New York: Wiley-Interscience; 1993. [Google Scholar]
- 28.Miles C.S., Manson F.D.C., Reid G.A., Chapman S.K. Substitution of a haem-iron axial ligand in flavocytochrome b2. Biochim. Biophys. Acta. 1993;1202:82–86. doi: 10.1016/0167-4838(93)90066-Z. [DOI] [PubMed] [Google Scholar]
- 29.Moore G. R., Pettigrew G. W. Cytochromes c: Evolutionary, Structural and Physicochemical Aspects. Heidelberg: Springer-Verlag; 1990. [Google Scholar]
- 30.Kurth J.M., Brito J.A., Reuter J., Flegler A., Koch T., Franke T., Klein E.M., Rowe S.F., Butt J.N. Denkmann K. Electron accepting units of the diheme cytochrome c TsdA, a bifunctional thiosulfate dehydrogenase/tetrathionate reductase. J. Biol. Chem. 2016 doi: 10.1074/jbc.M116.753863. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenthal R., Danson M.J., Hough D.W. Catalytic efficiency and kcat/KM: a useful comparator? Trends Biotechnol. 2007;25:247–249. doi: 10.1016/j.tibtech.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Girvan H.M., Seward H.E., Toogood H.S., Cheesman M.R., Leys D., Munro A.W. Structural and spectroscopic characterization of P450 BM3 mutants with unprecedented P450 heme iron ligand sets. New heme ligation states influence conformational equilibria in P450 BM3. J. Biol. Chem. 2007;282:564–572. doi: 10.1074/jbc.M607949200. [DOI] [PubMed] [Google Scholar]
- 33.Bradley J.M., Marritt S.J., Kihlken M.A., Haynes K., Hemmings A.M., Berks B.C., Cheesman M.R., Butt J.N. Redox and chemical activities of the hemes in the sulfur oxidation pathway enzyme SoxAX. J. Biol. Chem. 2012;287:40350–40359. doi: 10.1074/jbc.M112.396192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grabarczyk D.B., Chappell P.E., Eisel B., Johnson S., Lea S.M., Berks B.C. Mechanism of thiosulfate oxidation in the SoxA family of cysteine-ligated cytochromes. J. Biol. Chem. 2015;290:9209–9221. doi: 10.1074/jbc.M114.618025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abou Hamdan A., Dementin S., Liebgott P.P., Gutierrez-Sanz O., Richaud P., De Lacey A.L., Rousset M., Bertrand P., Cournac L., Léger C. Understanding and tuning the catalytic bias of hydrogenase. J. Am. Chem. Soc. 2012;134:8368–8371. doi: 10.1021/ja301802r. [DOI] [PubMed] [Google Scholar]
- 36.Hexter S.V., Esterle T.F., Armstrong F.A. A unified model for surface electrocatalysis based on observations with enzymes. Phys. Chem. Chem. Phys. 2014;16:11822–11833. doi: 10.1039/C3CP55230F. [DOI] [PubMed] [Google Scholar]
- 37.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983;166:557–580. doi: 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 38.Newell D.G., McBride H., Pearson A.D. The identification of outer membrane proteins and flagella of Campylobacter jejuni. J. Gen. Microbiol. 1984;130:1201–1208. doi: 10.1099/00221287-130-5-1201. [DOI] [PubMed] [Google Scholar]
- 39.Reuter M., van Vliet A.H. Signal balancing by the CetABC and CetZ chemoreceptors controls energy taxis in Campylobacter jejuni. PLoS One. 2013;8:e54390. doi: 10.1371/journal.pone.0054390. [DOI] [PMC free article] [PubMed] [Google Scholar]