Abstract
Background
Bone and soft tissue sarcomas (BSTS) are rare malignant tumors. Recently, the combination of gemcitabine and docetaxel (GD) was shown to have activity as second-line setting in BSTS. However, the efficacy as first-line and adjuvant settings and precise profiles of adverse events in Japanese patients are not known yet. In the present study, the feasibility and efficacy of GD in patients with BSTS were investigated.
Methods
Patients with BSTS treated with GD in our institutions were retrospectively analyzed. Information regarding clinical features, adverse events, and outcome was collected and statistically studied. Factors related to survival were analyzed using log-rank test and Cox proportional hazard regression method.
Results
A total of 134 patients were analyzed. GD was carried out as adjuvant setting in 9, first-line in 23, second-line in 56, and third-or-greater line in 46 patients. The response rate (RR) for all patients was 9.7%. RR for the patients treated as adjuvant or first-line setting was 18.8%, whereas that as second-or-greater line was 6.9%. The median progression-free survival (PFS) and overall survival (OS) of all patients were 4.8 (95% CI 3.5–6.1) and 16.4 (95% CI 9.8–22.9) months, respectively. Survival tended to be better in the patients treated as first-line than in those treated as second-or-greater line. Multivariate analysis demonstrated that history of prior chemotherapy (p = 0.046) and response to GD (p = 0.009) was significantly associated with PFS and OS, respectively. The leucopenia and neutropenia were the most frequent adverse events, and grade 3 or 4 leucopenia and neutropenia were observed in 69.4 and 72.4% of the patients. Grade 2 or 3 pneumonitis was observed in one (0.7%) and four (3.0%) patients, respectively. All the patients with pneumonitis had experienced prior chemotherapy and/or radiotherapy.
Conclusions
GD used as both first- and second/later line is effective chemotherapy for a proportion of patients with advanced BSTS. Higher response rate and better outcome was achieved in chemotherapy-naïve patients. This regimen is associated with high incidence of severe hematological toxicity, as well as the risk of severe pneumonitis, especially in pre-treated patients. GD is promising for further analysis by phase III study for the patients with BSTS.
Keywords: Gemcitabine, Docetaxel, Sarcoma, Adjuvant chemotherapy, Adverse events
Background
Bone and soft tissue sarcomas (BSTS) are very rare malignant tumors. BSTS account for approximately 1% of all malignancies. According to the Bone and Soft Tissue Tumor Registry reported by the Musculoskeletal Tumor Committee of the Japanese Orthopaedic Association, only 591 cases of bone sarcoma (BS) and 1509 cases of soft tissue sarcomas (STS) were registered in 2013 in Japan [1, 2]. Because of the rareness of BSTS, it is difficult to develop novel treatments for the tumors. Current standard chemotherapy for BSTS consists of old reagents such as doxorubicin (DOX) and ifosfamide (IFO) [3, 4]. DOX has remained a key drug for many years in the treatment of BSTS, and its response rate (RR) for sarcomas is approximately 25%. IFO is another key drug for BSTS with RR of approximately 30%. The combination of DOX with IFO has been shown to improve outcomes of the patients with localized STS [5], whereas the combination failed to show the improvement of prognosis of the patients with advanced STS [6].
Gemcitabine (GEM) is a fluorine-substituted pyrimidine analog, and is phosphorylated to the diphosphate and triphosphate metabolites. These active metabolites inhibit DNA synthesis and exhibit anti-tumor effects [7]. Docetaxel (DOC) has the activity to inhibit the depolymerization of microtubular bundles to free tubulin [8], resulting in the disruption of cell mitosis. The RR of GEM and DOC alone for sarcomas was reported to be approximately 3 and 0%, respectively, and each drug was inactive as single agent for BSTS [9, 10].
Recent studies have demonstrated the efficacy of the combination of GEM plus DOC (GD) [11–16]. GD regimen indicated the high response rates for the patients with advanced uterine leiomyosarcomas in both first-line [17] and second-line settings [18]. It has been also reported that GD exhibited higher response rates, progression-free survival (PFS), and overall survival (OS) than single-agent GEM in a randomized phase II trial for patients with advanced STS previously treated by up to three prior regimens [19]. The efficacy of GD as the second-line setting for advanced BSTS was also reported in the prospective and retrospective studies [20, 21]. However, only one study has been reported showing the effects of GD as adjuvant or first-line treatment on BSTS [22].
GD regimen is also known to be feasible and less toxic than DOX+IFO regimen [11–18]. Although pulmonary toxicity of both GEM and DOC should be noted, the combination of GEM and DOC would not increase the pulmonary toxicity [19]. On the other hand, it has been demonstrated that pulmonary toxicities by GD were severe among Japanese patients in a Japan Clinical Oncology Group (JCOG) trial. The clinical trial, JCOG0104, evaluated the efficacy of GD for non-small cell lung cancer as second-line setting and resulted in the early termination due to unexpected three treatment-related deaths with interstitial pneumonitis [23]. These observations suggest that the incidence of pulmonary toxicity might be high in Japanese patients. However, there is no study demonstrating the precise profiles of the adverse events of GD including the pulmonary toxicities in Japanese patients with BSTS.
In the present study, we analyzed the profiles of the adverse events and efficacy of GD, including as adjuvant and first-line settings, for 134 patients with BSTS treated in the institutions participating in the Bone and Soft Tissue Tumor Study Group (BSTTSG) of JCOG. This study is one of the largest series of the patients with BSTS treated by GD [11].
Methods
We retrospectively reviewed the records of the institutions of JCOG BSTTSG participating in the present study from July 2002 to September 2014. A total of 134 patients suffered from BSTS and treated by GD regimen in our institutions participating in JCOG BSTTSG were enrolled in the present study. This study was approved by the Institutional Review Board at Oita University, and a waiver of informed consent was provided.
The GD regimen consisted of GEM administrated in day 1 and 8 and DOC administrated in day 8. Basically, GEM was given intravenously in 30 min in 100 ml saline, and DOC was given intravenously over 60 min in 250 ml saline with premedication of 16 mg/day of dexamethasone for 3 days. Patients were given therapeutic and second-line prophylactic granulocyte colony stimulating factor if they had grade 4 neutropenia or febrile neutropenia. The median dose of GEM was 890 mg/m2/day (range 490–1000 mg/m2/day) and that of DOC was 70 mg/m2 (range 42–100 mg/m2). The chemotherapy was repeated until disease progression or intolerance to the regimen. The indication of adjuvant chemotherapy for BSTS was basically as follows: histologically high-grade sarcomas, larger than 5 cm in maximum diameter without metastasis, and deep-seated in tumor location. The median number of cycles of GD was three (range 1–14 cycles) for all patients. The mean follow-up period for 56 surviving patients was 18 months (range 1–75 months), and that for 78 patients who died was 14 months (range 1–72 months).
Toxicity was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0. The radiological evaluation of the response to the chemotherapy was assessed using Response Evaluation Criteria in Solid Tumor (RECIST) ver. 1.1. The progression-free survival (PFS) was defined as the time period from the day GD started until the day of the first evidence of disease progression or death. The overall survival (OS) was defined as the time period from the day GD started until the day of death or last follow-up. The PFS and OS were calculated using the Kaplan-Meier method. Differences in survivals were assessed by the log-rank test and Cox proportional hazard regression method. Differences were considered significant when p values were <0.05. Statistical analysis was done using IBM SPSS Statistics 22.0 software (IBM, Armonk, NY, USA).
Results
Patient characteristics
A total of 134 patients were analyzed in the present study (Table 1). The median age of the patients was 53 years old (range 10–78 years old) at the treatment by GD. The sites of primary lesions were soft tissues in 105 patients, and bones in 29 patients. The two most frequent histologic tumor types of STS were leiomyosarcoma (n = 33) and undifferentiated pleomorphic sarcoma (n = 18). The histologic tumor types of BS were osteosarcoma 15, Ewing sarcoma 3, leiomyosarcoma 5, undifferentiated pleomorphic sarcoma of bone 2, and other 4.
Table 1.
Patient characteristics (n = 134)
| Age | Median (years) | 53 |
| Range | 10–78 | |
| Bone tumor | 29 (21.6%) | |
| Primary site | Humerus | 2 (1.5%) |
| Spine | 6 (4.5%) | |
| Femur | 16 (11.9%) | |
| Tibia | 3 (2.2%) | |
| Others | 2 (1.5%) | |
| Soft tissue tumor | 105 (78.4%) | |
| Primary site | Head and neck | 4 (3.0%) |
| Shoulder girdle | 3 (2.2%) | |
| Upper arm | 3 (2.2%) | |
| Forearm | 6 (4.5%) | |
| Other upper extremity | 5 (3.7%) | |
| Chest wall | 4 (3.0%) | |
| Buttock | 6 (4.5%) | |
| Retroperitoneum | 14 (10.4%) | |
| Visceral | 10 (7.5%) | |
| Thigh | 25 (18.7%) | |
| Leg | 9 (6.7%) | |
| Other lower extremity | 9 (6.7%) | |
| Others | 7 (5.2%) | |
| Histological subtype | ||
| Leiomyosarcoma | 38 (28.4%) | |
| Undifferentiated pleomorphic sarcoma | 20 (14.9%) | |
| Osteosarcoma | 17 (12.7%) | |
| Liposarcoma | 9 (6.7%) | |
| Synovial sarcoma | 7 (5.2%) | |
| Malignant peripheral nerve sheath tumor | 7 (5.2%) | |
| Angiosarcoma | 4 (3.0%) | |
| Ewing sarcoma | 4 (3.0%) | |
| Epithelioid sarcoma | 4 (3.0%) | |
| Rhabdomyosarcoma | 3 (2.2%) | |
| Others | 21 (15.7%) | |
| Presentation status | ||
| Localized | 9 (6.7%) | |
| Metastatic or locally advanced | 125 (93.3%) | |
| Prior chemotherapy regimen | ||
| DOX+IFO | 38 (28.4%) | |
| DOX alone | 21 (15.7%) | |
| IFO alone | 18 (13.4%) | |
| IFO+VP16 | 12 (9.0%) | |
| IFO+CDBCA+VP16 | 11 (8.2%) | |
| DOX+CDDP | 6 (4.5%) | |
| None | 32 (23.9%) | |
| Prior radiation | 45 (33.6%) | |
Among 134 patients, the metastatic and/or locally advanced BSTS were observed in 125 patients, and GD was carried out as first-line setting in 23 patients, second-line in 56 patients, and third-or-greater line in 46 patients. On the other hand, GD regimen was carried out as adjuvant chemotherapy with resection of localize primary tumor in 9 patients. The frequent chemotherapy regimens prior to GD for the patients with advanced BSTS were DOX+IFO (38 patients), DOX alone (21 patients), and IFO alone (18 patients). The median number of lines of prior chemotherapy was 1 (range 1–5 regimens). The prior radiation therapy had been carried out for 45 patients. Median dose of radiation was 56 Gy.
The median doses of GEM and DOC were 890 mg/m2/day (range 490–1000 mg/m2/day) and 70 mg/m2 (range 42–100 mg/m2) for the patients with advanced BSTS, and 900 mg/m2/day (range 550–900 mg/m2/day) and 70 mg/m2 (range 50–100 mg/m2) for the non-advanced cases. The median number of cycles of GD was 3 (range 1–14 cycles) for the advanced cases, and 5 (range 2–9 cycles) for the non-advanced cases treated as adjuvant chemotherapy. The two most frequent reasons for the discontinuation of GD regimen were progression of disease (81%) and adverse events (10%).
Response and survival
The RECIST-assessed response rate was 9.7% (13/134) for all patients. Two patients were assessed as complete response (CR), 11 partial response (PR), 55 stable disease (SD), 55 progressive disease (PD), and 11 not evaluable (NE). The patients who had no prior chemotherapy, i.e., treated as adjuvant or first-line setting exhibited 1 CR and 5 PR, and RR was 18.8% (6/32). On the other hand, the patients treated as second-or-greater line had 1 CR and 6 PR, resulting in RR of 6.9% (7/102). Although the difference was not significant, RR of GD as adjuvant or first-line setting chemotherapy tended to be better than that for second-or-greater line setting chemotherapy. RR for the patients with BS was only 3.4% (1PR/29), whereas that for STS was 11.4% (2CR+10PR/105). Objective response by histological subtypes of BSTS was summarized in Table 2. The response (CR/PR) was observed in the patients with leiomyosarcoma, undifferentiated pleomorphic sarcoma, malignant peripheral nerve sheath tumor, angiosarcoma, and Ewing sarcoma. RR for leiomyosarcoma and undifferentiated pleomorphic sarcoma was 13.2% (5/38) and 15.0% (3/20), respectively (Table 2).
Table 2.
Response to GD by histological subtypes
| CR | PR | SD | PD | NE | RR (%) | |
|---|---|---|---|---|---|---|
| All | 2 | 11 | 55 | 55 | 11 | 9.7 |
| Leiomyosarcoma | 0 | 5 | 19 | 12 | 2 | 13.2 |
| Undifferentiated pleomorphic sarcoma | 1 | 2 | 11 | 4 | 2 | 15.0 |
| Osteosarcoma | 0 | 0 | 7 | 8 | 2 | 0 |
| Liposarcoma | 0 | 0 | 2 | 5 | 2 | 0 |
| Synovial sarcoma | 0 | 0 | 3 | 4 | 0 | 0 |
| Malignant peripheral nerve sheath tumor | 0 | 1 | 0 | 5 | 1 | 14.3 |
| Angiosarcoma | 0 | 1 | 1 | 1 | 1 | 25.0 |
| Ewing sarcoma | 0 | 1 | 1 | 2 | 0 | 25.0 |
| Epithelioid sarcoma | 0 | 0 | 1 | 3 | 0 | 0 |
| Rhabdomyosarcoma | 0 | 0 | 2 | 1 | 0 | 0 |
| Others | 1 | 1 | 8 | 10 | 1 | 9.5 |
CR complete response, PR partial response, SD stable disease, PD progressive disease, NE not evaluable, RR response rate
At the last follow-up time, 56 patients were alive and 78 patients were dead. The median PFS for all patients was 4.8 months (95% CI 3.5–6.1) (Fig. 1a). The median PFS for the patients treated by GD as adjuvant therapy (n = 9) was not reached yet (p < 0.001), and estimated 5-year PFS was 70%. The median PFS as first-line (n = 23) was 6.7 months (95% CI 5.9–7.5), that as second-line (n = 56) was 4.0 months (95% CI 2.5–5.6), and that as third-or-greater line (n = 46) was 2.0 months (95% CI 0.2–3.9), respectively.
Fig. 1.
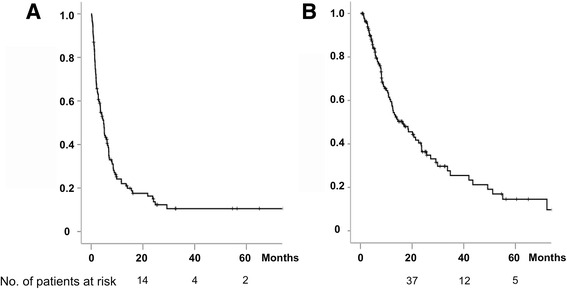
Kaplan-Meier estimates of survival of all patients. a Progression-free survival. b Overall survival
The median OS of all patients was 16.4 months (95% CI 9.8–22.9) (Fig. 1b). The median OS for the patients treated by GD as adjuvant therapy was not reached yet (p = 0.004), and estimated 5-year OS was 75%. The median OS as first-line was 22.5 months (95% CI 7.4–37.6), that as second-line was 14.1 months (95% CI 8.5–19.7), and that as third-or-greater line was 9.3 months (95% CI 6.6–12.0), respectively. Although the difference in OS between first-line and second-or-greater line setting was not significant, survival tended to be better in the patients treated as first-line setting (Fig. 2).
Fig. 2.
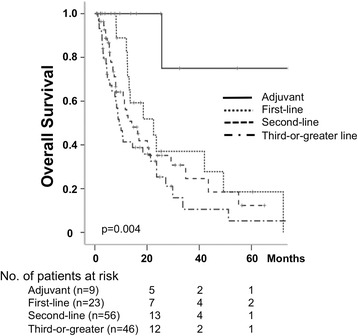
Kaplan-Meier estimates of overall survival of the patients treated with GD as adjuvant, first-line, second-line, and third-or-greater line setting
Next, the effects of doses on GD were investigated. The patients treated with GEM 900 mg/m2/day and DOC 70 mg/m2 (G900/D70) (n = 18), which was the same as the median dose, exhibited 1 CR and 2 PR, and RR rate was 16.7%. The patients treated with doses higher than G900/D70 (>G900/D70) (the mean dose of GEM 939 mg/m2/day and DOC 89 mg/m2) (n = 32) exhibited only 1 PR and RR was 3.1%. The median PFS and OS of patients with G900/D70 were 7.2 months (0.4–32.5 months) and 13.7 months (0.6–32.5 months), respectively, whereas those with >G900/D70 were 3.3 months (0.3–54.6 months) and 6.3 months (1.6–54.6 months), respectively.
Univariate analysis for the potential prognostic factors for PFS in 134 patients was carried out (Table 3). Histologic subtype (undifferentiated pleomorphic sarcoma vs. leiomyosarcoma vs. others) and history of prior chemotherapy (first-line setting vs. second-or-greater line setting) were significantly correlated with PFS. Age, the sites of primary lesions, bone or soft tissue tumors, response to GD (CR or PR vs. SD or PD), and doses of GD were not prognostic factors for PFS. Multivariate analysis also performed to demonstrate factors influencing to PFS. History of prior chemotherapy (p = 0.046) was a significant prognostic factor for PFS (Table 3).
Table 3.
Univariate and multivariate analyses for progression-free survival
| Univariate analysis | ||
|---|---|---|
| Factors | HR (95% CI) | p value |
| Age (years) | ||
| <50 (vs. ≥50) | 1.23 (0.82–1.84) | 0.322 |
| Tumor origin | ||
| Bone (vs. soft tissue) | 1.05 (0.65–1.69) | 0.843 |
| Primary site | 0.648 | |
| Trunk (vs. extremities) | 1.20 (0.67–2.15) | 0.542 |
| Retroperitoneum (vs. extremities) | 1.02 (0.56–1.85) | 0.957 |
| Visceral (vs. extremities) | 1.48 (0.78–2.83) | 0.231 |
| Histological subtype | 0.008 | |
| UPS (vs. leiomyosarcoma) | 1.20 (0.61–2.35) | 0.591 |
| Others (vs. leiomyosarcoma) | 2.01 (1.23–3.21) | 0.004 |
| Prior chemotherapy | 0.028 | |
| Second-line (vs. first-line) | 0.99 (0.55–1.78) | 0.974 |
| Third-or-greater line (vs. first-line) | 1.74 (0.97–3.12) | 0.064 |
| Response to GD | ||
| SD/PD (vs. CR/PR) | 2.05 (0.99–4.23) | 0.053 |
| Multivariate analysis | ||
| Factors | HR (95% CI) | p value |
| Age (years) | ||
| ≥50 (vs. <50) | ||
| Tumor origin | ||
| Bone (vs. soft tissue) | ||
| Primary site | ||
| Trunk (vs. extremities) | ||
| Retroperitoneum (vs. extremities) | ||
| Visceral (vs. extremities) | ||
| Histological subtype | ||
| UPS (vs. leiomyosarcoma) | ||
| Others (vs. leiomyosarcoma) | ||
| Prior chemotherapy | 0.043 | |
| Second-line (vs. first-line) | 1.02 (0.56–1.83) | 0.974 |
| Third-or-greater line (vs. first-line) | 1.71 (0.95–3.07) | 0.074 |
| Response to GD | ||
| SD/PD (vs. CR/PR) | ||
On the other hand, histologic subtype (p = 0.002) and response to GD (p = 0.010) were significant prognostic factors for OS in univariate analysis (Table 4). Multivariate analysis demonstrated that response to GD (p = 0.009) was significantly associated with OS (Table 4). The patients with leiomyosarcoma and UPS showed similar OS and PFS; however, the prognosis of leiomyosarcoma patients was significantly better than that of other histologic subtypes excluding UPS (for PFS, p = 0.004 and for OS, p = 0.001).
Table 4.
Univariate and multivariate analyses for overall survival
| Univariate analysis | ||
|---|---|---|
| Factors | HR (95% CI) | p value |
| Age (years) | ||
| <50 (vs. ≥50) | 1.20 (0.76–1.89) | 0.436 |
| Tumor origin | ||
| Bone (vs. soft tissue) | 1.32 (0.77–2.27) | 0.318 |
| Primary site | 0.633 | |
| Trunk (vs. extremities) | 0.85 (0.43–1.68) | 0.639 |
| Retroperitoneum (vs. extremities) | 0.65 (0.31–1.37) | 0.259 |
| Visceral (vs. extremities) | 1.15 (0.56–2.35) | 0.702 |
| Histological subtype | 0.002 | |
| UPS (vs. leiomyosarcoma) | 1.24 (0.55–2.81) | 0.608 |
| Others (vs. leiomyosarcoma) | 2.54 (1.46–4.42) | 0.001 |
| Prior chemotherapy | 0.126 | |
| Second-line (vs. first-line) | 1.38 (0.72–2.65) | 0.332 |
| Third-or-greater line (vs. first-line) | 1.90 (1.00–3.62) | 0.052 |
| Response to GD | ||
| SD/PD (vs. CR/PR) | 3.81 (1.37–10.55) | 0.010 |
| Multivariate analysis | ||
| Factors | HR (95% CI) | p value |
| Age (years) | ||
| ≥50 (vs. <50) | ||
| Tumor origin | ||
| Bone (vs. soft tissue) | ||
| Primary site | ||
| Trunk (vs. extremities) | ||
| Retroperitoneum (vs. extremities) | ||
| Visceral (vs. extremities) | ||
| Histological subtype | ||
| UPS (vs. leiomyosarcoma) | ||
| Others (vs. leiomyosarcoma) | ||
| Prior chemotherapy | ||
| Second-line (vs. first-line) | ||
| Third-or-greater line (vs. first-line) | ||
| Response to GD | ||
| SD/PD (vs. CR/PR) | 3.99 (1.42–11.22) | 0.009 |
Adverse events
There was no treatment-related death. The leucopenia and neutropenia were the most frequent adverse events by GD for BSTS (Table 5). Grade 3 or 4 leucopenia and neutropenia were observed in 93 (69.4%) and 97 (72.4%) patients. Grade 3 or 4 anemia and thrombocytopenia were observed in 25 (18.7%) and 37 (27.6%) patients.
Table 5.
Adverse events in all patients (n = 134)
| Adverse event | G0 | G1 | G2 | G3 | G4 | NE | All grades (%) | Grade 3/4 (%) |
|---|---|---|---|---|---|---|---|---|
| Leucocyte | 9 | 8 | 21 | 44 | 49 | 3 | 91.0 | 69.4 |
| Neutrophils | 9 | 7 | 17 | 30 | 67 | 4 | 90.3 | 72.4 |
| Hemoglobin | 27 | 41 | 38 | 23 | 2 | 3 | 77.6 | 18.7 |
| Platelets | 55 | 21 | 18 | 24 | 13 | 3 | 56.7 | 27.6 |
| AST | 87 | 38 | 3 | 3 | 0 | 3 | 32.8 | 2.2 |
| ALT | 82 | 35 | 9 | 5 | 0 | 3 | 36.6 | 3.7 |
| Anorexia | 52 | 59 | 15 | 2 | 0 | 6 | 56.7 | 1.5 |
| Nausea | 65 | 49 | 14 | 1 | 0 | 5 | 47.8 | 0.7 |
| Vomiting | 103 | 22 | 4 | 0 | 0 | 5 | 19.4 | 0.0 |
| Diarrhea | 112 | 13 | 2 | 2 | 0 | 5 | 12.7 | 1.5 |
| Fatigue | 50 | 59 | 15 | 6 | 0 | 4 | 59.7 | 4.5 |
| Febrile neutropenia | 120 | 0 | 0 | 13 | 0 | 1 | 9.7 | 9.7 |
| Fever | 97 | 22 | 12 | 1 | 0 | 2 | 26.1 | 0.7 |
| Infection | 118 | 4 | 5 | 5 | 1 | 1 | 11.2 | 4.5 |
| Dyspnea | 124 | 4 | 1 | 4 | 0 | 1 | 6.7 | 3.0 |
| Interstitial pneumonia | 124 | 4 | 1 | 4 | 0 | 1 | 6.7 | 3.0 |
| Neuropathy | 128 | 0 | 5 | 0 | 0 | 1 | 3.7 | 0.0 |
| Rash | 114 | 6 | 8 | 5 | 0 | 1 | 14.2 | 3.7 |
| Edema | 131 | 2 | 1 | 0 | 0 | 0 | 2.2 | 0.0 |
| Mucositis | 130 | 1 | 3 | 0 | 0 | 0 | 3.0 | 0.0 |
| Allergic reaction | 132 | 0 | 2 | 0 | 0 | 0 | 1.5 | 0.0 |
AST aspartate transaminase, ALT alanine aminotransferase
Febrile neutropenia was observed in 13 out of 134 patients (9.7%). The most frequent non-hematological toxicities were nausea and anorexia; however, these adverse events were modest. Grade 3 nausea and anorexia were observed only in two (1.5%) and one (0.7%) patients. Grade 4 non-hematological toxicity was found only in one patient (0.7%) as infection. Regarding lung toxicities, dyspnea and pneumonitis were observed in nine patients (6.7%) as all grades. Grade 2 or 3 pneumonitis was observed in one (0.7%) and four (3.0%) patients, respectively. All the five patients were assessed as interstitial pneumonitis and successfully treated by steroid-pulse therapy. All the patients with pneumonitis had experienced previous chemotherapy. Four out of five patients received GD as third-or-greater line and one as second-line chemotherapy. Three out of five patients with grade 2 or 3 pneumonitis had been treated by prior radiotherapy, and dose and site of radiation were 30 Gy to the chest wall, 54 Gy to the lung, and 50 Gy to the thigh.
Among 32 patients treated by GD as adjuvant or first-line therapy, grade 3 or 4 leucopenia and neutropenia were observed in 17 (53.1%) and 21 (65.6%) patients (Table 6). Grade 3 or 4 anemia and thrombocytopenia were observed in five (15.6%) and three (9.4%) patients. Grade 4 non-hematological toxicity was not observed. Grade 3 nausea and anorexia were not observed, whereas grade 3 dyspnea was observed in one patient (3.1%). The pneumonitis was observed in two patients (6.3%) only as grade 1.
Table 6.
Adverse events in patients treated as adjuvant/first-line setting (n = 32)
| Adverse event | G0 | G1 | G2 | G3 | G4 | NE | All grades (%) | Grade 3/4 (%) |
|---|---|---|---|---|---|---|---|---|
| Leucocyte | 3 | 3 | 8 | 10 | 7 | 1 | 87.5 | 53.1 |
| Neutrophils | 2 | 3 | 5 | 8 | 13 | 1 | 90.6 | 65.6 |
| Hemoglobin | 10 | 8 | 8 | 5 | 0 | 1 | 65.6 | 15.6 |
| Platelets | 19 | 3 | 6 | 3 | 0 | 1 | 37.5 | 9.4 |
| AST | 22 | 6 | 2 | 1 | 0 | 1 | 28.1 | 3.1 |
| ALT | 22 | 5 | 1 | 3 | 0 | 1 | 28.1 | 9.4 |
| Anorexia | 13 | 14 | 5 | 0 | 0 | 0 | 59.4 | 0.0 |
| Nausea | 17 | 11 | 4 | 0 | 0 | 0 | 46.9 | 0.0 |
| Vomiting | 26 | 5 | 1 | 0 | 0 | 0 | 18.8 | 0.0 |
| Diarrhea | 26 | 4 | 1 | 1 | 0 | 0 | 18.8 | 3.1 |
| Fatigue | 14 | 12 | 5 | 1 | 0 | 0 | 56.3 | 3.1 |
| Febrile neutropenia | 29 | 0 | 0 | 3 | 0 | 0 | 9.4 | 9.4 |
| Fever | 24 | 6 | 1 | 1 | 0 | 0 | 25.0 | 3.1 |
| Infection | 29 | 0 | 1 | 2 | 0 | 0 | 9.4 | 6.3 |
| Dyspnea | 31 | 0 | 0 | 1 | 0 | 0 | 3.1 | 3.1 |
| Interstitial pneumonia | 30 | 2 | 0 | 0 | 0 | 0 | 6.3 | 0.0 |
| Neuropathy | 32 | 0 | 0 | 0 | 0 | 0 | 0.0 | 0.0 |
| Rash | 24 | 4 | 3 | 1 | 0 | 0 | 25.0 | 3.1 |
| Edema | 30 | 2 | 0 | 0 | 0 | 0 | 6.3 | 0.0 |
| Mucositis | 31 | 0 | 1 | 0 | 0 | 0 | 3.1 | 0.0 |
| Allergic reaction | 32 | 0 | 0 | 0 | 0 | 0 | 0.0 | 0.0 |
AST aspartate transaminase, ALT alanine aminotransferase
For the patients treated as second-or-greater line (n = 102), grade 3 or 4 leucopenia and neutropenia were observed in both 76 patients (74.5%) (Table 7). Grade 3 or 4 anemia and thrombocytopenia were observed in 20 (19.6%) and 34 (33.3%) patients. The lung toxicities were observed as dyspnea and pneumonitis in eight (7.8%) and seven (6.9%) patients as all grades, respectively. Grade 3 pneumonitis was observed in four patients (3.9%). The incidence of grade 3 and 4 leucopenia (HR 0.214, 95% CI 0.021–0.406, p = 0.022) and thrombocytopenia (HR 0.24, 95% CI 0.103–0.376, p = 0.008) of the patients treated as adjuvant or first-line setting were significantly less frequent than those as second-or-greater line setting.
Table 7.
Adverse events in patients treated as second-or-greater line setting (n = 102)
| Adverse event | G0 | G1 | G2 | G3 | G4 | NE | All grades (%) | Grade 3/4 (%) |
|---|---|---|---|---|---|---|---|---|
| Leucocyte | 6 | 5 | 13 | 34 | 42 | 2 | 92.2 | 74.5 |
| Neutrophils | 7 | 4 | 12 | 22 | 54 | 3 | 90.2 | 74.5 |
| Hemoglobin | 17 | 33 | 30 | 18 | 2 | 2 | 81.4 | 19.6 |
| Platelets | 36 | 18 | 12 | 21 | 13 | 2 | 62.7 | 33.3 |
| AST | 65 | 32 | 1 | 2 | 0 | 2 | 34.3 | 2.0 |
| ALT | 60 | 30 | 8 | 2 | 0 | 2 | 39.2 | 2.0 |
| Anorexia | 39 | 45 | 10 | 2 | 0 | 6 | 55.9 | 2.0 |
| Nausea | 48 | 38 | 10 | 1 | 0 | 5 | 48.0 | 1.0 |
| Vomiting | 77 | 17 | 3 | 0 | 0 | 5 | 19.6 | 0.0 |
| Diarrhea | 86 | 9 | 1 | 1 | 0 | 5 | 10.8 | 1.0 |
| Fatigue | 36 | 47 | 10 | 5 | 0 | 4 | 60.8 | 4.9 |
| Febrile neutropenia | 91 | 0 | 0 | 10 | 0 | 1 | 9.8 | 9.8 |
| Fever | 73 | 16 | 11 | 0 | 0 | 2 | 26.5 | 0.0 |
| Infection | 89 | 4 | 4 | 3 | 1 | 1 | 11.8 | 3.9 |
| Dyspnea | 93 | 4 | 1 | 3 | 0 | 1 | 7.8 | 2.9 |
| Interstitial pneumonia | 94 | 2 | 1 | 4 | 0 | 1 | 6.9 | 3.9 |
| Neuropathy | 96 | 0 | 5 | 0 | 0 | 1 | 4.9 | 0.0 |
| Rash | 90 | 2 | 5 | 4 | 0 | 1 | 10.8 | 3.9 |
| Edema | 101 | 0 | 1 | 0 | 0 | 0 | 1.0 | 0.0 |
| Mucositis | 99 | 1 | 2 | 0 | 0 | 0 | 2.9 | 0.0 |
| Allergic reaction | 100 | 0 | 2 | 0 | 0 | 0 | 2.0 | 0.0 |
AST aspartate transaminase, ALT alanine aminotransferase
Discussion
Recent studies have demonstrated that the combination of GEM with DOC is effective for BSTS, and that GD regimen is supposed to have milder toxicity than DOX+IFO, the standard regimen for STS [11–16, 19]. In a clinical trial JCOG0304, in which DOX+IFO was administrated for high-grade STS, the incidences of grade 3 and 4 leucopenia, neutropenia, anemia, thrombocytopenia, and febrile neutropenia were 97.2, 98.6, 55.6, 15.3, and 18.2%, respectively [24, 25]. It has been reported that the incidences of grade 3 and 4 toxicities observed in GD were lower than those in DOX+IFO; leucopenia approximately 10–40%, neutropenia 10–70%, anemia 5–15%, thrombocytopenia 10–40%, and febrile neutropenia 0–10% [11–16, 19]. In the present study, the incidences of grade 3 and 4 adverse events by GD were consistent with the previous studies. Grade 3 or 4 neutropenia, anemia, and febrile neutropenia were observed in 72.4, 18.7, and 9.7%, respectively, of the patients received GD. Furthermore, when GD was administrated as adjuvant or first-line setting, the incidences of grade 3 or 4 leucopenia and thrombocytopenia were significantly lower than those as second-or-greater setting.
On the other hand, GD regimen is also known to have the risk of interstitial pneumonitis. In a randomized trial, JCOG0104, which was conducted to confirm the superiority of GD on survival of the patients with previously treated non-small cell lung cancer (NSCLC) over DOC alone, was early terminated due to the unexpectedly high incidence of interstitial pneumonia (grade 3 and 4 pneumonitis was observed in 12.3%) and three treatment-related deaths (4.6%) in the GD arm [23]. The risk of lung toxicities by GD could not be ignored especially in Japan, since the unexpected severe pneumonitis in JCOG0104 was observed in Japanese patients.
Since few interstitial pneumonia were described in the past studies of GD for BSTS [11–16, 19–22], it has been supposed that GD would be relatively safe regarding interstitial pneumonia in the treatment of BSTS. In the present study, interstitial pneumonia was observed 9 (6.7%) out of 134 patients for all grade and 4 (3.0%) for grade 3. All the patients (five out of 134) with symptomatic pneumonia (grade 2/3) were successfully treated by steroid-pulse therapy, and no regimen-related death was observed. It was noteworthy that all five patients were received prior chemotherapy, and median number of previous regimens was 3. Furthermore, three out of five patients had been treated with radiotherapy. When GD was given as first-line or adjuvant setting, symptomatic pneumonitis was not observed. Although definite risk factors for interstitial pneumonia caused by GD are still unknown, a careful attention to lung toxicity should be paid even in the treatment for BSTS, especially when the patient has been treated with prior chemotherapy and/or radiotherapy.
The feasibility and efficacy of GD for BSTS have been previously reported from the USA and Europe [11, 12, 16, 19–22]; however, those were not well analyzed in Asian population, especially in Japan. Only a few small studies of GD for BSTS were reported from Asian countries [13–15]. Furthermore, there was no description about interstitial pneumonia or lung toxicities by GD in these studies. Thus, the present study is the largest series of GD for Asian patients with BSTS, and is reporting the lung toxicities of GD in the treatment for BSTS in Asian patients for the first time.
In addition to the less toxicity, GD regimen is also thought to be as effective as DOX+IFO. In a randomized phase II trial comparing perioperative chemotherapy with GD and DOX+IFO for STS, 2-year PFS in the GD arm and the DOX+IFO arm were 74 and 57%, respectively [22]. The results suggested that GD is promising for a phase III trial.
In the present study, the results demonstrated promising effects of GD especially as adjuvant or first-line setting on survival of the patients with BSTS. Taken together, the JCOG BSTTSG is now conducting a randomized phase II/III study, JCOG1306, to elucidate the efficacy and safety of perioperative chemotherapy by GD comparing with DOX+IFO for operable high-grade STS [26].
It is noteworthy that the dose of GD and its efficacy were not paralleled in the present study. RR and survival in the patients treated with G900/D70 were better than those with >G900/D70. One possible explanation of the discrepancy was the influence of adverse effects caused by GD. The incidences of adverse events in the patients treated with >G900/D70 were more frequent than those with G900/D70. For instance, neutropenia and decrease in platelet were observed in 83.3 and 38.9% of the patients in G900/D70 group, whereas those in >G900/D70 group were 96.9 and 62.5%, respectively. Thus, it is possible that the higher incidence of adverse events in >G900/D70 group might associate with the inferior outcome because of discontinuation or dose reduction of GD. It has been reported that approximately half of the patients were required dose reduction of GD because of adverse effects in the previous randomized phase II trials using G900/D100 [18, 21]. Thus, such doses around G900/D100 might be too high for the patients with BSTS.
Another possibility was the influence of previous treatments. Since the number of chemotherapy-naïve patients was larger in G900/D70 group (50.0% of the patients) than that in >G900/D70 group (31.3%), there is a possibility that more drug-resistant tumors were included in the latter group, which might lead to the lower response to GD. In this regard, when the response to GD was compared only for the chemotherapy-naïve patients, RR and median OS were 0% (0/10) and 16.2 months in >G900/D70 group, whereas those were 33.3% (3/9) and 13.8 months comparably in G900/D70 group, respectively. These results suggest that G900/D70 might be useful for BSTS, especially in the treatment for chemotherapy-naïve patients.
In summary, it is suggested that the incidences of the severe adverse events including lung toxicities in the patients without prior chemotherapy or radiotherapy were lower than those with prior chemotherapy and/or radiation. GD might be effective not only as second-line therapy for advanced BSTS but also as adjuvant or first-line chemotherapy for BSTS. Since GD can be administered in an outpatient setting due to its lower toxicities, GD is promising for further investigation by phase III trials JCOG1306 for the patients with BSTS.
Conclusions
This is the first report demonstrating the precise profiles of the adverse events of GD for the Japanese patients with BSTS, and one of the largest series analyzing 134 patients with BSTS treated by GD. GD used as both first- and second/later line is effective chemotherapy for a proportion of patients with advanced BSTS. Higher response rate and better outcome were achieved in chemotherapy-naïve patients. This regimen is associated with high incidence of severe hematological toxicity, as well as the risk of severe pneumonitis, especially in pre-treated patients.
Acknowledgements
This work was supported in part by National Cancer Center Research and Development Fund (26-A-4) and Applied Research for Innovative Treatment of Cancer (H26-084, 15ck0106087h0002) from the Ministry of Health, Labour and Welfare and the Agency for Medical Research and Development, Japan.
Funding
This work was supported in part by National Cancer Center Research and Development Fund (26-A-4) and Applied Research for Innovative Treatment of Cancer (H26-084, 15ck0106087h0002) from the Ministry of Health, Labour and Welfare and the Agency for Medical Research and Development, Japan.
Availability of data and materials
Not applicable.
Authors’ contributions
KT and YI participated in the design of the study, searched the literature, and drafted the manuscript. KT performed the statistical analysis. SJ, HC, HHi, HM, HY, MH, MT, TK, HHa, MK, JT, YN, AN, and HT contributed to the analysis, interpretation of data, and revision of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Board at Oita University (Approval No. 915).
Abbreviations
- BS
Bone sarcomas
- BSTS
Bone and soft tissue sarcomas
- BSTTSG
Bone and Soft Tissue Tumor Study Group
- CR
Complete response
- DOC
Docetaxel
- DOX
Doxorubicin
- GD
Gemcitabine and docetaxel
- GEM
Gemcitabine
- IFO
Ifosfamide
- JCOG
Japan Clinical Oncology Group
- NE
Not evaluable
- OS
Overall survival
- PD
Progressive disease
- PFS
Progression-free survival
- PR
Partial response
- RECIST
Response Evaluation Criteria in Solid Tumor
- RR
Response rate
- SD
Stable disease
- STS
Soft tissue sarcomas
Contributor Information
Kazuhiro Tanaka, Phone: +81-97-586-5872, Email: ktanaka@oita-u.ac.jp.
Susumu Joyama, Email: joyamasu@kawati.or.jp.
Hirokazu Chuman, Email: hchuuman@ncc.go.jp.
Hiroaki Hiraga, Email: hhiraga@sap-cc.go.jp.
Hideo Morioka, Email: morioka.z3@keio.jp.
Hideki Yoshikawa, Email: yhideki@ort.med.osaka-u.ac.jp.
Masami Hosaka, Email: m-hosaka@med.tohoku.ac.jp.
Mitsuru Takahashi, Email: m.takahashi@scchr.jp.
Tadahiko Kubo, Email: kubot@hiroshima-u.ac.jp.
Hiroshi Hatano, Email: hatanoh@niigata-cc.jp.
Mitsunori Kaya, Email: kayamitsunori@yahoo.co.jp.
Junya Toguchida, Email: togjun@frontier.kyoto-u.ac.jp.
Yoshihiro Nishida, Email: ynishida@med.nagoya-u.ac.jp.
Akihito Nagano, Email: a-nagano@lucky.odn.ne.jp.
Hiroshi Tsumura, Email: htsumura@oita-u.ac.jp.
Yukihide Iwamoto, Email: yiwamoto@ortho.med.kyushu-u.ac.jp.
References
- 1.Japanese Orthopaedic Association Musculoskeletal Tumor Committee . Bone Tumor Registry in JAPAN 2013. Tokyo: National Cancer Center; 2013. [Google Scholar]
- 2.Japanese Orthopaedic Association Musculoskeletal Tumor Committee . Soft Tissue Tumor Registry in JAPAN 2013. Tokyo: National Cancer Center; 2013. [Google Scholar]
- 3.Weitz J, Antonescu CR, Brennan MF. Localized extremity soft tissue sarcoma: improved knowledge with unchanged survival over time. J Clin Oncol. 2003;21:2719–25. doi: 10.1200/JCO.2003.02.026. [DOI] [PubMed] [Google Scholar]
- 4.Demetri GD, Elias AD. Results of single-agent and combination chemotherapy for advanced soft tissue sarcomas. Hematol Oncol Clin North Am. 1995;9:765–85. [PubMed] [Google Scholar]
- 5.Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–81. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 6.Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–23. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 7.Huang P, Chubb S, Hertel LW, et al. Action of 2′,2′-difluorodeoxycytidine on DNA synthesis. Cancer Res. 1991;51:6110–7. [PubMed] [Google Scholar]
- 8.Eisenhauer E. Docetaxel: current status and future prospects. J Clin Oncol. 1995;13:2865–8. doi: 10.1200/JCO.1995.13.12.2865. [DOI] [PubMed] [Google Scholar]
- 9.Svancarova L, Blay JY, Judson I, et al. Gemcitabine in advanced adult soft-tissue sarcomas. A phase II study of the EORTC Soft tissue and Bone Sarcoma Group. Eur J Cancer. 2002;38:556–9. doi: 10.1016/S0959-8049(01)00408-7. [DOI] [PubMed] [Google Scholar]
- 10.Verweij J, Lee SM, Ruka W, et al. Randomized phase II study of docetaxel versus doxorubicin in first-line and second-line chemotherapy for locally advanced or metastatic soft tissue sarcomas in adults: a study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 2000;18:2081–6. doi: 10.1200/JCO.2000.18.10.2081. [DOI] [PubMed] [Google Scholar]
- 11.Bay JO, Ray-Coquard I, Fayette J, et al. Docetaxel and gemcitabine combination in 133 advanced soft-tissue sarcomas: a retrospective analysis. Int J Cancer. 2006;119:706–11. doi: 10.1002/ijc.21867. [DOI] [PubMed] [Google Scholar]
- 12.Leu KM, Ostruszka LJ, Shewach D, et al. Laboratory and clinical evidence of synergistic cytotoxicity of sequential treatment with gemcitabine followed by docetaxel in the treatment of sarcoma. J Clin Oncol. 2004;22:1706–12. doi: 10.1200/JCO.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 13.Lee EM, Rha SY, Lee J, et al. Phase II study of weekly docetaxel and fixed dose rate gemcitabine in patients with previously treated advanced soft tissue and bone sarcoma. Cancer Chemother Pharmacol. 2012;69:635–42. doi: 10.1007/s00280-011-1742-5. [DOI] [PubMed] [Google Scholar]
- 14.Lee HY, Shin SJ, Kim HS, et al. Weekly gemcitabine and docetaxel in refractory soft tissue sarcoma: a retrospective analysis. Cancer Res Treat. 2012;44:43–9. doi: 10.4143/crt.2012.44.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi WX, He AN, Tang LN, et al. Efficacy and safety of gemcitabine-docetaxel combination therapy for recurrent or refractory high-grade osteosarcoma in China: a retrospective study of 18 patients. Jpn J Clin Oncol. 2012;42:427–31. doi: 10.1093/jjco/hys030. [DOI] [PubMed] [Google Scholar]
- 16.Pautier P, Floquet A, Penel N, et al. Randomized multicenter and stratified phase II study of gemcitabine alone versus gemcitabine and docetaxel in patients with metastatic or relapsed leiomyosarcomas: a Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) French Sarcoma Group Study (TAXOGEM study) Oncologist. 2012;17:1213–20. doi: 10.1634/theoncologist.2011-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hensley ML, Ishill N, Soslow R, et al. Adjuvant gemcitabine plus docetaxel for completely resected stages I-IV high grade uterine leiomyosarcoma: results of a prospective study. Gynecol Oncol. 2009;112:563–7. doi: 10.1016/j.ygyno.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Hensley ML, Blessing JA, Degeest K, et al. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study. Gynecol Oncol. 2008;109:323–8. doi: 10.1016/j.ygyno.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected] J Clin Oncol. 2007;25:2755–63. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt T, Kosely F, Wuchter P, et al. Gemcitabine and docetaxel for metastatic soft tissue sarcoma—a single center experience. Onkologie. 2013;36:415–20. doi: 10.1159/000353564. [DOI] [PubMed] [Google Scholar]
- 21.Kaya AO, Büyükberber S, Ozkan M, et al. Efficacy and toxicity of gemcitabine plus docetaxel combination as a second line therapy for patients with advanced stage soft tissue sarcoma. Asian Pac J Cancer Prev. 2012;13:463–7. doi: 10.7314/APJCP.2012.13.2.463. [DOI] [PubMed] [Google Scholar]
- 22.Davis EJ, Chugh R, Zhao L, et al. A randomised, open-label, phase II study of neo/adjuvant doxorubicin and ifosfamide versus gemcitabine and docetaxel in patients with localised, high-risk, soft tissue sarcoma. Eur J Cancer. 2015;51:1794–802. doi: 10.1016/j.ejca.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Takeda K, Negoro S, Tamura T, et al. Phase III trial of docetaxel plus gemcitabine versus docetaxel in second-line treatment for non-small-cell lung cancer: results of a Japan Clinical Oncology Group trial (JCOG0104) Ann Oncol. 2009;20:835–41. doi: 10.1093/annonc/mdn705. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka K, Kawamoto H, Saito I, et al. Preoperative and postoperative chemotherapy with ifosfamide and adriamycin for adult high-grade soft-tissue sarcomas in the extremities: Japan Clinical Oncology Group Study JCOG0304. Jpn J Clin Oncol. 2009;39:271–3. doi: 10.1093/jjco/hyn153. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka K, Mizusawa J, Fukuda H, et al. Perioperative chemotherapy with ifosfamide and doxorubicin for high-grade soft tissue sarcomas in the extremities (JCOG0304) Jpn J Clin Oncol. 2015;45:555–61. doi: 10.1093/jjco/hyu220. [DOI] [PubMed] [Google Scholar]
- 26.Kataoka K, Tanaka K, Mizusawa J, et al. A randomized phase II/III trial of perioperative chemotherapy with adriamycin plus ifosfamide vs gemcitabine plus docetaxel for high-grade soft tissue sarcoma: Japan Clinical Oncology Group study JCOG1306. Jpn J Clin Oncol. 2014;44:765–9. doi: 10.1093/jjco/hyu080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


