Abstract
Venous thromboembolism (VTE) includes deep vein thrombosis and pulmonary embolism; a combination of environmental and genetic risk factors contributes to VTE risk. Within environmental risk factors, some are provoking (e.g. cancer, surgery, trauma or fracture, immobilization, pregnancy and the postpartum period, long distance travel, hospitalization, catheterization, and acute infection) and others are non-provoking (e.g. age, sex, race/ethnicity, body mass index and obesity, oral contraceptive or hormone therapy use, corticosteroid use, statin use, diet, physical activity, sedentary time, and air pollution). Additionally, VTE has a strong genetic basis, with approximately 50–60% of the variance in VTE incidence attributed to genetic effects. Some genetic susceptibility variants that contribute to risk have been identified in candidate genes, mostly related to the clotting system and responsible for inherited hypercoagulable states (e.g., factor V Leiden, prothrombin, fibrinogen gamma, or blood group non-O). Other susceptibility single nucleotide polymorphisms have been identified from genome-wide association studies, such as the two new loci in TSPAN15 (rs78707713) and SCL44A2 (rs2288904) genes. Risk factors are not always associated with VTE in isolation, however, and an understanding of how environmental and genetic factors interact may provide insight into the pathophysiology of VTE, possibly identifying opportunities for targeted prevention and treatment.
Keywords: Venous thromboembolism, Epidemiology, Genetics, Risk factors
Introduction
Venous thromboembolism (VTE) is a disease classification that includes clots that have formed in the veins of the legs and arms, known as deep vein thrombosis (DVT), as well as clots that have embolized and traveled to the lungs, known as pulmonary embolism (PE). Among persons of European ancestry, VTE is estimated to occur at an incidence rate of approximately 1 to 2 per 1,000 person-years, with approximately 60% all VTE cases presenting as DVTs-only and the other 40% presenting as PEs with or without DVT.1 After a first (incident) VTE event, the risk of a recurrent event is high, with approximately 30% of persons who experience an incident VTE event experiencing a recurrence within 10 years.1
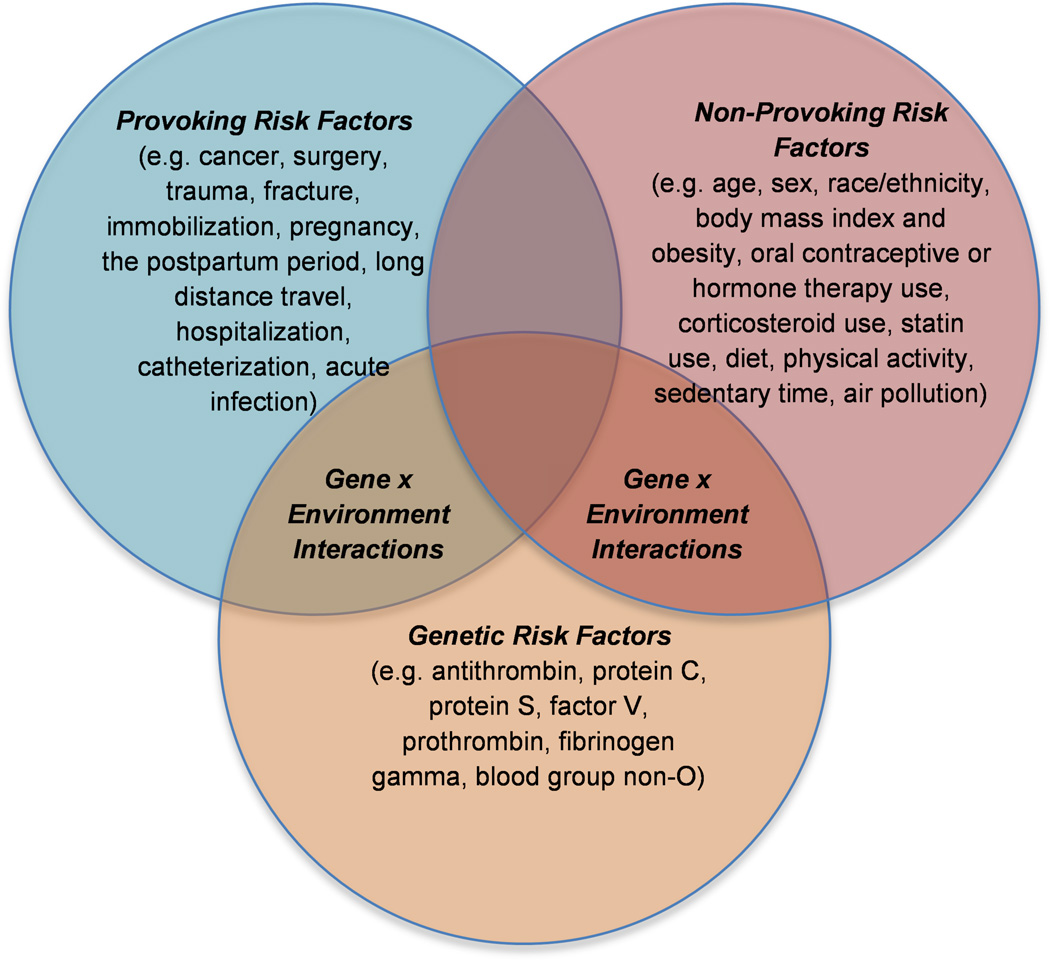
Although VTE is a type of cardiovascular disease, the etiology of VTE is unique from that of arterial thrombosis and likewise, only some traditional arterial thrombotic risk factors are thought to contribute to VTE risk.2 Multifactorial in nature, VTE includes both environmental and genetic risk factors. Within environmental risk factors for VTE, some are provoking (e.g. cancer, surgery, trauma or fracture, immobilization, pregnancy and the postpartum period, long distance travel, hospitalization, catheterization, and acute infection) and others are considered to be non-provoking (e.g. age, sex, race/ethnicity, BMI and obesity, oral contraceptive [OC] or hormone therapy [HT] use, corticosteroid use, statin use, diet, physical activity and sedentary time, and air pollution) (Figure 1).
Figure 1. Environmental and Genetic Risk Factors for Venous Thromboembolism.
Risk factors for venous thromboembolism are provoking, non-provoking, and genetic in nature.
In this review we will discuss a selection of environmental and genetic risk factors for VTE from a public health perspective. Importantly, our discussion will place special emphasis on risk factors that are controversial or of emerging interest. We will not attempt to be all-inclusive. In addition, while we acknowledge that “venous thrombosis” can include superficial thrombophlebitis, splenic, hepatic, ovarian vein, or cavernous sinus thrombosis, or other entities, this review will focus only on the most common forms of VTE: DVT and PE. Furthermore, our discussion in this review will focus on risk factors for incident and not for recurrent VTE. We have divided this review into several sections. In the first section we will discuss non-modifiable risk factors and their relationship to VTE, including: age, race/ethnicity, and sex. In the second section, we discuss the role of a selection of potentially modifiable environmental risk factors, including traditional risk factors for arterial thrombosis, medication use (exogenous hormones, corticosteroids, and statins), and emerging or controversial risk factors (diet, physical activity, and air pollution). While we acknowledge the importance of provoking environmental risk factors, these are well studied and characterized elsewhere, and thus will not be the focus of our review. In the third section we turn our attention to genetic risk factors for VTE, and in the final fourth section, we discuss gene-environment interactions in relation to VTE risk (Figure 1).
Non-Modifiable Risk Factors for VTE: Age, Sex, and Race/Ethnicity
Age
Although age, sex, and race/ethnicity are non-modifiable characteristics, understanding how VTE risk differs by these characteristics is critical to improving VTE diagnosis and treatment. While VTE can occur at any age, incident VTE more commonly occurs in older individuals. In young adult life until approximately midlife, VTE occurs at a low rate of 0.5 to 1 event per 1,000 person-years.3 This rate increases in midlife and by age 80, VTE incidence is substantially higher, occurring at a rate of approximately 5 to 7 VTE events per 1,000 person-years.3,4 Although the role of older age as an independent risk factor for VTE is not well understood, it has been proposed that blood coagulability may increase with age.5 In addition, age effects are likely mediated by a higher prevalence of provoking risk factors for VTE, such as cancer, immobility, hospitalization, and surgery.
Sex
VTE incidence across the lifespan also differs by sex, with a higher age-adjusted incidence rate among men (130 per 100,000 person-years) than women (110 per 100,000 person-years).3 However, it is controversial whether men are inherently at a greater risk of VTE than women. In younger adult life, the annual incidence of VTE is slightly higher among women than men,3 a difference that has been attributed to hormonal exposures that impact women in their childbearing years, such as pregnancy, the postpartum period and OC use.6 Following midlife, VTE incidence increases more rapidly among men than among women, resulting in a higher VTE incidence among older men than women.3 Although it is unclear as to why VTE incidence is higher among older men than women, it has been proposed that there may be differences in lifestyle-based risk factors between men and women. Alternatively, this difference may be mediated by body height.7
Race/Ethnicity
The risk of incident VTE is also thought to differ by race, with the highest risk thought to be among Black individuals, then White individuals, and the lowest risk among Asian or Hispanic individuals.8 Few studies have been able to appropriately evaluate the race-VTE association while controlling for appropriate confounders, however, and so the association of race with VTE remains controversial.9 Recently, a large study combined data from three United States-based cohorts: the Atherosclerosis Risk in Communities Study (ARIC), the Cardiovascular Health Study (CHS), and the Reasons for Geographic and Racial Differences in Stroke study (REGARDS). In the CHS, blacks had an 81% greater risk of incident VTE than did whites (hazard ratio [HR]=1.81; 95% confidence interval [CI]: 1.20–2.73) but in ARIC, there was no significant difference (HR=1.21; 95% CI: 0.96, 1.54).10 In REGARDS, the only one of the three cohort studies to include a substantial population of blacks residing outside of the Southeast, there was significant interaction between region of residence (Southeast vs. rest of country) and race (p=0.01), suggesting that regional differences in comorbid illnesses, environmental risk factors, or in quality and access to medical care may mediate a potential race-VTE association.10 Further complicating our understanding of a race-related gradient in VTE risk is the understanding that factor V Leiden (FVL) and prothrombin gene mutations (that will be discussed in detail in section 3) are both less common among persons of Black race than White race.8,11
Potentially Modifiable Environmental Risk Factors for VTE
In contrast to age, sex, and race/ethnicity, other risk factors for VTE may be modifiable, and are therefore relevant not only to diagnosis, but to VTE prevention. In this section, we will discuss potentially modifiable risk factors for VTE. We will focus on some risk factors that are common across both venous and arterial thrombosis. We will also discuss medications that are associated with VTE risk. Finally, we will discuss some risk factors for which the association with VTE is controversial or only just becoming clearer.
Traditional Arterial Thrombotic Risk Factors
Although venous and arterial thrombotic disease have been historically regarded as distinct diseases with differing etiologies, these two classes of thrombotic events share common characteristics. Both hypercoagulability and inflammation contribute to the development of arterial and venous thrombi and risk factors for the two diseases are not altogether dissimilar.2,12 A 2008 meta-analysis by Ageno et al. included 21 case-control and cohort studies that evaluated the association between traditional risk factors for arterial thrombosis and VTE risk. Obesity (odds ratio [OR]=2.33; 95% confidence interval [CI]: 1.68, 3.24), hypertension (OR=1.51; 95% CI: 1.23, 1.85), and diabetes mellitus (OR=1.42; 95% CI: 1.12, 1.77) were significantly associated with VTE risk, but there was no statistically significant evidence of an association between smoking (OR=1.18; 95% CI: 0.95, 1.46) or hypercholesterolemia (OR=1.42; 95% CI: 0.67, 2.02) and VTE risk.2 In this meta-analysis, there was no evidence that mean total and low-density lipoprotein cholesterol levels were associated with VTE risk, though mean high-density lipoprotein cholesterol levels were lower among VTE patients than controls (weighted mean difference=-2.86 mg/dL; 95% CI: −4.34, −1.38).2
The findings of Ageno et al. suggest that some traditional arterial thrombotic risk factors are also associated with VTE.2 However, the magnitude of VTE risk associated with some of these risk factors is not as high as with arterial disease, and in some studies do not reach statistical significance (for example, the borderline significant 18% greater VTE risk associated with smoking). Thus, while there are shared risk factors for arterial and venous disease, the etiologies of these two classes of thrombotic disease are distinct.
Medication Use
Exogenous Hormones
When evaluating medication use in relation to VTE risk, it can be difficult to disentangle risk associated with the medication from that associated with a medication’s underlying condition. However, the use of exogenous hormones (OC or HT) has been well established as positively associated with a 1.5 to >3-fold greater risk of incident VTE.13–16 Comparative studies of VTE risk associated with OC and HT type, dose, and formulation remain warranted especially as women’s use of OCs and indication for HTs remain common. Among women at risk for pregnancy, birth control use of any type ranges from 55% to 81% by state in the United States,17 and 30–80% of women experience hot flashes and night sweats at some point during the menopausal transition,18 which may prompt symptomatic treatment with HT. Moreover, different formulations are thought to be associated with different risks of VTE. In a 2014 systematic review and meta-analysis, OCs containing ethinyl estradiol with levonorgestrel were associated with a 50 to 80% lower risk of VTE than were OCs containing gestodene, desogestrel, cyproterone acetate, or drospirenone.19 Differences in VTE risk associated with the use of non-oral methods of contraception have also been reported. For example, in a Danish historical national registry-based cohort study, transdermal patch and the vaginal ring use were each separately associated with a greater risk of VTE than use of OCs containing levonorgestrel (relative risk [RR]=2.3; 95% CI: 1.0, 5.2, and RR=1.9; 95% CI: 1.3, 2.7, respectively).20 In contrast, the use of a levonorgestrel intrauterine device or an implant was associated with a lower risk of VTE (RR=0.18; 95% CI: 0.12, 2.6, and, RR=0.43; 95% CI: 0.18, 1.05, respectively) than the use of OCs containing levonorgestrel.20 Differences in VTE risk by oral HT type have also been identified. A population-based case-control study reported that among oral HT users, current use of conjugated equine estrogens was associated with a 2-fold greater risk of incident VTE as compared with current estradiol use (OR=2.08; 95% CI: 1.02, 4.27).21 In addition, a recent meta-analysis reported a greater VTE risk associated with oral estrogen HT as compared with transdermal HT use (RR=1.63; 95% CI: 1.40, 1.90).22 Given these reported differences in VTE risk by OC and HT type, dose, and formulations, additional research in this area is warranted.
Corticosteroids
Emerging research suggests that the use of corticosteroids may also be associated with VTE risk. Early studies of individuals with disease-based indications for corticosteroids reported a positive association between corticosteroid use and VTE. However, as corticosteroids are used to treat inflammation-associated conditions including asthma and arthritis, it has been difficult to control for residual confounding from the underlying disease in these studies. Only recently have studies evaluated VTE risk associated with corticosteroid use, either in relatively healthy populations or with improved adjustment for potential confounders. In a large (n=38,765 VTE cases) population-based case-control study using national databases in Denmark, glucocorticoid prescription fulfillment within the past 90 days was associated with more than a 2-fold greater risk of incident VTE (incidence rate ratio [IRR]=2.33; 95% CI: 2.18, 2.45), after adjustment for indicators of underlying disease severity.23 In a cohort study set in the British General Practice Research Database (n=6,550 VTE cases), current use of corticosteroids was associated with a 3-fold greater risk of incident VTE (OR=3.1; 95% CI: 2.5, 3.7).24 Of course, while the authors of these studies were able to adjust for cancer and for other inflammatory conditions, it is possible that residual confounding by inflammatory conditions may remain. However, one mechanism by which corticosteroids may increase VTE risk is via altered levels of hemostatic factors. In a study of 10-day randomized prednisolone vs. placebo use (total n=31) among healthy participants, levels of von Willebrand factor (VWF), plasminogen activator inhibitor type 1, and in vitro thrombin generation were all higher among prednisolone users.25
Statins
Statins, which are commonly prescribed to reduce low-density lipoprotein cholesterol levels for a reduction in the risk of arterial thrombotic events, have also been associated with a lower risk of incident VTE. In 2009, results from the Justification for the Use of Statins in Prevention: an Interpretation Trial Evaluating Rosuvastatin (JUPITER) suggested that rosuvastatin was associated with more than a 40% lower risk of incident VTE (HR=0.57; 95% CI: 0.37, 0.86) among apparently healthy persons.26 However, this association remains controversial.27 In a separate trial of pravastatin among men and women aged 70–82 years, the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER), randomization to pravastatin was not associated with a lower VTE risk (HR = 1.42; 95% CI: 0.80, 2.52), but confidence intervals were wide.
Emerging and/or Controversial Risk Factors of Interest
Diet
Although the role of diet in the risk of arterial thrombosis is commonly considered, the relation of diet to venous thrombosis is more controversial. Some studies have evaluated the relation between specific nutrients or food groups, and some evidence has suggested that fruits and vegetables,28 fish,28,29 whole grains,30 and alcohol30 may be negatively associated and that red/processed meat intake may be positively associated28,31 with VTE risk. Evidence remains inconsistent between studies, however.
Several studies have also evaluated patterns of diet in relation to VTE. The study of dietary patterns can be important since these patterns may more closely represent real-life food consumption than do studies of specific food groups of nutrients. Three studies have evaluated the Western Diet as compared to the Prudent Dietary Pattern in relation to VTE risk, but results have been inconsistent.28,30,31 The Western Diet, which is characterized by high intakes of refined grains, cured and red meats, desserts, sweets, French fries, and high-fat dairy products, is hypothesized to be associated with a greater VTE risk than is the Prudent Dietary Pattern, which is defined by high intakes of fruits, vegetables, fish, poultry, whole-grain products, and low-fat dairy products.31 Results from the ARIC study suggested (p-trend=0.04) that the Western Dietary Pattern was associated with a greater risk of VTE (top quintile of Western Diet Pattern score compared with bottom quintile HR: 1.6; 95% CI: 0.97, 2.66) although quintiles of Prudent Dietary Pattern intake were not linearly associated with VTE risk.28 In the Health Professionals Follow-up Study, the top two quintiles of Western dietary pattern intake were associated with an approximate 40% greater risk of incident VTE compared to the lowest value of intake (top quintile HR=1.43; 95% CI: 1.16, 1.78), and there was a significant linear trend by quintile (p<0.001).31 In contrast, analyses from the Iowa Women’s Health Study30 and the Nurses’ Health Study (NHS)31 suggested no significant association between the Western or Prudent Dietary Patterns and VTE risk. Inconsistent results have also been found when other dietary patterns have been studied. In an EPIC-NL analysis of the Mediterranean Diet Score, which assigns positive point values to above-median intake of vegetables, fruits, legumes/nuts, grains, fish/seafood, the ratio of unsaturated to saturated fatty acids, and to consumption of 1 or more alcoholic drinks per month, and negative point values for above-median intake of meat and dairy products, each 2-unit increase in intake was negatively associated with PE risk (HR=0.74; 95% CI: 0.49, 0.92).32 In contrast, studies have not reported strong evidence of an association between the Dietary Approaches to Stop Hypertension (DASH) dietary pattern33 and the Smart Diet Score34 with VTE risk. Thus, the relationship between diet and VTE risk remains challenging to understand.
Physical Activity and Sedentary Time
Physical activity is a well-characterized protective risk factor for arterial thrombosis, but like diet, its association with VTE is complex and uncertain.35–39 For example, in the CHS analysis of adults aged 65 years and older, self-reported exercise at baseline was not associated with the risk of VTE (HR=1.16; 95% CI: 0.84, 1.61), but when exercise was modeled in a time-varying fashion, the effect estimate shifted higher and nearly reached statistical significance, suggesting that physical activity among older adults may be associated with a greater risk of VTE (HR=1.38; 95% CI: 0.99, 1.91).39 There was also evidence that VTE risk may differ by intensity of physical activity. Whereas there was no association between mild intensity exercise and VTE risk (HR=0.75; 95% CI: 0.49, 1.16), strenuous exercise was associated with a greater risk of VTE than no physical activity (HR=1.75; 95% CI: 1.08, 2.83).39 In contrast, the Multiple Environmental and Genetic Assessment of risk factors for VTE (MEGA) case-control study, found that regular participation in leisure-time sports was associated with reduced risk of VTE (OR=0.71; 95% CI: 0.64, 0.78). However, investigators found no evidence of a dose-response relationship between sports intensity, frequency, and duration and risk of VTE.36 Other analyses, of the NHS,35 the Longitudinal Investigation of Thromboembolism Etiology (LITE) study,37 and the Tromsø study,38 reported no statistically significant evidence of an association between physical activity and VTE risk.
Contrasting findings between studies may stem from differences in the intensity, frequency, and duration of physical activity conducted by study participants, and how each study was able to evaluate these factors in relation to VTE risk. It is possible, for example, that physical activity may be associated with an increased short term VTE risk – perhaps by increasing the risk of injuries – whereas consistent physical activity may plausibly reduce VTE risk similarly to arterial thrombosis. It is also possible, especially given the results from the CHS that the association between physical activity and VTE varies depending on age.39
Air Pollution
Air pollution is emerging as a potential risk factor of interest, given its positive association with the risk of arterial thrombotic events40 and its proposed association with hypercoagulability and lung disease. However, like smoking, which is a strong risk factor for arterial thrombotic events, but a weak risk factor for VTE,2 the relationship between air pollution and VTE is inconsistent. Some studies suggest a positive association between air pollution and VTE risk,41–44 whereas others suggest no association.44–46 A recent systematic review of 11 studies concluded that there is a positive association between air pollution and VTE risk, but acknowledged that heterogeneity existed between studies.47 This heterogeneity likely stems from varied study settings41,42,45,46 with a wide range of pollutant levels.47 Like smoking, air pollution may be associated differently with venous versus arterial thrombotic risk, but further work is required to clarify this relationship.
Genetics
In this section, we will discuss genetic susceptibility for VTE. We will primarily focus on the genetic variants from candidate genes that have a large effect on VTE risk. We will also discuss the single nucleotide polymorphisms (SNPs) identified from genome-wide association studies that have been described to date.
Heritability and Family History
In addition to its association with environmental risk factors, VTE has a strong genetic basis: it has been shown that VTE is highly heritable and about 50–60% of the variance in VTE incidence is attributable to genetic effects.48–50 VTE inheritance follows a multifactorial, or non-Mendelian, inheritance model, with multiple genetic factors contributing to risk.48,51–54 The risk of VTE is greater among monozygotic twins (OR=13.5; 95% CI: 7.3, 24.8) than dizygotic twins (OR=3.8; 95% CI: 1.8, 8.3), providing further evidence of the importance of genetic influence to VTE risk.55 Family-based studies have confirmed these findings, and have reported that the risk for VTE in individuals with an affected sibling is 2.5 times higher than risk in the general population.48 Carriers of a familial thrombophilic genetic risk variant have a 0.8% risk per year of developing VTE.49,50
Susceptibility Variants from Candidate Gene Approaches
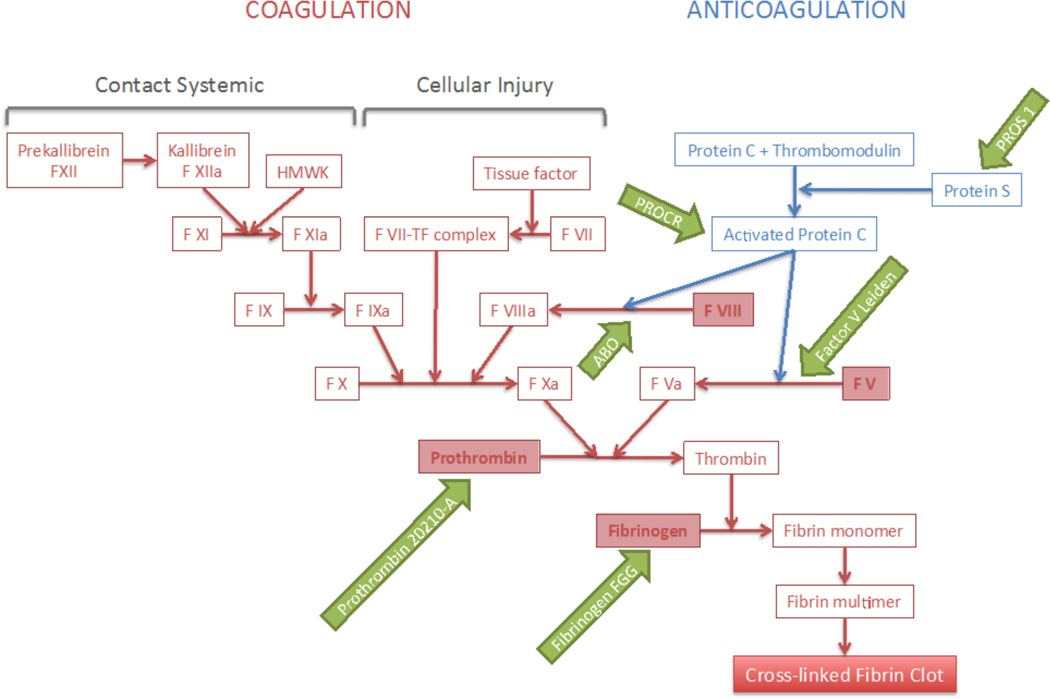
The genetic basis of VTE is only partly understood.56 At present 20–30 genetic VTE risk factors are known,56,57 and most of the identified genetic risk factors involve mutations in the clotting system (Figure 2). There are seven well-established genetic risk factors for VTE, all responsible for inherited hypercoagulable states. Four, which include variants in factor V (FV) (e.g. the FVL mutation), prothrombin (e.g. prothrombin 20210-A), fibrinogen gamma (FGG), and blood group non-O, are more frequent in the general population. The prevalence in European-descent individuals is around 5% for FVL and prothrombin 20210-A, and around 25% for FGG and non-0 blood group. The increase in VTE risk is about 3-fold for the FVL and prothrombin mutations, 1.5-fold for FGG, and 2-fold for non-O blood group.58–83 The other three are heterozygous deficiencies of the natural coagulation inhibitors (antithrombin, protein C, and protein S). Their effect is relatively large, increasing VTE risk by approximately 10 fold. However, these deficiencies are relatively rare, affecting less than 1% of the general population.
Figure 2. The Clotting Cascade.
HW=high-molecular-weight kininogen. TF=tissue factor.
Factor V
The strongest genetic variant identified to date in association with VTE risk is known as the FVL mutation. The rs6025 SNP represents an autosomal dominant genetic variant in the FV gene, encoding a change in the protein from an arginine at position 506 to a glutamine. This is found in 4–5% of individuals in most populations. FVL is a mutation in one of the genes involved in the intrinsic coagulation pathway (Figure 2), and causes resistance to activated protein C84,85. The missense variant rs6025 has been associated with a 2 to 7 fold increase in VTE risk for heterozygous carriers, and a 15- to 20-fold increase for homozygous carriers.86–90
Recently, a large meta-analysis successfully validated two missense variants located in FV; specifically the previously mentioned SNP rs6025, associated with an increased odds of VTE of 3.25 (p=1.10x10−96), and the SNP rs4524, associated with VTE risk independently of the previously described variant with an odds of VTE of OR=1.20, p=2.65x10−11.91
Other independent SNPs in FV gene (including rs3753305, rs9332695) have also been associated with alterations in FV, and consequently have been associated with increased VTE risk.92 Even though the risk allele frequencies for some of those SNPs might be higher in the general population, the effect sizes are lower than those at rs6025.
Prothrombin
A prothrombin gene mutation is the second most common cause of inherited thrombophilia. It is caused by mutation in the prothrombin gene (also known as Factor II), another key element in the blood clotting cascade (Figure 2). A prothrombin G to A SNP at position 20210 (rs1799963) in the untranslated 3’ region of the prothrombin gene leads to overproduction of prothrombin, consequently making carriers of this mutation prone to blood clots. This variant is present in 2–4% of the population, and is associated with a two-to-three fold increased risk of VTE for the heterozygous state and 5–10-fold for the homozygous state.87,93–96 The intronic variant in FII rs1799963 was also validated in the meta-analysis previously mentioned and with an odds ratio of 2.29 (p=1.73x10−9).91
ABO Blood Group
ABO blood group is known to be a major determinant of plasma levels of factor VIII, one of the proteins involved in the clotting cascade (Figure 2). Specifically, factor VIII and VWF undergo extensive post-translational modification by the ABO blood group-encoded glycosyltransferases.58,97 Increased plasma factor VIII and VWF levels are associated with an increased risk for VTE.98,99 ABO phenotype correlates with plasma levels of factor VIII and VWF, such that individuals with type O blood group have about 25% lower plasma factor VIII and VWF levels63,100 and accordingly, a lower risk of VTE. It is worth noting however, that non-O ABO blood type has been associated with an 86% (95% CI: 1.35, 2.57) increase in VTE risk independent of factor VIII, so there may be additional mechanisms behind this association as well.63
Different ABO SNPs have been independently associated with VTE. These include: rs8176719, an ABO exon 6 deletion determining type O blood group, and rs2519093, and ABO intron 1 tag SNP.101,102 Additionally, the SNP rs529565, an intronic variant in the ABO gene, was replicated on meta-analysis.91 The risk allele for this SNP is associated with a 55% increased risk of VTE (p=4.23×10−75).
The SNPs described above have a large effect on VTE risk. The joint population-attributable risk (PAR) for the four SNPs including FVL (rs6025), prothrombin (rs1799963), ABO non-O blood group (rs8176719) and ABO rs2519093 account for a large proportion of VTE among non-Hispanic adults of European-ancestry: population attributable risk (PAR)=0.40.103
Other Genes
Polymorphisms in other genes, including factors VIII, IX, XI and XIII also produce thrombophilia and consequently they have been associated with increased risk of VTE.83 One of the better established is FGG. Individuals with mutations in the FGG gene have a reduced FGG level, which increases binding of proteins that promote cleavage to fibrin monomers (Figure 2). Several studies have found the 455GA polymorphism of β-fibrinogen gene (rs2066865) to be associated with risk of VTE in Caucasian population. Carriers of the A allele have a slight (around 15%) protective effect against VTE.57 The large genome wide association study (GWAS) meta-analysis also validated the SNP rs2066865, located in the 3’ untranslated region (UTR) of the FGG gene, associated with odds of VTE of 1.24 (p=1.03×10−16).91
Moreover, deficiencies of protein C and antithrombin, two natural plasma anticoagulants, are known risk factors for VTE. Deficient protein C or antithrombin is typically present in 4% to 9% of the patients with familial thrombophilia. In contrast, the prevalence of protein C deficiency in the general population is less common and has been estimated to be between 0.2% and 0.4%.104,105 A number of mutations of the protein C and antithrombin genes contribute to deficiencies of these anticoagulant proteins.104 Hereditary protein S deficiency has also been associated with an increased risk of VTE.106 Protein S assists in the downregulation of thrombin formation by stimulating the activity of both activated protein C and tissue factor pathway inhibitor. The genetic basis of protein S deficiency is heterogeneous and several mutations in PROS1 have been described to date.
Other variants in genes coding for alpha and beta-fibrinogen, protein C, and plasminogen activator inhibitor, have been also associated with VTE risk. Some of these variants were validated in the aforementioned meta-analysis,91 specifically: the SNP rs4253417, an intronic variant in factor XI (FXI) gene, associated with VTE risk with an OR of 1.27 (p=1.21x10−23), and the SNP rs6087685, an intronic variant located in the protein C receptor (PROC) gene, associated with VTE risk with an OR of 1.15 (p=1.65x10−8). The majority of SNPs associated with VTE have been identified in populations of European ancestry. However, a genetic variant in the gene encoding for the methylenetetrahydrofolate reductase (MTHFR), the SNP rs1801133 (also known as C677T mutation) has been identified in Asian ancestry populations (OR=1.57; 95% CI: 1.23, 2.00); and the insertion/deletion polymorphism of the angiotensin-converting enzyme gene has been specifically described in African ancestry populations as associated with VTE risk (OR=1.50; 95% CI: 1.03, 2.18).
Susceptibility Variants from GWAS Approaches
Despite the accumulated evidence that genetic factors play a major role in the pathophysiology of VTE, only 35% of VTE patients undergoing testing for thrombophilia carry a polymorphism known to increase VTE risk.107 Given the high estimated heritability of VTE, additional genetic risk factors likely exist. All of the factors discussed above were identified based on their relevance to the clotting cascade, using candidate-gene approaches. GWAS are a powerful method to identify common SNPs associated with a complex disorder without a pre-specified hypothesis. GWAS have proven highly efficient in identifying novel susceptibility loci for other complex diseases.
Despite this promise, early GWAS were unable to identify any previously unknown variants associated with VTE.91,102,108–110 Most of these GWAS had limited samples sizes, and were underpowered to detect novel associations at the rigorous genome-wide-significance level. To overcome this limitation, the International Network on VENous Thrombosis (INVENT) Consortium came together to perform a meta-analysis of existing GWAS.91 The main objective was to identify additional common variants associated with VTE risk in the context of a large study. The INVENT network currently comprises 15 cohort and case-control studies worldwide and includes 10,676 VTE cases and more than 71,752 controls.
The INVENT meta-analysis is the largest investigation to date on the influence of common genetic variation on VTE risk. It included GWAS findings from 12 studies, including a total of 7,505 VTE cases and 52,632 controls and was the first study to identify new genetic loci involved in VTE susceptibility using modern, agnostic genotyping methods.91 Out of a total of 6,751,884 SNPs tested after quality-control measures, nine loci reached the genome-wide significance (5x10−8) and were successfully replicated in independent samples. Those included loci in six genes previously identified as associated with VTE: rs529565 in ABO, rs1799963 in FII, rs6025 and rs4524 in FV, rs4253417 in FXI, rs2066865 in FGG, rs6087685 in PROC; and two new loci in TSPAN15 (rs78707713) and SCL44A2 (rs2288904) genes. Interestingly, neither of the two new loci identified in this large meta-analysis belong to conventional pathways for thrombosis, nor have they been associated to other cardiovascular diseases. This suggests that they may represent novel pathophysiological mechanisms of VTE.91,102
The lead SNP at the TSPAN15 locus is the intronic variant rs78707713, and has an OR of 1.31 (p=1.67x10−16) for VTE. TSPAN15 codes for tetraspanin 15, a member of the tetraspanin superfamily that act as scaffolding proteins, anchoring multiple proteins to the cell membrane [reference]. Members of the tetraspanin family have roles in cells that regulate hemostasis. However, the TSPAN15 rs78707713 has not been linked to any regulatory elements supporting a functional role of this particular SNP. It is likely that the SNP is in strong linkage disequilibrium with yet unidentified functional variants.
The lead SNP at the SCL44A2 locus is the non-synonymous rs2288904, which has an OR of 1.21 (p=2.75x10−15) for VTE.91 The risk allele at the SLC44A2 locus (rs2288904-G) is probably the functional variant since it codes for the Arg154 isoform of the choline transporter-like protein 2 (CTL-2). CTL-2 has been associated with several human diseases, including transfusion-related acute lung injury (TRALI). TRALI is a life-threatening complication of blood transfusion and the leading cause of transfusion-associated mortality in developed countries. Severe TRALI is due to antibodies in blood components directed against the human neutrophil alloantigen-3a, which is determined by the Arg154 isoform.
Genetic Risk Scores
While specific genetic variants can have large effects on VTE risk, VTE is a complex disease. Thus, an individual’s risk of VTE may be better described by the sum of their genetic risk factors, or in other words, their overall genetic predisposition. In order to assess overall genetic predisposition, a few studies have constructed genetic risk scores (GRS) and assessed their association with VTE risk.
Soria et al. performed a systematic review and meta-analysis to select variants that contribute to VTE risk and created a GRS called Thrombo inCode (TiC).111 They concluded that TiC, which includes SNPs on FVL, FII, FXIII, SERPINC1, SERPINA10, and A1 blood group genes, improved VTE risk prediction compared to using variants in FVL and prothrombin genes alone. De Haan et al. also created a GRS based on 31 SNPs associated with VTE, identified through candidate gene approaches.112 In this case-control study of 2712 patients and 4634 controls, a GRS including five SNPs was highly associated with VTE risk (OR 7.48; 95% CI: 4.49, 12.46) and performed similarly to a GRS including all 31 studied SNPs. Both of these studies were performed in predominantly European ancestry subjects. Folsom et al113 recently tested the ability of a GRS based on five well-established VTE SNPs in the FVL, FII, ABO, FGG, and FXI genes to predict VTE incidence in African Americans. While this five-SNP GRS had identified white adults at risk of VTE, the GRS did not identify future VTE occurrence in African Americans. Recently, Crous-Bou et al114 constructed a GRS based on SNPs associated with VTE risk from previous GWAS. In this nested case-control study of 1,040 incident VTE cases and 16,637 controls, VTE risk increased with the number of risk alleles, and the risk of VTE among individuals with a high GRS was 1.93 times that of individuals with a low GRS (p for trend = 1.63x10−16).
In combination, these studies demonstrate that selected SNPs can be combined into a GRS with a strong, linear, positive association with VTE. In the coming era of personalized medicine, GRS may be incorporated into clinical decision making. Identifying individuals at high risk of VTE may provide opportunities for targeted prevention and testing of the most appropriate patients.
The Genetic Risk of DVT versus PE
Although much of the discussion thus far has focused on VTE (i.e. both DVT and PE), it is worth noting that genetic factors may affect the risk of DVT and PE differently. For example, studies repeatedly report that carriers of the FVL variant have a substantially (three-fold) increased risk of DVT, whereas the risk of PE is only mildly increased (up to two-fold) compared with non-carriers. The observation that patients at higher risk of VTE are more likely to present with the less severe manifestation of the disease has been called the “Factor V Leiden paradox”.115,116 Few studies have investigated possible mechanisms for this so-called paradox.115,117,118 Some of the proposed mechanisms take into account whether FVL affects thrombus location, number of affected veins, time until diagnosis, clot propagation speed or density and whether these factors differ in in patients with DVT compared to patients with PE.117 Further investigation into the FVL paradox is required.
Future Directions: Identification of Rare Variants associated with VTE Risk
It has been estimated that common variants captured by existing GWAS arrays explain 35% of the genetic variance underlying VTE susceptibility.109 However, as we have discussed, existing GWA studies for VTE have had limited success identifying previously undiscovered variants and a large proportion of variants associated with VTE are yet to be identified.108,109 Variants that have been discovered tend to be rare (<5% prevalence), functional, and have relatively strong effects.57,119–121 If heretofore-unknown risk factors for VTE are similar, an approach focused on low-frequency coding variants may outperform GWAS approaches at detecting potential susceptibility variants. Efforts to identify new rare variants associated with VTE risk are currently ongoing through a meta-analysis of exome-wide association studies of VTE being performed by the INVENT consortium. Future steps towards the identification of new genetic variants associated with VTE risk may also include approaches like targeted exome sequencing, or whole genome sequencing.
Gene-Environment Interactions
As we have discussed, both environmental and genetic risk factors contribute to VTE risk. However, these risk factors do not act in isolation. Previous research suggests that VTE risk is greatest when genetic predisposition is combined with an environmental risk factor.48,58,122–124 Understanding how genes and environmental risk factors interact may provide key insight into the pathophysiology of VTE and may identify opportunities for targeted prevention and treatment.125–127 However, few interactions have been explored in prospective cohort studies, with work focusing mostly on short-term environmental risk factors such as trauma or surgery.93,127–149
One of the first gene-environment interactions explored was the effect of exogenous hormone use (either OCs or HT) and VTE risk in FVL mutation carriers. Studies support gene-environment interaction between the FVL mutation and exogenous hormone use, with a 35-fold increased risk of VTE in women with the FVL mutation who also used hormones.130–133,136–139 One study also suggested that VTE risk associated with FXI risk alleles is blunted by statin use.150 However, in a case-control study of the association between FXI variants and VTE risk, Harrington et al. showed that VTE risks were similar across strata of statin use and that there was no statistical evidence of a FXI-statin interaction.151 In another analysis, Wolpin et al58 found that the risk of PE with non-O blood type was higher among smokers (HR=2.56; 95% CI: 1.60, 4.07) than never smokers (HR=1.31; 95% CI: 0.83, 2.07; p for interaction = 0.04).58 In order to build on those finding, Crous-Bou et al114 explored interactions between genetic risk factors and two key environmental risk factors, BMI and smoking. This study represented the first detailed exploration of interactions between BMI, smoking, and genetic risk factors for VTE. Although the authors did not find evidence of multiplicative interactions between genetic and environmental risk factors, they did demonstrate additive effects of both on VTE risk.114 Gene-environment interaction studies have the potential to provide key insight into the relative contribution of genetic and environmental risk factors for VTE, but result remain sparse. Further work into the interaction between genes and environment are needed.
Conclusions
In summary, both environmental and genetic factors contribute to the risk of VTE. Environmental factors can be characterized in a number of different ways, such as non-modifiable and modifiable factors; both of those have significant contribution in risk of VTE. Similarly, genetic risk factors influence the risk of VTE. Many of these were found through candidate gene studies, and only recently modern high-throughput techniques have increased our understanding of the genetic risk of VTE. Ultimately, however, VTE is a complex disease and is related to interactions between both environmental and genetic risk factors. This we are now beginning to understand, but future research should focus on a holistic approach that includes both genetic and environmental risk factors for VTE.
Acknowledgments
This work was conducted with support from the National Heart Lung and Blood Institute. Support for Harrington: T32 HL098048, PI: Eric B. Rimm. Support for Crous-Bou and Kabrhel: R01 HL116854, PI Christopher Kabrhel.
Contributor Information
Marta Crous-Bou, Email: nhmcr@channing.harvard.edu.
Laura B. Harrington, Email: lharring@hsph.harvard.edu.
References
- 1.Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41:3–14. doi: 10.1007/s11239-015-1311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 3.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 4.Bell EJ, Lutsey PL, Basu S, et al. Lifetime Risk of Venous Thromboembolism in Two Cohort Studies. Am J Med. 2016;129:339, e319–e326. doi: 10.1016/j.amjmed.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luxembourg B, Schmitt J, Humpich M, Glowatzki M, Seifried E, Lindhoff-Last E. Intrinsic clotting factors in dependency of age, sex, body mass index, and oral contraceptives: definition and risk of elevated clotting factor levels. Blood Coagul Fibrinolysis. 2009;20:524–534. doi: 10.1097/MBC.0b013e32832d9b58. [DOI] [PubMed] [Google Scholar]
- 6.Roach RE, Lijfering WM, Rosendaal FR, Cannegieter SC, le Cessie S. Sex difference in risk of second but not of first venous thrombosis: paradox explained. Circulation. 2014;129:51–56. doi: 10.1161/CIRCULATIONAHA.113.004768. [DOI] [PubMed] [Google Scholar]
- 7.Severinsen MT, Johnsen SP, Tjonneland A, Overvad K, Dethlefsen C, Kristensen SR. Body height and sex-related differences in incidence of venous thromboembolism: a Danish follow-up study. Eur J Intern Med. 2010;21:268–272. doi: 10.1016/j.ejim.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Buckner TW, Key NS. Venous thrombosis in blacks. Circulation. 2012;125:837–839. doi: 10.1161/CIRCULATIONAHA.111.073098. [DOI] [PubMed] [Google Scholar]
- 9.Goldhaber SZ. Race and venous thromboembolism: nature or nurture? Circulation. 2014;129:1463–1465. doi: 10.1161/CIRCULATIONAHA.114.008799. [DOI] [PubMed] [Google Scholar]
- 10.Zakai NA, McClure LA, Judd SE, et al. Racial and regional differences in venous thromboembolism in the United States in 3 cohorts. Circulation. 2014;129:1502–1509. doi: 10.1161/CIRCULATIONAHA.113.006472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zakai NA, McClure LA. Racial differences in venous thromboembolism. J Thromb Haemost. 2011;9:1877–1882. doi: 10.1111/j.1538-7836.2011.04443.x. [DOI] [PubMed] [Google Scholar]
- 12.Lijfering WM, Flinterman LE, Vandenbroucke JP, Rosendaal FR, Cannegieter SC. Relationship between venous and arterial thrombosis: a review of the literature from a causal perspective. Semin Thromb Hemost. 2011;37:885–896. doi: 10.1055/s-0031-1297367. [DOI] [PubMed] [Google Scholar]
- 13.Vessey M, Mant D, Smith A, Yeates D. Oral contraceptives and venous thromboembolism: findings in a large prospective study. Br Med J (Clin Res Ed) 1986;292:526. doi: 10.1136/bmj.292.6519.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manzoli L, De Vito C, Marzuillo C, Boccia A, Villari P. Oral contraceptives and venous thromboembolism: a systematic review and meta-analysis. Drug Saf. 2012;35:191–205. doi: 10.2165/11598050-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA. 2004;292:1573–1580. doi: 10.1001/jama.292.13.1573. [DOI] [PubMed] [Google Scholar]
- 16.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 17.Bensyl DM, Iuliano DA, Carter M, Santelli J, Gilbert BC. Contraceptive use--United States and territories, Behavioral Risk Factor Surveillance System, 2002. Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C.: 2002) 2005;54:1–72. [PubMed] [Google Scholar]
- 18.National Institutes of Health. National Institutes of Health State-of-the-Science Conference statement: management of menopause-related symptoms. Ann Intern Med. 2005;142:1003–1013. [PubMed] [Google Scholar]
- 19.de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014:Cd010813. doi: 10.1002/14651858.CD010813.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lidegaard O, Nielsen LH, Skovlund CW, Lokkegaard E. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001–10. BMJ. 2012;344:e2990. doi: 10.1136/bmj.e2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith NL, Blondon M, Wiggins KL, et al. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med. 2014;174:25–31. doi: 10.1001/jamainternmed.2013.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohammed K, Abu Dabrh AM, Benkhadra K, et al. Oral vs Transdermal Estrogen Therapy and Vascular Events: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2015;100:4012–4020. doi: 10.1210/jc.2015-2237. [DOI] [PubMed] [Google Scholar]
- 23.Johannesdottir SA, Horvath-Puho E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med. 2013;173:743–752. doi: 10.1001/jamainternmed.2013.122. [DOI] [PubMed] [Google Scholar]
- 24.Huerta C, Johansson S, Wallander MA, Garcia Rodriguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007;167:935–943. doi: 10.1001/archinte.167.9.935. [DOI] [PubMed] [Google Scholar]
- 25.Majoor CJ, Sneeboer MM, de Kievit A, et al. The influence of corticosteroids on hemostasis in healthy subjects. J Thromb Haemost. 2016;14:716–723. doi: 10.1111/jth.13265. [DOI] [PubMed] [Google Scholar]
- 26.Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–1861. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desai CS, Martin SS, Blumenthal RS. Non-cardiovascular effects associated with statins. Bmj. 2014;349:g3743. doi: 10.1136/bmj.g3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steffen LM, Folsom AR, Cushman M, Jacobs DR, Jr, Rosamond WD. Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology. Circulation. 2007;115:188–195. doi: 10.1161/CIRCULATIONAHA.106.641688. [DOI] [PubMed] [Google Scholar]
- 29.Hansen-Krone IJ, Enga KF, Sudduth-Klinger JM, et al. High fish plus fish oil intake is associated with slightly reduced risk of venous thromboembolism: the Tromso Study. J Nutr. 2014;144:861–867. doi: 10.3945/jn.113.189548. [DOI] [PubMed] [Google Scholar]
- 30.Lutsey PL, Steffen LM, Virnig BA, Folsom AR. Diet and incident venous thromboembolism: the Iowa Women’s Health Study. Am Heart J. 2009;157:1081–1087. doi: 10.1016/j.ahj.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varraso R, Kabrhel C, Goldhaber SZ, Rimm EB, Camargo CA., Jr Prospective study of diet and venous thromboembolism in US women and men. Am J Epidemiol. 2012;175:114–126. doi: 10.1093/aje/kwr377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoevenaar-Blom MP, Nooyens AC, Kromhout D, et al. Mediterranean style diet and 12-year incidence of cardiovascular diseases: the EPIC-NL cohort study. PLoS One. 2012;7:e45458. doi: 10.1371/journal.pone.0045458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzgerald KC, Chiuve SE, Buring JE, Ridker PM, Glynn RJ. Comparison of associations of adherence to a Dietary Approaches to Stop Hypertension (DASH)-style diet with risks of cardiovascular disease and venous thromboembolism. J Thromb Haemost. 2012;10:189–198. doi: 10.1111/j.1538-7836.2011.04588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen-Krone IJ, Enga KF, Njolstad I, Hansen JB, Braekkan SK. Heart healthy diet and risk of myocardial infarction and venous thromboembolism. The Tromso Study. Thromb Haemost. 2012;108:554–560. doi: 10.1160/TH11-11-0818. [DOI] [PubMed] [Google Scholar]
- 35.Kabrhel C, Varraso R, Goldhaber SZ, Rimm E, Camargo CA., Jr Physical inactivity and idiopathic pulmonary embolism in women: prospective study. BMJ. 2011;343:d3867. doi: 10.1136/bmj.d3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Stralen KJ, Le Cessie S, Rosendaal FR, Doggen CJ. Regular sports activities decrease the risk of venous thrombosis. J Thromb Haemost. 2007;5:2186–2192. doi: 10.1111/j.1538-7836.2007.02732.x. [DOI] [PubMed] [Google Scholar]
- 37.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162:1182–1189. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 38.Borch KH, Hansen-Krone I, Braekkan SK, et al. Physical activity and risk of venous thromboembolism. The Tromso study. Haematologica. 2010;95:2088–2094. doi: 10.3324/haematol.2009.020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Stralen KJ, Doggen CJ, Lumley T, et al. The relationship between exercise and risk of venous thrombosis in elderly people. J Am Geriatr Soc. 2008;56:517–522. doi: 10.1111/j.1532-5415.2007.01588.x. [DOI] [PubMed] [Google Scholar]
- 40.Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 41.Baccarelli A, Martinelli I, Zanobetti A, et al. Exposure to particulate air pollution and risk of deep vein thrombosis. Arch Intern Med. 2008;168:920–927. doi: 10.1001/archinte.168.9.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baccarelli A, Martinelli I, Pegoraro V, et al. Living near major traffic roads and risk of deep vein thrombosis. Circulation. 2009;119:3118–3124. doi: 10.1161/CIRCULATIONAHA.108.836163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dales RE, Cakmak S, Vidal CB. Air pollution and hospitalization for venous thromboembolic disease in Chile. J Thromb Haemost. 2010;8:669–674. doi: 10.1111/j.1538-7836.2010.03760.x. [DOI] [PubMed] [Google Scholar]
- 44.Pun VC, Hart JE, Kabrhel C, Camargo CA, Jr, Baccarelli AA, Laden F. Prospective Study of Ambient Particulate Matter Exposure and Risk of Pulmonary Embolism in the Nurses’ Health Study Cohort. Environ Health Perspect. 2015;123:1265–1270. doi: 10.1289/ehp.1408927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kan H, Folsom AR, Cushman M, et al. Traffic exposure and incident venous thromboembolism in the Atherosclerosis Risk in Communities (ARIC) Study. J Thromb Haemost. 2011;9:672–678. doi: 10.1111/j.1538-7836.2011.04210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shih RA, Griffin BA, Salkowski N, et al. Ambient particulate matter air pollution and venous thromboembolism in the Women’s Health Initiative Hormone Therapy trials. Environ Health Perspect. 2011;119:326–331. doi: 10.1289/ehp.1002256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franchini M, Mengoli C, Cruciani M, Bonfanti C, Mannucci PM. Association between particulate air pollution and venous thromboembolism: A systematic literature review. Eur J Intern Med. 2016;27:10–13. doi: 10.1016/j.ejim.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 48.Heit JA, Phelps MA, Ward SA, Slusser JP, Petterson TM, De Andrade M. Familial segregation of venous thromboembolism. Journal of thrombosis and haemostasis : JTH. 2004;2:731–736. doi: 10.1111/j.1538-7933.2004.00660.x. [DOI] [PubMed] [Google Scholar]
- 49.Vossen CY, Conard J, Fontcuberta J, et al. Familial thrombophilia and lifetime risk of venous thrombosis. Journal of thrombosis and haemostasis : JTH. 2004;2:1526–1532. doi: 10.1111/j.1538-7836.2004.00852.x. [DOI] [PubMed] [Google Scholar]
- 50.Vossen CY, Conard J, Fontcuberta J, et al. Risk of a first venous thrombotic event in carriers of a familial thrombophilic defect. The European Prospective Cohort on Thrombophilia (EPCOT) Journal of thrombosis and haemostasis : JTH. 2005;3:459–464. doi: 10.1111/j.1538-7836.2005.01197.x. [DOI] [PubMed] [Google Scholar]
- 51.Souto JC, Almasy L, Borrell M, et al. Genetic susceptibility to thrombosis and its relationship to physiological risk factors: the GAIT study. Genetic Analysis of Idiopathic Thrombophilia. Am J Hum Genet. 2000;67:1452–1459. doi: 10.1086/316903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Souto JC, Almasy L, Borrell M, et al. Genetic determinants of hemostasis phenotypes in Spanish families. Circulation. 2000;101:1546–1551. doi: 10.1161/01.cir.101.13.1546. [DOI] [PubMed] [Google Scholar]
- 53.Couturaud F, Kearon C, Leroyer C, et al. Incidence of venous thromboembolism in first-degree relatives of patients with venous thromboembolism who have factor V Leiden. Thromb Haemost. 2006;96:744–749. [PubMed] [Google Scholar]
- 54.Rosendaal FR, Reitsma PH. Genetics of venous thrombosis. Journal of thrombosis and haemostasis : JTH. 2009;7(Suppl 1):301–304. doi: 10.1111/j.1538-7836.2009.03394.x. [DOI] [PubMed] [Google Scholar]
- 55.Larsen TB, Sorensen HT, Skytthe A, Johnsen SP, Vaupel JW, Christensen K. Major genetic susceptibility for venous thromboembolism in men: a study of Danish twins. Epidemiology. 2003;14:328–332. [PubMed] [Google Scholar]
- 56.Reitsma PH. How to identify new genetic risk factors for VTE? Thromb Res. 2009;123(Suppl 4):S22–S24. doi: 10.1016/S0049-3848(09)70138-0. [DOI] [PubMed] [Google Scholar]
- 57.Gohil R, Peck G, Sharma P. The genetics of venous thromboembolism. A meta-analysis involving approximately 120,000 cases and 180,000 controls. Thromb Haemost. 2009;102:360–370. doi: 10.1160/TH09-01-0013. [DOI] [PubMed] [Google Scholar]
- 58.Wolpin BM, Kabrhel C, Varraso R, et al. Prospective study of ABO blood type and the risk of pulmonary embolism in two large cohort studies. Thromb Haemost. 2010;104:962–971. doi: 10.1160/TH10-05-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Canonico M, Olie V, Carcaillon L, Tubert-Bitter P, Scarabin PY. Synergism between non-O blood group and oral estrogen in the risk of venous thromboembolism among postmenopausal women: The ESTHER study. Thromb Haemost. 2008;99:246–248. doi: 10.1160/TH07-09-0536. [DOI] [PubMed] [Google Scholar]
- 60.Streiff MB, Segal J, Grossman SA, Kickler TS, Weir EG. ABO blood group is a potent risk factor for venous thromboembolism in patients with malignant gliomas. Cancer. 2004;100:1717–1723. doi: 10.1002/cncr.20150. [DOI] [PubMed] [Google Scholar]
- 61.Larsen TB, Johnsen SP, Gislum M, Moller CA, Larsen H, Sorensen HT. ABO blood groups and risk of venous thromboembolism during pregnancy and the puerperium. A population-based, nested case-control study. Journal of thrombosis and haemostasis : JTH. 2005;3:300–304. doi: 10.1111/j.1538-7836.2005.01195.x. [DOI] [PubMed] [Google Scholar]
- 62.Teixeira Mello TB, Machado TF, Montavao SA, Ozello MC, Annichino-Bizzacchi JM. Assessing the coagulation factor levels, inherited thrombophilia, and ABO blood group on the risk for venous thrombosis among Brazilians. Clin Appl Thromb Hemost. 2009;15:408–414. doi: 10.1177/1076029607311777. [DOI] [PubMed] [Google Scholar]
- 63.Ohira T, Cushman M, Tsai MY, et al. ABO blood group, other risk factors and incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE) Journal of thrombosis and haemostasis : JTH. 2007;5:1455–1461. doi: 10.1111/j.1538-7836.2007.02579.x. [DOI] [PubMed] [Google Scholar]
- 64.Morelli VM, de Visser MC, van Tilburg NH, et al. ABO blood group genotypes, plasma von Willebrand factor levels and loading of von Willebrand factor with A and B antigens. Thromb Haemost. 2007;97:534–541. [PubMed] [Google Scholar]
- 65.Morelli VM, De Visser MC, Vos HL, Bertina RM, Rosendaal FR. ABO blood group genotypes and the risk of venous thrombosis: effect of factor V Leiden. Journal of thrombosis and haemostasis : JTH. 2005;3:183–185. doi: 10.1111/j.1538-7836.2004.01071.x. [DOI] [PubMed] [Google Scholar]
- 66.Tirado I, Mateo J, Soria JM, et al. The ABO blood group genotype and factor VIII levels as independent risk factors for venous thromboembolism. Thromb Haemost. 2005;93:468–474. doi: 10.1160/TH04-04-0251. [DOI] [PubMed] [Google Scholar]
- 67.Minano A, Ordonez A, Espana F, et al. AB0 blood group and risk of venous or arterial thrombosis in carriers of factor V Leiden or prothrombin G20210A polymorphisms. Haematologica. 2008;93:729–734. doi: 10.3324/haematol.12271. [DOI] [PubMed] [Google Scholar]
- 68.Jick H, Slone D, Westerholm B, et al. Venous thromboembolic disease and ABO blood type. A cooperative study. Lancet. 1969;1:539–542. doi: 10.1016/s0140-6736(69)91955-2. [DOI] [PubMed] [Google Scholar]
- 69.Nefzger MD, Hrubec Z, Chalmers TC. Venous thromboembolism and blood-group. Lancet. 1969;1:887. doi: 10.1016/s0140-6736(69)91925-4. [DOI] [PubMed] [Google Scholar]
- 70.Hill H, Loudon NB, Pitcher CS, Pocock VM. Venous thromboembolic disease and ABO blood type. Lancet. 1969;1:623. doi: 10.1016/s0140-6736(69)91556-6. [DOI] [PubMed] [Google Scholar]
- 71.Talbot S, Wakley EJ, Ryrie D, Langman MJ. ABO blood-groups and venous thromboembolic disease. Lancet. 1970;1:1257–1259. doi: 10.1016/s0140-6736(70)91741-1. [DOI] [PubMed] [Google Scholar]
- 72.Allan TM. Venous thromboembolism and blood-group. Lancet. 1970;1:303. doi: 10.1016/s0140-6736(70)90670-7. [DOI] [PubMed] [Google Scholar]
- 73.Allan TM. ABO blood-groups and venous thromboembolism. Lancet. 1971;2:1209–1210. doi: 10.1016/s0140-6736(71)90533-2. [DOI] [PubMed] [Google Scholar]
- 74.Allan TM. ABO blood groups and age groups in surgical venous thromboembolism. Atherosclerosis. 1976;23:141–142. doi: 10.1016/0021-9150(76)90124-6. [DOI] [PubMed] [Google Scholar]
- 75.Bates M. Venous thromboembolic disease and ABO blood type. Lancet. 1971;1:239. doi: 10.1016/s0140-6736(71)90977-9. [DOI] [PubMed] [Google Scholar]
- 76.Westerholm B, Wiechel B, Eklund G. Oral contraceptives, venous thromboembolic disease, and ABO blood type. Lancet. 1971;2:664. doi: 10.1016/s0140-6736(71)80108-3. [DOI] [PubMed] [Google Scholar]
- 77.Talbot S, Wakley EJ, Langman MJ. A19 A29 B, and O blood-groups, Lewis blood-groups, and serum triglyceride and cholesterol concentrations in patients with venous thromboembolic disease. Lancet. 1972;1:1152–1154. doi: 10.1016/s0140-6736(72)91375-x. [DOI] [PubMed] [Google Scholar]
- 78.Jick H, Porter J. Thrombophlebitis of the lower extremities and ABO blood type. Arch Intern Med. 1978;138:1566–1567. [PubMed] [Google Scholar]
- 79.Parr JC, White LE. ABO blood-groups and the visual outcome of a retinal venous occlusion. Trans Ophthalmol Soc N Z. 1978;30:19–22. [PubMed] [Google Scholar]
- 80.Robinson WM, Roisenberg I. Venous thromboembolism and ABO blood groups in a Brazilian population. Hum Genet. 1980;55:129–131. doi: 10.1007/BF00329140. [DOI] [PubMed] [Google Scholar]
- 81.Wautrecht JC, Vincent G, Dereume JP. [Venous thromboembolic disease and Klinefelter’s syndrome] J Mal Vasc. 1986;11:125–127. [PubMed] [Google Scholar]
- 82.Mercier B, Oger E, Le Gal G, Mottier D, Ferec C. Phenotypic but not allelic ABO blood group association with risk of venous thrombosis. Thromb Haemost. 2005;93:388–389. [PubMed] [Google Scholar]
- 83.Morange PE, Tregouet DA. Current knowledge on the genetics of incident venous thrombosis. Journal of thrombosis and haemostasis : JTH. 2013;11(Suppl 1):111–121. doi: 10.1111/jth.12233. [DOI] [PubMed] [Google Scholar]
- 84.Folsom AR, Cushman M, Tsai MY, et al. A prospective study of venous thromboembolism in relation to factor V Leiden and related factors. Blood. 2002;99:2720–2725. doi: 10.1182/blood.v99.8.2720. [DOI] [PubMed] [Google Scholar]
- 85.Dowling NF, Austin H, Dilley A, Whitsett C, Evatt BL, Hooper WC. The epidemiology of venous thromboembolism in Caucasians and African-Americans: the GATE Study. Journal of thrombosis and haemostasis : JTH. 2003;1:80–87. doi: 10.1046/j.1538-7836.2003.00031.x. [DOI] [PubMed] [Google Scholar]
- 86.Heit JA, Sobell JL, Li H, Sommer SS. The incidence of venous thromboembolism among Factor V Leiden carriers: a community-based cohort study. Journal of thrombosis and haemostasis : JTH. 2005;3:305–311. doi: 10.1111/j.1538-7836.2004.01117.x. [DOI] [PubMed] [Google Scholar]
- 87.Herrington DM, Vittinghoff E, Howard TD, et al. Factor V Leiden, hormone replacement therapy, and risk of venous thromboembolic events in women with coronary disease. Arterioscler Thromb Vasc Biol. 2002;22:1012–1017. doi: 10.1161/01.atv.0000018301.91721.94. [DOI] [PubMed] [Google Scholar]
- 88.Major DA, Sane DC, Herrington DM. Cardiovascular implications of the factor V Leiden mutation. Am Heart J. 2000;140:189–195. doi: 10.1067/mhj.2000.108241. [DOI] [PubMed] [Google Scholar]
- 89.Marchiori A, Mosena L, Prins MH, Prandoni P. The risk of recurrent venous thromboembolism among heterozygous carriers of factor V Leiden or prothrombin G20210A mutation. A systematic review of prospective studies. Haematologica. 2007;92:1107–1114. doi: 10.3324/haematol.10234. [DOI] [PubMed] [Google Scholar]
- 90.Ridker PM, Miletich JP, Stampfer MJ, Goldhaber SZ, Lindpaintner K, Hennekens CH. Factor V Leiden and risks of recurrent idiopathic venous thromboembolism. Circulation. 1995;92:2800–2802. doi: 10.1161/01.cir.92.10.2800. [DOI] [PubMed] [Google Scholar]
- 91.Germain M, Chasman DI, de Haan H, et al. Meta-analysis of 65,734 individuals identifies TSPAN15 and SLC44A2 as two susceptibility loci for venous thromboembolism. Am J Hum Genet. 2015;96:532–542. doi: 10.1016/j.ajhg.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arellano AR, Bezemer ID, Tong CH, et al. Gene variants associated with venous thrombosis: confirmation in the MEGA study. Journal of thrombosis and haemostasis : JTH. 2010;8:1132–1134. doi: 10.1111/j.1538-7836.2010.03782.x. [DOI] [PubMed] [Google Scholar]
- 93.De Stefano V, Chiusolo P, Paciaroni K, et al. Prothrombin G20210A mutant genotype is a risk factor for cerebrovascular ischemic disease in young patients. Blood. 1998;91:3562–3565. [PubMed] [Google Scholar]
- 94.Hillarp A, Zoller B, Svensson PJ, Dahlback B. The 20210 A allele of the prothrombin gene is a common risk factor among Swedish outpatients with verified deep venous thrombosis. Thromb Haemost. 1997;78:990–992. [PubMed] [Google Scholar]
- 95.Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3’-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88:3698–3703. [PubMed] [Google Scholar]
- 96.Rosendaal FR, Vos HL, Poort SL, Bertina RM. Prothrombin 20210A variant and age at thrombosis. Thromb Haemost. 1998;79:444. [PubMed] [Google Scholar]
- 97.Matsui T, Titani K, Mizuochi T. Structures of the asparagine-linked oligosaccharide chains of human von Willebrand factor. Occurrence of blood group A, B, and H(O) structures. J Biol Chem. 1992;267:8723–8731. [PubMed] [Google Scholar]
- 98.Kamphuisen PW, Eikenboom JC, Rosendaal FR, et al. High factor VIII antigen levels increase the risk of venous thrombosis but are not associated with polymorphisms in the von Willebrand factor and factor VIII gene. Br J Haematol. 2001;115:156–158. doi: 10.1046/j.1365-2141.2001.03089.x. [DOI] [PubMed] [Google Scholar]
- 99.Tsai AW, Cushman M, Rosamond WD, et al. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE) Am J Med. 2002;113:636–642. doi: 10.1016/s0002-9343(02)01345-1. [DOI] [PubMed] [Google Scholar]
- 100.Smith NL, Chen MH, Dehghan A, et al. Novel associations of multiple genetic loci with plasma levels of factor VII, factor VIII, and von Willebrand factor: The CHARGE (Cohorts for Heart and Aging Research in Genome Epidemiology) Consortium. Circulation. 2010;121:1382–1392. doi: 10.1161/CIRCULATIONAHA.109.869156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu O, Bayoumi N, Vickers MA, Clark P. ABO(H) blood groups and vascular disease: a systematic review and meta-analysis. Journal of thrombosis and haemostasis : JTH. 2008;6:62–69. doi: 10.1111/j.1538-7836.2007.02818.x. [DOI] [PubMed] [Google Scholar]
- 102.Heit JA, Armasu SM, Asmann YW, et al. A genome-wide association study of venous thromboembolism identifies risk variants in chromosomes 1q24.2 and 9q. Journal of thrombosis and haemostasis : JTH. 2012;10:1521–1531. doi: 10.1111/j.1538-7836.2012.04810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heit JA, Cunningham JM, Petterson TM, Armasu SM, Rider DN, M DEA. Genetic variation within the anticoagulant, procoagulant, fibrinolytic and innate immunity pathways as risk factors for venous thromboembolism. Journal of thrombosis and haemostasis : JTH. 2011;9:1133–1142. doi: 10.1111/j.1538-7836.2011.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lane DA, Mannucci PM, Bauer KA, et al. Inherited thrombophilia: Part 1. Thromb Haemost. 1996;76:651–662. [PubMed] [Google Scholar]
- 105.Miletich J, Sherman L, Broze G., Jr Absence of thrombosis in subjects with heterozygous protein C deficiency. N Engl J Med. 1987;317:991–996. doi: 10.1056/NEJM198710153171604. [DOI] [PubMed] [Google Scholar]
- 106.Gandrille S, Borgel D, Sala N, et al. Protein S deficiency: a database of mutations--summary of the first update. Thromb Haemost. 2000;84:918. [PubMed] [Google Scholar]
- 107.Cushman M. Inherited risk factors for venous thrombosis. Hematology Am Soc Hematol Educ Program. 2005:452–457. doi: 10.1182/asheducation-2005.1.452. [DOI] [PubMed] [Google Scholar]
- 108.Tregouet DA, Heath S, Saut N, et al. Common susceptibility alleles are unlikely to contribute as strongly as the FV and ABO loci to VTE risk: results from a GWAS approach. Blood. 2009;113:5298–5303. doi: 10.1182/blood-2008-11-190389. [DOI] [PubMed] [Google Scholar]
- 109.Germain M, Saut N, Greliche N, et al. Genetics of venous thrombosis: insights from a new genome wide association study. PLoS One. 2011;6:e25581. doi: 10.1371/journal.pone.0025581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang W, Teichert M, Chasman DI, et al. A genome-wide association study for venous thromboembolism: the extended cohorts for heart and aging research in genomic epidemiology (CHARGE) consortium. Genet Epidemiol. 2013;37:512–521. doi: 10.1002/gepi.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Soria JM, Morange PE, Vila J, et al. Multilocus genetic risk scores for venous thromboembolism risk assessment. J Am Heart Assoc. 2014;3:e001060. doi: 10.1161/JAHA.114.001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Haan HG, Bezemer ID, Doggen CJ, et al. Multiple SNP testing improves risk prediction of first venous thrombosis. Blood. 2012;120:656–663. doi: 10.1182/blood-2011-12-397752. [DOI] [PubMed] [Google Scholar]
- 113.Folsom AR, Tang W, Weng LC, et al. Replication of a genetic risk score for venous thromboembolism in whites but not in African Americans. J Thromb Haemost. 2016;14:83–88. doi: 10.1111/jth.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Crous-Bou M, De Vivo I, Camargo CA, Jr, et al. Interactions of established risk factors and a GWAS-based genetic risk score on the risk of venous thromboembolism. Thromb Haemost. 2016:116. doi: 10.1160/TH16-02-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bounameaux H. Factor V Leiden paradox: risk of deep-vein thrombosis but not of pulmonary embolism. Lancet. 2000;356:182–183. doi: 10.1016/S0140-6736(00)02476-4. [DOI] [PubMed] [Google Scholar]
- 116.Girard P. Factor V Leiden paradox. Lancet. 2000;356:1028–1029. doi: 10.1016/S0140-6736(05)72645-3. [DOI] [PubMed] [Google Scholar]
- 117.van Stralen KJ, Doggen CJ, Bezemer ID, Pomp ER, Lisman T, Rosendaal FR. Mechanisms of the factor V Leiden paradox. Arterioscler Thromb Vasc Biol. 2008;28:1872–1877. doi: 10.1161/ATVBAHA.108.169524. [DOI] [PubMed] [Google Scholar]
- 118.van Langevelde K, Flinterman LE, van Hylckama Vlieg A, Rosendaal FR, Cannegieter SC. Broadening the factor V Leiden paradox: pulmonary embolism and deep-vein thrombosis as 2 sides of the spectrum. Blood. 2012;120:933–946. doi: 10.1182/blood-2012-02-407551. [DOI] [PubMed] [Google Scholar]
- 119.Leroyer C, Mercier B, Escoffre M, Ferec C, Mottier D. Factor V Leiden prevalence in venous thromboembolism patients. Chest. 1997;111:1603–1606. doi: 10.1378/chest.111.6.1603. [DOI] [PubMed] [Google Scholar]
- 120.Rees DC, Cox M, Clegg JB. World distribution of factor V Leiden. Lancet. 1995;346:1133–1134. doi: 10.1016/s0140-6736(95)91803-5. [DOI] [PubMed] [Google Scholar]
- 121.Mazoyer E, Ripoll L, Gueguen R, et al. Prevalence of factor V Leiden and prothrombin G20210A mutation in a large French population selected for nonthrombotic history: geographical and age distribution. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2009;20:503–510. doi: 10.1097/MBC.0b013e32832f5d7a. [DOI] [PubMed] [Google Scholar]
- 122.Rosendaal FR. Venous thrombosis: the role of genes, environment, and behavior. Hematology Am Soc Hematol Educ Program. 2005:1–12. doi: 10.1182/asheducation-2005.1.1. [DOI] [PubMed] [Google Scholar]
- 123.Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet. 1999;353:1167–1173. doi: 10.1016/s0140-6736(98)10266-0. [DOI] [PubMed] [Google Scholar]
- 124.Rosendaal FR. Venous thrombosis: prevalence and interaction of risk factors. Haemostasis. 1999;29(Suppl S1):1–9. doi: 10.1159/000054106. [DOI] [PubMed] [Google Scholar]
- 125.Kraft P, Hunter D. Integrating epidemiology and genetic association: the challenge of gene-environment interaction. Philos Trans R Soc Lond B Biol Sci. 2005;360:1609–1616. doi: 10.1098/rstb.2005.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kraft P, Yen YC, Stram DO, Morrison J, Gauderman WJ. Exploiting gene-environment interaction to detect genetic associations. Hum Hered. 2007;63:111–119. doi: 10.1159/000099183. [DOI] [PubMed] [Google Scholar]
- 127.van Boven HH, Vandenbroucke JP, Briet E, Rosendaal FR. Gene-gene and gene-environment interactions determine risk of thrombosis in families with inherited antithrombin deficiency. Blood. 1999;94:2590–2594. [PubMed] [Google Scholar]
- 128.Delluc A, Le Moigne E, Tromeur C, et al. Site of venous thromboembolism and prothrombotic mutations according to body mass index. Results from the EDITH study. Br J Haematol. 2011;154:486–491. doi: 10.1111/j.1365-2141.2011.08592.x. [DOI] [PubMed] [Google Scholar]
- 129.Martinelli I, Taioli E, Bucciarelli P, Akhavan S, Mannucci PM. Interaction between the G20210A mutation of the prothrombin gene and oral contraceptive use in deep vein thrombosis. Arterioscler Thromb Vasc Biol. 1999;19:700–703. doi: 10.1161/01.atv.19.3.700. [DOI] [PubMed] [Google Scholar]
- 130.Stiko-Rahm A, Wiman B, Hamsten A, Nilsson J. Secretion of plasminogen activator inhibitor-1 from cultured human umbilical vein endothelial cells is induced by very low density lipoprotein. Arteriosclerosis. 1990;10:1067–1073. doi: 10.1161/01.atv.10.6.1067. [DOI] [PubMed] [Google Scholar]
- 131.Juhan-Vague I, Vague P, Alessi MC, et al. Relationships between plasma insulin triglyceride, body mass index, and plasminogen activator inhibitor 1. Diabete Metab. 1987;13:331–336. [PubMed] [Google Scholar]
- 132.Juhan-Vague I, Alessi MC, Joly P, et al. Plasma plasminogen activator inhibitor-1 in angina pectoris. Influence of plasma insulin and acute-phase response. Arteriosclerosis. 1989;9:362–367. doi: 10.1161/01.atv.9.3.362. [DOI] [PubMed] [Google Scholar]
- 133.Hamsten A, Wiman B, de Faire U, Blomback M. Increased plasma levels of a rapid inhibitor of tissue plasminogen activator in young survivors of myocardial infarction. N Engl J Med. 1985;313:1557–1563. doi: 10.1056/NEJM198512193132501. [DOI] [PubMed] [Google Scholar]
- 134.Doggen CJ, Cats VM, Bertina RM, Rosendaal FR. Interaction of coagulation defects and cardiovascular risk factors: increased risk of myocardial infarction associated with factor V Leiden or prothrombin 20210A. Circulation. 1998;97:1037–1041. doi: 10.1161/01.cir.97.11.1037. [DOI] [PubMed] [Google Scholar]
- 135.Dudding TE, Attia J. The association between adverse pregnancy outcomes and maternal factor V Leiden genotype: a meta-analysis. Thromb Haemost. 2004;91:700–711. doi: 10.1160/TH03-10-0637. [DOI] [PubMed] [Google Scholar]
- 136.Ridker PM, Hennekens CH, Selhub J, Miletich JP, Malinow MR, Stampfer MJ. Interrelation of hyperhomocyst(e)inemia, factor V Leiden, and risk of future venous thromboembolism. Circulation. 1997;95:1777–1782. doi: 10.1161/01.cir.95.7.1777. [DOI] [PubMed] [Google Scholar]
- 137.Ridker PM, Glynn RJ, Miletich JP, Goldhaber SZ, Stampfer MJ, Hennekens CH. Age-specific incidence rates of venous thromboembolism among heterozygous carriers of factor V Leiden mutation. Ann Intern Med. 1997;126:528–531. doi: 10.7326/0003-4819-126-7-199704010-00005. [DOI] [PubMed] [Google Scholar]
- 138.van Boven HH, Reitsma PH, Rosendaal FR, et al. Factor V Leiden (FV R506Q) in families with inherited antithrombin deficiency. Thromb Haemost. 1996;75:417–421. [PubMed] [Google Scholar]
- 139.Vandenbroucke JP, Koster T, Briet E, Reitsma PH, Bertina RM, Rosendaal FR. Increased risk of venous thrombosis in oral-contraceptive users who are carriers of factor V Leiden mutation. Lancet. 1994;344:1453–1457. doi: 10.1016/s0140-6736(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 140.Andreassi MG, Botto N, Maffei S. Factor V Leiden, prothrombin G20210A substitution and hormone therapy: indications for molecular screening. Clin Chem Lab Med. 2006;44:514–521. doi: 10.1515/CCLM.2006.103. [DOI] [PubMed] [Google Scholar]
- 141.Gary JL, Barber RC, Reinert CM, Starr AJ. A Prospective Study of Thrombophilia in Trauma Patients With Pulmonary Embolism. J Trauma. 2011 doi: 10.1097/TA.0b013e31822f7d14. [DOI] [PubMed] [Google Scholar]
- 142.Westrich GH, Weksler BB, Glueck CJ, Blumenthal BF, Salvati EA. Correlation of thrombophilia and hypofibrinolysis with pulmonary embolism following total hip arthroplasty: an analysis of genetic factors. J Bone Joint Surg Am. 2002;84-A:2161–2167. doi: 10.2106/00004623-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 143.Wahlander K, Larson G, Lindahl TL, et al. Factor V Leiden (G1691A) and prothrombin gene G20210A mutations as potential risk factors for venous thromboembolism after total hip or total knee replacement surgery. Thromb Haemost. 2002;87:580–585. [PubMed] [Google Scholar]
- 144.Bowler DJ, Bale E, O’Byrne J. Factor V Leiden: prevalence and thromboembolic complications after total hip replacement in Ireland. Ir J Med Sci. 2007;176:273–277. doi: 10.1007/s11845-007-0095-x. [DOI] [PubMed] [Google Scholar]
- 145.Baba-Ahmed M, Le Gal G, Couturaud F, Lacut K, Oger E, Leroyer C. High frequency of factor V Leiden in surgical patients with symptomatic venous thromboembolism despite prophylaxis. Thromb Haemost. 2007;97:171–175. [PubMed] [Google Scholar]
- 146.Atluri P, Raper SE. Factor V Leiden and postoperative deep vein thrombosis in patients undergoing open Roux-en-Y gastric bypass surgery. Obes Surg. 2005;15:561–564. doi: 10.1381/0960892053723312. [DOI] [PubMed] [Google Scholar]
- 147.van Stralen KJ, Rosendaal FR, Doggen CJ. Minor injuries as a risk factor for venous thrombosis. Arch Intern Med. 2008;168:21–26. doi: 10.1001/archinternmed.2007.5. [DOI] [PubMed] [Google Scholar]
- 148.Ringwald J, Berger A, Adler W, Kraus C, Pitto RP. Genetic polymorphisms in venous thrombosis and pulmonary embolism after total hip arthroplasty: a pilot study. Clin Orthop Relat Res. 2009;467:1507–1515. doi: 10.1007/s11999-008-0498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bedair H, Berli M, Gezer S, Jacobs JJ, Della Valle CJ. Hematologic genetic testing in high-risk patients before knee arthroplasty: a pilot study. Clin Orthop Relat Res. 2011;469:131–137. doi: 10.1007/s11999-010-1514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bare LA, Arellano AR, Tong CH, et al. Genetic variants of F11, statin use and venous thrombosis. J Thromb Haemost. 2011;9:1249–1251. doi: 10.1111/j.1538-7836.2011.04289.x. [DOI] [PubMed] [Google Scholar]
- 151.Harrington LB, Wiggins KL, Sitlani CM, et al. The association of F11 genetic variants with the risk of incident venous thrombosis among women, by statin use. Thromb Haemost. 2016;115:682–684. doi: 10.1160/TH15-08-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]