Abstract
Azoles are widely used for controlling fungal growth in both agricultural and medical settings. The target protein of azoles is CYP51, a lanosterol 14-α-demethylase involved in the biosynthesis of ergosterol. Recently, a novel azole resistance mechanism has arisen in pathogenic fungal species Aspergillus fumigatus. Resistant strains contain a 34-bp or 46-bp tandem repeat (TR) in the promoter of cyp51A, and have disseminated globally in a short period of time. In this study, we investigated whether an azole-resistant strain with a 46-bp TR (TR46/Y121F/T289A) could be sensitised to azoles by deletion of srbA, encoding a direct regulator of cyp51A. The loss of SrbA did not affect colony growth or conidia production, but decreased expression of cyp51A. The srbA deletion strain showed hyper-susceptibility to medical azoles as well as azole fungicides, while its sensitivity to non-azole fungicides was unchanged. This is the first demonstration that deletion of a regulator of cyp51A can sensitise an azole-resistant A. fumigatus strain. This finding may assist in the development of new drugs to help combat life-threatening azole-resistant fungal pathogens.
Filamentous fungi (moulds) are ubiquitous in nature, and as well as causing significant crop damage, they can be pathogenic for humans. To prevent harmful fungal growth, a variety of antifungal chemicals are used to protect wood, plant materials, crops, food, and human health. Azoles are antifungals that are widely used in both agricultural and public health settings because of their excellent activity against a wide spectrum of plant and human pathogenic fungi. However, increasing levels of azole resistance have been noted over the last decade1,2. The situation is further complicated by the finding that some isolates of the major human pathogenic fungus Aspergillus fumigatus have acquired cross-resistance to medical azoles through exposure to azole fungicides in the environment3,4.
The target molecule of azoles is CYP51, a lanosterol 14-α-demethylase involved in the biosynthesis of ergosterol, which is an essential fungal membrane lipid. A. fumigatus has two Cyp51 proteins, Cyp51A and Cyp51B, inhibition of which by azoles results in a significant change in sterol profile in the cells. Ergosterol deficiency as well as production of toxic intermediates is thought to cause the antifungal effect5. Expression of cyp51A and cyp51B is regulated by a sterol element binding protein (SREBP), SrbA, in A. fumigatus6,7. A srbA deletion mutant showed decreased levels of cyp51A and cyp51B expression, as well as hyper-sensitivity to medical azoles such as itraconazole, voriconazole and posaconazole. It has been clearly demonstrated that SrbA regulates cyp51A expression in a direct manner; thus, SrbA is the only known direct regulator of intrinsic azole resistance among fungi8. Notably, the srbA deletion mutant showed impaired growth under hypoxic conditions, suggesting that proper regulation of the ergosterol biosynthesis pathway is necessary for fungi to adapt to oxygen limitation.
Azole-resistant A. fumigatus strains, with a combination of specific amino acid substitutions and a 34-bp or 46-bp tandem repeat (TR) in the promoter region of cyp51A (TR34/L98H and TR46/Y121F/T289A), have been isolated from patients regardless of azole treatment history, as well as from the environment3,9,10,11,12,13,14. It is widely accepted that these resistant strains are derived from exposure to azole fungicides in the environment4. This acquired azole resistance mechanism limits the choices for effective drug therapy of aspergillosis caused by Aspergillus fungi. Alarmingly, strains with the TR34/L98H and TR46/Y121F/T289A genotypes have rapidly spread across the globe since first reported in 2007 and 2012, respectively. The most recent epidemiological report from the Netherlands showed that 225 (23.6%) and 98 (10.3%) of 952 clinical A. fumigatus isolates harboured the TR34/L98H and TR46/Y121F/T289A mutations, respectively15. Thus, it is imperative to obtain a better understanding of the mechanisms involved in resistance to develop a strategy for combatting azole-resistant A. fumigatus strains.
Genetically reconstituted strains have been used to study the molecular mechanism of the environment-associated resistance mutations16,17. Strains carrying TR34 or TR46 showed a moderate upregulation (2–2.5-fold) of cyp51A expression. This increased expression was partly responsible for the lowered susceptibility to azoles. Notably, similar tandem repeats and insertions in the promoter region of cyp51 have been reported in several plant pathogens, including Penicillium digitatum18, Venturia inaequalis19, Mycosphaerella graminicola20 and Monilinia fructicola21. Some of these mutations have been associated with overexpression of cyp51. Collectively, these studies have shown that constitutive upregulation of cyp51A expression in strains with the tandem repeat or insertion seems to be a prerequisite for the azole resistance. Therefore, in the current study, we examined whether deletion of the direct regulator, SrbA, could reduce cyp51A expression in a TR46/Y121F/T289A A. fumigatus strain, and whether the deletion would affect susceptibility to azoles. Sensitisation of A. fumigatus strains harbouring the widespread resistance mechanisms would have significant implications for the effective control of human and plant fungal pathogens.
Results
Construction of the srbA deletion mutant
To investigate the role of SrbA in the azole resistant strains, we deleted srbA in an A. fumigatus strain containing TR46/Y121F/T289A mutations (IFM 63432)13. The deletion mutant strain was designated IFM 63432-ΔsrbA. We obtained two independent isolates (IFM 63432-ΔsrbA No. 1 and No. 2) from the transformation, and the two isolates showed virtually identical phenotypes. Thus, representative results are shown in the figures. A srbA deletion mutant was also successfully constructed in azole-susceptible A. fumigatus strain Af293 (designated Af293-ΔsrbA), and was used as a control throughout the study.
Colony growth and conidia production in the srbA deletion strains
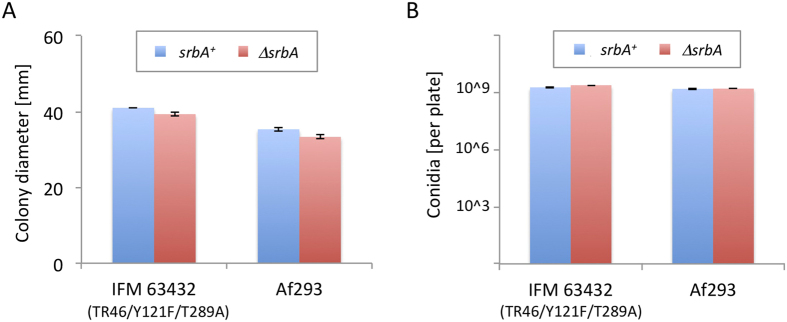
The deletion mutants were assayed for their ability to grow on potato dextrose agar (PDA) (Fig. 1A) and glucose minimal medium (GMM) (data not shown). There were no differences in growth rate between the parental strains (IFM 63432 and Af293) and the srbA deletion mutants. The amounts of conidia produced were also comparable between the strains (Fig. 1B).
Figure 1. Fungal growth and conidia production.
(A) Colony diameter was compared between wild-type strains IFM 63432 (TR46/Y121F/T289A) and Af293 and their corresponding srbA deletion mutants. All strains were grown on PDA for 48 h. (B) The number of produced conidia was compared between strains. Conidia were harvested from strains cultured for 3 days on PDA in 3.5-cm diameter plates. The mean values and standard deviations were obtained from three replicates.
qRT-PCR analysis of cyp51A expression
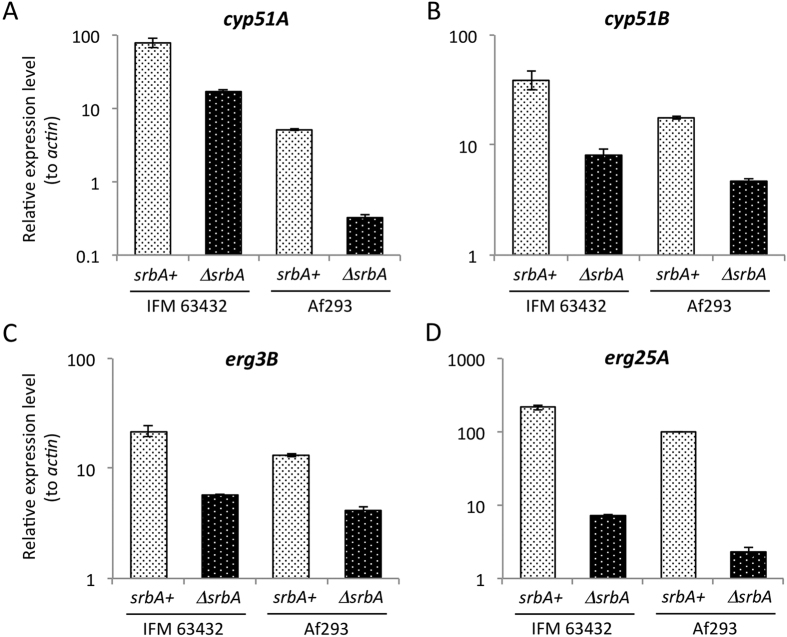
To assess the role of SrbA in the expression of genes related to azole resistance and/or ergosterol biosynthesis, we investigated the expression levels of cyp51A and cyp51B by qRT-PCR. As reported previously in other strains7,22, the levels of cyp51A and cyp51B expression in the Af293 background were decreased by 93.7% and 73.6%, respectively, in the ΔsrbA mutant compared with the wild-type (Fig. 2A and B). In the IFM 63432 background, deletion of srbA resulted in a reduction of cyp51A and cyp51B expression of 78.3% and 79.3%, respectively (Fig. 2A and B). It should be noted that cyp51A expression was markedly higher (15.3-fold) in IFM 63432 than in Af293, whereas cyp51B expression was only 2.2-fold higher in IFM 63432. This suggested that the presence of the TR46/Y121F/T289A mutations might cause increased cyp51A expression under the tested conditions.
Figure 2. Expression analysis of IFM 63432 and Af293 and the srbA deletion mutants.
Expression levels of cyp51A (A), cyp51B (B), erg3B (C) and erg25A (D) were determined by qRT-PCR. The strains were cultivated in PDB for 16 h. The expression levels of the genes of interest were normalised against those of the actin gene. Relative expression ratios are shown. Error bars represent the standard deviations based on three independent replicates.
In addition to Cyp51 genes, other ergosterol synthesis genes (erg3B and erg25A) are directly regulated by SrbA7. Hence, we also examined the expression levels of these genes in the current study. Expression levels of erg3B and erg25A were 1.7-fold and 2.2-fold higher, respectively, in IFM 63432 than in Af293 (Fig. 2C and D). In IFM 63432, erg3B expression was reduced by 73.7% in the ΔsrbA mutant compared with the parental strain, whereas erg25A expression was decreased by 96.7% in the mutant strain. Likewise, in Af293, the ΔsrbA mutant showed decreases of 68.5% and 97.7% in the expression levels of erg3B and erg25A, respectively, compared with the wild-type. These results indicated that deletion of srbA resulted in a marked reduction in the expression of cyp51A, erg3B and erg25A even in the IFM 63432 background.
Susceptibility of the IFM 63432-ΔsrbA strain to azoles
As demonstrated in our previous study, strain IFM 63432 shows multi-azole resistance (minimum inhibitory concentrations (MICs), fluconazole: >64; itraconazole: 2; voriconazole: >8; miconazole: 2; posaconazole: 4 mg/l). In the current study, the susceptibility of IFM 63432-ΔsrbA, Af293 and Af293-ΔsrbA to various antifungals was examined using the broth microdilution method (Table 1). While IFM 63432 showed a high MIC for voriconazole, the MIC was markedly lower in the IFM 63432-ΔsrbA strain. In addition, IFM 63432-ΔsrbA showed lowered MICs for fluconazole, itraconazole, miconazole and posaconazole compared with the wild-type strain. In the Af293 background, deletion of srbA resulted in relatively low MICs for all azoles except fluconazole. The MIC data indicated that SrbA plays a crucial role in azole resistance in strains with the TR46/Y121F/T289A mutations, as well as in Af293. The MICs for amphotericin B and 5-FC, along with the minimum effective concentrations for micafungin and caspofungin, were largely unchanged in the srbA deletion strains. The MICs for itraconazole and miconazole were comparable between IFM 63432 and Af293.
Table 1. Antifungal susceptibility test.
| Strain | MEC (mg/l) |
MIC (mg/l) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| MCFG | CPFG | AMPH-B | 5-FC | FCZ | ITCZ | VCZ | MCZ | PSCZ | |
| IFM 63432 | <=0.015 | 0.125 | 1 | 64 | >64 | 2 | >8 | 2 | 4 |
| IFM 63432-ΔsrbA No. 1 | <=0.015 | 0.25 | 2 | >64 | 8 | 0.25 | 0.125 | 0.125 | 0.125 |
| IFM 63432-ΔsrbA No. 2 | <=0.015 | 0.25 | 1 | 64 | 8 | 0.125 | 0.125 | 0.125 | 0.5 |
| Af293 | <=0.015 | 0.25 | 1 | >64 | >64 | 1 | 2 | 4 | 1 |
| Af293-ΔsrbA | <=0.015 | 0.25 | 2 | 64 | 16 | 0.25 | 0.125 | 0.0625 | 0.25 |
MEC: minimum effective concentration; MIC: minimum inhibitory concentration; MCFG: micafungin; CPFG: caspofungin; AMPH-B: amphotericin B; FCZ: fluconazole; VCZ: voriconazole; MCZ: miconazole; PSCZ: posaconazole.
We then examined the levels of growth inhibition by disc diffusion assay. On PDA (Fig. 3A), the parental strains showed similar levels of sensitivity to itraconazole and miconazole. In contrast, IFM 63432 showed much higher levels of resistance to azole fungicides that are widely used in agriculture compared with Af293 (Fig. 3B). As expected, IFM 63432-ΔsrbA and Af293-ΔsrbA exhibited hyper-susceptibility to the azole fungicides. Comparing the diameters of the inhibition halos, Af293-ΔsrbA was more sensitive to the azole fungicides than IFM 63432-ΔsrbA (Table 2). srbA deletion did not affect sensitivity to non-azole fungicides, including iprodione, fludioxonil and kresoxim-methyl, in either of the strain backgrounds (Figure S1).
Figure 3. Paper disc diffusion assays.
Conidia were streaked on PDA, and a paper disc was placed at the centre of each plate. A 10-μl aliquot of medical azoles itraconazole and miconazole (10 mg/ml) (A), or azole fungicides bromuconazole, difenoconazole, propiconazole and tebuconazole (10 mg/ml) (B), was spotted onto the paper disc. The plates were then incubated at 37 °C for 24 h before being photographed.
Table 2. Diameters of the inhibition halos produced by fungicide azoles.
| Strain | Mean diameter of the inhibition halo [mm] ± SD |
|||
|---|---|---|---|---|
| Bromuconazole | Difenoconazole | Propiconazole | Tebuconazole | |
| IFM 63432 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 10.0 ± 0.8 |
| IFM 63432-ΔsrbA | >80 | 46.7 ± 0.5 | 60.7 ± 0.5 | 75.7 ± 1.2 |
| Af293 | 33.3 ± 1.2 | 24.3 ± 0.5 | 25.3 ± 0.5 | 38.7 ± 0.9 |
| Af293-ΔsrbA | >80 | 78.3 ± 1.2 | >80 | >80 |
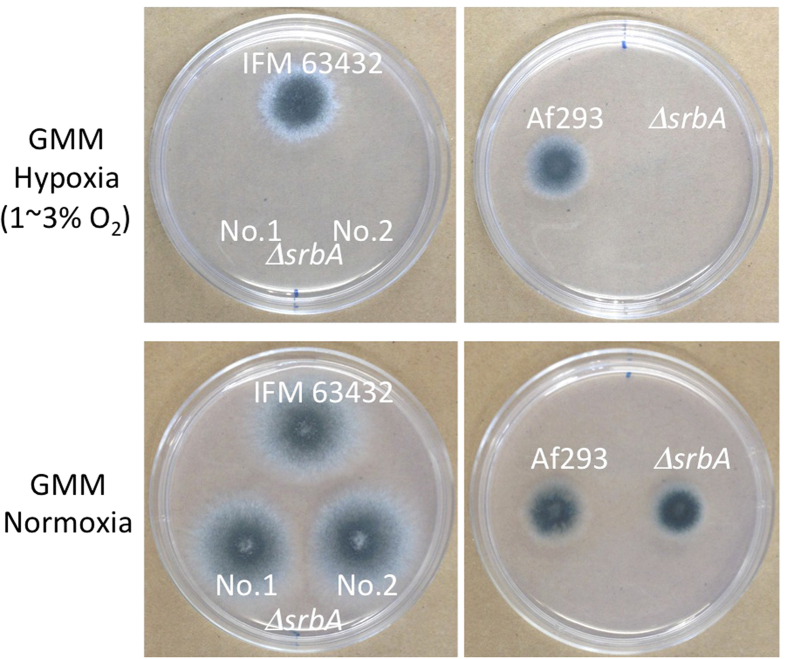
Growth under hypoxic conditions
SrbA is involved in growth under hypoxic conditions6. Therefore, we investigated the growth of IFM 63432 and Af293 and their srbA deletion mutants under hypoxic conditions. In both strain backgrounds, deletion of srbA abolished growth under hypoxic conditions (Fig. 4).
Figure 4. Growth under hypoxic conditions.
Conidia from each strain were inoculated onto GMM, and the plates were incubated at 37 °C for 3 days in an anaeropack system whereby the concentration of oxygen was controlled at 1–3% (hypoxia). The oxygen content was monitored by oxygen analyser.
Discussion
The Cyp51 proteins are essential for the biosynthesis of ergosterol in fungi. Thus, azoles, inhibitors of the Cyp51 proteins, are widely used for controlling fungal growth in both agricultural and clinical settings. Some azole fungicides can inhibit human pathogens such as A. fumigatus23; however, only a few azoles are approved for use in the treatment of aspergillosis. It is becoming evident that cross-resistance to medical azoles has developed from environmental exposure to azole fungicides, resulting in azole-resistant A. fumigatus strains with a tandem repeat sequence in the cyp51A promoter region24,25. We demonstrated in the present study that an environmental azole-resistant strain became markedly sensitized to azoles following deletion of the gene encoding SrbA, a SREBP that is responsible for the regulation of cyp51A.
The SREBPs are a highly conserved family of transcription factors containing a basic helix-loop-helix motif, and were first identified in mammals as regulators of cholesterol metabolism26. These proteins are involved in the regulation of fatty acid biosynthesis and development in cholesterol auxotrophs such as Drosophila melanogaster and Caenorhabditis elegans27,28. Yeasts Saccharomyces cerevisiae and Candida albicans have no apparent SREBP orthologues, whereas Schizosaccharomyces pombe and Cryptococcus neoformans contain functional SREBPs that regulate ergosterol biosynthesis and aid in adaptation to hypoxic conditions29,30,31. Amongst the filamentous fungi, SrbA from A. fumigatus is the only characterised SREBP, except for SrbA from Paracoccidioides, the coding sequence of which was shown to function heterologously in A. fumigatus cells32. Bioinformatic analysis suggests that important plant pathogen species Fusarium and Magnaporthe may contain SREBPs, although the physiological functions of these proteins are yet to be investigated. Our finding of an essential role for SrbA in azole resistance in A. fumigatus strains containing mutations in the cyp51A promoter region should encourage further studies of SREBPs in other filamentous fungi. Importantly, SrbA plays a crucial role in A. fumigatus virulence, most likely as a result of growth impairment under hypoxic conditions. Thus, it would be of interest to investigate whether the SREBPs from plant fungal pathogens play a role in infection as well as resistance to azole fungicides.
As shown in previous studies, SrbA is involved in multiple steps in the ergosterol biosynthesis pathway, regulating the expression of cyp51A, erg3B and erg25A7,8. Overexpression of cyp51A under the control of a niiA promoter did not fully restore voriconazole resistance and ergosterol content to wild-type levels in an A. fumigatus srbA mutant strain8. This implied that deletion of SrbA had a multimodal effect on azole resistance in A. fumigatus cells. Indeed, deletion of srbA resulted hyper-susceptibility to azoles in a strain containing the TR46/Y121F/T289A mutations (IFM 63432), although the relative expression of cyp51A was still higher in IFM 63432-ΔsrbA compared with that in the Af293 wild-type strain (Fig. 2A). The IFM 63432-ΔsrbA strain showed decreased expression of cyp51B, erg3B and erg25A, the combination of which might cause the sensitisation to azoles, possibly as a result of disordered sterol profiles. Regardless, deletion of srbA in IFM 63432 resulted in decreased MICs for azoles, meaning that the resultant mutant strain would be susceptible to conventional azole drug therapy.
Strain IFM 63432, containing the TR46/Y121F/T289A mutations, also showed high levels of resistance to fungicide azoles such as bromuconazole, difenoconazole, propiconazole and tebuconazole. This might support the hypothesis that the azole resistance was derived from environmental exposure to fungicide azoles, as these fungicides have a similar molecular structure to the medical azoles23. Further occurrences and dissemination of such resistance mechanisms in the human pathogenic fungus A. fumigatus would undoubtedly have significant implications for public health. However, it would be virtually impossible to eliminate the use of azole fungicides in current agricultural industries. Our findings may aid in the development of alternative strategies to address the increasing threat of drug resistance. SrbA is a potential target molecule for sensitisation of resistant strains to existing azole drugs. Compounds aimed at impairing SrbA function could overcome the cyp51A-related azole resistance when used in combination with azoles. Additionally, the IFM 63432-ΔsrbA mutant showed impaired growth under hypoxic conditions. As there is a close relationship between adaptation to hypoxia and fungal virulence, as shown by an attenuation of pathogenicity in a ΔsrbA strain6,31, inhibition of SrbA might affect in vivo growth of A. fumigatus, even in the azole-resistant strains.
In conclusion, deletion of the regulator of cyp51A transcription, SrbA, demonstrated that an environmentally-derived azole-resistant A. fumigatus strain could be greatly sensitized to azoles. This suggests the possibility of developing new drugs to combat the serious threat of azole-resistant fungal pathogens.
Methods
Strains and growth conditions
A. fumigatus strains Af293 and IFM 63432 were used to generate the srbA deletion mutants. Clinical strain IFM 63432 contains mutations TR46/Y121F/T289A within the cyp51A promoter region, and was isolated in Japan in 201313. All strains used in the present study were cultivated in PDA and GMM containing 1% glucose at 37 °C. Strains were grown on PDA to collect conidia. Commercially available antifungal chemicals itraconazole, miconazole, bromuconazole, difenoconazole, propiconazole and tebuconazole were obtained from Wako Pure Chemical Industries (Osaka, Japan). Iprodione, fludioxonil and kresoxim-methyl were generously provided by Dr. Keietsu Abe, Tohoku University, Sendai, Japan.
Construction of the srbA deletion mutant strains
A gene replacement cassette was generated to construct the deletion mutants. DNA manipulation was performed according to standard laboratory procedures. Genomic DNA was extracted using a DNeasy Plant Mini Kit (Qiagen, Hilden, Germany). To generate a replacement cassette for srbA, the 5′ and 3′ flanking regions were amplified using the primers srbA-U-F(pUC119E) and srbA-U-R(ptrA), and srbA-D-F(ptrA) and srbA-D-R(pUC119B), respectively. PrimeSTAR HS DNA polymerase (Takara BIO, Otsu, Japan) was used to amplify the flanking regions from genomic DNA. Primer sequences are listed in Supplementary Table S1. These flanking regions, along with a ptrA fragment amplified from pPTRI (Takara BIO) using primers ptrA-F and ptrA-R, were ligated into pUC119 using a Gene Art Seamless Cloning and Assembly Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The resulting plasmid was transformed into A. fumigatus using a protoplast-polyethylene glycol transformation method for Aspergillus33. Precise recombination and integration were confirmed by PCR using primers Check-srbA-F and Check-srbA-R, while the absence of srbA transcripts was confirmed by qRT-PCR analysis (described below).
Comparison of conidia numbers
Conidia production was examined as described previously34. Briefly, conidia were mixed into 2 ml of cooled liquid PDA, which was then poured into a 3.5-cm-diameter Petri plate and allowed to solidify (final concentration, 104 conidia/ml). Following incubation for 72 h, the agar was transferred to a 50-ml tube containing 10 ml of PBS supplemented with 0.1% Tween 20 and vigorously vortexed to dislodge the mycelia and the produced conidia. The number of conidia in the suspensions was counted using a haemocytometer, and the number per well was calculated.
Preparation of RNA and cDNA
Conidia from each strain were inoculated into potato dextrose broth (PDB) (final concentration, 105 conidia/ml) and incubated for 16 h at 37 °C. The mycelia were frozen in liquid nitrogen, and total RNA was isolated using a FastRNA Pro Red Kit (MP Biomedicals, Santa Ana, CA, USA) according to the manufacturer’s instructions. To obtain cDNA from the total RNA, reverse transcription was performed using ReverTra Ace qPCR RT Master Mix with gDNA remover (Toyobo, Osaka, Japan) according to the manufacturer’s instructions.
Quantitative RT-PCR
qRT-PCR was performed using SYBR Green detection as described previously33. Thunderbird SYBR qPCR Mix was used for reaction mixture preparation (Toyobo). Primer sets used are listed in Supplementary Table S1. The relative expression ratios were calculated using the comparative cycle threshold (Ct) (ΔΔCt) method33. The actin gene was used as a normalisation reference (internal control). Each sample was analysed in triplicate, and each experiment was reproduced at least twice.
Antifungal drug susceptibility tests
MIC analysis
The MIC of each antifungal drug against the various A. fumigatus strains was investigated as described previously35. Tests were performed in triplicate using micafungin, amphotericin B, 5-FC, fluconazole, itraconazole, voriconazole, miconazole and posaconazole in RPMI 1640 medium (pH 7.0) at 35 °C. Assays were performed according to the Clinical and Laboratory Standards Institute broth microdilution method, document M38-A2, with some modifications (dried plates for antifungal susceptibility testing; Eiken Chemicals, Tokyo, Japan).
Paper disc diffusion assay
For each assay, a paper disc was placed in the centre of a PDA plate, and the test strains were inoculated by streaking conidia towards the paper disc. A 10-μl aliquot of drug solution was then dropped onto the paper disc (miconazole: 10 mg/ml; itraconazole: 10 mg/ml; bromuconazole: 10 mg/ml; difenoconazole: 10 mg/ml; propiconazole: 10 mg/ml; tebuconazole: 10 mg/ml), and the plates were incubated at 37 °C for 24 h prior to being photographed. To compare drug susceptibility by diameter of inhibition halo, conidia of each strain were mixed with 20 ml of cooled liquid GMM agar, which was then allowed to solidify in 9-cm-diameter plates (final concentration, 104 conidia/ml). A 10-μl aliquot of drug solution was then dropped onto the paper disc (bromuconazole: 10 mg/ml; difenoconazole: 10 mg/ml; propiconazole: 10 mg/ml; tebuconazole: 10 mg/ml), and the plates were incubated at 37 °C for 48 h, followed by measurement of the diameter of the inhibition halo. Three replicates were carried out for each strain, and the mean and standard deviation were calculated.
Growth under hypoxic growth conditions
Conidia of each strain were inoculated onto GMM agar plates and incubated for 72 h at 37 °C in an anaeropack system (Mitsubishi Gas Chemical, Tokyo, Japan). In this system, the concentration of oxygen was controlled within the range of approximately 1–3% (hypoxia). Control plates were incubated at 37 °C under standard oxygen conditions (21% O2) for 72 h.
Additional Information
How to cite this article: Hagiwara, D. et al. Sensitisation of an Azole-Resistant Aspergillus fumigatus Strain containing the Cyp51A-Related Mutation by Deleting the SrbA Gene. Sci. Rep. 6, 38833; doi: 10.1038/srep38833 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was supported by the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT) Special Budget for Research Project: The Project on Controlling Aspergillosis and the Related Emerging Mycoses. The manuscript was edited for English by Edanz Group Ltd.
Footnotes
Author Contributions D.H. and A.W. performed the experiments and analysed the data. D.H., A.W. and K.K. designed the study. D.H. wrote the manuscript.
References
- Lelièvre L. et al. Azole resistant Aspergillus fumigatus: An emerging problem. Med. Maladies Infect. 43, 139–145 (2013). [DOI] [PubMed] [Google Scholar]
- Vermeulen E., Lagrou K. & Verweij P. E. Azole resistance in Aspergillus fumigatus: a growing public health concern. Curr. Opin. Infect. Dis. 26, 493–500 (2013). [DOI] [PubMed] [Google Scholar]
- Snelders E. et al. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 5, 1629–1637 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhary A., Kathuria S., Xu J. & Meis J. F. Emergence of azole-resistant Aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog. 9, e1003633 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcazar-Fuoli L. et al. Ergosterol biosynthesis pathway in Aspergillus fumigatus. Steroids 73, 339–47 (2008). [DOI] [PubMed] [Google Scholar]
- Willger S. D. et al. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog. 4, e1000200 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D. et al. ChIP-seq and In Vivo Transcriptome Analyses of the Aspergillus fumigatus SREBP SrbA Reveals a New Regulator of the Fungal Hypoxia Response and Virulence. PLoS Pathog. 10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blosser S. J. & Cramer R. A. SREBP-dependent triazole susceptibility in Aspergillus fumigatus is mediated through direct transcriptional regulation of erg11A (cyp51A). Antimicrob. Agents Chemother. 56, 248–257 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado E. et al. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob. Agents Chemother. 51, 1897–1904 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen E., Maertens J., Schoemans H. & Lagrou K. Azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation emerging in Belgium, July 2012. Eurosurveillance 17 (2012). [PubMed] [Google Scholar]
- Chowdhary A., Sharma C., Kathuria S., Hagen F. & Meis J. F. Azole-resistant Aspergillus fumigatus with the environmental TR46/Y121F/T289A mutation in India. J. Antimicrob. Chemother. 69, 555–557 (2014). [DOI] [PubMed] [Google Scholar]
- Wu C. J. et al. Azole-resistant Aspergillus fumigatus isolates carrying TR34/L98H mutations in Taiwan. Mycoses 58, 544–549 (2015). [DOI] [PubMed] [Google Scholar]
- Hagiwara D. et al. Multi-azole resistant Aspergillus fumigatus harboring Cyp51A TR46/Y121F/T289A isolated in Japan. J. Infect. Chemother, doi: 10.1016/j.jiac.2016.01.015 (2015). [DOI] [PubMed] [Google Scholar]
- Wiederhold N. P. et al. First Detection of TR34 L98H and TR46 Y121F T289A Cyp51 Mutations in Aspergillus fumigatus Isolates in the United States. J. Clin. Microbiol. 54, 168–171 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ingen J. et al. Azole, polyene and echinocandin MIC distributions for wild-type, TR34/L98H and TR46/Y121F/T289A Aspergillus fumigatus isolates in the Netherlands. J. Antimicrob. Chemother. 70, 178–181 (2015). [DOI] [PubMed] [Google Scholar]
- Snelders E. et al. The structure-function relationship of the Aspergillus fumigatus cyp51A L98H conversion by site-directed mutagenesis: The mechanism of L98H azole resistance. Fungal Genet. Biol. 48, 1062–1070 (2011). [DOI] [PubMed] [Google Scholar]
- Snelders E. et al. Genotype-phenotype complexity of the TR46/Y121F/T289A cyp51A azole resistance mechanism in Aspergillus fumigatus. Fungal Genet. Biol. 82, 129–35 (2015). [DOI] [PubMed] [Google Scholar]
- Hamamoto H. et al. Tandem repeat of a transcriptional enhancer upstream of the sterol 14a-demethylase gene (CYP51) in Penicillium digitatum. Appl. Environ. Microbiol. 66, 3421–3426 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel G. & Jones A. L. The 14α − Demethylase (CYP51A1) Gene is Overexpressed in Venturia inaequalis Strains Resistant to Myclobutanil. Phytopathology 91, 102–110 (2000). [DOI] [PubMed] [Google Scholar]
- Cools H. J., Bayon C., Atkins S., Lucas J. A. & Fraaije B. A. Overexpression of the sterol 14a-demethylase gene (MgCYP51) in Mycosphaerella graminicola isolates confers a novel azole fungicide sensitivity phenotype. Pest Manag. Sci. 68, 1034–1040 (2012). [DOI] [PubMed] [Google Scholar]
- Luo C. X. & Schnabel G. The cytochrome P450 lanosterol 14a-demethylase gene is a demethylation inhibitor fungicide resistance determinant in Monilinia fructicola field isolates from Georgia. Appl. Environ. Microbiol. 74, 359–366 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willger S. D. et al. Dsc orthologs are required for hypoxia adaptation, triazole drug responses, and fungal virulence in Aspergillus fumigatus. Eukaryot. Cell 11, 1557–1567 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelders E. et al. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS One 7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer P. & Denning D. W. Environmental fungicides and triazole resistance in Aspergillus. Pest Manag. Sci. 70, 173–178 (2014). [DOI] [PubMed] [Google Scholar]
- Verweij P. E., Chowdhary A., Melchers W. J. G. & Meis J. F. Azole Resistance in Aspergillus fumigatus: Can We Retain the Clinical Use of Mold-Active Antifungal Azoles? Clin. Infect. Dis. 62, 362–368 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espenshade P. J. SREBPs: sterol-regulated transcription factors. J. Cell Sci. 119, 973–976 (2006). [DOI] [PubMed] [Google Scholar]
- Rawson R. B. Control of lipid metabolism by regulated intramembrane proteolysis of sterol regulatory element binding proteins (SREBPs). Biochem. Soc. Symp. 221–31 (2003). [DOI] [PubMed] [Google Scholar]
- Nomura T., Horikawa M., Shimamura S., Hashimoto T. & Sakamoto K. Fat accumulation in Caenorhabditis elegans is mediated by SREBP homolog SBP-1. Genes Nutr. 5, 17–27 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. L., Todd B. L. & Espenshade P. J. SREBP pathway responds to sterols and functions as an oxygen sensor in fission yeast. Cell 120, 831–842 (2005). [DOI] [PubMed] [Google Scholar]
- Chang Y. C., Bien C. M., Lee H., Espenshade P. J. & Kwon-Chung K. J. Sre1p, a regulator of oxygen sensing and sterol homeostasis, is required for virulence in Cryptococcus neoformans. Mol. Microbiol. 64, 614–629 (2007). [DOI] [PubMed] [Google Scholar]
- Chun C. D., Liu O. W. & Madhani H. D. A link between virulence and homeostatic responses to hypoxia during infection by the human fungal pathogen Cryptococcus neoformans. PLoS Pathog. 3, 0225–0238 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima P. de S., Chung D., Bailão A. M., Cramer R. A. & Soares C. M. de A. Characterization of the Paracoccidioides Hypoxia Response Reveals New Insights into Pathogenesis Mechanisms of This Important Human Pathogenic Fungus. PLoS Negl. Trop. Dis. 9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara D. et al. NikA/TcsC histidine kinase is involved in conidiation, hyphal morphology, and responses to osmotic stress and antifungal chemicals in Aspergillus fumigatus. PLoS One 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara D., Suzuki S., Kamei K., Gonoi T. & Kawamoto S. The role of AtfA and HOG MAPK pathway in stress tolerance in conidia of Aspergillus fumigatus. Fungal Genet. Biol. 73, 138–149 (2014). [DOI] [PubMed] [Google Scholar]
- Kikuchi K. et al. Antifungal susceptibility of Aspergillus fumigatus clinical isolates collected from various areas in Japan. J. Infect. Chemother. 20, 336–338 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.