Abstract
Castration resistant prostate cancer (CRPC), the fatal form of prostate cancer, remains androgen dependent despite castrate levels of circulating testosterone (T) and 5α-dihydrotestosterone (DHT). To investigate mechanisms by which the tumor can synthesize its own androgens and develop resistance to abiraterone acetate and enzalutamide, methods to measure a complete androgen profile are imperative. Here, we report the development and validation of a stable isotope dilution liquid chromatography electrospray ionization tandem mass spectrometric (SID-LC-ESI–MS/MS) method to quantify nine human hydroxy-androgens as picolinates, simultaneously with requisite specificity and sensitivity. In the established method, the fragmentation patterns of all nine hydroxy-androgen picolinates were identified, and [13C3]-5α-androstane-3α, 17β-diol and [13C3]-5α-androstane-3β, 17β-diol used as internal standards were synthesized enzymatically. Intra-day and inter-day precision and accuracy corresponds to the U.S. Food and Drug Administration Criteria for Bioanalytical Method Validation. The lower limit of quantitation (LLOQ) of nine hydroxy-androgens is 1.0 pg to 2.5 pg on column. Diols which have been infrequently measured: 5-androstene-3β, 17β-diol and 5α-androstane-3α, 17β-diol can be determined in serum at values as low as 1.0 pg on column. The method also permits the quantitation of conjugated hydroxy-androgens following enzymatic digestion. While direct detection of steroid conjugates by electrospray-ionization tandem mass spectrometry has advantages the detection of unconjugated and conjugated steroids would require separate methods for each set of analytes. Our method was applied to pooled serum from male and female donors to provide reference values for both unconjugated and conjugated hydroxy-androgens. This method will allow us to interrogate the involvement of the conversion of 5-androstene-3β, 17β-diol to T, the backdoor pathway involving the conversion of 5α-androstane-3α, 17β-diol to DHT and the inactivation of DHT to 5α-androstane-3β, 17β-diol in advanced prostate cancer.
Keywords: Castration resistant prostate cancer, Androgen conjugates, Androgen diols, Enzymatic synthesis, Picolinic acid derivatization, LC-ESI-MS/MS
1. Introduction
Prostate cancer (CaP) is the most frequently diagnosed cancer and the second leading cause of cancer death in males in the United States [1]. Pathophysiological studies have shown that the development of CaP is initially dependent on androgens and mediated by the androgen receptor (AR) signaling axis [2–4]. Thus, androgen deprivation therapy (ADT) has been the primary clinical treatment for localized advanced or metastatic CaP [5,6]. However, progression of CaP occurs within 1–2 years in almost all patients receiving ADT despite castrate levels of circulating androgens (e.g. T and DHT), and is defined as castration resistant prostate cancer (CRPC), the fatal form of CaP [7,8]. CRPC is now treated with new drugs that either target androgen biosynthesis or antagonize the AR. Abiraterone acetate, which inhibits the activities of cytochrome P450 17α-hydroxylase/17,20-lyase (CYP17A1) to block the conversion of pregnenolone to DHEA (Scheme 1), significantly reduces circulating androgens, improves overall survival in CRPC patients and has been approved by FDA [9–11]. Enzalutamide, a second generation of AR antagonist, also offers efficacious treatment and survival benefit for patients with advanced prostate cancer [12,13]. The positive results obtained with these two agents in clinical trials indicate that CRPC remains androgen driven by the reactivation of AR signaling due in part to intratumoral androgen biosynthesis [5,14,15]. However, resistance to abiraterone and enzalutamide has been reported due to an elevated expression level of CYP17A1, AKR1C3, AR gene amplification or the emergence of AR splice variants which are constitutively active etc. [15–18]. In order to investigate the efficacy of new drug treatments, understand mechanisms of drug resistance and create precision treatment for CRPC, clinical chemistry requires methods to measure serum and intratumoral androgen levels with the requisite specificity, sensitivity, accuracy and precision.
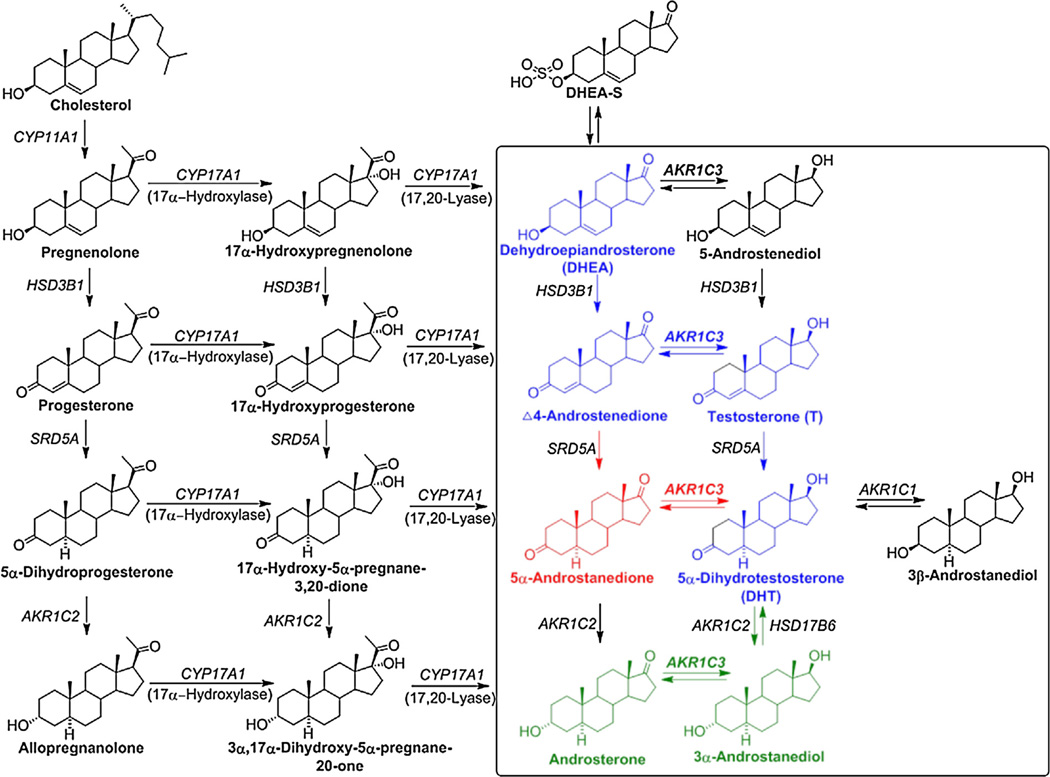
Scheme 1.
Intracrine androgen biosynthesis. Intraprostatic androgen metabolism is shown in the rectangle. Blue: classical pathway; Red: alternative pathway; Green: backdoor pathway. 3α-androstanediol: 5α-androstane-3α, 17β-diol; 3β-androstanediol: 5α-androstane-3β, 17β-diol; 5-androstenediol: 5-androstene-3β, 17β-diol; 5α-androstanedione: 5α-androstane-3,17-dione; Δ4-androstenedione: Δ4-androstene-3,17-dione; AKR1C1: 20α-hydroxysteroid dehydrogenase; AKR1C2: type 3 3α-hydroxysteroid dehydrogenase; AKR1C3: type 5 17β-hydroxysteroid dehydrogenase; CYP11A1: cytochrome P450 11A1; CYP17A1: cytochrome P450 17A1; DHEA-S: DHEA-sulfate; HSD3B1: type 1 3β-hydroxysteroid dehydrogenase; HSD17B6: type 6 17β-hydroxysteroid dehydrogenase; SRD5A: 5α-reductase. Enzymes are identified by gene names in italics.
T and DHT can be synthesized via four different pathways, where the enzymes involved in the prostatic androgen biosynthesis are shown in Scheme 1. The classical pathway involves DHEA → Δ4-androstenedione → T → DHT. An alternative pathway bypasses the formation of T and converts Δ4-androstenedione to 5α-androstanedione which is further reduced to DHT. Another pathway to DHT, also known as the backdoor pathway, converts androsterone to 3α-androstanediol which is subsequently oxidized to DHT. In addition, a potential route to testosterone involves the conversion of DHEA to 5-androstenediol and its subsequent dehydrogenation and isomerization to T [19]. The central role of AKR1C3 is also shown.
Several analytical methods exist to measure T and DHT and their precursors involved in intracrine androgen biosynthesis such as immunoassay, gas chromatography tandem mass spectrometry (GC–MS/MS) and liquid chromatography tandem mass spectrometry (LC–MS/MS) [20,21]. In comparison to traditional immuno-assays such as radioimmunoassay (RIA) and enzyme-linked immunosorbent assay (ELISA), mass spectrometry does not suffer from cross reactivity problems that can plague antibody based methods. In addition, LC–MS/MS provides accurate structural information of analytes (e.g. it can distinguish between different regio- and stereo-isomers), and can measure many analytes simultaneously using the selected reaction monitoring (SRM) mode and thus reduces the sample size of the biospecimen [21–29]. However, LC–MS/MS methods can be limited by insufficient sensitivity from poorly ionized steroids using soft ionization sources (e.g. ESI) [30,31]. To circumvent this problem, chemical derivatization techniques have been introduced to form easily ionized analytes prior to LC–MS/MS analysis [32]. Girard-T/P reagents targeting carbonyl groups (e.g. keto-androgens) have been successfully applied to improve detection sensitivity. LC-ESI–MS/MS coupled with Girard-T derivatization has been used by us to systematically quantify the keto-androgen profile in patients with CaP and CRPC [30,33–35]. Steroids which contain both a keto group and a hydroxyl group can be detected by either Girard-T derivatization of the keto group to form the oxime or by picolinic acid derivatization of the hydroxyl group to form the picolinate ester. However, hydroxy-androgens, which contain hydroxyl groups only (e.g. 5-androstenediol) cannot be measured by Girard-T derivatization. Currently, T, DHT, hydroxy-estrogens and corticosteroids in human saliva, serum, and tissue have been detected following picolinic acid derivatization using LC-ESI–MS/ MS [36–38]. This derivatization method improves the ionization efficiency of steroids with a hydroxyl group since the picolinic acid ester improves proton affinity and minimizes interferences from biological matrices (see Scheme 3) [37]. However, this method has not been systematically applied to a panel of hydroxy-androgens, specifically for the measurement of androgen diols.
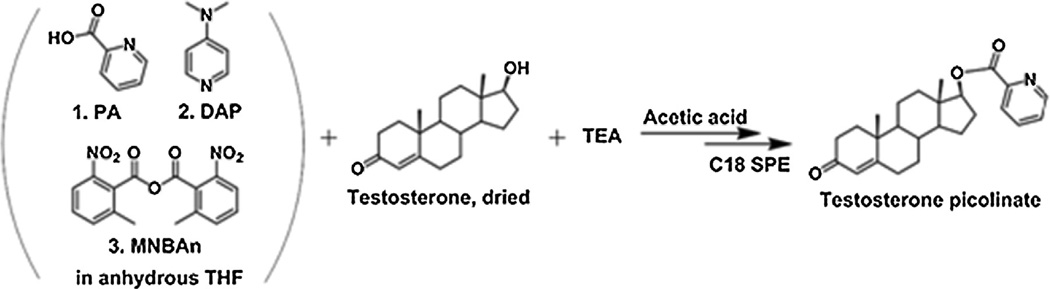
Scheme 3.
Testosterone derivatization bypicolinic acid reagents. PA: picolinic acid; DAP: 4-dimethylaminopyridine; MNBAn: 2-methyl-6-nitrobenzoic anhydride; SPE: solid phase extraction; TEA: triethylamine; THF: tetrahydrofuran.
Herein, we report the development and validation of a new stable isotope dilution liquid chromatography electrospray ionization tandem mass spectrometry (SID-LC-ESI–MS/MS) method using picolinic acid derivatization that allows us to simultaneously quantify nine human hydroxy-androgens and their glucuronide and sulfate conjugates, including a panel of hydroxy-androgens involved in androgen metabolism in prostate (rectangle circled in Scheme 1). While direct detection of steroid conjugates by electrospray-ionization tandem mass spectrometry has advantages the detection of unconjugated and conjugated steroids would require separate methods for each set of analytes. Moreover, our method can be adapted to measure the conversion of hydroxy-androgen precursors to DHT by four pathways including the canonical pathway, the alternative pathway from 5α-androstane-dione, the backdoor pathway from 3α-androstanediol and the conversion of 5-androstenediol to T. We also used an enzymatic method to synthesize internal standards (IS) of [13C]-labeled 3α-androstanediol and 3β-androstanediol for the quantitation of androgen diols (5-androstenediol, 3α-androstanediol and 3β-androstanediol). This method was used to determine nine hydroxy-androgens and their conjugates in pooled serum from male and female donors. For the first time, 5-androstenediol (a precursor of T), 3α-androstanediol involved in the backdoor pathway to DHT and 3β-androstanediol involved in DHT deactivation were determined simultaneously in human serum.
2. Materials and methods
2.1. Reagents and biological samples
Reagents were of ACS grade or higher and were purchased from Fisher Scientific (Pittsburgh, PA, USA) and used without further purification. T (17β-hydroxy-4-androsten-3-one), Epi-T (17α-hydroxy-4-androsten-3-one), DHEA (3β-hydroxy-5-androsten-17-one), DHEA-sulfate sodium salt (DHEA-S), DHEA-glucuronide (DHEA-G), androsterone (3α-hydroxy-5α-androstan-17-one), epi-androsterone (3β-hydroxy-5α-androstan-17-one), DHT (17β-hy-droxy-5α-androstan-3-one), 5-androstenediol (androst-5-ene-3β, 17β-diol), 3α-androstanediol (5α-androstane-3α,17β-diol) and 3β-androstanediol (5α-androstane-3β, 17β-diol) were purchased from Steraloids (Wilton, NH, USA). [2,3,4-13C3]-T ([13C3]-T) and [2,3,4-13C3]-DHT ([13C3]-DHT) were from C/D/N Isotopes (Point-Claire, Quebec, Canada) and Cambridge Isotopes (Andover, MA, USA), respectively. Nicotinamide adenine dinucleotide, Grade I reduced form (NADH) and nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) were obtained from Roche Diagnostics (Indianapolis, IN, USA). DHEA-S sodium salt in methanol (1 mg/mL), 4-dimethylaminopyridine (DAP), 2-methyl-6-nitrobenzoic anhydride (MNBAn), picolinic acid (PA), triethyl-amine (TEA), anhydrous tetrahydrofuran (THF), β-glucuronidase from E. coli and sulfatase from Abalone entrails were from Sigma-Aldrich (St. Louis, MO, USA). Recombinant rat liver 3α-hydroxysteroid dehydrogenase (AKR1C9, E.C. 1.1.1.213) and human steroid 5β-reductase mutant E120H (AKR1D1 E120H) were prepared and purified as previously described [39,40]. Charcoal dextran stripped fetal bovine serum (CD-FBS) was from Atlanta Biologicals (Lawrenceville, GA, USA). Pooled human serum collected from males and females was obtained from BioreclamationIVT (Westbury, NY, USA), shipped on dry ice, and stored at −80 °C until sample preparation. One pooled sample was purchased from male donors and one pooled sample was purchased from female donors.
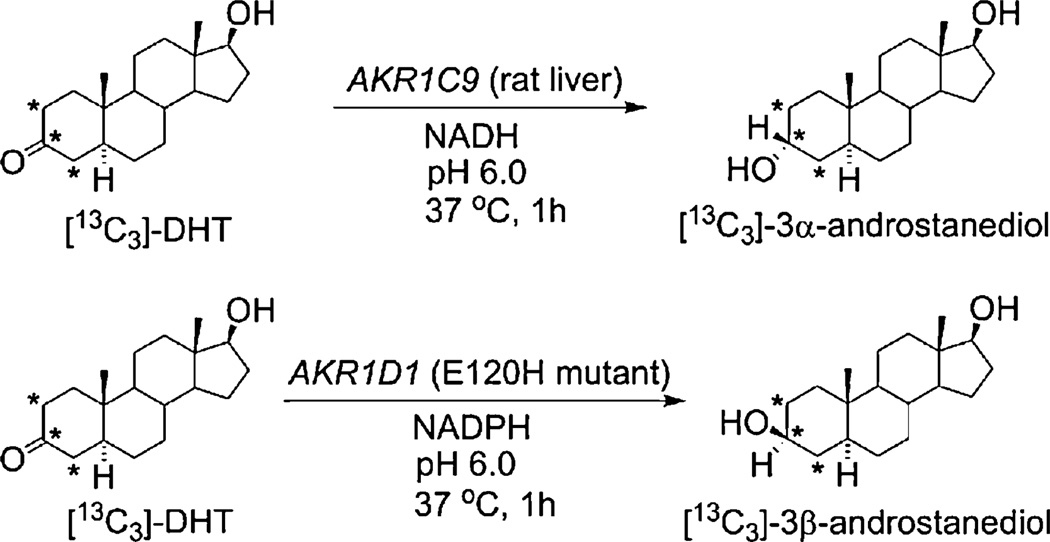
2.2. Enzymatic synthesis of [2,3,4-13C3]-3α-androstanediol and [2,3,4-13C3]-3β-androstanediol
[2,3,4-13C3]-3α-Androstanediol ([13C3]-3α-androstanediol) and [2,3,4-13C3]-3β-androstanediol ([13C3]-3β-androstanediol) were synthesized from [13C3]-DHT using rat liver AKR1C9 and human AKR1D1 E120H mutant, respectively (Scheme 2). AKR1D1 E120H mutant has been previously shown to be a soluble recombinant source of 3β-HSD [40]. This single point mutation in AKR1D1 is sufficient to eliminate the 5b-reductase activity of the enzyme and generate an enzyme that only has 3β-HSD activity [40]. For the synthesis of [13C3]-3α-androstanediol, the reaction contained 100 mM potassium phosphate buffer (pH 6.0), 4% acetonitrile (ACN, HPLC Grade), 651 mM NADH, AKR1C9 (9.3 ng/ µL) and [13C3]-DHT (4 pg/ µL). For the synthesis of [13C3]-3β-androstanediol, the reaction system was composed of 100 mM potassium phosphate buffer (pH 6.0), 4% ACN, 1 mM NADPH, AKR1D1 E120H mutant (14 ng/ mL) and [13C3]-DHT (4 pg/ µL). All the reactions were incubated at 37 °C for 1 h. After incubation, the solution was extracted with 1.5 mL of ethyl acetate by vortex followed by 20 min of centrifugation, and the ethyl acetate fraction was transferred into borosilicate tubes. The extraction step was repeated once. The extracts were combined and dried by a Savant SPD121P SpeedVac™ Concentrator (Thermo Scientific, San Jose, CA, USA). The products were reconstituted in 200-proof ethanol to ~50 pg/µL, and the amount of [13C3]-3α-androstanediol and [13C3]-3β-androstanediol were assessed by LC-ESI-SRM-MS using Dionex UltiMate 3000 UHPLC coupled with TSQ Quantum Ultra Triple Quadrupole mass spectrometer (Thermo Scientific, San Jose, CA, USA). For the quantitation of [13C3]-3α-androstanediol and [13C3]-3β-androstanediol, three aliquots (5 µL each) from [13C3]-3α-androstanediol and [13C3]-3β-androstanediol were analyzed after picolinic acid derivatization as described below, and the concentration was estimated from an external calibration curve constructed using a serial dilution of 3α-androstanediol and 3β-androstanediol picolinate solutions. The concentrations were then re-validated by combining the same amounts of 3α-androstanediol and [13C3]-3α-androstanediol or 3β-androstanediol and [13C3]-3β-androstanediol for derivatization and LC-ESI-SRM-MS analysis to check the peak area ratios (see Data-in-Brief in Ref. [41]). After quantitation, the solution was diluted to 20 pg/µL in ethanol and stored at −20°C until use. The methods were optimized by using unlabeled DHT to ensure that the reactions went to completion before its application to synthesize stable isotopically labeled internal standards (see Data-in-Brief in Ref. [41]).
Scheme 2.
Enzymatic synthesis of [13C3]-3α-androstanediol and [13C3]-3β-androstanediol from [13C3]-DHT. *: 13C position.
2.3. Preparation of derivatization reagent, stock solutions and calibrators
To prepare 1 mL of picolinic acid derivatization reagent, DAP (20 mg) and PA (50 mg) were first dissolved in anhydrous THF (1 mL). Next, MNBAn (40 mg) was added to the solution and dissolved with gentle shaking for derivatization (Scheme 3).
A standard stock solution containing a mixture of T, EpiT, DHEA, DHT, androsterone, epiandrosterone, 5-androstenediol, 3α-androstanediol and 3β-androstanediol in 200-proof ethanol at a concentration of 1 ng/µL was made. The IS solutions of [13C3]-T, [13C3]-DHT, [13C3]-3α-androstanediol and [13C3]-3β-androstanediol were also prepared in ethanol at a concentration of 20 pg/µL Solutions were stored at −20 °C.
CD-FBS or ethanol was used as the matrix for calibrators and quality control (QC) samples. An aliquot of CD-FBS (0.2 mL) in a borosilicate tube was mixed with a different amount of the standard mixture and IS mixture ([13C3]-T, [13C3]-DHT, [13C3]-3α-androstanediol and [13C3]-3β-androstanediol, 100 pg each). The mixtures were extracted with 2 mL of anhydrous diethyl ether by shaking for 15 min. The organic phase was separated by centrifugation using SVC-100H at 1500 rpm (~176 × g, Savant, Farmingdale, NY, USA) for 1 h. The organic fraction was transferred to another tube and evaporated to dryness under nitrogen in a VisiPrep 24DL manifold (Supelco, Bellefonte, PA, USA). The dried residue was dissolved in the prepared derivatization reagent (100 µL each) as described above, followed by the addition of 40 µL of TEA and incubated at room temperature with mild agitation for 90 min. The reaction was quenched by adding 1% aqueous acetic acid (1 mL, v/v) and the derivatives were further purified using Strata C18-E cartridge (Phenomenex, Torrance, CA, USA) coupled onto VisiPrep 24DL manifold for vacuum extraction (Scheme 3). The cartridges were first pre-conditioned with 2 mL of methanol followed by addition of 2 mL of water. After preconditioning, a solution of derivatives (~1.1 mL) was loaded onto the cartridge. After binding, the cartridges were washed with 2 mL of water and 3 mL of 30% (v/v) ACN in water. The derivatives were eluted with 3 mL of ACN and the eluent was dried on a Savant SPD121P SpeedVac™ Concentrator. The dried residues were stored at −20 °C until analysis by LC–ESI-SRM–MS.
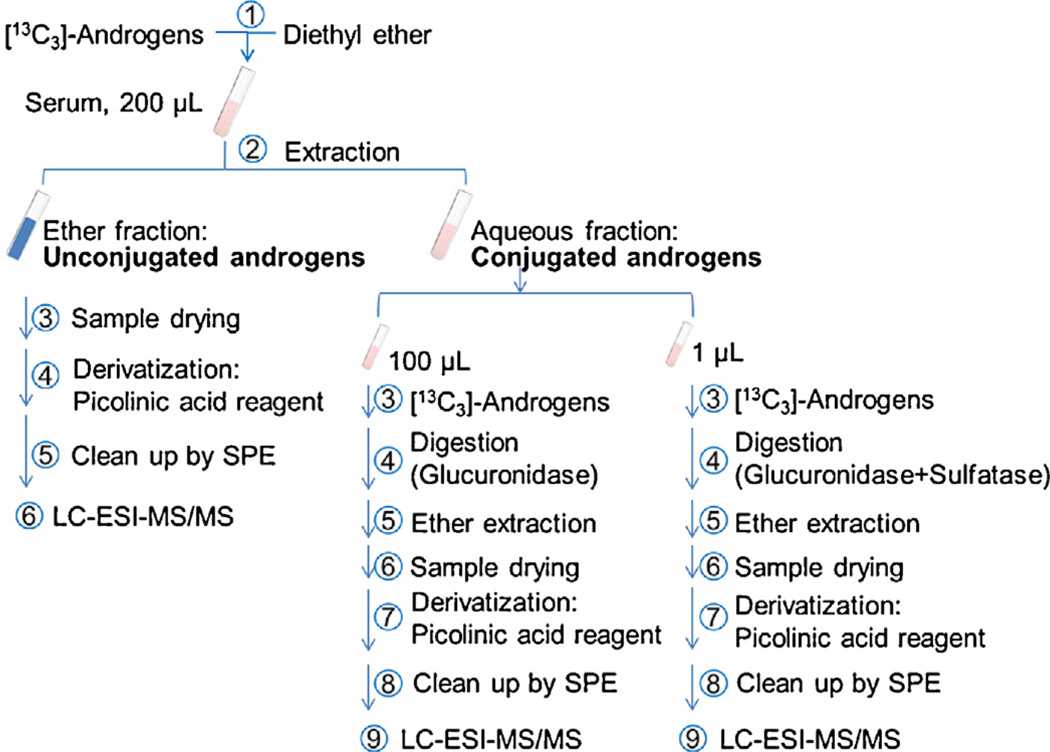
2.4. Sample preparation
Human serum (0.2 mL) was mixed with IS ([13C3]-T, [13C3]-DHT, [13C3]-3α-androstanediol and [13C3]-3β-androstanediol, 100 pg each) and extracted, and the organic fraction containing the unconjugated androgens was derivatized as described above, see Fig. 1. The aqueous solution containing the conjugated androgens was separated into two aliquots for enzymatic digestion to quantify glucuronidated and sulfated androgen metabolites using our published method with some slight modifications (Scheme S1 in Data-in-Brief: Ref. [41]) [33]. For the quantitation of glucuronidated hydroxy-androgens, 100 µL of the aqueous fraction was incubated with 100 mM (final concentration) sodium acetate buffer at pH 5.0, IS ([13C3]-T, [13C3]-DHT, [13C3]-3α-androstanediol and [13C3]-3β-androstanediol, 100 pg each) and 90 U of E. coli β-glucuronidase at 37 °C for 3–4 h. Then, another 90 U of E.coli β-glucuronidase was added to continue the digestion for a total of 16–18 h. For the quantitation of the combined sulfated and glucuronidated hydroxy-androgens, a 1 µL of the aqueous fraction was incubated with 100 mM (final concentration) sodium acetate buffer at pH 5.0, IS ([13C3]-T, [13C3]-DHT, [13C3]-3α-androstanediol and [13C3]-3β-androstanediol, 100 pg each), 30 U of Abalone entrails sulfatase and 90 U of E. coli β-glucuronidase at 37 °C for 3–4 h. Then, another 30 U of Abalone entrails sulfatase and 90 U of E. coli β-glucuronidase were added to continue the digestion for a total of 16–18 h. After digestion, sample extraction and derivatization followed the same procedure as described above. The dried residues were stored at −20 °C until analysis by LC-ESI-SRM-MS. The difference between the digestion using β-glucuronidase and β-glucuronidase plus sulfatase provided a quantitative estimate of the sulfate conjugates. Before digestion, both enzymes were titrated based on the protocols provided by the manufacturer. The validation of the digestion methods are described in the Data-in-Brief article (Tables S1–S3 in Ref. [41]).
Fig. 1.
Work-flow for the quantitation of conjugated and unconjugated hydroxy-androgens using picolinic acid derivatization by LC-ESI–MS/MS.
2.5. LC-ESI-MS/MS
LC-ESI–MS/MS was performed on a TSQ Quantum Ultra Triple Quadrupole mass spectrometer connected with Dionex UltiMate 3000 UHPLC. Kinetex C18 (Phenomenex, Torrance, CA, USA) 100 mm × 2.1 mm, 2.6 µm, 100 Å column with a C18 guard column (2.1 mm internal diameter) was used for the separation of hydroxy-androgen picolinates. The flow rate was 0.25 mL/min. The column was eluted with 0.05% (v/v) formic acid in water (mobile phase A) and 0.05% (v/v) formic acid in 40% acetonitrile: 60% methanol (v/v, mobile phase B), starting at 20% B for 1 min, then increased to 60% B over 5 min and maintained at 60% B for 20 min, then increased to 95% B over 15 min and maintained at 95% B for 5 min, and finally decreased to 20% B over 1 min and maintained at 20% B for 15 min. The mass spectrometer conditions were spray voltage, 4000 V; ion transfer capillary temperature, 350 °C; capillary offset voltage: 35V; sheath gas (nitrogen) pressure, 40 psi; auxiliary gas (nitrogen) pressure, 15 arbitrary units; collision gas: argon. All samples were reconstituted with 100 µL of 60% acetonitrile in water (v/v) and 20 µL of analyte was injected for mass spectrometric analysis. Data were analyzed by using the program Xcalibur 3.0.63 (Thermo Scientific).
2.6. Method validation
The quantitation of nine hydroxy-androgens in serum was validated based on linearity of calibration curves, matrix effects, detection specificity, determination of the lower limit of quantitation (LLOQ), and intra- and inter-day precision and accuracy. The efficiency, precision and accuracy of enzymatic digestion were also validated (see Table S3 and Data-in-Brief in Ref. [41]).
2.6.1. Calibration curves, matrix effects and specificity
Serial dilutions from the standard mixture were added into CD-FBS (0.2 mL) to yield concentrations of 5, 12.5, 25, 50, 125, 250, 500, 1250, 2500 and 5000 pg/0.2 mL, respectively. The IS mixture of [13C3]-T, [13C3]-DHT, [13C3]-3α-androstanediol and [13C3]-3β-androstanediol (100 pg each) was then spiked into each sample. The ratio of peak areas of PA derivatives of the standards to those of the corresponding IS versus amount of injected standards were plotted to yield calibration curves. For the quantitation of T, EpiT and DHEA, [13C3]-T was used as the IS; for the quantitation of DHT, androsterone and epiandrosterone, [13C3]-DHT was used as the IS; for the quantitation of 5-androstenediol and 3α-androstanediol, [13C3]-3α-androstanediol was used as the IS; for the quantitation of 3β-androstanediol, [13C3]-3β-androstanediol was used as IS. The same calibration curves were also constructed using ethanol (0.2 mL) as the matrix. The matrix effect from serum was evaluated by comparing the slopes of calibration curves acquired from CD-FBS and ethanol. The specificity of the method was tested by analyzing six individual blank CD-FBS samples.
2.6.2. Lower limit of quantitation, intra- and inter-day precision and accuracy
LLOQ was defined as the lowest concentration which can be measured with a signal to noise ratio ≥ 5:1, and the determined amount has a precision that does not exceed 20% of the coefficient of variation (CV) and accuracy is within 20% of the theoretical level. At least five individual samples were analyzed to determine LLOQ. The intra-day precision of the method was assessed by performing triplicate analysis of QC samples within one day. The inter-day precision of the method was assessed by performing triplicate analysis of QC samples on three consecutive days. Three CD-FBS samples (QC) spiked with low, medium and high concentrations (5 or 12.5, 250, 2500 pg/0.2 mL) of the standard mixture were prepared and analyzed, respectively. The accuracy of QC samples was calculated based on the ratio of the determined amount to the theoretical amount.
3. Results
3.1. Derivatization by picolinic acid and separation of hydroxy-androgen picolinates
The derivatization of nine hydroxy-androgens by the picolinic acid reagent was optimized (see Data-in-Brief in Ref. [41]). Hydroxy-androgens with mono-hydroxyl or dihydroxyl groups were completely converted to the corresponding mono- or bis-picolinates, respectively (Fig. 2 (text) and Fig. S8 in Ref. [41]). In addition, baseline separation for nine hydroxy-androgen picolinates was achieved by optimizing RP-HPLC conditions (Fig. 3 (text) and Fig. S6 in Ref. [41]).
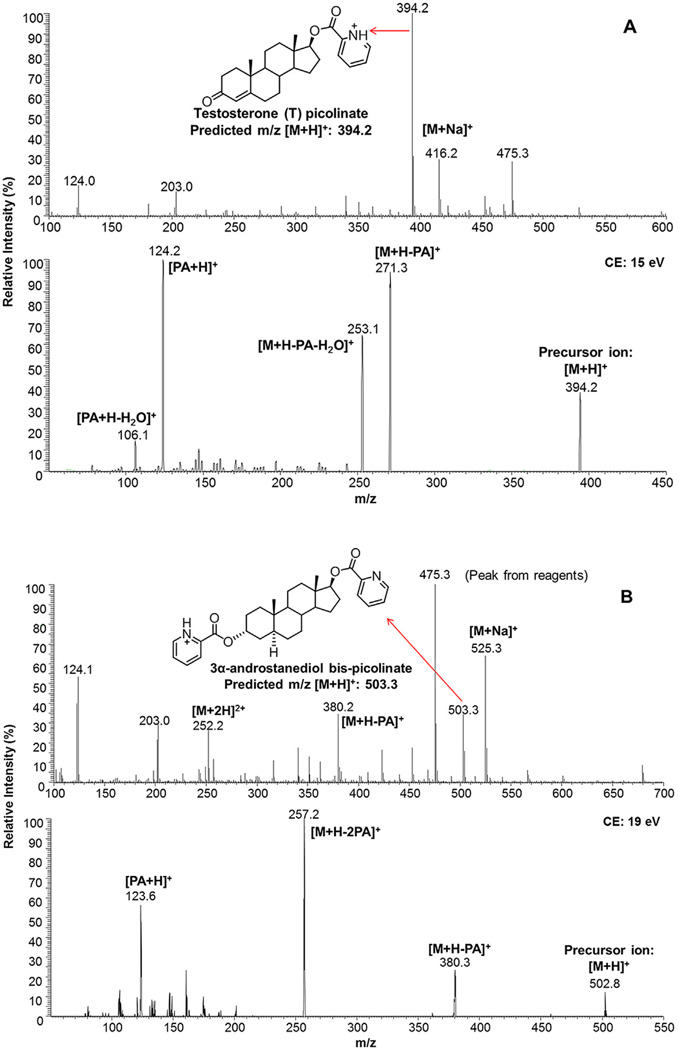
Fig. 2.
Representative mass spectra and tandem mass spectra of testosterone monopicolinate and 3α-androstanediol bis-picolinate. Testosterone mono-picolinate (A) and 3α-androstanediol bis-picolinate (B). No mono-picolinate ester with m/z of 398.3 was detected upon 3α-androstanediol derivatization. PA: picolinic acid; Na: sodium.
Fig. 3.
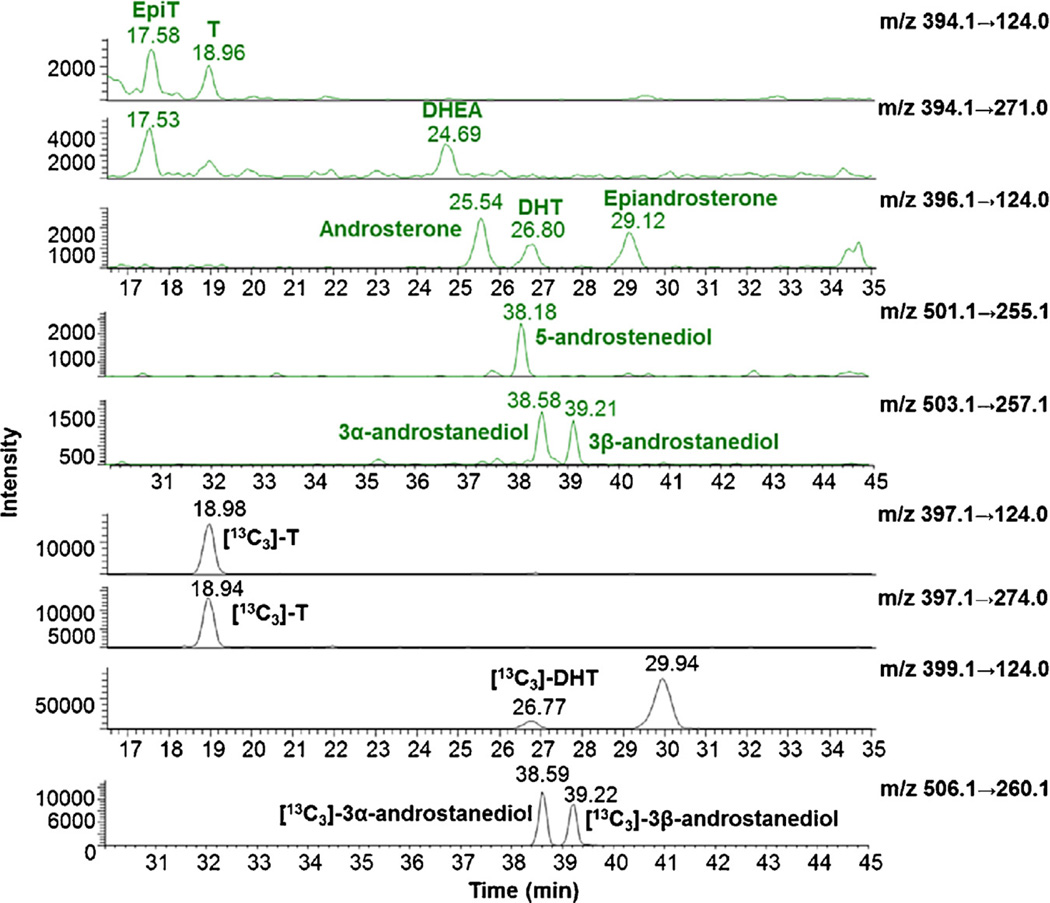
Selective reaction ion-chromatograms of hydroxy-androgen picolinates and four [13C3]-hydroxy-androgen picolinate internal standards. The sample is from a dilution of CD-FBS serum (0.2 mL) containing 12.5 pg of standard mixture and 100 pg of IS mixture. On-column injection is from: 2.5 pg of hydroxy-androgen picolinate and 20 pg of [13C3]-hydroxy-androgen picolinate prepared by extracting the unconjugated hydroxy-androgen from the matrix. 3α-androstanediol: 5α-androstane-3α, 17β-diol; 3β-androstanediol: 5α-androstane-3β, 17β-diol; 5-androstenediol: 5-androstene-3β, 17β-diol; DHEA: dehydroepiandrosterone; DHT: 5α-dihydrotestosterone; EpiT: epitestosterone; T: testosterone.
3.2. Enzymatic synthesis of [13C3]-3α-androstanediol and [13C3]-3β-androstanediol from [13C3]-DHT
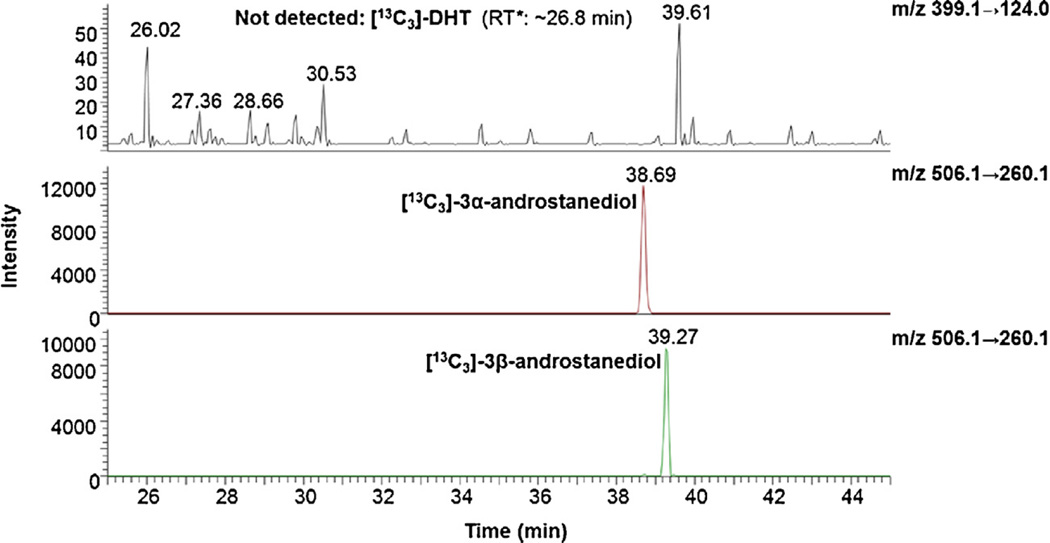
Following enzymatic reduction, the [13C3]-DHT substrate could not be detected by mass spectrometry (Fig. 4) and the synthesized [13C3]-3α-androstanediol and [13C3]-3β-androstanediol showed little interference upon analysis with other targeted hydroxy-androgen picolinates (Fig. S4 in Ref. [41]).
Fig. 4.
Selective reaction monitoring ion chromatograms for [13C3]-3α-androstanediol, and [13C3]-3β-androstanediol picolinates obtained from enzymatic reactions. ~30 pg of synthesized [13C3]-3α-androstanediol and [13C3]-3β-androstanediol picolinates were injected onto column for analysis, respectively. The top panel shows the absence of the [13C3]-DHT starting material.
3.3. ESI–MS, ESI–MS/MS and LC-ESI–MS/MS of hydroxy-androgen picolinates
The precursor and product ions of nine hydroxy-androgen picolinates were characterized by direct infusion ESI–MS and ESI–MS/MS in the positive-ion mode. Hydroxy-androgen monopicolinates (T, EpiT, DHEA, DHT, androsterone and epiandroster-one) displayed the predominant [M+H]+ as the base peaks; however, hydroxy-androgen bis-picolinates (5-androstenediol, 3α- and 3β-androstanediol) showed the presence of [M+2H]2+, [M+H]+ and [M+Na]+ (Fig. 2 (text) and Fig. S8 in Ref. [41]). The fragmentation of [M+H]+ of hydroxy-androgen picolinates gave a similar fragment pattern with relatively low collision energy (14–21 eV), which consisted of [M+H−C6H5NO2 (M+H-PA)]+, [M+H−C6H5NO2−H2O (M+H-PA−H2O)]+, [M+H−C12H10N2O4 (M + H-2PA from bis-picolinates)]+, [C6H6NO2 (PA+H)]+ and [C6H6NO2−H2O (PA+H−H2O)]+ (Fig. 2 (text) and Figs. S8 and S9 in Ref. [41]). In order to achieve the maximum sensitivity, the collision energies for product ions were further optimized by LC-ESI–MS/MS analysis. Ion transitions of m/z 394.1 → 124.0 for T and EpiT; m/z 394.1 → 271.0 for DHEA; m/z 396.1 → 124.0 for DHT, androsterone and epiandrosterone; m/z 501.1 → 255.1 for 5-androstenediol; m/z 503.1 → 257.1 for 3α - and 3β -androstane-diol; m/z 397.1 → 124.0 and m/z 397.1 → 274.0 for [13C3]-T; m/z 399.1 → 124.0 for [13C3]-DHT; m/z 506.1 → 260.1 for [13C3]-3α - and [13C3]-3β-androstanediol were chosen for LC-ESI-SRM-MS in quantitation of nine hydroxy-androgen picolinates (Fig. 3 (text) and Table S4 in Ref. [41]).
3.4. Method validation
3.4.1. Linearity of calibration curves, matrix effects and specificity
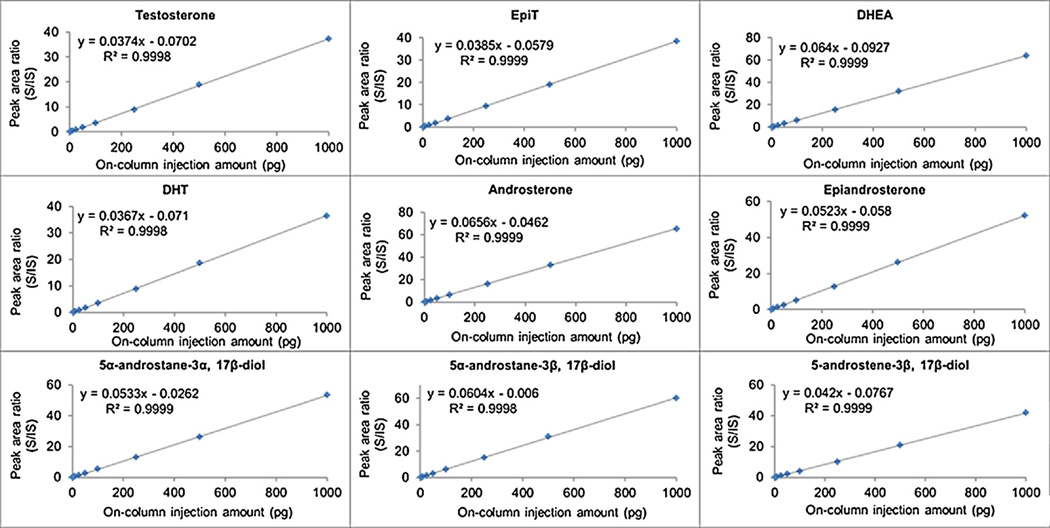
Calibration curves of T, EpiT, DHEA, DHT, androsterone, epiandrosterone, 5-androstenediol, 3α- and 3β-androstanediol standards were constructed in CD-FBS (0.2 mL) or ethanol (0.2 mL) based on the peak area ratios between targeted hydroxy-androgen picolinates and the corresponding IS picolinates. At least six concentration levels in the range of 5–5000 pg/0.2 mL were analyzed, and the calibration curves were linear based on their slopes and gave high correlation coefficients (r2) of 0.9988–0.9999 (Fig. 5 (text) and Fig. S10 in Ref. [41]). Matrix effects were assessed for nine hydroxy-androgen picolinates, and no significant difference was observed by comparing calibration curve slopes between CD-FBS and ethanol (coefficient of variation < 20%, see Fig. 5 (text) and Fig. S10 in Ref. [41]).
Fig. 5.
Calibration curves for nine hydroxy-androgens using charcoal dextran stripped fetal bovine serum (CD-FBS) as matrix. S: unlabeled hydroxy-androgen standard. IS: stable isotope labeled hydroxy-androgen standard. Curves were constructed following the extraction of unconjugated hydroxy-androgens from the matrix. R2: correlation coefficient of linearity. EpiT: epitestosterone; DHEA: dehydroepiandrosterone; DHT: 5α-dihydrotestosterone.
In addition, CD-FBS (0.2 mL) and IS spiked CD-FBS (0.2 mL) as control samples were analyzed to evaluate the specificity of detection. The mass spectrometric analysis indicated that there was little interference from other compounds that co-eluted with the targeted hydroxy-androgen picolinates or IS picolinates (Fig. S11 in Ref. [41]).
3.4.2. Lower limit of quantitation, intra- and inter-day precision and accuracy
LLOQ was defined as the lowest amount of targeted hydroxy-androgen picolinates which could be determined with an accuracy within 20% of theoretical value and a precision less than 20% of the CV. The determined LLOQs are listed in Table 1 (see SRM chromatograms in Fig. 3 (text) and Fig. S12 in Ref. [41]). LLOQs of T, 5-androstenediol and 3α-androstanediol in CD-FBS were 1.0 pg on column (Fig. S12 in Ref. [41]), and LLOQs of EpiT, DHEA, DHT, androsterone, epiandrosterone and 3β-androstanediol in CD-FBS were 2.5 pg on column (Fig. 3). Since some analytes are at relatively high concentrations we all estimated the Upper Limit of Quantification (ULOQ) for which DHEA was 15 ng on column. QC samples were prepared and analyzed three times in one day and on three consecutive days to assess the precision and accuracy of targeted analytes. Precision and accuracy in CD-FBS were analyzed at three different QC concentration levels (LLOQ, medium and high). The intra- and inter-day accuracy were within 15% of the theoretical amount and the precision was less than 15% of CV, except at the LLOQ level, where values still corresponded to U.S. Food and Drug Administration (FDA) Criteria for Bioanalytical Method Validation (Table 1).
Table 1.
Inter- and intra-assay accuracy and precision from determination of nine hydroxy-androgen picolinates.
| T | EpiT | DHEA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Theoretical (pg) | 1.0 | 50 | 500 | 2.5 | 50 | 500 | 2.5 | 50 | 500 |
| Inter-assay | |||||||||
| Determined(± SD, pg) | 1.11 ± 0.06 | 50.5 ± 0.7 | 497 ± 7 | 2.40 ± 0.07 | 52.6 ± 1.5 | 505 ± 6 | 2.47 ± 0.18 | 51.9 ± 2.8 | 503 ± 3 |
| Accuracy (%) | 111 | 101 | 99.5 | 96.0 | 105 | 101 | 98.8 | 104 | 101 |
| Precision (%) | 5.4 | 1.4 | 1.4 | 2.9 | 2.8 | 1.2 | 7.3 | 5.4 | 0.6 |
| Inter-assay | |||||||||
| Determined(± SD, pg) | 1.07 ± 0.08 | 51.1 ± 0.9 | 502 ± 7 | 2.46 ± 0.14 | 52.0 ± 1.6 | 498 ± 10 | 2.36 ± 0.19 | 50.8 ± 1.6 | 505 ± 11 |
| Accuracy (%) | 107 | 102 | 10 0 | 106 | 104 | 99.6 | 94.4 | 102 | 101 |
| Precision (%) | 7.5 | 1.8 | 1.4 | 5.7 | 3.1 | 2.0 | 8.1 | 3.1 | 2.2 |
| DHT | Androsterone | Epiandrosterone | |||||||
| Theoretical (pg) | 2.5 | 50 | 500 | 2.5 | 50 | 500 | 2.5 | 50 | 500 |
| Inter-assay | |||||||||
| Determined(± SD, pg) | 2.24 ± 0.23 | 50.4 ± 0.7 | 498 ± 2 | 2.40 ± 0.30 | 49.4 ± 1.4 | 506 ± 17 | 2.63 ± 0.45 | 52.1 ± 2.6 | 503 ± 8 |
| Accuracy (%) | 89.6 | 101 | 99.6 | 96.0 | 98.8 | 101 | 105 | 104 | 101 |
| Precision (%) | 10 | 1.4 | 0.2 | 12.5 | 2.8 | 3.4 | 17 | 5.0 | 1.6 |
| Inter-assay | |||||||||
| Determined(± SD, pg) | 2.10 ± 0.09 | 50.8 ± 0.6 | 497 ± 2 | 2.56 ± 0.29 | 49.9 ± 0.6 | 497 ± 7 | 2.95 ± 0.18 | 50.8 ± 0.7 | 494 ± 6 |
| Accuracy (%) | 84.0 | 102 | 99.4 | 102 | 99.8 | 99.4 | 118 | 102 | 98.8 |
| Precision (%) | 4.3 | 1.2 | 0.4 | 11 | 1.2 | 1.4 | 6.1 | 1.4 | 1.2 |
| 3α–androstanediol | 3β–androstanediol | 5-androstenediol | |||||||
| Theoretical (pg) | 1.0 | 50 | 500 | 2.5 | 50 | 500 | 1.0 | 50 | 500 |
| Inter-assay | |||||||||
| Determined(± SD, pg) | 1.02 ± 0.16 | 50.0 ± 2.2 | 506 ± 7 | 2.52 ± 0.26 | 50.3 ± 0.9 | 510 ± 4 | 1.07 ± 0.08 | 50.5 ± 2.3 | 505 ± 9 |
| Accuracy (%) | 102 | 100 | 101 | 101 | 101 | 102 | 107 | 101 | 101 |
| Precision (%) | 15.7 | 4.4 | 1.4 | 10.3 | 1.4 | 0.8 | 7.5 | 4.6 | 1.8 |
| Inter-assay | |||||||||
| Determined(± SD, pg) | 0.96 ± 0.03 | 52.6 ± 1.1 | 504 ± 7 | 2.32 ± 0.10 | 49.4 ± 1.1 | 506 ± 3 | 1.01 ± 0.03 | 51.8 ± 0.3 | 501 ± 7 |
| Accuracy (%) | 96.0 | 105 | 101 | 92.8 | 98.8 | 101 | 101 | 104 | 100 |
| Precision (%) | 3.1 | 2.1 | 1.4 | 4.3 | 2.2 | 0.6 | 3.0 | 0.6 | 1.4 |
All data are presented as a mean with standard deviation in pg on column and obtained using matrix (CD-FBS) matched standards. Precision is given as the percentage of coefficient of variation. SD: standard deviation. LLOQ is highlighted in bold (1.0 pg on column: 2.5 ng ng/dL; 2.5 pg on column: 6.25 ng/dL).T: testosterone; EpiT: epitestosterone; DHEA: dehydroepiandrosterone; DHT: 5α-dihydrotestosterone; 3α-androstanediol: 5-androstane-3α, 17β-diol; 3β-androstanediol: 5-androstane-3β, 17β-diol; 5-androstenediol: 5-androstene-3β, 17β-diol.
3.4.3. Measurement of hydroxyandrogens in human serum
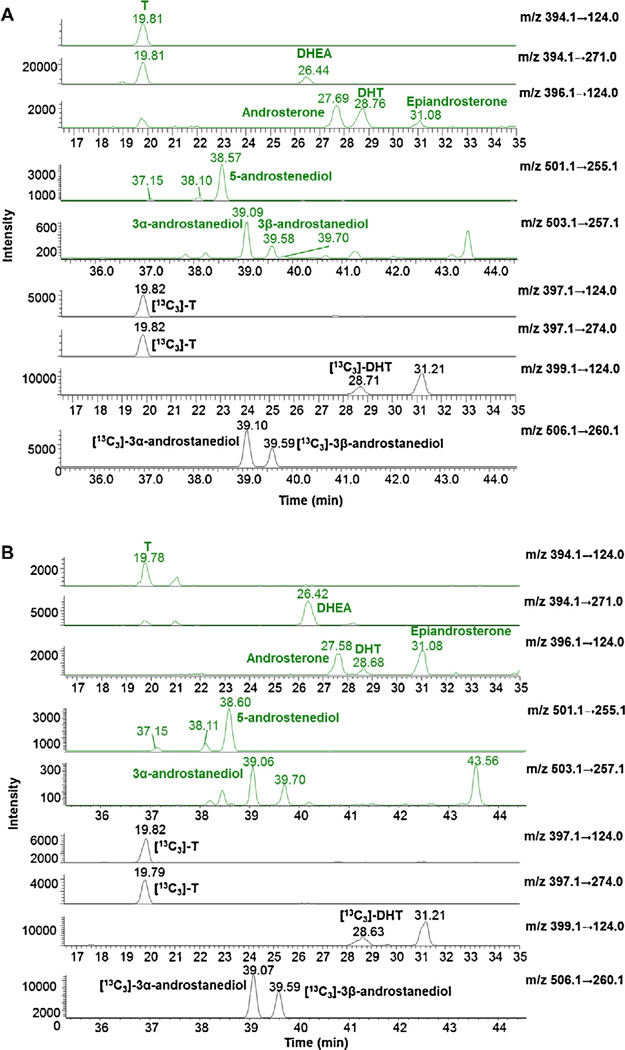
The validated method was applied to commercially available pooled serum from male and female donors for the determination of unconjugated and conjugated hydroxy-androgens (Fig. 6 (text) and Figs. S14–S17 in Ref. [41]). Interference from peaks in human serum that co-elute with the IS picolinates could affect the accuracy of quantitation. By comparing with the control samples without adding IS, a S/N value (>200) showed that the peaks coeluting with IS picolinates had no significant interference in the method (Figs. S18 and S19 in Ref. [41]). Unconjugated hydroxy-androgens, T, DHEA, DHT, androsterone, epiandrosterone, 5-androstenediol were quantitated in serum from both males and females (Table 2). Comparison by gender showed that levels of T (450 ng/dL) and DHT (35.2 ng/dL) were higher (~15-fold and 3-fold) in serum from males than the levels of T (33.0 ng/dL) and DHT (10.8 ng/dL) in serum from females respectively. The level of epiandrosterone (18.5 ng/dL) in serum from females was 2-fold higher than its level (9.1 ng/dL) in serum from males. DHEA, androsterone, 5-androstenediol showed comparable levels in serum from males and females, respectively. 3α-Androstanediol was quantified in serum from males; however, it was not quantifiable in serum from females, because its level was below the LLOQ. 3β-Androstanediol was not quantifiable in serum from males, and was absent (or not detected) in serum from females.
Fig. 6.
Selective reaction monitoring (SRM) ion chromatograms of hydroxy-androgen picolinates of unconjugated androgens obtained by organic extraction of human serum. Ion chromatograms for pooled male’s serum (A) and ion chromatogram for pooled female’s serum (B).
Table 2.
Quantitation of hydroxy-androgens and the corresponding conjugates in serum from male and female.
| Type | Hydroxy-androgen (± SD, ng/dL), in triplicate from pooled serum (200 µL each) |
|||||
|---|---|---|---|---|---|---|
| Male |
Female |
|||||
| Unconjugated | Sulfate (−S) | Glucuronide(−G) | Unconjugated | Sulfate (−S) | Glucuronide(−G) | |
| T | 450 ± 16 | ND | 123 ± 6 | 33.0 ± 2.6 | ND | 36.7 ± 1.1 |
| EpiT | ND | ND | ND | ND | ND | ND |
| DHEA | 118 ± 4 | 119507 ± 1823 | 31.1 ± 1.3 | 97.8 ± 0.8 | 122799 ± 602 | 43.9 ± 0.4 |
| DHT | 35.2 ± 2.6 | ND | ND | 10.8 ± 0.3 | ND | ND |
| Androsterone | 20.7 ± 1.0 | 724 ± 58 | 4868 ± 66 | 14.5 ± 0.8 | ND | 4176 ± 32 |
| Epiandrosterone | 9.1 ± 0.6 | 3886 ± 117 | 3591 ± 63 | 18.5 ± 0.9 | 4578 ± 122 | 3679 ± 17 |
| 5-androstenediol | 50.0 ± 4.7 | 6606 ± 391 | NQ | 43.7 ± 2.1 | 7982 ± 780 | NQ |
| 3α-androstanediol | 6.9 ± 0.7 | ND | 520 ± 35 | NQ | ND | 242 ± 4 |
| 3β-androstanediol | NQ | ND | ND | NQ | ND | ND |
ND: not detected. Limit of detection (LOD, S/N≥3) of T, 5-androstenediol and 3α-androstanediol: ~0.4 pg on column (or 1.0 ng/dL); LOD of EpiT, DHEA, DHT, androsterone, epiandrosterone and 3β-androstanediol: ~1.0 pg on column (or 2.5 ng/dL). NQ: not quantifiable (below LLOQ). SD: standard deviation. Triplicate represents three independent determinations.
DHEA-S, epiandrosterone-S and 5-androstenediol-S were determined in serum from both males and females. Almost no sex difference was observed in their levels indicating their adrenal origin. Androsterone-S was quantified in serum from males and was not detected in serum from females. The other hydroxy-androgen (T, DHT, 3α- and 3β-androstanediol) sulfate conjugates were not detected.
T-G, DHEA-G, androsterone-G, epiandrosterone-G and 3α-androstanediol-G were determined in serum from males and females. Levels of T-G (123 ng/dL) and 3α-androstanediol-G (520 ng/dL) in serum from males were higher than the levels of T-G (36.7 ng/dL) and 3α-androstanediol-G (242 ng/dL) in serum from females. Levels of DHEA-G, androsterone-G, epiandroster-one-G did not show differences between males and females. 5-Androstenediol-G was not quantifiable and 3β-androstanediol-G was not detected in serum from either males or females. EpiT and its conjugates (sulfate and glucuronide) were absent (or not detected) in serum from males or females. Three unknown peaks at retention time of 37.1, 38.1 (ion transition m/z: 501.1 → 255.1) and 39.7 min (ion transition m/z: 503.1 → 257.1) remain to be identified (Fig. 6).
Within each gender group, the level of DHEA-S observed was three orders of magnitude higher than the level of unconjugated DHEA. Levels of androsterone-G, epiandrosterone-S and -G, and 5-androstenediol-S were two orders of magnitude higher than their unconjugated forms. 3α-Androstanediol-G showed a higher level in both male (520 ng/dL) and female (242 ng/dL) when compared to their corresponding levels of unconjugated 3α-androstanediol, respectively. In addition, the level of androsterone-S (724 ng/dL) in serum from males was thirty times higher than free androsterone (20.7 ng/dL).
4. Discussion
Measurement of clinically relevant androgens is necessary for the diagnosis and treatment of CaP and CRPC patients. We developed and validated a SID-LC-ESI–MS/MS method for the simultaneous determination of nine hydroxy-androgens and their conjugates following picolinic acid derivatization. Thus we can potentially detect and quantify 27 analytes in all. This method reliably quantifies 1 pg on column of T, 5-androstenediol and 3α-androstanediol, and 2.5 pg on column of EpiT, DHEA, DHT, androsterone, epiandrosterone and 3β-androstanediol in serum (Table 1).
4.1. Derivatization by picolinic acid and separation of hydroxy-androgen picolinates
Hydroxy-androgen picolinates have a maximum UV absorption at 265 nm, so the efficiency of derivatization and separation of the derivatized hydroxy-androgens were investigated by HPLC-UV/Vis at the beginning of methods development (see Data-in-Brief in Ref. [41]). We found that hydroxy-androgens with two hydroxyl groups (e.g. 3α-androstanediol) could not be completely converted to hydroxy-androgen bis-picolinates by following the reported derivatization conditions [37], which would compromise the quantitation of androgen diols. As such, we optimized the conditions for the derivatization of hydroxy-androgens. Kinetic assays (Figs. S1 and S2 in Ref. [41]) showed that 3α-androstanediol (10 µg) was completely converted to 3α-androstanediol bis-picolinate under the optimized conditions described in Materials and Methods. Mass spectrometric analyses showed that all hydroxy-androgens with mono- or di-hydroxyl groups were converted to one single mono-picolinate or bis-picolinate, respectively (Fig. 2 (text) and Fig. S8 in Ref. [41]). Thus, this reaction condition was used for the derivatization of the targeted hydroxy-androgen mixture.
Another critical consideration is the baseline separation for nine hydroxy-androgen picolinates. Several hydroxy-androgen picolinates with the same molecular weight (e.g.T and EpiT, DHT and androsterone) will share the same ion transitions during SRM mass spectrometric analysis. An incomplete separation of these hydroxy-androgen picolinates will affect the specificity of detection and the accuracy of quantitation. The LC-ESI–MS/MS method coupled with picolinic acid derivatization for quantitation of T and DHT in human serum has been reported previously, with a LLOQ of 0.2 pg on column [37]. However, using an identical chromatographic separation method we found that the isomeric T and EpiT picolinates and the isomeric DHT and androsterone picolinates could not be separated [37], and this would result in an overestimation of T and DHT. By optimizing the separation conditions (Fig. 3 (text) and Fig. S6 in Ref. [41]), complete separation of nine hydroxy-androgen picolinates was accomplished, which improved the specificity of detection and the accuracy of quantitation in human serum.
4.2. Enzymatic synthesis of [13C3]-3α-androstanediol and [13C3]-3β-androstanediol
To accurately quantitate the androgen diols (5-androstenediol, 3α-androstanediol and 3β-androstanediol), it is necessary to use stable isotope labeled internal standards. Deuterium labeled internal standards are usually more cost-effective than [13C] or [15N]- labeled internal standards; however, deuterium labeled internal standards can either cause hydrogen/deuterium exchange during the ionization process or cause a shift in retention time during separation, which affects the accurate quantitation and experimental reproducibility of a method [42]. By comparison, standards containing [13C] or [15N] are more stable and resistant to exchange. Since [13C]- labeled 3α-androstanediol, 3β-androstanediol and 5-androstenediol are not commercially available, we developed enzymatic methods to synthesize [13C3]-3α-androstanediol and [13C3]-3β-androstanediol using the NAD(P)H-dependent reduction of [13C3]-DHT catalyzed by selected recombinant aldo-kteo reductases (Scheme 2). These reactions can be driven to > 99% completion and are highly selective for the stereospecific reduction of DHT under mild acidic conditions (pH 6.0) (Fig. S3 in Ref. [41]) [40,43]. The use of recombinant enzymes reduces the cost, and the synthesis is performed in aqueous solution under ambient conditions with a low percentage of recycled solvent, which is a good application of Green Chemistry for the production of stable isotope labeled steroids. After a one-step extraction, mass spectrometric analysis showed that the synthesized [13C3]-3α-androstanediol and [13C3]-3β-androstanediol did not display any significant interference with either the analysis of targeted hydroxy-androgen picolinates or the [13C3] T/DHT picolinates (Fig. S4 in Ref. [41]).
4.3. Enzymatic hydrolysis of conjugated hydroxy-androgens
The development of LC-Electrospray Ionization (ESI)-MS/MS in the negative ion mode permits androgen conjugates (glucuronide and sulfate) to be detected directly without deconjugation and derivatization, and reduces the time and cost of sample preparation [44,45]. It also allows the separation and detection of regioisomeric conjugates. However, hydrolysis of conjugated androgen using enzymatic digestion is still commonly performed in the laboratories equipped with GC–MS/MS because of the low volatility and low thermal stability of the conjugated analytes [46]. In addition, ion suppression due to matrix effects affects the direct quantitation of steroid conjugates (e.g. accuracy of measurement), as reflected in the determination of steroid glucuronides by LC–MS/MS, and the effect of the matrix on ion suppression and hence quantification can be exacerbated by the lack of the corresponding conjugate internal standards [45,47]. Furthermore, in order to determine both free and conjugated androgens, two or three separate LC–MS/MS methods must be developed and validated, including optimization of separation methods and tuning protocols etc. which could increase the time and cost for methods development [48]. Based on these considerations enzymatic hydrolysis of conjugated steroids was chosen by our group and others to allow the quantitation of both unconjugated and conjugated androgens using a single LC–MS/MS method [33,49–53]. One important factor that affects the quantitation of conjugated androgens is the efficiency of enzymatic digestion, which was validated using DHEA-S and DHEA-G glucuronide.
In order to apply the developed method to measure hydroxy-androgen conjugates, efficient de-conjugation is a prerequisite for an accurate quantitation. The protocol for enzymatic hydrolysis of conjugated androgens has been reported by us for the quantitation of keto-androgen conjugates and was modified in the current method [33]. Method validation was performed using DHEA-S and DHEA-G, and the precision (<15% of the CV) and accuracy within 15% of theoretical value were also performed (Tables S2 and S3 in Ref. [41]). We find it necessary to conduct a dual digestion procedure using both Abalone entrails sulfatase and E.coli β-glucuronidase, since the commercial source of aryl sulfatase is contaminated with β-glucuronidase, which can lead to an overestimate of sulfate conjugates. Determination of hydroxy-androgen sulfate levels was performed by subtracting the amount of hydroxy-androgen glucuronides obtained from the β-glucuronidase digestion alone.
Another consideration is the volume of the aqueous fraction for digestion. DHEA-S is the most abundant androgen sulfate in human serum [54,55]. In order to prevent signal saturation of the mass detector and maintain the values of de-conjugated DHEA from DHEA-S within the linear range of the calibration curve, only 1 µL of the aqueous fraction was used for the dual digestion procedure (sulfatase plus glucuronidase) [33]. However, this low sample volume may affect the determination of other hydroxy-androgen sulfates which are present at lower levels in serum and therefore larger volumes of the aqueous phase for the dual digestion would provide more informative data in future studies.
4.4. Method application to human serum
This method was successfully applied to determine the level of nine hydroxy-androgens and their conjugates in pooled serum from male and female donors, respectively (Table 2). This method can simultaneously determine 5-androstenediol (a precursor of T), 3α-androstanediol involved in the backdoor pathway to DHT, and 3β-androstanediol involved in DHT deactivation. Up until now, few LC–MS/MS methods have been developed for the simultaneous quantitation of 5-androstenediol, 3α-androstanediol and 3β-androstanediol in human samples [19,56]. As shown in Scheme 1, AKR1C3 is required to make potent androgens via several pathways including the little studied conversion of DHEA to 5-androstenediol. The quantitation of 5-androstenediol, 3α-androstanediol and 3β-androstanediol can thus determine the efficacy of new agents that target AKR1C3 or HSD17B6 for inhibition [14]. In addition, although analytical methods for the quantitation of 5-androstenediol have been developed, 5-androstenediol has not been determined in human serum by LC-ESI–MS/MS [57]. By using picolinic acid derivatization, 5-androstenediol was detected in the serum from both males and females (Table 2), which indicates that 5-androstenediol could be an important source of T in prostate and breast.
Hydroxy-androgens undergoing phase II metabolism involving sulfotransferases or glucuronosyltransferases will become either sulfate or glucuronide conjugates [58,59]. These conjugates are usually considered to be inactive, but they can be converted back to unconjugated androgens through enzymatic hydrolysis (e.g. steroid sulfatase), which can in turn regulate androgen pools. Quantitation of androgen-conjugates will help interrogate the role of the conjugates in androgen biosynthesis and elimination and the enzyme activities involved in their interconversion. In comparison to the levels of unconjugated DHEA, androsterone, epiandrosterone, 5-androstenediol and 3α-androstanediol (Table 2), much higher concentrations of conjugated androgens including DHEA-S, androsterone-G, epiandrosterone-S and epiandrosterone–G, 5-androstenediol-S and 3α-androstanediol-G were observed in serum from males and females, which agrees with previous reports [60–64]. Levels of conjugates showed no gender differences except for androsterone-S and 3α-androstanediol-G. Conjugates that show gender independent levels likely result from adrenal steroidogenesis while other sources of androsterone-S and 3α-androstanediol-G may exist. Of the sulfate conjugates seen, serum levels of DHEA-S can remain at sufficiently high levels to feed intratumoral synthesis of T and DHT in CaP after androgen deprivation therapy. It has been proposed that the DHEA-S reservoir that remains after abirater-one treatment contributes to drug resistance [34,35,65]. Serum levels of 5-androstenediol-S were two orders of magnitude lower than the level of DHEA-S, but levels were reported to have a strong correlation with DHEA-S, suggesting that either DHEA-S or DHEA is the source of 5-androstenediol-S [66,67]. Combined levels of androsterone and epiandrosterone-S were the most abundant 5α-reduced hydroxy-androgens in the circulation and have been reported to be decreased in people who took finasteride, an inhibitor of 5α-reductase type 2. It has been proposed that levels of androsterone and epiandrosterone-S could be used as biomarkers of 5α-reductase activity and its response to inhibitors [63,68].
So far, serum levels of androsterone-G and 3α-androstanediol-G have also been proposed as good biomarkers to monitor the activity of 5α-reductase which catalyzes the conversion of T to DHT and predict prostate volume in adult men [69–71]. In prostate, uridine glucuronsyl transferase (UGT 2B15/B17) can catalyze glucuronidation of androsterone, 3α- and 3β-androstanediol, which competes with the conversion of 3α- or 3β-androstanediol to DHT [8,58,72]. Low expression of UGT 2B15/17 could cause a significant increase in DHT levels in CaP cell lines, so activities of UGT 2B15/17 are critical for the deactivation of androgens [58,62,72,73]. Thus, quantitation of androsterone-G and 3α-androstanediol-G in serum is important to investigate the activities of 5α-reductase and UGT. However, 3β-androstanediol and its glucuronide and sulfate were not quantified in serum using our assay (below LOD or LLOQ).
Interestingly, three unknown peaks (retention time: 37.15, 38.10 and 39.70 min) were observed in serum from both males and females (Fig. 6). Glucuronide conjugates of these unknown steroids were also detected at much higher levels (Figs. S14 and S16 in Ref. [41]). Based on the ion transition pairs, these peaks could be the stereoisomers of 5-androstenediol (retention time: 37.15 and 38.10 min) and 3α- or 3β-androstanediol (retention time: 39.70 min). The structures of these unknown hydroxy-androgens will be further investigated by analyzing some standard stereoisomers such as 5-androstene-3β, 17α-diol, 5β-androstane-3α, 17α(β)-diol, 5β-androstane- 3β, 17α(β)-diol [61,74–76].
So far, no single method can measure all androgens of clinical relevance. We have previously developed a SID-LC-ESI–MS/MS method coupled with Girard-T derivatization to quantitate ketoandrogens with high sensitivity and specificity [33]. When this method is combined with the quantitation of hydroxy-androgens with picolinic acid derivatization, specifically androgen diols, the complete androgen metabolome shown in Scheme 1 can be quantified.
5. Conclusions
We have developed a sensitive and specific LC-ESI–MS/MS method to simultaneously quantify nine hydroxy-androgens and their corresponding conjugates in human serum. Determination of accuracy and precision demonstrated that this method could provide reliable and reproducible analyses for hydroxy-androgens in serum. More importantly, this method can simultaneously determine the serum levels of 5-androstenediol, 3α-androstanediol and 3β-androstanediol, which can be used to interrogate the roles of each androgen diol in androgen biosynthesis. The applicability of the method in human serum suggests that it can be used for the diagnosis and investigation of treatment efficacy for CaP and CRPC patients.
Supplementary Material
Acknowledgments
We thank Dr. Mo Chen for her assistance with enzymatic reactions and Dominic Ciccimaro in the laboratory of Prof. Ian A. Blair for his assistance with mass spectrometry. This work was supported by a NCI Grant 1P01-CA163227-03 and P30-ES013508 awarded to TMP, and a NCI Cancer Pharmacology Training Grant R25 CA101871 awarded to DT.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jsbmb.2016.08.001.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics 2015. CA Cancer J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J. Urol. 2002;168:9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 3.Wong YN, Ferraldeschi R, Attard G, de Bono J. Evolution of androgen receptor targeted therapy for advanced prostate cancer. Nat. Rev. Clin. Oncol. 2014;11:365–376. doi: 10.1038/nrclinonc.2014.72. [DOI] [PubMed] [Google Scholar]
- 4.Lonergan PE, Tindall DJ. Androgen receptor signaling in prostate cancer development and progression. J. Carcinog. 2011;10:20. doi: 10.4103/1477-3163.83937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai C, Balk SP. Intratumoral androgen biosynthesis in prostate cancer pathogenesis and response to therapy. Endocr. Relat. Cancer. 2011;18:R175–R182. doi: 10.1530/ERC-10-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat. Rev. Cancer. 2002;2:389–396. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards J, Bartlett JM. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 2: androgen-receptor cofactors and bypass pathways. BJU Int. 2005;95:1327–1335. doi: 10.1111/j.1464-410X.2005.05527.x. [DOI] [PubMed] [Google Scholar]
- 8.Mostaghel EA. Steroid hormone synthetic pathways in prostate cancer. Transl. Androl. Urol. 2013;2:212–227. doi: 10.3978/j.issn.2223-4683.2013.09.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.COU-AA-301 Investigators. de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Flechon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logothetis CJ, Efstathiou E, Manuguid F, Kirkpatrick P. Abiraterone acetate. Nat. Rev. Drug Discov. 2011;10:573–574. doi: 10.1038/nrd3516. [DOI] [PubMed] [Google Scholar]
- 11.Yin L, Hu Q. CYP17 inhibitors-abiraterone, C17,20-lyase inhibitors and multi-targeting agents. Nat. Rev. Urol. 2014;11:32–42. doi: 10.1038/nrurol.2013.274. [DOI] [PubMed] [Google Scholar]
- 12.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Flechon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS, Investigators A. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 13.Menon MP, Higano CS. Enzalutamide, a second generation androgen receptor antagonist: development and clinical applications in prostate cancer. Curr. Oncol. Rep. 2013;15:69–75. doi: 10.1007/s11912-013-0293-9. [DOI] [PubMed] [Google Scholar]
- 14.Penning TM. Androgen biosynthesis in castration-resistant prostate cancer. Endocr. Relat. Cancer. 2014;21:T67–T78. doi: 10.1530/ERC-14-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong CM, Gao AC. Drug resistance in castration resistant prostate cancer: resistance mechanisms and emerging treatment strategies. Am. J. Clin. Exp. Urol. 2015;3:64–76. [PMC free article] [PubMed] [Google Scholar]
- 16.Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, Marck B, Matsumoto AM, Simon NI, Wang H, Chen S, Balk SP. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, Nelson PS, Montgomery RB. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin. Cancer Res. 2011;17:5913–5925. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Lou W, Zhu Y, Yang JC, Nadiminty N, Gaikwad NW, Evans CP, Gao AC. Intracrine androgens and AKR1C3 activation confer resistance to enzalutamide in prostate cancer. Cancer Res. 2015;75:1413–1422. doi: 10.1158/0008-5472.CAN-14-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitamura K, Nakagawa T, Shimada K, Namiki M, Koh E, Mizokami A, Honma S. Identification of dehydroepiandrosterone metabolites formed from human prostate homogenate using liquid chromatography-mass spectrometry and gas chromatography-mass spectrometry. J. Chromatogr. A. 2002;961:97–105. doi: 10.1016/s0021-9673(02)00134-6. [DOI] [PubMed] [Google Scholar]
- 20.Krone N, Hughes BA, Lavery GG, Stewart PM, Arlt W, Shackleton CH. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS) J. Steroid Biochem. Mol. Biol. 2010;121:496–504. doi: 10.1016/j.jsbmb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol. Biomarkers Prev. 2007;16:1713–1719. doi: 10.1158/1055-9965.EPI-06-0765. [DOI] [PubMed] [Google Scholar]
- 22.Moal V, Mathieu E, Reynier P, Malthiery Y, Gallois Y. Low serum testosterone assayed by liquid chromatography-tandem mass spectrometry. Comparison with five immunoassay techniques. Clin. Chim. Acta. 2007;386:12–19. doi: 10.1016/j.cca.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J. Clin. Endocrinol. Metab. 2004;89:534–543. doi: 10.1210/jc.2003-031287. [DOI] [PubMed] [Google Scholar]
- 24.Titus M, Tomer KB. Androgen quantitation in prostate cancer tissue using liquid chromatography tandem mass spectrometry. Methods Mol. Biol. 2011;776:47–57. doi: 10.1007/978-1-61779-243-4_3. [DOI] [PubMed] [Google Scholar]
- 25.Penning TM, Lee SH, Jin Y, Gutierrez A, Blair IA. Liquid chromatography-mass spectrometry (LC-MS) of steroid hormone metabolites and its applications. J. Steroid Biochem. Mol. Biol. 2010;121:546–555. doi: 10.1016/j.jsbmb.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soldin SJ, Soldin OP. Steroid hormone analysis by tandem mass spectrometry. Clin. Chem. 2009;55:1061–1066. doi: 10.1373/clinchem.2007.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SH, Williams MV, DuBois RN, Blair IA. Targeted lipidomics using electron capture atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2003;17:2168–2176. doi: 10.1002/rcm.1170. [DOI] [PubMed] [Google Scholar]
- 28.Ahonen L, Fasciotti M, Gennas GB, Kotiaho T, Daroda RJ, Eberlin M, Kostiainen R. Separation of steroid isomers by ion mobility mass spectrometry. J. Chromatogr. A. 2013;1310:133–137. doi: 10.1016/j.chroma.2013.08.056. [DOI] [PubMed] [Google Scholar]
- 29.Juang YM, She TF, Chen HY, Lai CC. Comparison of CID versus ETD-based MS/MS fragmentation for the analysis of doubly derivatized steroids. J. Mass Spectrom. 2013;48:1349–1356. doi: 10.1002/jms.3300. [DOI] [PubMed] [Google Scholar]
- 30.Athanasiadou I, Angelis YS, Lyris E, Georgakopoulos C. Chemical derivatization to enhance ionization of anabolic steroids in LC-MS for doping-control analysis. Trac.-Trend Anal. Chem. 2013;42:137–156. [Google Scholar]
- 31.Star-Weinstock M, Williamson BL, Dey S, Pillai S, Purkayastha S. LC-ESI-MS/ MS analysis of testosterone at sub-picogram levels using a novel derivatization reagent. Anal. Chem. 2012;84:9310–9317. doi: 10.1021/ac302036r. [DOI] [PubMed] [Google Scholar]
- 32.Marcos J, Pozo OJ. Derivatization of steroids in biological samples for GC-MS and LC-MS analyses. Bioanalysis. 2015;7:2515–2536. doi: 10.4155/bio.15.176. [DOI] [PubMed] [Google Scholar]
- 33.Tamae D, Byrns M, Marck B, Mostaghel EA, Nelson PS, Lange P, Lin D, Taplin ME, Balk S, Ellis W, True L, Vessella R, Montgomery B, Blair IA, Penning TM. Development, validation and application of a stable isotope dilution liquid chromatography electrospray ionization/selected reaction monitoring/ mass spectrometry (SID-LC/ESI/SRM/MS) method for quantification of ketoandrogens inhuman serum. J. Steroid Biochem. Mol. Biol. 2013;138:281–289. doi: 10.1016/j.jsbmb.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mostaghel EA, Nelson PS, Lange P, Lin DW, Taplin ME, Balk S, Ellis W, Kantoff P, Marck B, Tamae D, Matsumoto AM, True LD, Vessella R, Penning T, Hunter Merrill R, Gulati R, Montgomery B. Targeted androgen pathway suppression in localized prostate cancer: a pilot study. J. Clin. Oncol. 2014;32:229–237. doi: 10.1200/JCO.2012.48.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taplin ME, Montgomery B, Logothetis CJ, Bubley GJ, Richie JP, Dalkin BL, Sanda MG, Davis JW, Loda M, True LD, Troncoso P, Ye H, Lis RT, Marck BT, Matsumoto AM, Balk SP, Mostaghel EA, Penning TM, Nelson PS, Xie W, Jiang Z, Haqq CM, Tamae D, Tran N, Peng W, Kheoh T, Molina A, Kantoff PW. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J. Clin. Oncol. 2014;32:3705–3715. doi: 10.1200/JCO.2013.53.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamashita K, Okuyama M, Watanabe Y, Honma S, Kobayashi S, Numazawa M. Highly sensitive determination of estrone and estradiol in human serum by liquid chromatography-electrospray ionization tandem mass spectrometry. Steroids. 2007;72:819–827. doi: 10.1016/j.steroids.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita K, Miyashiro Y, Maekubo H, Okuyama M, Honma S, Takahashi M, Numazawa M. Development of highly sensitive quantification method for testosterone and dihydrotestosterone in human serum and prostate tissue by liquid chromatography-electrospray ionization tandem mass spectrometry. Steroids. 2009;74:920–926. doi: 10.1016/j.steroids.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita K, Takahashi M, Tsukamoto S, Numazawa M, Okuyama M, Honma S. Use of novel picolinoyl derivatization for simultaneous quantification of six corticosteroids by liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. A. 2007;1173:120–128. doi: 10.1016/j.chroma.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Schlegel BP, Jez JM, Penning TM. Mutagenesis of 3 alpha-hydroxysteroid dehydrogenase reveals a push-pull mechanism for proton transfer in aldoketo reductases. Biochemistry. 1998;37:3538–3548. doi: 10.1021/bi9723055. [DOI] [PubMed] [Google Scholar]
- 40.Chen M, Drury JE, Christianson DW, Penning TM. Conversion of human steroid 5beta-reductase (AKR1D1) into 3beta-hydroxysteroid dehydrogenase by single point mutation E120H: example of perfect enzyme engineering. J. Biol. Chem. 2012;287:16609–16622. doi: 10.1074/jbc.M111.338780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zang T, Tamae D, Mesaros C, Wang Q, Huang M, Blair IA, Penning TM. Supporting data for the quantitation of hydroxy-androgens by stable isotope dilution liquid chromatography mass spectrometry. Data in Brief. 2016 doi: 10.1016/j.jsbmb.2016.08.001. (Submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Copper J, Jian H, Johnson D, Dilek I, Sreenivasan U. Evaluation of LCMS/MS scrambling ratios for deuterium-labeled Vitamin D metabolites, steroids and other compounds of clinical significance. Clin. Chem. 2011;57:A1–A235. [Google Scholar]
- 43.Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM. Human cytosolic 3alpha-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3beta-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J. Biol. Chem. 2004;279:10784–10795. doi: 10.1074/jbc.M313308200. [DOI] [PubMed] [Google Scholar]
- 44.Gomes RL, Meredith W, Snape CE, Sephton MA. Conjugated steroids: analytical approaches and applications. Anal. Bioanal. Chem. 2009;393:453–458. doi: 10.1007/s00216-008-2451-8. [DOI] [PubMed] [Google Scholar]
- 45.Penning TM, Lee SHY, Jin Gutierrez A, Blair IA. Liquid chromatography (LC-MS) of steroid hormone metabolites and its applications. J. Steroid Biochem. Mol. Biol. 2010;121:546–555. doi: 10.1016/j.jsbmb.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadanala KC, Lee J, Chung BC, Choi MH. Targeted metabolite profiling: sample preparation techniques for GC–MS based steroid analysis. Mass Spectrom. Lett. 2012;3:04–09. [Google Scholar]
- 47.Bowers LD. Analytical goals in therapeutic drug monitoring. Clin. Chem. 1998;44:375–380. [PubMed] [Google Scholar]
- 48.Labrie F, Belanger A, Belanger P, Berube R, Martel C, Cusan L, Gomez J, Candas B, Chaussade V, Castiel I, Deloche C, Leclaire J. Metabolism of DHEA in postmenopausal women following percutaneous administration. J. Steroid Biochem. Mol. Biol. 2007;103:178–188. doi: 10.1016/j.jsbmb.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 49.Hauser B, Deschner T, Boesch C. Development of a liquid chromatography-tandem mass spectrometry method for the determination of 23 endogenous steroids in small quantities of primate urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008;862:100–112. doi: 10.1016/j.jchromb.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Wang Q, Rangiah K, Mesaros C, Snyder NW, Vachani A, Song H, Blair IA. Ultrasensitive quantification of serum estrogens in postmenopausal women and older men by liquid chromatography-tandem mass spectrometry. Steroids. 2015;96:140–152. doi: 10.1016/j.steroids.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal. Chem. 2007;79:7813–7821. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 52.Dallal CM, Lacey JV, Jr, Pfeiffer RM, Bauer DC, Falk RT, Buist DS, Cauley JA, Hue TF, LaCroix AZ, Tice JA, Veenstra TD, Xu X, Brinton LA. B-FIT Research Group, Estrogen metabolism and risk of postmenopausal endometrial and ovarian cancer: the B approximately FIT cohort. Horm. Cancer. 2016;7:49–64. doi: 10.1007/s12672-015-0237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Falk RT, Brinton LA, Dorgan JF, Fuhrman BJ, Veenstra TD, Xu X, Gierach GL. Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res. 2013;15:R34. doi: 10.1186/bcr3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Charalampopoulos I, Alexaki VI, Tsatsanis C, Minas V, Dermitzaki E, Lasaridis I, Vardouli L, Stournaras C, Margioris AN, Castanas E, Gravanis A. Neurosteroids as endogenous inhibitors of neuronal cell apoptosis in aging. Ann. N. Y. Acad. Sci. 2006;1088:139–152. doi: 10.1196/annals.1366.003. [DOI] [PubMed] [Google Scholar]
- 55.Pru JK. A spectrum of serum dehydroepiandrosterone and sex steroid levels in postmenopausal women. Menopause. 2011;18:11–12. doi: 10.1097/gme.0b013e318200498f. [DOI] [PubMed] [Google Scholar]
- 56.Kim SH, Cha EJ, Lee KM, Kim HJ, Kwon OS, Lee J. Simultaneous ionization and analysis of 84 anabolic androgenic steroids in human urine using liquid chromatography-silver ion coordination ionspray/triple-quadrupole mass spectrometry. Drug Test Anal. 2014;6:1174–1185. doi: 10.1002/dta.1747. [DOI] [PubMed] [Google Scholar]
- 57.Ke Y, Bertin J, Gonthier R, Simard JN, Labrie F. A sensitive, simple and robust LC–MS/MS method for the simultaneous quantification of seven androgen-and estrogen-related steroids in postmenopausal serum. J. Steroid Biochem. Mol. Biol. 2014;144(Pt B):523–534. doi: 10.1016/j.jsbmb.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 58.Barbier O, Belanger A. Inactivation of androgens by UDP-glucuronosyltransferases in the human prostate. Best Pract. Res. Clin. Endocrinol. Metab. 2008;22:259–270. doi: 10.1016/j.beem.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front. Neuroendocrinol. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trabert B, Xu X, Falk RT, Guillemette C, Stanczyk FZ, McGlynn KA. Assay reproducibility of serum androgen measurements using liquid chromatography-tandem mass spectrometry. J. Steroid Biochem. Mol. Biol. 2016;155:56–62. doi: 10.1016/j.jsbmb.2015.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill M, Parizek A, Kancheva R, Duskova M, Velikova M, Kriz L, Klimkova M, Paskova A, Zizka Z, Matucha P, Meloun M, Starka L. Steroid metabolome in plasma from the umbilical artery umbilical vein, maternal cubital vein and in amniotic fluid in normal and preterm labor. J. Steroid Biochem. Mol. Biol. 2010;121:594–610. doi: 10.1016/j.jsbmb.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 62.Tagawa N, Takano T, Fukata S, Kuma K, Tada H, Izumi Y, Kobayashi Y, Amino N. Serum concentration of androstenediol and androstenediol sulfate in patients with hyperthyroidism and hypothyroidism. Endocr. J. 2001;48:345–354. doi: 10.1507/endocrj.48.345. [DOI] [PubMed] [Google Scholar]
- 63.Mitamura K, Setaka M, Shimada K, Honma S, Namiki M, Koh E, Mizokami A. Determination of sulfates of androsterone and epiandrosterone in human serum using isotope diluted liquid chromatography-electrospray ionizationmass spectrometry. Biomed. Chromatogr. 2005;19:796–801. doi: 10.1002/bmc.522. [DOI] [PubMed] [Google Scholar]
- 64.Sanchez-Guijo A, Oji V, Hartmann MF, Traupe H, Wudy SA. Simultaneous quantification of cholesterol sulfate, androgen sulfates, and progestagen sulfates in human serum by LC–MS/MS. J. Lipid Res. 2015;56:1843–1851. doi: 10.1194/jlr.D061499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamae D, Mostaghel E, Montgomery B, Nelson PS, Balk SP, Kantoff PW, Taplin ME, Penning TM. The DHEA-sulfate depot following P450c17 inhibition supports the case for AKR1C3 inhibition in high risk localized and advanced castration resistant prostate cancer. Chem. Biol. Interact. 2015;234:332–338. doi: 10.1016/j.cbi.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitamura K, Nagaoka Y, Shimada K, Honma S, Namiki M, Koh E, Mizokami A. Simultaneous determination of androstenediol 3-sulfate and dehydroepiandrosterone sulfate in human serum using isotope diluted liquid chromatography-electrospray ionization-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003;796:121–130. doi: 10.1016/j.jchromb.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 67.Brind JL. Direct radioimmunoassay of androstenediol-3-sulfate in the serum of normal men. Steroids. 1991;56:320–324. doi: 10.1016/0039-128x(91)90054-y. [DOI] [PubMed] [Google Scholar]
- 68.Lewis JG, George PM, Elder PA. Plasma androsterone/epiandrosterone sulfates as markers of 5 alpha-reductase activity: effect of finasteride in normal men (vol 62 pg 632, 1997) Steroids. 1997;62:795–795. doi: 10.1016/s0039-128x(97)00048-2. [DOI] [PubMed] [Google Scholar]
- 69.Vandenput L, Labrie F, Mellstrom D, Swanson C, Knutsson T, Peeker R, Ljunggren O, Orwoll E, Eriksson AL, Damber JE, Ohlsson C. Serum levels of specific glucuronidated androgen metabolites predict BMD and prostate volume in elderly men. J. Bone Miner. Res. 2007;22:220–227. doi: 10.1359/jbmr.061018. [DOI] [PubMed] [Google Scholar]
- 70.Belanger A, Brochu M, Cliche J. Plasma levels of steroid glucuronides in prepubertal, adult and elderly men. J. Steroid Biochem. 1986;24:1069–1072. doi: 10.1016/0022-4731(86)90361-4. [DOI] [PubMed] [Google Scholar]
- 71.Belanger A, Brochu M, Cliche J. Levels of plasma steroid glucuronides in intact and castrated men with prostatic cancer. J. Clin. Endocrinol. Metab. 1986;62:812–815. doi: 10.1210/jcem-62-5-812. [DOI] [PubMed] [Google Scholar]
- 72.Chouinard S, Barbier O, Belanger A. UDP-glucuronosyltransferase 2B15 (UGT2B15) and UGT2B17 enzymes are major determinants of the androgen response in prostate cancer LNCaP cells. J. Biol. Chem. 2007;282:33466–33474. doi: 10.1074/jbc.M703370200. [DOI] [PubMed] [Google Scholar]
- 73.Chouinard S, Yueh MF, Tukey RH, Giton F, Fiet J, Pelletier G, Barbier O, Belanger A. Inactivation by UDP-glucuronosyltransferase enzymes: the end of androgen signaling. J. Steroid Biochem. Mol. Biol. 2008;109:247–253. doi: 10.1016/j.jsbmb.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 74.Ha YW, Moon JY, Jung HJ, Chung BC, Choi MH. Evaluation of plasma enzyme activities using gas chromatography-mass spectrometry based steroid signatures. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009;877:4125–4132. doi: 10.1016/j.jchromb.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 75.Eriksson H, Gustafsson JA. Excretion of steroid hormones in adults. Steroids in faeces from adults. Eur. J. Biochem. 1971;18:146–150. doi: 10.1111/j.1432-1033.1971.tb01225.x. [DOI] [PubMed] [Google Scholar]
- 76.Schulze JJ, Lorentzon M, Ohlsson C, Lundmark J, Roh HK, Rane A, Ekstrom L. Genetic aspects of epitestosterone formation and androgen disposition: influence of polymorphisms in CYP17 and UGT2 B enzymes. Pharmacogenet. Genom. 2008;18:477–485. doi: 10.1097/FPC.0b013e3282fad38a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.