Chitramuthu and Bateman highlight recent work proposing that the receptor tyrosine kinase EphA2 functions as receptor for the growth factor progranulin.
Abstract
Progranulin is a secreted protein with roles in tumorigenesis, inflammation, and neurobiology, but its signaling receptors have remained unclear. In this issue, Neill et al. (2016. J. Cell Biol. https://doi.org/10.1083/jcb.201603079) identify the tyrosine kinase EphA2 as a strong candidate for such a receptor, providing insight into progranulin and EphA2 signaling.
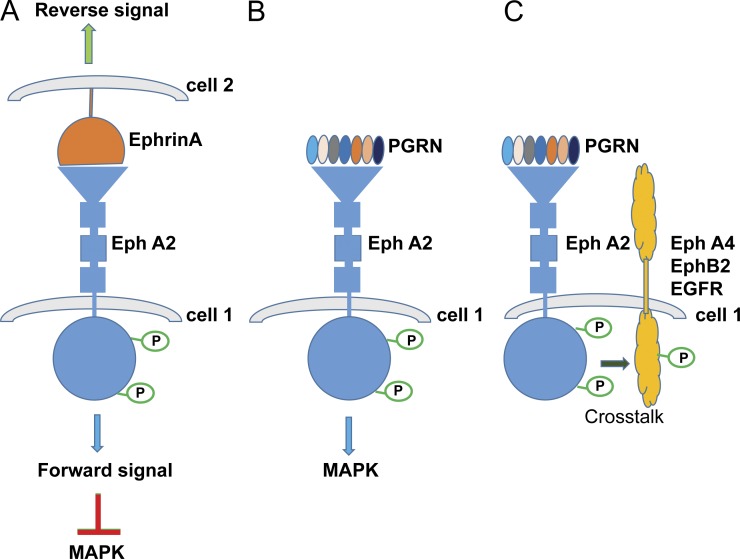
Occasionally, a paper brings together two seemingly unrelated strands of research in a new and illuminating fashion. In this issue, Neill et al. do just that by demonstrating that the EphA2 receptor tyrosine kinase is a functional receptor for the secreted glycoprotein progranulin, a protein that is totally unrelated to the ephrins that are the classic ligands for the Eph receptors. The Eph receptors and progranulin take part in many overlapping biological processes and the possibility raised in this paper that at least some of their activities converge on a progranulin–EphA2 receptor interaction poses many questions for future study. Eph receptors, of which 14 members are known in mammals, are divided into two classes, EphA and EphB, depending on whether they ligate A- or B-type ephrins, both of which are cell surface proteins, although A-type ephrins may also exist as soluble monomers (Lisabeth et al., 2013). The dominant mode of interaction between ephrins and the Eph receptors is juxtacrine with ephrins on the surface of one cell engaging the corresponding Eph receptor in the plasma membrane of a neighboring cell (Fig. 1 A). Ephrin/Eph pairings regulate cell movement, survival, proliferation, morphology, adhesion, and other cellular behaviors and play critical roles in embryonic development, vasculogenesis, angiogenesis, axon guidance, and synaptogenesis (Lisabeth et al., 2013). Particularly pertinent in the context of the study by Neill et al. (2016), EphA2 is important in tumorigenesis and angiogenesis. It is often overexpressed across a broad spectrum of solid tumors and is typically associated with a more aggressive cancer phenotype (Wykosky and Debinski, 2008; Boyd et al., 2014).
Figure 1.
EphrinA and progranulin signal through the EphA2 receptor. (A) Ephrin-A and EphA2 engage in a cell–cell interaction, with forward signaling from EphA2 typically suppressing MAPK signaling. This is likely to be tumor suppressive. (B) Neill et al. (2016) propose that the binding of progranulin (PGRN) with EphA2 results in the activation of MAPK signaling and it is likely that this will prove to be tumorigenic. (C) One consequence of the progranulin–EphA2 interaction is to trigger receptor cross talk with other members of the Eph receptor family and EGFR.
In contrast to the complexity of the Eph/ephrin regulatory system, with its many receptors and ligands, progranulin appears at first remarkably simple. It is composed of a chain of seven and a half disulfide-rich granulin modules (sometimes called epithelin modules) aligned along the protein like beads on a string (Bhandari et al., 1992). In mammals, it is represented by only a single gene (GRN). This simplicity is deceptive. As with Eph/ephrins, progranulin contributes to a striking range of biological processes (Toh et al., 2011). It has growth factor–like properties, stimulating cell proliferation, motility, and survival. It promotes tumorigenesis in vivo, is overexpressed in many cancers, and, as with EphA2, its expression often correlates with more invasive tumors and poor patient outcomes (Zhang and Bateman, 2011). It is angiogenic. Progranulin modulates inflammation, with progranulin knockout mice displaying a highly overblown inflammatory response. The loss of a single allele of GRN in humans results in progressive loss of neurons in the frontal and temporal cortex that manifests clinically as frontotemporal dementia (Petkau and Leavitt, 2014). Surprisingly, the loss of both GRN alleles does not result in worsened frontotemporal dementia, as might be expected, but instead in a neuronal lysosomal storage disease called neuronal ceroid lipofuscinosis (Smith et al., 2012). Given this range of biological properties and their clinical significance, understanding how progranulin transmits its signal to the target cell is of great interest.
Many progranulin binding partners have been identified. Most prominent among them is the lysosome trafficking protein sortilin (Hu et al., 2010), which controls extracellular progranulin levels and tumor necrosis factor-α receptors (Tang et al., 2011), for which, albeit with some controversy, progranulin is reportedly an antagonist. Sortilin also binds to proteoglycans, extracellular matrix proteins, and matrix metalloproteinases (Toh et al., 2011). The problem has been that none of the many proteins to which progranulin binds fully explains its ability to stimulate the signal transduction processes needed for its growth factor–like or neuroprotective actions.
By using a nonbiased antibody-based screen for differential tyrosine phosphorylation of receptor tyrosine kinases, Neill et al. (2016) found that progranulin rapidly increased tyrosine phosphorylation of EphA2 in a human urinary bladder carcinoma cell line (Fig. 1 B). The ability of progranulin to bind EphA2-Fc, which is a soluble fusion protein of the ectodomain of EphA2 with the Fc region of immunoglobulin, was confirmed in solid-phase cell-free assays with a dissociation constant of around 35 nM. Microscale thermophoresis, a technique that measures binding affinities of biomolecules in solution, determined a dissociation constant for the progranulin–EphA2-Fc interaction of 1.2 nM. These numbers are important as they lie within the biological potency range of progranulin when it is assayed as a growth factor for epithelial cancer cells. When added exogenously to living cells, progranulin rapidly colocalized with EphA2, and they were internalized together, whereas soluble EphA2-Fc competed for cell surface binding of progranulin, an interaction that was reduced by lithocholic acid, an inhibitor of the ephrin/eph interaction, as well as by RNAi silencing of EphA2. Progranulin is known to promote the activation of the MAPK and Akt pathways and Neill et al. (2016) found that the depletion of EphA2 in a prostate cancer cell line inhibited the ability of progranulin to stimulate these pathways. In a similar manner, RNA silencing of EphA2 blunted the ability of progranulin to accelerate capillary-like tube formation by endothelial cells in culture. Progranulin regulates its own expression, and the work by Neill et al. (2016) indicates that this also appears to be mediated through EphA2.
Taking all the data together, the case for progranulin acting at the EphA2 receptor is strong (Fig. 1 B), with the receptor binding studies and bioassay data both supporting this conclusion, but there are inevitable caveats and questions. The experiments were conducted on cell lines and, especially given the juxtacrine nature of physiological Eph receptor signaling, it will be important to study how well progranulin and EphA2 signal together in an intact tissue. It is unknown, as yet, whether progranulin interacts with EphA2 in the juxtacrine space, and if it does so, whether it acts in conjunction with ephrins or in opposition to them. Ephrin/Eph signaling is unusual in that in addition to forward signaling through the Eph receptor, there is also reverse signaling from the ligated ephrin back into its own cell (Fig. 1 A; Lisabeth et al., 2013). Therefore, progranulin might influence both ephrin-mediated forward and reverse signaling, either negatively by competition for EphA2 or positively by engaging in a productive three-way interaction involving progranulin, Eph2A, and ephrin. If progranulin does not act in the juxtacrine space, it might engage “free” EphA2, thereby establishing a novel paracrine axis where the EphA2 receptor of one cell is activated not by its immediately adjacent juxtacrine cellular partner but rather by progranulin secreted from a more distant cell (Fig. 1 B).
The role of EphA2 in cancer is complicated (Wykosky and Debinski, 2008). Unlike many other receptor tyrosine kinases, Eph receptors are often tumor suppressive, their binding with ephrins resulting in suppression of Ras-MAPK activity (Lisabeth et al., 2013). However, Neill et al. (2016) suggest that progranulin does the opposite: it stimulates tumorigenic Ras-MAPK and Akt pathways. Despite the tumor-suppressive actions of the ephrin–EphA2 coupling, the overexpression of EphA2 in a context of low ephrin levels is often tumorigenic, and this is thought to be due to EphA2 signaling independently of ephrin (Wykosky and Debinski, 2008; Lisabeth et al., 2013; Boyd et al., 2014). Given that progranulin is overexpressed in many cancers, it is easy to envisage mechanisms through which it might activate a tumorigenic signaling pathway by engaging ephrin-free EphA2 and thereby counteract the tumor suppressive activity ascribed to the ephrin-EphA2 coupling. In many instances, however, ephrin-independent EphA2 signaling is mediated by phosphorylation of a serine residue (S897; Lisabeth et al., 2013) and not through the tyrosine phosphorylation reported by Neill et al. (2016) after progranulin stimulation. Further study into how progranulin fits into the already existing knowledge of ephrin-independent EphA2 signaling in cancers should prove informative.
Neill et al. (2016) showed that phosphorylation of EphA2 by progranulin led to tyrosine phosphorylation of other tyrosine kinases, namely, EphA4, EphB2, and the epidermal growth factor receptor (EGFR) and this was prevented by silencing EphA2. Thus, the engagement of EphA2 by progranulin triggers extensive cross talk among receptor tyrosine kinases (Fig. 1 C), but how this occurs was not reported. Eph receptors are known to form multimeric signaling clusters upon binding to their ephrin ligands. These clusters may include more than one receptor type that, even when not bound to ephrin, become phosphorylated (Wimmer-Kleikamp et al., 2004). Progranulin is secreted as a dimer, and, in principle, up to 14 granulin modules are available per dimer for protein–protein interaction. Speculatively, this might enable it to bridge several receptors and serve as a scaffold for the assembly of a multireceptor signaling complex interaction. The cross talk with EGFR is particularly intriguing. It might amplify the Ras-MAPK and/or Akt signaling originating from the progranulin–EphA2 interaction. It will be interesting to discover to what extent the MAPK activity stimulated by progranulin emanated directly from EphA2 and how much MAPK activity was a result of cross talk with EGFR in these experiments. Studies with EphA2 knockout mice show that EphA2 cooperates with ErbB2, which is a member of the EGFR family, to promote mammary tumorigenesis and metastasis (Brantley-Sieders et al., 2008). This cooperation is a result of augmented activation of the Ras-MAPK and Rho-GTPase signaling pathways. The possibility exists, therefore, that progranulin-mediated cross talk between EphA2 and members of the EGFR family, or other growth factor receptors, will have a significant role in tumor progression. Ephs, including EphA2, are important targets in anti-cancer drug development (Wykosky and Debinski, 2008; Boyd et al., 2014), and, regardless of the exact details, by situating progranulin action within the context of EphA2 signaling, Neill et al. (2016) have opened exciting new routes for future therapeutic intervention in the treatment of EphA2 and progranulin-sensitive cancers.
Progranulin contributes to a great many processes other than tumorigenesis (Toh et al., 2011). The work of Neill et al. (2016) suggests that progranulin exerts its angiogenic activity through EphA2 receptors. Haploinsufficency of GRN results in neurodegeneration. Ephs and ephrins are known for their roles in axon guidance during development by acting as attraction–repulsion signals and regulating migration and adhesion. They have key roles in synaptogenesis and dendritic spine dynamics (Martínez and Soriano, 2005; Lisabeth et al., 2013). Progranulin influences neuronal cell morphology (Petkau and Leavitt, 2014), raising the question of whether a progranulin–EphA2 interaction has a role in mediating progranulin action in the nervous system. In general, however, EphA2 seems less involved in neurodevelopmental regulation than other members of the Eph receptor family. A critical question is, therefore, to what extent the progranulin–EphA2 partnership contributes to progranulin neurobiology and, if it does, whether its disruption in GRN haploinsufficient individuals contributes to their loss of frontotemporal lobe neurons. Mutation of both copies of GRN results in lysosome storage defects (Smith et al., 2012), and it is likely that the neurodegeneration taking place in GRN haploinsufficient patients is also attributable in some measure to disturbances in normal lysosome activity. It is not immediately obvious how EphA2 signaling would contribute to this phenotype. Progranulin knockout mice display a very prominent exaggeration of their inflammatory reactions. Eph receptors have roles in the immune system but it is uncertain whether these are sufficient to understand the inflammatory sequelae of progranulin deletion. In future work, it will be important to probe how much of the diverse biology of progranulin is determined by signaling through EphA2.
Acknowledgments
The authors declare no competing financial interests.
References
- Bhandari V., Palfree R.G., and Bateman A.. 1992. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc. Natl. Acad. Sci. USA. 89:1715–1719. 10.1073/pnas.89.5.1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A.W., Bartlett P.F., and Lackmann M.. 2014. Therapeutic targeting of EPH receptors and their ligands. Nat. Rev. Drug Discov. 13:39–62. 10.1038/nrd4175 [DOI] [PubMed] [Google Scholar]
- Brantley-Sieders D.M., Zhuang G., Hicks D., Fang W.B., Hwang Y., Cates J.M., Coffman K., Jackson D., Bruckheimer E., Muraoka-Cook R.S., and Chen J.. 2008. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J. Clin. Invest. 118:64–78. 10.1172/JCI33154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F., Padukkavidana T., Vægter C.B., Brady O.A., Zheng Y., Mackenzie I.R., Feldman H.H., Nykjaer A., and Strittmatter S.M.. 2010. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron. 68:654–667. 10.1016/j.neuron.2010.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisabeth E.M., Falivelli G., and Pasquale E.B.. 2013. Eph receptor signaling and ephrins. Cold Spring Harb. Perspect. Biol. 5:a009159 10.1101/cshperspect.a009159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez A., and Soriano E.. 2005. Functions of ephrin/Eph interactions in the development of the nervous system: Emphasis on the hippocampal system. Brain Res. Brain Res. Rev. 49:211–226. 10.1016/j.brainresrev.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Neill T., Buraschi S., Goyal A., Sharpe C., Natkanski E., Schaefer L., Morrione A., and Iozzo R.V.. 2016. EphA2 is a functional receptor for the growth factor progranulin. J. Cell Biol. 10.1083/jcb.201603079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkau T.L., and Leavitt B.R.. 2014. Progranulin in neurodegenerative disease. Trends Neurosci. 37:388–398. 10.1016/j.tins.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Smith K.R., Damiano J., Franceschetti S., Carpenter S., Canafoglia L., Morbin M., Rossi G., Pareyson D., Mole S.E., Staropoli J.F., et al. . 2012. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am. J. Hum. Genet. 90:1102–1107. 10.1016/j.ajhg.2012.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Lu Y., Tian Q.Y., Zhang Y., Guo F.J., Liu G.Y., Syed N.M., Lai Y., Lin E.A., Kong L., et al. . 2011. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 332:478–484. 10.1126/science.1199214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh H., Chitramuthu B.P., Bennett H.P., and Bateman A.. 2011. Structure, function, and mechanism of progranulin; The brain and beyond. J. Mol. Neurosci. 45:538–548. 10.1007/s12031-011-9569-4 [DOI] [PubMed] [Google Scholar]
- Wimmer-Kleikamp S.H., Janes P.W., Squire A., Bastiaens P.I., and Lackmann M.. 2004. Recruitment of Eph receptors into signaling clusters does not require ephrin contact. J. Cell Biol. 164:661–666. 10.1083/jcb.200312001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykosky J., and Debinski W.. 2008. The EphA2 receptor and ephrinA1 ligand in solid tumors: Function and therapeutic targeting. Mol. Cancer Res. 6:1795–1806. 10.1158/1541-7786.MCR-08-0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., and Bateman A.. 2011. The glycoprotein growth factor progranulin promotes carcinogenesis and has potential value in anti-cancer therapy. J. Carcinog. Mutagen. S2:001 10.4172/2157-2518.S2-001 [DOI] [Google Scholar]