Synopsis
Organic anion transporters (OATs) encoded by solute carrier 22 (SLC22) family are localized in the epithelia of multiple organs, where they mediate the absorption, distribution, and excretion of a diverse array of negatively charged environmental toxins and clinically important drugs. Alterations in the expression and function of OATs play important roles in intra- and inter-individual variability of the therapeutic efficacy and the toxicity of many drugs. As a result, the activity of OATs must be under tight regulation so as to carry out their normal functions. The regulation of OAT transport activity in response to various stimuli can occur at several levels such as transcription, translation, and posttranslational modification. Posttranslational regulation is of particular interest, because it usually happens within a very short period of time (minutes to hours) when the body has to deal with rapidly changing amounts of substances as a consequence of variable intake of drugs, fluids, or meals as well as metabolic activity. This review article highlights the recent advances from our laboratory in uncovering several posttranslational mechanisms underlying OAT regulation. These advances offer the promise of identifying targets for novel strategies that will maximize therapeutic efficacy in drug development.
Keywords: Membrane transporter, Drug transporter, Posttranslational modification, regulation
1. Introduction
The organic anion transporter (OAT) family of 10 membrane proteins belongs to the SLC22 (solute carrier 22) subfamily of the major facilitator superfamily (MFS); the SLC22 subfamily also includes the organic cation transporters (OCTs) and organic carnitine (zwitterion) transporters (OCTNs)1–5. Computer modeling and biochemical studies revealed that OAT family members have 12 putative membrane spanning segments and multiple sites for posttranslational modifications such as glycosylation, phosphorylation, and ubiquitination6–8. The major physiological functions of OATs are to facilitate the transfer of nutrients or endogenous necessities across the cell membrane, such as endogenous metabolites and signaling molecules. However, the specificity of these transporters is not strictly constrained to their physiological substrates in that exogenous drugs that bear similar structural features can also be recognized and transported. OATs are thus referred to the term of “drug transporters”9.
OAT family members play critical roles in the handling of common drugs (antibiotics, antivirals, diuretics, nonsteroidal anti-inflammatory drugs), toxins (mercury, aristolochic acid), and nutrients (vitamins, flavonoids)1–5. OAT isoforms are expressed in many tissues, including kidney, liver, brain, and placenta. In the kidney, OAT1 and OAT3 utilize a tertiary transport mechanism to move organic anions/drugs across the basolateral membrane into the proximal tubule cells for subsequent exit across the apical membrane into the urine for elimination. This tertiary transport mechanism involves three transport systems: Na+/K+-ATPase actively pumps Na+ out of the proximal tubule cells, establishing an inwardly directed (blood to cell) Na+ gradient. The potential energy stored in this Na+ gradient is used by a second transport protein, Na+/dicarboxylate cotransporter, to import the dicarboxylate α-ketoglutarate (α-KG), maintaining an outwardly directed (cell to blood) α-KG gradient. α-KG then serves as the physiological counterion for the third transport protein in this chain, a dicarboxylate/organic anion exchanger, namely OAT, which translocates organic anion substrates into the cells10–13. Once inside cells, the organic anions then move across the apical membrane into the urine for secretion via other transporters, such as the multidrug resistance-associated proteins MRP2/MRP4, OAT4, and urate transporter 17 (Figure 1).
Figure 1.
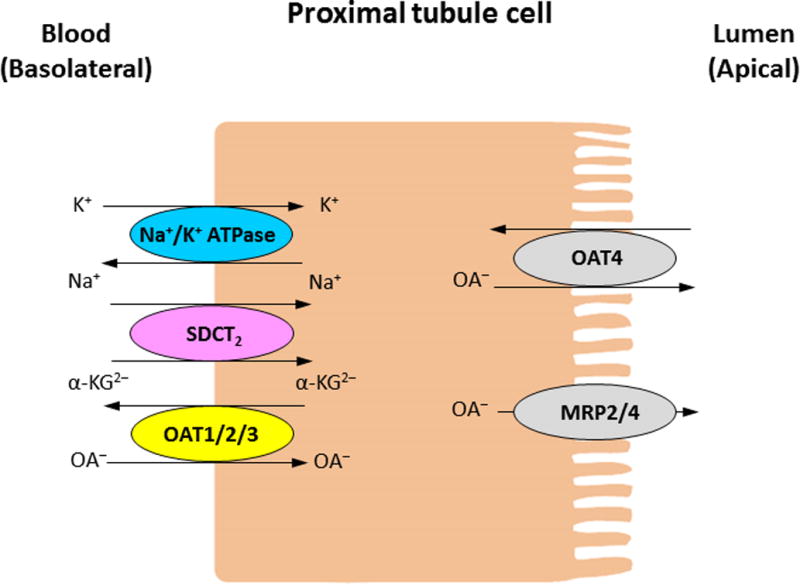
Simplified model of organic anion transport in the kidney proximal tubule cells.
Recent studies from our laboratory using pharmacophore modeling identified two common structural features associated with inhibitors for OAT1 and OAT3, viz., an anionic hydrogen-bond acceptor atom, and an aromatic center separated by ~5.7 Å14. Because of the characteristics of OATs in their wide range of substrate recognition, co-administered drugs may compete for the same transporters, causing serious side effects through drug-drug interaction, and therefore affecting the pharmacokinetics and pharmacodynamics of the drug profile. In recognizing these facts, the International Transporter Consortium in conjunction with the United States Food and Drug Administration (FDA) issued guidance/recommendations for the assessment of OAT- and several other transporter-mediated drug-drug interactions during drug development15,16.
Alterations in OAT expression and function have been observed with several disease states (Table 1), which can have a significant impact on OAT-mediated drug disposition and therefore affect drug efficacy and toxicity. A number of relevant studies have been conducted in animal models. The decreased rOat1 and rOat3 levels and function have been reported in rats with chronic renal failure17,18 and acute renal failure19,20. In a rat model of bilateral ureteral obstruction (BUO), a disease that blocks the urine to pass from kidney to bladder, the function and expression of renal rOat1/3 were also decreased21. In addition, the relationship between OATs and diabetes has been extensively studied and the results seem disparate with species difference22. Studies conducted in type I diabetic rats indicated a decreased function and expression level of rOat3, but not rOat123, but mRNA and protein expression of mOat1, mOat2 and mOat3 were all found to be decreased in type I diabetic mice24. At the same time, type II diabetic rat showed elevated protein expression of rOat225, however, mRNA expression of mOat2 was found to be reduced in type II diabetic mice26,27. In some cases, liver impairment directly affected renal functions with varying OAT expression; however, the reported results were not consistent, which may be due to the use of different methods and models28. Cholestasis rat model induced by administration with alpha-naphthylisothiocyanate (ANIT) or by bile duct ligation (BDL), both showed reduced protein expression for rOat1, while rOat3 was reduced in ANIT model but increased in BDL model28,29.
Table 1.
Disease status and OAT expression
| Disease status | Models | Transporters | RNA/protein | Change | Ref | |
|---|---|---|---|---|---|---|
| Chronic renal failure (CRF) | 5/6 Nephrectomized rat | rOat1, rOat2, rOat3 | mRNA/protein | ↓ | 17,18 | |
| Acute renal failure (ARF) | Rat with renal ischemia | rOat1, rOat3 | mRNA/protein | ↓ | 19,20 | |
| Bilateral ureteral obstruction (BUO) | Rat with bilateral obstruction of the proximal ureters | rOat1, rOat3 | Surface protein | ↓ | 21 | |
| Diabetes | Type I diabetes | streptozotocin (STZ) induced diabetic rat | rOat3 | protein | ↓ | 23 |
| Ins2Akita mouse | mOat1, mOat2 mOat3, mOat5 | mRNA/protein | ↓ | 24 | ||
| Type II diabetes | Ob/Ob (Obese) mouse | mOat2 | mRNA | ↓ | 26 | |
| Db/Db (diabetic) mouse | mOat2 | mRNA | ↓ | 27 | ||
| STZ induced diabetic rat with high-fat diet | rOat2 | protein | ↑ | 25 | ||
| Cholestasis | ANIT induce intrahepatic cholestasis rat | rOat1, rOat3 | mRNA/protein | ↓ | 28 | |
| Bile duct ligation (BDL) rat | rOat1 | protein | ↓ | 29 | ||
| rOat3 | protein | ↑ | ||||
Given the critical roles OATs play in determining the effects of therapeutics and toxic chemicals, understanding the molecular and cellular mechanisms underlying OAT regulation is of clinical and pharmacological importance.
2. Regulation of Organic Anion Transporters OATs
OAT activity must be delicately controlled in order to carry out their normal activity. Like other proteins, OAT activity can be regulated at multiple levels from gene to protein, including transcriptional regulation (when and how often a gene encoding the transporter is transcribed), post-transcriptional regulation (how the primary RNA transcript for the transporter is cleaved or processed), translational regulation (which mRNA for the transporter in the cytoplasm is translated by ribosomes), and post-translational regulation (how the transporter is covalently modified and assembled after its synthesis).
The regulations at the levels of transcription, post-transcription, and translation are usually referred to as long-term or chronic regulation, which happens within hours to days. Long-term regulation usually occurs when the body undergoes massive change, for example, during development or the occurrence of disease. Several excellent review articles have described the long-term regulation of drug transporters1,4,22,30. While post-translational modifications belong to short-term or acute regulation and the time frame ranges from minutes to hours. Short-term regulation often takes place when the body has to deal with rapidly changing amounts of substrates in the case of variable intake of drugs, fluids, ions, meals, and metabolism processes.
Post-translational modification is a process where new functional group(s) are conjugated to the amino acid side chains in a target protein through reversible or irreversible biochemical reactions. The common modifications include glycosylation, phosphorylation, ubiquitination, sulfation, methylation, acetylation, and hydroxylation31. Post-translational modification contributes significantly to the structural complexity and functional diversity of the proteins beyond the coding capacity of the genome, affecting the physical and chemical properties of the transporters, their folding, conformation, distribution, trafficking, stability, and activity.
During the past couple of years, our laboratory has uncovered several mechanisms underlying the post-translational regulation of OATs. We have reported that OAT activity can be regulated by glycosylation, phosphorylation, and ubiquitination-mediated membrane trafficking. Glycosylation is a process, in which a sugar chain is covalently added to the NH2 group on the side chain of an asparagine residue (N-linked glycosylation) of the target protein. Our mutagenesis study combined with biochemical analyses showed that simultaneous elimination of all the glycosylation sites resulted in OAT being trapped in an intracellular compartment, suggesting that glycosylation plays a critical role in the targeting of these transporters to the plasma membrane32,33. Phosphorylation was another important mechanism in the regulation of OAT activity. Phosphorylation is a process in which a negatively charged phosphate group is added to the target protein, consequently influencing the conformation and charge of the target protein, thereby also its activity. We showed that the treatment of OAT-expressing cells with okadaic acid resulted in an increased level of phosphorylation in OAT, which paralleled, in time and concentration, the decrease of OAT1-mediated transport of para-aminohippurate (PAH), a prototypical organic anion34. Okadaic acid is a potent inhibitor of protein phosphatase 1 and protein phosphatase 2A, and it enhances the phosphorylation of many cellular proteins by preventing dephosphorylation35. The detailed description of OAT regulation by glycosylation and phosphorylation has been reviewed previously5,36–38. Here, we will focus on the recent progress made from our laboratory in delineating the molecular mechanism underlying ubiquitination-mediated OAT trafficking and transport activity.
3. Regulation of OAT by Ubiquitination
3.1 Constitutive and Regulated Membrane Trafficking of OAT
The amount of OATs at the cell surface is critical for their drug transport activity. Rather than being statically resident plasma membrane proteins, our laboratory has shown that members of OAT family constitutively internalize from and recycle back to cell surface. The rate of OAT internalization is equal to the rate of OAT recycling being ~ 10% per 5 min39. The dynamic characteristic of OAT rather than a static state would make it more effective for the transporter to initiate trafficking in response to stimuli such as activation of protein kinases, therefore providing quick and efficient fine-tuning in response to environmental changes. We further demonstrated that the activation of protein kinase C (PKC) inhibits OAT transport activity by accelerating the internalization of these transporters from cell surface to EEA1-positive early endosomes, without changes in the rate of OAT recycling. As a result, the amount of OAT at the cell surface is reduced and OAT transport activity is decreased39,40. Prolonged PKC activation results in the degradation of OAT in both proteasome and lysosome40. Evidence from our studies also pointed out that both constitutive and PKC-modulated OAT internalization occurred partly through a dynamin- and clathrin-dependent pathway39,41,42. PKC-induced direct phosphorylation has been reported for other membrane proteins43–46. Interestingly, our results showed that a range of PKC activators failed to elevate the phosphorylation level of OATs under various experimental conditions34. This suggests that direct phosphorylation of OATs is unlikely to be the cause for PKC-induced OAT trafficking and inhibition of OAT activity.
3.2 Ubiquitination of OAT
Modification of receptors and channels by ubiquitin conjugation has emerged as the major regulatory mechanism of internalization, intracellular sorting, and turnover of many membrane proteins47,48. Ubiquitin moieties can be recognized by the components of plasma membrane internalization and endosomal sorting machinery.
Ubiquitin is a highly conserved 8-kDa polypeptide. Ubiquitination occurs in a sequence of three enzymatic steps. The initial step is the formation of a thioester bond between the COOH terminus of ubiquitin and the active cysteine residue of an ubiquitin-activating enzyme (E1). Next, the activated ubiquitin is transferred to an ubiquitin-conjugating enzyme (E2). The E2 enzymes are catalytically similar to E1 in that a thioester bond is formed with ubiquitin. The third and last step in ubiquitination is a reaction catalyzed by an ubiquitin-protein ligase (E3) in which an isopeptide bond between the COOH-terminal glycine of ubiquitin and the amino group of a lysine residue on the target protein is formed. It is the E3 that is responsible for substrate recognition. An ubiquitin molecule itself has seven lysine residues (Lys6, Lys11, Lys27, Lys29, Lys33, Lys48, and Lys63) that can serve as a base for chain elongation. Therefore, a substrate can be modified by different types of ubiquitin conjugation: monoubiquitination (conjugation of one single ubiquitin to one single lysine on the substrate), multiubiquitination (conjugation of several monoubiquitin molecules to multiple lysine residues on the substrate), and polyubiquitination (extended polyubiquitin chain)49,50. In addition, a polyubiquitin chain can bear different linkages such as Lys48 linkage (Lys48 in the ubiquitin serves as a base for chain elongation) and Lys63 linkage (Lys63 in the ubiquitin serves as a base for chain elongation). It has been shown that different type and linkage of ubiquitination have different physiologic outcome for the ubiquitinated substrates51–54. Recent evidences have shown that proteins that are ubiquitinated in the plasma membrane are internalized into early endosomes, where these proteins are then deubiquitinated by deubiquitinating enzymes and recycle back to the plasma membrane. Alternatively, these ubiquitinated proteins can interact with the endosomal sorting complexes required for transport machinery and are sorted to late endosomes, and ultimately, to the lysosomes for degradation50,55. Thus, the balance between ubiquitination, mediated by E3 ligases and deubiquitination, mediated by deubiquitinating enzymes, regulates the abundance of membrane proteins on plasma membrane.
Our investigation of OATs in cultured cells and in rat kidney slices demonstrated that the PKC-induced acceleration of OAT internalization is due to an elevation in OAT ubiquitination42. Ubiquitination-mediated functional regulation has been observed with other members of solute carrier (SLC) family of drug transporters56–58. Mass spectroscopy analysis revealed that the ubiquitination of OAT occurs through the conjugation of a lysine 48-linked polyubiquitin chain to the transporter42. Three important ubiquitin-accepting lysine residues Lys297, Lys303, and Lys315 were identified within the large intracellular loop between transmembrane domains 6 and 7 of OAT159. These lysine residues play a synergistic role in PKC-regulated OAT1 ubiquitination, as mutating any one of the three lysines prevented the ubiquitin conjugation to the other two lysines59. Our unpublished results also indicated that lysine 48-linked polyubiquitin chain plays an important role in the long-term PKC regulation of OAT1 stability.
3.3 Ubiquitination Enzymes for OAT
As mentioned above, ubiquitination involves the sequential reaction catalyzed by a cascade of enzymes, including the ubiquitin-activating enzyme E1, the ubiquitin-conjugating enzyme E2, and the ubiquitin-protein ligase E3. It is the E3 ubiquitin ligase that is responsible for the substrate recognition. There are two major families of E3 ubiquitin ligases: RING (Really Interesting New Gene) and HECT (Homology to E6AP C-Terminus) E3 ligases. Nedd4 (neural precursor cell expressed, developmentally down-regulated 4) is a member of the HECT family of E3 ligases60. It was first identified in a set of genes with developmentally down-regulated expression in the mouse brain61. There are nine members in Nedd4 subfamily, including Nedd4-1 (Nedd4), Nedd4-2 (Nedd4L), Itch, Smurf1, Smurf2, WWP1, WWP2, Nedl1, and Nedl2, of which Nedd4-1 is the prototype62–64. Structurally, Nedd4 family members contain three functional domains: catalytic HECT domain in their carboxyl termini, the C2 (Ca2+/lipid binding) domain at the amino termini, and 2–4 protein-protein-interacting WW domains, each of which contains two highly conserved tryptophan residues65. The Nedd4 ubiquitin ligases bind to their substrates via WW domains that recognize the (L/P)PxY motif on the substrates66,67. However, exception also exists that many Nedd4 family substrates do not contain this motif and interact with the E3 ligases either through other mechanism or indirectly via an adaptor protein containing (L/P)PxY sequences, like the arrestin-related trafficking (ART) proteins68,69.
Many studies suggested that Nedd4 family members are involved in the PKC regulation of membrane transporters, such as dopamine transporter (DAT)70,71, cationic amino acid transporter (CAT-1)72, and glutamate transporter GLT-173. Recent investigation from our laboratory identified ubiquitin ligases Nedd4-1 and Nedd4-2 as important regulators for OAT17. Overexpression of Nedd4-1 or Nedd4-2 enhanced OAT1 ubiquitination, reduced OAT1 expression at the cell surface, and suppressed OAT1 transport activity. Furthermore, we discovered that PKC-dependent changes in OAT1 ubiquitination, expression, and transport activity were significantly blocked in cells transfected with the ligase-dead mutant of Nedd4-2 (Nedd4-2/C821A) or with Nedd4-2-specific siRNA to knockdown endogenous Nedd4-2, but not in cells transfected with the ligase-dead mutant of Nedd4-1 (Nedd4-1/C867S) or with Nedd4-1-specific siRNA to knockdown endogenous Nedd4-1. These observations demonstrated that both Nedd4-1 and Nedd4-2 are important regulators for OAT1 ubiquitination, expression, and function. Yet they play distinct roles, as Nedd4-2 but not Nedd4-1 can block the response of OAT1 to PKC-induced ubiquitination, expression, and transport activity. An interesting question that remains to be addressed is how PKC discriminates between the two very similar ubiquitin ligases? The study carried out by Garcia-Tardon, et al73 showed that PKC activation induces Nedd4-2 phosphorylation and the formation of GLT-1·Nedd4-2 complexes. Our unpublished work also revealed that activation of PKC by phorbol 12-myristate 13-acetate (PMA) induced phosphorylation of Nedd4-2 on serine and/or threonine residues. Thus, we propose that PKC exerts its effect through directly phosphorylating Nedd4-2 but not Nedd4-1. Supporting such a hypothesis is our preliminary analysis, which indicates that Nedd4-2 contains several conserved PKC phosphorylation sites (Ser-325, Ser-327, Thr-352 and Ser-417) that are not present in Nedd4-1. This area of investigation is currently underway in our laboratory.
In addition to OAT1, our recent study also adds OAT3 to the list of membrane transporters that are recognized and ubiquitinated by Nedd4-28. Overexpression of Nedd4-2 enhanced OAT3 ubiquitination, decreased its expression at the cell surface, and inhibited its transport activity, while the ubiquitin ligase-dead mutant Nedd4-2/C821A or Nedd4-2 siRNA had a dominant negative effect. OATs do not have conventional WW domain-interacting (L/P)PXY motifs, yet we observed physical interaction between Nedd4-2 and OATs (OAT17/OAT38/OAT474), suggesting that other unidentified motif(s) or adaptor proteins containing (L/P)PxY sequences must be present to interact with Nedd4-1/Nedd4-2. This is in line with the previous reports that Nedd4-1 has physical association with β2-adrenergic receptor (β2AR)75 and mammalian Na+/H+ exchanger (NHE)76, and Nedd4-2 could directly interact with dopamine transporter (DAT)71, all of which do not contain the conventional WW domain-binding motifs.
3.4 SGK Regulates OATs through Nedd4-2
In addition to PKC, we recently demonstrated that the serum- and glucocorticoid-inducible kinase 2 (sgk2) also regulates OAT activity via Nedd4-274. The sgk family of protein kinases has three isoforms: sgk1, sgk2, and sgk3 that are involved in controlling diverse cellular processes including sodium Na+ homeostasis, osmoregulation, cell survival, and cell proliferation77–82. Sgk1 and sgk3 are ubiquitously expressed, whereas sgk2 expression seems to be restricted primarily to liver, kidney, pancreas, and brain. It has been shown that sgk2 within the mammalian kidney has distinct expression, regulation, and role from sgk1 and sgk383. Unlike sgk1 and sgk3, sgk2 expression in the kidney was not subjected to the regulation by aldosterone. Renal expression characterized by immunocytochemistry localized sgk1 protein to distal convoluted tubule, cortical, and medullary collecting duct, whereas sgk2 protein was highly expressed in kidney proximal tubule cells83. Based on the distinct characteristics of sgk2, we investigated whether OATs, also highly expressed in proximal tubule cells, are regulated by sgk2. We showed that in contrast to the inhibitory effect of PKC, sgk2 stimulated OAT1 and OAT4 transport activity. Such stimulation mainly resulted from an increased cell surface expression of the transporter, kinetically revealed as an increased maximal transport velocity Vmax without significant change in substrate-binding affinity Km74,84. We further showed that the regulation of OAT4 activity by sgk2 was mediated by ubiquitin ligase Nedd4-2. Overexpression of Nedd4-2 enhanced OAT4 ubiquitination and inhibited OAT4 transport activity, whereas overexpression of ubiquitin ligase-dead mutant Nedd4-2/C821A or siRNA knockdown of endogenous Nedd4-2 had dominant negative effects on sgk2 stimulation of OAT4 activity. Our co-immunoprecipitation experiment revealed that sgk2 weakened the association between OAT4 and Nedd4-2. Therefore, sgk2 stimulates OAT4 transport activity by abrogating the inhibition effect of Nedd4-2 on the transporter74.
Studies from other laboratories showed that Sgk1, an isoform of sgk2, stimulates the activity of the epithelial sodium channel ENaC by phosphorylating Nedd4-2 at Ser221, Thr246, and Ser32785,86. Such phosphorylation leads to the inhibition of the Nedd4-2·ENaC interaction and further results in the loss of the ability of Nedd4-2 to ubiquitinate and to suppress ENaC activity87. Our unpublished data showed that sgk2 also enhanced Nedd4-2 phosphorylation possibly at same sites as that in response to sgk1.
3.5 Ubiquitin Ligase Nedd4-2 may Serve as A Central Switch for Protein Kinase-Regulated OAT Expression and Transport Activity
Our published and unpublished work showed that the inhibitory effect of PKC vs. stimulatory effect of sgk2 on OAT expression and transport activity seem to converge on the same molecule: the ubiquitin ligase Nedd4-2. This observation suggests that the opposite regulation of OATs by PKC (negative regulation) and sgk2 (positive regulation) is exerted through the dynamic phosphorylation at distinct sites on Nedd4-2, a central switch, thereby inducing distinct conformational changes of Nedd4-2 and modifying its binding (association/dissociation) to OAT, which leads to a change (promotion/demotion) in OAT ubiquitination, trafficking, and transport activity. Our laboratory is currently testing this novel mechanism (Figure 2), the outcome of which will provide significant insights into our understanding of how protein kinase-Nedd4-2 signaling network transduces diverse physiological stimuli to OAT-mediated drug transport in vivo. Indeed, we and others have previously shown that OAT activity was suppressed by physiological hormones such as angiotensin II and parathyroid hormone via activation of PKC88–90. We therefore speculate that Nedd4-2-mediated OAT ubiquitination plays critical role in these pathways.
Figure 2.
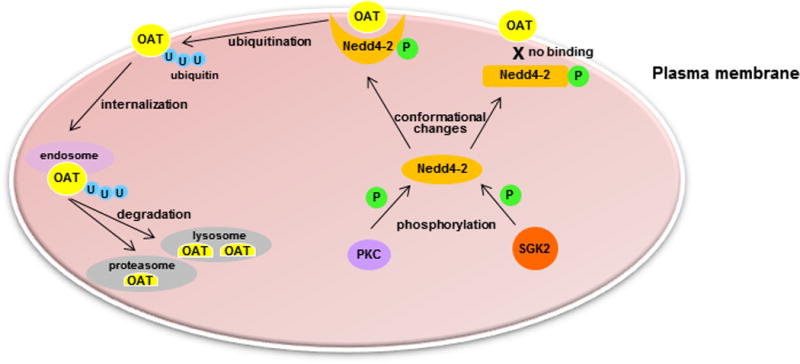
Hypothetical model of ubiquitin ligase Nedd4-2 as a central switch for protein kinase-regulated OAT activity.
4. Conclusion and future perspectives
In this review, we updated recent advances from our laboratory in uncovering the loops and layers of posttranslational mechanisms controlling OAT expression and function (Table 2). Ubiquitination is one of the post-translational modifications that has attracted more and more attention recently, and its role in affecting the trafficking and stability of drug transporters is evident at least for OATs as revealed by our laboratory. Ubiquitination is a highly dynamic process that can be reversed by the action of specialized enzymes known as deubiquitinases, which are a large group of proteases that remove monoubiquitin and polyubiquitin chain from target proteins. Identification of the specific deubiquitinases in the regulation of OATs will be an interesting research area to pursue. In clinics, several anti-cancer drugs target the ubiquitin-proteasome system. Bortezomib (Velcade; Millennium Pharmaceuticals) is the first approved proteasome inhibitor and used for multiple myeloma and mantle cell lymphoma91. Carfilzomib (Kyprolis; Onyx Pharmaceuticals)92 and Marizomib (NPI-0052; Nereus Pharmaceuticals)93 are examples of second generation proteasome inhibitors which provide irreversible proteasome inhibition with sustained response. These drugs are used for the treatment of hematologic tumors, with possible extensive application to solid tumors93. It is therefore interesting to investigate whether these drugs can interfere with the regulation of OATs by ubiquitination. Thorough understanding of the posttranslational modifications of OATs will provide significant insights into the regulation of OAT-mediated drug transport in various physiological and pathophysiological conditions.
Table 2.
Post-translational modifications (PTM) of OATs
| Type of PTM | OATs | Details | Ref. |
|---|---|---|---|
| Phosphorylation | mOat1 | Treatment with phosphatase inhibitor okadaic acid promotes serine/threonine phosphorylation and inhibits mOat1 transport activity. | 34 |
| Glycosylation | mOat1 hOAT1 hOAT4 |
Treatment with N-linked glycosylation inhibitor, tunicamycin, or mutagenesis of all glycosylation sites impaires the targeting of OATs to the plasma membrane and inhibits OAT transport activity. | 32,33,94 |
| hOAT4 | Expression of OAT4 in cells defective in processing of oligosaccharides from mannose-rich type to complex type decreases the affinity of OAT4 for its substrates. | 33 | |
| Ubiquitination | rOat1 hOAT1 hOAT3 |
Treatment of OAT-expressing cells or rat kidney slides with protein kinase C activator, PMA, increased OAT ubiquitination, while PKC inhibitor staurosporin blocked OAT ubiquitination. | 8,42 |
| hOAT1 hOAT3 hOAT4 |
Overexpression of Nedd4-2 increased OAT ubiquitination and decreased OAT activity. siRNA knockdown of endogenous Nedd4-2 abolished PKC-stimulated OAT1/OAT3 ubiquitination, reversed PKC-induced decrease of OAT activity. Knockdown of endogenous Nedd4-2 also abolished sgk2-stimulated OAT4 activity. | 7,8,74 | |
| hOAT1 | LC-MS/MS detected Lys48-linked polyubiquitin peptides conjugated to OAT. Ubiquitin mutant Ub-K48R prevents the formation of polyubiquitin chains, abolishes PKC-stimulated OAT1 ubiquitination, internalization and PKC-induced decrease in OAT expression at the cell expression. | 42 | |
| Mutagenesis of Lys297, Lys303 and Lys315 abolished PKC-stimulated OAT1 ubiquitination. | 59 | ||
| Overexpression of Nedd4-1 increased OAT1 ubiquitination and decreased OAT transport activity. | 7 |
Acknowledgments
This work was supported by grants (to Dr. Guofeng You) from National Institute of General Medical Sciences (R01-GM079123 and R01-GM097000).
Biographies
Da Xu is currently a Ph.D. student in the Department of Pharmaceutics, the Ernest Mario School of Pharmacy, Rutgers University, USA. He obtained his master’s degree in Biochemistry and Molecular Biology from Nankai University, China, in 2010, and bachelor’s degree in Biology from Southern Yangtze University, China, in 2007.
Haoxun Wang is currently a Ph.D. student in the Department of Pharmaceutics, the Ernest Mario School of Pharmacy, Rutgers University, USA. He obtained his bachelor’s degree in Pharmaceutics from China Pharmaceutical University, China in 2012.
Guofeng You is a Distinguished Professor in the Department of Pharmaceutics, the Ernest Mario School of Pharmacy, Rutgers University, USA. Her research interest focuses on the elucidation of the molecular, cellular and functional characteristics of drug/xenobiotic transporters, their implications in human physiology and diseases, and their applications to drug therapy. Dr. You has trained many Ph.D., M.S. students, and postdoctoral fellows, and has published numerous original research articles in the field of drug transport. Dr. You has been serving on several NIH panels, and is on the Editorial Board of Pharmaceutical Research and International Journal of Biochemistry and Molecular Biology. She is also the coeditor for the first and second editions of the book “Drug Transporters – Molecular Characterization and Role in Drug Disposition” (Wiley, 2007 and 2014).
References
- 1.Wang L, Sweet DH. Renal organic anion transporters (SLC22 family): expression, regulation, roles in toxicity, and impact on injury and disease. AAPS J. 2013;15(1):53–69. doi: 10.1208/s12248-012-9413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn SY, Nigam SK. Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol Pharmacol. 2009;76(3):481–490. doi: 10.1124/mol.109.056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelis RM, Wright SH. Renal transport of organic anions and cations. Compr Physiol. 2011;1(4):1795–1835. doi: 10.1002/cphy.c100084. [DOI] [PubMed] [Google Scholar]
- 4.Terada T, Inui K. Gene expression and regulation of drug transporters in the intestine and kidney. Biochem Pharmacol. 2007;73(3):440–449. doi: 10.1016/j.bcp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 5.You G. Structure, function, and regulation of renal organic anion transporters. Med Res Rev. 2002;22(6):602–616. doi: 10.1002/med.10019. [DOI] [PubMed] [Google Scholar]
- 6.Hong M, Tanaka K, Pan Z, Ma J, You G. Determination of the external loops and the cellular orientation of the N- and the C-termini of the human organic anion transporter hOAT1. Biochem J. 2007;401(2):515–520. doi: 10.1042/BJ20061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu D, Wang H, Zhang Q, You G. Nedd4-2 but not Nedd4-1 is Critical for Protein Kinase C-Regulated Ubiquitination, Expression and Transport Activity of Human Organic Anion Transporter 1. Am J Physiol Renal Physiol. 2016 doi: 10.1152/ajprenal.00522.2015. ajprenal 00522 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu D, Wang H, You G. An Essential Role of Nedd4-2 in the Ubiquitination, Expression, and Function of Organic Anion Transporter-3. Mol Pharm. 2015 doi: 10.1021/acs.molpharmaceut.5b00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.You G, Morris ME, Wang B. Drug Transporters: Molecular Characterization and Role in Drug Disposition. Second. Wiley; 2014. [Google Scholar]
- 10.Shimada H, Moewes B, Burckhardt G. Indirect coupling to Na+ of p-aminohippuric acid uptake into rat renal basolateral membrane vesicles. Am J Physiol. 1987;253(5 Pt 2):F795–801. doi: 10.1152/ajprenal.1987.253.5.F795. [DOI] [PubMed] [Google Scholar]
- 11.Pritchard JB. Coupled transport of p-aminohippurate by rat kidney basolateral membrane vesicles. Am J Physiol. 1988;255(4 Pt 2):F597–604. doi: 10.1152/ajprenal.1988.255.4.F597. [DOI] [PubMed] [Google Scholar]
- 12.Pritchard JB, Miller DS. Mechanisms mediating renal secretion of organic anions and cations. Physiol Rev. 1993;73(4):765–796. doi: 10.1152/physrev.1993.73.4.765. [DOI] [PubMed] [Google Scholar]
- 13.Dantzler WH, Wright SH. The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules. Biochim Biophys Acta. 2003;1618(2):185–193. doi: 10.1016/j.bbamem.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Duan P, Li S, Ai N, Hu L, Welsh WJ, You G. Potent inhibitors of human organic anion transporters 1 and 3 from clinical drug libraries: discovery and molecular characterization. Mol Pharm. 2012;9(11):3340–3346. doi: 10.1021/mp300365t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9(3):215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillgren KM, Keppler D, Zur AA, Giacomini KM, Stieger B, Cass CE, Zhang L. Emerging transporters of clinical importance: an update from the International Transporter Consortium. Clin Pharmacol Ther. 2013;94(1):52–63. doi: 10.1038/clpt.2013.74. [DOI] [PubMed] [Google Scholar]
- 17.Monica Torres A, Mac Laughlin M, Muller A, Brandoni A, Anzai N, Endou H. Altered renal elimination of organic anions in rats with chronic renal failure. Biochim Biophys Acta. 2005;1740(1):29–37. doi: 10.1016/j.bbadis.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Naud J, Michaud J, Beauchemin S, Hebert MJ, Roger M, Lefrancois S, Leblond FA, Pichette V. Effects of chronic renal failure on kidney drug transporters and cytochrome P450 in rats. Drug Metab Dispos. 2011;39(8):1363–1369. doi: 10.1124/dmd.111.039115. [DOI] [PubMed] [Google Scholar]
- 19.Matsuzaki T, Watanabe H, Yoshitome K, Morisaki T, Hamada A, Nonoguchi H, Kohda Y, Tomita K, Inui K, Saito H. Downregulation of organic anion transporters in rat kidney under ischemia/reperfusion-induced acute [corrected] renal failure. Kidney Int. 2007;71(6):539–547. doi: 10.1038/sj.ki.5002104. [DOI] [PubMed] [Google Scholar]
- 20.Schneider R, Sauvant C, Betz B, Otremba M, Fischer D, Holzinger H, Wanner C, Galle J, Gekle M. Downregulation of organic anion transporters OAT1 and OAT3 correlates with impaired secretion of para-aminohippurate after ischemic acute renal failure in rats. Am J Physiol Renal Physiol. 2007;292(5):F1599–1605. doi: 10.1152/ajprenal.00473.2006. [DOI] [PubMed] [Google Scholar]
- 21.Villar SR, Brandoni A, Anzai N, Endou H, Torres AM. Altered expression of rat renal cortical OAT1 and OAT3 in response to bilateral ureteral obstruction. Kidney Int. 2005;68(6):2704–2713. doi: 10.1111/j.1523-1755.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 22.Yacovino LL, Aleksunes LM. Endocrine and metabolic regulation of renal drug transporters. J Biochem Mol Toxicol. 2012;26(10):407–421. doi: 10.1002/jbt.21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phatchawan A, Chutima S, Varanuj C, Anusorn L. Decreased renal organic anion transporter 3 expression in type 1 diabetic rats. Am J Med Sci. 2014;347(3):221–227. doi: 10.1097/MAJ.0b013e3182831740. [DOI] [PubMed] [Google Scholar]
- 24.Xu C, Zhu L, Chan T, Lu X, Shen W, Gillies MC, Zhou F. The altered renal and hepatic expression of solute carrier transporters (SLCs) in type 1 diabetic mice. PLoS One. 2015;10(3):e0120760. doi: 10.1371/journal.pone.0120760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowicki MT, Aleksunes LM, Sawant SP, Dnyanmote AV, Mehendale HM, Manautou JE. Renal and hepatic transporter expression in type 2 diabetic rats. Drug Metab Lett. 2008;2(1):11–17. doi: 10.2174/187231208783478425. [DOI] [PubMed] [Google Scholar]
- 26.Cheng Q, Aleksunes LM, Manautou JE, Cherrington NJ, Scheffer GL, Yamasaki H, Slitt AL. Drug-metabolizing enzyme and transporter expression in a mouse model of diabetes and obesity. Mol Pharm. 2008;5(1):77–91. doi: 10.1021/mp700114j. [DOI] [PubMed] [Google Scholar]
- 27.More VR, Wen X, Thomas PE, Aleksunes LM, Slitt AL. Severe diabetes and leptin resistance cause differential hepatic and renal transporter expression in mice. Comp Hepatol. 2012;11(1):1. doi: 10.1186/1476-5926-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu T, Guo X, Meng Q, Wang C, Liu Q, Sun H, Ma X, Kaku T, Liu K. Effect of JBP485 on obstructive jaundice is related to regulation of renal Oat1, Oat3 and Mrp2 expression in ANIT-treated rats. Peptides. 2012;36(1):78–85. doi: 10.1016/j.peptides.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Brandoni A, Anzai N, Kanai Y, Endou H, Torres AM. Renal elimination of p-aminohippurate (PAH) in response to three days of biliary obstruction in the rat. The role of OAT1 and OAT3. Biochim Biophys Acta. 2006;1762(7):673–682. doi: 10.1016/j.bbadis.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Srimaroeng C, Perry JL, Pritchard JB. Physiology, structure, and regulation of the cloned organic anion transporters. Xenobiotica. 2008;38(7–8):889–935. doi: 10.1080/00498250801927435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karve TM, Cheema AK. Small changes huge impact: the role of protein posttranslational modifications in cellular homeostasis and disease. J Amino Acids. 2011;2011:207691. doi: 10.4061/2011/207691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka K, Xu W, Zhou F, You G. Role of glycosylation in the organic anion transporter OAT1. J Biol Chem. 2004;279(15):14961–14966. doi: 10.1074/jbc.M400197200. [DOI] [PubMed] [Google Scholar]
- 33.Zhou F, Xu W, Hong M, Pan Z, Sinko PJ, Ma J, You G. The role of N-linked glycosylation in protein folding, membrane targeting, and substrate binding of human organic anion transporter hOAT4. Mol Pharmacol. 2005;67(3):868–876. doi: 10.1124/mol.104.007583. [DOI] [PubMed] [Google Scholar]
- 34.You G, Kuze K, Kohanski RA, Amsler K, Henderson S. Regulation of mOAT-mediated organic anion transport by okadaic acid and protein kinase C in LLC-PK(1) cells. J Biol Chem. 2000;275(14):10278–10284. doi: 10.1074/jbc.275.14.10278. [DOI] [PubMed] [Google Scholar]
- 35.Haystead TA, Sim AT, Carling D, Honnor RC, Tsukitani Y, Cohen P, Hardie DG. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989;337(6202):78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- 36.You G. Towards an understanding of organic anion transporters: structure-function relationships. Med Res Rev. 2004;24(6):762–774. doi: 10.1002/med.20014. [DOI] [PubMed] [Google Scholar]
- 37.Zhou F, You G. Molecular insights into the structure-function relationship of organic anion transporters OATs. Pharm Res. 2007;24(1):28–36. doi: 10.1007/s11095-006-9144-9. [DOI] [PubMed] [Google Scholar]
- 38.Duan P, You G. Short-term regulation of organic anion transporters. Pharmacol Ther. 2010;125(1):55–61. doi: 10.1016/j.pharmthera.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Q, Hong M, Duan P, Pan Z, Ma J, You G. Organic anion transporter OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway. J Biol Chem. 2008;283(47):32570–32579. doi: 10.1074/jbc.M800298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q, Suh W, Pan Z, You G. Short-term and long-term effects of protein kinase C on the trafficking and stability of human organic anion transporter 3. Int J Biochem Mol Biol. 2012;3(2):242–249. [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Q, Pan Z, You G. Regulation of human organic anion transporter 4 by protein kinase C and NHERF-1: altering the endocytosis of the transporter. Pharm Res. 2010;27(4):589–596. doi: 10.1007/s11095-009-9983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q, Li S, Patterson C, You G. Lysine 48-linked polyubiquitination of organic anion transporter-1 is essential for its protein kinase C-regulated endocytosis. Mol Pharmacol. 2012;83(1):217–224. doi: 10.1124/mol.112.082065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramamoorthy S, Giovanetti E, Qian Y, Blakely RD. Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J Biol Chem. 1998;273(4):2458–2466. doi: 10.1074/jbc.273.4.2458. [DOI] [PubMed] [Google Scholar]
- 44.Li D, Cheng SX, Fisone G, Caplan MJ, Ohtomo Y, Aperia A. Effects of okadaic acid, calyculin A, and PDBu on state of phosphorylation of rat renal Na+-K+-ATPase. Am J Physiol. 1998;275(6 Pt 2):F863–869. doi: 10.1152/ajprenal.1998.275.6.F863. [DOI] [PubMed] [Google Scholar]
- 45.Mehrens T, Lelleck S, Cetinkaya I, Knollmann M, Hohage H, Gorboulev V, Boknik P, Koepsell H, Schlatter E. The affinity of the organic cation transporter rOCT1 is increased by protein kinase C-dependent phosphorylation. J Am Soc Nephrol. 2000;11(7):1216–1224. doi: 10.1681/ASN.V1171216. [DOI] [PubMed] [Google Scholar]
- 46.Ciarimboli G, Koepsell H, Iordanova M, Gorboulev V, Durner B, Lang D, Edemir B, Schroter R, Van Le T, Schlatter E. Individual PKC-phosphorylation sites in organic cation transporter 1 determine substrate selectivity and transport regulation. J Am Soc Nephrol. 2005;16(6):1562–1570. doi: 10.1681/ASN.2004040256. [DOI] [PubMed] [Google Scholar]
- 47.Miranda M, Sorkin A. Regulation of receptors and transporters by ubiquitination: new insights into surprisingly similar mechanisms. Mol Interv. 2007;7(3):157–167. doi: 10.1124/mi.7.3.7. [DOI] [PubMed] [Google Scholar]
- 48.Staub O, Rotin D. Role of ubiquitylation in cellular membrane transport. Physiol Rev. 2006;86(2):669–707. doi: 10.1152/physrev.00020.2005. [DOI] [PubMed] [Google Scholar]
- 49.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 50.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82(2):373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 51.Varghese B, Barriere H, Carbone CJ, Banerjee A, Swaminathan G, Plotnikov A, Xu P, Peng J, Goffin V, Lukacs GL, Fuchs SY. Polyubiquitination of prolactin receptor stimulates its internalization, postinternalization sorting, and degradation via the lysosomal pathway. Mol Cell Biol. 2008;28(17):5275–5287. doi: 10.1128/MCB.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar KG, Barriere H, Carbone CJ, Liu J, Swaminathan G, Xu P, Li Y, Baker DP, Peng J, Lukacs GL, Fuchs SY. Site-specific ubiquitination exposes a linear motif to promote interferon-alpha receptor endocytosis. J Cell Biol. 2007;179(5):935–950. doi: 10.1083/jcb.200706034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bomberger JM, Barnaby RL, Stanton BA. The deubiquitinating enzyme USP10 regulates the post-endocytic sorting of cystic fibrosis transmembrane conductance regulator in airway epithelial cells. J Biol Chem. 2009;284(28):18778–18789. doi: 10.1074/jbc.M109.001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou R, Patel SV, Snyder PM. Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC. J Biol Chem. 2007;282(28):20207–20212. doi: 10.1074/jbc.M611329200. [DOI] [PubMed] [Google Scholar]
- 55.Bonifacino JS, Weissman AM. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu Rev Cell Dev Biol. 1998;14:19–57. doi: 10.1146/annurev.cellbio.14.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warsi J, Hosseinzadeh Z, Elvira B, Pelzl L, Shumilina E, Zhang DE, Lang KS, Lang PA, Lang F. USP18 Sensitivity of Peptide Transporters PEPT1 and PEPT2. PLoS One. 2015;10(6):e0129365. doi: 10.1371/journal.pone.0129365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chothe PP, Swaan PW. Resveratrol promotes degradation of the human bile acid transporter ASBT (SLC10A2) Biochem J. 2014;459(2):301–312. doi: 10.1042/BJ20131428. [DOI] [PubMed] [Google Scholar]
- 58.Liu Z, Sneve M, Haroldson TA, Smith JP, Drewes LR. Regulation of Monocarboxylic Acid Transporter 1 Trafficking by the Canonical Wnt/beta-Catenin Pathway in Rat Brain Endothelial Cells Requires Cross-talk with Notch Signaling. J Biol Chem. 2016;291(15):8059–8069. doi: 10.1074/jbc.M115.710277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li S, Zhang Q, You G. Three ubiquitination sites of organic anion transporter-1 synergistically mediate protein kinase C-dependent endocytosis of the transporter. Mol Pharmacol. 2013;84(1):139–146. doi: 10.1124/mol.113.086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sadowski M, Sarcevic B. Mechanisms of mono- and poly-ubiquitination: Ubiquitination specificity depends on compatibility between the E2 catalytic core and amino acid residues proximal to the lysine. Cell Div. 5:19. doi: 10.1186/1747-1028-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar S, Tomooka Y, Noda M. Identification of a set of genes with developmentally down-regulated expression in the mouse brain. Biochem Biophys Res Commun. 1992;185(3):1155–1161. doi: 10.1016/0006-291x(92)91747-e. [DOI] [PubMed] [Google Scholar]
- 62.Harvey KF, Kumar S. Nedd4-like proteins: an emerging family of ubiquitin-protein ligases implicated in diverse cellular functions. Trends Cell Biol. 1999;9(5):166–169. doi: 10.1016/s0962-8924(99)01541-x. [DOI] [PubMed] [Google Scholar]
- 63.Ingham RJ, Gish G, Pawson T. The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene. 2004;23(11):1972–1984. doi: 10.1038/sj.onc.1207436. [DOI] [PubMed] [Google Scholar]
- 64.Yang B, Kumar S. Nedd4 and Nedd4-2: closely related ubiquitin-protein ligases with distinct physiological functions. Cell Death Differ. 17(1):68–77. doi: 10.1038/cdd.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vina-Vilaseca A, Sorkin A. Lysine 63-linked polyubiquitination of the dopamine transporter requires WW3 and WW4 domains of Nedd4-2 and UBE2D ubiquitin-conjugating enzymes. J Biol Chem. 285(10):7645–7656. doi: 10.1074/jbc.M109.058990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J. 1996;15(10):2371–2380. [PMC free article] [PubMed] [Google Scholar]
- 67.Goulet CC, Volk KA, Adams CM, Prince LS, Stokes JB, Snyder PM. Inhibition of the epithelial Na+ channel by interaction of Nedd4 with a PY motif deleted in Liddle’s syndrome. J Biol Chem. 1998;273(45):30012–30017. doi: 10.1074/jbc.273.45.30012. [DOI] [PubMed] [Google Scholar]
- 68.Rauch S, Martin-Serrano J. Multiple interactions between the ESCRT machinery and arrestin-related proteins: implications for PPXY-dependent budding. J Virol. 85(7):3546–3556. doi: 10.1128/JVI.02045-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nabhan JF, Pan H, Lu Q. Arrestin domain-containing protein 3 recruits the NEDD4 E3 ligase to mediate ubiquitination of the beta2-adrenergic receptor. EMBO Rep. 11(8):605–611. doi: 10.1038/embor.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sorkina T, Miranda M, Dionne KR, Hoover BR, Zahniser NR, Sorkin A. RNA interference screen reveals an essential role of Nedd4-2 in dopamine transporter ubiquitination and endocytosis. J Neurosci. 2006;26(31):8195–8205. doi: 10.1523/JNEUROSCI.1301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vina-Vilaseca A, Sorkin A. Lysine 63-linked polyubiquitination of the dopamine transporter requires WW3 and WW4 domains of Nedd4-2 and UBE2D ubiquitin-conjugating enzymes. J Biol Chem. 2010;285(10):7645–7656. doi: 10.1074/jbc.M109.058990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vina-Vilaseca A, Bender-Sigel J, Sorkina T, Closs EI, Sorkin A. Protein kinase C-dependent ubiquitination and clathrin-mediated endocytosis of the cationic amino acid transporter CAT-1. J Biol Chem. 2011;286(10):8697–8706. doi: 10.1074/jbc.M110.186858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia-Tardon N, Gonzalez-Gonzalez IM, Martinez-Villarreal J, Fernandez-Sanchez E, Gimenez C, Zafra F. Protein kinase C (PKC)-promoted endocytosis of glutamate transporter GLT-1 requires ubiquitin ligase Nedd4-2-dependent ubiquitination but not phosphorylation. J Biol Chem. 2012;287(23):19177–19187. doi: 10.1074/jbc.M112.355909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang H, Xu D, Toh MF, Pao AC, You G. Serum- and glucocorticoid-inducible kinase SGK2 regulates human organic anion transporters 4 via ubiquitin ligase Nedd4-2. Biochem Pharmacol. 2015;102:120–129. doi: 10.1016/j.bcp.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shenoy SK, Xiao K, Venkataramanan V, Snyder PM, Freedman NJ, Weissman AM. Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting, and degradation of the beta2-adrenergic receptor. J Biol Chem. 2008;283(32):22166–22176. doi: 10.1074/jbc.M709668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simonin A, Fuster D. Nedd4-1 and beta-arrestin-1 are key regulators of Na+/H+ exchanger 1 ubiquitylation, endocytosis, and function. J Biol Chem. 2010;285(49):38293–38303. doi: 10.1074/jbc.M110.115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(5):2514–2519. doi: 10.1073/pnas.96.5.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Naray-Fejes-Toth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Toth G. sgk is an aldosterone-induced kinase in the renal collecting duct. Effects on epithelial na+ channels. The Journal of biological chemistry. 1999;274(24):16973–16978. doi: 10.1074/jbc.274.24.16973. [DOI] [PubMed] [Google Scholar]
- 79.Rozansky DJ, Wang J, Doan N, Purdy T, Faulk T, Bhargava A, Dawson K, Pearce D. Hypotonic induction of SGK1 and Na+ transport in A6 cells. American journal of physiology Renal physiology. 2002;283(1):F105–113. doi: 10.1152/ajprenal.00176.2001. [DOI] [PubMed] [Google Scholar]
- 80.Waldegger S, Barth P, Forrest JN, Jr, Greger R, Lang F. Cloning of sgk serine-threonine protein kinase from shark rectal gland - a gene induced by hypertonicity and secretagogues. Pflugers Archiv: European journal of physiology. 1998;436(4):575–580. doi: 10.1007/s004240050674. [DOI] [PubMed] [Google Scholar]
- 81.Leong ML, Maiyar AC, Kim B, O’Keeffe BA, Firestone GL. Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. The Journal of biological chemistry. 2003;278(8):5871–5882. doi: 10.1074/jbc.M211649200. [DOI] [PubMed] [Google Scholar]
- 82.Buse P, Tran SH, Luther E, Phu PT, Aponte GW, Firestone GL. Cell cycle and hormonal control of nuclear-cytoplasmic localization of the serum- and glucocorticoid-inducible protein kinase, Sgk, in mammary tumor cells. A novel convergence point of anti-proliferative and proliferative cell signaling pathways. The Journal of biological chemistry. 1999;274(11):7253–7263. doi: 10.1074/jbc.274.11.7253. [DOI] [PubMed] [Google Scholar]
- 83.Pao AC, Bhargava A, Di Sole F, Quigley R, Shao X, Wang J, Thomas S, Zhang J, Shi M, Funder JW, Moe OW, Pearce D. Expression and role of serum and glucocorticoid-regulated kinase 2 in the regulation of Na+/H+ exchanger 3 in the mammalian kidney. American journal of physiology Renal physiology. 2010;299(6):F1496–1506. doi: 10.1152/ajprenal.00075.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu D, Huang H, Toh M, You G. Serum- and Glucocorticoid-Inducible Kinase Sgk2 Stimulates the Transport Activity of Human Organic Anion Transporters 1 by Enhancing the Stability of the Transporter. International Journal of Biochemistry and Molecular Biology. 2016 [PMC free article] [PubMed] [Google Scholar]
- 85.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na(+) channel cell surface expression. EMBO J. 2001;20(24):7052–7059. doi: 10.1093/emboj/20.24.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Snyder PM, Steines JC, Olson DR. Relative contribution of Nedd4 and Nedd4-2 to ENaC regulation in epithelia determined by RNA interference. J Biol Chem. 2004;279(6):5042–5046. doi: 10.1074/jbc.M312477200. [DOI] [PubMed] [Google Scholar]
- 87.Rotin D, Staub O. Nedd4-2 and the regulation of epithelial sodium transport. Front Physiol. 2012;3:212. doi: 10.3389/fphys.2012.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li S, Duan P, You G. Regulation of human organic anion transporter 1 by ANG II: involvement of protein kinase Calpha. Am J Physiol Endocrinol Metab. 2009;296(2):E378–383. doi: 10.1152/ajpendo.90713.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duan P, Li S, You G. Angiotensin II inhibits activity of human organic anion transporter 3 through activation of protein kinase Calpha: accelerating endocytosis of the transporter. Eur J Pharmacol. 2010;627(1–3):49–55. doi: 10.1016/j.ejphar.2009.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nagai J, Yano I, Hashimoto Y, Takano M, Inui K. Inhibition of PAH transport by parathyroid hormone in OK cells: involvement of protein kinase C pathway. Am J Physiol. 1997;273(5 Pt 2):F674–679. doi: 10.1152/ajprenal.1997.273.5.F674. [DOI] [PubMed] [Google Scholar]
- 91.Chen D, Frezza M, Schmitt S, Kanwar J, Dou QP. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr Cancer Drug Targets. 2011;11(3):239–253. doi: 10.2174/156800911794519752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khan ML, Stewart AK. Carfilzomib: a novel second-generation proteasome inhibitor. Future Oncol. 2011;7(5):607–612. doi: 10.2217/fon.11.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Potts BC, Albitar MX, Anderson KC, Baritaki S, Berkers C, Bonavida B, Chandra J, Chauhan D, Cusack JC, Jr, Fenical W, Ghobrial IM, Groll M, Jensen PR, Lam KS, Lloyd GK, McBride W, McConkey DJ, Miller CP, Neuteboom ST, Oki Y, Ovaa H, Pajonk F, Richardson PG, Roccaro AM, Sloss CM, Spear MA, Valashi E, Younes A, Palladino MA. Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011;11(3):254–284. doi: 10.2174/156800911794519716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuze K, Graves P, Leahy A, Wilson P, Stuhlmann H, You G. Heterologous expression and functional characterization of a mouse renal organic anion transporter in mammalian cells. J Biol Chem. 1999;274(3):1519–1524. doi: 10.1074/jbc.274.3.1519. [DOI] [PubMed] [Google Scholar]


