Abstract
Background
Aromatic L-amino acid decarboxylase (AADC) enzymes catalyze the synthesis of biogenic amines, including the neurotransmitters serotonin and dopamine, throughout the animal kingdom. These neurotransmitters typically perform important functions in both the nervous system and other tissues, as illustrated by the debilitating conditions that arise from their deficiency. Studying the regulation and evolution of AADC genes is therefore desirable to further our understanding of how nervous systems function and evolve.
Results
In the nematode C. elegans, the bas-1 gene is required for both serotonin and dopamine synthesis, and maps genetically near two AADC-homologous sequences. We show by transformation rescue and sequencing of mutant alleles that bas-1 encodes an AADC enzyme. Expression of a reporter construct in transgenics suggests that the bas-1 gene is expressed, as expected, in identified serotonergic and dopaminergic neurons. The bas-1 gene is one of six AADC-like sequences in the C. elegans genome, including a duplicate that is immediately downstream of the bas-1 gene. Some of the six AADC genes are quite similar to known serotonin- and dopamine-synthetic AADC's from other organisms whereas others are divergent, suggesting previously unidentified functions. In comparing the AADC genes of C. elegans with those of the congeneric C. briggsae, we find only four orthologous AADC genes in C. briggsae. Two C. elegans AADC genes – those most similar to bas-1 – are missing from C. briggsae. Phylogenetic analysis indicates that one or both of these bas-1-like genes were present in the common ancestor of C. elegans and C. briggsae, and were retained in the C. elegans line, but lost in the C. briggsae line. Further analysis of the two bas-1-like genes in C. elegans suggests that they are unlikely to encode functional enzymes, and may be expressed pseudogenes.
Conclusions
The bas-1 gene of C. elegans encodes a serotonin- and dopamine-synthetic AADC enzyme. Two C. elegans AADC-homologous genes that are closely related to bas-1 are missing from the congeneric C. briggsae; one or more these genes was present in the common ancestor of C. elegans and C. briggsae. Despite their persistence in C. elegans, evidence suggests the bas-1-like genes do not encode functional AADC proteins. The presence of the genes in C. elegans raises questions about how many 'predicted genes' in sequenced genomes are functional, and how duplicate genes are retained or lost during evolution. This is another example of unexpected retention of duplicate genes in eukaryotic genomes.
Background
Aromatic L-amino acid decarboxylase (E.C. 4.1.1.28, AADC) catalyzes the second enzymatic step in synthesis of the neurotransmitters dopamine and serotonin, which are found in neurons of all animals (Figure 1). Alteration in the normal expression of these transmitters is associated with human neurological disorders such as Parkinson's disease and depression [1,2]. In mammals, AADC is expressed in many tissues beside the nervous system, associated with additional regulatory roles of dopamine and serotonin in a wide range of tissues [3]. In insects, AADC is further required to produce amines for cuticle synthesis and pigmentation [4]. Because of its role in the synthesis of both transmitters, by decarboxylation of L-dopa and 5-hydroxytryptophan, AADC is also known as dopa decarboxylase or 5-hydroxytryptophan decarboxylase (reviewed in [3]). AADC belongs to the α family (subgroup II) of pyridoxal-5'-phosphate (PLP) dependent enzymes. Other subgroup II enzymes include histidine, tyrosine, tryptophan and glutamate decarboxylases [5]; in animals some of these enzymes mediate synthesis of other biogenic amines (e.g., histamine, tyramine, octopamine) and GABA. In mammals and in Drosophila, a single gene encodes the serotonin- and dopamine-synthetic AADC [6,7], although tissue-specific isoforms of the protein are generated by alternative splicing [8,9]. Different genes encode PLP-dependent decarboxylase enzymes for histamine, octopamine and GABA synthesis.
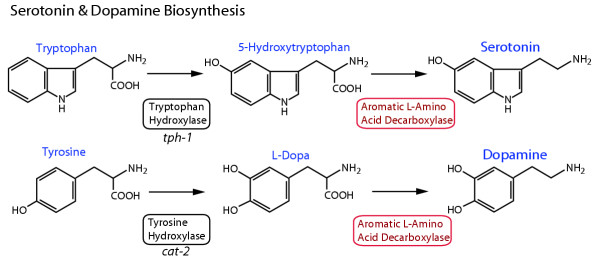
Figure 1.
Serotonin and dopamine biosynthetic pathways. Serotonin and dopamine are synthesized from the aromatic amino acids tryptophan and tyrosine, respectively. The first and rate-limiting step in synthesis is carried out by a neurotransmitter-specific aromatic amino acid hydroxylase enzyme, either tryptophan or tyrosine hydroxylase. In C. elegans, these genes are encoded by the tph-1 and cat-2 genes, respectively [19, 25]. The second synthetic step for both neurotransmitters shares the aromatic L-amino acid decarboxylase (AADC) enzyme, which has a relatively broad substrate specificity, and is also known as 5-hydroxytryptophan decarboxylase or dopa decarboxylase.
In the nematode Caenorhabditis elegans, serotonin is expressed in at least nine neurons in the hermaphrodite and nineteen in the male; dopamine is found in eight neurons in the hermaphrodite and fourteen in the male [10]. By examining the behavior of worms in which specific neurons have been ablated and examining mutants lacking serotonin and/or dopamine, we have learned that serotonin is involved in behaviors including egg laying [11-13], pharyngeal pumping [14,15], male mating [16], and experience-dependent regulation of locomotion [17,18]. Serotonin-deficient mutants also display abnormalities in entry into the diapause-like dauer stage and in fat storage, mediated via an insulin-related signaling pathway [19,20]. Dopamine plays roles in male mating [21], in regulating locomotion via mechanosensation [17,22], and in foraging behavior [23].
Identification of genes involved in neurotransmitter synthesis and related aspects of signaling in C. elegans was greatly accelerated by genomic sequencing, which was essentially completed in 1998 [23,24]. For genes identified originally by mutants via a traditional genetic approach, a candidate gene approach often allowed rapid confirmation of a gene's identity; for predicted genes identified from the genomic sequence by homology, a reverse genetic approach has been taken. Many components of the serotonin and dopamine synthesis and transport pathways in C. elegans have now been identified by these traditional and reverse genetic approaches, including tyrosine hydroxylase (cat-2; [25]), tryptophan hydroxylase (tph-1; [19]), serotonin reuptake transporter (mod-5; [26]), dopamine reuptake transporter (dat-1; [27]) and vesicular monoamine transporter (cat-1; [28]). Postsynaptic components have also been identified, including various receptors [29-32] and intracellular G protein signaling components [33-36].
Further analysis of gene function, regulation and evolution in C. elegans is being facilitated by genomic sequencing of related nematodes. A whole genome shotgun sequence of Caenorhabditis briggsae was recently completed; the sequence is estimated to be 98% complete [37]. The divergence of C. briggsae and C. elegans is estimated between 80 – 110 million years ago [37,38], although it should be noted that these estimates lack a fossil record to anchor the dates [39]. This is considered to be a favorable evolutionary distance to identify conserved non-coding regulatory sequences, although the sequences from only two orthologous genes from related species is often inadequate to identify such sequences unambiguously. Genomic sequencing is planned or underway of three additional congeneric relatives of C. elegans that are more closely related than C. briggsae, which will enhance our ability to analyze the genes of C. elegans. We have used genomic sequences of both C. elegans and C. briggsae to help identify and characterize another component of the serotonin and dopamine signaling systems – the bas-1 gene – and to examine the evolution of this and related genes.
The bas-1 [biogenic amine synthesis abnormal] mutant is serotonin- and dopamine-deficient, and displays several behavioral abnormalities [12,16,17]. Unlike wildtype and other serotonin-deficient mutant worms, bas-1 mutants are unable to convert exogenous 5-hydroxytryptophan (5HTP) into serotonin (5-hydroxytryptamine, 5HT), as assessed by serotonin antiserum staining. Because of this phenotype, we have previously proposed that the bas-1 gene likely encoded the AADC enzyme of C. elegans [16].
Results
Rescue of the bas-1 mutant with an AADC-homologous sequence
The bas-1 gene maps to chromosome III, between dpy-17 and unc-32. When this region was sequenced by the C. elegans Genome Sequencing Consortium, two AADC-homologous predicted genes, designated C05D2.4 and C05D2.3, were found to be located close together on a single cosmid, C05D2 (Fig. 2A). This suggested that one (or both) of these sequences comprised the gene mutated in bas-1 mutants. To test this hypothesis, we injected bas-1 mutants with the cosmid C05D2 plus rol-6 (dom) plasmid DNA as a co-injection marker. We isolated transgenic Roller progeny (expressing the rol-6 (dom) marker phenotype) of the injected worm and propagated strains that transmitted the marker, then tested these worm strains using serotonin antibody staining. We found that 3 of 3 independent Roller transgenic lines were rescued for serotonin immunoreactivity, confirming that the bas-1 gene was located within this 46 kb of genomic DNA (Fig. 2B). We then injected plasmid subclones of C05D2, each of which still contained both the predicted C05D2.4 and C05D2.3 genes. A 15.1 kb plasmid subclone (C05D2XN) also rescued bas-1 mutants (n = 4/4), as did smaller subclones of C05D2XN, including an 11.3 kb subclone (pCL3001, n = 11/11) and an 8.8 kb subclone (pCL7001, n = 1/1). These results confirm that at least one of AADC-homologous genes likely corresponds to the bas-1 gene.
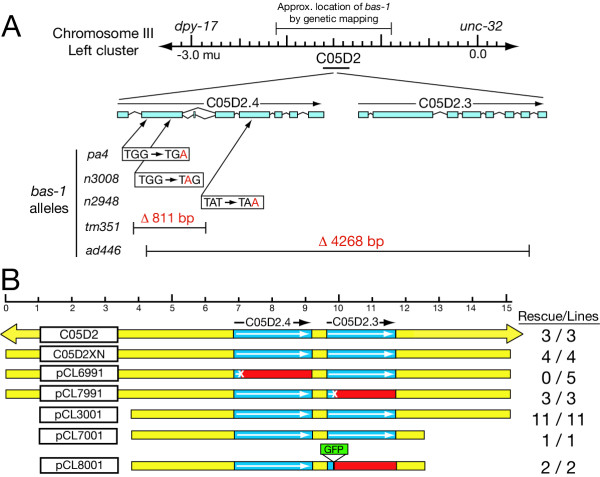
Figure 2.
Molecular genetics and transformation rescue of bas-1. (A) Genetic and physical map of bas-1 region and bas-1 mutant alleles. Locations and extent of mutations for each bas-1 allele are shown to scale with respect to C05D2.4 and C05D2.3 coding sequences, based on known splicing patterns or Genefinder predictions. Exons are indicated by rectangular bars; an alternatively spliced 27 bp exon is also indicated after exon 2 in C05D2.4. Four of five bas-1 mutants affect only C05D2.4; ad446 is a larger deletion removing most coding sequence of both C05D2.4 and C05D2.3. (B) Genomic DNA constructs that rescue or fail to rescue bas-1 mutants. Constructs are shown to scale (top), based on the 15.1 kbp insert of the plasmid clone C05D2XN; construct names are indicated in the box on the left. The cosmid C05D2 is larger, as indicated by the arrows. Clones below are subclones or modifications of C05D2XN. Coding regions for the two predicted AADC genes C05D2.4 and C05D2.3 are indicated by the blue boxes; intergenic regions are shown in yellow. C05D2XN upstream of C05D2.4 contains two other predicted genes, one complete (C05D2.8) and one partial (C05D2.5). There are no predicted genes in the 3 kb downstream of C05D2.3. In the constructs with the least upstream sequence, only a portion of C05D2.8 remains. Constructs mutated to introduce premature stop codons are indicated with a X in the coding sequence, and red downstream of the introduced stop codon. The construct pCL8001 has a GFP gene inserted in a manner that would inactivate the C05D2.3 gene, so is comparable to the pCL7991 construct. [No GFP expression was seen in the C05D2.3::GFP reporter construct lines.]
To determine which of the two predicted AADC sequences was needed to rescue bas-1 mutants, we prepared two constructs from C05D2XN, one mutated in C05D2.4, the other in C05D2.3 (Fig. 2B). In each case, a mutation was created by eliminating a unique restriction site early in the predicted coding region, creating a frameshift resulting in premature stop codons. We found that constructs mutated in C05D2.3 when injected rescued serotonin immunoreactivity in bas-1 mutants (n = 3/3), whereas the construct mutated in C05D2.4 failed to rescue (n = 0/5 rescued). A construct containing a GFP gene inserted into the C05D2.3 coding sequence (and disrupting the gene) also rescued bas-1 mutants (n = 2/2). In Roller transgenic lines lacking rescue, we confirmed the presence of the injected construct by PCR. Therefore, an intact C05D2.4 gene is necessary to rescue bas-1 mutants, whereas the C05D2.3 gene is not. In all rescued transgenic lines, we saw the complete set of known serotonergic neurons, although not necessarily all cells in every animal – mosaicism from somatic loss of extrachromosomal DNA is expected in these transgenics. This result suggests that no critical cell-specific regulatory sequences were missing from even the smallest construct we injected.
To confirm further that C05D2.4 is the bas-1 gene, we identified the mutations in four bas-1 mutant alleles; we also examined the phenotypes of deletion mutants in C05D2.4 and C05D2.3 generated by the C. elegans Gene Knockout Consortium (GKC). We found that the bas-1 alleles pa4, n2948, and n3008 contained point mutations in C05D2.4 coding sequence resulting in premature stop codons (Fig. 2A). We found that the original bas-1 allele (ad446) had a 4268 bp deletion from the second exon of C05D2.4 to the final intron of C05D2.3; therefore, ad446 is a knockout of both predicted genes. We examined the phenotypes of GKC-generated deletion mutants in each predicted gene. The C05D2.4 knockout (tm351) removes the entire predicted second exon. We found that both tm351 homozygotes and tm351/ad446 worms were deficient in serotonin immunoreactivity. On the other hand, a knockout of C05D2.3 (ok703) is wildtype for serotonin staining. Therefore, tm351 is a fifth mutant allele of the bas-1 gene, and C05D2.4 corresponds to the gene bas-1.
Transcripts from the bas-1 gene and the predicted BAS-1 protein
To continue our characterization of the bas-1 gene, we isolated cDNAs using RT-PCR; we also obtained cDNA clones from the C. elegans EST/Transcriptome project (courtesy of Yuki Kohara) and the ORFeome project [40]. We found that C05D2.4/bas-1 cDNAs are trans-spliced to SL1 just 15 nucleotides upstream of the predicted translation start site. The consensus sequence from our clones and others we examined predicts a 514 amino acid, 58 kDa protein product (Fig. 3A). This is similar in size to other known AADC/dopa decarboxylase proteins such as those of Drosophila (510 aa) and human (480 aa). The predicted protein possesses a conserved lysine PLP binding site at residue 343, and has other amino acids identical to those shown to be essential for rat AADC function [5,41,42]. A number of possible phosphorylation sites can be predicted, including three serines and one tyrosine that are conserved in all known AADCs and HisDCs (Fig. 3A).
Figure 3.

C. elegans bas-1 cDNA sequences. (A) Consensus cDNA sequence and translation for C05D2.4/bas-1, based on the most common splice form. Nucleotide numbering is shown on the left side and amino acid numbering on the right side of the sequence. SL1 spliced leader sequence is overlined in blue in the top line. Intron locations are indicated with blue arrowheads; the phase at all intron locations is 0 (between codons; see also Fig 6). The conserved lysine (K) pyridoxal 5-phosphate binding site at amino acid 343 is boxed in black. Red amino acids in the predicted Bas-1 protein (T286, D292, H309, D311, S336, K343, K357, V378, R393, and W401) are identical to those shown to be essential for rat AADC function [5, 41, 42]. Possible phosphorylation sites that are absolutely conserved in known DDC and HisDC proteins are boxed in green (Y37, S149, S229, S230). The polyadenylation signal in the final line is underlined. Mutations found in bas-1 alleles are indicated with the allele designation and the changed base over the wildtype sequence. The allele tm351 deletion, which removes the entire second exon, is indicated by a red line over the missing sequence. The wildtype cDNA sequence shown is consistent with our RT-PCR clones (primers SL1B, C05D2-B), those we sequenced from the ORFeome project (from predicted translation start to stop), and C. elegans EST project 'YK clones' ends (used to determine the 3' end, including the site of polyadenylation). (B) Alternatively spliced 27 bp exon and surrounding genomic sequence in C. elegans and C. briggsae. The additional exon is found in a fraction of C. elegans bas-1 transcripts, and the sequence is conserved in genomic sequence from C. briggsae as shown. [We did not isolate a cDNA containing this exon among our C. briggsae bas-1 cDNAs, S. DePaul & C. Loer, unpublished results.] Predicted translation of the exon is shown above or below the nucleotide sequence. Consensus splice signals are overlined in blue, and identical nucleotides are indicated by vertical lines between the two nucleotide sequences.
We found two splice variants different from the Genefinder-predicted cDNA described above, which was the predominant form. About 20% of clones we sequenced had a 27 bp microexon inserted between the predicted exons 2 and 3 (Fig. 3B). The 27 bp microexon is found within what is the second intron in the more commmon splice form. This intron is not conserved among other AADCs, and is inserted within a region of the BAS-1 protein that is not conserved among AADC proteins. Modeling of BAS-1 protein structure, based on a recent crystal structure of porcine DDC [43], indicates that this region is located at the surface of the protein where it would not interfere with the conserved enzymatic function of the protein (data not shown). We observed that this additional exon is conserved in the C. briggsae ortholog of bas-1 in genomic sequence (Fig. 3B), although we did not isolate any splice variants with this exon among C. briggsae bas-1 cDNAs we sequenced (see also below). We found a single clone that used an alternative splice acceptor 60 bp upstream of the usual splice site for exon 3; this alternate splice introduces a premature stop codon in the coding sequence. This transcript may be a rare, aberrant splice form without functional significance.
Expression of a bas-1::GFP reporter fusion in transgenic worms
We examined the pattern of expression of a GFP reporter construct with ~4500 bp upstream of the predicted bas-1 translation start site and an in-frame fusion with the 2nd exon, injected with rol-6(dom) plasmid into wild-type worms (kindly provided by Ian Hope). Two independent transgenic Roller lines with extrachromosomal arrays had the same pattern of expression. The reporter was reliably expressed in several easily identified cells including the paired serotonergic neurons NSM and HSN and the dopaminergic PDE postdeirid sensory neurons (Fig. 4). NSM processes studded with varicosities were apparent in the isthmus of the pharynx labeled with GFP (Fig. 4A,4D). The egg-laying neuron HSN normally expresses serotonin only in adulthood, and we found the reporter to be expressed in adult hermaphrodites and sometimes late L4 larvae. Often the HSN processes were apparent extending to vulval muscles and anteriorly within the ventral nerve cord (Fig. 4C,4F). We saw a cell we identified as PDE, which is born during L2, only after this stage. In some worms, we saw a PDE process and dendrite, confirming our identification (Fig. 4F).
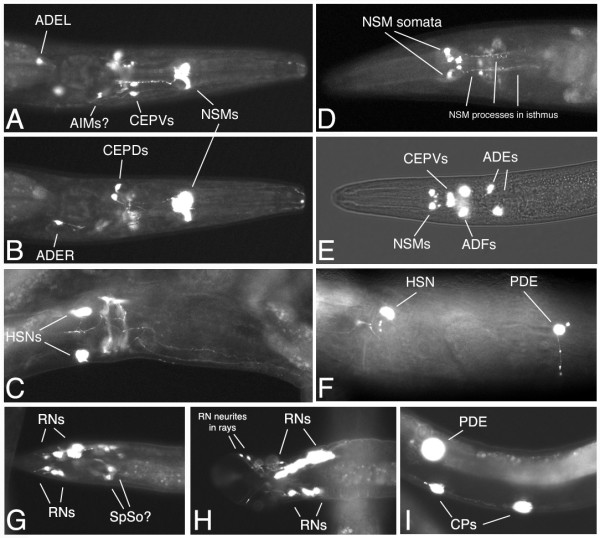
Figure 4.
Expression pattern of a bas-1::GFP reporter fusion in transgenic Roller worms. (Panels A-C are from the same adult hermaphrodite. Ventral is down and anterior to the right.) A. Ventral, slightly oblique view of the head, showing NSMs, CEPDs, ADEL and likely AIMs. B. Same head, higher (more dorsal) focal plane, showing CEPDs and ADER. C. Photomontage showing ventral oblique view of HSNs and their processes in the ventral nerve cord; note also apparent labeling of muscles associated with the vulva. A second worm is immediately adjacent above, obscuring the edge of the worm shown. (Panels D-F: Anterior is to the left.) D. Adult hermaphrodite head, ventral view, chosen to show the characteristic highly varicose processes of the NSM cells within the isthmus of the pharynx. E. Larval head, ventral view with fluorescence and brightfield. This clearly shows the location of the NSM somata in the ventral pharynx, anterior bulb; it also shows the serotonergic ADF neurons not seen in A, B. CEPDs would be seen in a dorsal focal plane in this worm. F. Adult hermaphrodite lateral view of body wall. Ventral is down. Shows HSN and PDE; note PDE process extending ventrally toward the ventral nerve cord and dendrite extending dorsally into postdeirid sensillum. Twisting of the body axis associated with Roller phenotype makes HSN and PDE somata appear at the same lateral level when HSN is actually located sublateral and PDE subdorsal; twisting also takes ventral nerve cord out of plane of focus in the right of the panel. (Panels G – I are from males; anterior is to the right.) G. Late L4 male tail showing ray neurons (RNs) with processes extending into the rays. In some males we saw spicule cell staining likely belonging to spicule socket cells (SpSo). Ventral, slightly oblique view. H. Adult male tail showing RNs and their neurites in rays 7 and 9 on the right side, view ventral, slightly oblique. I. Male-specific ventral nerve cord motoneurons CP5 and CP6, the CP neurons most commonly expressing the transgene. The PDE soma in the lateral body wall is out of the plane of focus.
The bas-1::GFP reporter was also expressed in other neurons in the head, around the nerve ring. We believe that all of these cells are known serotonergic and dopaminergic neurons. It was somewhat more difficult, however, to be certain about these identifications since we saw few processes, and even when present we could not always unambiguously associate a process with a particular neuronal soma. Nevertheless, the reporter was expressed in probable dorsal and ventral cephalic sensilla neurons CEPD and CEPV; we sometimes observed as many as four processes extending to the tip of the nose (Fig 4A,4B,4E). We also saw expression in the anterior deirid sensory neurons ADE (Fig. 4A,4B,4E). Less frequently we saw expression in probable ADF and AIM neurons (Fig 4A,4E). We saw as many as 12 neurons (6 bilateral pairs) expressing the reporter in the head of young larvae. This includes all the identified serotonergic (NSM, ADF, AIM) and dopaminergic (CEPD, CEPV, ADE) head neurons excepting the unpaired RIH neuron [10]. In a small number of males examined, we saw expression in male-specific serotonergic and dopaminergic neurons, including up to 6 pairs of ray sensory neurons (RNs) in both adults and late L4 larvae (Fig. 4G,4H). (There are three pairs of serotonergic, and three pairs of dopaminergic RNs among the 18 RNs.) Expression in CP neurons, male-specific ventral cord motoneurons controlling tail curling during mating, was limited and usually weak in the male worms we examined. Six CP neurons are strongly serotonin-immunoreactive in males [16]. At most we saw three posterior cells staining, and usually only one or two posterior cells (CP5, CP6) weakly stained, when expression was present at all (Fig. 4I). We never saw CP staining in L4 animals, and often none even in male worms expressing GFP strongly in the RNs.
C05D2.4 (bas-1) and its downstream homolog C05D2.3
Just downstream of the bas-1/C05D2.4 gene is C05D2.3, the product of an ancient tandem duplication event. The two genes have diverged considerably – being only 59% identical at the amino acid level (Table 1). The genomic structures of the two genes have also diverged. The two genes share four introns, but C05D2.4 has one and C05D2.3 has three introns not found in the other (Fig. 7). Nevertheless, comparisons with other AADC proteins showed that bas-1/C05D2.4 is most similar to C05D2.3 and the predicted gene F12A10.3 (Fig. 5, Table 1). The predicted amino acid sequence of C05D2.3 contains one noteworthy gap: it is missing six amino acids from a highly conserved region found in all other PLP-dependent decarboxylases. This sequence, the consensus of which is VDAAYA, contains an aspartate (D) residue that is absolutely essential for function of Rat DDC. Substitution of an alanine or asparagine completely abolishes enzymatic activity, and even the conservative substitution of a glutamate at this site reduces activity to 2% of wildtype [5]. It is therefore unlikely that a C05D2.3 protein could function enzymatically as a typical AADC.
Table 1.
Pairwise BLAST comparisons with C. elegans AADCs.
| C05D2.4 | C05D2.3 | F12A10.3* | K01C8.3 | ZK829.2 | C09G9.4 | Ce GAD | |
| C05D2.4 (bas-1) |
Score %Id / %Sim |
- | - | - | - | - | - |
| C05D2.3 | 1674 59 / 75 |
- | - | - | - | - | - |
| F12A10.3* | 1756 60 / 77 |
1793 63 / 78 |
- | - | - | - | - |
| K01C8.3 (tdc-1) |
970 37 / 57 |
844 34 / 54 |
733 33 / 51 |
- | - | - | - |
| ZK829.2 | 583 29 / 47 |
541 27 / 46 |
559 27 / 49 |
1147 44 / 66 |
- | - | - |
| C09G9.4 | 224 22 / 40 |
150 18 / 38 |
190 19 / 40 |
272 22 / 42 |
275 23 / 44 |
- | - |
|
Ce GAD (unc-25) |
210 22 / 38 |
164 20 / 36 |
216 23 / 37 |
299 25 / 43 |
250 24 / 41 |
129 20 / 40 |
- |
| Dm DDC |
1095 41 / 60 |
909 36 / 56 |
984 38 / 58 |
1390 50 / 69 |
883 37 / 57 |
256 22 / 42 |
328 24 / 40 |
| Hs DDC | 1067 41 / 60 |
912 36 / 55 |
949 38 / 58 |
1458 55 / 73 |
935 39 / 59 |
233 20 / 42 |
322 26 / 44 |
| Dm HisDC | 996 39 / 57 |
816 33 / 53 |
807 32 / 54 |
1348 52 / 70 |
910 40 / 59 |
278 23 / 42 |
339 26 / 44 |
| Hs HisDC | 988 38 / 56 |
802 33 / 53 |
876 34 / 56 |
1290 48 / 69 |
920 39 / 59 |
231 21 / 42 |
306 26 / 42 |
| Dm G30446 | 971 38 / 57 |
845 34 / 53 |
686 30 / 50 |
1671 64 / 77 |
1008 40 / 62 |
254 20 / 44 |
n.s. |
| Dm AMD | 961 38 / 57 |
799 35 / 53 |
668 36 / 53 |
1124 44 / 63 |
741 33 / 51 |
201 20 / 40 |
278 25 / 41 |
| Dm G30445 | 837 34 / 52 |
797 35 / 53 |
814 33 / 53 |
1407 55 / 71 |
905 39 / 57 |
259 22 / 42 |
n.s. |
| Cr TrpDC | 743 30 / 51 |
674 28 / 50 |
735 30 / 52 |
916 37 / 59 |
269 31 / 51 |
272 24 / 43 |
333 25 / 42 |
| Dm GAD | 226 22 / 39 |
207 21 / 38 |
232 22 / 41 |
374 27 / 43 |
226 22 / 41 |
99 25 / 55 |
1146 44 / 64 |
| Hs GAD67 | 215 22 / 36 |
173 19 / 36 |
192 21 / 36 |
324 24 / 44 |
228 24 / 41 |
86 16 / 41 |
1535 56 / 73 |
Comparisons of bas-1/C05D2.4 and other pyridoxal-phosphate dependent decarboxylase amino acid sequences were made using "BLAST 2 Sequences" [version 2.2.6, http://www.ncbi.nlm.nih.gov/blast/bl2seq/bl2.html [73]; Settings (largely default): Matrix – BLOSUM62, Open gap penalty – 11, extension gap penalty – 1, low complexity filtering – OFF). As shown in the table above, on the top line, each comparison shows the blast score; below is the percent identity and percent similarity for the 'alignable' sequence. The highest scoring match (excluding among C. elegans AADCs) is indicated in bold. Sequences in the left column are arranged in order of blast score in comparison to C05D2.4 C. elegans AADCs are indicated by their predicted gene designation: C05D2.4, C05D2.3, F12A10.3, K01C8.3, ZK829.2 and C09G9.4. Abbreviations: Ce – C. elegans, Cr – Caranthus roseus (periwinkle plant), Dm – Drosophila melanogaster, Hs – Homo sapiens, DDC – dopa decarboxylase, HisDC – histidine decarboxylase, AMD – Alpha-methyl dopa hypersensitive protein, TrpDC – tryptophan decarboxylase, GAD – glutamate decarboxylase. *An amino acid sequence for F12A10.3 was generated from cDNA sequence by introducing 2 frameshifts to preserve AADC homology in the predicted aa sequence merely for sake of comparison to other AADC's (see text); this sequence is different from predicted sequences in found in Genbank and Wormbase which are based on incorrect cDNA predictions.
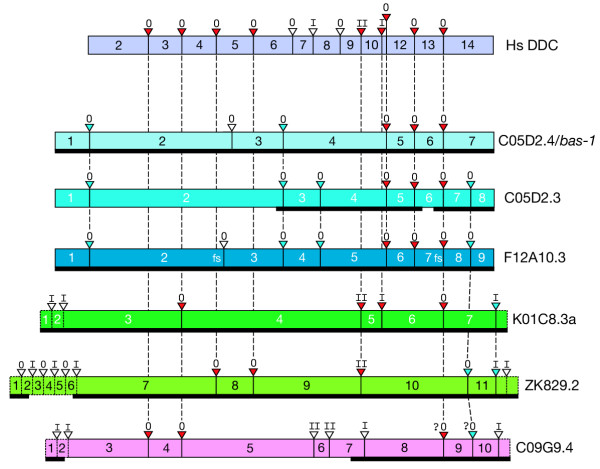
Figure 7.
Genomic structure of C. elegans AADC genes compared with Human DDC. Rectangular blocks represent coding exons of the genes indicated (relative size of exons is approximate). Red triangles indicate ancient conserved introns found in both Human DDC and at least one of the C. elegans AADC genes; blue triangles indicate introns conserved among C. elegans AADC genes; and open triangles indicate non-conserved splice sites (comparing only among the genes shown). Roman numerals above triangles indicate the phase of the intron. Vertical dashed lines between solid triangles indicate splice sites conserved between at least two genes. Diagonal dashed lines indicate probable conserved sites that are shifted by 2–3 amino acids relative to the other splice site. Alignments of homologous splice sites are based on amino acid multiple alignments of the predicted proteins; insertions and deletions are ignored in the drawing. No alternative splicing is indicated; the most readily "alignable" version of each gene was used in cases with multiple splice variants. Dashed boxes at the ends of genes indicate non-AADC homologous extensions unique to the given gene. The most divergent AADC, C09G9.4, is more difficult to align; assignments of splice sites on either side of exon 9 as conserved are more tentative (indicated by question marks). In a few cases where gaps occur in the protein sequence alignments at intron-exon boundaries, introns marked as homologous only begin or end at an homologous location. The splicing pattern shown is fully supported by cDNA sequences for C05D2.4/bas-1, F12A10.3, K01C8.3, ZK829.2 ; the pattern is supported by partial cDNA sequences for C05D2.3 and C09G9.4. The extent of supporting cDNA sequence is shown by the heavy black line beneath the colored blocks. F12A10.3 is a special case in that frameshifts (indicated by 'fs') occur in the cDNA relative to the AADC homologous sequence. The first frameshift occurs at a site where many AADCs are spliced (and where a splice is incorrectly predicted by gene prediction programs), and the second at a splice junction.
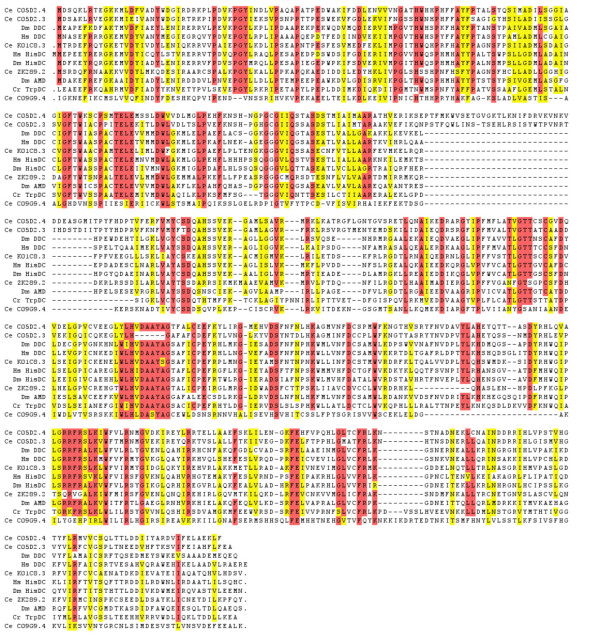
Figure 5.
Alignments of AADC protein sequences with C. elegans BAS-1 predicted protein. Gaps are indicated with a dash (-); at the beginning or end of a sequence, periods indicate additional sequence upstream or downstream that is not shown. Alignments were performed with CLUSTALW. Abbreviations for species and gene names are the same as listed in the legend for Table 1. For genes with multiple splice forms, the most readily aligned sequence was chosen. Red shading indicates amino acids are identical in ≥ 90% of the aligned sequences. Yellow shading indicates similar amino acids found in that position in ≥ 90% of the aligned sequences.
Because the bas-1 and C05D2.3 genes are so close together – only 369 bp from predicted translation stop to predicted translation start – we considered whether they might be expressed as an operon. In C. elegans and other nematodes, genes that are very close together (and often functionally related) may be expressed from a single promoter initially as a single primary transcript [44]. Operon transcripts are subsequently processed to yield separate mRNAs. The first gene in an operon is trans-spliced to the leader sequence SL1; downstream genes are typically spliced to a slightly different leader sequence termed SL2. We would expect to find C05D2.3 transcripts trans-spliced to SL2 if it is a downstream gene in an operon with bas-1. We were unable to isolate either SL1 or SL2-spliced transcripts from C05D2.3 by RT-PCR, although we did isolate a partial cDNA using internal primers. DNA microarray experiments suggest the gene is not expressed above background levels, unlike C05D2.4/bas-1 (Table 2). Furthermore, a global analysis of expression specifically designed to identify operons did not select C05D2.4 and C05D2.3 as likely members of an operon [45]. Since genes comprising an operon should be expressed at similar levels, these data provide no support for the idea that bas-1 and C05D2.3 constitute an operon.
Table 2.
C. elegans AADC genes expression and C. briggsae orthologs.
| C. elegans AADC | C.e. cDNAs | C.e. Microarray | C. briggsae ortholog | C.b. cDNAs |
| C05D2.4 (bas-1) | +(3) a,b,c,d | + | FPC2187 (84,978 / - strand) | + a |
| C05D2.3 | + a,c | - | none | NA |
| C09G9.4 | + d | - | FPC4079 (~28,330 / + strand) | - |
| F12A10.3 | +(2) b,d | - | none | NA |
| K01C8.3 (tdc-1) | +(2) c,d | + | FPC0011 (663,153 / + strand) | + |
| Y37D8A.23 (unc-25) | +(3) c,d | + | FPC4030 (765,572 / - strand) | - |
| ZK289.2 | + c,d | - | FPC0143 (1,747,392 / - strand) | - |
C. e. cDNAs: Parentheses indicate number of splice forms found. acDNAs found via our RT-PCR experiments and bour sequencing of ORFeome project clones. cC. elegans EST project. dWorm ORFeome project. Microarray: Expression levels at all developmental stages as shown by C. elegans microarray experiments found in Wormbase. "+" indicates significant expression at some stage; "-" indicates no expression above background detected at any stage. C. briggsae AADC orthologs: We did TBLASTN searches of the C. briggsae whole genome shotgun assembly (cb25.agp8) on the Sanger Centre C. briggsae blast server using complete predicted amino acid sequences for each C. elegans AADC gene. C. briggsae genes are designated by contig location, first nucleotide of predicted coding sequence, and strand, based on predicted C. elegans sequence. In each case, alignments showed extended regions of 100% or near 100% amino acid identity beginning at the site indicated. (We did not locate the beginning of the C.b. C09G9.4 ortholog; alignments did not identify a matching site for the first 11 C. elegans amino acids). For C05D2.3 and F12A10.3, the best matches in C. briggsae were the C05D2.4 ortholog (FPC2187), followed by the K01C8.3 ortholog (FPC0011); these two genes appear to be absent from C. briggsae. We have isolated an SL1-spliced C. briggsae bas-1 cDNA by RT-PCR (S. DePaul & C. Loer, unpublished); C. briggsae tdc-1 ESTs are in GenBank. NA – not applicable.
The bas-1-AADC and other AADC genes in C. elegans
We compared the predicted amino acid sequences of five other C. elegans AADC-like genes revealed by deletion mapping [46] and by whole genomic sequencing [24], along with a previously identified C. elegans glutamate decarboxylase (GAD) gene, unc-25 [47] to related PLP-dependent decarboxylases from other organisms. Some of the C. elegans genes are clearly closely related to other AADCs, whereas others are more divergent (Fig 5, Table 1). All contain the core conserved domain (PFAM 00282) defining this group of PLP-dependent decarboxylases. None of the AADC or GAD predicted proteins in C. elegans appears to have a signal sequence.
The protein predicted from K01C8.3 is now believed to encode a tyrosine decarboxylase (tdc-1) used for tyramine and octopamine synthesis, which both appear to be used as neurotransmitters in C. elegans [48,49]. The best match to K01C8.3/tdc-1 is a predicted Drosophila AADC-homologous protein of unknown function (G30446). Interestingly, K01C8.3/tdc-1 shows a stronger match to known DDCs than any of the other C. elegans AADCs, including C05D2.4 (Table 1), although it is equally similar to known histidine decarboxylases (HisDCs). The strongest match of C05D2.4/bas-1 (outside of nematodes) is to insect and mammalian DDCs, but again the match is only slightly better than to HisDCs. The predicted genes C05D2.3, F12A10.3 and ZK829.2 also have about the same level of identity and similarity to known AADCs and HisDCs. The ZK829.2 predicted protein, however, is much larger (830 AA) than a typical AADC, having extended N- and C-terminal domains not found in other PLP-dependent DCs. Most of ZK829.2 predicted coding sequence is confirmed by cDNA sequences, suggesting that the predicted protein 'extensions' likely are real.
The predicted gene C09G9.4 is the most divergent from known AADC's with only 20 – 24% amino acid identity; it is even more divergent than C.e. GAD/unc-25. It also appears to lack the absolutely conserved Lys of PLP-dependent decarboxylases, although it otherwise retains considerable homology with the conserved domain of this family of proteins. There are no similar proteins among other organisms to provide clues about a possible function for this gene; C09G9.4 is a truly novel member of the group II PLP-dependent enzyme family. Proteins with a similar level of divergence with AADC (~20% identity over a few hundred amino acids) include other group II PLP-dependent enzymes such as sphingosine-1-phosphate lyase and cysteine sulfinic acid decarboxylase. C09G9.4, however, has very little or no significant similarity to these other enzymes. The C. elegans GAD/unc-25 predicted protein has a strong match to identified Drosophila and mammalian GADs (Table 1), and is found as a single copy. There are no other GAD-like genes in C. elegans such as cysteine sulfinate decarboxylase, which is the rate-limiting enzyme in taurine synthesis, and the closest non-AADC relative to GAD in the vertebrates [50].
Comparison of C. elegans and C. briggsae AADC genes
We performed BLAST searches of a C. briggsae whole genome shotgun assembly using predicted protein sequences of all six C. elegans AADC genes and the unc-25/GAD gene. We found five orthologous genes in C. briggsae – four AADC homologs and one GAD homolog (Table 2). All of these matches included 100% or near 100% identity over extended regions of aligned predicted amino acid sequences, and were paired with high confidence in phylogenies (Fig. 6). Using a core AADC sequence for alignments and tree building, we found that the bas-1 orthologs have evolved more quickly than some of the other AADC's. The C. elegans gene K01C8.3 and its ortholog, for example, are 98% identical in this core region (vs. 91% identity for bas-1 orthologs). Most of the divergence between K01C8.3 and its ortholog is in N- and C-terminal extensions that are not found in other AADC's. The C. elegans C09G9.4 and C. briggsae ortholog are even less similar to one another than are the bas-1 orthologs.
Figure 6.
Phylogenetic trees of AADC protein and nucleotide sequences. Trees were made from sequences aligned with CLUSTALW. Species and gene names are abbreviated as listed in the legend for Table 1. (A) The single minimum-length tree resulting from a heuristic search using parsimony from alignments of core protein sequences (531 characters) of selected C. elegans, C. briggsae, Human and Drosophila AADCs. C. roseus (periwinkle plant) TrpDC was used as an outgroup. Branch lengths are indicated, with bootstrap values using the same search conditions (1000 replicates) in parentheses. The search used the tree-bisection-reconnection (TBR) branch-swapping algorithm; characters were equally weighted. An identical tree topologically was obtained by a branch-and-bound search. C. elegans F12A10.3 was excluded from this analysis since it lacks a functional protein sequence (see Fig. 7 and text). Trees determined by distance methods were similar, but rearranged some of branches with low bootstrap values in the tree shown. (B) The single minimum-length tree resulting from a heuristic search using parsimony (same settings as above) of nucleotide sequence alignments (1608 characters) from a subset of AADCs above, with the addition of C. elegans F12A10.3. Dm DDC was used as an outgroup. Branch lengths and bootstrap values using the same search conditions (1000 replicates) are shown as in A. An identical tree topologically was obtained by a branch-and-bound search.
Our most striking observation is that C. briggsae appears to lack orthologs for the C. elegans predicted genes C05D2.3 and F12A10.3. This suggests that gene duplications giving rise to these two genes, which are most closely related to bas-1/C05D2.4, occurred either in the C. elegans lineage after its split with the C. briggsae lineage, or that C. briggsae lost both C05D2.3 and F12A10.3 orthologs (or their common ancestor) following the split. Using phylogenetic analysis of aligned amino acid and nucleotide sequences, we found that C05D2.3 and F12A10.3 share a common ancestor and that the gene duplication giving rise to bas-1 and C05D2.3/F12A10.3 likely occurred prior to the C. elegans/C. briggsae divergence. This is also suggested by the pattern of introns in the genes. [We have confirmed the splicing pattern of C. briggsae bas-1 by isolating a cDNA (S. DePaul & C. Loer, unpublished data).] The C. elegans and C. briggsae bas-1 genes have identical genomic structure which differs from that of C05D2.3 and F12A10.3, which are more similar to one another (Fig. 7). Therefore C05D2.3 and F12A10.3 (or their common ancestor) were retained in the line leading to C. elegans but lost in the C. briggsae line. The original duplication event giving rise to the tandem copies of C05D2.4 and C05D2.3 on chromosome III probably occurred via an unequal crossing-over or similar event. The duplication creating F12A10.3, which is found on chromosome II, presumably occurred subsequently. We noted no homology of other predicted genes downstream of F12A10.3 and C05D2.3 that might suggest an event duplicating more than the AADC gene.
The retention of the genes and their expression in C. elegans suggests that they may have acquired a new function that is under selection, retain a subfunction of the AADC, or instead that they are still in the process of being lost. After sequencing F12A10.3 cDNAs (courtesy of the ORFeome Project), we found that current splicing predictions for the gene were incorrect. We sequenced six F12A10.3 clones and found two slightly different splicing patterns, both different from Genefinder and Intronerator predictions. The two types of clones differed only in whether a final intron was removed or not. We found 4 clones with 9 exons, and 2 clones with 8 exons. The failure of the gene prediction programs in this case is likely to be due to their preference for creating functional transcripts. All F12A10.3 cDNAs instead appear to be non-functional: they have frameshifts relative to AADC-homologous reading frames. The first frameshift occurs in the second exon and quickly leads to a premature stop codon. At best F12A10.3 transcripts would result in a 158 amino acid protein that could not function as an AADC. F12A10.3 therefore appears to be an expressed pseudogene. DNA microarray experiments and representation in cDNA sequencing projects suggest that F12A10.3, like C05D2.3, is likely expressed at a low level (Table 2).
In order to assess whether the bas-1-like genes C05D2.3 and F12A10.3 might be under reduced selection pressure, we calculated the ratio of non-synonymous to synonymous substitutions (KA/KS) comparing the bas-1 orthologs and bas-1-like genes. We also calculated these values for the other AADC ortholog pairs from C. elegans and C. briggsae (Table 3). KA/KS < 1 indicates purifying (negative) selection, KA/KS = 1 indicates no selection (as in true pseudogenes), and KA/KS < 1 indicates Darwinian (positive) selection. KA/KS for 8179 C. elegans and C. briggsae ortholog pairs had a mean value of 0.06, indicating most genes are under purifying selection [37]. We found that the bas-1 genes are under purifying selection (KA/KS = 0.039), but the bas-1-like genes appear to be under reduced selective pressure; the average KA/KS for comparisons with bas-1-like genes was 0.148, more than three times the value of the bas-1 ortholog comparison. The proportion of observed to potential non-synonymous substitutions (pN) among the bas-1-like gene comparisons was similarly much higher than for the bas-1 orthologs.
Table 3.
Synonymous vs. non-synonymous codon substitution between C. elegans and C. briggsae AADC orthologs and bas-1-like paralogs.
| Ce, Cb AADCs compared | Codons | pS | pN | KA/KS |
| bas-1 | 521 | 0.68 | 0.07 | 0.039 |
| C05D2.3, F12A10.3, bas-1* | 521 | 0.71 ± 0.03 | 0.26 ± 0.02 | 0.148 ± 0.044 |
| ZK289.2 | 833 | 0.71 | 0.09 | 0.043 |
| C09G9.4 | 507 | 0.74 | 0.12 | 0.037 |
| tdc-1 full length | 626 | 0.79 | 0.03 | NA |
| tdc-1 core | 474 | 0.82 | 0.02 | NA |
| tdc-1 N, C terminals | 152 | 0.71 | 0.07 | 0.035 |
Nucleotide alignments of C. elegans and C. briggsae genes were analyzed by SNAP software (see Methods). Only the C. elegans member of the ortholog pair is named. pS = proportion of observed/potential synonymous substitutions; pN = proportion of observed/potential nonsynonymous substitutions. NA – not applicable (cannot be calculated when pS > 0.75). *Includes all pairwise comparisons (n = 5) except C.e. vs. C.b. bas-1. Values are mean ± SD (strict statistical comparison with other values is not intended, as KA/KS values are not distributed normally).
Two other AADC ortholog pairs showed strong purifying selection at work, with values like that calculated for the bas-1 orthologs (Table 3), but a value could not readily be calculated for the tdc-1 orthologs. In all the AADC ortholog comparisons, the proportion of observed to potential synonymous substitutions (pS) was near mutational saturation (pS > 0.75); KA/KS cannot be calculated when pS > 0.75. Thus, a value could not be calculated either for full-length tdc-1 alignments, or using a tdc-1 core sequence. We were able to calculate a value by aligning the N- and C-terminals sequence of the tdc-1 orthologs (Table 3). These regions of the protein are under levels of selection like the other AADCs, whereas the core has a very low rate of non-synonymous substitution, consistent with the high level of amino acid conservation in this region of the protein.
Discussion
Our experiments demonstrate that the predicted gene C05D2.4, which encodes an aromatic L-amino acid decarboxylase (AADC), corresponds to the genetically-defined bas-1 gene. Serotonin immunoreactivity is restored in bas-1 mutants by DNA containing an intact C05D2.4 gene, but not with DNA mutated in C05D2.4. The adjacent AADC-homologous gene, C05D2.3, is not needed to rescue bas-1 mutants. The bas-1 gene is therefore likely to encode the serotonin- and dopamine-synthetic AADC of C. elegans. Although we did not test for rescue of dopamine expression, it is likely that bas-1 encodes the same AADC required for DA synthesis. Mutants with point mutations in C05D2.4 – bas-1 alleles n2948 and n3008 – have been shown previously to be DA-deficient [17], and neither of these appears to contain mutations in the C05D2.3 gene. Furthermore, AADC proteins from other animals have been consistently shown to catalyze both 5HTP and L-dopa decarboxylation reactions [3]. Finally, a bas-1 reporter construct is expressed both in identified serotonergic and dopaminergic cells.
The bas-1 gene is expressed in at least two alternatively spliced forms, one of which appears to be less common and contains a small additional 27 nucleotide exon. The short segment of protein encoded by the additional exon, and the surrounding region are not found in other AADC proteins, suggesting a novel function for this region of the AADC protein. In other organisms, the serotonin- and dopamine-synthetic AADC genes have alternative splicing that result in tissue-specific protein isoforms. Currently we have no indication that bas-1 is expressed in any cells other than serotonergic and dopaminergic neurons, and no information about the functional significance of this alternative splicing.
AADC has received somewhat less attention with respect to the regulation of serotonin and dopamine synthesis than the specific, rate-limiting synthetic enzymes tryptophan hydroxylase and tyrosine hydroxylase [51]. This is in part due to the view that AADC activity is not limiting, and that its activity is not regulated. Regulation of AADC activity by protein kinase A-dependent phosphorylation has recently been proposed based on in vitro experiments [52], although its functional significance has been questioned [53]. Our examination of the predicted BAS-1 protein revealed several potential phosphorylation sites that are highly conserved, although none fit the consensus sequence for PKA phosphorylation. Any possible regulation of C. elegans AADCs by phosphorylation remains speculation.
Possible functions of other AADC homologous genes in C. elegans
We compared the protein sequences of other predicted AADCs in C. elegans with those of other organisms in order to guess about their possible functions. This is particularly relevant because all bas-1 mutants retain weak, residual serotonin immunoreactivity ([13]; C. Loer, unpublished) suggesting that other enzymes may be able to carry out the same reaction. This would not be surprising since animal AADCs tend to have broad specificity [3]. Based purely on sequence homology, it seems that predicted genes K01C8.3 and ZK829.2 could act as AADCs or as HisDCs. In fact, the predicted gene K01C8.3 is now believed to be a tyrosine decarboxylase and has been named tdc-1 [48]. If correct, then its best match in Drosophila (G30446), an uncharacterized AADC homolog, is likely to encode the fly's octopamine-synthetic tyrosine decarboxylase. It has long been known that a separate gene encoded this enzymatic activity in flies, since the activity is still detectable in Ddc deletion mutants [4]. It will be interesting to see whether such tyrosine decarboxylases in animals have more restricted substrate specificity, such as the tyrosine and tryptophan decarboxylases in plants [54], or are more similar to typical animal AADCs with a broad specificity. Tighter substrate specificity of a tdc-1 protein could be reflected in the much slower rate of amino acid substitution seen in its C. elegans & briggsae orthologs than in the bas-1 orthologs which encode more 'promiscuous' enzymes.
Whether C. elegans or other nematodes make the neurotransmitter histamine, and therefore need a HisDC enzyme, is unclear. Although histamine has been reportedly isolated from C. elegans [55], this observation is unique among nematodes, and has not subsequently been confirmed. There is no particularly good candidate for a HisDC in C. elegans. The ZK829.2 predicted protein may be most closely related to tdc-1 in its core sequence, although its long N- and C-terminal extensions are perhaps suggestive of a new function. Unfortunately, transgenics with reporter fusions of this gene to date have shown no expression, the pattern of which might suggest a function (C. Loer, unpublished; M. Alkema, personal communication). As with tdc-1, C. elegans ZK829.2 and its C. briggsae ortholog have also evolved more slowly than the bas-1 orthologs. A recent analysis of eukaryotic AADC sequences that includes the C. elegans ZK829.2 and its C. briggsae ortholog as the only nematode representatives clearly demonstrates that AADC genes can evolve at very different rates, and that a constant "molecular clock" cannot be assumed in phylogenetic analyses [56].
Finally, since the C09G9.4 predicted protein is so highly divergent from the typical AADC, and lacks a critical lysine residue that binds the PLP cofactor, it is unlikely to be an AADC enzyme. It has a similar level of divergence from genuine AADCs as do other group II PLP-dependent enzymes such as cysteine sulfinic acid decarboxylase, to which it has little or no similarity. Whatever the function of a C09G9.4-encoded protein, it appears to represent a new PLP-DC-related protein; sequencing of more genomes may yet reveal additional members.
Duplicate gene retention and loss in Caenorhabditis
We found that the closest relatives of C05D2.4/bas-1 in C. elegans, the genes C05D2.3 and F12A10.3, are missing from C. briggsae. Furthermore, phylogenetic analysis indicates the two extra genes did not arise in the C. elegans line, but were present (or their commmon ancestor was present) in the species that gave rise to both the C. elegans and C. briggsae lines. Finally, careful examination of the cDNAs and predicted protein sequences of C05D2.3 and F12A10.3 reveals that neither is likely to be functional as an AADC: the former lacks critical amino acids and the latter can encode only a truncated protein. Both are expressed, based on the presence of cDNAs, but probably at a very low level, which is not above background in microarray experiments. It is possible that the duplicate genes are functionally 'lost' in C. elegans as well.
The features of C05D2.3 and F12A10.3 raise a number of interesting questions about the fate of duplicate genes, and the true nature of many 'predicted genes' in C. elegans. Taking a random sampling of predicted genes and generating transgenics with reporter fusion constructs (in order to determine a pattern of expression), Mounsey and colleagues [57] found that a much higher percentage of recently duplicated genes than conserved or unique genes failed to show expression. Assuming that failure of expression was no more likely among recently duplicated genes for technical reasons, this meant that many more of these are in reality not expressed. The numbers suggested that up to 20% of annotated, predicted genes in C. elegans may be pseudogenes. In fact, careful inspection of recently duplicated genes showed that many were actually pseudogenes, like we found to be the case for F12A10.3. Overall, close inspection of predicted genes revealed at least 4% were pseudogenes.
So, why are C05D2.3 and F12A10.3 still present in C. elegans if they lack a function? C. briggsae and C. elegans may have diverged 80 – 110 million years ago [37,38]. Since the bas-1-like gene or genes were likely present in the common ancestor of C. elegans and C. briggsae, then there seems to have been ample time for loss in the C. elegans line. Under a simple model of gene loss following duplication, only a few million generations would be the mean time to fix a null allele of the gene duplicate [58]. In Caenorhabditis, a million generations could be completed in 10,000 years or less. This seems to suggest that the downstream duplicate of bas-1 (ancestor of C05D2.3) may have continued to function for a considerable time after the duplication, perhaps by gene conversion which might have continued until sufficient divergence from bas-1 [59]. Loss of the critical six amino acids occurred after the second duplication giving rise to the ancestor of F12A10.3, since the appropriate sequence is still present there (although frame-shifted). It is also possible that the C05D2.3 gene retains some function. The gene still encodes a respectable protein, albeit one that seems unable to function as an AADC. It has diverged considerably from bas-1, but has not accumulated stop codons and frameshifts expected for a pseudogene. Walsh [60] has proposed that fixation of an allele with an advantageous new function, vs. becoming a pseudogene, may be the fate of many duplicate genes even when such mutations are rare, given a population that is sufficiently large.
C05D2.3 and F12A10. 3 seem to have been retained longer than expected. Lynch and Force [61] proposed that the unexpectedly high rate of gene duplicate retention in eukaryotic genomes is due to 'subfunctionalization' – the retention of a portion of the original single gene's function by each of the duplicates, which then complement one another. Although this was suggested to occur primarily by regulatory mutations that partition expression of the genes spatially, other forms of subfunctionalization could also occur. Another possible reason for retaining such genes is the presence of non-coding regulatory functions associated with transcription and splicing of these sub-functional transcripts that affect the transcription of other nearby genes, although a bas-1::GFP construct is expressed well without such sequences in cis.
Our analysis of synonymous vs. non-synonymous substitutions indicates that the bas-1-like genes C05D2.3 and F12A10.3 are under relaxed selection relative to bas-1 and other AADCs. It should be noted that precise quantitative comparisons cannot be made with the results presented in the C. briggsae whole genome analysis [37], since we used a different method of calculating KA/KS; however our calculations indicate that bas-1 and the other AADC's, like most genes in the Caenorhabditis genomes, are under strong purifying selection. Even if both C05D2.3 and F12A10.3 are now pseudogenes, some significant period of time during which they functioned and were under purifying selection could act to obscure this fact in an analysis of KA/KS. Even if C05D2.3 has acquired a new, adaptive function, such a new function might result from changes in only a few sites in the protein, and so again this could be obscured by a majority of sites under purifying selection. With the sequencing of three related Caenorhabditis species it will be interesting to learn of the fates of bas-1 and the bas-1-like genes in other lines.
Conclusions
The bas-1 gene encodes a serotonin- and dopamine-synthetic AADC enzyme in C. elegans. The C. elegans genome possesses five other AADC-homologous genes, two of which are closely related to bas-1. These bas-1-like genes are missing, however, from the congeneric C. briggsae, and evidence suggests that, despite their persistence in C. elegans, the genes do not encode functional AADC proteins. Since one or more of the bas-1-like genes was likely present in the common ancestor of C. elegans and C. briggsae- which may have diverged over 80 million years ago – it is unclear why the bas-1-like genes have been retained in the C. elegans line. This is another example of unexpected retention of duplicate genes in eukaryotic genomes.
Methods
Routine culturing of Caenorhabditis elegans was performed as described by Brenner [62]. Nomenclature used here for C. elegans genetics conforms to the conventions set forth by Horvitz et al. [63]. Strains used include N2 (wild type); CB1490: him-5(e1490)V; MT7988: bas-1(ad446)III; MT7990: bas-1(n2948)III; MT8002: bas-1(n3008)III; LC7: bas-1(pa4)III; LC33: bas-1(tm351)III. The him-5(e1490) strain generates approximately 30% males by increased X chromosome non-disjunction [64], but is otherwise essentially wild-type.
Mutant allele sequencing
Mutations were identified by PCR-amplifying small regions from genomic DNA, then sequencing purified PCR product. We isolated genomic DNA (Puregene Kit, Gentra Systems) from four bas-1 mutant strains (alleles ad446, n2948, n3008, pa4) and then PCR-amplified small segments of C05D2.4 and C05D2.3 protein-coding sequence. Five pairs of primers were used to survey the C05D2.4 gene (A+B, E+F, G+H, P+Q, R+S; see below for primer sequences) and 4 pairs for C05D2.3 (C+D, K+L, M+N, P+Q). Bands of the predicted size were excised from 2% agarose gels and purified with GeneClean (Bio101/Qbiogene), then sequenced. All mutations were confirmed by sequencing both strands for two independent PCR reactions. All PCR and sequencing primers were designed using the program Primer3 (http://frodo.wi.mit.edu; [65]). Primer sequences were as follows. Within C05D2.4: C05D2-A: gaggaaactcaaggcgacac; C05D2-B: tgttgatggaaccaagtgga; C05D2-E: cgtccttttctctttgcgac; C05D2-F: tggctccgacttgattctct ; C05D2-G: ttacaattaggccgcaaacc; C05D2-H: ccacctgaactgtggtgatg; C05D2-P: ggactcacatgtttccgattg; C05D2-R: ttagacgttggttgcacgag; C05D2-S: attggcgagcagtcaaagtt ; C05D2-T: tcttatgggattaccagaac; C05D2-U: ctacataaagctggaatggt; C05D2-V: gtttcctaaaaatccacgtg; C05D2-W: atgatcgattgatagctgag. Within C05D2.3: C05D2-C: ctaggtgcctttgccttctg; C05D2-D: caagagacgctcgttgtcag; C05D2-K: gccatctaatcctccaacca; C05D2-L: acattgctcccttttcaacg [note that primer L can also prime within C05D2.4]; C05D2-M: ccatcaactttccaatggct; C05D2-N: tctcgacgcccatatttctc; C05D2-Q: ccaattccagcggagaagta.
Microinjection to create transgenics
All DNAs for microinjection were purified with Qiagen tip20 columns. Experimental DNAs were co-injected with the dominant marker plasmid pRF4 containing the mutant gene rol-6(su1006) [66] into N2 (wildtype), CB1490, or bas-1 mutant worms. Progeny of injectees that express the rol-6 dominant plasmid have the easy-to-identify Roller phenotype which results in worms with a helically-twisted body along the anterior-posterior axis. Roller transgenic worms typically carry co-injected DNAs. Transgenic Roller progeny were isolated and propagated; rescue of bas-1 mutants was scored by staining with serotonin antiserum as described previously[16,67].
Mutant rescue with subclones of cosmid C05D2 and plasmid C05D2XN; GFP Reporter Construct
The rescuing plasmid C05D2XN contains a 15.8 kbp genomic DNA insert (XhoI to NheI) derived from the cosmid C05D2, and contains both C05D2.4 and C05D2.3 predicted genes. A number of deletions of C05D2XN were made to test for rescue of bas-1 mutants. Clones were analyzed by restriction digests. We also made C05D2XN derivatives in which either C05D2.4 and C05D2.3 was mutated to introduce a premature stop in coding sequence. Clones were sequenced to determine the nature of the introduced mutation. Clone pCL6991 had a 4 bp deletion in the second exon of C05D2.4; pCL7991 had a 2 bp insertion in the first exon of C05D2.3; each causing a frameshift mutation. In the clone pCL7003, derived from the rescuing plasmid pCL3001, almost the entire C05D2.3 coding sequence is deleted. The bas-1 GFP reporter construct (within transgenics) was kindly provided by Ian Hope, and consists of a PCR-generated fragment of C05D2 with 4595 bp upstream of the predicted translation start site and 403 bp protein coding region to make an in-frame protein fusion in the 2nd exon. The construct was created by a multi-site recombination reaction with the C05D2 fragment, PCR-generated GFP, and vector using the Invitrogen Gateway cloning system (I. Hope, personal communication) as continuation of a project to determine expression patterns for C. elegans genes through reporter gene technology [57].
RT-PCR and cDNA clones
We isolated RNA for RT-PCR from mixed stage CB1490 worms. (The CB1490 strain has ~30% males, whereas wildtype N2 has ~0.2% males. Since adult males have 13 more serotonergic neurons and 6 more dopaminergic neurons than hermaphrodites, we reasoned that these worms might express more bas-1 mRNA.) Worms were isolated from six 100 mm NGM plates, washed several times with M9 buffer, and pooled to form a ~100 μl pellet of worms. The pellet was mixed with 175 μl RNA lysis buffer (SV Total RNA Isolation System, Promega), frozen and ground to a fine powder with a pestle and mortar cooled with liquid nitrogen. The powder was recovered in a fresh microfuge tube, mixed with 350 μl SV RNA dilution buffer and centrifuged to remove debris. The cleared lysate was transferred to a new tube and precipitated with 200 μl 95% ethanol and applied to the SV spin column assembly. The remaining RNA purification was performed exactly as described for the SV System "RNA Purification by Centrifugation." Purified RNA was eluted from the spin column with 100 μl nuclease-free water. RNA was converted to cDNA in a 80 μl reaction containing 800 Units of M-MLV Reverse Transcriptase (Life Technologies/BRL), 16 μl 5X RT buffer, 25 mM dNTPs, 80 Units RNAsin (Promega) 2.0 μg random hexamer primers (Life Tech), and 50 μl purified RNA (from above). Bas-1(C05D2.4) and C05D2.3 cDNAs were amplified by PCR from him-5 cDNA produced as described above, typically using Failsafe PCR mixes (Epicentre Technologies). Conditions for PCR were 94°C (1 min.), 50°C (1 min.), 72°C (3 min.) for 40 cycles, then 72°C (10 min.). Primers used to amplify C05D2.4 cDNAs were SL1-B + C05D2-L and SL1-B + C05D2-B; a partial C05D2.3 cDNA was amplified with primers C05D2-M and -N. Spliced leader primer sequences were as follows: SL1-B: AAAGGATCCTTTAATTACCCAAGTTTGAG; SL2-B: AAAGGATCCTTTTAACCCAGTTACTCAAG. Appropriately-sized bands were isolated with GeneClean or Ultrafree-DA (Millipore), then cloned directly into the pCRII-TOPO vector using the TOPO-TA Cloning kit (Invitrogen). From seven of our RT-PCR derived clones we sequenced, we found two different splice variants different form the Genefinder prediction (see results).
We also obtained cDNA clones from the ORFeome project [40] and the C. elegans EST project (courtesy Yuki Kohara). DNA we received from the ORFeome project is purified from a pool of transformants derived from ligation and transformation of their original RT-PCR, thereby allowing the isolation of internal splice variants from the mix. We transformed with this DNA and isolated several clones. We sequenced two ORFeome clones completely; six more clones were partially sequenced. Interestingly, each of the 8 clones appeared to have at least one mutation (compared to known genomic sequence). Since we found one splice variant containing the 27 bp microexon from among the eight clones that we sequenced, we analyzed 29 additional ORFeome project-derived clones by PCR from single isolated bacterial colonies. Amplification of the region between primers C05D2-T and -L allowed us to distinguish between clones with or without the 27 bp microexon based on product size (464 bp versus 437 bp). Seven of 29 clones were the larger size, so likely contained the 27 bp microexon. None of the ORFeome clones analyzed by sequence or PCR appeared to use the alternative exon 3 splice acceptor noted above. Finally, the two 'YK' cDNA clones from the C. elegans EST project that we examined are from a 'full-length' capped library, and each has an SL1 leader and a poly-A tail. We found that each bas-1 'YK' clone, however, contained a different internal deletion (overall abnormality of these clones is reported at ~5% – J. Theirry-Meig, personal communication). Each of the deletions was adjacent to a short repeated sequence.
Sequence Analyses
C. elegans genomic and predicted cDNA sequences were retrieved from ACeDB, WormBase, and/or GenBank. Blast searches and Blast2 comparisons were performed using the NCBI Blast server. C. briggsae genomic sequence was searched using TBLASTN of the 7/12/02 shotgun assembly http://www.sanger.ac.uk/Projects/C_briggsae/blast_server.shtml. Assembly and consensus sequence determination of our own cDNA sequences was done using the program SeqMan (DNAStar, Madison, WI). Some information about ORFeome clones was retrieved from WorfDB (http://worfdb.dfci.harvard.edu; [68]). Phosphorylation predictions were made with NetPhos 2.0 http://www.cbs.dtu.dk/services/NetPhos/ and PhosphoBase http://www.cbs.dtu.dk/databases/PhosphoBase/predict/predict.html. Signal sequence predictions were performed with SignalP v.1.1 (http://www.cbs.dtu.dk/services/SignalP/, [69]). Multiple sequence alignments were performed primarily with CLUSTALW http://www.ebi.ac.uk/clustalw/, with some manual adjustments. Phylogenetic analyses were performed with PAUP* version 4.0b10 (Sinauer Associates, [70]). Some alignments of cDNAs with genomic DNA to determine intron locations were performed with SIM4 [71]. Estimates of rates of synonymous and non-synonymous substitutions were made with SNAP (Synonymous/Non-synonymous Analysis Program – http://www.hiv.lanl.gov/content/hiv-db/SNAP/WEBSNAP/SNAP.html using pairwise or multiple sequence nucleotide alignments generated with CLUSTALW and adjusted manually. This program uses the method of Nei and Gojobori [72].
List of Abbreviations
AADC – aromatic amino acid decarboxylase
DDC – dopa decarboxylase
HisDC – histidine decarboxylase
GAD – glutamic acid decarboxylase
TrpDC – tryptophan decarboxylase
PLP – pyridoxal 5'-phosphate
NSM – neurosecretory motoneuron
HSN – hermaphrodite-specific neuron
PDE – postdeirid sensory neuron
ADE – anterior deirid sensory neuron
RN – ray sensory neuron
CEPD, CEPV – dorsal or ventral cephalic sensory neuron
ADF – amphid sensory neuron, dual cilia, designation F
AIM – ring interneuron, designation M
RIH – ring interneuron (unpaired), designation H
CP – posterior daughter of designation C cell, male-specific ventral cord motoneuron
Authors' contributions
EH prepared deletion and expression constructs, cloned and sequenced bas-1 cDNAs and mutant alleles, participated in anti-serotonin staining and genetics, and drafted the manuscript. CL conceived and directed the study, generated transgenics by microinjection, performed sequence and phylogenetic analyses, and completed the manuscript. Both authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We wish to thank the following persons: Willy Christian and Patrick Merritt for technical assistance; Raffi Aroian for advice regarding RT-PCR; Fred Wolf and Gian Garriga for the C05D2XN plasmid and sharing unpublished information; Beth Sawin, Mark Alkema and H. Robert Horvitz for sharing unpublished information; and other members of the Loer lab for various contributions to this project, including Susan Anderton (isolated the bas-1(pa4) mutant), Scott DePaul (sequenced the C. briggsae bas-1 cDNA), Tonya Lebet (isolated and sequenced F12A10.3 cDNAs), Nicola Marsden-Haug, Nailah Thompson, Cristina Ramirez, and Laura Rivard (for comments on the manuscript). C. briggsae bas-1 cDNAs and genomic clones were originally isolated by members of the Spring 2002 Biology 182 (Molecular Biology) class at USD. Ian Hope generously provided two bas-1::GFP reporter transgenic strains made in his lab. Michael Mayer provided significant help and guidance with phylogenetic analyses using PAUP*.
Some of the strains used were obtained from the Caenorhabditis Genetics Center, which is funded by the NIH National Centers for Research Resources. The C05D2.4 and C05D2.3 deletion mutants were generated by the C. elegans Gene Knockout Consortium (tm351 was specifically provided by the labs of Shohei Mitani and Yuji Kohara; ok704 by the Barstead lab). Two bas-1 cDNAs were provided by the Kohara lab (C. elegans EST/transcriptome project); bas-1 and F12A10.3 cDNAs by J. Reboul and the M. Vidal lab (C. elegans ORFeome project).
CL was supported by a National Science Foundation RUI grant (Developmental Neuroscience, IBN-9796217), an NIHGMS Area Grant (R15 GM60203), start-up funds from the University of San Diego, and the Fletcher Jones Foundation. Some of this work was performed when CL was at Lafayette College, and so was also supported by start-up funds from that institution, some of which came from a Howard Hughes Medical Institute grant.
Contributor Information
Emily E Hare, Email: ehare@berkeley.edu.
Curtis M Loer, Email: cloer@sandiego.edu.
References
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- Deakin JF. Depression and antisocial personality disorder: two contrasting disorders of 5HT function. J Neural Transm Suppl. 2003;64:79–93. doi: 10.1007/978-3-7091-6020-6_5. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Juorio AV. Aromatic L-amino acid decarboxylase: biological characterization and functional role. Gen Pharmac. 1995;26:681–696. doi: 10.1016/0306-3623(94)00223-A. [DOI] [PubMed] [Google Scholar]
- Wright TRF. The genetics of biogenic amine metabolism, sclerotization, and melanizaton in Drosophila melaogaster. Adv Genet. 1987;24:127–222. [PubMed] [Google Scholar]
- Poupon A, Jebai F, labesse G, Gros F, Thibault J, Mornon J, Krieger M. Structure modelling and site-directed mutagenesis of the rat aromatic L-amino acid pyridoxal 5'-phosphate-dependent decarboxylase: a functional study. Proteins. 1999;37:191–203. doi: 10.1002/(SICI)1097-0134(19991101)37:2<191::AID-PROT5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Tempel BL. Genetic dissection of monoamine neurotransmitter synthesis in Drosophila. Nature. 1983;303:67–70. doi: 10.1038/303067a0. [DOI] [PubMed] [Google Scholar]
- Albert VR, Allen JM, Joh TH. A single gene codes for aromatic L-amino acid decarboxylase in both neuronal and non-neuronal tissues. J Biol Chem. 1987;262:9404–9411. [PubMed] [Google Scholar]
- Eveleth DD, Gietz RD, Spencer CA, Nargang FE, Hodgetts RB, Marsh JL. Sequence and structure of the dopa decarboxylase gene of Drosophila : evidence for novel RNA splicing variants. EMBO J. 1986;5:2663–2672. doi: 10.1002/j.1460-2075.1986.tb04549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley K, Harmon S, Moffat M, Uhland-Smith A, Wong S. The human aromatic L-amino acid decarboxylase gene can be alternatively spliced to generate unique protein isoforms. J Neurochem. 1995;65:2409–2416. doi: 10.1046/j.1471-4159.1995.65062409.x. [DOI] [PubMed] [Google Scholar]
- Rand JB, Nonet ML. Appendix 2. Neurotransmitter assignments for specific neurons. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editor. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 1049–1052. [Google Scholar]
- Desai C, Horvitz HR. Caenorhabditis elegans mutants defective in the functioning of the motor neurons responsible for egg laying. Genetics. 1989;121:703–721. doi: 10.1093/genetics/121.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner LE, Zhou GT, Schafer RW, Schafer WR. Control of alternative behavioral states by serotonin in Caenorhabditis elegans. Neuron. 1998;21:203–214. doi: 10.1016/S0896-6273(00)80527-9. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Garriga G, Thomas JH. Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J Neurosci. 1995;15:6975–6985. doi: 10.1523/JNEUROSCI.15-10-06975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J Exp Zool. 1990;253:263–270. doi: 10.1002/jez.1402530305. [DOI] [PubMed] [Google Scholar]
- Niacaris T, Avery L. Serotonin regulates repolarization of the C. elegans pharyngeal muscle. J Exp Biol. 2003;206:223–231. doi: 10.1242/jeb.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loer CM, Kenyon CJ. Serotonin-deficient mutants and male mating behavior in the nematode Caenorhabditis elegans. J Neurosci. 1993;13:5407–5417. doi: 10.1523/JNEUROSCI.13-12-05407.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/S0896-6273(00)81199-X. [DOI] [PubMed] [Google Scholar]
- Nuttley WM, Atkinson-Leadbeater KP, Van Der Kooy D. Serotonin mediates food-odor associative learning in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:12449–12454. doi: 10.1073/pnas.192101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze JY, Victor M, Loer CM, Yang S, Ruvkun G. Feeding and metabolic signaling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–564. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–272. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- Liu KS, Sternberg PW. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron. 1995;14:79–89. doi: 10.1016/0896-6273(95)90242-2. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Wintle RF, Kindt KS, Nuttley WM, Arvan R, Fitzmaurice P, Bigras E, Merz DC, Hebert TE, Van Der Kooy D, et al. Dopamine modulates the plasticity of mechanosensory responses in Caenorhabditis elegans. EMBO J. 2004;23:473–482. doi: 10.1038/sj.emboj.7600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- The C.elegans Geonome Sequencing Consortium Genome sequence of the nematode C. elegans : a platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- Lints R, Emmons SW. Patterning of dopaminergic neurotransmitter identity among Caenorhabditis elegans ray sensory neurons by a TGFb family signaling pathway and a Hox gene. Development. 1999;126:5819–5831. doi: 10.1242/dev.126.24.5819. [DOI] [PubMed] [Google Scholar]
- Ranganathan R, Sawin ER, Trent C, Horvitz HR. Mutations in the Caenorhabditis elegans serotonin reuptake transporter MOD-5 reveal serotonin-dependent and -independent activities of fluoxetine. J Neurosci. 2001;21:5871–5884. doi: 10.1523/JNEUROSCI.21-16-05871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R, Hall DH, Miller DM, Blakely RD. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Nat Acad Sci. 2002;99:3264–3269. doi: 10.1073/pnas.042497999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr JS, Frisby DL, Gaskin J, Duke A, Asermely K, Huddleston D, Eiden LE, Rand JB. The cat-1 gene of Caenorhabditis elegans encodes a vesicular monoamine transporter required for specific monoamine-dependent behaviors. J Neurosci. 1999;19:72–84. doi: 10.1523/JNEUROSCI.19-01-00072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olde B, McCombie WR. Molecular cloning and functional expression of a serotonin receptor from Caenorhabditis elegans. J Molec Neurosci. 1997;8:53–62. doi: 10.1007/BF02736863. [DOI] [PubMed] [Google Scholar]
- Hamdan FF, Ungrin MD, Abramovitz M, Ribeiro P. Characterization of a novel serotonin receptor from Caenorhabditis elegans : Cloning and expression of two splice variants. J Neurochem. 1999;72:1372–1383. doi: 10.1046/j.1471-4159.1999.721372.x. [DOI] [PubMed] [Google Scholar]
- Suo S, Sasagawa N, Ishiura S. Identification of a dopamine receptor from Caenorhabditis elegans. Neurosci Lett. 2002;319:13–16. doi: 10.1016/S0304-3940(01)02477-6. [DOI] [PubMed] [Google Scholar]
- Suo S, Sasagawa N, Ishiura S. Cloning and characterization of a Caenorhabditis elegans D2-like dopamine receptor. J Neurochem. 2003;86:869–878. doi: 10.1046/j.1471-4159.2003.01896.x. [DOI] [PubMed] [Google Scholar]
- Segalat L, Elkes DA, Kaplan JM. Modulation of serotonin-controlled behaviors by Go in Caenorhabditis elegans. Science. 1995;267:1648–1651. doi: 10.1126/science.7886454. [DOI] [PubMed] [Google Scholar]
- Brundage L, Avery L, Katz A, Kim UJ, Mendel JE, Sternberg PW, Simon MI. Mutations in a C. elegans Gqalpha gene disrupt movement, egg laying, and viability. Neuron. 1996;16:999–1009. doi: 10.1016/S0896-6273(00)80123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiani CA, Gharib S, Simon MI, Sternberg PW. Caenorhabditis elegans G(alpha)(q) regulates egg-laying behavior via a PLC(beta)-independent and serotonin-dependent signaling pathway and likely functions both in the nervous system and in muscle. Genetics. 2003;165:1805–1822. doi: 10.1093/genetics/165.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G, Thijssen KL, Werner P, van der Horst M, Hazendonk E, Plasterk RHA. The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat Genet. 1999;21:414–419. doi: 10.1038/7753. [DOI] [PubMed] [Google Scholar]
- Stein LD, Bao Z, Blasiar D, Blumenthal T, Brent M, Chen N, Chinwalla A, Clarke L, Clee C, Coghlan A, et al. The Genome sequence of Caenorhabditis briggsae : A platform for comparative genomics. PLoS Biol. 2003;1:E45. doi: 10.1371/journal.pbio.0000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan A, Wolfe KH. Fourfold faster rate of genome rearrangement in nematodes than in Drosophila. Genome Res. 2002;16:857–867. doi: 10.1101/gr.172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graur D, Martin W. Reading the entrails of chickens: molecular timescales of evolution and the illusion of precision. Trends Genet. 2004;20:80–86. doi: 10.1016/j.tig.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Reboul J, Vaglio P, Tzellas N, Thierry-Mieg N, Moore T, Jackson C, Shin-i T, Kohara Y, Thierry-Mieg D, Thierry-Mieg J, et al. Open-reading-frame sequence tags (OSTs) support the existence of at least 17,300 genes in C. elegans. Nat Genet. 2001;27:332–336. doi: 10.1038/85913. [DOI] [PubMed] [Google Scholar]
- Ishii S, Mizuguchi H, Nishino J, Hayashi H, Kagamiyama H. Functionally important residues of aromatic L-amino acid decarboxylase probed by sequence alignment and site-directed mutagenesis. J Biochem. 1996;120:369–376. doi: 10.1093/oxfordjournals.jbchem.a021422. [DOI] [PubMed] [Google Scholar]
- Nishino J, Hayashi H, Ishii S, Kagamiyama H. An anomalous side reaction of the Lys303 mutant aromatic L-amino acid decarboxylase unravels the role of the residue in catalysis. J Biochem (Tokyo) 1997;121:604–611. doi: 10.1093/oxfordjournals.jbchem.a021628. [DOI] [PubMed] [Google Scholar]
- Burkhard P, Dominici P, Borri-Voltattorni C, Jansonius JN, Malashkevich VN. Structural insight into Parkinson's disease treatment from drug-inhibited DOPA decarboxylase. Nat Struct Biol. 2001;8:963–967. doi: 10.1038/nsb1101-963. [DOI] [PubMed] [Google Scholar]
- Blumenthal T, Steward K. RNA processing and gene structure. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editor. C elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 117–145. [PubMed] [Google Scholar]
- Blumenthal T, Evans D, Link CD, Guffanti A, Lawson D, Thierry-Mieg J, Thierry-Mieg D, Chiu WL, Duke K, Kiraly M, et al. A global analysis of Caenorhabditis elegans operons. Nature. 2002;417:851–854. doi: 10.1038/nature00831. [DOI] [PubMed] [Google Scholar]
- Marra MA, Prasad SS, Baillie DL. Molecular analysis of two genes between let-653 and let-56 in the unc-22(IV) region of Caenorhabditis elegans. Mol Gen Genet. 1993;236:289–298. doi: 10.1007/BF00277125. [DOI] [PubMed] [Google Scholar]
- Jin Y, Jorgensen E, Hartwieg E, Horvitz HR. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J Neurosci. 1999;19:539–548. doi: 10.1523/JNEUROSCI.19-02-00539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkema M, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine modulates foraging behavior and head movements in C. elegans. Soc Neurosci Abstr. 2003. p. 44.10.
- Rex E, Komuniecki RW. Characterization of a tyramine receptor from. Caenorhabditis elegans. 2002;82:1352–1359. doi: 10.1046/j.1471-4159.2002.01065.x. [DOI] [PubMed] [Google Scholar]
- Park E, Park SY, Wang C, Xu J, LaFauci G, Schuller-Levis G. Cloning of murine cysteine sulfinic acid decarboxylase and its mRNA expression in murine tissues. Biochim Biophys Acta. 2002;1574:403–406. doi: 10.1016/S0167-4781(01)00364-5. [DOI] [PubMed] [Google Scholar]
- Berry MD, JA V, Li X-M, Boulton AA. Aromatic L-amino acid decarboxylase: a neglected and misunderstood enzyme. Neurochem Res. 1996;21:1075–1087. doi: 10.1007/BF02532418. [DOI] [PubMed] [Google Scholar]
- Duchemin A, Berry MD, Neff NH, Hadjiconstantinou M. Phosphorylation and activation of brain aromatic L-amino acid decarboxylase by cyclic AMP-dependent protein kinase. J Neurochem. 2000;75:725–731. doi: 10.1046/j.1471-4159.2000.0750725.x. [DOI] [PubMed] [Google Scholar]
- Waymire JC, Haycock JW. Lack of regulation of aromatic L-amino acid decarboxylase in intact bovine chromaffin cells. J Neurochem. 2002;81:589–593. doi: 10.1046/j.1471-4159.2002.00849.x. [DOI] [PubMed] [Google Scholar]
- Facchini PJ, Huber-Allanach KL, Tari LW. Plant aromatic L-amino acid decarboxylases: evolution, biochemistry, regulation, and metabolic engineering applications. Phytochem. 2000;54:121–138. doi: 10.1016/S0031-9422(00)00050-9. [DOI] [PubMed] [Google Scholar]
- Pertel R, Wilson SH. Histamine content of the nematode, C. elegans. Comp Gen Pharmacol. 1974;5:83–85. doi: 10.1016/s0306-3623(74)80011-x. [DOI] [PubMed] [Google Scholar]
- Saenz-de-Miera LE, Ayala FJ. Complex evolution of orthologous and paralogous decarboxylase genes. J Evol Biol. 2004;17:55–66. doi: 10.1046/j.1420-9101.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- Mounsey A, Bauer P, Hope IA. Evidence suggesting that a fifth of annotated Caenorhabditis elegans genes may be pseudogenes. Genome Res. 2002;12:770–775. doi: 10.1101/gr208802. 10.1101/gr208802. Article published online before print in April 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force A, Lynch M, Pickett FB, Amores A, Yan Y-L, Postlethwait J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151:1531–1545. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh BJ. Sequence-dependent gene conversion: can duplicated genes diverge fast enough to escape conversion? Genetics. 1987;117:543–557. doi: 10.1093/genetics/117.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh BJ. How often do duplicated genes evolve new functions? Genetics. 1995;139:421–428. doi: 10.1093/genetics/139.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Force A. The probability of duplicate gene preservation by subfunctionalization. Genetics. 2000;154:459–473. doi: 10.1093/genetics/154.1.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of C. elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz HR, Brenner S, Hodgkin J, Herman RK. A uniform genetic nomenclature for the nematode C. elegans. Mol Gen Genet. 1979;175:129–133. doi: 10.1007/BF00425528. [DOI] [PubMed] [Google Scholar]
- Hodgkin J, Horvitz HR, Brenner S. Nondisjunction mutants of the nematode C. elegans. Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans : extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai C, Garriga G, McIntire SL, Horvitz HR. A genetic pathway for the development of the Caenorhabditis elegans HSN motor neurons. Nature. 1988;336:638–646. doi: 10.1038/336638a0. [DOI] [PubMed] [Google Scholar]
- Vaglio P, Lamesch PE, Reboul J, Rual J, Martinez M, Hill DE, Vidal M. WorfDB: the Caenorhabditis elegans ORFeome Database. Nucleic Acids Res. 2003;31:237–240. doi: 10.1093/nar/gkg092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- Swofford DL. In Sunderland, Massachusetts: Sinauer Associates. 4 2000. PAUP*. Phylogenetic analysis using parsimony (*and other methods) [Google Scholar]
- Florea L, Hartzell G, Zhang Z, Rubin GM, Miller W. A computer program for aligning a cDNA sequence with a genomic DNA sequence. Genome Research. 1998;8:967–974. doi: 10.1101/gr.8.9.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- Tatusova TA, Madden TL. BLAST 2 Sequences – a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett. 1999;174:247–250. doi: 10.1016/S0378-1097(99)00149-4. [DOI] [PubMed] [Google Scholar]