Abstract
Background:
While most recent evidence does not support a role for pregnancy in accelerating HIV disease progression, very little information is available on the effects of incident pregnancy in response to antiretroviral therapy (ART). Hormonal, immune, and behavioral changes during pregnancy may influence response to ART. We sought to explore the effects of incident pregnancy (after ART initiation) on virologic, immunologic, and clinical response to ART.
Methods:
Data were collected from HIV-infected women participating in 3 prospective studies (Partners in Prevention Herpes simplex virus/HIV Transmission Study, Couples Observational Study, and Partners Preexposure Prophylaxis Study) from 7 countries in Africa from 2004 to 2012. Women were included in this analysis if they were ≤45 years of age, were started on ART during the study and were not pregnant at ART initiation. Pregnancy was treated as a time-dependent exposure variable covering the duration of pregnancy, including all pregnancies occurring after ART initiation. Virologic failure was defined as a viral load (VL) greater than 400 copies per milliliter ≥6 months after ART initiation and viral suppression was defined as VL ≤400 copies per milliliter. Multivariable Cox proportional hazards models were used to assess the association between pregnancy and time to viral suppression, virologic failure, World Health Organization clinical stage III/IV, and death. Linear mixed-effects models were used to assess the association between pregnancy and CD4+ count and VL. All analyses were adjusted for confounders, including pre-ART CD4+ count and plasma VL.
Results:
A total of 1041 women were followed, contributing 1196.1 person-years of follow-up. Median CD4+ count before ART initiation was 276 cells per cubic millimeter (interquartile range, 209–375); median pre-ART VL was 17,511 copies per milliliter (interquartile range, 2480–69,286). One hundred ten women became pregnant after ART initiation. Pregnancy was not associated with time to viral suppression (adjusted hazard ratio [aHR], 1.20, 95% confidence interval [CI]: 0.82 to 1.77), time to virologic failure (aHR, 0.67, 95% CI: 0.37 to 1.22), time to World Health Organization clinical stage III or IV (aHR, 0.79, 95% CI: 0.19 to 3.30), or time to death (aHR, 2.04, 95% CI: 0.25 to 16.8). Incident pregnancy was associated with an adjusted mean decrease in CD4+ T-cell count of 47.3 cells per cubic millimeter (P < 0.001), but not with difference in VL (P = 0.06).
Conclusions:
For HIV-infected women on ART, incident pregnancy does not affect virologic control or clinical HIV disease progression. A modest decrease in CD4+ T-cell count could be due to physiologic effects of pregnancy.
Key Words: pregnancy, HIV, antiretroviral therapy, response, African
The question of whether pregnancy influences HIV type 1 disease progression has been the subject of several investigations.1–15 However, very limited information is available on the effects of pregnancy in response to antiretroviral therapy (ART). There are several mechanisms by which pregnancy may affect response to ART, both biologic and behavioral, that can affect drug intake and adherence. Pregnancy related physiological changes may affect absorption, distribution, metabolism, or excretion of drugs.16 Pregnancy may also alter immune responses.17
Information on potential effects of incident pregnancy in response to ART is particularly relevant for resource-limited settings, as plans for ART rollout among all HIV-infected women of reproductive age are currently in progress in many such countries. The few studies addressing this question from sub-Saharan African settings have led to differing conclusions of either no change in risk or of increased risk of virologic failure on ART with pregnancy.18–21 Studying the effects of pregnancy in response to ART has, nonetheless, great importance for policy, particularly with regards to counseling HIV-infected women who are on ART about their fertility desires and subsequent family planning or optimizing prepregnancy health.
We examined whether pregnancy affects response to ART among HIV-infected women followed in 3 prospective studies of HIV-serodiscordant couples in Africa.
METHODS
Study Population
Enrollment and follow-up for the Partners in Prevention Herpes simplex virus (HSV)/HIV Transmission Study (2004–2008), the Couples Observational Study (2008–2010), and the Partners Preexposure Prophylaxis (PrEP) Study (2008–2013) has been previously described.22–27 Briefly, HIV-serodiscordant heterosexual couples from 7 African countries (Botswana, Kenya, Rwanda, South Africa, Tanzania, Uganda, and Zambia) were enrolled and followed between 2004 and 2013. Overall in 63% of couples, the HIV-infected partner was female. At the time of enrollment, HIV-infected partners did not meet national guideline eligibility criteria for ART use; during follow-up, clinical and immunologic status was monitored and ART was recommended and initiated according to national guidelines.
Demographic information was collected at enrollment, whereas data on ART use were collected quarterly with follow-up continuing for a maximum of 1 year (Couples Observational Study), 2 years (Partners in Prevention HSV/HIV Transmission Study), or 3 years (Partners PrEP Study). CD4+ T-cell counts were measured every 6 months; HIV viral load (VL) was measured every 6 months in the Partners in Prevention Study and Couples Observational Study and annually in the Partners PrEP Study. Pregnancy status was ascertained at each study visit by history; urine pregnancy tests were performed quarterly in the Partners in Prevention HSV/HIV Transmission Study and as clinically indicated in the other 2 studies. Date of last menstrual period (LMP), estimated date of delivery, and pregnancy information were collected with standardized questionnaires. All women gave written informed consent. The studies were approved by the University of Washington Human Subjects Review Committee and local ethic review boards at each study site.
Statistical Analysis
For this analysis, women were included if they (1) were enrolled as the HIV-infected partner in the HIV-serodiscordant couple, (2) were started on ART during study follow-up, (3) were not pregnant at the time of ART initiation, (4) were aged 45 years or younger, and (5) had at least one study visit at which plasma was obtained for HIV VL assessment after starting ART (N = 1041). Pregnancies prevalent at the time of ART initiation were excluded, as many pregnant women are started on ART to prevent mother-to-child transmission of HIV, and not for their own clinical indications. The exposure was pregnancy occurring after ART initiation, defined as a time-dependent variable, which could include multiple pregnancies occurring after ART initiation. Start of pregnancy was defined as the date of LMP and the end of pregnancy as the date of delivery or pregnancy loss. Complete data on LMP and date of delivery were available for 96% of pregnancies; for the remaining pregnancies, start and end dates were estimated using the reported duration of the pregnancy based on maternal history and either the LMP or date of delivery. Virologic failure was defined as plasma HIV RNA greater than 400 copies per milliliter 6 months or later after ART initiation. This included both failure to achieve virologic suppression within 6 months of initiating ART and virologic rebound after initial suppression. To meet this outcome, women had to have at least one plasma HIV VL result 6 months or later after ART initiation (n = 769).
We compared baseline characteristics between women who became pregnant after ART initiation and those who did not using Wilcoxon rank-sum tests or χ2 tests. Multivariable Cox proportional hazards models stratified by study and extended Kaplan–Meier curves28 were used to assess the effect of incident pregnancy after ART initiation on time to virologic suppression (HIV VL ≤400 copies per milliliter), time to virologic failure, time to World Health Organization (WHO) clinical stage III or IV, and time to death. Linear mixed-effects models were used to assess the effect of time-varying incident pregnancy on CD4+ T-cell count and plasma HIV VL after ART initiation. Age, study year, education, income, marital status, parity, CD4+ T-cell count before ART initiation, HIV VL before ART initiation, and diagnosis of tuberculosis at ART initiation were considered as potential confounders, and were included in final models if the hazard ratio for incident pregnancy changed by greater than 10%. All analyses were conducted in SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
Participant Characteristics
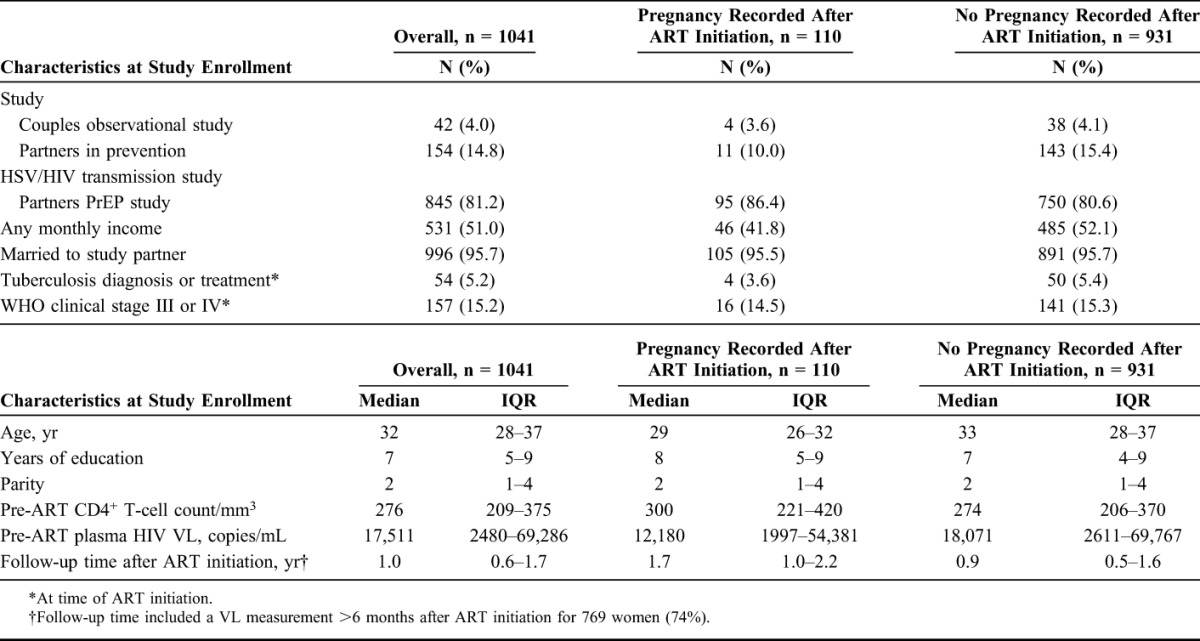
Among the 1041 women included in this analysis, most (81.2%) were enrolled in the Partners PrEP Study. The great majority (95.7%) were married, whereas about half reported any monthly income (Table 1). Their median age was 32 years, median duration of education was 7 years, and median number of children was 2 (Table 1). The median CD4+ T-cell count before ART initiation was 276 cells per cubic millimeter (interquartile range [IQR], 209–375 cells per cubic millimeter), and the median HIV VL before ART initiation was 17,511 copies per milliliter (IQR, 2480–69,286 copies per milliliter). Approximately 5% had a diagnosis of or had been treated for tuberculosis at ART initiation (Table 1). The most common first-line ART regimens included zidovudine or stavudine or tenofovir, lamivudine, and nevirapine or efavirenz.
TABLE 1.
Baseline Characteristics for 1041 HIV-Infected Women Included in the Analysis, by Pregnancy Status After Initiating ART
Effect of Pregnancy in Response to ART
The 1041 women contributed 1196.1 person-years of follow-up, with a median follow-up time of 1.0 year (IQR, 0.6–1.7). One hundred ten women (10.6%) became pregnant at least once after ART initiation. Women who became pregnant were younger (median age, 29 vs. 33 years, P < 0.001) and less likely to have any monthly income (41.8% vs. 52.1%, P = 0.04) than those who did not become pregnant (Table 1). Overall, the proportion of women achieving viral suppression was 82.9%; virologic failure occurred in 24.7% of women, 5.9% progressed to WHO clinical stage III or IV, and 1.2% died. Pregnancy was not associated with time to viral suppression (adjusted hazard ratio, [aHR], 1.20, 95% confidence interval [CI]: 0.82 to 1.77), time to virologic failure (aHR, 0.67, 95% CI: 0.37 to 1.22), time to WHO clinical stage III or IV (aHR, 0.79, 95% CI: 0.19 to 3.30), or time to death (aHR, 2.04, 95% CI: 0.25 to 16.8) (Table 2). The median time to viral suppression for those who became pregnant was 4.29 months compared with 4.26 months for those who did not become pregnant. Incident pregnancy was associated with an adjusted mean decrease in CD4+ T-cell count of 47.3 cells per cubic millimeter (P < 0.001), but not with difference in HIV plasma VL (P = 0.06) (Tables 3 and Fig. 1).
TABLE 2.
Associations Between Incident Pregnancy and Clinical/Virologic Outcomes After Initiation of ART: The Couples Observational Study, the Partners in Prevention Study, and the Partners PrEP Study
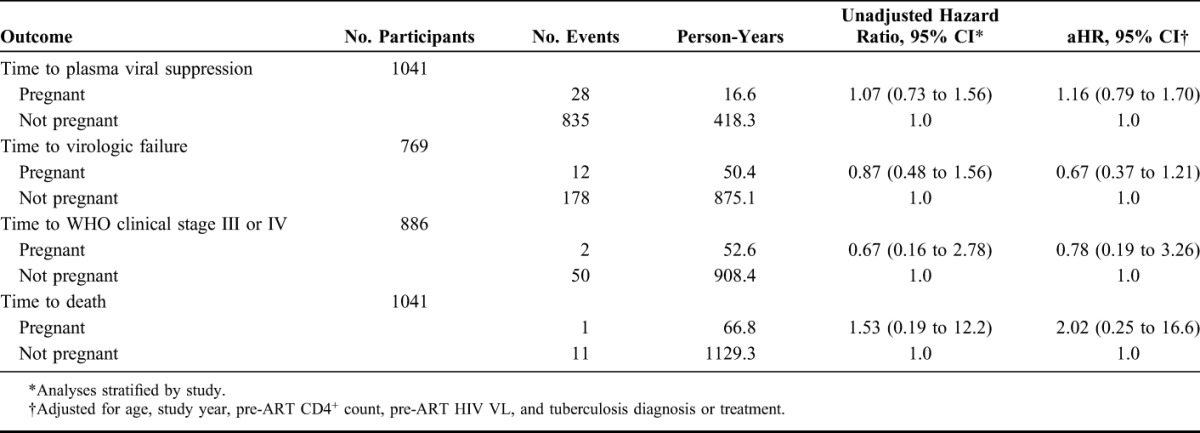
TABLE 3.
Associations Between Incident Pregnancy and CD4+ T-Cell Count and Plasma HIV VL After Initiation of ART: The Couples Observational Study, the Partners in Prevention Study, and the Partners PrEP Study
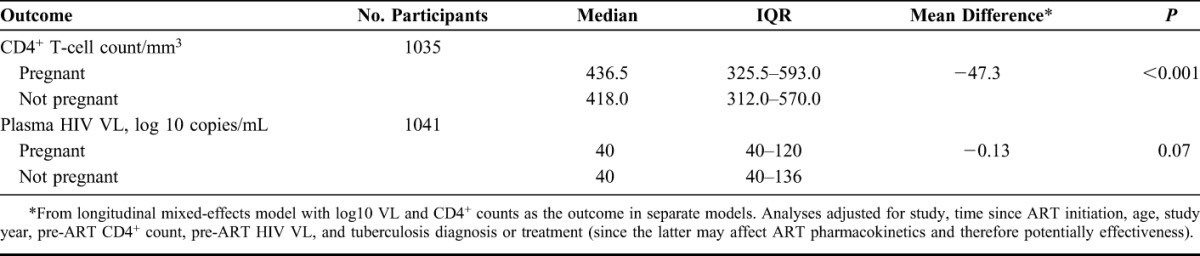
FIGURE 1.
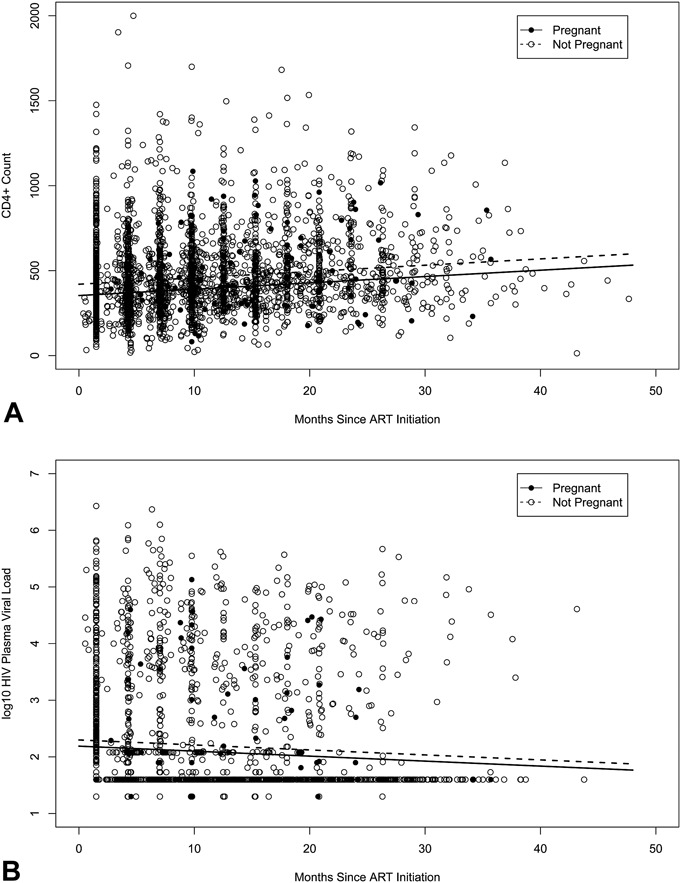
CD4+ T-cell count (A) and Plasma HIV VL (B) from initiation of ART by time-varying incident pregnancy.
Because the studies did not have information about attendance at clinical care visits outside the research clinics, we used study retention as a proxy to evaluate overall compliance with care. This was to see whether any differences by pregnancy status may be explained by differences in follow-up, additional testing, and care of pregnant women compared with nonpregnant women. There was no difference in the odds of attendance at the next regular study visit between pregnant and nonpregnant women (odds ratio, 0.79, 95% CI: 0.59 to 1.06).
DISCUSSION
In this analysis of prospective data from HIV-infected women who initiated ART in 7 African countries, we found that incident pregnancy after ART initiation was not associated with a delay in time to virologic suppression, increased risk of virologic failure of therapy during follow-up, or increased risk of clinical progression of HIV disease to WHO clinical stage III or IV, or death. A modest decrease of CD4+ T-cell count was seen among those with incident pregnancies (mean decrease of 47.7 cells per cubic millimeter compared with nonpregnant women) over the course of follow-up.
Pregnancy-induced changes in the immune system may increase susceptibility to and severity of infectious diseases.29 Although most evidence does not support a role for pregnancy in accelerating HIV disease progression, mainly from studies from the United States and Europe,2–4,7–15 some data from developing countries have suggested progression of HIV disease in pregnancy.5,6 Differences in the impact of pregnancy on HIV disease across geographic areas may reflect differences in other concomitant factors, such as poverty, nutrition, concurrent infections, or stage of HIV disease at the time of pregnancy.
Limited information is available about the effects of pregnancy in response to ART. Physiologic changes during pregnancy are multifaceted and may be compounded by the introduction of ART. When a meta-analysis of studies on pregnancy and HIV disease progression published through 2013 was stratified by ART availability, the pooled estimates for the effect of pregnancy on accelerating progression to low CD4+ T-cell count, HIV-related illness, AIDS-defining illness, and death were attenuated when ART was available, with significant effect modification by ART availability on the effects of pregnancy on AIDS-defining illness and death.1 For most of these studies, ART, if available, was initiated during pregnancy. Data on the effect of incident pregnancy among women already taking ART are even more limited. It is however important to make this distinction, as women who initiate ART during pregnancy do so mainly for prevention of mother-to-child transmission and are more likely to be healthier, whereas women who are on ART when they become pregnant are more likely to have initiated ART for their own immunologic or clinical indications. In a single study with the primary aim of evaluating incident pregnancy on clinical HIV disease progression among HIV-infected women who became pregnant in South Africa, incident pregnancy after ART initiation was not associated with an increased hazard of a new AIDS event or death.30
Unlike our study's findings of no effect of incident pregnancy on virologic failure, a study of 541 pregnancies during follow-up of 5954 women in South Africa found incident pregnancy after combination ART initiation was associated with a modest increase in risk of virologic failure (aHR, 1.34, 95% CI: 1.02 to 1.78).18 That study's strength was its large sample size and careful statistical analysis; however, it was based on analysis of observational data from a single clinic's database that lacked baseline HIV VL for most women, and confounding cannot be ruled out. In 3 much smaller studies, there was no evidence of an effect of incident pregnancy on HIV RNA change or virologic failure.19–21 The largest of these studies was among 128 women who became pregnant after 3 or more months of combination ART in the Swiss Mother and Child HIV Cohort Study, which found no difference in the frequency of virologic failure during pregnancy compared with a period of equal duration before and after pregnancy (adjusted odds ratio = 1.04, 95% CI:0.48 to 2.28).20
Our finding of a modest decrease of the CD4+ T-cell count among women with incident pregnancies (mean decrease of 47.7 cells per cubic millimeter) was not unexpected. Cellular immunity and CD4+ lymphocyte levels are expected to decline during pregnancy in all women but eventually return to prepregnancy levels.31 In a pooled analysis of 4 studies,3,9,11,12 HIV-infected pregnant women exposed to no or partial ART had marginally increased risk (1.41, 95% CI: 0.99 to 2.02) of progressing to a low CD4+ T-cell count compared with nonpregnant HIV-infected women.1 Although these studies counted the postpartum period in the pregnancy person-time, other studies have compared the prepregnancy, pregnancy, and postpartum periods and found that CD4+ T-cell count declines during pregnancy are temporary effects, likely due to physiologic effects of pregnancy, and rebound in the postpartum period.15,19
Our analysis has some limitations. The follow-up time was not long enough to allow separate evaluation of pregnancy versus any postpartum effects. As most of the women were on a standard first-line ART regimen, we were also unable to examine possible effects of pregnancy in response to different ART regimens. Despite our relatively large sample size, the power of this study to detect small effects of pregnancy in response to ART may be limited. Strengths of our study include the prospective, systematic, and detailed data collection across all clinical study participants, statistical analysis to account for time-varying variables, and recruitment from several countries in Africa, which may enhance generalizability of the results.
Rapid adoption of the WHO's recommendation of lifelong ART for all pregnant and breastfeeding women living with HIV regardless of CD4+ T-cell count or clinical stage of disease has led to large increases in the proportion of HIV-infected pregnant women using and seeking ART. Moreover, with the recommendation of maintaining ART for life and the low risk of HIV transmission to their infants when HIV viremia is suppressed, more women will remain healthy and experience repeat incident pregnancies while using ART. Indeed, emerging data suggest that use of ART is associated with heightened feelings of hope, health, fertility desires, and a 70% higher rate of subsequent pregnancy.32–35 It is thus important to understand how pregnancy may affect a woman's response to ART. Our findings add to the emerging body of evidence that incident pregnancy does not seem to affect virologic, immunologic, and clinical response to ART. These findings have implications for counseling HIV-infected women of reproductive age who desire pregnancy about the potential for a healthy pregnancy and child when ART is used. However, intensive counseling about the importance of antenatal and postnatal adherence to ART for the mothers' and infant's health and continued careful monitoring of long-term clinical outcomes for both women and their infants is important.
ACKNOWLEDGMENTS
The authors thank the women who participated in these studies. Partners in Prevention HSV/HIV Transmission Study Team included University of Washington Coordinating Center and Central Laboratories, Seattle, WA: C.C. (principal investigator), Anna Wald (protocol co-chair), J.R.L. (medical director), J.M.B., Mary Campbell, Lawrence Corey, Robert W. Coombs, James P. Hughes, Amalia Magaret, M. Juliana McElrath, Rhoda Morrow, and James I. Mullins. Study sites and site principal investigators: Cape Town, South Africa (University of Cape Town): David Coetzee; Eldoret, Kenya (Moi University, Indiana University): Kenneth Fife, Edwin Were; Gaborone, Botswana (Botswana Harvard Partnership): Max Essex, Joseph Makhema; Kampala, Uganda (Infectious Disease Institute, Makerere University): Elly Katabira, Allan Ronald; Kigali, Rwanda (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Kayitesi Kayitenkore, Etienne Karita; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig Cohen; Kitwe, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, William Kanweka; Lusaka, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Bellington Vwalika; Moshi, Tanzania (Kilimanjaro Christian Medical College, Harvard University): Saidi Kapiga, Rachel Manongi; Nairobi, Kenya (University of Nairobi, University of Washington): Carey Farquhar, Grace John-Stewart, James Kiarie; Ndola, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Mubiana Inambao; Orange Farm, South Africa (Reproductive Health Research Unit, University of the Witwatersrand): Sinead Delany-Moretlwe, Helen Rees; Soweto, South Africa (Perinatal HIV Research Unit, University of the Witwatersrand): Guy de Bruyn, Glenda Gray, James McIntyre; Thika, Kenya (Kenyatta Nationa Hospital University of Washington): N.R.M., Kenneth Ngure Partners PrEP Study Team included University of Washington Coordinating Center and Central Laboratories, Seattle, WA: C.C. (principal investigator, protocol cochair), J.M.B. (medical director, protocol cochair), D.D. (protocol statistician), Robert W. Coombs, J.R.L., M. Juliana McElrath.Study sites and site principal investigators: Eldoret, Kenya (Moi University, Indiana University): Kenneth H. Fife, Edwin Were; Kabwohe, Uganda (Kabwohe Clinical Research Center): Elioda Tumwesigye; Jinja, Uganda (Makerere University, University of Washington): Patrick Ndase, Elly Katabira; Kampala, Uganda (Makerere University): Elly Katabira, Allan Ronald; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig R. Cohen; Mbale, Uganda (The AIDS Support Organization, CDC-Uganda): Jonathan Wangisi, James D. Campbell, Jordan W. Tappero; Nairobi, Kenya (University of Nairobi, University of Washington): James Kiarie, Carey Farquhar, Grace John-Stewart; Thika, Kenya, (Kenyatta National Hospitali, University of Washington): N.R.M., Kenneth Ngure; Tororo, Uganda (CDC-Uganda, The AIDS Support Organization): James D. Campbell, Jordan W. Tappero, Jonathan Wangisi. Data management was provided by DF/Net Research, Inc. (Seattle, WA) and site laboratory oversight was provided by Contract Lab Services (University of the Witwatersrand, Johannesburg, South Africa).
Footnotes
The sources of support included Health Promotion and Disease Prevention Research Center supported by Cooperative Agreement from the Centers for Disease Control and Prevention (U48 DP 005013 SIP 14-023), the Eunice Kennedy Shriver National Institute of Child Health and Development of the United States National Institutes of Health (R21 HD074439) and the Bill & Melinda Gates Foundation (OPP1056051, 26469, and 41185).
The authors have no funding or conflicts of interest to disclose.
The opinions expressed in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Calvert C, Ronsmans C. Pregnancy and HIV disease progression: a systematic review and meta-analysis. Trop Med Int Health. 2015;20:122–145. [DOI] [PubMed] [Google Scholar]
- 2.Berrebi A, Kobuch WE, Puel J, et al. Influence of pregnancy on human immunodeficiency virus disease. Eur J obstet Gynecol Reprod Biol. 1990;37:211–217. [DOI] [PubMed] [Google Scholar]
- 3.Weisser M, Rudin C, Battegay M, et al. Does pregnancy influence the course of HIV infection? Evidence from two large Swiss cohort studies. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:404–410. [DOI] [PubMed] [Google Scholar]
- 4.Minkoff HL, Willoughby A, Mendez H, et al. Serious infections during pregnancy among women with advanced human immunodeficiency virus infection. Am J obstet Gynecol. 1990;162:30–34. [DOI] [PubMed] [Google Scholar]
- 5.Kumar RM, Uduman SA, Khurrana AK. Impact of pregnancy on maternal AIDS. J Reprod Med. 1997;42:429–434. [PubMed] [Google Scholar]
- 6.Deschamps MM, Pape JW, Desvarieux M, et al. A prospective study of HIV-seropositive asymptomatic women of childbearing age in a developing country. J Acquir Immune Defic Syndr. 1993;6:446–451. [PubMed] [Google Scholar]
- 7.Bessinger R, Clark R, Kissinger P, et al. Pregnancy is not associated with the progression of HIV disease in women attending an HIV outpatient program. Am J Epidemiol. 1998;147:434–440. [DOI] [PubMed] [Google Scholar]
- 8.Tai JH, Udoji MA, Barkanic G, et al. Pregnancy and HIV disease progression during the era of highly active antiretroviral therapy. J Infect Dis. 2007;196:1044–1052. [DOI] [PubMed] [Google Scholar]
- 9.Alliegro MB, Dorrucci M, Phillips AN, et al. Incidence and consequences of pregnancy in women with known duration of HIV infection. Italian Seroconversion Study Group. Arch Intern Med. 1997;157:2585–2590. [PubMed] [Google Scholar]
- 10.Buskin SE, Diamond C, Hopkins SG. HIV-infected pregnant women and progression of HIV disease. Arch Intern Med. 1998;158:1277–1278. [DOI] [PubMed] [Google Scholar]
- 11.Hocke C, Morlat P, Chene G, et al. Prospective cohort study of the effect of pregnancy on the progression of human immunodeficiency virus infection. The Groupe d'Epidemiologie Clinique Du SIDA en Aquitaine. Obstet Gynecol. 1995;86:886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van der Paal L, Shafer LA, Todd J, et al. HIV-1 disease progression and mortality before the introduction of highly active antiretroviral therapy in rural Uganda. AIDS. 2007(21 suppl 6):S21–S29. [DOI] [PubMed] [Google Scholar]
- 13.Saada M, Le Chenadec J, Berrebi A, et al. Pregnancy and progression to AIDS: results of the French prospective cohorts. SEROGEST and SEROCO Study Groups. AIDS. 2000;14:2355–2360. [DOI] [PubMed] [Google Scholar]
- 14.Allen S, Stephenson R, Weiss H, et al. Pregnancy, hormonal contraceptive use, and HIV-related death in Rwanda. J Womens Health (Larchmt). 2007;16:1017–1027. [DOI] [PubMed] [Google Scholar]
- 15.Heffron R, Donnell D, Kiarie J, et al. A prospective study of the effect of pregnancy on CD4 counts and plasma HIV-1 RNA concentrations of antiretroviral-naive HIV-1-infected women. J Acquir Immune Defic Syndr. 2014;65:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loebstein R, Lalkin A, Koren G. Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet. 1997;33:328–343. [DOI] [PubMed] [Google Scholar]
- 17.Sappenfield E, Jamieson DJ, Kourtis AP. Pregnancy and susceptibility to infectious diseases. Infect Dis obstet Gynecol. 2013;2013:752852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westreich D, Cole SR, Nagar S, et al. Pregnancy and virologic response to antiretroviral therapy in South Africa. PLoS One. 2011;6:e22778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayanja BN, Shafer LA, Van der Paal L, et al. Effect of pregnancy on immunological and virological outcomes of women on ART: a prospective cohort study in rural Uganda, 2004-2009. Trop Med Int Health. 2012;17:343–352. [DOI] [PubMed] [Google Scholar]
- 20.Keiser O, Gayet-Ageron A, Rudin C, et al. Antiretroviral treatment during pregnancy. AIDS. 2008;22:2323–2330. [DOI] [PubMed] [Google Scholar]
- 21.Melekhin VV, Shepherd BE, Stinnette SE, et al. Antiretroviral therapy initiation before, during, or after pregnancy in HIV-1-infected women: maternal virologic, immunologic, and clinical response. PLoS One. 2009;4:e6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pyra M, Heffron R, Mugo NR, et al. Effectiveness of hormonal contraception in HIV-infected women using antiretroviral therapy. AIDS. 2015;29:2353–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lingappa JR, Baeten JM, Wald A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010;375:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mujugira A, Baeten JM, Donnell D, et al. Characteristics of HIV-1 serodiscordant couples enrolled in a clinical trial of antiretroviral pre-exposure prophylaxis for HIV-1 prevention. PLoS One. 2011;6:e25828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snapinn SM, Jiang Q, Iglewicz B. Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimator. Am Stat. 2005;59:301–307. [Google Scholar]
- 29.Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;371:1077. [DOI] [PubMed] [Google Scholar]
- 30.Westreich D, Maskew M, Evans D, et al. Incident pregnancy and time to death or AIDS among HIV-positive women receiving antiretroviral therapy. PLoS One. 2013;8:e58117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landers DV, Martinez de Tejada B, Coyne BA. Immunology of HIV and pregnancy. The effects of each on the other. Obstet Gynecol Clin North Am. 1997;24:821–831. [DOI] [PubMed] [Google Scholar]
- 32.Myer L, Carter RJ, Katyal M, et al. Impact of antiretroviral therapy on incidence of pregnancy among HIV-infected women in Sub-Saharan Africa: a cohort study. PLoS Med. 2010;7:e1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper D, Harries J, Myer L, et al. “Life is still going on”: reproductive intentions among HIV-positive women and men in South Africa. Soc Sci Med. 2007;65:274–283. [DOI] [PubMed] [Google Scholar]
- 34.Cooper D, Moodley J, Zweigenthal V, et al. Fertility intentions and reproductive health care needs of people living with HIV in Cape Town, South Africa: implications for integrating reproductive health and HIV care services. AIDS Behav. 2009(13 suppl 1):38–46. [DOI] [PubMed] [Google Scholar]
- 35.Kaida A, Matthews LT, Kanters S, et al. Incidence and predictors of pregnancy among a cohort of HIV-positive women initiating antiretroviral therapy in Mbarara, Uganda. PLoS One. 2013;8:e63411. [DOI] [PMC free article] [PubMed] [Google Scholar]


