Abstract
We investigate the association between phosphorylated histone H3 (PhH3) and Oncotype DX recurrence score (RS). All invasive breast carcinoma with RS results from our city between 2007 and 2010 (n=47) were reviewed. Whole-tumor sections were stained for PhH3. Mitotic and PhH3 counts were performed and clinical charts reviewed. PhH3 correlated well with RS (r=0.69, P<0.001). Other correlations were: PhH3 versus mitotic count (r=0.87, P<0.001), PhH3 versus mitotic score (r=0.71, P<0.001), PhH3 versus modified Bloom-Richardson-Elston (MBR) grade (r=0.65, P<0.001), RS versus mitotic count (r=0.62, P<0.001), RS versus mitotic score (r=0.44, P=0.002), and RS versus MBR grade (r=0.49, P=0.001). Significant correlation between PhH3 and RS remained after controlling for mitotic count (r=0.39, P=0.007), mitotic score (r=0.60, P<0.001), MBR grade (r=0.56, P<0.001), and all 3 (r=0.37, P=0.014) by partial correlation. Two patients died of metastasis at 12 and 38 months after diagnosis. One had intermediate RS, and 1 high RS; both were in the top-third of PhH3 count. All other patients are alive and recurrence free. Correlation between PhH3 and RS was statistically significant in our cohort, and remained significant after controlling for traditional measures of proliferation. Given that RS has an established strong relationship with prognosis and therapy responsiveness, PhH3 may thus also be an important prognostic/predictive marker in breast cancer.
Key Words: breast neoplasms, immunohistochemistry, biomarker
Breast carcinoma is the most common cancer in women, comprising 25% of cancers and 15% of cancer-related deaths.1 The evaluation of breast cancer prognosis and guidance for treatment has traditionally been based on several clinicopathologic factors including age, TNM tumor stage, histologic subtype, tumor grade [modified Bloom-Richardson-Elston (MBR)], lymph-vascular invasion, and margin status. It was later discovered that the expression of certain proteins was also relevant, first the estrogen receptor (ER) in the 1970s, and later progesterone receptor (PR) and receptor tyrosine-protein kinase erbB-2 [human epidermal growth factor receptor 2 (HER2)].2 However, because adjuvant chemotherapy results in acute and chronic toxicities,3 further refinement of individual patient prognosis was required to limit chemotherapy exposure to those whose risk of recurrence warranted the adverse effects.
In the 2000s, molecular profiling was developed in an attempt to predict recurrence risk and thus guide chemotherapy use. One product is Oncotype DX (Genomic Health Inc., Redwood City, CA), which assesses the expression of 21 genes and, using an algorithm heavily weighted toward cell proliferation markers, generates a recurrence score (RS) that estimates the probability of tumor recurrence.4 Although useful as a prognostic and predictive factor with extensive validation in large random-controlled studies,4–7 it remains expensive. There are potential drawbacks with molecular testing, including the problem of tumor heterogeneity, “contamination” by nontumoral elements found in the stroma,8 and long turnaround times. Evidence also suggests that histologic and immunohistochemical features of breast carcinoma may provide similar recurrence information.9,10
The recent introduction of PhH3 immunohistochemistry (IHC) augments our ability to evaluate cell proliferation. Phosphorylation of histone H3 at serines 10 or 28 is crucial for chromosome condensation and cell-cycle progression during mitosis and meiosis.11 Phosphorylation begins in late G2 phase, is maximal at metaphase, and dephosphorylates at the beginning of telophase.12 Because PhH3 expression overlaps with the majority of mitotic phases, and because telophase nuclei are probably not included in mitotic counts by most observers, immunohistochemical evaluation of this marker is a reasonable surrogate for mitosis. However, because PhH3 does not exactly correlate with mitosis, variances between PhH3 and morphology-based mitotic counts can be expected.13,14 Whether or not these variances might provide meaningful insight into the predictive or prognostic value of PhH3 and other markers of proliferation has only recently been the focus of study, and results to date have been limited.10,15–19
With this in mind, the objective of our study is to determine the relationship between PhH3 IHC and the Oncotype DX RS in patients with breast carcinoma, with the goal of developing a foundation for the prospective evaluation of PhH3 as a potentially cost-effective alternative to RS.
MATERIALS AND METHODS
Case and Slide Selection
All invasive breast carcinoma cases with RS results from all hospitals of the Calgary metropolitan area between 2007 and 2010 were selected. Blinded to the original diagnosis, all cases were reviewed and regraded by a breast pathologist to confirm initial assessment. The section previously sent for Oncotype DX testing was selected for mitotic count and PhH3 IHC. If the tissue block for that section was unavailable, the adjacent tissue section containing the highest mitotic activity was used. Chart review was performed on these cases to obtain clinical data, including age, sex, RS score, treatment, time to recurrence, and status at last follow-up.
Mitotic Count
Mitotic counts were performed on the original hematoxylin and eosin (H&E) slides targeting the region with the highest mitotic activity. Ten adjacent high-power fields (HPFs) (Nikon Eclipse E600 microscope, ×40 objective, 0.65 aperture; Nikon Corporation, Tokyo) were counted, with the average of 2 independent counts by 2 pathologists used in analyses. Mitotic score was derived from the mitotic count according to the standard cut-offs for field diameter.
PhH3 IHC and Count
Four-μm-thick sections were made from the selected tissue block and stained for PhH3 (rabbit polyclonal, 1/200 dilution; Cell Marque Corp., Rocklin, CA). Antigens were retrieved using heat-induced epitope retrieval with the EDTA-based Leica Bond Epitope Retrieval Solution 2 (Leica Microsystems GmbH, Wetzlar, Germany) for 20 minutes at 100°C and pH 9.0. They were processed on the Leica Bond III stainer using the Leica Bond Polymer Refine Detection utilizing a poly-HRP anti-mouse IgG reagent that localizes the primary antibody, DAB chromogen, and haematoxylin counterstain. Tonsils were used as positive controls. Negative controls were run simultaneously with the primary antibody replaced with an antibody against non-human antigens (IgG1×0931, mouse monoclonal; Dako Corp, Carpinteria, CA). PhH3 count was performed in a manner similar to mitotic count in the region with the highest PhH3 concentration. Nuclei with weak fine granular PhH3 were not counted as these are not in mitotic or G2 phases.20 The average of 2 independent counts by 2 pathologists was used.
Statistical Analysis
Correlations between RS and PhH3, RS and traditional proliferation variables (grade, mitotic count, and mitotic score), and PhH3 and the traditional proliferation variables were plotted with corresponding Pearson correlation coefficient (r). Variables were analyzed as continuous; in particular, the numerical RS was used rather than the risk group categorization. Correlations between RS and PhH3 count were also controlled for confounding variables—mitotic score, mitotic count, and MBR grade, using partial correlations, and correlations between RS and the traditional proliferation variables were controlled for PhH3 as a comparison. Patient survival was calculated with Kaplan-Meier survival curves and log-rank test. The level of significance was selected as P≤0.05. Statistical analyses were performed using SPSS 19.0 (IBM Corporation, Armonk, NY).
This study was approved by our institutional ethics board.
RESULTS
Forty-seven cases were available for retrieval and review (25 low RS, 15 intermediate RS, and 7 high RS). Clinicopathologic characteristics are summarized in Table 1.
TABLE 1.
Patient Characteristics (N=47)
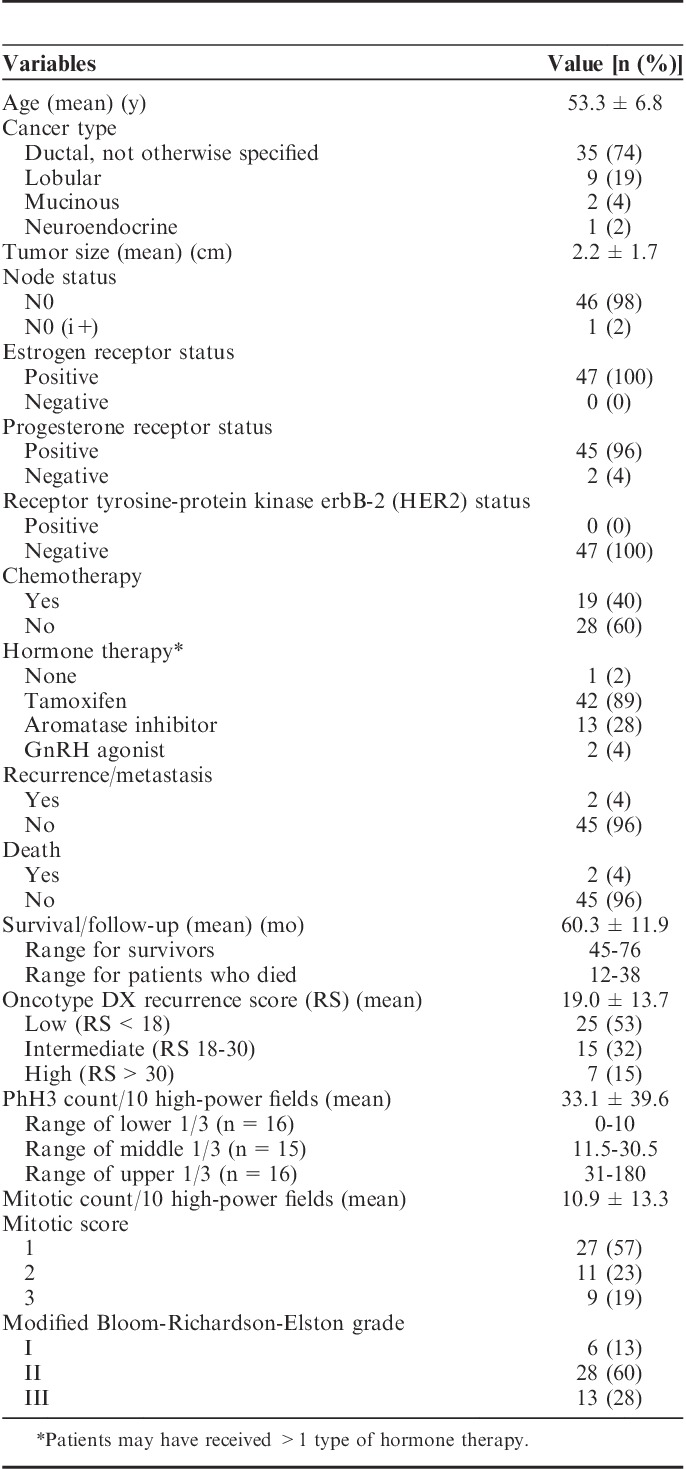
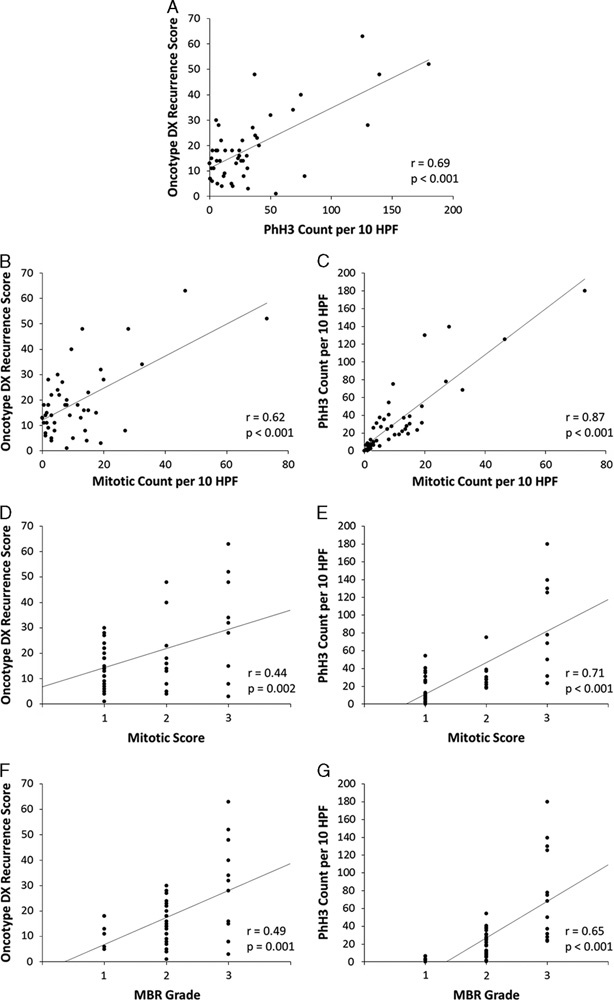
Correlations between all variables are summarized in Figure 1. Correlations between RS and PhH3 were strongly positive and statistically significant (Table 2). A positive and significant correlation remained after controlling for each of the confounding variables as well as all 3. Performing correlations between RS and each confounding variable, but controlling for PhH3 reveals that each variable loses its significance (Table 3).
FIGURE 1.
Correlations between (A) Oncotype DX RS and PhH3 count per 10 HPF (B, D, F) Oncotype DX RS and each confounding factor, and (C, E, G) PhH3 count per 10 HPF and each confounding factor. HPF indicates high-power field; MBR, modified Bloom-Richardson-Elston; PhH3, phosphorylated histone H3; RS, recurrence score.
TABLE 2.
Correlation Between Oncotype DX RS and PhH3, and Partial Correlations Controlling for Traditional Proliferation Variables
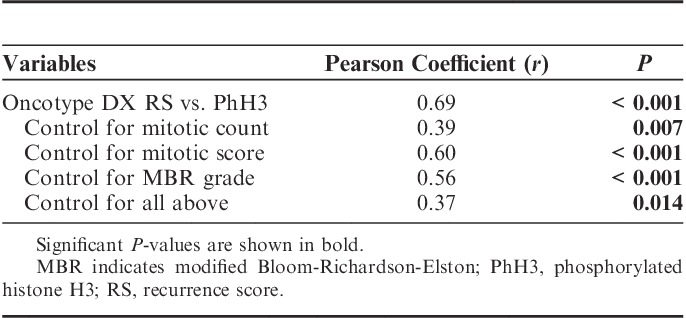
TABLE 3.
Correlation Between Oncotype DX RS and Traditional Proliferation Variables, and Partial Correlation Controlling for PhH3
Two patients died. Patient 1 died of metastatic disease 12 months after diagnosis. Patient 2 died of an intracranial hemorrhage 38 months after diagnosis, presumably due to metastatic disease (no autopsy was performed). Patient 1 had intermediate RS (RS=20) and patient 2 had high RS (RS=32). Both were in the top-third of PhH3 counts (patient 1 PhH3 count=40.5, patient 2 PhH3 count=50). Survival analysis did not show difference in survival between low, intermediate, and high RS risk group (χ2(2)=2.93, P=0.232), or between lower, middle, and upper PhH3 counts (χ2(2)=4.00, P=0.135), although clinical follow-up is limited by the short time Oncotype DX has been available to patients in Alberta.
DISCUSSION
MBR grade has long been an important prognostic and predictive factor in breast cancer. However, 34% of breast cancers fall into the grade II category,21 which when hormone positive, HER2 negative, and node negative, continues to be challenging for clinicians in their decision of whether to offer adjuvant chemotherapy.22 Thus, the application of molecular testing such as Oncotype DX to predict who will most benefit from chemotherapy has become increasingly popular. However, there is increasing evidence that histologic evaluation of proliferation markers may provide similar prognostic and predictive information.9,10 We found that PhH3 has a strong correlation with Oncotype DX RS score, according to Dancey and Reidy’s23 categorization of correlation strength. The strongest correlation was between PhH3 and mitotic count; similar correlations between these 2 counts have been found in other studies of both breast and nonbreast carcinomas.13,24,25 As would be expected, correlation of PhH3 with mitotic score was weaker due to loss of detail when binning counts, and correlation of PhH3 with MBR grade was weakest because grade includes nonproliferative information. Correlations between RS and mitotic count, score, and MBR grade were moderate in strength, but weaker than RS versus PhH3, suggesting that PhH3 expression is a more reliable indicator of proliferative activity than morphologic assessment of mitotic activity or tumor grade. This suggestion is supported by the observation in our study that a positive, statistically significant correlation between PhH3 and RS remains after controlling for the traditional proliferation variables.
Interestingly, while each confounder has a significant correlation with RS, all lose their correlation when controlled for PhH3. Several factors likely contribute to this observation: PhH3 IHC likely improves the accuracy of the mitotic count, notably through significantly improved signal-to-noise ratio compared with H&E mitotic figures that allows for easy identification of high proliferative areas; PhH3 stains more readily guide the pathologist to areas best suited for standard counts of 10 consecutive HPFs; accuracy may also be improved because PhH3 does not stain mimickers of mitoses such as hyperchromatic, karyorrhectic, and apoptotic nuclei, although rare nonspecific staining can occur.19,26 Standard practice in the morphologic assessment of mitosis requires that only clearly identifiable mitotic figures in metaphase, anaphase, and telophase should be counted on H&E to prevent the counting of the mimickers.21 This not only ignores cells in prophase, but also misses the mitoses that are not clear mitotic figures as is common in malignancies. PhH3 would identify these (Fig. 2). On balance, the inclusion of cells in late G2 phase by PhH3 and the lack of prophase in H&E mitotic counts accounts for higher PhH3 counts compared with mitotic counts. Aside from the issue of accuracy, because PhH3 IHC does not correspond precisely to the mitotic phase, there may be inherently more information, or more important information, in the phases PhH3 labels as compared with the H&E mitoses.
FIGURE 2.
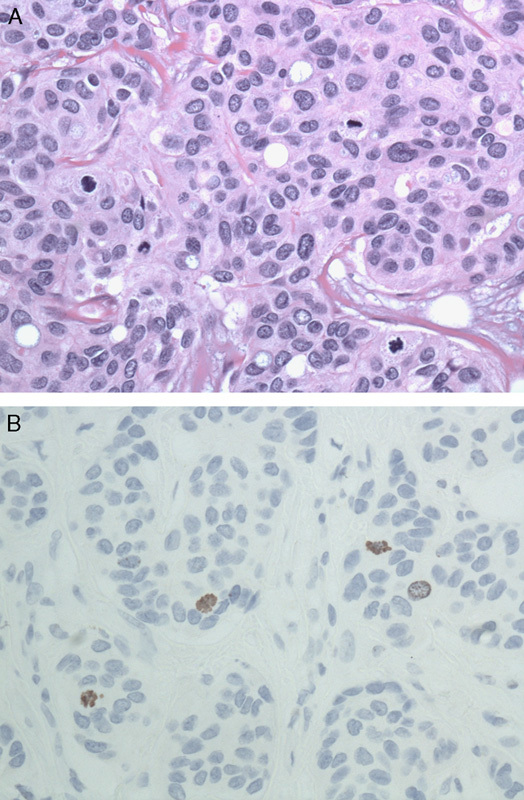
A, It may be difficult to determine if a potential mitotic figure on hematoxylin and eosin is truly a mitotic figure (×400). B, Immunohistochemistry stain for PhH3 highlights mitotic figures (×400). PhH3 indicates phosphorylated histone H3.
The clinical importance of our findings is grounded in the demonstration that Oncotype DX has an established strong relationship with prognosis and therapy responsiveness, a conclusion that has been validated through adhoc retrospective analyses of the RS of patients in several large randomized control trials. Analyses of the National Surgical Adjuvant Breast and Bowel Project B14 and B20 patients showed that RS could predict the likelihood of distant recurrence and magnitude of chemotherapy benefit in tamoxifen-treated, node-negative, estrogen-positive breast cancer patients.4,5 The strong correlation between PhH3 count and RS in our study suggests that PhH3 may provide a level of prognostic and predictive ability. At minimum, it should contribute to current efforts in place to predict RS through a combination of various clinical, histologic, and immunohistochemical indices.9,27–29
Because of the predictive and prognostic value of RS, other tests that triage patients for adjuvant therapy have been compared with RS. Adjuvant! (Adjuvant! Inc.) is computer tool incorporating classic clinicopathologic factors (age, comorbidities, ER status, tumor grade, tumor size, nodal status, and endocrine or chemotherapy treatment) to predict 10-year breast cancer outcome.30 However, RS was found to provide a more consistent and better estimate of chemotherapy benefit than Adjuvant!.6 The “IHC4” combination IHC test, evaluating ER, PR, HER2, and Ki-67 antigen, has also been compared with RS and found to provide prognostic information similar to that provided by RS.27,28 These IHC markers were also combined with standard clinicopathologic variables to create equations, known as the Magee equations, in an attempt to predict the RS score.9,29 These equations provided concordance with the risk category of RS in 54% to 60% of cases. Interestingly, when the intermediate-risk category was removed, concordance ranged from 97% to 100%. These findings raise the question about the relative value of the intermediate-risk category, but also suggest that a novel marker that independently correlates with RS—such as PhH3—might provide an interesting variation on the Magee equations with respect to intermediate risk.
Two previous studies have compared PhH3 with Oncotype DX results. Williams et al31 compared PhH3 labeling, categorized into low (<2%), intermediate (2% to 5%), high (>5%) percent of cells staining, with RS, categorized as low, intermediate, and high. PhH3 labeling was assessed by digital analysis of 10 random ×20 power “hot spot” fields. They found the 2 variables were related (χ2 test, P=0.027). In a separate study, they then assessed the use of PhH3 as a replacement for mitotic count in determining the mitotic score component of the MBR grade and compared mitotic score with RS.13 PhH3 was counted both digitally and manually in 10 HPFs. PhH3 mitotic score correlated with H&E mitotic score: H&E versus manual PhH3 (Spearman coefficient ρ=0.39, P<0.001) and H&E versus automated PhH3 (ρ=0.33, P<0.001). In addition, mitotic score correlated with RS: H&E mitotic score versus RS (ρ=0.30, P<0.001), and manual PhH3 mitotic score versus RS (ρ=0.28, P<0.001). Unfortunately, correlation between automated PhH3 mitotic score and RS was not reported. Both of these studies were performed assuming that PhH3 would replace H&E mitotic count or score. This differed from our assumption, and thus their goals and counting methods differed from ours. The automated counts in the 2 studies cited above counted 10 random fields and the percent of cells staining was used for analyses. In their second study, they did not specify how the 10 HPFs for manual PhH3 counts were chosen. In neither study was the relative strength of the PhH3 to RS correlation controlled for confounding variables (ie, mitotic count, score, grade). Interestingly, their second study showed marginally better correlation between H&E mitotic score and RS than between PhH3 mitotic score and RS, which is opposite of our findings. It is notable, however, that their correlation was a Spearman rank correlation, which should not be overinterpreted as a measure of the strength of association between 2 variables.
Assessing the prognosis of patients in our study was uninformative as only 2 patients had cancer recurrence, and both died. However, several studies, all done in part by 1 group, have assessed the prognostic ability of PhH3 directly. Skaland et al15 evaluating the prognosis of early-stage, node-negative invasive breast cancer in patients less than 5515 or 71 years old16 found that PhH3 had the strongest prognostic ability of the variables measured (including age, size, grade, tubular formation, nuclear atypia, mitotic index, ER, PR). Furthermore, no variable offered prognostic value additional to PhH3. An extension of the study by including multiple IHC markers (PhH3, Ki-67, ER, PR, HER2, CK5/6, CK14) still found that PhH3 was the strongest prognostically.17 A further study evaluated mitotic score, PhH3, cyclin B1, cyclin A, and Ki-67.18 Interestingly, mitosis was the strongest prognostic proliferation factor, and only mitosis and cyclin A were independently prognostic. However, this may be the result of methodology because the IHC markers were assessed on TMAs taken from representative tumor areas rather than highest proliferation areas, whereas mitotic count was performed on whole slides in the region of highest proliferation. More recently, their group compared the prognostic ability of mitosis, PhH3, and Ki-67 as well as the gene profiles used in Oncotype DX and MammaPrint (Agendia Inc., Amsterdam).10 PhH3 was again the strongest independent prognostic factor. It must be noted that they did not use the commercial Oncotype DX and MammaPrint services, but instead replicated those tests in their own laboratory and possibly introducing technical differences from the commercial service. Even so, the above studies show that PhH3 is a powerful prognostic marker. Large validation trials utilizing PhH3, similar to those done for Oncotype DX, have not been performed, making it difficult to adopt PhH3 and replace Oncotype DX. Furthermore, these studies have not studied the ability of PhH3 to predict treatment response. Thus, our finding that PhH3 may independently assist in predicting the value of RS is important.
Our study has several limitations. Although we are determining whether PhH3 has prognostic or predictive abilities by comparing with RS, the gold standard is patient outcome. Because Oncotype Dx has only been available to patients in Alberta for a relatively short time, retrospective accrual of additional patients with known clinical endpoints is not possible. Hence, prospective PhH3 assessment and follow-up will be required to validate our observations. Admittedly, our study set is not mature: our mean clinical follow-up time is only 60.3 months, but with 89.2% five-year-survival rates in breast cancer, and 98.6% if node negative,32 significantly longer follow-up is required to assess the prognostic value of PhH3 directly. However, this is perhaps not a liability. Studies note that Oncotype DX does not accurately predict recurrence in all cases suggesting that additional prospective studies are required to fully understand the implications of RS. On the basis of our findings, we would expect that the addition of PhH3 to data sets developed for ongoing follow-up studies may be of particular benefit. Future steps for our study population might also reasonably include a larger sample size, as well as an analysis of the prognostic and predictive value of PhH3 in node-positive patients, a patient population in which Oncotype DX may also be useful.33
In conclusion, we have shown that PhH3 is strongly correlated with Oncotype DX RS, and maintains correlation even when controlling for the contributions of mitotic count, mitotic score, or MBR grade. It thus has potential to be a simple and elegant prognostic and predictive marker for invasive breast carcinoma and a cost-effective alternative to more expensive gene expression profiles. Although these expectations will require longer follow-up to be confirmed, the role of PhH3 as an addition to established prognostic factors (including clinicopathologic factors and proliferation markers such as Ki-67 and cyclin A) and its value as a more accurate measure of mitotic activity have immediate applications.
Footnotes
Supported by Calgary Laboratory Services.
The authors declare no conflict of interest.
REFERENCES
- 1.Ferlay J, Soefjomataram I, Ervik M, et al. GLOBOCAN 2012 v10, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available at: http://globocan.iarc.fr. [Google Scholar]
- 2.Lakhani SR, Ellis IO, Schnitt SJ, et al. WHO Classification of Tumours of the Breast, 4th ed Lyon: World Health Organization; 2012. [Google Scholar]
- 3.Gianni L, Norton L, Wolmark N, et al. Role of anthracyclines in the treatment of early breast cancer. J Clin Oncol. 2009;27:4798–4808. [DOI] [PubMed] [Google Scholar]
- 4.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. [DOI] [PubMed] [Google Scholar]
- 5.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. [DOI] [PubMed] [Google Scholar]
- 6.Tang G, Shak S, Paik S, et al. Comparison of the prognostic and predictive utilities of the 21-gene Recurrence Score assay and Adjuvant! for women with node-negative, ER-positive breast cancer: results from NSABP B-14 and NSABP B-20. Breast Cancer Res Treat. 2011;127:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyman GH, Cosler LE, Kuderer NM, et al. Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: an economic analysis based on prognostic and predictive validation studies. Cancer. 2007;109:1011–1018. [DOI] [PubMed] [Google Scholar]
- 8.Acs G, Esposito NN, Kiluk J, et al. A mitotically active, cellular tumor stroma and/or inflammatory cells associated with tumor cells may contribute to intermediate or high Oncotype DX Recurrence Scores in low-grade invasive breast carcinomas. Mod Pathol. 2012;25:556–566. [DOI] [PubMed] [Google Scholar]
- 9.Klein ME, Dabbs DJ, Shuai Y, et al. Prediction of the Oncotype DX recurrence score: use of pathology-generated equations derived by linear regression analysis. Mod Pathol. 2013;26:658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonsdottir K, Assmus J, Slewa A, et al. Prognostic value of gene signatures and proliferation in lymph-node-negative breast cancer. PloS One. 2014;9:e90642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong Z, Bode AM. The role of histone H3 phosphorylation (Ser10 and Ser28) in cell growth and cell transformation. Mol Carcinog. 2006;45:416–421. [DOI] [PubMed] [Google Scholar]
- 12.Hendzel MJ, Wei Y, Mancini MA, et al. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. [DOI] [PubMed] [Google Scholar]
- 13.Zbytek B, Cohen C, Wang J, et al. Nottingham-defined mitotic score: comparison with visual and image cytometric phosphohistone H3 labeling indices and correlation with Oncotype DX recurrence score. Appl Immunohistochem Mol Morphol. 2013;21:48–53. [DOI] [PubMed] [Google Scholar]
- 14.Lee LH, Yang H, Bigras G. Current breast cancer proliferative markers correlate variably based on decoupled duration of cell cycle phases. Sci Rep. 2014;4:5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skaland I, Janssen EAM, Gudlaugsson E, et al. Phosphohistone H3 expression has much stronger prognostic value than classical prognosticators in invasive lymph node-negative breast cancer patients less than 55 years of age. Mod Pathol. 2007;20:1307–1315. [DOI] [PubMed] [Google Scholar]
- 16.Skaland I, Janssen EAM, Gudlaugsson E, et al. Validating the prognostic value of proliferation measured by Phosphohistone H3 (PPH3) in invasive lymph node-negative breast cancer patients less than 71 years of age. Breast Cancer Res Treat. 2009;114:39–45. [DOI] [PubMed] [Google Scholar]
- 17.Gudlaugsson E, Klos J, Skaland I, et al. Prognostic comparison of the proliferation markers (mitotic activity index, phosphohistone H3, Ki67), steroid receptors, HER2, high molecular weight cytokeratins and classical prognostic factors in T1-2N0M0 breast cancer. Pol J Pathol. 2013;64:1–8. [DOI] [PubMed] [Google Scholar]
- 18.Klintman M, Strand C, Ahlin C, et al. The prognostic value of mitotic activity index (MAI), phosphohistone H3 (PPH3), cyclin B1, cyclin A, and Ki67, alone and in combinations, in node-negative premenopausal breast cancer. PloS One. 2013;8:e81902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voss SM, Riley MP, Lokhandwala PM, et al. Mitotic count by phosphohistone H3 immunohistochemical staining predicts survival and improves interobserver reproducibility in well-differentiated neuroendocrine tumors of the pancreas. Am J Surg Pathol. 2015;39:13–24. [DOI] [PubMed] [Google Scholar]
- 20.Bossard C, Jarry A, Colombeix C, et al. Phosphohistone H3 labelling for histoprognostic grading of breast adenocarcinomas and computer-assisted determination of mitotic index. J Clin Pathol. 2006;59:706–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. [DOI] [PubMed] [Google Scholar]
- 22.NCCN Clinical Practice Guidelines in Oncology: Breast Cancer (Version 30.2014) [Internet]. National Comprehensive Cancer Network Inc.; 2014. Available at: http://www.nccn.org. Accessed April 22, 2014.
- 23.Dancey CP, Reidy J. Statistics Without Maths for Psychology. Harlow, England: New York: Prentice Hall/Pearson; 2011. [Google Scholar]
- 24.Kim Y-J, Ketter R, Steudel W-I, et al. Prognostic significance of the mitotic index using the mitosis marker anti-phosphohistone H3 in meningiomas. Am J Clin Pathol. 2007;128:118–125. [DOI] [PubMed] [Google Scholar]
- 25.Tetzlaff MT, Curry JL, Ivan D, et al. Immunodetection of phosphohistone H3 as a surrogate of mitotic figure count and clinical outcome in cutaneous melanoma. Mod Pathol. 2013;26:1153–1160. [DOI] [PubMed] [Google Scholar]
- 26.Tapia C, Kutzner H, Mentzel T, et al. Two mitosis-specific antibodies, MPM-2 and phospho-histone H3 (Ser28), allow rapid and precise determination of mitotic activity. Am J Surg Pathol. 2006;30:83–89. [DOI] [PubMed] [Google Scholar]
- 27.Dowsett M, Salter J, Zabaglo L, et al. Predictive algorithms for adjuvant therapy: TransATAC. Steroids. 2011;76:777–780. [DOI] [PubMed] [Google Scholar]
- 28.Cuzick J, Dowsett M, Pineda S, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol. 2011;29:4273–4278. [DOI] [PubMed] [Google Scholar]
- 29.Flanagan MB, Dabbs DJ, Brufsky AM, et al. Histopathologic variables predict Oncotype DX recurrence score. Mod Pathol. 2008;21:1255–1261. [DOI] [PubMed] [Google Scholar]
- 30.Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. [DOI] [PubMed] [Google Scholar]
- 31.Williams DJ, Cohen C, Darrow M, et al. Proliferation (Ki-67 and phosphohistone H3) and oncotype DX recurrence score in estrogen receptor-positive breast cancer. Appl Immunohistochem Mol Morphol. 2011;19:431–436. [DOI] [PubMed] [Google Scholar]
- 32.Howlander N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2011 [Internet]. Bethesda, MD: National Cancer Institute; 2013. Available at: http://seer.cancer.gov/csr/1975_2011/. [Google Scholar]
- 33.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]


