Abstract
Objective
Galunisertib (LY2157299 monohydrate), an inhibitor of the transforming growth factor β (TGFβ) pathway, is currently under investigation in several clinical trials involving multiple tumor types. The primary objective of this study was to assess relative bioavailability of two new galunisertib formulations developed using the roller compaction (RC) dry-milled (RCD) and RC slurry-milled (RCS) processes, compared with the existing formulation developed using the high-sheer wet granulation (HSWG) process. The secondary objective was to report the safety profile after a single dose of the three formulations.
Methods
Patients with advanced or metastatic cancer were enrolled into this single-center, 3-period, 6-sequence crossover study. Patients were assigned sequentially to 1 of 6 sequences in blocks of 6 to ensure that all 6 sequences have the same number of completers. A patient entering a sequence received a different galunisertib formulation as a single 150 mg dose orally during each of the 3 periods. Each period was separated from the next by a washout interval of at least 48 hours. Pharmacokinetic (PK) parameters, including area under curve (AUC) and Cmax, were computed using standard non-compartmentalized methods of analysis. For comparison of exposures between formulations, log-transformed AUC and Cmax values were analyzed using a linear mixed-effects model. Safety assessments included adverse event monitoring, physical examinations, and laboratory tests.
Results
Of the 14 patients who entered and completed the study, 13 patients were included in the final statistical analysis. AUC(0-tlast), AUC(0–48 h), and AUC(0-∞) for the RC formulations and the HSWG formulation were similar. Cmax was reduced by approximately 22% and tmax was longer by at least 1.00 h for the RCD and RCS formulations compared with the HSWG formulation. The RC formulations demonstrated a safety profile after a single dose similar to the HSWG formulation.
Conclusions
In this relative bioavailability study comparing galunisertib formulations after a single dose, RCD and RCS formulations had similar exposure and safety profile compared with the HSWG formulation.
Keywords: LY2157299, transforming growth factor beta, area under curve, biological availability, half-life, pharmacokinetics, neoplasms, adverse drug event
Introduction
Transforming growth factor-β ligands (TGFβ1, TGFβ2, and TGFβ3) play an important role in the tumorigenesis and progression of several tumors [1,2]. These ligands signal via the TGFβRI and TGFβRII receptors to activate a signaling cascade involving SMAD proteins [3]. TGFβ signaling is activated in several malignancies including gliomas [4], hepatocellular carcinoma [5] and pancreatic cancer [6]. Galunisertib (LY2157299 monohydrate), an oral small- molecule inhibitor of TGFβRI and TGFβRII, is currently under investigation in several clinical trials involving multiple tumor types [7]. Galunisertib is well absorbed with peak plasma concentrations attaining around 0.5–2 hours following administration and with mean terminal half-life of 8 hours [8]. The effect of gut pH on galunisertib absorption has been studied previously, where we reported that galunisertib in solution or tablet products maintained supersaturation during transit in the gastrointestinal tract [9]. To reduce the impact of change in pH after a meal, galunisertib has thus far been administered to patients only in fasted state. Based on a pharmacokinetic (PK)/ pharmacodynamics (PD) model, a therapeutic window for galunisertib administration (160–300 mg/day) has been defined for phase II/III studies [10]. At 150 mg BID on an intermittent dosing schedule (14 days on/14 days off on a 28-day cycle), galunisertib has a favorable safety profile with very few grade 3/4 adverse events (AEs) [8,10].
The galunisertib tablet formulation used for early clinical studies was initially developed and manufactured using a high-sheer wet granulation (HSWG) process. The HSWG process was developed initially to minimize the possible impact of physical property variability of the incoming active pharmaceutical ingredient (API) that may be observed early in development. A roller compaction (RC) dry granulation process was subsequently developed to maintain sufficient tablet strength to allow film coating. RC tablets were manufactured using API that was dry milled (RCD) to achieve particle size reduction (x90 of 50 μm). A separate batch of RC tablets was manufactured using API that was slurry milled (RCS, x90 of 80 μm). These RC tablets were compared in a Relative Bioavailability (RBA) study to a single batch of HSWG tablets manufactured with only dry milled API (x90 of 50 μm). Therefore, the RBA study compared API of two different particles sizes (x90 of 50 μm and x90 of 80 μm) and two different oral solid manufacturing platforms (HSWG and RC). It was expected that the in vivo PK profile of the 3 tablet presentations would be similar based on in vitro experiments [9]. However, a clinical evaluation was necessary for further clinical development of these galunisertib formulations. The objective of this study was to assess the PK profile and safety after a single dose of these two RC formulations relative to the HSWG formulation in patients with advanced or metastatic cancer.
Methods
Study design and study drug administration
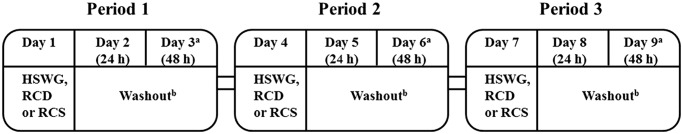
This relative bioavailability study is an addendum to the first-in-human dose (FHD) study of galunisertib in patients with advanced or metastatic cancer, results from which have been reported previously [8,11]. The study was an open-label, 3-period, 6-sequence crossover study conducted at a single investigational site in patients with advanced or metastatic cancer who had exhausted all available therapeutic options. Patients were grouped into sets of 6 with each patient in a set being assigned sequentially to 1 of 6 possible treatment sequences (Supplementary Table S1). Patients received galunisertib formulations as RCS 150 mg (3 × 50 mg), RCD 150 mg or HWSG 150 mg, orally on the first day of each of the 3 treatment periods (Figure 1). If a patient discontinued from the study treatment in any period, another patient was enrolled into that sequence starting from period 1. A washout interval of at least 48 hours and up to a maximum of 5 days separated each period. During each period, approximately 4 mL of venous blood and the resultant plasma samples were used for measurement of galunisertib concentrations using a liquid chromatography/mass spectrometry (LC/MS) method. The samples were collected at intervals up to 48 hours following each dose. Patients were monitored for safety throughout the study. Patients who completed the study were allowed to take part in the main protocol of the FHD study, in which they received galunisertib 150 mg BID in the HSWG formulation as monotherapy.
Figure 1.
Study design.
aTo allow for site scheduling flexibility, an extra 1-day interval between dosing periods was allowed (ie, if the third day of a period fell on a Saturday, the next period may have begun on Monday).
bMinimum of 2 days, maximum of 5 days.
Abbreviations: h = hour; HSWG = high-sheer wet granulation; PK = pharmacokinetic; RCD = roller compaction dry-milled (test formulation 2); RCS = roller compaction slurry-milled (test formulation 1).
The study was conducted in accordance with the principles as defined in the most recent version of the Declaration of Helsinki for human experimentation. The study protocol was approved by the Institutional Review Board of the investigational site. Informed consent declaration (ICD) was obtained from each patient after they had been made aware of the potential risks and benefits, as well as the investigational nature of the study. All patients were given the option to roll-over to the main protocol of the study and be treated with galunisertib until disease progression.
Bioanalytical methods
Plasma samples were analyzed for galunisertib using 2 validated liquid chromatography methods coupled with tandem mass spectrometry [8]. For the high-range method, the lower and upper limit of quantification was 5.000 ng/mL and 1000.000 ng/mL, respectively. For samples above the upper limit of quantification of the high-range method, reanalysis was done after dilution to yield results within the calibrated range. For the low-range method, the lower and upper limits of quantifications were 0.050 ng/mL and 10.000 ng/mL, respectively. The intra-assay accuracy and precision during validation have been described previously [8].
Pharmacokinetic analyses
Primary PK parameters were maximum plasma drug concentration (Cmax) and area under the curve (AUC). Other parameters included half-life (t1/2), volume of distribution (Vz/F), and clearance (CL/F). All these parameters were computed using standard non-compartmental methods of analysis.
Statistical analyses of pharmacokinetic parameters
A sample size of 12 patients was planned for this study, which is the minimum number recommended in the USFDA guidance for any cross-over bioavailability study [12]. Log-transformed values of AUC and Cmax were analyzed using a linear mixed-effects model with sequence, period and treatment groups being treated as fixed effects, and subject treated as a random effect. Least squares mean (LSM) for each treatment group and 90% confidence interval (CI) for the mean of the pairwise differences were estimated using this model. After back transformation from the log scale, estimates of geometric LSM and 90% CI for the ratio of means were calculated. Times to maximum plasma drug concentration (tmax) values were analyzed using the non-parametric Wilcoxon signed-rank test to obtain the medians of pairwise differences and their 90% CI.
Safety analyses
Safety assessments included monitoring for AEs and serious adverse events (SAEs), physical examinations, vital sign measurements, laboratory tests, and review of concomitant medications. AEs were assessed according to the Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3.0) and treatment-emergent adverse events (TEAEs) were included in the safety analyses. All patients receiving at least 1 dose of galunisertib were included in the safety analyses.
Results
Patient disposition
All 14 patients who entered the study received at least 1 dose of the study drug and completed 3 periods.
Patient demographics
The mean age of patients was 59.8 years; all patients were White (100%) and majority were female (64%). Of the total number of patients, 86% of the patients had a baseline Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1, 79% had recurrent disease at study entry, and 64% had a pathological diagnosis of glioma, namely, glioblastoma and astrocytoma (Table 1).
Table 1.
Patient baseline characteristics.
| Variable | Galunisertib (any formulation) N=14 |
|---|---|
| Age, years | |
| Mean | 59.8 |
| Median (range) | 56.5 (34–76) |
|
| |
| Sex, n (%) | |
| Female | 9 (64.0) |
| Male | 5 (36.0) |
|
| |
| Origin, n (%) | |
| Caucasian | 14 (100) |
|
| |
| ECOG performance status, n (%) | |
| 0 | 4 (28.6) |
| 1 | 8 (57.1) |
| 2 | 2 (14.3) |
|
| |
| Initial pathological diagnosis, n (%) | |
| Astrocytoma | 2 (14.3) |
| Choroidal melanoma | 1 (7.1) |
| Colon adenocarcinoma | 1 (7.1) |
| Glioblastoma | 7 (50.0) |
| Hepatocellular carcinoma | 2 (14.2) |
| Pancreatic adenocarcinoma | 1 (7.1) |
|
| |
| Stage of disease at study entry, n (%) | |
| Metastatic | 3 (21.4) |
| Recurrent | 11 (78.6) |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; N = number of patients who received drug.
Pharmacokinetic analysis
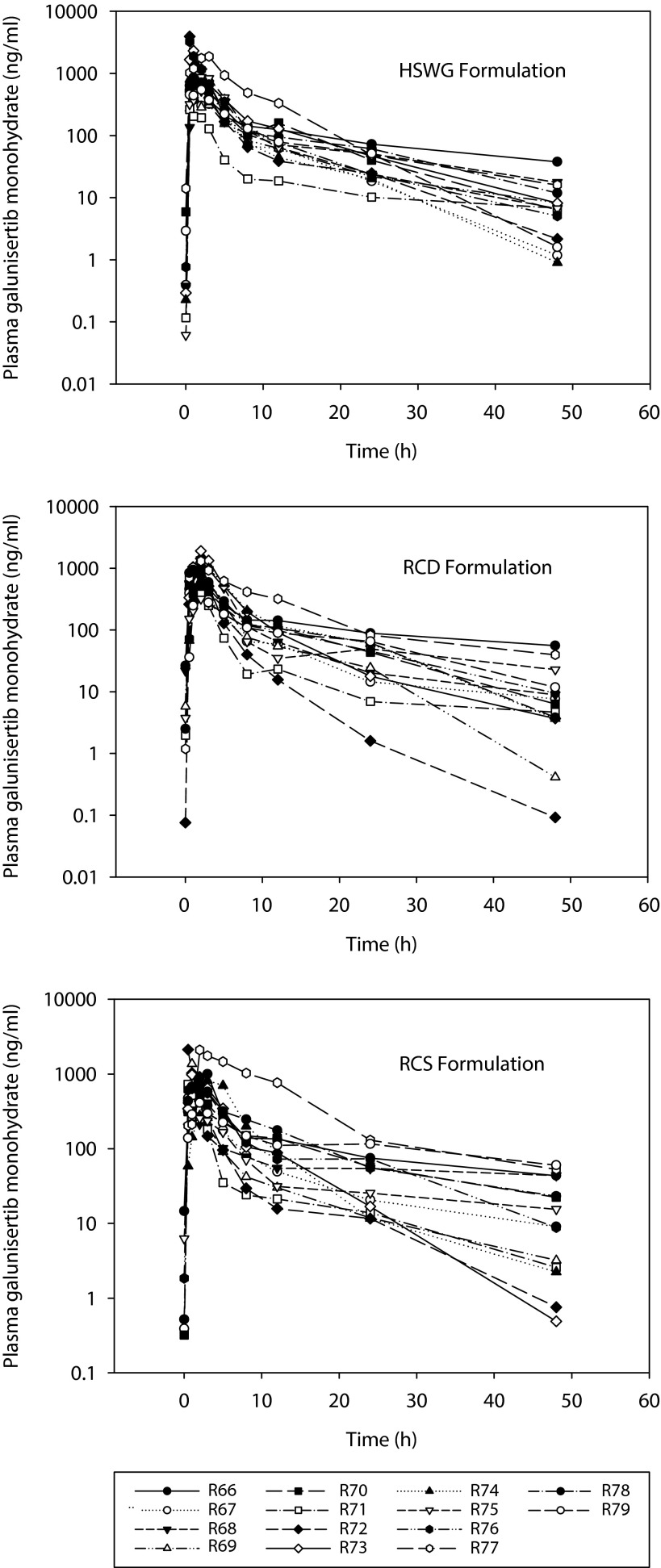
Plasma concentrations of galunisertib from all patients were included in the PK evaluations. The final statistical analysis of AUC, Cmax, and tmax excluded a patient (R77) with colon adenocarcinoma as the primary tumor, who was recovering from an underlying infection with associated inflammation. This patient showed substantially higher exposures that varied considerably as the inflammation resolved (assessed using CRP biomarker, not shown) across the 3 study periods (Figure 3). The patient had AUC0-∞ values of 12,500, 10,900 and 20,100 g*h/L for HSWG, RCD and RCS formulations, respectively (Figure 3). In comparison, the geometric mean of AUC0-∞ values in the study were 4,740, 4,490 and 4,790 μg*h/L for HSWG, RCD and RCS formulations, respectively (Table 2). The statistical analysis including the data from this patient (N=14) is presented in Supplementary Table S2 and Supplementary Table S3.
Figure 3.
Individual plasma concentration-time profiles for formulations of galunisertib.
Individual plasma concentration-time profiles following a single 150 mg oral dose of galunisertib given as either high-sheer wet granulation (HSWG), roller compaction dry-milled (RCD), or roller compaction slurry-milled (RCS) formulations.
Table 2.
Non-compartmental pharmacokinetic parameters of the three formulations of galunisertib.
| Parametersa | Galunisertib (150 mg) | ||
|---|---|---|---|
| HSWG (N=14) | RCD (N=14) | RCS (N=14) | |
| tmaxb (h) | 1.00 (0.50–3.08) | 2.00 (0.50–3.17) | 2.00 (0.50–3.00) |
| Cmax (μg/L) | 954 (89.7) | 734 (68.0) | 769 (67.8) |
| AUC(0–12) (μg·h/L) | 3,430 (64.6) | 3,170 (56.1) | 2,970 (63.0) |
| AUC(0–24) (μg·h/L) | 4,070 (61.5) | 3,840 (53.7) | 3,660 (64.3) |
| AUC(0-tlast) (μg·h/L) | 4,520 (58.5) | 4,360 (52.0) | 4,350 (63.9) |
| AUC(0–48) (μg·h/L) | 4,520 (58.6) | 4,360 (52.0) | 4,340 (63.8) |
| AUC(0-∞) (μg·h/L) | 4,740 (55.6) | 4,490c (53.3) | 4,790d (70.9) |
| CL/F (L/hr) | 31.7 (55.6) | 33.4c (53.3) | 31.3d (70.9) |
| Vz/F (L) | 505 (88.0) | 473c (76.0) | 511d (81.9) |
| t1/2 (h) | 11.1 (47.0) | 9.81c (41.1) | 11.3d (42.4) |
All pharmacokinetic parameters except tmax evaluated after a single dose on Day 1 of each period are geometric mean (% CV) (unless stated otherwise).
tmax is median (range);
N=12;
N=11. AUC(0-∞), CL/F, Vz/F, and t1/2 not reported for patients where extrapolated AUCtlast-inf>20%.
Abbreviations: AUC = area under the plasma concentration versus time curve; AUC(0-∞) = AUC from zero to infinity; AUC(0-tlast) = AUC from time zero to time t where t is the last time point with a measurable concentration; AUC(0-x) = AUC from time zero to time x where x is the last time point with a measurable concentration; CL/F = clearance; Cmax = maximum plasma drug concentration; CV = coefficient of variation; HSWG = high-sheer wet granulation; N = number of subjects used in pharmacokinetic analysis; RCD = roller compaction dry-milled; RCS = roller compaction slurry-milled; t1/2 = half-life associated with the terminal rate constant in non-compartmental analysis; tmax = time of maximum plasma drug concentration; Vz/F = volume of distribution.
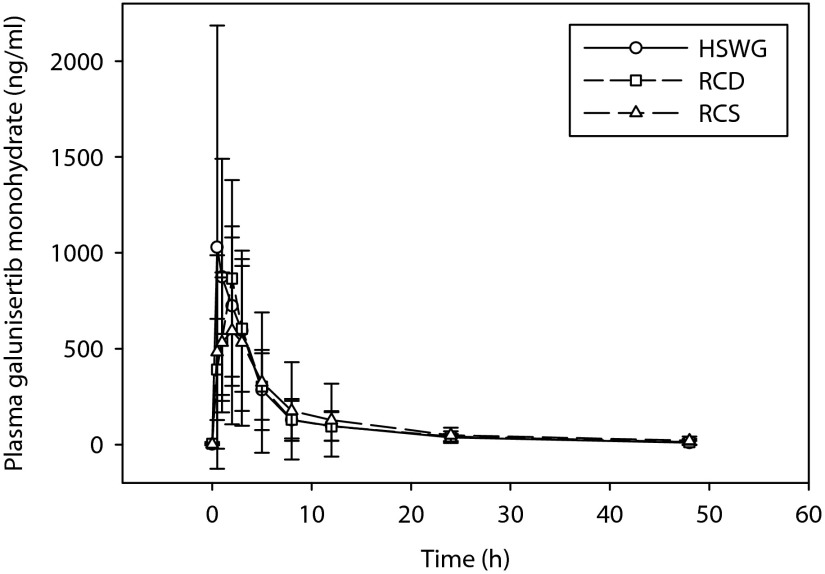
Values for CL/F, Vz/F, and t1/2 parameters of galunisertib were similar for all three formulations (Table 2). Exposures as defined by AUC [0-tlast], AUC [0–48 h], and AUC [0-∞] were comparable between the RCD and RCS formulations, and the HSWG formulation (Table 3). Cmax of galunisertib was lower for the RCD and RCS formulations by approximately 22% compared with the HSWG formulation (Table 3). Additionally, the median tmax of galunisertib was longer for the RCD and RCS formulations (2.00 h) compared with the HSWG formulation (1.00 h) (Table 4). The mean and individual plasma concentrations of galunisertib over a period of 48 hours are shown in Figures 2 and 3, respectively.
Table 3.
Analysis of AUC and Cmax of galunisertib.
| Parameter | Formulation, 150 mg | N | Geometric LSM (90% CI) | Ratio of geometric LSM RCD/RCS:HSWG (90% CI) |
|---|---|---|---|---|
| AUC(0-tlast) (μg·h/L) | HSWG | 13 | 3,795 (3,267, 4,410) | |
| RCD | 13 | 3,704 (3,188, 4,304) | 0.98 (0.83, 1.15) | |
| RCS | 13 | 3,497 (3,009, 4,065) | 0.92 (0.78, 1.08) | |
| AUC(0–48) (μg·h/L) | HSWG | 13 | 3,797 (3,268, 4,413) | |
| RCD | 13 | 3,702 (3,186, 4,302) | 0.97 (0.83, 1.14) | |
| RCS | 13 | 3,489 (3,001, 4,056) | 0.92 (0.78, 1.08) | |
| AUC(0-∞) (μg·h/L) | HSWG | 13 | 4,026 (3,449, 4,699) | |
| RCD | 11 | 3,921 (3,328, 4,621) | 0.97 (0.84, 1.13) | |
| RCS | 10 | 3,842 (3,246, 4,547) | 0.95 (0.81, 1.12) | |
| Cmax (μg/L) | HSWG | 13 | 859 (618, 1,194) | |
| RCD | 13 | 666 (479, 925) | 0.77 (0.57, 1.06) | |
| RCS | 13 | 667 (479, 927) | 0.78 (0.57, 1.06) |
One patient (R77) who showed substantially higher exposures as explained in results was excluded from analysis. For AUC(0-∞), 2 and 3 patients for RCD and RCS, respectively had extrapolated AUC(tlast-inf) values >20% and could not be included in the analysis.
Abbreviations: AUC = area under the plasma concentration versus time curve; AUC(0-∞) = AUC from zero to infinity; AUC(0–48) = AUC from zero to 48 hours postdose; AUC(0-tlast) = AUC from time zero to time t where t is the last time point with a measurable concentration; CI = confidence interval; Cmax = maximum plasma drug concentration; HSWG = high-sheer wet granulation; LSM = least squares means; N = number of patients; PK = pharmacokinetic; RCD = roller compaction dry-milled; RCS = roller compaction slurry-milled.
Table 4.
Analysis of tmax of galunisertib.
| Parameter | Formulation, 150 mg | N | Median (min, max) | Median difference RCD/RCS – HSWG (90% CI) |
|---|---|---|---|---|
| tmax (h) | HSWG | 13 | 1.00 (0.50, 3.00) | |
| RCD | 13 | 2.00 (0.50, 3.17) | 0.59 (0.00, 1.25) | |
| RCS | 13 | 2.00 (0.50, 3.00) | 0.00 (0.00, 0.75) |
One patient (R77) who showed substantially higher exposures as explained in results was excluded from analysis.
Abbreviations: CI = confidence interval; HSWG = high-sheer wet granulation; max = maximum; min = minimum; N = number of patients; RCD = roller compaction dry-milled; RCS = roller compaction slurry-milled; tmax = time of maximum plasma drug concentration.
Figure 2.
Mean plasma concentration-time profiles of formulations of galunisertib.
Mean ± standard deviation of plasma concentration as a function of time profiles following a 150 mg single oral dose of galunisertib administered as either high-sheer wet granulation (HSWG), roller compaction dry-milled (RCD), or roller compaction slurry-milled (RCS) formulations.
Safety and tolerability
All 14 patients were included in the safety analysis in this single dose study. Five of the 14 patients reported 7 TEAEs regardless of causality. The most common TEAE was grade 3 lymphopenia which was experienced by 2 patients. All other TEAEs were of grade 1 severity (Table 5). None of the TEAEs were determined to be related to galunisertib by the investigator. There were no study discontinuations due to TEAEs. There were also no SAEs or deaths during this study.
Table 5.
Number of patients with treatment-emergent adverse events regardless of causality.
| Preferred term | Galunisertib (150 mg), N=14 | |||
|---|---|---|---|---|
| HSWG | RCD | RCS | All | |
| Lymphopenia (Grade 3) | 1 | 0 | 1 | 2 |
| Pyrexia (Grade 1) | 0 | 1 | 0 | 1 |
| Vulvovaginitis (Grade 1) | 0 | 1 | 0 | 1 |
| Hyperglycemia (Grade 1) | 1 | 0 | 0 | 1 |
| Convulsion (Grade 1) | 1 | 0 | 0 | 1 |
| Dizziness (Grade 1) | 1 | 0 | 0 | 1 |
Abbreviations: HSWG = high-sheer wet granulation; N = number of patients; RCD = roller compaction dry-milled; RCS = roller compaction slurry-milled.
Discussion
The HSWG process has been used to manufacture galunisertib tablet formulations for use in early clinical trials. The RCD and RCS processes for galunisertib tablets were developed to minimize the risk of physical instability often associated with wet granulation. The RC processes are also easier to scale up which is important for commercialization. A potential disadvantage of a roller-compacted process is the loss of compactability, resulting in tablets with lower than acceptable tensile strength. However, this risk was mitigated through the use of extragranular powders in both the RCD and RCS process, producing tablets of adequate hardness, low friability, and fast disintegration/dissolution, meeting all critical quality attributes. The PK profile for galunisertib tablets (150 mg BID) manufactured using the HSWG process has been established previously [10]. The purpose of this relative bioavailability study was to confirm that with the new manufacturing processes, the PK profiles of galunisertib tablets administered as a 150 mg, single dose, do not change. This may help decide if the new formulations can be taken forward for further clinical development.
In this relative bioavailability study, exposures as defined by AUC over different time periods (AUC[0-tlast], AUC[0–48], and AUC[0-∞]) of RCD and RCS formulations were comparable with the HSWG formulation (Table 3). The ratios of geometric LSM of AUC[0-∞] values of RCD and RCS formulations relative to the HSWG formulation (i.e. RCD/RCS:HSWG) were 0.97 (90% CI:0.84, 1.13) and 0.95 (90% CI:0.81, 1.12) for RCD and RCS, respectively (Table 3). However, Cmax was at least 22% lower and tmax occurred at least 1.00 h longer in the RCD and RCS formulations, compared with the HSWG formulation (Tables 3 and 4). The dosing schedule for galunisertib has been established previously as 150 mg twice daily,14 day on/14 day off [8,10]. For this schedule, with multiple dosing and a small inter-dosing interval, the potential impact of lower Cmax and longer tmax on exposure, and hence on efficacy and safety, will be diminished at steady state.
These clinical findings are consistent with our previous findings using the artificial stomach–duodenum dissolution model. In these in vitro experiments, the PK profiles of the RC formulations were similar to that of the HSWG formulation [9].
The safety profile of galunisertib dosed at 150 mg BID has been established previously [8,10]. In the current study, patients were monitored only for the management of adverse events. The RC formulations at a single 150 mg dose demonstrated a safety profile similar to the HSWG formulation (Table 5). Grade 3 lymphopenia was the most severe AE in the study experienced by 2 patients; one administered with the HSWG formulation and another administered with the RCS formulation.
Overall, the findings from this relative bioavailability study suggest that the new processes for manufacturing galunisertib tablets should not affect the dosing regimen of galunisertib in future clinical trials.
Supplementary Material
Supplementary Table S1.
Study treatment sequences.
| Treatment sequence | Period 1 | Period 2 | Period 3 |
|---|---|---|---|
| Sequence 1 | HSWG | RCS | RCD |
| Sequence 2 | RCD | HSWG | RCS |
| Sequence 3 | RCS | RCD | HSWG |
| Sequence 4 | HSWG | RCD | RCS |
| Sequence 5 | RCD | RCS | HSWG |
| Sequence 6 | RCS | HSWG | RCD |
Abbreviations: HSWG = high-sheer wet granulation; PK = pharmacokinetic; RCD = roller compaction dry-milled; RCS = roller compaction slurry-milled.
Supplementary Table S2.
Statistical analysis of AUC and Cmax of LY2157299 including all patients.
| Parameter | Formulation, 150 mg | N | Geometric LSM (90% CI) | Ratio of geometric LSM RCD/RCS:HSWG (90% CI) |
|---|---|---|---|---|
| AUC(0-tlast) (μg·h/L) | HSWG | 14 | 4,516 (3,320, 6,143) | |
| RCD | 14 | 4,348 (3,196, 5,915) | 0.96 (0.82, 1.13) | |
| RCS | 14 | 4,344 (3,193, 5,909) | 0.96 (0.82, 1.13) | |
| AUC(0–48) (μg·h/L) | HSWG | 14 | 4,518 (3,321, 6,148) | |
| RCD | 14 | 4,347 (3,195, 5,915) | 0.96 (0.82, 1.13) | |
| RCS | 14 | 4,334 (3,185, 5,897) | 0.96 (0.82, 1.13) | |
| AUC(0-∞) (μg·h/L) | HSWG | 14 | 4,766 (3,516, 6,462) | |
| RCD | 12 | 4,631 (3,402, 6,303) | 0.97 (0.83, 1.13) | |
| RCS | 11 | 4,816 (3,531, 6,567) | 1.01 (0.86, 1.19) | |
| Cmax (μg/L) | HSWG | 14 | 960 (686, 1,345) | |
| RCD | 14 | 735 (525, 1,029) | 0.77 (0.57, 1.03) | |
| RCS | 14 | 770 (550, 1,078) | 0.80 (0.60, 1.07) |
For AUC(0-∞), 2 and 3 patients for RCD and RCS, respectively had extrapolated AUCtlast-inf values >20% and could not be included in the analysis.
Abbreviations: AUC = area under the plasma concentration versus time curve; AUC(0-∞) = AUC from zero to infinity; AUC(0–48) = AUC from zero to 48 hours post dose; AUC(0-tlast) = AUC from time zero to time t, where t is the last time point with a measurable concentration; CI = confidence interval; Cmax = maximum plasma drug concentration; LSM = least squares means; N = number of patients; HSWG = high-sheer wet granulation; PK = pharmacokinetic; RCD = roller compaction dry-milled; RCS = roller compaction slurry-milled.
Supplementary Table S3.
Statistical analysis of tmax of LY2157299 including all patients.
| Parameter | Formulation, 150 mg | N | Median (min, max) | Median difference RCD/RCS – HSWG (90% CI) |
|---|---|---|---|---|
| tmax (h) | HSWG | 14 | 1.00 (0.50, 3.08) | |
| RCD | 14 | 2.00 (0.50, 3.17) | 0.54 (0.00, 1.09) | |
| RCS | 14 | 2.00 (0.50, 3.00) | 0.00 (0.00, 0.71) |
Abbreviations: CI = confidence interval; HSWG = high- sheer wet granulation; max = maximum; min = minimum; RCD = roller compaction dry-milled; N = number of patients; tmax = time of maximum plasma drug concentration; RCS = roller compaction slurry-milled.
Acknowledgements
Sriram Govindan, PhD, an employee of Eli Lilly provided medical writing assistance for this manuscript. The authors would like to thank James A. Wesley and David C. Sperry, both employees of Eli Lilly, for their advice on the formulations used in the study. The authors would like to thank all patients and all the site staff for their participation in the study.
Abbreviations
- AE
adverse events
- API
active pharmaceutical ingredient
- AUC
area under curve
- Cmax
maximum plasma drug concentration
- CI
confidence interval
- CTCAE v3.0
common terminology criteria for adverse events version 3.0
- CV
coefficient of variation
- ECOG
Eastern Cooperative Oncology Group
- FHD
first-in-human dose
- HSWG
high-sheer wet granulation
- ICD
informed consent declaration
- LC/MS
liquid chromatography/mass spectrometry
- LSM
least squares mean
- PD
pharmacodynamics
- PK
pharmacokinetic
- PS
performance status
- RBA
relative bioavailability
- RC
roller compaction
- RCD
roller compaction dry-milled
- RCS
roller compaction slurry-milled
- SAE
serious adverse events
- TEAEs
treatment-emergent adverse events
- TGFβ
transforming growth factor β
Footnotes
Disclosure and potential conflicts of interest: Dr. Rodon has served as consultant on Eli Lilly advisory boards and received financial compensation. Drs. Azaro, Seoane, Brana, and Sicart have no conflict of interest. Dr. Gueorguieva, Dr. Desaiah, Mr. Miles, and Ms. Cleverly are employees of Eli Lilly and Company, Indianapolis, IN, USA and Erl Wood, UK and may hold company stocks. Drs. Lahn and Mitchell were former employees of Eli Lilly and Company and may hold company stock. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors are available for download at: http://www.drugsincontext.com/wp-content/uploads/2016/12/dic.212303-COI.pdf.
Funding declaration: The study was sponsored by Eli Lilly and Company, IN, USA.
ClinicalTrials.Gov: NCT01682187
Correct attribution: Copyright © 2016 Gueorguieva I, Cleverly A, Desaiah D, Azaro A, Seoane J, Braña I, Sicart E, Miles C, Lahn MM, Mitchell MI, Rodon J. http://doi.org/10.7573/dic.212303. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 3.0.
Peer review comments to author: 20 October 2016;
Drugs in Context is published by Bioexcel Publishing Ltd. Registered office: 14 Weller Street, London, SE1 1QU, UK
Bioexcel Publishing Limited is registered in England Number 10038393. VAT GB 252772009
For all manuscript and submissions enquiries, contact Julia Savory, Head of Digital Publishing and Submissions Management julia.savory@bioexcelpublishing.com
References
- 1.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10(6):415–24. doi: 10.1038/nrc2853. http:dx.doi.org/10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 2.Pickup M, Novitskiy S, Moses HL. The roles of TGFbeta in the tumour microenvironment. Nat Rev Cancer. 2013;13(11):788–99. doi: 10.1038/nrc3603. http:dx.doi.org/10.1038/nrc3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yingling JM, Blanchard KL, Sawyer JS. Development of TGF–beta signalling inhibitors for cancer therapy. Nat Rev Drug Discov. 2004;3(12):1011–22. doi: 10.1038/nrd1580. http:dx.doi.org/10.1038/nrd1580. [DOI] [PubMed] [Google Scholar]
- 4.Bruna A, Darken RS, Rojo F, Ocana A, Penuelas S, Arias A, Paris R, Tortosa A, Mora J, Baselga J, Seoane J. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11(2):147–60. doi: 10.1016/j.ccr.2006.11.023. http:dx.doi.org/10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Fransvea E, Angelotti U, Antonaci S, Giannelli G. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology. 2008;47(5):1557–66. doi: 10.1002/hep.22201. http:dx.doi.org/10.1002/hep.22201. [DOI] [PubMed] [Google Scholar]
- 6.Melisi D, Ishiyama S, Sclabas GM, Fleming JB, Xia Q, Tortora G, Abbruzzese JL, Chiao PJ. LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Cancer Ther. 2008;7(4):829–40. doi: 10.1158/1535-7163.MCT-07-0337. http:dx.doi.org/10.1158/1535-7163.MCT-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbertz S, Sawyer JS, Stauber AJ, Gueorguieva I, Driscoll KE, Estrem ST, Cleverly AL, Desaiah D, Guba SC, Benhadji KA, Slapak CA, Lahn MM. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther. 2015;9:4479–99. doi: 10.2147/DDDT.S86621. http:dx.doi.org/10.2147/DDDT.S86621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodon J, Carducci M, Sepulveda-Sanchez JM, Azaro A, Calvo E, Seoane J, Brana I, Sicart E, Gueorguieva I, Cleverly A, Pillay NS, Desaiah D, Estrem ST, Paz-Ares L, Holdhoff M, Blakeley J, Lahn MM, Baselga J. Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-beta receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Invest New Drugs. 2015;33(2):357–70. doi: 10.1007/s10637-014-0192-4. http:dx.doi.org/10.1007/s10637-014-0192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding X, Gueorguieva I, Wesley JA, Burns LJ, Coutant CA. Assessment of in vivo clinical product performance of a weak basic drug by integration of in vitro dissolution tests and physiologically based absorption modeling. AAPS J. 2015;17(6):1395–406. doi: 10.1208/s12248-015-9797-6. http:dx.doi.org/10.1208/s12248-015-9797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gueorguieva I, Cleverly AL, Stauber A, Sada Pillay N, Rodon JA, Miles CP, Yingling JM, Lahn MM. Defining a therapeutic window for the novel TGF-beta inhibitor LY2157299 monohydrate based on a pharmacokinetic/pharmacodynamic model. Br J Clin Pharmacol. 2014;77(5):796–807. doi: 10.1111/bcp.12256. http:dx.doi.org/10.1111/bcp.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodon J, Carducci MA, Sepulveda-Sanchez JM, Azaro A, Calvo E, Seoane J, Brana I, Sicart E, Gueorguieva I, Cleverly AL, Pillay NS, Desaiah D, Estrem ST, Paz-Ares L, Holdhoff M, Blakeley J, Lahn MM, Baselga J. First-in-human dose study of the novel transforming growth factor-beta receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res. 2015;21(3):553–60. doi: 10.1158/1078-0432.CCR-14-1380. http:dx.doi.org/10.1158/1078-0432.CCR-14-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FDA Guidance for Industry. Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations. 2003. [Last accessed: May, 2016]. http://www.fda.gov/ohrms/dockets/ac/03/briefing/3995B1_07_GFI-BioAvail-BioEquiv.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1.
Study treatment sequences.
| Treatment sequence | Period 1 | Period 2 | Period 3 |
|---|---|---|---|
| Sequence 1 | HSWG | RCS | RCD |
| Sequence 2 | RCD | HSWG | RCS |
| Sequence 3 | RCS | RCD | HSWG |
| Sequence 4 | HSWG | RCD | RCS |
| Sequence 5 | RCD | RCS | HSWG |
| Sequence 6 | RCS | HSWG | RCD |
Abbreviations: HSWG = high-sheer wet granulation; PK = pharmacokinetic; RCD = roller compaction dry-milled; RCS = roller compaction slurry-milled.
Supplementary Table S2.
Statistical analysis of AUC and Cmax of LY2157299 including all patients.
| Parameter | Formulation, 150 mg | N | Geometric LSM (90% CI) | Ratio of geometric LSM RCD/RCS:HSWG (90% CI) |
|---|---|---|---|---|
| AUC(0-tlast) (μg·h/L) | HSWG | 14 | 4,516 (3,320, 6,143) | |
| RCD | 14 | 4,348 (3,196, 5,915) | 0.96 (0.82, 1.13) | |
| RCS | 14 | 4,344 (3,193, 5,909) | 0.96 (0.82, 1.13) | |
| AUC(0–48) (μg·h/L) | HSWG | 14 | 4,518 (3,321, 6,148) | |
| RCD | 14 | 4,347 (3,195, 5,915) | 0.96 (0.82, 1.13) | |
| RCS | 14 | 4,334 (3,185, 5,897) | 0.96 (0.82, 1.13) | |
| AUC(0-∞) (μg·h/L) | HSWG | 14 | 4,766 (3,516, 6,462) | |
| RCD | 12 | 4,631 (3,402, 6,303) | 0.97 (0.83, 1.13) | |
| RCS | 11 | 4,816 (3,531, 6,567) | 1.01 (0.86, 1.19) | |
| Cmax (μg/L) | HSWG | 14 | 960 (686, 1,345) | |
| RCD | 14 | 735 (525, 1,029) | 0.77 (0.57, 1.03) | |
| RCS | 14 | 770 (550, 1,078) | 0.80 (0.60, 1.07) |
For AUC(0-∞), 2 and 3 patients for RCD and RCS, respectively had extrapolated AUCtlast-inf values >20% and could not be included in the analysis.
Abbreviations: AUC = area under the plasma concentration versus time curve; AUC(0-∞) = AUC from zero to infinity; AUC(0–48) = AUC from zero to 48 hours post dose; AUC(0-tlast) = AUC from time zero to time t, where t is the last time point with a measurable concentration; CI = confidence interval; Cmax = maximum plasma drug concentration; LSM = least squares means; N = number of patients; HSWG = high-sheer wet granulation; PK = pharmacokinetic; RCD = roller compaction dry-milled; RCS = roller compaction slurry-milled.
Supplementary Table S3.
Statistical analysis of tmax of LY2157299 including all patients.
| Parameter | Formulation, 150 mg | N | Median (min, max) | Median difference RCD/RCS – HSWG (90% CI) |
|---|---|---|---|---|
| tmax (h) | HSWG | 14 | 1.00 (0.50, 3.08) | |
| RCD | 14 | 2.00 (0.50, 3.17) | 0.54 (0.00, 1.09) | |
| RCS | 14 | 2.00 (0.50, 3.00) | 0.00 (0.00, 0.71) |
Abbreviations: CI = confidence interval; HSWG = high- sheer wet granulation; max = maximum; min = minimum; RCD = roller compaction dry-milled; N = number of patients; tmax = time of maximum plasma drug concentration; RCS = roller compaction slurry-milled.