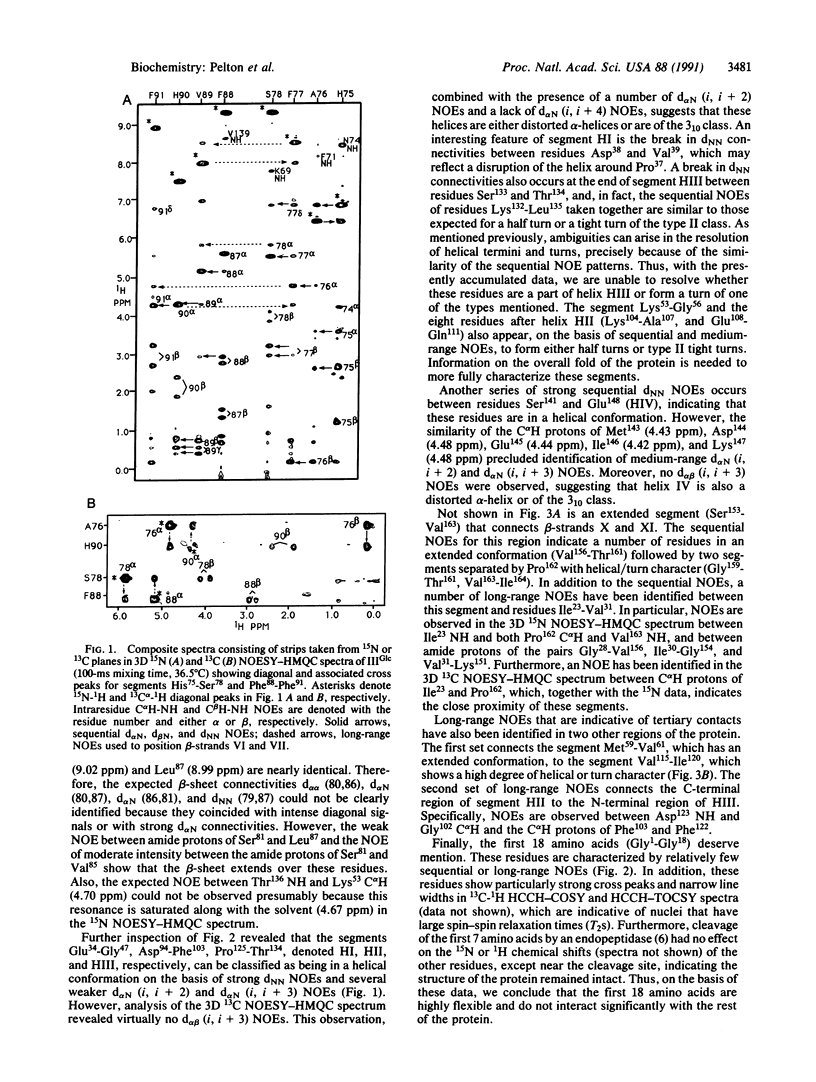
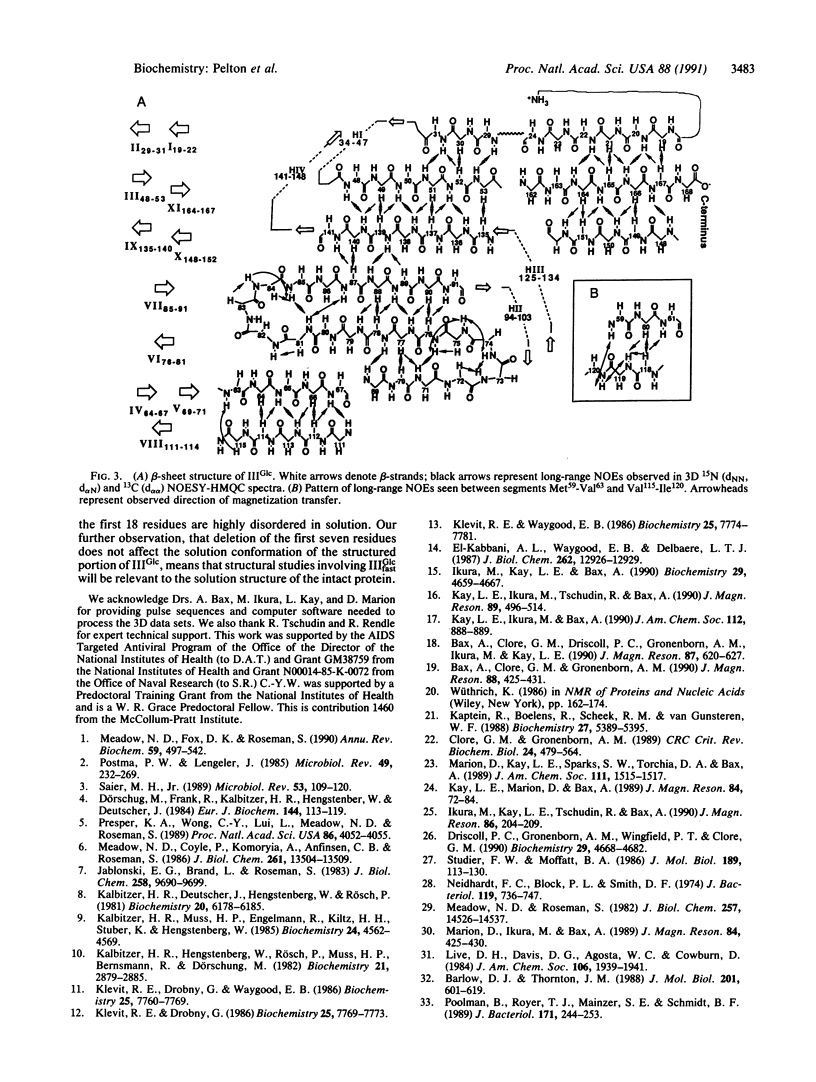
Abstract
IIIGlc is a signal-transducing phosphocarrier protein of the phosphoenolpyruvate:glycose phosphotransferase system of Escherichia coli. The secondary structure of IIIGlc is determined by heteronuclear (15N, 13C) three-dimensional NMR spectroscopy. Sequential, medium-range, and long-range nuclear Overhauser effects seen in NMR spectra are used to elucidate 11 antiparallel beta-strands and four helical segments. The medium-range nuclear Overhauser effect patterns suggest that the helices are either distorted alpha-helices or are of the 3(10) class. The amino acids separating the active-site histidine residues (His75 and His90) form two strands (Ala76-Ser81 and Val85-Phe91) of a six-stranded antiparallel beta-sheet that brings His90 and His75 in close proximity. Sequence similarities in IIIGlc and several other sugar-transport proteins suggest that the histidine residues within these proteins may be arranged in a similar manner. The 18-residue N-terminal peptide that precedes beta-strand Thr19-Ile22 in native IIIGlc is disordered and does not interact with the rest of the protein. Furthermore, removal of the N-terminal heptapeptide by a specific endopeptidase does not affect the structure of the remaining protein, thus explaining the phospho-acceptor activity of modified IIIGlc with the phospho-histidine-containing phosphocarrier protein of this system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow D. J., Thornton J. M. Helix geometry in proteins. J Mol Biol. 1988 Jun 5;201(3):601–619. doi: 10.1016/0022-2836(88)90641-9. [DOI] [PubMed] [Google Scholar]
- Driscoll P. C., Gronenborn A. M., Wingfield P. T., Clore G. M. Determination of the secondary structure and molecular topology of interleukin-1 beta by use of two- and three-dimensional heteronuclear 15N-1H NMR spectroscopy. Biochemistry. 1990 May 15;29(19):4668–4682. doi: 10.1021/bi00471a023. [DOI] [PubMed] [Google Scholar]
- Dörschug M., Frank R., Kalbitzer H. R., Hengstenberg W., Deutscher J. Phosphoenolpyruvate-dependent phosphorylation site in enzyme IIIglc of the Escherichia coli phosphotransferase system. Eur J Biochem. 1984 Oct 1;144(1):113–119. doi: 10.1111/j.1432-1033.1984.tb08438.x. [DOI] [PubMed] [Google Scholar]
- Ikura M., Kay L. E., Bax A. A novel approach for sequential assignment of 1H, 13C, and 15N spectra of proteins: heteronuclear triple-resonance three-dimensional NMR spectroscopy. Application to calmodulin. Biochemistry. 1990 May 15;29(19):4659–4667. doi: 10.1021/bi00471a022. [DOI] [PubMed] [Google Scholar]
- Jablonski E. G., Brand L., Roseman S. Sugar transport by the bacterial phosphotransferase system. Preparation of a fluorescein derivative of the glucose-specific phosphocarrier protein IIIGlc and its binding to the phosphocarrier protein HPr. J Biol Chem. 1983 Aug 25;258(16):9690–9699. [PubMed] [Google Scholar]
- Kalbitzer H. R., Deutscher J., Hengstenberg W., Rösch P. Phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus: 1H nuclear magnetic resonance studies on phosphorylated and unphosphorylated factor IIIlac and its interaction with the phosphocarrier protein HPr. Biochemistry. 1981 Oct 13;20(21):6178–6185. doi: 10.1021/bi00524a041. [DOI] [PubMed] [Google Scholar]
- Kalbitzer H. R., Hengstenberg W., Rösch P., Muss P., Bernsmann P., Engelmann R., Dörschug M., Deutscher J. HPr proteins of different microorganisms studied by hydrogen-1 high-resolution nuclear magnetic resonance: similarities of structures and mechanisms. Biochemistry. 1982 Jun 8;21(12):2879–2885. doi: 10.1021/bi00541a012. [DOI] [PubMed] [Google Scholar]
- Kalbitzer H. R., Muss H. P., Engelmann R., Kiltz H. H., Stüber K., Hengstenberg W. Phosphoenolpyruvate-dependent phosphotransferase system. 1H NMR studies on chemically modified HPr proteins. Biochemistry. 1985 Aug 13;24(17):4562–4569. doi: 10.1021/bi00338a012. [DOI] [PubMed] [Google Scholar]
- Kaptein R., Boelens R., Scheek R. M., van Gunsteren W. F. Protein structures from NMR. Biochemistry. 1988 Jul 26;27(15):5389–5395. doi: 10.1021/bi00415a001. [DOI] [PubMed] [Google Scholar]
- Klevit R. E., Drobny G. P., Waygood E. B. Two-dimensional 1H NMR studies of histidine-containing protein from Escherichia coli. 1. Sequential resonance assignments. Biochemistry. 1986 Nov 18;25(23):7760–7769. doi: 10.1021/bi00371a071. [DOI] [PubMed] [Google Scholar]
- Klevit R. E., Waygood E. B. Two-dimensional 1H NMR studies of histidine-containing protein from Escherichia coli. 3. Secondary and tertiary structure as determined by NMR. Biochemistry. 1986 Nov 18;25(23):7774–7781. doi: 10.1021/bi00371a073. [DOI] [PubMed] [Google Scholar]
- Meadow N. D., Coyle P., Komoryia A., Anfinsen C. B., Roseman S. Limited proteolysis of IIIGlc, a regulatory protein of the phosphoenolpyruvate:glycose phosphotransferase system, by membrane-associated enzymes from Salmonella typhimurium and Escherichia coli. J Biol Chem. 1986 Oct 15;261(29):13504–13509. [PubMed] [Google Scholar]
- Meadow N. D., Fox D. K., Roseman S. The bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu Rev Biochem. 1990;59:497–542. doi: 10.1146/annurev.bi.59.070190.002433. [DOI] [PubMed] [Google Scholar]
- Meadow N. D., Roseman S. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of a glucose-specific phosphocarrier protein (IIIGlc) from Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14526–14537. [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Royer T. J., Mainzer S. E., Schmidt B. F. Lactose transport system of Streptococcus thermophilus: a hybrid protein with homology to the melibiose carrier and enzyme III of phosphoenolpyruvate-dependent phosphotransferase systems. J Bacteriol. 1989 Jan;171(1):244–253. doi: 10.1128/jb.171.1.244-253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presper K. A., Wong C. Y., Liu L., Meadow N. D., Roseman S. Site-directed mutagenesis of the phosphocarrier protein. IIIGlc, a major signal-transducing protein in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4052–4055. doi: 10.1073/pnas.86.11.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr Protein phosphorylation and allosteric control of inducer exclusion and catabolite repression by the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Microbiol Rev. 1989 Mar;53(1):109–120. doi: 10.1128/mr.53.1.109-120.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- el-Kabbani O. A., Waygood E. B., Delbaere L. T. Tertiary structure of histidine-containing protein of the phosphoenolpyruvate:sugar phosphotransferase system of Escherichia coli. J Biol Chem. 1987 Sep 25;262(27):12926–12929. [PubMed] [Google Scholar]



