Abstract
Hydrophilic albumin microspheres are proposed as a new delivery system for amphotericin B (AMB; AMB microspheres). The acute toxicity of AMB microspheres was lower than that of the AMB-deoxycholate (AMB-Doc) reference formulation in hamsters. Lethal doses in healthy and infected animals were improved at least eight times. Intravenous bolus administration of doses of AMB microspheres up to 40 mg/kg of body weight did not produce acute symptoms of toxicity. The efficacy of this new formulation was tested against Leishmania infantum-infected hamsters at doses of 2, 10, 20, and 40 mg/kg. With the 2-mg/kg dose, the activity of AMB, as assessed through the parasite load reductions in the liver and spleen and the evolution of antibody levels, was also improved (P < 0.05) by use of the AMB microsphere system. At the higher doses of 10, 20, and 40 mg/kg, reductions in parasite levels of more than 99% were achieved in the liver and spleen after the administration of AMB microspheres. A pharmacokinetic study was performed to study the serum, liver, and spleen AMB concentrations after administration of AMB microspheres and the reference formulation. Interestingly, a significant accumulation of AMB in the spleen and liver was observed after AMB microsphere administration. Our results suggest that this new formulation is a promising alternative to the conventional AMB-Doc formulation for the treatment of visceral leishmaniasis.
Visceral leishmaniasis is a disease caused by different species of the genus Leishmania both in the Old World and in the New World (23), with an estimated annual incidence of 500,000 cases (44). In Mediterranean countries this infection is considered an emerging disease closely linked to human immunodeficiency virus infection (2, 8, 9). In the Mediterranean Basin, wild canines and domestic dogs are the main reservoirs of zoonotic infections caused by Leishmania infantum (16), with L. infantum infections affecting about 5% of the total dog population in that region. Despite the availability of several drugs, successful treatment of visceral leishmaniasis has not yet been achieved. The most prevalent treatment consists of the administration of pentavalent antimony in the form of sodium stibogluconate (Pentostam) or meglumine antimonate (Glucantime) (26), but there is growing evidence that the rates of response to the antimonials are declining due to the emergence of resistance, and relapses are frequent (24, 38). Amphotericin B (AMB) provides significant leishmanicidal activity as well, and its use results in fewer treatment failures and relapses. However, the important side effects, mainly nephrotoxicity, produced by this drug when it is administered at therapeutic doses have often led to its rejection as a first-choice treatment (28).
New drug delivery systems, such as liposomes, nanospheres, and microspheres, can result in higher concentrations of AMB in the liver and spleen but lower concentrations in the kidneys and lungs (41), thus decreasing the toxicity of AMB (36). Moreover, the administration of AMB through these delivery systems can enhance the accessibility of the drug to organs and tissues (e.g., bone marrow) otherwise inaccessible to the free drug (1).
During the last few years, new AMB formulations (AmBisome, Abelcet, and Amphotec) with improved efficacy/toxicity ratios have been marketed (4, 27). Nevertheless, the utility of these new products is greatly limited by their high costs (34, 43), so they generally have little value for the treatment of humans in many areas where leishmaniasis is endemic and for the treatment of animals in veterinary clinics. Under these circumstances the development of low-cost vehicles is required. It is a matter of interest to design new formulations that, like the recently commercialized AMB alternatives, can grant a better ratio between activity and toxicity compared with that of the classically used formulation Fungizone (AMB-deoxycholate [AMB-Doc]), but with economical advantages in terms of the cost of the formulation compared with the costs of the AMB formulations already marketed.
Thus, a new formulation consisting of albumin microspheres containing AMB (AMB microspheres), elaborated by a spray-drying process, has been developed (40). Albumin is a biocompatible and biodegradable carrier (35). The composition of the formulation and manufacturing procedures make feasible the production of a stable final product that could be an economically interesting alternative to the recently marketed lipid AMB formulations. In the present paper, the activity of this new drug delivery system against L. infantum, which was tested in an experimental model of visceral leishmaniasis in hamsters and whose activity was compared to the activity of the AMB-Doc reference formulation, is presented. Moreover, toxicity and pharmacokinetic studies with both formulations were carried out with hamsters.
MATERIALS AND METHODS
Materials.
AMB was a gift from Bristol-Myers Squibb, Barcelona, Spain. Sodium deoxycholate was purchased from Fluka Chemie AG, Buchs, Switzerland. Dibasic sodium phosphate, monobasic sodium phosphate, and dimethyl sulfoxide were supplied by Panreac S.A., Barcelona, Spain. Methanol was purchased from Labscan, Dublin, Ireland. Human serum albumin (20%) was obtained from Aventis Behring, Barcelona, Spain. Fetal bovine serum was bought from Cansera (Toronto, Ontario, Canada). Goat anti-hamster immunoglobulin G was supplied by Oxford Biotechnology (Oxford, England).
Preparation and characterization of AMB formulations.
Two different AMB formulations were prepared: AMB microspheres and AMB-Doc.
(i) Preparation of AMB formulations for intravenous administration. (a) AMB microspheres.
AMB microspheres were prepared by spray drying. AMB (50 mg) was dispersed in 4.49 ml of an aqueous dissolution containing sodium deoxycholate (41 mg), dibasic sodium phosphate (10 mg), and monobasic sodium phosphate (0.9 mg). The resulting dispersion was subjected to moderate stirring with a magnetic stirrer until a homogeneous suspension was achieved. A 20% serum albumin solution (4.49 ml) was added, and the final mixture was spray dried (inlet temperature, 165°C; feed rate, 3.0 ml/min) with a B-191 spray drier (Büchi, Flawil, Switzerland). Microspheres were collected and heated at 60°C for 1 h. Before drug injection, the AMB microspheres were dispersed in a 5% glucose-water solution.
(b) Preparation of AMB-Doc.
AMB (50 mg) was dispersed in a solution of sodium deoxycholate (41 mg), dibasic sodium phosphate (10 mg), and monobasic sodium phosphate (0.9 mg) in water previously adjusted to pH 12.0 with 2 N sodium hydroxide. Once the drug was homogeneously dispersed, it was acidified to pH 7.4 with 2 N ortho-phosphoric acid. Water was added to the resulting mixture up to a final volume of 10 ml and later diluted in a 5% glucose-water solution.
(ii) Physicochemical characterization of AMB microspheres. (a) Particle size.
The mean particle size of the microspheres was determined on a Galai Cis laser diffractometer just after dispersion in distilled water.
(b) AMB content.
In order to quantify the amount of AMB loaded, a certain quantity of microspheres was dispersed in dimethyl sulfoxide in triplicate, vortexed for 15 min, and centrifuged for 20 min. Afterwards, 1 part of the supernatant was mixed with 19 parts of methanol, and the AMB in the resulting mixture was quantified by spectrophotometry at 405 nm. The sediment was redispersed in dimethyl sulfoxide and subjected to the same procedure twice more.
(c) Molecular aggregation state.
AMB microspheres were dispersed in phosphate-buffered saline (PBS) to form two different dilutions containing 10−4 and 10−5 M AMB, respectively. The absorption spectra between 300 and 450 nm were recorded with a Beckman DU-7 spectrophotometer. The same process was completed with AMB-Doc.
Animals.
Male Syrian golden hamsters were used according to the principles of animal protection amended by Directive 86/609/EU of European Union legislation. They were purchased from Harlan Interfauna Ibérica S.A. (Barcelona, Spain) and housed in plastic cages, food and water were provided ad libitum, and before the experiments the animals were kept for a week to provide them with an adaptation period.
Parasite.
The autochthonous isolate M/CAN/ES/96/BCN150 (zymodeme MON-1) of L. infantum was kindly provided by C. Alonso and J. M. Requena (Centro de Biología Molecular “Severo Ochoa,” UAM, Madrid, Spain) in 1999. Since then, it has been maintained in our laboratory by periodic passage in golden hamsters.
Preparation of parasites for experimental infection.
Amastigotes harvested from the spleens of infected hamsters were cultured in Novy-MacNeal-Nicolle (NNN) medium supplemented with penicillin (200 IU), gentamicin (200 μg/ml), and streptomycin (2 mg/ml) for 2 days up to the time of transformation into promastigotes. Thereafter, they were transferred to C-199 medium supplemented with 1% 10 mM adenine in 50 mM HEPES, 0.25% hemin in 50% triethanolamine, 0.348 g of sodium bicarbonate per liter, 25 mM HEPES, 20% heat-inactivated fetal bovine serum, 100 μg of penicillin per ml, and 100 μg of streptomycin per ml at pH 7.2. Under these conditions the maximum number of metacyclic forms, as determined by resistance to complement lysis, was achieved at about day 7. After 7 days (stationary phase), promastigotes were harvested from the primary culture by centrifugation at 2,000 × g for 15 min, washed with PBS at pH 7.2, and finally resuspended in PBS. Promastigotes were counted with a Neubauer hemocytometer, and the final suspension was adjusted to provide the appropriate number of promastigotes per inoculum. Each hamster was infected with 107 promastigotes, given by the intracardiac route.
Acute toxicity assay. (i) Infected animals.
Hamsters were infected via the heart with 107 L. infantum M/CAN/ES/96/BCN 150 promastigotes. Six weeks after the infection, they were divided into similar groups depending on the specific antibody concentrations in serum, determined as described below. On day 69 postinfection these groups, consisting of six animals each, were injected via the heart with one of the following preparations over 1 min: AMB microspheres or AMB-Doc (both formulations were used at increasing concentrations of 2.5, 5, 10, 20, and 40 mg of AMB per kg of body weight until the 50% lethal dose was reached). One group was left untreated as a control. The animals were examined for mortality over the next 48 h.
(ii) Healthy animals.
The same preparations and doses mentioned above were injected into uninfected animals.
Subacute nephrotoxicity assay.
Creatinine and urea concentrations in plasma were quantified with an autoanalyzer (Ra-500; Bayer), according to the instructions of the manufacturer. To this end, the concentrations in the serum samples of all the hamsters included in the activity tests were measured during the activity studies 1 week before treatment and 5 weeks later.
Activity assays.
Activity experiments were divided into three assays. In all of them, male Syrian golden hamsters were infected and classified as described above for the acute toxicity test with infected animals.
(i) First activity assay.
Both formulations were tested by use of the same drug dose. Groups of five or six animals each were used. A serum sample was taken each week in order to determine the antibody response, as described below. On days 69, 71, and 73 postinfection, three doses of 2 mg of AMB/kg were administered as AMB microspheres via the heart over 1 min to one of the groups. A second group was given the same doses of AMB in the form of the reference AMB-Doc formulation. A third group received no treatment (control group). On day 150 postinfection, all animals were killed and the parasite burdens in the spleens and livers as well as the antibody titers were quantified as described below. The existence of statistically significant differences was determined by applying a one-way analysis of variance with log10-transformed data.
(ii) Second activity assay.
The activities of the AMB microspheres were studied by use of a dose of 40 mg of AMB per kg, which was the maximum quantity administered in the toxicity assays. A smaller group formed by three animals was treated with empty albumin microspheres with the same amount of albumin used in the formulation when AMB was injected at 2 mg/kg in order to verify the effect produced by the formulation without drug. An untreated group was included as well. The second activity test was performed as described above for the first activity assay.
(iii) Third activity assay.
AMB microspheres were tested at two intermediate drug doses, 10 and 20 mg/kg, as described above for the first two assays.
Antigen preparation and analysis of antibody responses.
Crude saline extracts were prepared from promastigotes of the strain coded as MHOM/FR/78/LEM75, obtained from cultures in Schneider medium supplemented with HEPES (4 g/liter), sodium bicarbonate (0.4 g/liter), 10% fetal bovine serum (FBS), and antibiotics, as described above. After the parasite suspension was washed several times in PBS (pH 7.2 to 7.4), the parasite suspension, which was kept on ice, was sonicated until a visible decrease in viscosity was detected. Following overnight extraction at 4°C in PBS containing 1 mM phenylmethylsulfonyl fluoride as a protease inhibitor, the extract was centrifuged at 18,000 × g for 1 h and the supernatant was collected and dialyzed overnight at 4°C against PBS. Protein content was measured by the Bradford method, and the aliquoted samples were stored at −80°C until use. Serum antibody levels were measured by an enzyme-linked immunosorbent assay. Crude saline extracts at a concentration of 2.5 μg/ml were used to coat 96-well microtiter plates. Bovine serum albumin at 0.1% in PBS was used as postcoating agent. Sera from hamsters were assayed at 1:100 dilutions, and goat anti-hamster immunoglobulin G coupled to peroxidase was used at a 1:4,500 dilution. o-Phenylenediamine was used as the substrate.
Estimation of parasite burdens.
Immediately after the animals were killed (on day 150 postinfection), the liver and spleen from each animal were removed and weighed. Afterwards, samples of these organs were homogenized in cold PBS-glucose-EDTA solution with a stainless steel tissue grinder. Cell debris was eliminated by passage through a glass wool column. The suspension obtained was centrifuged at 2,000 × g for 15 min at 4°C. Thereafter, the supernatants were discarded and the pellets were resuspended in C-199 medium supplemented as described above. A total of 200 μl of the suspension was transferred to the first well of a 96-well microtiter plate containing NNN medium supplemented with antibiotics. Parasite burdens were estimated by the limiting dilution assay described by Hill et al. (18) and Titus et al. (39). Briefly, 100 μl from the first well was added to the second well with 100 μl of medium C-199. This procedure was repeated to obtain serial dilutions. The plates were incubated at 28°C for 10 days. The last positive dilution at which mobile promastigotes could be detected was considered to contain one parasite cell.
Pharmacokinetics.
Five groups consisting of five healthy hamsters each were administered a single dose of either AMB microspheres or AMB-Doc, each of which contained AMB at a dose of 2 mg/kg, as a bolus into the heart. At different times (5, 30, 90, 360, and 1,440 min) all the animals included in each of these groups were killed; and the AMB concentrations in serum, spleen, and liver were quantified.
(i) Preparation of samples.
Blood was collected in heparinized tubes, and plasma was immediately separated by mild centrifugation. One part plasma was mixed with 4 parts methanol. The spleens and livers were weighed and homogenized. Samples of these organs were sonicated in water for 20 min, and 4 parts methanol was added to 1 part homogenate. The resulting mixtures obtained from the plasma, spleen, and liver were mixed with a vortex mixer and centrifuged (8,000 × g). Supernatants were stored at −20°C until analysis.
(ii) Quantification of AMB.
The levels of AMB in plasma and tissues were quantified by high-performance liquid chromatography. Samples were filtered through syringe filters (Millex HV; Millipore) and analyzed. The chromatography system consisted of a Gilson 306 isocratic pump, an automatic sampler (231 XL; Gilson), a variable-wavelength detector (116; Gilson) set at 380 nm, and an integrator (SP-270; Spectra-Physics). Separation was achieved with a Kromasil 100 C18 reverse-phase column (200 by 4.6 mm; particle size, 5 μm; Teknokroma, Barcelona, Spain) preceded by a guard precolumn (KJO-4282; Phenomenex, Torrance, Calif.). The mobile phase was methanol-5 mM disodium EDTA (80:20; vol/vol), delivered at a flow rate of 1.2 ml/min. The assay was linear from 0.1 to 4.0 μg/ml (correlation coefficient, 0.997). Repeatability (coefficient of variation, <10%), accuracy (91.3 to 99.2%), lower limit of detection (0.03 μg/ml), lower limit of quantification (0.1 μg/ml), resolution (1.12), and selectivity (1.70) were determined previously.
A one-way analysis of variance test was applied to compare the results for serum and tissues, depending on the formulation injected. Significance was set at a P value <0.05.
RESULTS
Physicochemical characterization of AMB formulations.
The mean particle size (mean diameter) of the AMB microspheres was 1.0 μm, and the standard deviation was 0.7 μm. The AMB content in the final product was 4.0%. The absorption spectrum of the AMB microspheres presented maxima at 420, 392, 370, and 358 nm. This conformation would correspond to associated or aggregated molecules, as indicated in previous work (6). The absorption spectrum of AMB-Doc showed only one important peak, at 328 nm, indicating the prevalence of a different molecular disposition, probably oligomeric water-soluble AMB (22).
Assessment of AMB formulation toxicities. (i) Acute toxicity.
Important differences in acute toxicity were observed, depending on the formulation injected and the existence of infection (Table 1). At a dose of 5 mg of AMB/kg, all of the animals infected with L. infantum and treated with the AMB-Doc formulation died in the first 48 h following the injection. All of the healthy hamsters survived after having been administered the same dose (5 mg/kg), but showed acute adverse reactions (instability and arrhythmia) after having been injected with 10 mg/kg, and all of them died after AMB-Doc administration at a dose of 20 mg/kg. However, none of the hamsters, healthy or sick, treated with AMB microspheres (with AMB at 2.5, 5, 10, 20, or 40 mg/kg) appeared to experience adverse effects.
TABLE 1.
Mortality in hamsters during the 48 h after administration of a single dose of AMB
| AMB dose (mg/kg) | No. of animals that died/total no. of animals tested (%)
|
|||
|---|---|---|---|---|
| AMB-Doc
|
AMB microspheres
|
|||
| Infected | Healthy | Infected | Healthy | |
| 0 (untreated) | 0/6 (0) | 0/6 (0) | 0/6 (0) | 0/6 (0) |
| 2.5 | 0/6 (0) | 0/6 (0) | 0/6 (0) | 0/6 (0) |
| 5 | 6/6 (100) | 0/6 (0) | 0/6 (0) | 0/6 (0) |
| 10 | 0/6 (0)a | 0/6 (0) | 0/6 (0) | |
| 20 | 6/6 (100) | 0/6 (0) | 0/6 (0) | |
| 40 | 0/6 (0) | 0/6 (0) | ||
All the animals showed uncoordinated movements or abnormal behavior.
(ii) Subacute nephrotoxicity.
Apparently, under our experimental conditions, none of the formulations seemed to cause subacute nephrotoxicity, because creatinine and urea concentrations were not significantly altered (data not shown).
Antileishmanial activity.
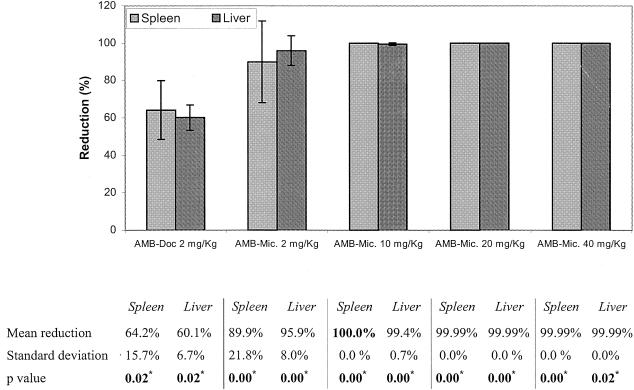
The reductions in the parasite burdens in the spleens and livers of animals treated with AMB-Doc or AMB microspheres in relation to the burdens in the control groups are shown in Fig. 1. The AMB microspheres displayed higher levels of activity than the reference AMB-Doc formulation. The AMB microspheres with a dose of 40 mg of AMB per kg apparently eliminated the infection in the spleens and achieved almost complete removal of the parasites from the livers. Moreover, treatment with the same microspheres with 10 mg of AMB per kg proved to be effective enough to produce almost complete clearance of the parasite burdens in both organs. Albumin microspheres containing no drug did not significantly reduce the parasite burdens compared with the burdens in the control group (data not shown).
FIG. 1.
Reductions of parasite burdens in spleens and livers of treated hamsters in relation to those in the control (untreated) groups. The standard deviation (bars) was calculated by comparing individual data for the treated animals with the average value for the control group. *, statistically significant differences; Mic., microspheres.
Kinetics of antibody responses in treated and control animals.
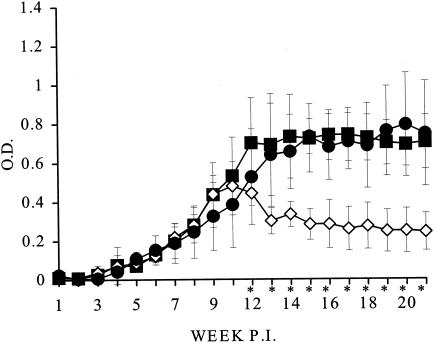
Mean optical density values for the antibody levels in treated and control animals were measured for 21 weeks postinfection. From week 14 onward the patterns of the response clearly differed between the control and the treated animals. In the control animals the antibody levels kept increasing to optical density values greater than 0.6 to 0.7 at about week 21. However, in animals treated with 2 mg of AMB/kg as AMB microspheres or AMB-Doc, these levels tended to gradually decrease to values of about 0.4 and followed similar patterns in both groups.
The antibody responses in animals treated with AMB microspheres containing AMB at higher doses showed greater differences compared with those in the controls. The kinetics in animals treated with AMB microspheres with AMB at 40 mg/kg and empty microspheres compared with those in the untreated controls are represented in Fig. 2.
FIG. 2.
Kinetics of antibody responses in untreated control animals (▪) and animals treated with AMB microspheres containing 40 mg of AMB/kg (◊) or empty microspheres (•) given on days 69, 71, and 73 days (week 11) postinfection (P.I.) by the intracardiac route. Animals were infected with 107 stationary-phase promastigotes of L. infantum, and serum samples were taken weekly for 21 weeks. Bars, standard deviations; *, significant difference between the control group and the group treated with AMB microspheres; O.D., optical density.
In animals treated with AMB microspheres with AMB at doses of 10 and 20 mg/kg, the antibody response patterns were similar, although the decrease in antibody levels from week 14 onward was more important for the higher doses administered.
Pharmacokinetics.
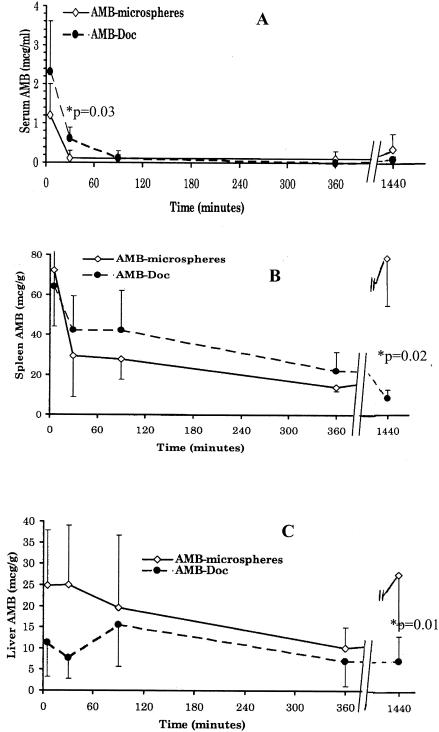
Differences in AMB distributions were observed depending on the formulation, as can be seen in Fig. 3A to C. When the drug was administered via microspheres, AMB was cleared from the bloodstream faster (see drug concentrations in serum 30 min after the injection; P = 0.03) and accumulation was observed in the spleen and the liver 24 h after administration.
FIG. 3.
AMB concentration (average values and standard errors [bars]) versus time in serum (A), spleen (B), and liver (C) after the injection of AMB microspheres or AMB-Doc with a dose of 2 mg of AMB/kg by the intracardiac route. The P values are indicated for the points with significant differences.
DISCUSSION
Toxicity limits the clinical use of AMB formulations. Due to its low cost AMB-Doc is usually the first choice for the AMB treatment of leishmaniasis, although it provides a low therapeutic margin for the treatment of this disease. Therefore, in most cases other drugs are used in both human and animal medicine. Often, the other compounds are effective but do not preclude a high incidence of relapses.
In recent years several safer, although expensive, lipid AMB formulations (AmBisome, Abelcet, and Amphotec) have been commercialized. Other alternatives to the marketed AMB formulations have been explored in the search for a better ratio between safety and manufacturing cost. Some of the strategies used by different investigators have been the inclusion of AMB in lecithin-based microemulsions (25), lipid emulsions (21), lipid nanospheres (28), or ɛ-caprolactone nanospheres (11); conjugation to arabinogalactan (12, 15); or heat treatment (20, 32).
The present study was designed to achieve an advantageous formulation by consideration of in vivo toxicity, antileishmanial activity, and economic cost. With this aim in view, a new drug delivery system consisting of hydrophilic albumin microspheres (AMB microspheres) has been developed. Leishmaniae are located inside phagocytes, and the inclusion of the drug in microparticles tends to enhance the amount that arrives inside reticuloendothelial system cells. Another aim is to hinder the interaction between AMB molecules and the biological membranes, against which they can cause adverse effects. From an industrial point of view, the components and production procedures for AMB microspheres allow more reasonable manufacturing costs compared to those for the expensive lipid-AMB complex formulations.
In order to study the lethal toxicity of the new microsphere formulation, different doses were administered to hamsters and the findings were compared with those obtained with the reference formulation, AMB-Doc. AMB-Doc was demonstrated to have lethal effects when it was administered to mice as a therapeutic dose with 1.0 mg of AMB/kg (32). Under our experimental conditions, the lethal toxicity of the reference formulation was observed with doses higher than 2.5 mg/kg in hamsters (Table 1), so our toxicity results are more similar to those previously described by Souza and Campa (37) for rats, for which the 50% lethal dose was 4.2 mg/kg. Interestingly, no lethal toxicity with the new AMB microsphere formulation was observed even when it was administered at the highest dose with 40 mg of AMB/kg. From the data reported in Table 1, it can be concluded that in hamsters the lethal toxicity of this new formulation in relation to that of the reference AMB-Doc formulation is decreased at least eightfold. An important improvement in AMB toxicity was also previously obtained and reported for AmBisome (5). The use of heated Fungizone and mixtures of AMB with emulsions decreased the acute toxicity of AMB about three times compared with that of the conventional formulation (32, 37).
It should also be pointed out that hamsters infected with L. infantum are more susceptible to the acute toxicity of AMB than healthy hamsters. These data suggest that the use of the less toxic formulations would be especially important at the most advanced stages of the disease.
Nephrotoxicity is an important limitation to the clinical use of AMB. Under our experimental conditions changes in creatinine and urea plasma values were not found in any group of animals. This could be because only three AMB doses of both formulations were administered. The use of more doses would probably have led to renal toxicity.
In relation to the activity studies, when AMB was given at a dose of 2 mg/kg the use of AMB microspheres resulted in significantly higher levels of antileishmanial efficacy in the spleens and livers (decreases in parasite levels of 72% [P = 0.02] and 90% [P = 0.01], respectively) than the use of AMB-Doc. Moreover, treatment with the drug in albumin microspheres not only resulted in a higher level of activity at the same dose but also allowed the administration of drug at much higher doses, which were tolerated well. AMB at 5 mg/kg applied as AMB-Doc killed all the infected hamsters. Interestingly, three doses of 40 mg of AMB/kg each injected (on days 69, 71, and 73 after infection) as AMB microspheres produced an apparent complete clearance of the leishmaniae in the spleens and their almost total removal from the livers without producing observable adverse effects. The application of the same treatment with 10 mg of AMB/kg as AMB microspheres also provided almost complete elimination (99.99%) of the parasite burdens from the spleens and livers (P = 0.00 for both organs if the results for both organs are compared to those for the control groups).
The improved activity/toxicity ratio for AMB microspheres in comparison with that for AMB-Doc could be related to different drug biodistributions. Dissimilar pharmacokinetic behaviors have certainly been observed. The results of this work indicate that the levels of clearance of the drug from plasma and accumulation in tissues seem to be higher when AMB is administered in microspheres. The faster clearance from plasma could have to do with the lower acute toxicity, as well as the interaction of the drug with the albumin matrix. Previous reports (10, 30, 33) have shown higher levels of AMB accumulation in the spleen and liver when AMB is injected in other microparticulate delivery systems, particularly liposomes and nanospheres, compared to the levels of accumulation when the drug is administered as AMB-Doc. Our results suggest the same behavior, but one that is even more pronounced, when AMB is given in AMB microspheres. We hypothesize that the differences in biodistributions could be related to different particle sizes and aggregation of the drug at the molecular level.
Previous work has reported on the important influence of particle size on biodistribution. The faster uptake of drugs included in microparticles by organs containing numerous phagocytes has been described before. Inclusion of drugs in microparticles can enhance their levels of entry into the lymphatic stream from tissues (29) and a subsequent slow arrival to the spleen and liver from the lymph system (42). It has been proposed that the use of a particle size between 1 and 15 μm is ideal to increase the level of drug access to the lymph system (13). The AMB microspheres have a mean size of 1.0 μm, so once they are administered by the intravenous route they should be cleared from the blood by the mononuclear phagocyte system (19).
The aggregation state of AMB molecules brings about the particle size once the drug has left the albumin matrix and, consequently, could affect the biodistribution of the drug. Besides, some work has concluded that the particle size has an influence on toxicity (3, 17, 22) and on the activity of this drug for the treatment of different diseases (6, 7, 14, 31). Therefore, it is probable that the molecular disposition of AMB could account for the different activities, toxicities, and pharmacokinetics observed. Perhaps the existence of large multiaggregates makes it easier for the formulation to reach splenic and hepatic macrophages. An extensive study is being developed to evaluate this.
In conclusion, treatment with AMB microspheres resulted in an improved therapeutic index, with a safe profile detected in hamsters with up to 40 mg AMB per kg and a higher degree of efficacy compared to that of the reference AMB-Doc formulation for therapy for visceral leishmaniasis. The demonstration of these advantages in terms of activity and toxicity suggests that AMB microspheres have a strong potential for further studies, not only as a treatment for kala azar but also as a treatment for other diseases that can be treated with AMB. Trials with this new formulation against natural visceral leishmaniasis in dogs are in progress.
Acknowledgments
This work was supported by grants from Science and Technology Interministerial Commission, Government of Spain (project AGL2002-02175 GAN).
We thank Bristol-Myers Squibb for supplying us with AMB and C. Alonso and J. M. Requena for providing us the L. infantum isolate.
REFERENCES
- 1.Agrawal, A. K., A. Agrawal, A. Pal, P.Y. Guru, and C. M. Gupta. 2002. Superior chemotherapeutic efficacy of amphotericin B in tuftsin-bearing liposomes against Leishmania donovani infection in hamsters. J. Drug Target. 10:41-45. [DOI] [PubMed] [Google Scholar]
- 2.Alvar, J., C. Cañavate, B. Gutiérrez-Solar, M. Jiménez, F. Laguna, R. López-Vélez, R. Molina, and J. Moreno. 1997. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin. Microbiol. Rev. 10:298-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barwicz, J., C. Christian, and I. Gruda. 1992. Effects of the aggregation state of amphotericin B on its toxicity to mice. Antimicrob. Agents Chemother. 36:2310-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman, J. D. 1997. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin. Infect. Dis. 24:684-703. [DOI] [PubMed] [Google Scholar]
- 5.Boswell, G. W., I. Bekersky, D. Buell, R. Hiles, and T. J. Walsh. 1998. Toxicological profile and pharmacokinetics of a unilamellar liposomal vesicle formulation of amphotericin B in rats. Antimicrob. Agents Chemother. 42:263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brajtburg, J., S. Elberg, G. S. Kobayashi, and J. Bolard. 1994. Amphotericin B incorporated into egg lecithin-bile salt mixed micelles: molecular and cellular aspects relevant to therapeutic efficacy in experimental mycoses. Antimicrob. Agents Chemother. 38:300-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheron, M., C. Petit, J. Bolard, and F. Gaboriau. 2003. Heat-induced reformulation of amphotericin B-deoxycholate favours uptake by the macrophage-like cell line J774. J. Antimicrob Chemother. 52:904-910. [DOI] [PubMed] [Google Scholar]
- 8.Dedet, J. P., and F. Pratlong. 2000. Leishmania, Trypanosoma and monoxenous trypanosomatids as emerging opportunistic agents. J. Eukaryot. Microbiol. 47:37-39. [DOI] [PubMed] [Google Scholar]
- 9.Desjeux, P. 1995. Leishmania/HIV co-infections. Afr. Health 18:20-22. [PubMed] [Google Scholar]
- 10.Echevarría, I., C. Barturen, M. J. Renedo, I. F. Trocóniz, and M. C. Dios-Viéitez. 2000. Comparative pharmacokinetics, tissue distribution and effects on renal function of novel polymeric formulations of amphotericin B-deoxycholate in rats. Antimicrob. Agents Chemother. 44:898-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espuelas, M. S., P. Legrand, P. M. Loiseau, C. Bories, G. Barrat, and J. M. Irache. 2002. In vitro antileishmanial activity of amphotericin B loaded in poly(epsilon-caprolactone) nanospheres. J. Drug Target. 10:593-599. [DOI] [PubMed] [Google Scholar]
- 12.Falk, R., A. J. Domb, and I. Polacheck. 1999. A novel injectable water-soluble amphotericin B-arabinogalactan conjugate. Antimicrob. Agents Chemother. 43:1975-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faries, M. B., I. Bedrosian, C. Reynolds, H. Q. Nguyen, A. Alavi, and B. J. Czerniecki. 2000. Active macromolecule uptake by lymph node antigen-presenting cells: a novel mechanism in determining sentinel lymph node status. Ann. Surg. Oncol. 7:98-105. [DOI] [PubMed] [Google Scholar]
- 14.Gaboriau, F., M. Cheron, C. Petit, and J. Bolard. 1997. Heat-induced superaggregation of amphotericin B reduces its in vitro toxicity: a new way to improve its therapeutic index. Antimicrob. Agents Chemother. 41:2345-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golenser, J., S. Frankenburg, T. Ehrenfreund, and A. J. Domb. 1999. Efficacious treatment of experimental leishmaniasis with amphotericin B-arabinogalactan water-soluble derivatives. Antimicrob. Agents Chemother. 43:2209-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gradoni, L. 1999. Epizootiology of canine leishmaniasis in southern Europe, p. 32-39. In R. Killick-Kendrick (ed.), Canine leishmaniasis: an update. Hoechst Roussel Vet, Wiesbaden, Germany.
- 17.Gruda, I., D. Milette, M. Brother, G. S. Kobayashi, G. Medoff, and J. Brajtburg. 1991. Structure-activity study of inhibition of amphotericin B (Fungizone) binding to sterols, toxicity to cells, and lethality to mice by esters of sucrose. Antimicrob. Agents Chemother. 35:24-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill, J. O., R. J. North, and F. M. Collins. 1983. Advantages of measuring changes in the number of viable parasites in murine models of experimental cutaneous leishmaniasis. Infect. Immun. 39:1087-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janknegt, R., S. de Marie, I. A. J. M. Bakker-Woudenberg, and D. J. A. Crommelin. 1992. Liposomal and lipid formulations of amphotericin B. Clin. Pharmacokinet. 23:279-291. [DOI] [PubMed] [Google Scholar]
- 20.Kwong, E. H., M. Ramaswamy, E. A. Bauer, S. C. Hartsel, and K. M. Wasam. 2001. Heat treatment of amphotericin B modifies its serum pharmacokinetics, tissue distribution, and renal toxicity following administration of a single intravenous dose to rabbits. Antimicrob. Agents Chemother. 45:2060-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamothe, J. 2001. Activity of amphotericin B in lipid emulsion in the initial treatment of canine leishmaniasis. J. Small Anim. Pract. 42:170-175. [DOI] [PubMed] [Google Scholar]
- 22.Legrand, P., E. A. Romero, B. E. Cohen, and J. Bolard. 1992. Effects of aggregation and solvent on the toxicity of amphotericin B to human erythrocytes. Antimicrob. Agents Chemother. 36:2518-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauricio, I. L., J. R. Stothard, and M. A. Miles. 2000. The strange case of Leishmania chagasi. Parasitol. Today 16:188-189. [DOI] [PubMed] [Google Scholar]
- 24.Melby, P. C. 2002. Recent developments in leishmaniasis. Curr. Opin. Infect. Dis. 15:485-490. [DOI] [PubMed] [Google Scholar]
- 25.Moreno, M. A., P. Frutos, and M. P. Ballesteros. 2001. Lyophilized lecithin based oil-water microemulsions as a new and low toxic delivery system for amphotericin B. Pharm. Res. 18:344-351. [DOI] [PubMed] [Google Scholar]
- 26.Mullen, A. B., A. J. Baillie, and K. C. Carter. 1998. Visceral leishmaniasis in the BALB/c mouse: a comparison of the efficacy of a nonionic surfactant formulation of sodium stibgluconate with those of three proprietary formulations of amphotericin B. Antimicrob. Agents Chemother. 42:2722-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullen, A. B., K. C. Carter, and A. J. Baillie. 1997. Comparison of the efficacies of various formulations of amphotericin B against murine visceral leishmaniasis. Antimicrob. Agents Chemother. 41:2089-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otsubo, T., S. Maesaki, M. A. Hossain, Y. Yamamoto, K. Tomono, T. Tashiro, J. Seki, Y. Tomii, S. Sonoke, and S. Kohno. 1999. In vitro and in vivo activities of NS718, a new lipid microsphere incorporating amphotericin B, against Aspergillus fumigatus. Antimicrob. Agents Chemother. 43:471-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oussoren, C., J. Zuidema, D. J. Crommelin, and G. Storm. 1997. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. II. Influence of liposomal size, lipid composition and lipid dose. Biochim. Biophys. Acta 4:261-272. [DOI] [PubMed] [Google Scholar]
- 30.Paul, M., R. Durand, H. Fessi, D. Rivollet, R. Houin, A. Astier, and M. Deniau. 1997. Activity of a new liposomal formulation of amphotericin B against two strains of Leishmania infantum in a murine model. Antimicrob. Agents Chemother. 41:1731-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petit, C., M. Cheron, V. Joly, J. M. Rodrigues, J. Bolard, and F. Gaboriau. 1998. In-vivo therapeutic efficacy in experimental murine mycoses of a new formulation of deoxycholate-amphotericin B obtained by mild heating. J. Antimicrob. Chemother. 42:779-785. [DOI] [PubMed] [Google Scholar]
- 32.Petit, C., V. Yardley, F. Gaboriau, J. Bolard, and S. L. Croft. 1999. Activity of a heat-induced reformulation of amphotericin B deoxycholate (Fungizone) against Leishmania donovani. Antimicrob. Agents Chemother. 43:390-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proffitt, R. T., A. Satorius, S. M. Chiang, L. Sullivan, and J. P. Adler-Moore. 1991. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AMBisome) in rodents. J. Antimicrob. Chemother. 28(Suppl. B):49-61. [DOI] [PubMed] [Google Scholar]
- 34.Rex, J. H., and T. J. Walsh. 1999. Estimating the true cost of amphotericin B. Clin. Infect. Dis. 29:1408-1410. [DOI] [PubMed] [Google Scholar]
- 35.Santhi, K., S. A. Dhanaraj, S. D. Rajendran, K. Raja, S. Ponnusankar, and B. Suresh. 1999. Nonliposomal approach: a study of preparation of egg albumin nanospheres containing amphotericin B. Drug Dev. Ind. Pharm. 25:547-551. [DOI] [PubMed] [Google Scholar]
- 36.Saxena, S., and P. C. Ghosh. 2000. Biodistribution of amphotericin B when delivered through cholesterol hemisuccinate vesicles in normal and A. fumigatus infected mice. Pharm. Res. 17:1236-1242. [DOI] [PubMed] [Google Scholar]
- 37.Souza, L. C., and A. Campa. 1999. Pharmacological parameters of intravenously administered amphotericin B in rats: comparison of the conventional formulation with amphotericin B associated with a triglyceride-rich emulsion. J. Antimicrob. Chemother. 44:77-84. [DOI] [PubMed] [Google Scholar]
- 38.Sundar, S., and M. Rai. 2002. Advances in the treatment of leishmaniasis. Curr. Opin. Infect. Dis. 15:593-598. [DOI] [PubMed] [Google Scholar]
- 39.Titus, R. G., M. Marchand, T. Boon, and J. A. Louis. 1985. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 7:545-555. [DOI] [PubMed] [Google Scholar]
- 40.Torrado, J. J., S. Torrado, J. A. Sánchez-Brunete, F. Bolás, M. A. Dea, S. Rama, and J. M. Alunda. January 2003. Microesferas de anfotericina B. Spanish patent P20030089.
- 41.Townsed, R. W., A. Zutshi, and I. Bekersky. 2001. Biodistribution of 4-[(14)C]cholesterol-Ambisome following a single intravenous administration to rats. Drug Metab. Dispos. 29:681-685. [PubMed] [Google Scholar]
- 42.Urban, R. M., J. J. Jacobs, M. J. Tomlinson, J. Gavrilovic, J. Black, and M. Peoc'h. 2000. Dissemination of wear particles to the liver, spleen, and abdominal nodes of patients with hip or knee replacement. J. Bone Joint Surg. Am. 82:455-456. [DOI] [PubMed] [Google Scholar]
- 43.Wong-Beringer, A., R. A. Jacobs, and B. J. Guglielmo. 1998. Lipid formulations of amphotericin B: clinical efficacy and toxicities. Clin. Infect. Dis. 27:603-618. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. 4 April 2002, posting date. Leishmaniasis. http://www.who.int/tdr/diseases/leish/files/leish-poster.pdf.