Abstract
Alpha interferon and ribavirin are required in combination to achieve a sustained virological response in the treatment of hepatitis C virus (HCV) infection. Alpha interferon has direct antiviral activity and also enhances HCV-specific T-cell responses. Ribavirin has little direct activity against HCV but reduces hepatic inflammation. It is therefore likely that these drugs in combination have hitherto unidentified immunological effects. In the present study we investigated the effects of alpha interferon and ribavirin on dendritic cell (DC) maturation and cytokine production induced by double-stranded RNA in vitro. Alpha interferon alone enhanced the expression of HLA class I, HLA class II, and CD86 on immature DCs but did not stimulate full DC maturation, which requires the expression of CD83. Alpha interferon enhanced the production of interleukin 12 p70 [IL-12(p70)] and tumor necrosis factor alpha (TNF-α) but had no effect on IL-10 production. In contrast, ribavirin at physiological doses had no effect on DC maturation but markedly suppressed the production of TNF-α, IL-10, and IL-12(p70). The suppression of cytokines by ribavirin cannot be explained by the induction of DC apoptosis or cell death. Quantitative PCR confirmed that cytokine suppression occurs at the level of mRNA. The suppression of IL-12(p70) and TNF-α in maturing DCs may explain the reduction in hepatic inflammation observed during ribavirin monotherapy. Combination alpha interferon-ribavirin therapy may alter the cytokine profile of maturing DCs overall by suppressing IL-10 production but maintaining IL-12(p70) and TNF-α production, a pattern that would favor viral elimination through downstream effects on T cells.
Hepatitis C virus (HCV) infection is a global public health problem and a significant cause of patient morbidity and mortality (14). Alpha interferon (IFN-α) in combination with ribavirin (1-β-d-ribofuranosyl-1H-1,2,4-triazole-3 carboxamide) is now the mainstay of treatment of HCV infection and leads to sustained viral eradication in 46 to 76% of infected patients, depending on the HCV genotype (13). The mechanisms of action of these drugs are poorly understood. IFN-α monotherapy leads to sustained viral eradication in only 8% of infected patients (28), while ribavirin monotherapy has a minimal effect on the viral load but significantly reduces hepatic inflammation (11, 15, 29). Clearly, then, these drugs have effects, hitherto unidentified, which in combination promote HCV control.
During persistent HCV infection, HCV-specific T-cell responses are weak and very difficult to detect ex vivo (19). However, these responses are enhanced during combination therapy (6), and the HCV-specific lymphoproliferative capacity has been associated with a sustained virological response to therapy (9). Furthermore, the enhanced virological response rates seen with pegylated IFN-α for the treatment of HCV have been attributed to sustained HCV-specific CD4+-T-cell responses (16). These studies clearly support the hypothesis that long-term viral control during therapy for HCV is, at least in part, T-cell mediated.
Given that IFN-α and ribavirin are required in combination to achieve a sustained virological response and that ribavirin has little direct activity against HCV, the combination of these drugs must have immunological effects that neither drug can achieve independently. In support of this, it has been shown that while therapy with IFN-α alone enhances HCV-specific proliferative T-cell responses in vitro, combination therapy suppresses the production of interleukin 10 (IL-10) during these proliferative responses (9).
Despite these findings, it remains unclear how IFN-α therapy exerts its effects on cellular immune responses during therapy for HCV infection. There are several possibilities, including a direct effect through the stimulation of HCV-specific T cells, or an indirect effect, such as through a decrease in the HCV load, which allows exhausted T cells to recover. It is also possible that IFN-α therapy exerts its effects indirectly on professional antigen-presenting cells, as IFN-α has been shown to promote the terminal differentiation of dendritic cells (DCs) in vitro (21). To add to this complexity, none of these hypotheses are mutually exclusive. The contribution of ribavirin to viral eradication is still more perplexing, given that ribavirin has very weak effects against HCV.
DCs play a crucial role in the coordination of cellular immune responses (5). The effects of ribavirin on DC maturation and function are completely unknown. In the study described here we assessed the effects of IFN-α and ribavirin, alone and in combination, on DC function and maturation.
MATERIALS AND METHODS
Generation of immature DCs.
Peripheral blood mononuclear cells were isolated from healthy donors by centrifugation on Ficoll. Monocytes were purified by positive selection with anti-CD14 conjugated magnetic microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). Immature DCs were generated by culturing monocytes in RPMI-10% low-endotoxin fetal calf serum (Invitrogen) supplemented with penicillin-streptomycin, 2 mM l-glutamine, 1% nonessential amino acids, 1% pyruvate, 5 × 10−5 M 2-mercaptoethanol (Sigma), granulocyte-macrophage colony-stimulating factor (50 ng/ml; Leucomax; Schering-Plough), and IL-4 (250 IU/liter; Pharmingen) for 6 days, as described previously (30). DCs were cultured in 6- or 24-well plates at a concentration of 250,000 to 560,000 DCs/ml of medium (higher concentrations of 480,000 to 560,000 DCs/ml were used for the detection of IL-10 secretion).
Maturation of immature DCs.
Immature DCs were stimulated with double-stranded RNA (dsRNA; poly I:C; Sigma) at 50 μg/ml to generate mature DCs, as described previously (7).
Ribavirin and IFN-α.
Immature DCs were matured in the presence or absence of ribavirin (ICN Pharmacuticals) at a physiological concentration (25) of 120 μM (30 μl of ribavirin at 1 mg/ml) and/or IFN-α2b (Schering-Plough) at 2,000 IU/ml.
FACS analysis.
DC maturation was evaluated by staining the surfaces of the DCs with the following antibodies: for isotype controls, immunoglobulin G1 (IgG1)-fluorescein isothiocyanate (FITC) and IgG2a-phycoerythrin (PE) (Dako); for HLA class I, PE (clone W6/32; Dako); and for HLA Class II, FITC (Pharmingen), CD83-FITC (Pharmingen), and CD86-FITC (Dako). 7-Amino-actinomycin D (Pharmingen) was used to exclude dead cells from the analysis. The samples were analyzed on a FACSCalibur fluorescence-activated cell sorter (FACS; Becton Dickinson) with Cell Quest software.
Determination of DC death and apoptosis.
Immature DCs were matured in the presence of poly I:C (dsRNA) with and without ribavirin (240 μM for 54 h). Cell death was determined by FACS analysis with annexin V-FITC in combination with propidium iodide (Pharmingen), according to the instructions of the manufacturer.
Cytokine determination.
The cytokines produced by maturing DCs were initially assessed in cell culture supernatants at multiple time points. Cytokine concentrations were determined by using the cytometric bead array [which determines the concentrations of IL-2, IL-5, IL-6, IL-8, IL-10, IL-12(p70), tumor necrosis factor alpha (TNF-α), IFN-γ, and IL-1β; Becton Dickinson], according to the instructions of the manufacturer, with analysis with Becton Dickinson cytometric bead array software. The supernatants were also analyzed by enzyme-linked immunosorbent assay (ELISA) with antibodies specific for IL-10 and IL-12(p70), according to the instructions of the manufacturer (Pharmingen).
Nuclear run-on assay (polymerases II and III).
The activities of polymerases II and III were determined with the same number of either untreated cells or cells incubated with ribavirin (120 μM) for 18 h by the nuclear isolation and run-on analysis of cultured HeLa cells, as described previously (2). For analysis of the U2 gene, a single-stranded M13 probe containing sequences complementary to the U2 gene sequences from pTP18 (DDBJ/EMBL/GenBank accession no. U57614) was used. By taking the first base pair of the U2 coding sequence as base +1, the probe is complementary to bases +20 to +180. The 5S RNA probe contains the whole RNA-coding region cloned into M13. Control probes for M13 and those for the histone H4 gene were used as described previously (26). For the analysis of the β-actin gene (DDBJ/EMBL/GenBank accession no. E01452), two contiguous 80-mer oligonucleotides complementary to bases +74 to +234 of the β-actin-coding region, as described previously (23), were used.
Quantitative PCR of TNF-α mRNA.
Competitive PCR for quantification of the TNF-α mRNA in DCs matured with dsRNA in the presence and absence of ribavirin was performed according to the instructions of the manufacturer (Maxim Biotech, San Francisco, Calif.). In brief, immature DCs were stimulated with dsRNA. Total RNA was isolated from the DCs (RNeasy; Qiagen) and immediately frozen at −80°C.
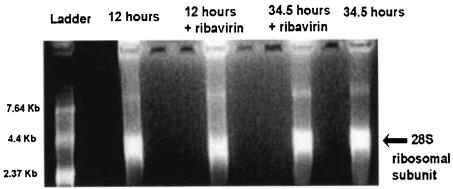
Following RNA extraction, the integrity of the RNA in untreated cells and cells treated with ribavirin was confirmed by denaturing agarose gel electrophoresis with ethidium bromide staining and visualization of the 28S rRNAs transcribed by polymerase I.
cDNA was derived from 5 μg of total RNA by reverse transcription (RT). To determine the amount of TNF-α competitor to be used in the fine-tuned PCR, a preliminary PCR was performed; RT-PCR was performed with cDNA in the presence of 10-fold increasing concentrations of TNF-α competitor. A 325-bp PCR fragment was generated by amplification of the TNF-α target gene, and a 301-bp PCR fragment was generated by amplification of the TNF-α PCR competitor. In this way, the concentration of competitor that gave a competitor band on a 2% agarose gel equivalent in size and density to the target gene band was determined, and fine-tuning of the PCR was performed with twofold serial dilutions of competitor.
RESULTS
Effects of IFN-α on DC maturation.
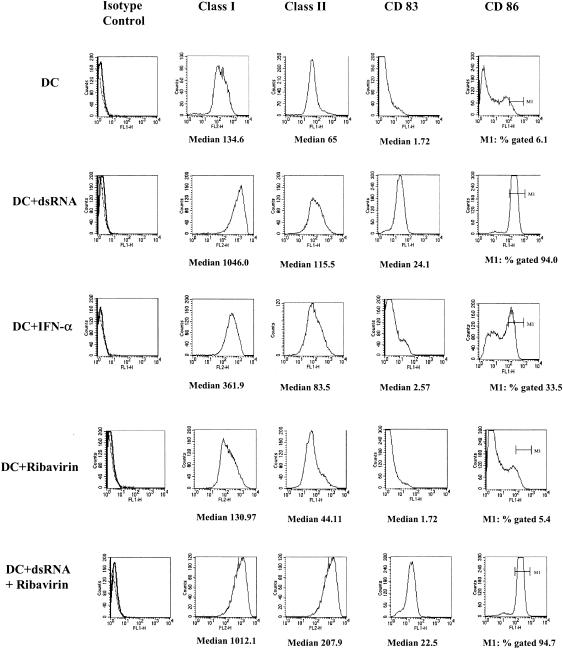
Immature DCs were cultured with dsRNA for 36 h, which induced the maturation of DCs, as shown by the increased expression of HLA class I and class II, CD83, and CD86 (Fig. 1). When IFN-α was added to immature DCs in the presence of dsRNA, it did not further enhance the expression of these markers (data not shown).
FIG. 1.
Effects of IFN-α and ribavirin on phenotypic expression of markers associated with maturation. Immature DCs were cultured in the presence of control medium (DC), dsRNA (DC + dsRNA), IFN-α (DC + IFN-α), ribavirin alone (DC + Ribavirin), and dsRNA together with ribavirin (DC + dsRNA + Ribavirin) for 36 h; and their phenotypes were determined.
As shown previously (7), IFN-α alone induced partial maturation of immature DCs, as shown by the up-regulation of HLA class I and class II and CD86 but not CD83 (Fig. 1).
Effects of ribavirin on DC maturation.
Ribavirin had no effect on the expression of HLA class I or class II, CD86, or CD83 when it was cultured with immature DCs for 36 h (Fig. 1), nor did it affect the maturation induced by dsRNA (Fig. 1).
Furthermore, ribavirin had no effect on the up-regulation of HLA class I and class II and CD86 on immature DCs cultured with IFN-α for 36 h (data not shown).
Effects of IFN-α on cytokine production during DC maturation.
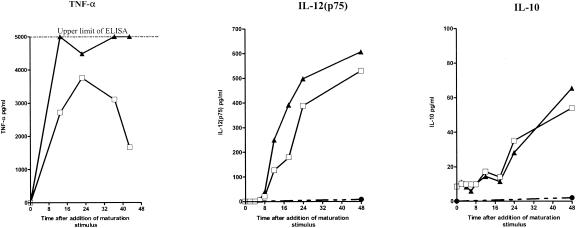
Immature DCs were cultured with dsRNA and/or IFN-α for 48 h, and the culture supernatants were sampled at multiple time points. Using the cytometric bead assay, we screened for the cytokines that are produced when immature DCs are stimulated with dsRNA. dsRNA stimulated the production of IL-10, IL-12(p70), TNF-α, IL-6, and IL-8, as shown previously (18), but not that of IL-1β, IL-2, IL-5, or IFN-γ (data not shown). We next studied the effects of ribavirin and IFN-α on the stimulation of IL-10, IL-12(p70),and TNF-α production by ELISA. In a series of preliminary experiments we found that IFN-α does not stimulate the production of any of these cytokines when IFN-α was added alone to immature DCs (data not shown). However, dsRNA and IFN-α together enhanced the production of IL-12(p70) and TNF-α but had no effect on IL-10 secretion (Fig. 2). Each of these cytokines had a distinct kinetic profile (as described previously in the absence of IFN-α [18]), with TNF-α, IL-12, and IL-10 levels peaking at different time points. This experiment was repeated four times with DCs from four different donors. The kinetics of cytokine production and the effects of IFN-α were similar for DCs from all donors, although the amounts of cytokines produced varied considerably between DCs from different individuals.
FIG. 2.
Kinetics of cytokine production in maturing DCs in the presence and the absence of IFN-α. The concentrations of the cytokines TNF-α, IL-12(p70), and IL-10 in the culture supernatants were determined at sequential time points after dsRNA stimulation with and without IFN-α. The results of one representative of four experiments are shown. The data shown are the means of duplicate ELISAs. The standard error was negligible. □, immature DCs plus dsRNA; ▴, immature DCs plus dsRNA and IFN-α; •, immature DCs plus medium.
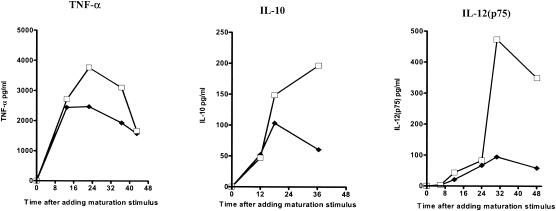
Effects of ribavirin on cytokine production during DC maturation.
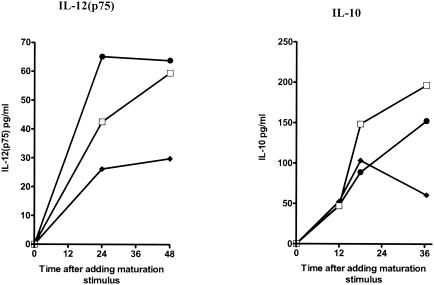
Immature DCs were cultured with dsRNA for 48 h in the presence or absence of ribavirin, and the culture supernatants were sampled at multiple time points. Ribavirin inhibited the production of IL-10, IL-12, and TNF-α (Fig. 3). Titration of the dose of ribavirin confirmed that the suppressive effects of ribavirin could be detected at doses as low as 12 μM (data not shown)). IFN-α partially restored the suppressive effects of ribavirin on IL-10 and completely restored the suppressive effects of ribavirin on IL-12(p70) production in maturing DCs (Fig. 4).
FIG. 3.
Kinetics of cytokine production in maturing DCs in the presence and the absence of ribavirin. The concentrations of the cytokines TNF-α, IL-12(p75), and IL-10 in the culture supernatants were determined at sequential time points after dsRNA stimulation with and without ribavirin. The results of one representative of four experiments are shown. The datum points are the means of duplicate ELISAs. □, immature DCs plus dsRNA; ♦, immature DCs plus dsRNA and ribavirin.
FIG. 4.
Effects of IFN-α on the suppressive effects of ribavirin in maturing DCs. The concentrations of the cytokines IL-12(p75) and IL-10 in the culture supernatants were determined at sequential time points after dsRNA stimulation in the presence of dsRNA alone, dsRNA in addition to ribavirin, and dsRNA in addition to ribavirin and IFN-α. The data shown are the means of duplicate ELISAs. The standard error was negligible. □, immature DCs plus dsRNA; ♦, immature DCs plus dsRNA and ribavirin; •, DCs plus dsRNA, ribavirin, and IFN-α.
Ribavirin does not suppress cytokines through the induction of cell death or apoptosis.
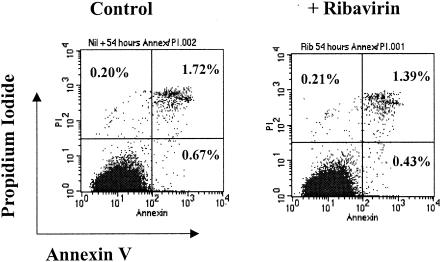
To ensure that the suppressive effects of ribavirin on cytokine production of maturing DCs is not due to the induction of cell death or apoptosis, DCs were stained with annexin V and propidium iodide (Fig. 5). After 54 h of culture without ribavirin, 0.67% of DCs were undergoing apoptosis and 2.39% were dead. Addition of ribavirin did not increase the percentages of apoptotic or dead cells in the cultures (0.43 and 1.82%, respectively).
FIG. 5.
Effects of ribavirin (Rib) on DC apoptosis and cell death. DCs were matured with dsRNA with and without ribavirin (240 μM) for 54 h. Cell death and apoptosis were determined by FACS analysis, following staining with annexin V and propidium iodide (PI).
Ribavirin is not a general inhibitor of RNA polymerases.
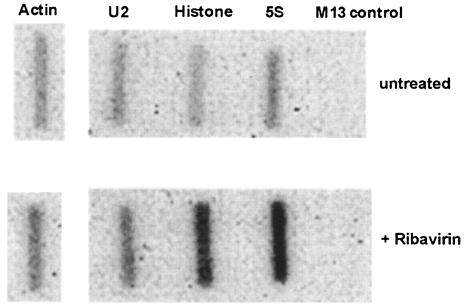
Having shown that ribavirin inhibits cytokine production in immature DCs stimulated with dsRNA and that this is not through the induction of apoptosis, we attempted to further define the mechanism of this inhibition. Ribavirin is a nucleoside analogue. We therefore determined whether ribavirin might act as a general inhibitor of RNA polymerase I, II, or III. The quantity of 28S rRNA, which is transcribed by RNA polymerase I, was determined by denaturing agarose gel electrophoresis, following stimulation of DCs with dsRNA for 12 and 34.5 h in the presence and absence of ribavirin. There was no difference in the 28S ribosomal bands for the ribavirin-treated and untreated DCs (Fig. 6). To assess whether ribavirin is a general inhibitor of polymerase II, which is the enzyme responsible for the transcription of cytokine genes, or polymerase III, which synthesizes small noncoding RNAs such as 5S rRNA, a nuclear run-on assay was performed with HeLa cells incubated with ribavirin for 18 h. The levels of transcription of the U2 polymerase II-dependent snRNA-, β-actin-, and histone protein-coding genes and the polymerase III-dependent 5S rRNA gene were determined (Fig. 7). Ribavirin did not inhibit the transcription of any of these genes but appeared, rather, to activate transcription of the histone H4 and 5S RNA genes.
FIG. 6.
Effects of ribavirin on polymerase I activity in maturing DCs. Following RNA extraction, the activities of polymerase I in DCs matured with dsRNA for 12 and 34.5 h with and without ribavirin were determined by denaturing agarose gel electrophoresis with ethidium bromide staining and visualization of the 28S rRNA band.
FIG. 7.
Effects of ribavirin on polymerase II activity in HeLa cells. The polymerase II activities were determined with and without ribavirin by nuclear isolation and run-on analysis of cultured HeLa cells. Probes complementary to the U2, actin, and histone genes and an M13 control probe were used.
Ribavirin decreases TNF-α mRNA levels in maturing DCs.
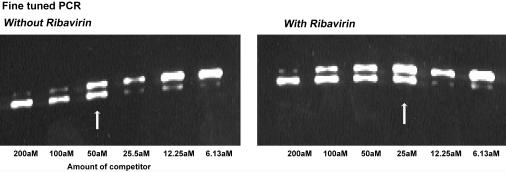
The levels of TNF-α mRNA transcribed in maturing DCs peak very early following stimulation, and TNF-α is undetectable 12 h following stimulation (18). Using competitive PCR, we found that ribavirin clearly inhibits the amount of TNF-α mRNA produced by maturing DCs. In the fine-tuned PCR, at 3 h after stimulation, 50 aM (Fig. 8) of competitor was required to achieve bands equal in intensity to those for DCs cultured in the absence of ribavirin, and 25 aM (Fig. 8) was required in the presence of ribavirin. There was therefore a twofold decrease in the amount of TNF-α mRNA in the presence of ribavirin.
FIG. 8.
Effects of ribavirin on TNF-α mRNA in maturing DCs. Competitive PCR to quantify TNF-α mRNA levels in DCs matured with dsRNA for 3 h with and without ribavirin following RNA extraction and RT-PCR. A fine-tuned PCR was performed with twofold serial dilutions of a TNF-α competitor (301 bp of a TNF-α competitor that competes with the 325-bp TNF-α gene fragment during PCR amplification) in DCs that had been stimulated with and without ribavirin. The white arrows highlight bands of equal size and intensity that were identified in the fine-tuned PCR.
DISCUSSION
For the treatment of HCV infection, IFN-α and ribavirin are required in combination to achieve a sustained virological response. T-cell responses are enhanced during combination therapy (6) and are associated with a sustained response to therapy (9, 16). Ribavirin has little direct activity against HCV but reduces liver inflammation (11). Therefore, it is likely that the combination of these drugs has immunological effects that neither drug can achieve independently.
In this study we show that IFN-α and ribavirin may exert distinct effects on the phenotype and function of maturing DCs in vitro. Although the relevance of these findings in vivo are not established, our data suggest that modulation of DC function may account for some of the previously described effects of combination therapy for the treatment of HCV.
dsRNA was used to induce the maturation of immature DCs throughout our experiments, as this is the ligand that exists during maturation stimulates Toll-like receptor 3 (1) and is associated with the replication of positive- and negative-strand RNA viruses. It is therefore particularly relevant to HCV infection, although other DC maturation factors may also be present during HCV infection. Following T-cell-mediated hepatocyte lysis, dsRNA would be available to circulating DCs in both hepatic sinusoids and local lymph nodes. The cytokine profile produced by DCs when they are stimulated with dsRNA in the presence of exogenous ribavirin and high doses of IFN-α may be quite different from the cytokine profile produced in the absence of these drugs. This would have downstream effects on both HCV-specific T cells and bystander T-cell recruitment. Further analysis of the ability of DCs matured in the presence of ribavirin to prime and stimulate T cells would be interesting, but interpretation of the results of such experiments would be difficult, as ribavirin inhibits T-cell proliferation in vitro (27, 32).
dsRNA induces the up-regulation of HLA class I and class II, CD83, and CD86, as described previously (7, 35), and induces the production of IL-10, IL-12(p70), TNF-α, IL-6, and IL-8 but not IL-1β, IL-2, IL-5, or IFN-γ. Although the up-regulation and cytokine profile of maturing DCs stimulated with lipopolysaccharide has been thoroughly investigated, the effects of dsRNA on cytokine production during DC maturation are less clear, and studies of these effects have provided inconsistent results (18, 35), with earlier reports suggesting that dsRNA induced the production of IL-12(p70) but not IL-10 in maturing DCs (35). However, IL-10 is produced in relatively small amounts, so that culture media with low DC concentrations might allow the detection of IL-12(p70) but not IL-10, perhaps explaining these conflicting results.
The pattern of cytokine production was very similar between DCs from different individuals, although the absolute amount of cytokines produced varied considerably. Others have also described this phenomenon (18). As shown previously (7), the addition of exogenous IFN-α alone to maturing DCs induces the up-regulation of HLA class I and class II and CD86 but does not induce full DC maturation, for which the up-regulation of CD83 (the marker particularly associated with the high stimulatory capacity of fully mature DCs [36]) is required. However, IFN-α enhances the production of IL-12(p70) and TNF-α, but not that of IL-10, in DCs stimulated with dsRNA. It has previously been shown that IFN-α enhances the production of IL-10 and IL-12(p70) in CD40L-mediated DC maturation (20). This suggests that IFN-α has different effects on different cytokines, depending on the maturation stimulus used. This would be an appropriate phenomenon in vivo, as different pathogenic stimuli are likely to require different cytokine profiles in maturing DCs for maximum effect.
The mechanism of action of ribavirin for the treatment of HCV is not understood. Certainly, ribavirin has broad-spectrum activities against both DNA and RNA viruses, and multiple mechanisms have been proposed to account for these activities. Ribavirin has been shown to inhibit IMP dehydrogenase, which depletes the intracellular pools of GMP, which is then unavailable to viral polymerases (33). This would impair viral transcription. The induction of error catastrophe that occurs through the accumulation of mutations through mutagenesis during viral replication has also been proposed as a mechanism (10), and some evidence obtained with an HCV replicon system supports this hypothesis (8). Further hypotheses include the direct inhibition of viral polymerase (12) and inhibition of the capping of viral RNA transcripts (31).
It is possible to dissect these hypotheses further as they apply to HCV infection: HCV is not a capped virus, so the inhibition of viral capping as a potential mechanism cannot apply. Moreover, as ribavirin monotherapy has a minimal effect on the HCV load, inhibitory effects on HCV viral polymerase or the induction of error catastrophe cannot fully account for the activity of ribavirin against HCV. Furthermore, and most importantly, none of these hypotheses explain the reduction in hepatic inflammation (11, 29) that arises during ribavirin treatment in the absence of significant decreases in the HCV load.
A number of studies have reported that ribavirin has diverse effects on the cytokines produced by peripheral blood mononuclear cells stimulated with nonspecific mitogens (22, 24, 32, 34). The results of these studies suggest that ribavirin enhances the production of some cytokines and inhibits the production of others. Accordingly, it has been suggested that the alteration in the cytokine profile accounts for the effects of ribavirin against HCV. Although these studies may indicate that ribavirin can modulate cellular immune responses, interpretation has been confounded by the fact that ribavirin has antiproliferative effects, which may indirectly account for cytokine inhibition. Furthermore, several of the assays used peripheral blood mononuclear cells, clearly a mixed cell population containing T cells, monocytes, B cells, and DCs.
We show here that ribavirin inhibits the production of cytokines IL-10, IL-12(p70), and TNF-α in maturing DCs, a single-cell population that does not proliferate following stimulation with dsRNA. For IL-10 and IL-12(p70), these effects were most pronounced after 24 h of stimulation with dsRNA, whereas the effect on TNF-α was observed as early as 12 h. Ribavirin was used at physiologically relevant doses (25) and did not appear to be generally toxic, since the DCs that matured in the presence of ribavirin appeared to be phenotypically normal. In addition, there was no evidence of apoptosis after incubation with ribavirin at high doses for 54 h.
A competitive PCR for TNF-α indicated that ribavirin inhibits the amount of cytokine mRNA detected in maturing DCs. Nuclear run-on analysis showed that ribavirin does not generally inhibit transcription of protein-coding genes by RNA polymerase II or III in rapidly dividing tissue culture cells (HeLa cells). This observation for HeLa cells should be applied cautiously to a DC population, as we cannot rule out the possibility that ribavirin inhibits the very high rates of polymerase II transcription that occur in stimulated DCs, either directly or through the depletion of intracellular pools of guanosine. This could explain why the inhibition of IL-10 and IL-12(p70) occurs at later time points, when transcription rates are highest, and earlier for TNF-α, which is maximally transcribed in the first 6 h of DC stimulation. In addition, it is possible that the transcription of some cytokine genes is specifically inhibited.
While the relevance of our in vitro observations to the treatment of HCV with ribavirin in vivo remains unclear, our results may explain the seemingly paradoxical effects of ribavirin, which, although clearly anti-inflammatory to the HCV-infected liver (11), is also associated with enhanced HCV-specific T-cell responses (6, 9, 17) and sustained viral eradication (28) when it is used in combination with IFN-α. The suppression of IL-12(p70) and TNF-α in maturing DCs may explain the reduction in hepatic inflammation observed during ribavirin monotherapy. Combination IFN-α-ribavirin therapy may alter the cytokine profile of maturing DCs by, overall, suppressing IL-10 production but maintaining IL-12(p70) and TNF-α production. This may have downstream influences on the induction of T-cell responses, as observed previously (18), as well as effects on innate responses, such as NK cell function.
A number of studies have suggested that HCV may replicate within a DC population and impair DC function in HCV-infected individuals (3, 4). The impact of this, in combination with the effect of IFN-α and ribavirin therapy on DCs, is not clear. Interestingly, these studies have usually included patients that have received or who were actually receiving ribavirin at the time of the investigation. In light of the findings that we present here, it is possible that the alteration in DC function was due not to HCV infection but to ribavirin therapy.
Finally, the finding that ribavirin may suppress the production of cytokines from maturing DCs and alter the cytokine balance when it is given with IFN-α may have ramifications that extend beyond the treatment of HCV infection. Patients with conditions in which an alteration in cytokine production toward a Th1-like profile might be beneficial, such as leishmaniasis, leprosy, schistosomiasis, and tuberculosis, could benefit from combination therapy, while inflammatory autoimmune conditions, such as rheumatoid arthritis, could theoretically benefit from ribavirin therapy. The role of ribavirin monotherapy for the long-term treatment of IFN-α-resistant HCV and the effect of this on progressive liver fibrosis deserve further exploration, given the anti-inflammatory effects of ribavirin.
Acknowledgments
E.B. is an MRC clinical training fellow, P.K. is a Wellcome Trust senior clinical fellow, M.S. and V.C. are supported by CRUK grant C399/A2991, S.M. is an MRC senior fellow, and J.M. is an MRC graduate student.
REFERENCES
- 1.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll- like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 2.Ashe, M. P., L. H. Pearson, and N. J. Proudfoot. 1997. The HIV-1 5′ LTR poly(A) site is inactivated by U1 snRNP interaction with the downstream major splice donor site. EMBO J. 16:5752-5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auffermann-Gretzinger, S., E. B. Keeffe, and S. Levy. 2001. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 97:3171-3176. [DOI] [PubMed] [Google Scholar]
- 4.Bain, C., A. Fatmi, F. Zoulim, J. P. Zarski, C. Trepo, and G. Inchauspe. 2001. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 120:512-524. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 6.Barnes, E., G. Harcourt, D. Brown, M. Lucas, R. Phillips, G. Dusheiko, and P. Klenerman. 2002. The dynamics of T-lymphocyte responses during combination therapy for chronic hepatitis C virus infection. Hepatology 36:743-754. [DOI] [PubMed] [Google Scholar]
- 7.Cella, M., M. Salio, Y. Sakakibara, H. Langen, I. Julkunen, and A. Lanzavecchia. 1999. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J. Exp. Med. 189:821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contreras, A. M., Y. Hiasa, W. He, A. Terella, E. V. Schmidt, and R. T. Chung. 2002. Viral RNA mutations are region specific and increased by ribavirin in a full-length hepatitis C virus replication system. J. Virol. 76:8505-8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cramp, M. E., S. Rossol, S. Chokshi, P. Carucci, R. Williams, and N. V. Naoumov. 2000. Hepatitis C virus-specific T-cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology 118:346-355. [DOI] [PubMed] [Google Scholar]
- 10.Crotty, S., D. Maag, J. J. Arnold, W. Zhong, J. Y. Lau, Z. Hong, R. Andino, and C. E. Cameron. 2000. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat. Med. 6:1375-1379. [DOI] [PubMed] [Google Scholar]
- 11.Dusheiko, G., J. Main, H. Thomas, O. Reichard, C. Lee, A. Dhillon, S. Rassam, A. Fryden, H. Reesink, M. Bassendine, G. Norkrans, T. Cuypers, N. Lelie, P. Telfer, J. Watson, C. Weegink, P. Sillikens, and O. Weiland. 1996. Ribavirin treatment for patients with chronic hepatitis C: results of a placebo-controlled study. J. Hepatol. 25:591-598. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson, B., E. Helgstrand, N. G. Johansson, A. Larsson, A. Misiorny, J. O. Noren, L. Philipson, K. Stenberg, G. Stening, S. Stridh, and B. Oberg. 1977. Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob. Agents Chemother. 11:946-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried, M. W., M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Goncales, Jr., D. Haussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, A. Lin, J. Hoffman, and J. Yu. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975-982. [DOI] [PubMed] [Google Scholar]
- 14.Heintges, T., and J. Wands. 1997. Hepatitis C virus: epidemiology and transmission. Hepatology 26:521-526. [DOI] [PubMed] [Google Scholar]
- 15.Hoofnagle, J. H., M. G. Ghany, D. E. Kleiner, E. Doo, T. Heller, K. Promrat, J. Ong, F. Khokhar, A. Soza, D. Herion, Y. Park, J. E. Everhart, and T. J. Liang. 2003. Maintenance therapy with ribavirin in patients with chronic hepatitis C who fail to respond to combination therapy with interferon alfa and ribavirin. Hepatology 38:66-74. [DOI] [PubMed] [Google Scholar]
- 16.Kamal, S. M., L. Bianchi, A. Al Tawil, M. Koziel, K. El Sayed Khalifa, T. Peter, and J. W. Rasenack. 2001. Specific cellular immune response and cytokine patterns in patients coinfected with hepatitis C virus and Schistosoma mansoni. J. Infect. Dis. 184:972-982. [DOI] [PubMed] [Google Scholar]
- 17.Kamal, S. M., J. Fehr, B. Roesler, T. Peters, and J. W. Rasenack. 2002. Peginterferon alone or with ribavirin enhances HCV-specific CD4 T-helper 1 responses in patients with chronic hepatitis C. Gastroenterology 123:1070-1083. [DOI] [PubMed] [Google Scholar]
- 18.Langenkamp, A., M. Messi, A. Lanzavecchia, and F. Sallusto. 2000. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat. Immunol. 1:311-316. [DOI] [PubMed] [Google Scholar]
- 19.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luft, T., P. Luetjens, H. Hochrein, T. Toy, K. A. Masterman, M. Rizkalla, C. Maliszewski, K. Shortman, J. Cebon, and E. Maraskovsky. 2002. IFN-alpha enhances CD40 ligand-mediated activation of immature monocyte-derived dendritic cells. Int. Immunol. 14:367-380. [DOI] [PubMed] [Google Scholar]
- 21.Luft, T., K. C. Pang, E. Thomas, P. Hertzog, D. N. Hart, J. Trapani, and J. Cebon. 1998. Type I IFNs enhance the terminal differentiation of dendritic cells. J. Immunol. 161:1947-1953. [PubMed] [Google Scholar]
- 22.Martin, J., S. Navas, J. A. Quiroga, M. Pardo, and V. Carreno. 1998. Effects of the ribavirin-interferon alpha combination on cultured peripheral blood mononuclear cells from chronic hepatitis C patients. Cytokine 10:635-644. [DOI] [PubMed] [Google Scholar]
- 23.Medlin, J. E., P. Uguen, A. Taylor, D. L. Bentley, and S. Murphy. 2003. The C-terminal domain of pol II and a DRB-sensitive kinase are required for 3′ processing of U2 snRNA. EMBO J. 22:925-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meier, V., E. Burger, S. Mihm, B. Saile, and G. Ramadori. 2003. Ribavirin inhibits DNA, RNA, and protein synthesis in PHA-stimulated human peripheral blood mononuclear cells: possible explanation for therapeutic efficacy in patients with chronic HCV infection. J. Med. Virol. 69:50-58. [DOI] [PubMed] [Google Scholar]
- 25.Page, T., and J. D. Connor. 1990. The metabolism of ribavirin in erythrocytes and nucleated cells. Int. J. Biochem. 22:379-383. [DOI] [PubMed] [Google Scholar]
- 26.Pavelitz, T., L. Rusche, A. G. Matera, J. M. Scharf, and A. M. Weiner. 1995. Concerted evolution of the tandem array encoding primate U2 snRNA occurs in situ, without changing the cytological context of the RNU2 locus. EMBO J. 14:169-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peavy, D. L., W. C. Koff, D. S. Hyman, and V. Knight. 1980. Inhibition of lymphocyte proliferative responses by ribavirin. Infect. Immun. 29:583-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poynard, T., P. Marcellin, S. S. Lee, C. Niederau, G. S. Minuk, G. Ideo, V. Bain, J. Heathcote, S. Zeuzem, C. Trepo, J. Albrecht, et al. 1998. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 352:1426-1432. [DOI] [PubMed] [Google Scholar]
- 29.Reichard, O., J. Andersson, R. Schvarcz, and O. Weiland. 1991. Ribavirin treatment for chronic hepatitis C. Lancet 337:1058-1061. [DOI] [PubMed] [Google Scholar]
- 30.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245-3253. [DOI] [PubMed] [Google Scholar]
- 31.Scheidel, L. M., and V. Stollar. 1991. Mutations that confer resistance to mycophenolic acid and ribavirin on Sindbis virus map to the nonstructural protein nsP1. Virology 181:490-499. [DOI] [PubMed] [Google Scholar]
- 32.Shiffman, M. L., S. B. Verbeke, and P. M. Kimball. 2000. Alpha interferon combined with ribavirin potentiates proliferative suppression but not cytokine production in mitogenically stimulated human lymphocytes. Antivir. Res. 48:91-99. [DOI] [PubMed] [Google Scholar]
- 33.Streeter, D. G., J. T. Witkowski, G. P. Khare, R. W. Sidwell, R. J. Bauer, R. K. Robins, and L. N. Simon. 1973. Mechanism of action of 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc. Natl. Acad. Sci. USA 70:1174-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tam, R. C., B. Pai, J. Bard, C. Lim, D. R. Averett, U. T. Phan, and T. Milovanovic. 1999. Ribavirin polarizes human T cell responses towards a type 1 cytokine profile. J. Hepatol. 30:376-382. [DOI] [PubMed] [Google Scholar]
- 35.Verdijk, R. M., T. Mutis, B. Esendam, J. Kamp, C. J. Melief, A. Brand, and E. Goulmy. 1999. Polyriboinosinic polyribocytidylic acid (poly(I:C)) induces stable maturation of functionally active human dendritic cells. J. Immunol. 163:57-61. [PubMed] [Google Scholar]
- 36.Zhou, L. J., and T. F. Tedder. 1995. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J. Immunol. 154:3821-3835. [PubMed] [Google Scholar]