Abstract
Surface temperature measured by an infrared temperature-scanning thermometer was used to evaluate disease severity and predict imminent death in a murine model of pneumococcal pneumonia. We showed that a decrease in temperature was associated with increasing severity of disease and concomitant histological changes and also that a temperature of 30°C or less was a predictor of death. Furthermore, viable bacterial counts in the lungs of mice euthanized at a temperature of ≤ 30°C were not significantly different from those seen in the lungs of mice allowed to die without intervention. These data support temperature change as a more subtle indicator of outcome than death and demonstrate that this could be used as a reliable end point for euthanasia. To test the utility of our model in a drug trial, we examined the efficacies of moxifloxacin and levofloxacin by using temperature as a measure of disease severity prior to and during treatment. Regardless of the antibiotic used, mice assessed as moderately ill (temperature ≥ 32°C) at the start of treatment had better clinical and bacteriological outcomes than mice assessed as severely ill (temperature < 32°C). However, moxifloxacin offered better protection and greater bacterial clearance than did levofloxacin in all infected mice independent of disease severity. This model not only allows a more subtle evaluation of drug efficacy but also ensures a better degree of standardization and a more humane approach to drug efficacy studies involving animals.
Experimental animal models are fundamental to drug development and are often the only available means of testing the safety, potency, and efficacy of new compounds prior to human clinical trials (7). Several murine infection models, such as the thigh model, have been used to study and evaluate new antimicrobials against respiratory pathogens (6). Other murine models include sepsis models and, more recently, a proliferation of pneumonia models following the increased availability of new antimicrobials with enhanced potency against gram-positive respiratory pathogens such as Streptococcus pneumoniae.
In most rodent trials, drug efficacy is measured by the survival of infected animals following treatment. The use of death as an end point subjects the animals to a great deal of trauma and pain, and animal ethics committees are seeking the establishment of alternate end points for determining efficacy. Unfortunately, specific criteria for evaluating the clinical condition of the animal and predicting death are poorly described, and studies to refine such end points are often not supported by funding agencies (9).
Although changes in the physical appearance of a rodent, including piloerection and a hunched posture, are indicators of distress (4, 5, 14), researchers are reluctant to use such observations as a surrogate for death, on the basis that the subjectivity of these measurements would likely compromise the scientific outcomes of their research. Alternatively, a decrease of 4°C or more in core body temperature is an important sign of deterioration in an animal's condition following challenge with an infectious agent and has therefore been regarded as a more reliable and objective measure (11, 16, 18). However, temperature is also seldom used, since the equipment available to measure it, i.e., rectal thermometers and microchip implant systems, are problematic and expensive, respectively. Measurement of rectal temperature at least twice a day adds further distress for the animal, and the costs and facility requirements associated with the microchip implant system are beyond the scope of most investigators despite its published success (11).
While it is unrealistic to correlate animal model outcomes to clinical situations, it is important to design experiments that permit a reasonable estimation of a drug's activity during a particular stage of infection. With current models, it is difficult to determine if a drug is equally or less effective at advanced and early stages of an infection. Also, treatment start times are chosen arbitrarily under the assumptions that all animals are equally susceptible to the infection and that disease progression occurs in a consistent manner, which is unrealistic and does not reflect the situation in human infections.
In our laboratory we have developed a murine model of pneumonia that closely mimics the human disease and which is used to test the efficacy of new antimicrobials for respiratory diseases. Similar to other models of pneumonia, this infection results in 100% mortality, with death occurring as early as 41 h postinoculation and as late as 89 h if the animal is left untreated (median time to death, 65 h). In order to better monitor disease progression before and during drug treatment, we evaluated a change in surface temperature as an unbiased predictor of imminent death. Surface temperature was measured using a noncontact infrared temperature-scanning thermometer, an affordable, noninvasive, and practical means by which to measure temperature in small animals. Unlike conventional electronic and bulb thermometers, the infrared thermometer measures skin temperature by detecting and quantifying emitted infrared radiation without making physical contact.
To test the utility of our model, we assessed the efficacy of moxifloxacin versus levofloxacin against advancing stages of pneumococcal pneumonia by using surface temperature as a measure of disease severity prior to and during treatment.
MATERIALS AND METHODS
Animals.
Immunocompetent female Swiss Webster mice aged 4 to 6 weeks and weighing 20 to 22 g (Charles River, Raleigh, N.C.) were used. All procedures were performed in accordance with the guidelines on the care and use of laboratory animals put forth by the Canadian Council on Animal Care and approved by Mount Sinai Hospital's Ethics Committee for Animal Experimentation. Animals were acclimated to the facility 7 days prior to each experiment and housed in an adequately ventilated and temperature-controlled environment for the duration of the experiments.
Experimental pneumococcal pneumonia.
Pneumococcal pneumonia was induced by using virulent serotype 3 strain ATCC 6303 (American Type Culture Collection, Manassas, Va.). ATCC 6303 is fully susceptible in vitro to all quinolones, including moxifloxacin (MIC, 0.12 μg/ml) and levofloxacin (MIC, 0.5 μg/ml) and shows no efflux or mutations in the quinolone resistance-determining region of parC/E or gyrA/B. As previously described (2), mice were anesthetized by inhalation with 3% isofluorane USP (Abbott Laboratories, Montreal, Canada) and inoculated endotracheally via the mouth with 50 μl of a phosphate-buffered saline suspension containing 105 log-phase CFU of S. pneumoniae. Control mice were given 50 μl of phosphate-buffered saline and were considered healthy.
Surface temperature studies.
Surface temperatures of infected and healthy mice were monitored twice daily at 0900 h and 1600 h for 96 h or until death occurred to determine the extent of temperature change during the course of an infection. The surface temperature of the abdominal region was measured by the Raynger MX4 series high-performance infrared temperature-scanning thermometer (Raytek, Santa Cruz, Calif.). Briefly, the hindquarters of the mouse were gently raised to expose the abdominal region and provide an easily accessible and unobstructed surface area. The instrument was then held approximately 6 in. from the surface of the mouse, and the device was activated by disengaging the trigger. A circular laser-sighting beam emitted from the instrument provided the user with a clear visual of the targeted area. The displayed reading is an average of three successive temperature readings. Under ambient operating temperatures of 22 to 25°C, the standard deviation of measurements taken repeatedly is ±0.3°C. This experiment was performed twice.
Viable counts in the lungs.
Surface temperatures of infected mice were monitored as before, and the lungs were excised at death, in order to determine if surface temperature correlates with numbers of CFU in the lung. Representative numbers of infected mice were euthanized when their temperatures decreased to ≤30°C, whereas others were allowed to die naturally. Additional mice were euthanized at 24 h in order to evaluate the CFU in lungs from mice with temperatures of >30°C. The total numbers of CFU recovered from whole-lung homogenates were determined by serial twofold dilutions plated onto Columbia blood agar.
Histology.
Twenty mice were infected with S. pneumoniae strain ATCC 6303, and surface temperature was monitored as before. Ten mice each were euthanized at 24 and 35 h postinoculation, and a gross morphological examination of the lungs, including the pleural surfaces, was conducted. In addition, five uninfected mice were used as a negative control. Bacterial counts of the left lung were obtained as described above, whereas right lungs were fixed in 10% buffered formalin, embedded in paraffin, and processed for light microscopy to quantify the extent of lung pathology, including consolidation, acute inflammatory infiltration of lung tissue, and the degree of pleuritis. Scoring for evaluation of histopathological changes is shown in Table 1. This experiment was performed twice.
TABLE 1.
Scoring grid for histopathologic examination of lung tissue
| Parameter | Criterion | Score | Interpretation |
|---|---|---|---|
| Collapse-consolidation | Percent replacement of air with fluid and cellular material | 0 | None |
| 1 | 1-10% | ||
| 2 | 11-50% | ||
| 3 | 51-100% | ||
| Alveolar hemorrhage | Number of erythrocytes per alveolus | 0 | None |
| 1 | 1-5 | ||
| 2 | 6-10 | ||
| 3 | >10 | ||
| Infiltration | Number of PMN leukocytes per 10 alveolar spaces in areas of consolidation | NA | |
| Alveolar edema | Degree of filling of alveolus with proteinaceous material | 0 | None |
| 1 | Mild | ||
| 2 | Moderate | ||
| 3 | Severe | ||
| Bronchial epithelial lesions | Number of bronchi that show necrosis and sloughing of epithelium, epithelial inflammation, and inflammatory exudate within the bronchial space | 0 | None |
| 1 | 1 | ||
| 2 | 2 | ||
| 3 | ≥3 | ||
| Pleuritis | Degree of fibrin deposition and entrapped PMN leukocytes on the pleural surface | 0 | None |
| 1 | Mild | ||
| 2 | Moderate | ||
| 3 | Severe |
Pharmacokinetic studies and analysis.
Concentrations of moxifloxacin and levofloxacin in serum and lung were determined after administration of a single subcutaneous dose of 50 mg/kg of body weight to uninfected mice. Samples of serum and lung were collected from groups of three mice at 0, 5, 10, 15, and 30 min and at 1, 1.5, 2, 4, and 5 h postdosing. Animals were euthanized by intraperitoneal injection of sodium pentobarbital and exsanguinated by intracardiac puncture. Blood and lung samples were processed accordingly and frozen at −80°C. The experiment was performed twice. Drug concentrations were determined by reverse-phase high-performance liquid chromatography (Beckman C18 Ultrasphere column) as previously reported, with modifications (15, 17). Fluorescence detection was performed for moxifloxacin at an excitation wavelength of 296 nm and an emission wavelength of 488 nm, producing a lower limit of quantification of 125 ng/ml. Levofloxacin was also detected by using fluorescence (excitation, 282 nm; emission, 442 nm), and the limit of quantification was 50 ng/ml. Area under the drug concentration-time curve (AUC) and elimination half-life were calculated using the linear trapezoidal rule of the concentration-versus-time data and regression of the semilogarithmic concentration-versus-time data, respectively (Table 2).
TABLE 2.
Pharmacokinetic parameters of moxifloxacin and levofloxacin in serum and lungs of Swiss Webster mice after subcutaneous single-dose administrationa
| Fluoroquinolone | Specimen | Tmax (h)b | Cmax (μg/ml)c | AUC0-24 (μg · h/ml) | Half-life (h) | Elimination rate |
|---|---|---|---|---|---|---|
| Moxifloxacin | Serum | 0.27 | 19.4 | 17.4 | 1.2 | 0.6 |
| Whole-lung homogenate | 0.20 | 0.05 | 0.06 | 1.3 | 0.6 | |
| Levofloxacin | Serum | 0.25 | 25.9 | 25.7 | 0.9 | 0.8 |
| Whole-lung homogenate | 0.63 | 0.06 | 0.06 | 1.2 | 0.6 |
Values are calculated from mean concentrations in serum and whole-lung tissue samples taken at 0, 5, 10, 15 and 30 min and at 1, 1.5, 2, 4, and 5 h postinjection. All values are means for at least five or a total of six mice.
Tmax, time to maximum concentration of drug in serum and lung.
Cmax, maximum concentration of drug observed in serum and lung.
Efficacy trials.
Pneumococcal pneumonia was induced and surface temperature was monitored as before. Each trial was performed twice. At 35 h, all mice were assessed with regard to clinical condition and categorized by surface temperature into two groups: moderate and severe. The disease severity of a mouse was considered moderate if its surface temperature was ≥32°C and severe if its surface temperature was between 30 and 32°C (Table 3). All mice with temperatures of ≤30°C were excluded from the trial, as surface temperature studies showed death to be imminent within 24 h. Moxifloxacin or levofloxacin at a dose of 50 mg/kg was administered subcutaneously twice daily to each cohort, beginning at 35 h and continuing for up to 5 days. Antimicrobial doses were chosen to achieve AUC/MIC ratios greater than 40, as this value is considered optimal to ensure clinical improvement in human-modeled in vitro studies as previously described (1, 12, 13). The ability of a drug to treat gravely ill mice (≤30°C) was not evaluated in this study. Consequently, mice whose temperature fell to 30°C or lower and continued to drop over the next 24 h were euthanized following the 24-h period and were considered to have failed therapy. All mice remaining at the end of the study were euthanized, and their lungs were harvested and homogenized in 1 ml of saline. The total number of CFU recovered from whole-lung homogenates was determined by serial twofold dilutions plated onto Columbia blood agar.
TABLE 3.
Mean numbers of CFU and corresponding surface temperatures of mice infected with ATCC 6303
| Mouse group and temp (°C) at death (n) | Temp (°C)
|
Log10 no. of CFU (mean) | Time to death (h)
|
||
|---|---|---|---|---|---|
| Mean | Range | Median | Range | ||
| Euthanasia | |||||
| ≥32 (23) | 32.8 ± 0.4 | 32-33.7 | 6.0 ± 0.6 | 24 | |
| <32->30 (16) | 31 ± 0.3 | 30.4-31.9 | 6.6 ± 0.6 | 24 | |
| ≤30 (14) | 28.3 ± 1.3 | 26.1-30 | 8 ± 0.3 | 41 | 41-65 |
| Death without intervention | |||||
| >30 (5) | 30.8 ± 0.6 | 30.1-31.7 | 7.5 ± 0.6 | 41 | 41-65 |
| ≤30 (15) | 24.9 ± 3.1 | 19.2-29.6 | 7.5 ± 0.9 | 65 | 41-96 |
Statistical analysis.
CFU data for infected mice were compared between surface temperature groups by using Dunnett's multiple comparison test. Simultaneous 95% confidence limits were used, and as a result a P value of <0.015 was considered significant. Histological variables were compared with temperatures and numbers of CFU by linear regression techniques. Survival and eradication data were compared between antimicrobials for each isolate by using Fisher's exact test and the chi-square test. A P value of 0.05 was considered significant.
RESULTS
Surface temperature change during infection.
Surface temperatures of 21 mice infected with strain 6303 and 10 uninfected mice were monitored for 96 h or until death occurred to determine the extent of temperature change during the course of an infection. Temperatures of uninfected mice were for the most part unchanged over 96 h (mean, 33.1 ± 0.7°C). By comparison, all 21 infected mice showed significant decreases in temperature. Prior to death, 18 (86%) mice had a temperature of ≤30°C (mean, 26.1 ± 2.3°C; range, 22.8 to 30°C) and 3 (14%) mice had a temperature of 30.1 to 30.8°C (mean, 30.4 ± 0.3°C). We found that a 3-to-5°C decrease in surface temperature occurred as early as 24 h postinoculation, and in most cases temperature fell an additional 5°C until death. Death ensued as early as 12 h and as late as 24 h after a temperature of ≤30°C was recorded.
Viable counts in the lungs.
Bacterial counts in the lungs of mice infected with strain 6303 that were euthanized at temperatures of ≤30°C (mean, 8 ± 0.3 log10 CFU) were significantly greater than those in mice euthanized at a temperature of >30°C (6.0 ± 0.6 log10 and 6.6 ± 0.6 log10 CFU) (P < 0.001) (Table 3). In contrast, the counts from mice euthanized at a temperature of ≤30°C (8 ± 0.3 log10 CFU) were not statistically different from the counts of mice that died without intervention and had a temperature ≤30°C (7.5 ± 0.9 log10) (P < 0.04). Equally high were the counts from five mice that died prior to a recorded temperature of ≤30°C (mean count, 7.5 ± 0.6 log10 CFU; mean temperature, 30.8 ± 0.6°C). We speculate that the temperatures of these mice were ≤30°C before death as was observed for other infected mice, since these mice died during the night following an end-of-day mean recorded temperature of 30.8°C.
Histology.
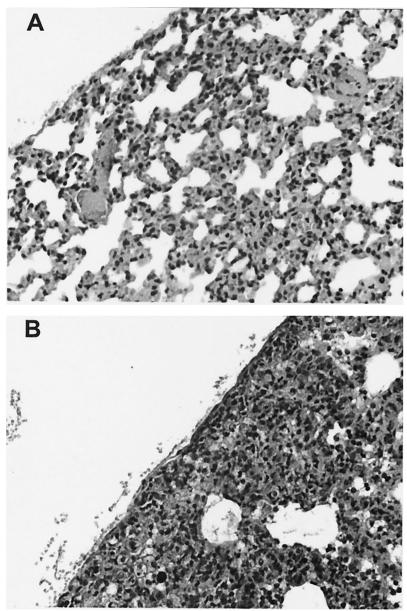
Though pertinent histological features such as consolidation, alveolar hemorrhage and edema, infiltration of polymorphonuclear (PMN) leukocytes, and bronchial epithelial changes were semiquantitatively assessed, the severity of pleuritis was the only histological parameter which showed significant correlation with temperature (r = −0.492, P = 0.02, df = 22) and with CFU (r = 0.604, P = 0.017, df = 15) (Table 4). Minimal congestion and no evidence of pneumonia or pleuritis were observed for mice with temperatures of ≥32°C (Fig. 1A), whereas marked consolidation of alveoli, marked infiltration by PMN leukocytes, and marked pneumonic and pleuritic changes were typical of mice with temperatures between 30 and 32°C (Fig. 1B).
TABLE 4.
Correlations of temperature with lung histology of infected mice
| Parameter |
P value for correlation with:
|
|||||
|---|---|---|---|---|---|---|
| Collapse- consolidation | Alveolar hemorrhage | PMN leukocyte infiltration | Alveolar edema | Bronchial epithelial lesions | Pleuritis | |
| Temperature | NSa | NS | NS | NS | NS | 0.017 |
| Collapse-consolidation | 0.005 | 0.003 | 0.002 | 0.003 | 0.019 | |
| Alveolar hemorrhage | NS | NS | NS | 0.002 | ||
| PMN leukocyte infiltration | 0.001 | 0.006 | NS | |||
| Alveolar edema | 0.15 | NS | ||||
| Bronchial epithelial lesions | NS | |||||
NS, no significance.
FIG. 1.
Light microscopy of lung architecture of an infected mouse with a temperature of ≥32°C (A) and an infected mouse with a temperature of <32 to >30°C (B). Magnification, ×20.
Efficacy trials.
Infected mice were grouped into moderately and severely ill groups as described above and were treated at 35 h with either moxifloxacin or levofloxacin. Clinical and bacteriological responses were assessed by monitoring temperature change and the extent of bacterial eradication in the lungs. As shown in Tables 5 and 6, moxifloxacin-treated mice demonstrated better clinical improvement and greater bacterial clearance than those treated with levofloxacin independent of disease severity. However, statistically significant differences between moxifloxacin and levofloxacin were noted only for percent eradication (P ≤ 0.05) and not percent survival (P > 0.05). Clinical cure rates and bacteriological success rates correlated with surface temperatures at the start of treatment. Regardless of the antibiotic used, mice that had surface temperatures of ≥32°C at the start of treatment had better clinical and bacteriological outcomes than mice with temperatures of <32°C.
TABLE 5.
Clinical outcomes for mice infected with ATCC 6303 and treated with moxifloxacin and levofloxacin
| Disease severitya | Moxifloxacin
|
Levofloxacin
|
||||
|---|---|---|---|---|---|---|
| Sample size | Temp (°C)b | Survival (%) | Sample size | Temp (°C)b | Survival (%) | |
| Moderate | 31 | 33.3 ± 0.7 | 31/31 (100) | 42 | 33.5 ± 0.7 | 37/42 (88)c |
| Severe | 27 | 31 ± 0.7 | 21/27 (78) | 17 | 31 ± 0.2 | 12/17 (70)d |
Moderate surface temperature of ≥32°C; Severe, surface temperature of <32 to >30°C.
Temperature prior to treatment. Values are means ± standard deviations.
P = 0.07 compared with the value for the moxifloxacin group.
P = 0.72 compared with the value for the moxifloxacin group.
TABLE 6.
Bacteriological response outcomes for mice infected with ATCC 6303 and treated with moxifloxacin and levofloxacin
| Disease severitya | Moxifloxacin
|
Levofloxacin
|
||||
|---|---|---|---|---|---|---|
| No. with eradication/total (%) | Viable count in mice with no eradication (log10 CFU)
|
No. with eradication/total (%) | Viable count in mice with no eradication (log10 CFU)
|
|||
| Meanb | Range | Meanb | Range | |||
| Moderate | 31/31 (100) | 31/42 (74)c | 7.0 ± 1.5 | 4.3-8.9 | ||
| Severe | 24/27 (89) | 7.6 ± 0.4 | 7.0-7.9 | 9/17 (53)d | 6.6 ± 1.9 | 3.8-8.7 |
Moderate surface temperature of ≥32°C; Severe, surface temperature of <32 to >30°C.
Values are means ± standard deviations.
P = 0.05 compared with the value for the moxifloxacin group.
P = 0.01 compared with the value for the moxifloxacin group.
DISCUSSION
Over the years, a variety of murine infection models have been used to evaluate the efficacies of new respiratory antimicrobials. In this study we used an existing murine lung infection model but incorporated the use of surface temperature change as a means by which to monitor disease progression and drug efficacy. In doing so, we not only eliminated the use of death as an end point but also established a novel means by which to assess and classify the clinical condition of a mouse prior to drug treatment. Drug intervention in animal trials can now more closely resemble that which occurs in human infections.
We showed that a 3-to-5°C decrease in surface temperature occurred as early as 24 h postinoculation, and in most cases the temperature fell an additional 5°C until death approximately 24 h later. Furthermore, a temperature of 30°C or less proved to be lethal, thus providing a more subtle indicator of outcome than death and serving as an appropriate end point for euthanasia. We also showed that the viable count in the lungs of a mouse euthanized at a skin temperature of ≤30°C was not significantly different from that of a mouse allowed to die naturally. Consequently, euthanasia of a mouse based on skin temperature is not likely to jeopardize the numbers of CFU in the lungs following a bacterial challenge and subsequent drug treatment.
A decrease in core body temperature has been recognized previously as a sign of the deteriorating health of an animal challenged with an infectious agent (11, 16, 18). Kerr et al. showed that peak pulmonary inflammation immediately precedes a 4°C drop in core body temperature in mice infected with pneumococci (10). Similarly, we show increasing severity of pleuritis with decreasing temperature, thereby suggesting that surface temperature accurately mirrors the health of the animal and reflects core temperature. Our data demonstrate that measurement of surface temperature is a reliable and reproducible measure for monitoring disease progression. Further, use of an infrared thermometer is an inexpensive and noninvasive alternative to other devices used for measuring temperature.
Our use of temperature in animal drug trials was tested by comparing the efficacies of moxifloxacin and levofloxacin, both of which have been recommended for treatment of pneumococcal pneumonia. As expected, we observed significantly better clinical cure rates and bacteriological success rates for mice with higher surface temperatures at the start of treatment, regardless of the antibiotic used. We also demonstrated that moxifloxacin offered significantly better protection against both moderate and severe pneumococcal lung infections than levofloxacin, although the interpretation of these data may be limited by the pharmacokinetics of each agent in mice. Given the concentration-dependent nature of fluoroquinolone activity, both the maximum concentration (Cmax)/MIC and the AUC/MIC ratios, which are important predictors of clinical and bacteriological eradication success, were used to determine dosing. For both drugs, the Cmax/MIC ratios (161.6 and 51.8 for moxifloxacin and levofloxacin, respectively) were much higher than can possibly be achieved in humans and greater than the suggested breakpoint of >10. Similarly, for both drugs, the AUC/MIC ratio exceeded the breakpoint of 40, although that of moxifloxacin (145) was much greater than that of levofloxacin (51.8). Regardless of dosing issues, however, this model can discern a difference in efficacy at different severities of illness. Since in human clinical trials, patients with pneumonia are classified and treated according to their severity of illness, animal trials should also reflect this distinction (8).
The ability to assess disease severity prior to initiation of treatment not only allows a more subtle evaluation of drug efficacy but also ensures a better degree of standardization. In studies where the clinical condition of the mouse prior to starting treatment is not considered, the overall survival among different trials could vary significantly. It is our hope that our observations will prompt other investigators to explore the utility of infrared technology for their animal research, particularly considering the gains in accuracy and reproducibility of the experiment and the well-being of the animal.
Acknowledgments
This work was funded by the Bayer AG Corporation.
Special thanks go to the following individuals for their contributions to this project: A. Dalhoff, J. Ambler, K. Ibrahim, C. Costigan, and K. Gravelle.
REFERENCES
- 1.Ambrose, P. G., and D. M. Grasela. 2000. The use of Monte Carlo simulation to examine pharmacodynamic variance of drugs: fluoroquinolone pharmacodynamics against Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 38:151-157. [DOI] [PubMed] [Google Scholar]
- 2.Azoulay-Dupuis, E., E. Vallee, B. Veber, J. P. Bedos, J. Bauchet, and J. J. Pocidalo. 1992. In vivo efficacy of a new fluoroquinolone, sparfloxacin, against penicillin-susceptible and -resistant and multiresistant strains of Streptococcus pneumoniae in a mouse model of pneumonia. Antimicrob. Agents Chemother. 36:2698-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedos, J. P., V. Rieux, J. Bauchet, M. Muffat-Joly, C. Carbon, and E. Azoulay-Dupuis. 1998. Efficacy of trovafloxacin against penicillin-susceptible and multiresistant strains of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob. Agents Chemother. 42:862-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canadian Council on Animal Care. 1993. Guide to the care and use of experimental animals. Bradda Printing Services Inc., Ottawa, Canada.
- 5.Canadian Council on Animal Care. 1998. CCAC guidelines on: choosing an appropriate endpoint in experiments using animals for research, teaching and testing. Canadian Council on Animal Care, Ottawa, Canada.
- 6.Craig, W. A., J. Redington, and S. C. Ebert. 1991. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J. Antimicrob. Chemother. 27(Suppl. C):29-40. [DOI] [PubMed] [Google Scholar]
- 7.Druilhe, P., P. Hagan, and G. A. Rook. 2002. The importance of models of infection in the study of disease resistance. Trends Microbiol. 10:S38-S46. [DOI] [PubMed] [Google Scholar]
- 8.Fine, M. J., T. E. Auble, D. M. Yealy, B. H. Hanusa, L. A. Weissfeld, D. E. Singer, C. M. Coley, T. J. Marrie, and W. N. Kapoor. 1997. A prediction rule to identify low-risk patients with community-acquired pneumonia. N. Engl. J. Med. 336:243-250. [DOI] [PubMed] [Google Scholar]
- 9.Foltz, C. J., and M. Ullman-Cullere. 1999. Guidelines for assessing the health and condition of mice. Lab. Anim. 28:28-32. [PubMed] [Google Scholar]
- 10.Kerr, A. R., J. J. Irvine, J. J. Search, N. A. Gingles, A. Kadioglu, P. W. Andrew, W. L. McPheat, C. G. Booth, and T. J. Mitchell. 2002. Role of inflammatory mediators in resistance and susceptibility to pneumococcal infection. Infect. Immun. 70:1547-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kort, W. J., J. M. Hekking-Weijma, M. T. TenKate, V. Sorm, and R. VanStrik. 1998. A microchip implant system as a method to determine body temperature of terminally ill rats and mice. Lab. Anim. 32:260-269. [DOI] [PubMed] [Google Scholar]
- 12.Lacy, M. K., W. Lu, X. Xu, P. R. Tessier, D. P. Nicolau, R. Quintiliani, and C. H. Nightingale. 1999. Pharmacodynamic comparisons of levofloxacin, ciprofloxacin, and ampicillin against Streptococcus pneumoniae in an in vitro model of infection. Antimicrob. Agents Chemother. 43:672-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lister, P. D., and C. C. Sanders. 1999. Pharmacodynamics of levofloxacin and ciprofloxacin against Streptococcus pneumoniae. J. Antimicrob. Chemother. 43:79-86. [DOI] [PubMed] [Google Scholar]
- 14.Olfert, E. D., and D. L. Godson. 2000. Humane endpoints for infectious disease animal models. ILAR J. 41:99-104. [DOI] [PubMed] [Google Scholar]
- 15.Siefert, H. M., A. Domdey-Bette, K. Henninger, F. Hucke, C. Kohlsdorfer, and H. H. Stass. 1999. Pharmacokinetics of the 8-methoxyquinolone, moxifloxacin: a comparison in humans and other mammalian species. J. Antimicrob. Chemother. 43(Suppl. B):69-76. [DOI] [PubMed] [Google Scholar]
- 16.Soothill, J. S., D. B. Morton, and A. Ahmad. 1992. The HID50 (hypothermia-inducing dose 50): an alternative to the LD50 for measurement of bacterial virulence. Int. J. Exp. Pathol. 73:95-98. [PMC free article] [PubMed] [Google Scholar]
- 17.Stass, H., and D. Kubitza. 1999. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J. Antimicrob. Chemother. 43(Suppl. B):83-90. [DOI] [PubMed] [Google Scholar]
- 18.Wong, J. P., E. G. Saravolac, J. G. Clement, and L. P. Nagata. 1997. Development of a murine hypothermia model for study of respiratory tract influenza virus infection. Lab. Anim. Sci. 47:143-147. [PubMed] [Google Scholar]