Abstract
Human tear lipocalin (TL; also known as Lcn1) is a secretory protein present in large amounts in fluids that cover epithelial surfaces such as tears and respiratory secretions. It is supposed to act as a physiological scavenger of hydrophobic, potentially harmful molecules, but there is evidence that it also inhibits bacterial growth. In the present study, we reconsidered the possibility that TL might interfere with microbial growth by scavenging of siderophores, as described for human neutrophil gelatinase-associated lipocalin (NGAL). Indeed, our experiments revealed that TL binds to microbial siderophores with high affinities. In contrast to NGAL, which was shown to have some specificity for bacterial catecholate-type siderophores, TL binds to a broad array of siderophores, including bacterial catecholate-type enterobactin and hydroxamate-type desferrioxamine B, and all major classes of fungal siderophores. By adding exogenous TL, bacterial and fungal growth could be inhibited under iron-limiting conditions. Thus, TL might be a novel member of the innate immune system especially involved in mucosal defense against fungal infections.
Several epithelial surfaces are continuously exposed to potential pathogens but rarely become infected because they are protected by specific secretions. These secretions contain a number of antimicrobial components and are constantly cleared by mechanical action. For instance, the human cornea has an elaborate system of defense provided by the tear film, which contains an assortment of proteins that derive antimicrobial activity by a variety of mechanisms. Human tear lysozyme hydrolyzes the glycosidic linkages in the polysaccharide components of the cell wall and kills Micrococcus leisodeikticus (6) and Bacillus subtilis (36). Secretory phospholipase A2 hydrolyzes phospholipids found mainly in microbial membranes and is the principal bactericide in tears for staphylococci and other gram-positive bacteria (32). Lactoferrin induces bacteriostasis by competing for free iron, but it also has bactericidal activity against several bacteria (2). Secretory immunoglobulin A inhibits the adhesion of specific bacteria and protozoa to the corneal epithelium (1). Defensin peptides, which have a wide range of potential antimicrobial activities as a result of their formation of pores in the microbial cell membrane (16), have also been found in human tear fluid (14).
There is circumstantial and somewhat controversial evidence for the antibacterial activity of another protein found in high concentrations in tear fluid and other secretions, namely, tear lipocalin (TL), also called von Ebner's gland protein or Lcn1 (33, 35). TL is a member of the protein superfamily of lipocalins and is typically characterized by its ability to bind to a large variety of hydrophobic compounds, including fatty acids, phospholipids, glycolipids, cholesterol, retinol, arachidonic acid, and other lipid peroxidation products (10, 20, 33). By scavenging these molecules, TL may act as a general protection factor for epithelial surfaces (35).
Several facts prompted us to reconsider the antimicrobial activity of TL. First, there were some indications that its antibacterial activity depends on the ligands bound (10). TL purified by gel filtration showed antibacterial activity, which was not present after purification by reversed-phase high-pressure liquid chromatography (7, 36). Second, two other properties of TL might be relevant for antibacterial activity: cysteine proteinase inhibition and nonspecific magnesium-dependent endonuclease activity (38, 42). Third, very recently it was found that human neutrophil gelatinase-associated lipocalin (NGAL), another member of the lipocalin superfamily, avidly binds to the bacterial siderophore enterobactin and inhibits bacterial growth by interference with siderophore-mediated iron acquisition by members of the family Enterobacteriaceae (12).
Here we show that TL exhibits its antimicrobial activity by binding to microbial siderophores similarly to NGAL. However, in contrast to NGAL, TL was found to bind to a broad spectrum of siderophores, including all major classes of fungal siderophores. Growth inhibition tests showed that this siderophore binding results in a strong reduction of bacterial and fungal growth under iron-limiting conditions.
MATERIALS AND METHODS
Chemicals, siderophores, strains, and media.
The fluorescent fatty acid derivative 11-({[5-(dimethylamino)-1-naphthalenyl]sulfonyl}amino) undecanoic acid (DAUDA) was purchased from Molecular Probes (Leiden, The Netherlands). The recombinant TL used for the binding studies was produced in Escherichia coli as described previously (15) and was delipidated by chromatography on Lipidex 1000 (Sigma, Vienna, Austria) (11). The siderophores enterobactin, pyoverdine, aerobactin, and coprogen were purchased from Biophore Research Products (Tübingen, Germany). Ferricrocin and triacetylfusarinine C (TAFC) were purified from Aspergillus nidulans by high-pressure liquid chromatography by standard methods (18). Desferrioxamine B, rhodotorulic acid, and ferrichrome were obtained from Sigma.
The following organisms were used for microbial growth inhibition tests: E. coli (XL1-Blue), Pseudomonas aeruginosa (ATCC 9027), and A. nidulans ΔsidA (29). All bacteria were cultivated in M9 minimal medium (which contained the following per liter: 200 ml of 5× M9 minimal medium [64 g of Na2HPO4 · 7H2O, 15 g of KH2PO4, 2.5 g of NaCl, 5 g of NH4Cl], 2 ml of 1 M MgSO4, 20 ml of 20% glucose, and 0.1 ml of 1 M CaCl2) at 37°C. The fungi were cultivated as described by Pontecorvo et al. (30) at 30°C in minimal medium (MM) containing 1% glucose as the carbon source and 20 mM glutamine as the nitrogen source.
Microbial growth inhibition tests.
To test the bacteriostatic activity of TL, 500 μl of M9 minimal medium in 48-well dishes (Nunc, Wiesbaden, Germany) was inoculated with a 1% overnight culture of E. coli or P. aeruginosa. TL was added at 5 μM, and the optical density at 600 nm (OD600) was measured after 10, 25, and 36 h. Assays for antifungal activity were performed on MM agar in six-well plates (Techno Plastic Products, Trasadingen, Switzerland) containing 50 nM TAFC or 50 nM TAFC that had been preincubated with 500 nM TL in potassium phosphate (pH 7.2) for 15 min and then added to the agar. Afterwards, 5 μl of a spore suspension (105 conidia/ml) was point inoculated. The plates were incubated at 30°C.
Ligand binding assays.
Binding of siderophores to TL was analyzed by displacement of the fluorescent fatty acid DAUDA as described previously (8). Experiments were carried out in a Hitachi F4500 fluorescence spectrophotometer with slits set at bandwidths of 1 and 5 nm for emission and excitation, respectively, and at a constant temperature (22°C). The excitation wavelength was 345 nm, and fluorescence was measured at 490 nm. The assay was carried out in a final volume of 250 μl (20 mM potassium phosphate [pH 7.2]) containing 5 μM TL and 4 μM DAUDA. Exactly 10 μl of an appropriately diluted competitor (from a 0.5 mM stock solution of siderophores dissolved in potassium phosphate) was added. Determination of the kinetics of binding of TAFC to TL was performed by internal tryptophan fluorescence quenching analysis essentially as described previously for other lipocalins (25). Experiments were carried out in the same fluorescence spectrophotometer described above. Tryptophan fluorescence of TL was excited at 295 nm, and emission was recorded at between 330 and 350 nm. For each measurement 250 μl of protein solution (5 μM) in 20 mM potassium phosphate (pH 7.2) was used, and the solution was mixed with 1 μl of a diluted ligand. The set of datum points was analyzed by standard mathematical methods, and a curve was fitted by nonlinear hyperbolic regression.
Endpoint saturation.
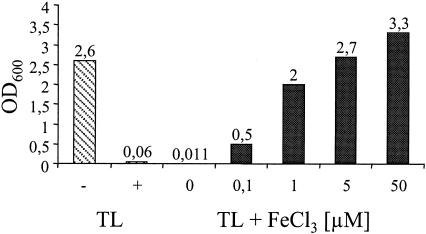
M9 minimal medium (500 μl) was inoculated with a 1% overnight E. coli culture containing 5 μM TL. Iron (FeCl3) was added in increasing concentrations: 0.1, 1, 5, and 50 μM. The OD600 was measured after incubation for 16 h at 37°C.
Atomic absorption measurements.
All atomic absorption measurements were performed with a Unicam 939 QZ atomic absorption spectrophotometer. Before the iron content of the probe was measured, a solution containing 2.4 nM TAFC, 0.8 nM Fe (FeCl3), and 2.4 nM TL was dialyzed in a Slide-A-Lyzer dialysis cassette (molecular weight cutoff, 3,500) obtained from Pierce (Rockford, Ill.) to remove the free iron in the buffer. Probes containing 2.4 nM TL in buffer and 2.4 nM TAFC in buffer (20 mM potassium phosphate buffer [pH 7.2]) were used as a control.
RESULTS
Binding of bacterial siderophores to TL.
In general, the binding of siderophores was measured by competitive DAUDA displacement assays. DAUDA, a fluorescent fatty acid analogue, exhibits little fluorescence in buffer but shows enhanced fluorescence and a blue shift when it is bound to TL. It is known that DAUDA binds to TL entirely within the hydrophobic pocket, at a single binding site, with a calculated dissociation constant (Kd) of 2.8 μM (10). When DAUDA is displaced from TL, there is a corresponding decrease in intensity and a redshift of the maximum peak.
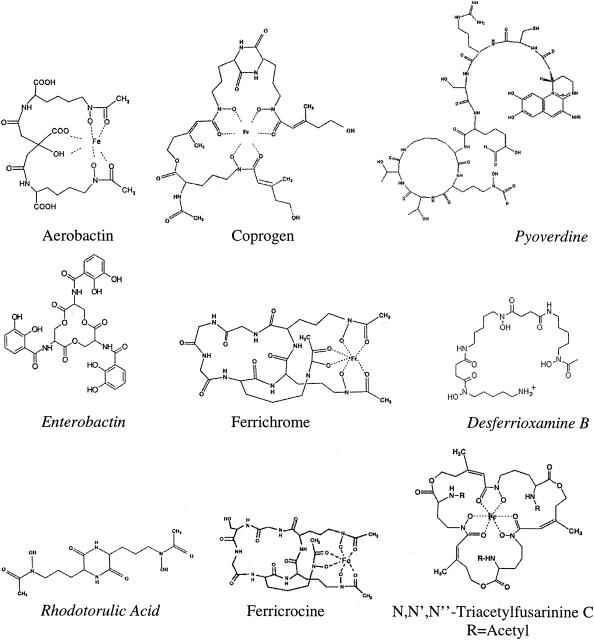
Most bacterial siderophores are divided into two groups on the basis of their chemical compositions: hydroxamates and catecholates (27). Therefore, we tested enterobactin (26), the major catecholate-type siderophore of E. coli, and desferrioxamine B (13), a hydroxamate-type siderophore of Streptomyces (Fig. 1). In addition, aerobactin (9), another hydroxamate-type siderophore of enterobacteria, and pyoverdine (31), the hydroxamate-type siderophore of P. aeruginosa, were included in this study. Pyoverdins differ from other hydroxamates by the additional attachment of a conserved dihydroxyquinoline chromophore (31).
FIG. 1.
Schematic structures of bacterial and fungal siderophores. Italicized siderophores are the desferri forms.
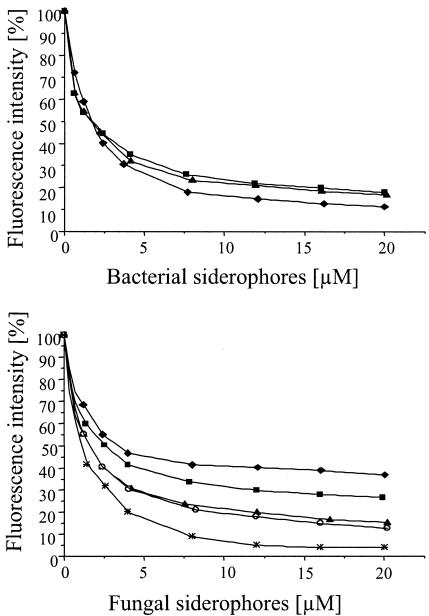
The displacement curves for various concentrations of ferric enterobactin, ferric aerobactin, and ferrioxamine B (iron-saturated desferrioxamine B) are shown in Fig. 2, top panel. All three siderophores were found to bind to TL. Ferric enterobactin and desferrioxamine B showed similar affinities, which were higher than that of ferric aerobactin. Pyoverdine could not be investigated by this assay due to the intrinsic fluorescence of the attached chromophore. Therefore, we investigated the binding of pyoverdine by obtaining atomic absorption measurements with Fe-saturated pyoverdine. Whereas the binding of Fe-saturated enterobactin could be easily detected by this method, no binding of pyoverdine was found (data not shown). Pyoverdine does not bind to TL due to the additional bulky chromophore. In addition, no significant binding of free iron to TL could be detected by atomic absorption.
FIG. 2.
(Top panel) Competitive binding assays of bacterial siderophores determined by measurement of displacement of DAUDA (4 μM) from TL (5 μM) by increasing concentrations of aerobactin (♦), enterobactin (▪), and desferrioxamine B (▴). (Bottom panel) Competitive binding assays of fungal siderophores determined by measurement of displacement of DAUDA (4 μM) from TL (5 μM) by increasing concentrations of ferricrocin (♦), ferrichrome (○), rhodotorulic acid (*), coprogen (▴), and TAFC (▪).
Binding of fungal siderophores to TL.
All of the most relevant fungal siderophores belong to the group of hydroxamates (Fig. 1). Therefore, rhodotorulic acid from Rhodotorula pilimanae (24), TAFC from A. nidulans (4, 29), coprogen from Neurospora crassa (37), and two ferrichromes, namely, ferrichrome from Ustilago maydis and ferricrocin from A. nidulans (4, 21, 29), were used in our binding studies. From the DAUDA displacement curves in Fig. 2 (bottom panel), it is evident that all fungal siderophores tested bind to TL. Rhodotorulic acid showed the highest affinity to TL; TAFC, coprogen, and ferrichrome showed slightly lower affinities; and ferricrocin showed the lowest affinity.
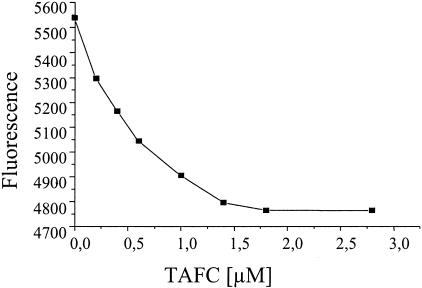
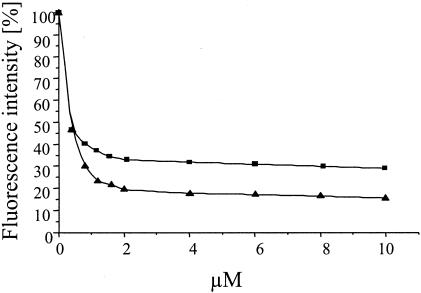
Since it is known that fatty acids are physiological ligands of TL, we compared the Kd of TAFC, which shows an affinity to TL in the medium range, to the Kds of fatty acids. By analysis with internal tryptophan fluorescence quenching, a Kd of 0.5 ± 0.1 μM was obtained for TAFC (Fig. 3). One of the most strongly TL-binding fatty acids is stearic acid; however, its affinity to TL was not measured directly but was calculated from relative displacement assays (10). Therefore, we performed a direct comparison of TAFC and stearic acid by displacement analysis in the same series of experiments. The results of our experiments demonstrate that TAFC has only a slightly lower affinity than the strongly binding ligand stearic acid (Fig. 4).
FIG. 3.
Determination of the binding affinity of TL for TAFC by internal tryptophan fluorescence quenching experiments. The ligand concentration was plotted against the relative decrease in fluorescence.
FIG. 4.
Comparison of DAUDA displacement from TL by increasing concentrations of TAFC (▪) and stearic acid (▴).
We found no significant difference in the binding of TL to iron-depleted or iron-saturated siderophores either for desferrioxamine B or for TAFC. Together with the results from atomic absorption analysis that no free iron is bound by TL, it is evident that iron is bound to TL only in a complex with a siderophore and that siderophore binding to TL is independent of iron.
Inhibition of bacterial growth by TL.
In order to test the interference of TL with siderophore-mediated iron uptake by microorganisms, we first explored its effect on the growth of E. coli in M9 minimal medium under iron-limiting conditions. The M9 minimal medium was not supplemented with iron, but trace amounts present in glassware and medium were sufficient for the bacteria to grow to saturation. However, after the addition of exogenous TL at a concentration of 5 μM, drastic inhibition of growth was observed (Fig. 5). This growth inhibition could be reversed by adding free iron, as indicated from endpoint saturation experiments (Fig. 5). In addition, no growth inhibition could be detected in non-iron-limited medium (data not shown). Therefore, our experiments clearly suggest that the bacteriostatic effect of TL is directly related to the inhibition of siderophore-mediated iron sequestration.
FIG. 5.
Results of endpoint saturation experiments of bacterial growth. Hatched columns, growth (OD600) of E. coli XL-1 Blue cultures grown in M9 minimal medium at 37°C after 36 h, without and with 5 μM TL; black columns, growth in M9 minimal medium containing 5 μM TL supplemented with increasing concentrations of iron.
The same experiments were performed with P. aeruginosa, but no inhibition of growth could be detected (data not shown), thus supporting the results of our binding studies.
Inhibition of fungal growth by TL.
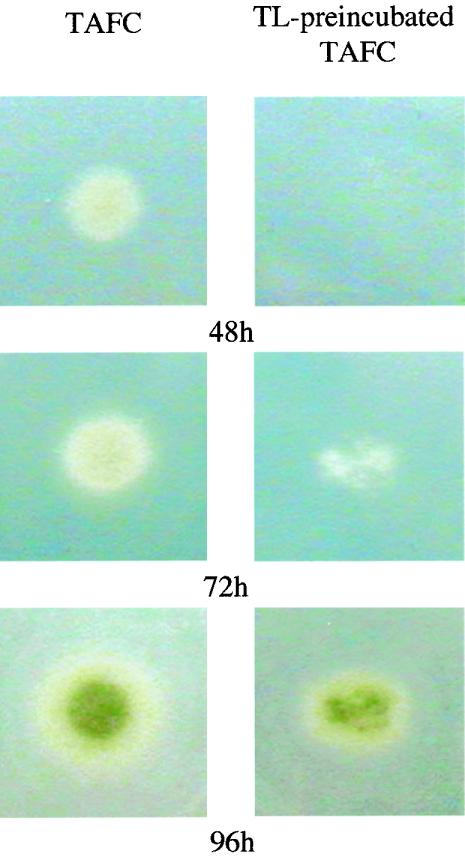
Investigations concerning the inhibitory effects of TL on fungal growth were performed with a mutant strain of A. nidulans that contains a deletion of the l-ornithine N5-monooxygenase-encoding gene sidA (ΔsidA), which completely blocks siderophore biosynthesis. Thus, this mutant strain is unable to grow on minimal medium without supplementation with siderophores (5). A. nidulans ΔsidA was grown on a medium supplemented with TAFC or a medium containing TAFC preincubated with TL. As shown in Fig. 6, significant inhibition of growth was apparent after 48 and 72 h of growth on the medium containing the TL-preincubated TAFC compared to the growth on the TAFC-supplemented medium. TL does not have any antifungal activity beyond siderophore sequestration, since no growth inhibition was observed when a surplus of TAFC was used (data not shown). It is evident from the data for the 72- and 96-h time points that growth is not completely blocked but is retarded. The growth after prolonged incubation on the medium containing TL-preincubated TAFC was probably due to protease activity, since increased degradation of TL could be detected between 72 and 96 h by Western blot analysis (data not shown).
FIG. 6.
Effect of TL addition on growth of siderophore-deficient strain A. nidulans ΔsidA. Conidia (5 × 102) were point inoculated on MM supplemented with 50 nM TAFC (left column) or TL preincubated with TAFC (right column) and incubated for the indicated times.
DISCUSSION
In the present study we demonstrated that the antimicrobial activity of TL depends on its ability to bind to microbial siderophores, thereby interfering with the iron acquisition systems of microorganisms. Since iron is an essential nutrient for microorganisms but is strongly bound to host proteins in mammals, microorganisms must compete with these proteins for this essential ion. Microbes have evolved powerful acquisition systems by producing highly specific iron chelators (siderophores) in response to iron deficiency. Thus, inhibition of this iron acquisition process by scavenging microbial siderophores is a powerful mechanism in the defense against microbial infections. Indeed, two human proteins have been found to interfere with microbial siderophores so far. The first one is serum albumin, which was described to inhibit the transfer of iron from ferric enterobactin to E. coli (19). The other one is NGAL, a member of the lipocalins produced by neutrophiles and epithelial cells during inflammation and enhanced cell proliferation (17). NGAL could be isolated and crystallized with bound ferric enterobactin (12). Our results show that TL is an additional member of this group.
Similar to NGAL, siderophores seem to be bound within the hydrophobic calyx of TL, since they are able to displace DAUDA, which is known to have a specific binding site within this pocket (10). Nevertheless, TL shows some significant differences from NGAL in siderophore binding. In contrast to NGAL, which binds to bacterial catecholate type siderophores, TL also binds to bacterial and fungal hydroxamate-type siderophores. NGAL has an extremely high affinity for ferric enterobactin, with a Kd of about 0.4 nM (12). TL has a much lower affinity (Kd, about 0.5 μM for TAFC) but binds to a broad spectrum of siderophores, consistent with a general scavenger function (20). The action of TL against fungal organisms would complement the numerous bacterial defenses already described for the eye and would be valuable in the mouth, trachea, and other locations where TL is expressed (3, 34).
Growth inhibition tests showed that TL has strong bacteriostatic and fungistatic effects in vitro. However, since TL is not a siderophore-specific binding protein but, rather, a scavenger with a broad ligand specificity, several aspects concerning its antimicrobial action in vivo should be considered. Most of the TL-containing secretions also contain other potential ligands of TL, e.g., fatty acids. Therefore, the affinity of TL for siderophores must be at least as high as the affinity for the other ligands. 16-(9-Anthroyloxy)palmitic acid, a derivative of palmitic acid, has been shown to bind to TL, with a Kd of 0.8 μM (11). The Kd of stearic acid, which was described to be one of the ligands that binds to TL most strongly in vivo, was not measured directly but was calculated from relative displacement assays, which suggested a Kd of about 0.3 μM (10). Our results demonstrated that TL indeed has an affinity for siderophores comparable to that of strongly binding fatty acids. Next, the concentration and affinity of TL must be high enough to compete with microbial siderophore uptake systems. TL is constantly produced by several glands and is present in large amounts in tear fluid, nasal mucus, and tracheal secretions, e.g., tear fluid contains up to 3 mg of TL per ml (7, 22). In addition, its concentration increases during inflammation and/or infection (22, 34). The Kd values of fungal siderophore transporters were found to range from 0.9 to 2.3 μM, which were for ferrichromes and TAFC, respectively (23, 41). Since we found comparable affinities of TL for these fungal siderophores, TL should be specific enough to efficiently outcompete all of the fungal siderophore receptors. Bacterial siderophore transporters are known to have higher affinities; e.g., enterobactin binds to its receptor with a Kd in the range of 0.4 to 100 nM (28). Therefore, TL might be a more efficient inhibitor of fungal growth than of bacterial growth in vivo.
Recent investigations demonstrated that TL binds to LIMR, a novel endocytic receptor (39) which targets the TL-ligand complex and places them into specific intracellular vesicles, probably late endosomes or lysosomes (40). Targeting of the TL-siderophore-iron complex to these cellular compartments will be an important mechanism in the clearance process; furthermore, the acidic conditions of these vesicles might lead to ligand removal and degradation of the siderophores.
Acknowledgments
We thank E. Artner-Dworzak for providing access to atomic absorption analysis, G. Gstraunthaler for advice on fluorescence measurements, and F. Marx for helpful discussions.
M.F. and B.R. were supported by Austrian Science Foundation (FWF) grant P14850, and B.J.G. was supported by NIH grant EY-11224.
REFERENCES
- 1.Alizadeh, H., S. Apte, M. S. El-Agha, L. Li, M. Hurt, K. Howard, H. D. Cavanagh, J. P. McCulley, and J. Y. Niederkorn. 2001. Tear IgA and serum IgG antibodies against Acanthamoeba in patients with Acanthamoeba keratitis. Cornea 20:622-627. [DOI] [PubMed] [Google Scholar]
- 2.Arnold, R. R., M. Brewer, and J. J. Gauthier. 1980. Bactericidal activity of lactoferrin: sensitivity of a variety of microorganisms. Infect. Immun. 28:893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaker, M., K. Kock, C. Ahlers, F. Buck, and H. Schmale. 1993. Molecular cloning of human von Ebner's gland protein, a member of the lipocalin superfamily highly expressed in lingual salivar glands. Biochim. Biophys. Acta 1172:131-137. [DOI] [PubMed] [Google Scholar]
- 4.Charlang, G., B. Ng, N. H. Horowitz, and R. M. Horowitz. 1981. Cellular and extracellular siderophores of Aspergillus nidulans and Penicillium chrysogenum. Mol. Cell. Biol. 1:94-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisendle, M., H. Oberegger, I. Zadra, and H. Haas. 2003. The siderophore system is essential for viability of Aspergillus nidulans: functional analysis of two genes encoding l-ornithine N5-monooxygenase (SidA) and a non-ribosomal peptide synthetase (SidC). Mol. Microbiol. 49:359-375. [DOI] [PubMed] [Google Scholar]
- 6.Fleming, A. 1922. On a remarkable bacteriolytic ferment found in tissue and secretions. Proc. R. Soc. Ser. B 93:306-317. [Google Scholar]
- 7.Fullard, R. J., and D. M. Kissner. 1991. Purification of the isoforms of tear specific prealbumin. Curr. Eye Res. 10:613-628. [DOI] [PubMed] [Google Scholar]
- 8.Gasymov, O. K., A. R. Abduragimov, T. N. Yusifov, and B. J. Glasgow. 1999. Binding studies of tear lipocalin: the role of the conserved tryptophan in maintaining structure, stability and ligand affinity. Biochim. Biophys. Acta 1433:307-320. [DOI] [PubMed] [Google Scholar]
- 9.Gibson, F., and D. I. Magrath. 1969. The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes 62-I. Biochim. Biophys. Acta 192:175-184. [DOI] [PubMed] [Google Scholar]
- 10.Glasgow, B. J., A. R. Abduragimov, Z. T. Farahbakhsh, K. F. Faull, and W. L. Hubbell. 1995. Tear lipocalins bind a broad array of lipid ligands. Curr. Eye Res. 14:363-372. [DOI] [PubMed] [Google Scholar]
- 11.Glatz, J. F., and J. H. Veerkamp. 1983. A radiochemical procedure for the assay of fatty acid binding by proteins. Anal. Biochem. 132:89-95. [DOI] [PubMed] [Google Scholar]
- 12.Goetz, D. H., M. A. Holmes, N. Borregaard, M. E. Bluhm, K. N. Raymond, and R. K. Strong. 2002. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell 10:1033-1043. [DOI] [PubMed] [Google Scholar]
- 13.Gunter, K., C. Toupet, and T. Schupp. 1993. Characterization of an iron-regulated promoter involved in desferrioxamine B synthesis in Streptomyces pilosus: repressor-binding site and homology to the diphtheria toxin gene promoter. J. Bacteriol. 175:3295-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes R. J., P. J. Tighe, and H. S. Dua. 1999. Antimicrobial defensin peptides of the human ocular surface. Br. J. Ophthalmol. 83:737-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzfeind, P., P. Merschak, H. Rogatsch, J. Feichtinger, H. Klocker, and B. Redl. 1996. Expression of the gene for tear lipocalin/von Ebner's gland protein in human prostate. FEBS Lett. 395:95-99. [DOI] [PubMed] [Google Scholar]
- 16.Kagan, B. L., M. E. Selsted, T. Ganz, and R. I. Lehrer. 1990. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc. Natl. Acad. Sci. USA 87:210-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kjeldsen, L., J. B. Cowland, and N. Borregaard. 2000. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim. Biophys. Acta 1482:272-283. [DOI] [PubMed] [Google Scholar]
- 18.Konetschny-Rapp, S., H. G. Huschka, G. Winkelmann, and G. Jung. 1988. High-performance liquid chromatography of siderophores from fungi. Biol. Met. 1:9-17. [DOI] [PubMed] [Google Scholar]
- 19.Konopka, K., and J. B. Neilands. 1984. Effect of serum albumin on siderophore-mediated utilization of transferrin iron. Biochemistry 22:2122-2127. [DOI] [PubMed] [Google Scholar]
- 20.Lechner, M., P. Wojnar, and B. Redl. 2001. Human tear lipocalin acts as an oxidative-stress-induced scavenger of potentially harmful lipid peroxidation products in a cell culture system. Biochem. J. 356:129-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leong, S. A., and G. Winkelmann. 1998. Molecular biology of iron transport in fungi. Met. Ions Biol. Syst. 35:147-186. [PubMed] [Google Scholar]
- 22.Lindahl, M., B. Stahlbom, and C. Tagesson. 1999. Newly identified proteins in human nasal and bronchoalveolar lavage fluids: potential biomedical and clinical applications. Electrophoresis 20:3670-3676. [DOI] [PubMed] [Google Scholar]
- 23.Moore, R. E., Y. Kim, and C. C. Philpott. 2003. The mechanism of ferrichrome transport through Arn1p and its metabolism in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 100:5664-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller, G., S. J. Barclay, and K. N. Raymond. 1985. The mechanism and specificity of iron transport in Rhodotorula pilimanae probed by synthetic analogs of rhodotorulic acid. J. Biol. Chem. 260:13916-13920. [PubMed] [Google Scholar]
- 25.Müller, H. N., and A. Skerra. 1993. Functional expression of the uncomplexed serum retinol-binding protein in Escherichia coli. Ligand binding and reversible unfolding characteristics. J. Mol. Biol. 220:725-732. [DOI] [PubMed] [Google Scholar]
- 26.Nahlik, M. S., T. P. Fleming, and M. A. McIntosh. 1987. Cluster of genes controlling synthesis and activation of 2,3-dihydroxybenzoic acid in production of enterobactin in Escherichia coli. J. Bacteriol. 169:4163-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neilands, J. B. 1981. Microbial iron compounds. Annu. Rev. Biochem. 50:715-731. [DOI] [PubMed] [Google Scholar]
- 28.Newton, S. M., J. D. Igo, D. C. Scott, and P. E. Klebba. 1999. Effect of loop deletions on the binding and transport of ferric enterobactin by FepA. Mol. Microbiol. 32:1153-1165. [DOI] [PubMed] [Google Scholar]
- 29.Oberegger, H., M. Schoeser, I. Zadra, B. Abt, and H. Haas. 2001. SREA is involved in regulation of siderophore biosynthesis, utilization and uptake in Aspergillus nidulans. Mol. Microbiol. 41:1077-1089. [DOI] [PubMed] [Google Scholar]
- 30.Pontecorvo, G., J. A. Roper, L. M. Hemmons, K. D. MacDonald, and A. W. Bufton. 1953. The genetics of Aspergillus nidulans. Adv. Genet. 5:141-238. [DOI] [PubMed] [Google Scholar]
- 31.Poole, K., and G. A. McKay. 2003. Iron acquisition and its control in Pseudomonas aeruginosa: many roads lead to Rome. Front. Biosci. 8:661-686. [DOI] [PubMed] [Google Scholar]
- 32.Qu, X. D., and R. I. Lehrer. 1998. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect. Immun. 66:2791-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redl, B., P. Holzfeind, and F. Lottspeich. 1992. cDNA cloning and sequencing reveals human tear prealbumin to be a member of the lipophilic-ligand carrier protein superfamily. J. Biol. Chem. 267:20282-20287. [PubMed] [Google Scholar]
- 34.Redl, B., P. Wojnar, H. Ellemunter, and H. Feichtinger. 1998. Identification of a lipocalin in mucosal glands of the human tracheobronchial tree and its enhanced secretion in cystic fibrosis. Lab. Investig. 78:1121-1129. [PubMed] [Google Scholar]
- 35.Redl, B. 2000. Human tear lipocalin. Biochim. Biophys. Acta 1482:241-248. [DOI] [PubMed] [Google Scholar]
- 36.Selsted, M. E., and R. J. Martinez. 1982. Isolation and purification of bactericides from human tears. Exp. Eye Res. 34:305-318. [DOI] [PubMed] [Google Scholar]
- 37.van der Helm, D., and G. Winkelmann. 1994. Hydroxamates and polycarbonates as iron transport agents (siderophores) in fungi, p. 139-148. In G. Winkelmann and D. R. Winge (ed.), Metal ions in fungi. Marcel Decker, Inc., New York, N.Y.
- 38.van't Hof, W., M. F. Blankenvoorde, E. C. Veerman, and A. V. Amerongen. 1997. The salivary lipocalin von Ebner's gland protein is a cysteine proteinase inhibitor. J. Biol. Chem. 272:1837-1841. [DOI] [PubMed] [Google Scholar]
- 39.Wojnar, P., M. Lechner, P. Merschak, and B. Redl. 2001. Molecular cloning of a novel lipocalin-1 interacting human cell membrane receptor (LIMR) using phage-display. J. Biol. Chem. 276:20206-20212. [DOI] [PubMed] [Google Scholar]
- 40.Wojnar, P., M. Lechner, and B. Redl. 2003. Antisense downregulation of the lipocalin-interacting-membrane-receptor expression inhibits cellular internalization of lipocalin-1 in human NT2 cells. J. Biol. Chem. 278:16209-16215. [DOI] [PubMed] [Google Scholar]
- 41.Yun, C. W., J. S. Tiedeman, R. E. Moore, and C. C. Philpott. 2000. Siderophore-iron uptake in Saccharomyces cerevisiae. Identification of ferrichrome and fusarinine transporters. J. Biol. Chem. 275:16354-16359. [DOI] [PubMed] [Google Scholar]
- 42.Yusifov, T. N., A. R. Abduragimov, O. K. Gasymov, and B. J. Glasgow. 2000. Endonuclease activity in lipocalins. Biochem. J. 347:815-819. [PMC free article] [PubMed] [Google Scholar]