Abstract
The effect of protein binding on the antimicrobial activity of ertapenem was evaluated using the bacterial kill rate and concentration-response studies. Various proportions of human serum were utilized to determine the total and free-drug concentrations using a validated high-performance liquid chromatography assay. The MICs and kill curves were determined for test isolates of Enterobacter cloacae and Staphylococcus aureus at various percentages of human serum. The killing of bacteria was analyzed in relation to the free and total concentrations of ertapenem at various proportions of human serum. It was determined that unbound ertapenem was responsible for the antimicrobial activity against the test isolates.
Protein binding is important for antibacterial drugs, due to its effects on pharmacokinetics and bioactivity. Reductions in antibacterial effects are evident in serum-containing media compared to standard microbiological media for drugs that are extensively bound to serum proteins (7, 8, 14). Most studies examining changes in bioactivity in relation to protein binding have utilized changes in the MIC and have limited the human serum concentration to 50% (4). Limiting the serum concentration to ≤50% is often necessary to avoid the antibacterial effects of serum itself. Serum may inhibit bacterial growth or cause the killing of bacteria even at concentrations of <50%, requiring that serum be heat treated to destroy complement and other serum components (10, 19). There are also comparatively small molecules in serum that possess or modify antibacterial activity, as demonstrated in serum ultrafiltrates (11). Only one investigator has managed to use 100% serum for determination of the MIC (8).
Using the MIC as a bioactivity endpoint presents several problems. When serum is added to broth medium, the resulting solution is cloudy, and this impairs the ability to distinguish turbidity changes related to bacterial growth. In addition, the MIC is defined discretely by fixed twofold dilutions, leading to poor accuracy in defining the effect on bioactivity. Other investigators have attempted to use bactericidal rates as an endpoint (9, 12, 16). However, in one study, changes in pH in the presence of human serum led to different effects on the activities of teicoplanin and of vancomycin (1). The authors were also unable to elicit a dose response for vancomycin and teicoplanin. Assessment of the killing rate in another study was complicated by a diminished bactericidal effect associated with increasing antibacterial concentrations, which was called a “paradoxical effect” (16).
Whether antibacterial effects are determined solely by the concentration of free, unbound antibacterial drugs is controversial. Some investigators have disputed the importance of protein binding except with drugs that exhibit very high binding levels (17). Others have asserted that only free-drug concentrations are important and that pharmacodynamic activity is determined solely by the free-drug concentration (3, 5, 6). The purpose of this study was to explicitly define the effect of protein binding of ertapenem by using the bacterial kill rate and concentration-response curves. Human serum mixtures ranging from 10 to 90% were utilized with cation-adjusted Mueller-Hinton broth (CAMHB) as the growth medium, and the test isolates were acclimated with human serum to minimize the antibacterial effects of the serum itself. Total and free-drug concentrations were measured using a validated high-performance liquid chromatography (HPLC) assay.
MATERIALS AND METHODS
Chemicals and reagents.
MES (2-[N-morpholino]ethanesulfonic acid), HPLC-grade methanol, and American Chemical Society-grade citric acid monohydrate were purchased from Sigma (St. Louis, Mo.). HPLC-grade tetrahydrofuran and acetonitrile were purchased from Sigma-Aldrich (Milwaukee, Wis.). CAMHB, tryptic soy agar, and tryptic soy agar with 5% sheep blood (blood agar) were obtained from Remel (Lenexa, Kans.). Frozen human serum was purchased from Biological Specialty Corp. (Colmar, Pa.). Human serum albumin (USP; 25% solution), provided by Baxter Healthcare Corp. (Glendale, Calif.), and 20% human albumin, distributed by Sigma, were obtained. All reagents were used as received. Ertapenem sodium was obtained from Merck & Co. (Whitehouse Station, N.J.). Stock solutions and quality controls were prepared in 10 mM MES buffer (pH 6.5) and stored at −80°C.
Bacteria.
Two blood culture isolates of Enterobacter cloacae and Staphylococcus aureus were obtained from human blood cultures originating from a patient with more than one positive culture. Clinical isolates were chosen, assuming that the strains would better adapt to growth in human serum. The purity of the test strain was ensured by subculturing isolates at least three times before freezing the stock cultures. Each organism was serially trained to grow in 0 to 90% serum by alternating growth in human serum-CAMHB solutions with blood agar plates. The serum-acclimated organisms were maintained with growth in 50% human serum. MICs for each organism were determined using approved procedures of the NCCLS (National Committee for Clinical Laboratory Standards) (15). To ensure stability, MIC testing was performed weekly over a 3-month period for each organism in 0 to 90% human serum with CAMHB. In the case of S. aureus, the organism was grown in alternating media (blood agar and 50% human serum-CAMHB), and serum resistance was maintained with up to 90% human serum. For the E. cloacae isolate, some serum resistance was acquired, but there was initially up to a 2-log10-unit reduction in the inoculum in the test culture before growth was apparent. Thus, a subpopulation that rapidly lost serum resistance was present. The procedure was modified to use an overnight culture in 50% human serum-CAMHB for the inoculum. Since the inoculum could not be verified turbidimetrically, the conditions were standardized and the procedures were adjusted to produce the correct inoculum in the test cultures. It was determined that a fresh culture needed to be started in 50% human serum 1 h prior to inoculation of the test culture to achieve logarithmic growth and avoid killing due to human serum.
Killing of bacteria.
Kill rate experiments were performed in triplicate in media containing human serum at concentrations between 0 and 90% (10% increments). At baseline, 10-ml volumes of test solutions were inoculated with ∼106 organisms per ml. Immediately after inoculation, various concentrations of ertapenem (0 to 32 × MIC) were added to the test solutions. Each culture was gently agitated during incubation at 35°C. Viable-colony counts were determined at 0 (baseline), 4, 6, and 24 h by serial dilution coupled with a pour plate technique. In order to obtain a lower limit of sensitivity of 30 colonies per ml, a centrifuge technique followed by saline washes was used to remove residual drug. Higher colony counts were determined following simple dilution of the culture suspension with sterile saline.
Overnight cultures of the S. aureus isolate grown on blood agar were used to prepare the inoculum. For the E. cloacae organism, 100 μl of an overnight inoculum in 50% human serum was added to 9.9 ml of test solution and reincubated for 1 h to achieve logarithmic growth. In both cases, the suspensions were adjusted based on turbidity to provide bacterial counts of ∼106 organisms/ml. The procedures were checked experimentally and adjusted to result in the correct final inoculum concentration.
Analytical study of ertapenem.
A previously described HPLC assay was modified and validated for determination of free and total ertapenem concentrations in human serum, CAMHB, and mixtures of the two (20). The procedure utilized a reverse-phase column (Synergi Hydro-RP 80A; 250- by 4.6-mm inside diameter; 0.4-μm particle size) and a C18 guard column obtained from Phenomenex (Torrance, Calif.) with UV detection at 315 nm. The mobile phase consisted of a 10:1:89 ratio of acetonitrile, tetrahydrofuran, and 0.025 M citrate buffer (pH 4.5). The assay range was 0.4 to 100 μg/ml, and the between-day precision was 4.47% at 2.5 μg/ml, 9.92% at 40 μg/ml, and 6.46% at 80 μg/ml.
Human serum solutions with concentrations ranging from 0 to 100% using 10% increments were prepared with CAMHB diluent. Portions of these 11 different solutions were supplemented with 12 and 50 μg of ertapenem/ml. Each ertapenem-containing solution was split to determine total and free-ertapenem concentrations. To determine the total concentration, the samples were mixed 1:1 with stabilizer (0.2 M citrate buffer, pH 6.5) and frozen at −80°C until they were analyzed. To determine the free concentration, the samples were ultrafiltered using an Amicon Centrifree YM-30 cartridge (Millipore, Burlington, Mass.) with centrifugation at 1,500 × g for 30 min (18, 20). The ultrafiltrate was then mixed 1:1 with stabilizer and frozen at −80°C. All samples were prepared and assayed in triplicate. Quality control samples were prepared in a 1:3 mixture of human serum-CAMHB ultrafiltrate after determining that the ratio did not affect the recovery of ertapenem after ultrafiltration. Standard samples were all prepared in CAMHB ultrafiltrate. Standards and quality control samples were mixed 1:1 with stabilizer prior to assay or storage.
All samples were extracted using solid-phase extraction columns (Strata-X; 33-μm polymeric sorbent; 60 mg/3 ml; Phenomenex). The columns were conditioned using acetonitrile and 0.0025 M citrate buffer (pH 4.5). Thawed samples (0.5 ml) were mixed 1:1 with 0.25 M citrate buffer (pH 4.1) and immediately loaded onto the column. The column was washed with 0.0025 M citrate buffer (pH 4.5). The sample was eluted with 0.5 ml of 10% acetonitrile in 0.1 M MES buffer (pH 6.5). The eluent was centrifuged at 9,000 × g for 5 min, and the supernatant was injected into the HPLC system.
Pharmacodynamic analysis.
The data from the in vitro and analytical studies were analyzed for the rate and extent of killing of bacteria in relation to total and free-ertapenem concentrations at various percentages of human serum. The growth rate at a given ertapenem concentration and medium condition was defined as follows: [ln(final colony count) − ln(baseline colony count)]/time interval. Thus, the growth rate was positive when bacterial counts increased and negative when the counts decreased. The adjusted kill rate (KR) was defined as the growth rate (without antibacterial agent) minus the test growth rate (at specific ertapenem concentrations) under the same medium conditions.
Concentration-effect profiles were fitted with nonlinear regression analysis based on the Sigmoid Emax equation and WinNonlin standard edition version 2.1 (Pharsight Corp., Cary, N.C.) software. Derived parameters included the maximum adjusted kill rate (KRmax), the concentration at the half-maximum adjusted kill rate (50% effective concentration [EC50]), and Hill's coefficient (h), where C is the concentration: KR = (KRmax × Ch)/(EC50h + Ch).
The primary objective of this investigation was to determine if the unbound concentration of ertapenem was solely responsible for the antibacterial activity observed against the test organisms. This was determined by modeling the relationship between the killing of bacteria and the unbound- and total ertapenem concentrations.
RESULTS
MICs monitored over 3 months remained stable for the E. cloacae and S. aureus test isolates. The relationships between geometric mean MICs and percent human serum concentrations are provided in Table 1. As expected, the MIC increased when higher concentrations of human serum were present. Although many of the human serum and CAMHB mixtures were turbid, growth could be distinguished when it was present. In cases where discrimination between growth and inhibition was difficult, a semiquantitative subculture was used. Growth was defined as >1 log10 unit increase in the bacterial count compared to the baseline.
TABLE 1.
Pharmacodynamic parameters (based on 4-h endpoint) for ertapenem associated with various percentages of human serum in CAMHB
| Test isolate | % Human serum | MICa | KRmax | EC50 | h | EC50, unboundb |
|---|---|---|---|---|---|---|
| E. cloacae | 0 | 1.0 | 3.93 | 1.15 | 2.28 | 1.10 |
| 10 | 1.0 | 5.06 | 2.34 | 1.25 | 1.90 | |
| 20 | 1.0 | 4.78 | 2.70 | 1.18 | 1.80 | |
| 30 | 1.5 | 4.63 | 2.80 | 1.41 | 1.37 | |
| 40 | 2.0 | 3.49 | 2.40 | 1.52 | 0.93 | |
| 50 | 2.0 | 4.80 | 4.82 | 1.48 | 1.28 | |
| 60 | 2.0 | 3.65 | 5.00 | 1.31 | 0.96 | |
| 70 | 4.0 | 3.84 | 10.62 | 2.33 | 1.80 | |
| 80 | 4.0 | 3.62 | 8.03 | 1.61 | 1.05 | |
| 90 | 4.0 | 4.02 | 17.51 | 1.59 | 1.83 | |
| S. aureus | 0 | 0.07 | 2.99 | 0.14 | 4.96 | 0.13 |
| 10 | 0.09 | 2.98 | 0.15 | 3.62 | 0.12 | |
| 20 | 0.14 | 3.02 | 0.18 | 3.92 | 0.12 | |
| 30 | 0.27 | 2.28 | 0.38 | 3.62 | 0.19 | |
| 40 | 0.28 | 2.86 | 0.48 | 5.10 | 0.19 | |
| 50 | 0.34 | 3.00 | 0.48 | 2.98 | 0.13 | |
| 60 | 0.50 | 2.49 | 1.02 | 5.61 | 0.20 | |
| 70 | 0.50 | 2.52 | 1.64 | 4.41 | 0.28 | |
| 80 | 0.50 | 3.11 | 3.15 | 1.85 | 0.41 | |
| 90 | 0.50 | 2.26 | 2.47 | 6.98 | 0.26 |
Geometric mean MIC.
EC50 based on free-drug concentration.
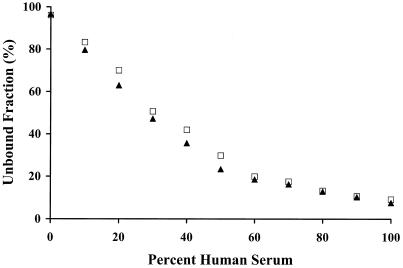
Unbound- and total ertapenem concentrations were determined in various proportions of human serum and CAMHB. The samples were supplemented at ertapenem concentrations of 12 and 50 μg/ml, which were shown to be in the linear range of protein binding. Figure 1 shows the unbound fraction relative to the percentage of human serum. When ertapenem was added to CAMHB only, a small amount of ertapenem was lost (3.6% bound). Since there was minimal loss to the extraction column, there could have been binding to some component of CAMHB or loss due to degradation. The protein binding was 91.6% in 100% human serum.
FIG. 1.
Unbound concentrations of ertapenem at 12 (▴) and 50 (□) μg/ml at various human serum proportions.
As expected, the adjusted kill rates determined at 24 h did not discriminate effects very well. Either the counts were below the detection limit, or growth was extensive. Adjusted kill rates based on 4- or 6-h counts provided good discrimination of the concentration-effect profile. Since regrowth was apparent in a few of the 6-h profiles, the 4-h endpoints were used to assess the impact of protein binding.
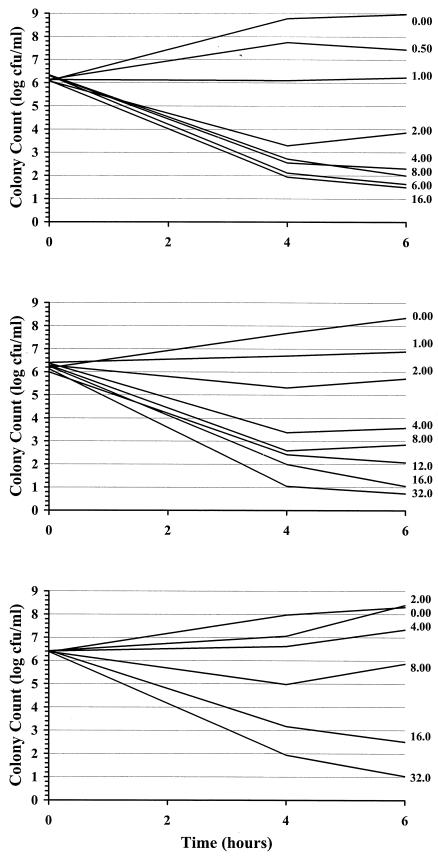
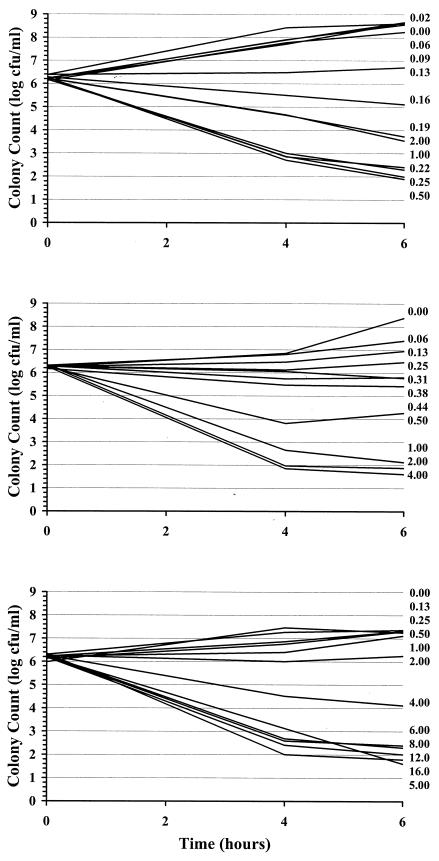
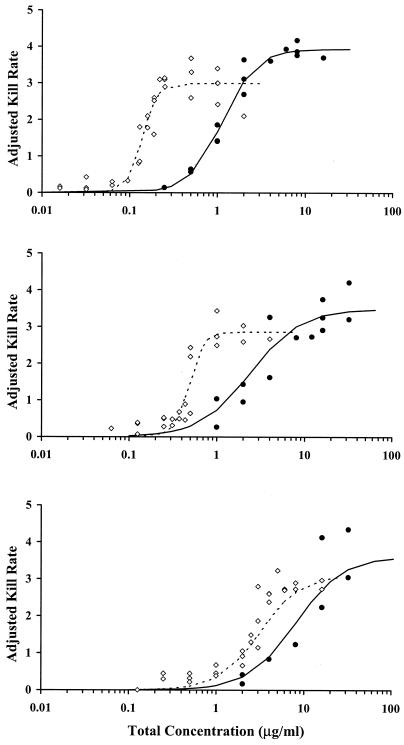
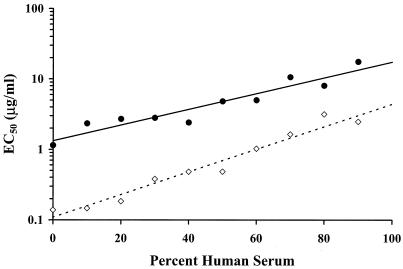
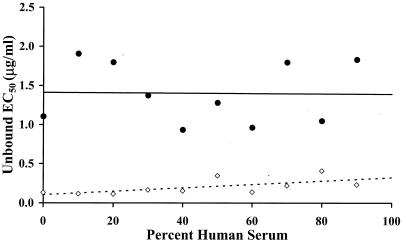
Examples of kill curves at 0, 40, and 80% human serum in CAMHB for the E. cloacae and S. aureus organisms are shown in Fig. 2 and 3, respectively. A concentration-effect relationship was established at all human serum concentrations. Table 1 provides the mean pharmacodynamic parameters for each concentration of human serum. Increased proportions of human serum were associated with increased concentrations of ertapenem required to achieve the same adjusted kill rates. Figure 4 shows examples of concentration-effect curves for E. cloacae and S. aureus using human serum concentrations of 0, 40, and 80%. The profiles show similar adjusted-kill-rate-versus-concentration curves that are shifted to the right with increasing percentages of human serum. KRmax values were variable (coefficient of variation, 12 to 14%), but there was no trend in values according to the percentage of human serum. Figure 5 shows the relationship between the EC50 and the percentage of human serum for the two test isolates. The EC50 (log scale) increased fairly linearly with the percentage of human serum. Thus, an exponentially higher concentration is required to produce the same effect in the presence of increasing human serum concentrations. Once EC50 is expressed in terms of the free-ertapenem concentration, the unbound EC50 is constant over the range of human serum concentrations tested for E. cloacae (Fig. 6). This result indicates that the antibacterial activity is explained solely by the free-ertapenem concentration. However, a slightly positive slope is observed with the S. aureus isolate. The increase in unbound EC50 with increasing percentages of human serum is minimal in comparison to that seen with the total ertapenem concentration. One can conclude that the majority of ertapenem activity against S. aureus is explained by the free-ertapenem concentration; however, the protein binding could have been higher in the kill rate test cultures or a product of the growth in human serum mixtures could have slightly impaired killing.
FIG. 2.
E. cloacae kill curves with various ertapenem concentrations in 0 (top), 40 (middle), and 80% (bottom) human serum in CAMHB.
FIG. 3.
S. aureus kill curves with various ertapenem concentrations in 0 (top), 40 (middle), and 80% (bottom) human serum in CAMHB.
FIG. 4.
E. cloacae (•) and S. aureus (⋄) adjusted kill rates versus concentration profiles for ertapenem in CAMHB with 0 (top), 40 (middle), and 80% (bottom) human serum.
FIG. 5.
Relationships between EC50 and percent human serum for the two test isolates of E. cloacae (•) and S. aureus (⋄).
FIG. 6.
EC50 based on free ertapenem concentration versus percent human serum. Data are shown for E. cloacae (•) and S. aureus (⋄). The lines represent the best fit lines by least-squares linear regression.
DISCUSSION
The effect of protein binding on the antimicrobial activities of antibiotics has been a source of controversy. This experiment supports the theory that only the free, unbound portion of antibacterial drugs is active. The adjusted kill rates of ertapenem in the absence and presence of various concentrations of human serum were determined. For both organisms, the maximum adjusted kill rate remained constant with increasing human serum concentrations, while the EC50 increased relative to the fraction of drug that was protein bound. Growth rates for this study are the average growth rates over a 4-h period. The lag time until initiation of killing was not considered. The lag time for carbapenems is relatively short (≤0.5 h), and the use of the average adjusted kill rate provides similar EC50 values. Adjusted kill rates are expected to be slightly lower than if the lag time was considered. However, omitting the lag time allows more efficient testing, given the large number of concentrations and medium conditions examined.
When the EC50 based on free-ertapenem concentrations is examined relative to the percentage of human serum, the importance of the free-drug concentration becomes readily apparent. For the E. cloacae test isolate, the bioactivity was clearly explained by the free-drug concentration. The bioactivity was slightly less well explained by the free-drug concentration for the S. aureus isolate. Bioactivity was based on bacterial killing, which for β-lactam agents occurs in actively dividing bacteria. The growth rate for S. aureus was reduced in media containing higher percentages of human serum. This could have changed the potency (EC50), although the KRmax was not affected. Also, the concentration of free drug in the test cultures could have been lower than estimated. However, the majority of the differences in bioactivity are still explained by binding to serum proteins.
Human serum itself contains substances that possess antibacterial activity. Although “serum-resistant” bacterial strains have been described, they are not routinely encountered. Untrained strains of E. cloacae and S. aureus that grow in serum-CAMHB mixtures exceeding 50% serum were not encountered in this study in searches for candidate test isolates. Many investigators have used heat-treated human serum to reduce antimicrobial activity; however, this rarely eliminates all antibacterial activity. Given the relatively frequent occurrence of bacteremia and the experience that blood culture isolates are not serum resistant after in vitro culturing, it was hypothesized that bacterial strains adapt to grow in human blood. Consequently, the test strains were conditioned in media containing increasing concentrations of human serum. The organisms adapted to growth in the human serum; however, they would lose their serum resistance after several passages in microbiological media. Initially, one isolate of Klebsiella pneumoniae was also subcultured, but the MIC was repeatedly and intermittently elevated 30-fold. Distinct subpopulations could not be isolated. Perhaps the organisms carried a resistance trait that was expressed only occasionally. The susceptibilities of the two test isolates that were used for this study remained stable over the 3-month assessment period.
Protein binding of ertapenem is nonlinear, but saturation of binding sites occurs at relatively high concentrations. Susceptible pathogens are inhibited at concentrations that are low enough to remain in the range of linear protein binding. This study included only ertapenem. Thus, the results would need to be confirmed with other highly protein-bound antibacterial agents. As shown in Table 2, ertapenem exhibited only moderate binding in various preparations of 4.5-g/dl albumin solutions in buffered saline. The binding increased only slightly when the albumin was diluted in human serum ultrafiltrate. Others have reported poor binding to albumin (S. Kiem and W. A. Craig, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-493, 2002). To our knowledge, the protein(s) responsible for ertapenem binding has not been determined, but alpha-1 acid glycoprotein has been excluded. It is possible that slight conformational changes in purified albumin could explain the reduced binding and that albumin is the important binding protein in vivo.
TABLE 2.
Protein binding of ertapenem in various preparations of 4.5 g of human albumin/dl and CAMHB
| Albumin preparation | Ertapenem concn (μg/ml) | % Fraction bound |
|---|---|---|
| Sigma, fresh | 200 | 48.2 |
| 50 | 59.1 | |
| Baxter, fresh | 200 | 15.7 |
| 50 | 26.6 | |
| Baxter, frozen | 200 | 10.8 |
| 50 | 36.4 |
The use of integrated pharmacokinetic-pharmacodynamic (PK-PD) relationships to develop more rational dose regimens has been gaining momentum over the last 15 years. The PK-PD characteristics of an antibacterial drug suggest that the optimal dose intensity and dosing intervals for target organisms are based on their MICs (2). On the other hand, given established dose regimens that have been demonstrated to be safe, PK-PD can be used to establish susceptibility breakpoints and to determine which pathogens are likely to respond to treatment with a specific antibacterial drug (13). The results of this study support the commonly stated belief that antibacterial activity is explained by the free-, unbound-drug concentration. The implication of this finding is that PK-PD exposure parameters should be based on the free-drug concentration, i.e., free Cmax/MIC, free AUC0-24/MIC, and the time that the free concentration exceeds the MIC (2). The use of parameters based on the free-drug concentration is paramount when extrapolating the results of one study to another drug in the same class that has different protein binding. The free-drug concentration and exposure parameters should be considered in interpreting PK-PD relationships, as well as in determining susceptibility breakpoints (13). This investigation has controlled for many of the variables that have confounded other studies. Kill rate studies were performed over a limited concentration range, thus avoiding the paradoxical effect that was previously described (16). The MIC and preliminary studies were used to designate the range of concentrations appropriate to describe the concentration-effect for each test isolate and medium condition. Kill rate studies provide a more accurate characterization of the effect of protein binding than MICs alone.
Acknowledgments
This work was supported by a Medical School Grant from Merck, Inc.
REFERENCES
- 1.Bailey, E. M., M. J. Rybak, and G. W. Kaatz. 1991. Comparative effect of protein binding on the killing activities of teicoplanin and vancomycin. Antimicrob. Agents Chemother. 35:1089-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig, W. A. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of β-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 17:479-501. [DOI] [PubMed] [Google Scholar]
- 3.Craig, W. A., and S. C. Ebert. 1989. Protein binding and its significance in antibacterial therapy. Infect. Dis. Clin. N. Am. 3:407-421. [PubMed] [Google Scholar]
- 4.Craig, W. A., and B. Suh. 1996. Protein binding and the antimicrobial effects: methods for determination of protein binding, p. 477-514. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, Md.
- 5.Drusano, G., H. Standiford, P. Ryan, W. McNamee, B. Tatem, and S. Schimpff. 1986. Correlation of predicted serum bactericidal activities and values measured in volunteers. Eur. J. Clin. Microbiol. 5:88-92. [DOI] [PubMed] [Google Scholar]
- 6.Dudley, M. N., J. Blaser, D. Gilbert, and S. H. Zinner. 1990. Significance of “extravascular” protein binding for antimicrobial pharmacodynamics in an in vitro capillary model of infection. Antimicrob. Agents Chemother. 34:98-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones, R. N., and A. L. Barry. 1987. Antimicrobial activity of ceftriaxone, cefotaxime, desacetylcefotaxime, and cefotaxime-desacetylcefotaxime in the presence of human serum. Antimicrob. Agents Chemother. 31:818-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunun, C. M. 1965. Clinical pharmacology of the new penicillins. I. The importance of serum protein binding in determining antimicrobial activity and concentration in serum. Clin. Pharmacol. Ther. 7:166-179. [DOI] [PubMed] [Google Scholar]
- 9.Lam, Y. W. F., M. H. Duroux, J. G. Gambertoglio, S. L. Barriere, and B. J. Guglielmo. 1988. Effect of protein binding on serum bactericidal activities of ceftazidime and cefoperazone in healthy volunteers. Antimicrob. Agents Chemother. 32:298-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang, S. D. R., G. L. Cameron, and P. R. Mullins. 1981. Anomalous effect of serum on antimicrobial activity of cefoperazone. Drugs 22(Suppl. 1):52-59. [DOI] [PubMed] [Google Scholar]
- 11.Leggett, J. E., and W. A. Craig. 1989. Enhancing effect of serum ultrafiltrate on the activity of cephalosporins against gram-negative bacilli. Antimicrob. Agents Chemother. 33:25-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merrikin, D. J., J. Briant, and G. N. Rolinson. 1983. Effect of protein binding on antibiotic activity in vivo. J. Antimicrob. Chemother. 11:233-238. [DOI] [PubMed] [Google Scholar]
- 13.Mouton, J. W. 2003. Impact of pharmacodynamics on breakpoint selection for susceptibility testing. Infect. Dis. Clin. N. Am. 17:579-598. [DOI] [PubMed] [Google Scholar]
- 14.Nath, S. K., G. A. Foster, L. A. Mandell, and C. Rotstein. 1994. Antimicrobial activity of ceftriaxone versus cefotaxime: negative effect of serum albumin binding of ceftriaxone. J. Antimicrob. Chemother. 33:1239-1243. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 5th ed. M07-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 16.Odenholt, I., S. E. Holm, and O. Cars. 1987. Effect of antibiotic protein binding on the killing rate of Staphylococcus aureus and on the paradoxical phenomenon. Chemotherapy 33:331-339. [DOI] [PubMed] [Google Scholar]
- 17.Perl, T. M., M. A. Pfaller, A. Houston, and R. P. Wenzel. 1990. Effect of serum on the in vitro activities of 11 broad-spectrum antibiotics. Antimicrob. Agents Chemother. 34:2234-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson, L. R., W. H. Hall, H. H. Zinneman, and D. N. Gerding. 1977. Standardization of a preparative ultracentrifuge method for quantitative determination of protein binding of seven antibiotics. J. Infect. Dis. 136:778-783. [DOI] [PubMed] [Google Scholar]
- 19.Reller, B. L., and C. W. Stratton. 1977. Serum dilution test for bactericidal activity. II. Standardization and correlation with antimicrobial assays and susceptibility tests. J. Infect. Dis. 136:196-204. [DOI] [PubMed] [Google Scholar]
- 20.Wong, B. K., P. J. Bruhin, and J. H. Lin. 1999. Dose-dependent plasma clearance of MK-826, a carbapenem antibiotic, arising from concentration-dependent plasma protein binding in rats and monkeys. J. Pharm. Sci. 88:277-280. [DOI] [PubMed] [Google Scholar]