Abstract
In this study, novel hyaluronic acid-pH stimuli-responsive lipid membrane mesoporous silica nanoparticles (HA-PL-MSNs) were designed and assembled, with the chemotherapeutic agent doxorubicin (DOX) as the model drug. HA-PL-MSNs exhibited a well-defined mesostructure covered by lipid bilayer and particle size of ~150 nm. The drug loading capacity was up to ~18.2%. DOX release could be effectively retained by the lipid bilayer in pH 7.4 buffer and exhibited a pH-triggered burst release in the acidic condition. Confocal laser scanning microscopy and fluorescence-activated cell sorting showed that HA-PL-MSNs exhibited higher cellular uptake efficiency via CD44 receptor-mediated endocytosis compared with PL-MSNs in HeLa cells. In vitro cytotoxicity studies demonstrated that HA-PL-MSNs could effectively enhance the targeted delivery of DOX and restrain the growth of HeLa cells. This might provide a promising alternative for the development of a targeted anticancer drug delivery system.
Keywords: mesoporous silica nanoparticles, hyaluronic acid, pH-sensitive lipid membrane, CD44 receptor, HeLa cells
Introduction
Cancer is now the leading cause of morbidity and mortality in the world. The principal cancer treatment in clinics is still chemotherapy; however, the efficacy of many chemotherapeutic agents is often hindered by their low aqueous solubility, drug resistance, toxicity, and side effects, which have limited clinical efficacy because of nonspecific biodistribution in the body.1,2 To change this phenomenon, researchers started to develop a lot of nanomaterials to overcome the limitations. In recent decades, nanomaterials have been widely used in nanomedicine as potential diagnostic and therapeutic agents for cancer imaging and treatment, with less toxicity and improved efficacy.3,4 Targeted delivery of more efficient cancer treatments has been well documented and is vital to distribute the drug throughout the body to minimize the side effects, which is desirable in order to increase the anticancer drug concentration at the target sites.5
The major goal of chemotherapy is to ensure the safe and efficient transportation of drug molecules to the targeted sites.2 Over the past two decades, advances in biotechnology and nanotechnology have brought about a paradigm shift to design more sophisticated drug deliveries approaches.6 Recent reports on the design of capped and gated mesoporous silica nanoparticles (MSNs)-based systems show they are attractive carriers for drug delivery and have shown promise in preventing premature release of drugs and controlled release of guest molecules from MSNs. They offer unique properties such as straightforward synthesis, tunable pore sizes, high-loading capacity from the large surface area and pore volumes, good chemical stability, excellent biocompatibility, ease of surface modification, multifunctionality for high drug loading capacity, and efficient encapsulation of a wide variety of cargo molecules.7,8 The MSNs system is an extremely robust nanoparticle system used for drug delivery; recent studies also supported the use of MSNs as a delivery system for hydrophobic anticancer drugs to overcome their insolubility problem.9
When these nanomaterials are developed as drug delivery systems, drawbacks are encountered such as drug leakage,10 nonspecific targeting effects,11 difficulties in monitoring cellular events after drug delivery, etc.12 Therefore, the design and development of smart drug delivery systems with multiple functions, such as simultaneous stimuli-responsive drug release and targeting to tumor cell, are becoming increasingly urgent. Various stimuli, such as temperature, photoirradiation, enzymes, and pH levels have been applied as triggers to release encapsulated drugs.13,14 The pH-responsive system is of particular interest for cancer therapy because both extracellular tumors (pH 6.8) and endosomes (pH 5.5) are more acidic than normal tissues (pH 7.4), which enables the carriers to release anticancer drugs in a pH-dependent manner. Many methods have been developed to synthesize pH-responsive MSNs systems and pH-sensitive lipid membrane as drug carriers to deliver drugs and trigger the release of encapsulated drugs in tumor cells, an important role in all drug delivery systems.14–17 The pH-sensitive lipid membrane that target tumors are stable at physiological pH values, but are designed to become unstable under acidic conditions, resulting in the release of their drug cargo.18 Some studies have shown that coated nanoparticles of pH-sensitive lipid membrane can protect the drug and increase the drug concentration, which is favored to decrease systemic toxicity and enhance antitumor activity.14,15
Hyaluronic acid (HA) is a kind of functional material that acts as a drug carrier or targeting moiety, which is a natural ligand for cancer cells overexpressing CD44 and that could interact with a CD44 receptor excessively expressed in most tumor cells.19 HA is one of the major components of vertebrate tissue and body fluid; it interacts with the CD44 receptors that trigger intracellular signals influencing cellular proliferation, differentiation, and migration. CD44 overexpression is associated with tumor angiogenesis and progression to metastasis, and so HA has been used to target cancer cells with various nanovectors in vitro.20–22
We designed HA-pH stimuli-responsive lipid membrane MSNs (HA-PL-MSNs) to deliver antitumor drugs. Doxorubicin hydrochloride (DOX), a cytotoxic drug that inhibits cellular mitochysis by interfering with the normal breakdown of microtubules during cell division, was chosen as the model drug.3 This system, after being intravenously injected into the blood circulation, could deliver drugs into the tumor cells and avoid the clearance of the reticuloendothelial system, which could prolong the time of circulation and prevent the drug from being leaked. As for the prolonged blood circulation time, MSNs were accumulated in the tumor tissue via the enhanced permeation and retention effect, and by HA and CD44 receptors interacting, the nanoparticles would enrich in the target. The carrier-coated pH-sensitive material would then release DOX quickly when the particle was situated in the acidic conditions and DOX played a cytotoxic role in tumor cells. The nanocarriers retained and protected guest molecules before reaching the target cells, and the coated layer was readily disrupted to release anti-tumor drugs after cellular uptake. Thus, compared to bare MSN, the MSN coated by the lipids could minimize toxicity and maximize the effectiveness of drugs (Figure 1).
Figure 1.
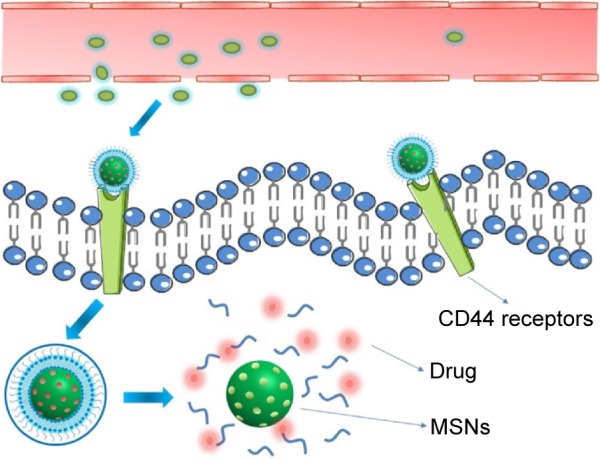
Schematic illustration of cellular uptake through a CD44 receptor-mediated interaction of DOX@HA-PL-MSNs and the responsive intracellular storm release of DOX.
Abbreviations: DOX, doxorubicin; HA, hyaluronic acid-functionalized; MSN, mesoporous silica nanoparticle; PL, pH stimuli-responsive lipid membrane.
Materials and methods
Materials
Hydrogenated soybean phospholipids, distearoyl phosphoethanolamine (DSPE), dioleoyl phosphoethanolamine, and HA (200–400 kDa) were purchased from Shanghai Advanced Vehicle Technology Co., Ltd. (Shanghai, People’s Republic of China). Cholesteryl hemisuccinate was obtained from XiYa Chemical Industry Co., Ltd. (Shanghai, People’s Republic of China). DOX was purchased from Beijing Huafeng United Technology Co., Ltd. (Beijing, People’s Republic of China). Cholesterol (AR), cetyltrimethyl ammonium bromide (CTAB), and tetraethyl orthosilicate (TEOS) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, People’s Republic of China) and 1-ethyl-3-(3-(dimethylamino)propyl) carbodiimide hydrochloride and N-hydroxysuccinimide were obtained from Shanghai Titan technology Co., Ltd. (Shanghai, People’s Republic of China). All of the other purchased chemicals were of analytical grade and obtained from a variety of vendors. HeLa cells and NIH3T3 cells came from Shanghai Institutes for Biological Sciences of the Chinese Academy of Sciences (Shanghai, People’s Republic of China).
Preparation of DOX@HA-PL-MSNs
Synthesis of MSNs
MSNs were synthesized by CTAB-templated, base-catalyzed condensation reaction of TEOS.5,9,20,23 Briefly, 2.4 g of CTAB was dissolved in deionized water (800 mL) with vigorous stirring at 80°C. Then 3.6 mL of NaOH (2 mol/L) was dissolved into the solution and stirred vigorously for 2 continual hours. Finally, 8 mL TEOS as the silicon resource was added drop-wise and the solution was stirred at this temperature for 8 h. After the reaction mixture continuously reacted for 8 h, it was then centrifuged to collect the synthesized materials. The organic template (CTAB) was completely removed through refluxing in a mixed solution of ethanol and hydrochloric acid (9:1) at 80°C for 12 h, and the same reflux procedure was repeated three times. The productive MSNs were dried for 12 h at room temperature in a vacuum.
Drug loading
For the preparation of DOX@MSNs, MSNs were dispersed into DOX aqueous solution.9,24 The mixture was gently stirred under light-sealed conditions for 12 h. The DOX@ MSNs were collected by centrifugation (13,000 rpm, 20 min) and washed with phosphate buffered solution (PBS, pH 7.4) to remove the dissociative DOX. To evaluate the loading efficiency of DOX, the supernatant was collected, and the residual DOX content was determined by using a UV-Vis spectrometer at a wavelength of 480 nm. To measure the drug loading capacity, 5 mg of DOX was dissolved in different pH solutions (3.0, 4.0, 5.0, 6.0, 6.5, and 7.4) containing 10 mg MSNs, and then we found the most suitable pH condition.
The particle size was determined by dynamic laser scattering using a Malvern Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) at 25°C, and morphology was observed by transmission electron microscopy (TEM, JEOL [Tokyo, Japan], and JEM-2100). The narrow pore size distribution and surface area were measured by the small-angle X-ray powder diffraction (D8 Advance, Bruker AXS, Billerica, MA, USA) and N2 adsorption (ASAP 2020 HD88, Micromeritics, Norcross, GA, USA). We used Fourier transform infrared spectroscopy (FTIR, Thermo Fisher Scientific [Waltham, MA, USA], Nicolet 6700) to investigate if DOX was loaded in the MSNs.
Preparation of DOX@LP-MSNs
Dioleoyl phosphoethanolamine, cholesteryl hemisuccinate, and DSPE at molar ratio of 6:4:0.3 were dissolved in CH2Cl2. A thin lipid film was formed by rotary evaporation at 30°C. DOX@MSNs (15.0 mg) dissolved into 40 mL PBS (pH 7.4) was added to rehydrate the thin film at 60°C to attach a pH-sensitive lipid-bilayer coating. The solution was then subjected to a high-pressure extruder (AH-BASICI, ATS Engineering, Shanghai, People’s Republic of China) at 1,000 bar five times. By the same method, hydrogenated soybean phospholipids, DSPE, and cholesterol at a molar ratio of 14:8:1 were used to make DOX@MSNs coated general lipid-bilayer (L-MSNs) (DOX@L-MSNs).17,25
Modification of PL-MSNs with HA
To prepare DOX@HA-PL-MSNs, HA (26 mg) was hydrated in water overnight and then activated by 1-ethyl-3-(3-(dimethylamino)propyl) carbodiimide hydrochloride (12 mg) and N-hydroxysuccinimide (6.8 mg) for 2 h, in which the pH was adjusted to 4 by 1 mol/L hydrochloric acid. Then the solution of HA (quality is 10% of the PL-MSNs) was added to the PL-MSNs solution overnight and the pH was adjusted to 8.6 by borate buffer solution. Finally, the HA-modified PL-MANs were collected after centrifugation at 8,000 rpm for 10 min and the unreacted HA was removed.3,26
In vitro DOX release
To study the drug release, the DOX released from various nanoparticles were investigated in PBS at different pH values (pH 7.4 and 5.0).17 Briefly, 1 mL of a solution (1 mg/mL) that included DOX@MSNs, DOX@L-MSNs, DOX@PL-MSNs, DOX@HA-L-MSNs, and DOX@HA-PL-MSNs was introduced into dialysis bags (MWCO 3,500 Da, Spectrum Laboratories, Inc., Rancho Dominguez, CA, USA) and sealed with dialysis bag holders. The dialysis bags were then submerged in 20 mL PBS at 37°C and shaken at a speed of 100 rpm. At desired time intervals, 1 mL of release media was taken out from the PBS dialysis solution to measure the concentration of the released drug by high-performance liquid chromatography (Agilent Technologies [Santa Clara, CA, USA], 1260 Infinity). At the same time, an equal volume of fresh PBS solution was replenished to keep the volume constant. To demonstrate that the lipid layer could prevent the premature release of guest molecules from the mesostructure, after 6 h incubation of DOX@HA-PL-MSNs in a pH 7.4 PBS, the membrane-disrupting agent Triton X-100 (Sigma-Aldrich, St Louis, MO, USA) was added into the dialysis tube at 1% of the total volume, and the released DOX was determined. In addition, to show the pH-triggered release feature, DOX@HA-PL-MSNs were shaken in simulated body fluid (pH 7.4 PBS) and tumor microenvironment condition (pH 6.8 PBS) for 12 h, and the dialysis bag was transferred to PBS pH 5.0 (simulated endosomal/lysosomal acidic condition in tumor cells). The amount of DOX released was determined by high-performance liquid chromatography. The mobile phase consisted of a mixture of acetonitrile, methanol, and water (40/50/10, v/v/v), and the detection wavelength of DOX was 233 nm.27,28
Cellular uptake
To track the cellular uptake of various nanoparticles, the MSNs were labeled with fluorescein-5-isothiocyanate (FITC). On the surface of the MSNs, free NH2 moieties were labeled with FITC and the functional group of FITC, thiocyanate, is highly amino reactive; therefore, the prepared MSNs can be conjugated with FITC. Specifically, we added 50 mg MSNs into 10 mL deionized solution and mixed it with 10 mL FITC ethanol solution (0.3 mg/mL). After stirring overnight, the nanoparticles were centrifuged and washed three times until the supernatants were colorless. The sample was then made according to the aforementioned methods.3,8,29
HeLa cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Thermo Fisher Scientific) supplemented with fetal calf serum (10%) in 5% CO2 at 37°C. HeLa cells were seeded in a 12-well plate (5×104 cells per well) and incubated for 8 h. After washing twice with PBS, the cells were incubated with FITC-labeled MSNs, PL-MSNs, or HA-PL-MSNs in 2 mL of the serum-free DMEM medium for 5 h. The cells were subsequently washed three times with 4°C PBS to remove the remaining nanoparticles and dead cells, and the cells were investigated under a confocal laser scanning microscope (CLSM, Leica [Wetzlar, Germany], TCS SP8).3,23
For competitive inhibition studies, the medium was replaced with 2 mL serum-free culture medium containing HA polymer (10 mg/mL) and then FITC-labeled HA-PL-MSNs (20 mg/mL) in HeLa cells,28,29 followed by the same treatment as described earlier. In order to further confirm competitive inhibition studies of HA to CD44, NIH3T3 cells5,6,19 were cultured in the same way. We then added FITC-labeled HA-PL-MSNs (20 mg/mL). Finally, we viewed the NIH3T3 cells under a CLSM.
A quantitative determination of the cellular uptake was performed on a fluorescence-activated cell sorter (FACS). HeLa cells were seeded in 12-well plates at a density of 2×104 per well. The cells, after 8 h washing with PBS, were incubated with 30 μg/mL FITC-labeled DOX@MSNs, DOX@PL-MSNs, DOX@L-MSNs, and DOX solution in 2 mL serum-free DMEM medium. The cells in each well were subsequently rinsed with cold PBS three times, trypsinized, centrifuged, and resuspended in 0.5 mL PBS. The mean fluorescence intensity was measured by flow cytometry (FACS, BD).24,30
In vitro antitumor activity of DOX@HA-PL-MSNs
HeLa cells were seeded in a 96-well plate at a density of 2×104 cells per well and cultured in 5% CO2 at 37°C. After 8 h, free DOX, DOX@MSNs, DOX@L-MSNs, DOX@ PL-MSNs, and DOX@HA-PL-MSNs were added to the cells in DMEM medium at different DOX concentrations of 0, 0.25, 0.5, 1, 2, 4, and 8 μg/mL, respectively, and the cells were further incubated in 5% CO2 at 37°C for 24 and 48 h. Next, 10 μL MTT solution was added to each well of the plate and the plate was incubated in the CO2 incubator for an additional 4 h. The cells were then lysed by the addition of dimethylsulfoxide. Absorbance values of formazan were determined with a microplate reader at 490 nm. Three replicates were done for each treatment group.3,19,29
Results and discussion
Characterization of MSNs and PL-MSNs
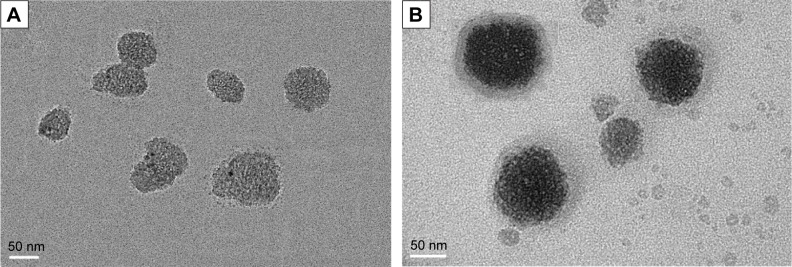
Ordered MSNs were successfully synthesized following a previously reported approach using TEOS as the silica source and surfactant CTAB as the pore template. TEM imaging (Figure 2A) shows that the resulting spherical MSNs and exhibited uniform well-defined mesostructures, and from Figure 2B, it is obvious that the MSN nanoparticles were covered in bright layers of lipid membrane. This was the most direct evidence to prove the coating of the lipid membrane around the MSNs matrix.
Figure 2.
TEM images of MSNs (A) and PL-MSNs (B).
Abbreviations: TEM, transmission electron microscopy; MSNs, mesoporous silica nanoparticles; PL, pH stimuli-responsive lipid membrane.
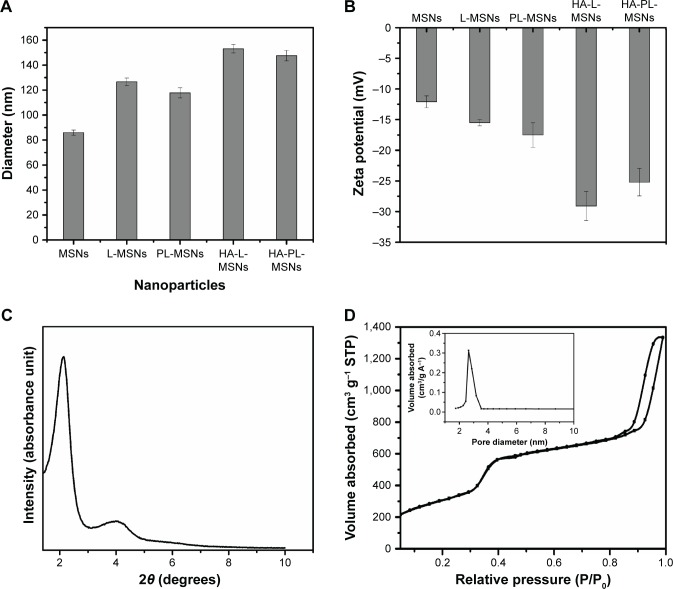
As can be seen from Figure 3A, the result showed MSNs mean size was around 85 nm and the size of nanoparticles coated with lipid bilayer was around 120 nm (polymer dispersity index <0.25). When the nanoparticles were modified with HA, the size grew to 150 nm; these results were consistent with that of other studies.17,29 The resulting picture of zeta potential (Figure 3B) shows that the MSNs exhibited a negative zeta potential. After the coating of lipid bilayer, the zeta potential changed from −12 to −16 mV, and with the modification of HA, the zeta potential dropped to approximately −28 mV.17,31
Figure 3.
(A) Particle size distribution determined by DLS of different nanoparticles. (B) Zeta potential of different nanoparticles. (C) Small-angle X-ray powder diffraction pattern of MSNs. (D) Nitrogen adsorption–desorption isotherms and pore diameter distribution of MSNs (mean ± SD, n=5).
Abbreviations: DLS, dynamic light scattering; HA, hyaluronic acid-functionalized; L-MSNs, MSNs coated general lipid-bilayer; MSN, mesoporous silica nanoparticle; PL, pH stimuli-responsive lipid membrane; SD, standard deviation; STP, standard temperature and pressure.
The small-angle X-ray diffraction pattern and N2 adsorption–desorption isotherm (Figure 3C and D) revealed that MSNs had two-dimensional hexagonal symmetry, with uniform mesoporous channels and a relatively narrow pore size distribution. MSNs were characterized by pore diameters of 2.8 nm,8,31,32 a large surface area of 1134.57 m2/g, and cumulative pore volumes of 2.06 cm3/g,9,17 which contributed to high DOX loading in MSNs.
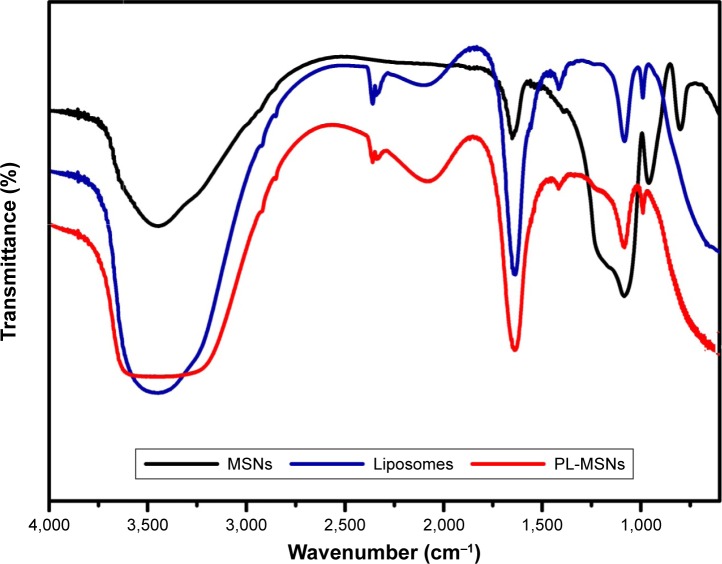
To verify MSNs having been coated by the lipid bilayer, FTIR spectra were employed, and Figure 4 shows the FTIR spectra of MSNs, liposomes, and PL-MSNs, respectively. From the spectrum of MSNs, the absorption peaks near 776 cm−1 and 1,076 cm−1 were the Si–O–Si29,33 and Si–OH31 stretching vibrations of MSNs, respectively. And the characteristic peaks of liposomes were present at 2,900–2,850 cm−1 and 1,730 cm−1, which corresponded to the stretching vibrations of alkyl groups and ester groups, respectively.34 When the MSNs were coated by the lipid bilayer, we could find the disappearance of the peak around 776 cm−1 and diminishment around 1,076 cm−1. And at the same time, there were the appearance of a minor peak around 2,900–2,850 cm−1 and the change of the peak at 1,730 cm−1, which indicated the formation of PL-MSNs. As seen in the FTIR images (Figure 4), the MSNs were encapsulated in the lipid membrane, which was consistent with the TEM results (Figure 2B).
Figure 4.
FTIR spectra of MSNs, liposomes, and PL-MSNs.
Abbreviations: FTIR, Fourier transform infrared spectroscopy; MSN, mesoporous silica nanoparticle; PL, pH stimuli-responsive lipid membrane.
Drug loading and pH stimuli-responsive drug release
To demonstrate the optimum pH condition for drug loading, the collected supernatant solution was measured by UV-vis to quantify the DOX after drug loading. Looking at the image (Figure 5), we found that the amount of the drug loaded in the MSNs increased with the increase of the pH value. When the pH of the solution was 3.0, the drug loading was ~9.28%, and when the pH increased to 6.5, the drug loading plateau occurred and the drug was ~18.2%. The maximum drug loaded was 18.61 mg/100 mg when the pH condition was at 7.4, by which the following experiments were designed and performed. The reason was as the pH value of solution increased, the solubility of DOX was also increased accordingly, which means that more of the drug could be physically absorbed on MSNs.
Figure 5.
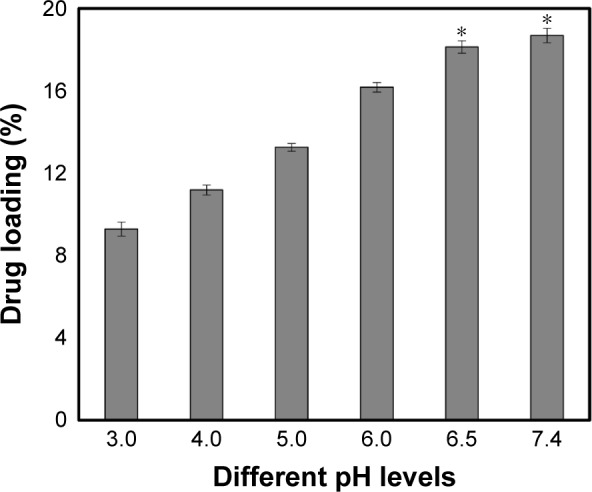
The amount of drug loaded at different pH conditions and the maximum of loaded.
Notes: DOX was 18.62 mg/100 mg MSNs. Data are represented as mean ± SD (n=5). *Statistically significant difference versus the pH 3.0 group (P<0.01).
Abbreviations: DOX, doxorubicin; MSN, mesoporous silica nanoparticle; SD, standard deviation.
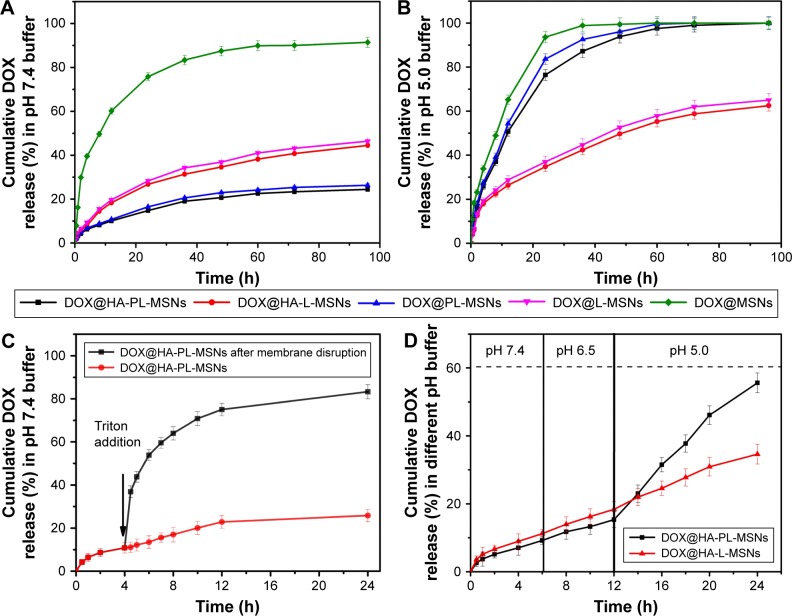
To demonstrate the pH-triggered release properties of HA-PL-MSNs, nanoparticles were placed in a dialysis bag and immersed in dissolution media (PBS) with different pH values. As shown in Figure 6A, the drug loaded in the PL-MSNs and HA-PL-MSNs leaked ~20% in the solution when maintained in simulated body fluid (pH 7.4) at 37°C, whereas uncoated MSNs leaked 90% of drug loaded within 96 h.17 The premature release of encapsulated cargo may result in off-target toxicity to normal bystander cells, which means the protective effect of lipid bilayer is effective. Exposing HA-PL-MSNs to a pH 5.0 PBS that simulates the endosomal–lysosomal system in tumor cells and destabilizes the supported lipid layer and promoted the rapid release of drugs loaded within the mesoporous core. As shown in Figure 6B, HA-PL-MSNs released more than 80% of their encapsulated DOX within 36 h, but the HA-L-MSNs released ~60% DOX into the solution, which means the pH-sensitive lipid bilayer destabilized in the acidic condition and was more beneficial to the release in the tumor cells.17 Ideal nanocarriers are desired to burst-release encapsulated drugs into cancer cells to enhance antitumor efficacy, but maintain stability in bodily fluids to avoid toxicity to healthy cells. In addition, HA is favored to avoid nanoparticles being eliminated after intravenous injection by the reticuloendothelial system, which promotes the longer circulation of nanoparticles and concentrate in the tumor site by the enhanced permeation and retention effect. Finally, the DOX@PL-HA-MSNs could be taken up into the tumor cells by the CD44 receptor and rapidly release the drug in a short time while suppressing tumor growth. From the aforementioned result, it was obvious that HA-PL-MSNs exhibited an ideal release behavior, so we chose HA-PL-MSNs for further studies.
Figure 6.
(A) The time-dependent release of DOX from MSNs, L-MSNs, PL-MSNs, HA-L-MSNs, and HA-PL-MSNs when exposed to a simulated body fluid (pH 7.4) and (B) in a pH 5.0 buffer at 37°C. (C) DOX release profiles versus the time of DOX@HA-PL-MSNs with or without lipid membrane disruption. (D) DOX release profiles of DOX@HA-PL-MSNs and DOX@HA-L-MSNs by lowering the pH to 5.0 after exposure to pH 7.4 and pH 6.5 buffers for 12 h. Data are represented as mean ± SD (n=3; P<0.01).
Abbreviations: DOX, doxorubicin; HA, hyaluronic acid-functionalized; L-MSNs, MSNs coated general lipid-bilayer; MSN, mesoporous silica nanoparticle; PL, pH stimuli-responsive lipid membrane; SD, standard deviation.
To demonstrate that lipid bilayer could retain the release of DOX from HA-PL-MSNs, we determined the DOX released from HA-PL-MSNs in pH 7.4 buffer. After 4 h of monitoring (Figure 6C), the drug released from HA-PL-MSNs was ~10%, which indicated DOX could be held within HA-PL-MSNs without obvious premature release. A membrane-disrupting agent, Triton X-100, was then added into the dialysis bag, which resulted in a burst drug release, and the amount of drug released from HA-PL-MSNs reached ~80% in 24 h.17 It is indicated that pH-sensitive lipid bilayer could effectively prevent the premature release of the absorbed drug molecules. In addition, the pH of the solution was lowered to 5.0 to confirm that DOX released from HA-PL-MSNs was indeed triggered by the pH conditions. The release of DOX in simulated conditions of bodily fluid (pH 7.4) and tumor microenvironments (pH 6.5) was monitored for 12 h, followed by a lowering of the pH of dissolution medium to 5.0. As shown in Figure 6D, the DOX release profiles of HA-PL-MSNs in the pH 6.5 buffer were ~15% at 12 h, which followed a similar trend with that in the pH 7.4 buffer; when the pH changed to 5.0, a burst release of DOX was observed. Evidently, HA-PL-MSNs showed a more rapid release rate in pH 5.0 buffer and the drug released from HA-PL-MSNs could reacĥ55% in 24 h. Compared with HA-PL-MSNs, the rate of the drug release of HA-L-MSNs did not appear to be much different from Figure 6B and the drug released finally reached 30%, which means traditional lipid bilayer could not facilitate pH-sensitive burst drug release. This in vitro experiment strongly suggested the potential of HA-PL-MSNs as an intracellular pH-triggered storm drug release system.
Cellular uptake
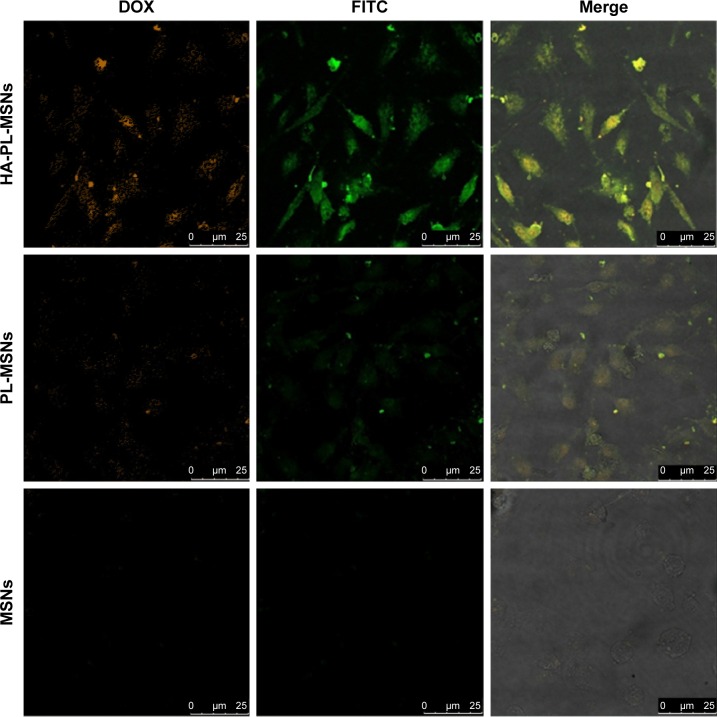
To visualize the cellular uptake of various nanoparticles, MSNs, PL-MSNs, and HA-PL-MSNs were labeled with FITC and studied by CLSM. In Figure 7, it can be seen that the red and green fluorescence represented the DOX in the cells and the FITC-labeled MSNs, respectively. We could easily observe the strongest green fluorescence and red fluorescence in the group of HA-PL-MSNs (as shown in Figure 7), which demonstrated that the HA-PL-MSNs were more easily taken in HeLa and that the DOX loaded in the MSNs could release more in the tumor cells than the particles of PL-MSNs unlinked with HA when HA-PL-MSNs were used to treat the cells. And the most brilliant fluorescence of HA-PL-MSNs, compared with cells incubated with FITC-labeled MSNs and PL-MSNs, illustrated the highest intracellular uptake of nanoparticles and the efficient transportation of DOX into the Hela cells.
Figure 7.
Confocal microscopy images of HeLa cells treated with the FITC-labeled HA-PL-MSNs, MSNs, and PL-MSNs, respectively.
Notes: Green fluorescence arises from FITC dyes that are conjugated to silica nanoparticles and red fluorescence shows the DOX intake in cells.
Abbreviations: DOX, doxorubicin; FITC, fluorescein-5-isothiocyanate; HA, hyaluronic acid-functionalized; MSN, mesoporous silica nanoparticle; PL, pH stimuli-responsive lipid membrane.
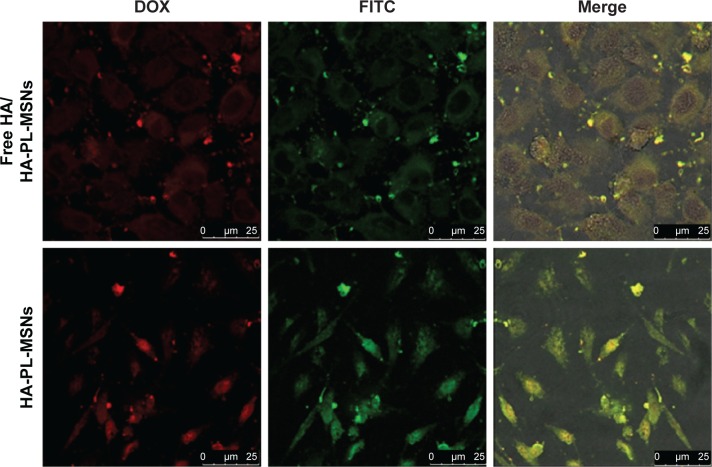
Furthermore, as shown in Figure 8, when HeLa cells were pretreated with free HA (10 mg/mL) to block CD44 prior to FITC-labeled HA-PL-MSNs treatment, the green and red intracellular fluorescence was also weaker compared with the HA-PL-MSNs group. The result demonstrates that pretreatment of the HeLa cells with HA molecules to block HA receptors on the surface of the cells drastically decreased the target efficiency of the nanocarriers and thus reduced the fluorescence intensity in the inhibition assay, corroborating that the uptake was mainly induced by the specific interaction between HA and CD44.3
Figure 8.
Confocal microscopy images of HeLa cells treated FITC-labeled HA-PL-MSNs and free HA (10 mg/mL) together with FITC-labeled HA-PL-MSNs.
Abbreviations: DOX, doxorubicin; FITC, fluorescein-5-isothiocyanate; HA, hyaluronic acid-functionalized; MSN, mesoporous silica nanoparticle; PL, pH stimuli-responsive lipid membrane.
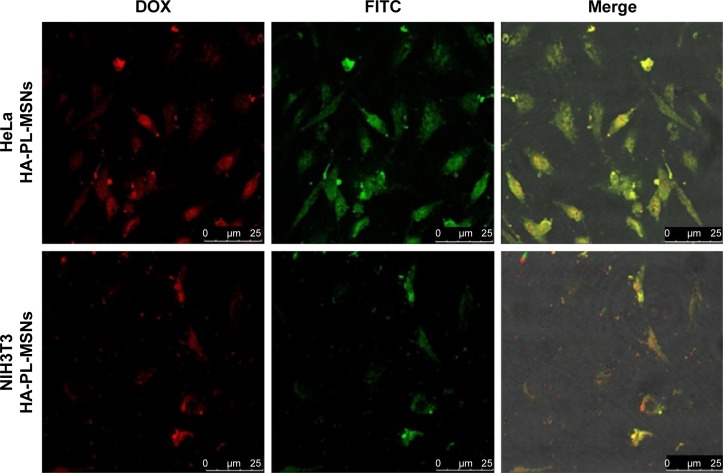
The NIH3T3 cells, CD44 receptor-negative cells, were also incubated in the aforementioned way, and the result suggested that the HA-PL-MSNs taken into NIH3T3 cells was under the Hela cells group. As seen in Figure 9, comparing NIH3T3 cells with the HeLa cells, there was a higher fluorescence emission of HeLa cells (CD44 overexpressed) than NIH3T3 cells (normal cells) due to the specific interaction between HA and CD44 overexpressed on HeLa cells, leading to an enhanced uptake of the nanoparticles. The result of this experiment indicated that the DOX@HA-PL-MSNs drug delivery system could increase the amount of drug by the targeted function of HA to the tumor cell.3,19
Figure 9.
Confocal microscopy images of HeLa cells and NIH3T3 cells treated with the FITC-labeled HA-PL-MSNs.
Abbreviations: DOX, doxorubicin; FITC, fluorescein-5-isothiocyanate; HA, hyaluronic acid-functionalized; MSN, mesoporous silica nanoparticle; PL, pH stimuli-responsive lipid membrane.
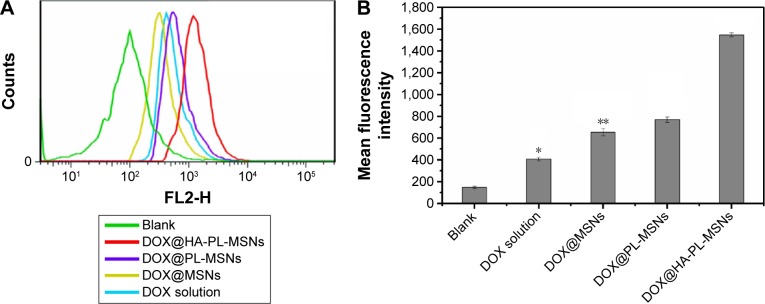
To provide a quantitative comparison, the cellular uptake performance of HA-PL-MSNs, PL-MSNs, MSNs, and DOX solution in HeLa cells was further studied by FACS analysis. As seen in Figure 10A and B, compared to the control group, the cells incubated with each group showed a much higher florescence intensity of DOX, which confirmed the results seen in Figure 7 and further demonstrated Hela cells could achieve targeted intake HA-PL-MSNs. The results showed that the DOX florescence intensity of the DOX@HA-PL-MSNs group exhibited the highest level, which is ~3 times higher than those that were treated with DOX solution and over 2 times higher than those that were treated with DOX@ MSNs and DOX@PL-MSNs. This phenomenon demonstrated that HeLa cells could intake HA-PL-MSNs, because of the existence of a high level of CD44 on the surface, and that DOX could release into the cells, consistent with a previous study.30
Figure 10.
(A) Flow cytometry histogram profiles of DOX florescence within HeLa cells and (B) quantitative analysis of mean fluorescence intensity when incubated with a series of particles for 6 h.
Notes: Data are represented as mean ± SD (n=3). *Statistical difference versus the DOX@HA-PL-MSNs group (P<0.05); **statistically significant difference versus the DOX@HA-PL-MSNs group (P<0.01).
Abbreviations: DOX, doxorubicin; HA, hyaluronic acid-functionalized; MSN, mesoporous silica nanoparticle; PL, pH stimuli-responsive lipid membrane; SD, standard deviation.
In vitro cytotoxicity studies of DOX@ HA-PL-MSNs
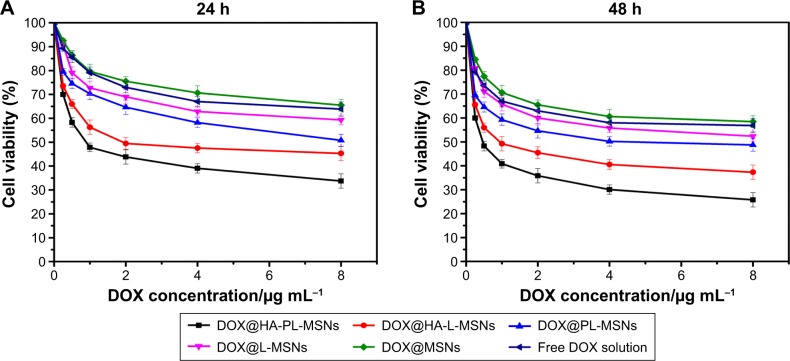
To demonstrate that the DOX@HA-PL-MSNs system could target CD44-overexpressing cancer cells and facilitate the cell-killing activity, the MTT assay was used for quantitative testing of the cell viability. As shown in Figure 11A and B, the cell viability decreases with increasing DOX concentration in the range of 0.25–8 μg/mL.35 After being treated by DOX@HA-PL-MSNs for 24 and 48 h, the cell viability was only 33% and 25%, indicating that this drug delivery system could restrain the growth of HeLa cells. In the same concentration range of DOX, HA-PL-MSNs showed the highest anticancer efficacy among all of the experimental groups after incubation, consistent with the CLSM observation. Compared with the PL-MSNs groups, what we found was HeLa cells could intake more HA-PL-MSNs and DOX and induce cells apoptosis ~2 times more, which implied that HA could promote nanoparticles to be taken in by CD44-overexpressing cancer cells.
Figure 11.
In vitro cytotoxicity assay curves for HeLa cells obtained by plotting the cell viability percentage against the concentration of DOX for 24 h (A) or 48 h (B), which was loaded in the DOX@HA-PL-MSNs, DOX@HA-L-MSNs, DOX@L-MSNs, DOX@PL-MSNs, DOX@MSNs, and free DOX solution, respectively.
Note: Data are represented as mean ± SD (n=4; P<0.05).
Abbreviations: DOX, doxorubicin; HA, hyaluronic acid-functionalized; L-MSNs, MSNs coated general lipid-bilayer; MSN, mesoporous silica nanoparticle; PL, pH stimuli-responsive lipid membrane; SD, standard deviation.
Compared with free DOX solution or DOX@MSNs group, cell viability was slightly lowered to ~60% after treatment with PL-MSNs and L-MSNs for 24 h, which proved that the lipid-bilayer coated on the MSNs could prevent the premature release of DOX from MSNs. HeLa cells were more sensitive to DOX@HA-PL-MSNs than DOX@PL-MSNs, as seen in the corresponding cell viability, which is presumably due to the improved intracellular uptake of nanoparticles through the interaction between the CD-44 receptor and the HA ligand. From the results, HA-PL-MSNs were shown to be more effective than the HA-L-MSNs; this could be explained due to the fact that the pH stimuli-responsive lipid membrane could both prevent premature drug leakage into the culture medium and facilitate intracellular burst drug release to achieve effective high drug concentration.17 The result demonstrated that HA-PL-MSNs were better suited to the targeted delivery of the chemotherapeutic agent DOX and enhanced the drug accumulation and the cytotoxity of the nanocarriers.17
Conclusion
In this investigation, we successfully produced and characterized pH-sensitive liposome-supported MSNs decorated by HA. This drug delivery system could be used as an efficient drug nanocarrier with a high-loading capacity of up to 18.6% at pH 7.4. In vitro DOX release showed that pH-sensitive lipid membrane could prevent premature drug leakage when the pH is 7.4 and that the loaded drug could be rapidly released in an acidic environment. In vitro targeting studies showed that DOX@HA-PL-MSNs exhibited higher uptake efficiency in CD44 receptor overexpressed HeLa cells via CD44 receptor-mediated endocytosis; so this nanocarrier could promote intracellular delivery of an antitumor drug and could show a response to the intracellular pH of tumor cells but not to the normal pH of bodily fluids. In vitro cytotoxicity studies showed that DOX@HA-PL-MSNs were the most cytotoxic toward HeLa cells due to the protective function of pH-sensitive lipid membrane and the increased cellular uptake because of the targeted function of HA. Thus, DOX@HA-PL-MSNs are promising vectors for the treatment of tumors, and they may be used to produce a new more effective approach to targeted cancer treatments.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Baek S, Singh RK, Khanal D, et al. Smart multifunctional drug delivery towards anticancer therapy harmonized in mesoporous nanoparticles. Nanoscale. 2015;7(34):14191–14216. doi: 10.1039/c5nr02730f. [DOI] [PubMed] [Google Scholar]
- 2.Arpicco S, Milla P, Stella B, Dosio F. Hyaluronic acid conjugates as vectors for the active targeting of drugs, genes and nanocomposites in cancer treatment. Molecules. 2014;19(3):3193–3230. doi: 10.3390/molecules19033193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu M, Jambhrunkar S, Thorn P, Chen J, Gu W, Yu C. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale. 2013;5(1):178–183. doi: 10.1039/c2nr32145a. [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Zhang Q, Hou Y, Zhang J, Liang X-J. Nanomaterials in medicine and pharmaceuticals: nanoscale materials developed with less toxicity and more efficacy. Eur J Nanomed. 2013;5(2):61–79. [Google Scholar]
- 5.Zhao Q, Geng H, Wang Y, et al. Hyaluronic acid oligosaccharide modified redox-responsive mesoporous silica nanoparticles for targeted drug delivery. ACS Appl Mater Interfaces. 2014;6(22):20290–20299. doi: 10.1021/am505824d. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Li Z, Lin Y, Yin M, Ren J, Qu X. Bioresponsive hyaluronic acid-capped mesoporous silica nanoparticles for targeted drug delivery. Chemistry. 2013;19(5):1778–1783. doi: 10.1002/chem.201202038. [DOI] [PubMed] [Google Scholar]
- 7.Popat A, Hartono SB, Stahr F, Liu J, Qiao SZ, Qing Max Lu G. Mesoporous silica nanoparticles for bioadsorption, enzyme immobilisation, and delivery carriers. Nanoscale. 2011;3(7):2801–2818. doi: 10.1039/c1nr10224a. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Q, Liu J, Zhu W, et al. Dual-stimuli responsive hyaluronic acid-conjugated mesoporous silica for targeted delivery to CD44-overexpressing cancer cells. Acta Biomater. 2015;23:147–156. doi: 10.1016/j.actbio.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Zou Z, He D, He X, et al. Natural gelatin capped mesoporous silica nanoparticles for intracellular acid-triggered drug delivery. Langmuir. 2013;29(41):12804–12810. doi: 10.1021/la4022646. [DOI] [PubMed] [Google Scholar]
- 10.Xu Q, Tanaka Y, Czernuszka JT. Encapsulation and release of a hydrophobic drug from hydroxyapatite coated liposomes. Biomaterials. 2007;28(16):2687–2694. doi: 10.1016/j.biomaterials.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Chan JM, Zhang L, Yuet KP, et al. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials. 2009;30(8):1627–1634. doi: 10.1016/j.biomaterials.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Hu K, Cao W, et al. pH-responsive biocompatible fluorescent polymer nanoparticles based on phenylboronic acid for intracellular imaging and drug delivery. Nanoscale. 2014;6(22):13701–13709. doi: 10.1039/c4nr04054f. [DOI] [PubMed] [Google Scholar]
- 13.Zhu CL, Wang XW, Lin ZZ, Xie ZH, Wang XR. Cell microenvironment stimuli-responsive controlled-release delivery systems based on mesoporous silica nanoparticles. J Food Drug Anal. 2014;22(1):18–28. doi: 10.1016/j.jfda.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng J, Tian X, Sun Y, Lu D, Yang W. pH-sensitive poly(glutamic acid) grafted mesoporous silica nanoparticles for drug delivery. Int J Pharm. 2013;450(1–2):296–303. doi: 10.1016/j.ijpharm.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 15.She Z, Zhang T, Wang X, et al. The anticancer efficacy of pixantrone-loaded liposomes decorated with sialic acid-octadecylamine conjugate. Biomaterials. 2014;35(19):5216–5225. doi: 10.1016/j.biomaterials.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Yuba E, Tajima N, Yoshizaki Y, Harada A, Hayashi H, Kono K. Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy. Biomaterials. 2014;35(9):3091–3101. doi: 10.1016/j.biomaterials.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Li F, Guo S, et al. Biofunctionalized polymer-lipid supported mesoporous silica nanoparticles for release of chemotherapeutics in multidrug resistant cancer cells. Biomaterials. 2014;35(11):3650–3665. doi: 10.1016/j.biomaterials.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Zhong, Chen, Sun, et al. Complexes containing cationic and anionic pH-sensitive liposomes: comparative study of factors influencing plasmid DNA gene delivery to tumors. Int J Nanomed. 2013;8:1573–1593. doi: 10.2147/IJN.S42800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou L, Dong K, Chen Z, Ren J, Qu X. Near-infrared absorbing mesoporous carbon nanoparticle as an intelligent drug carrier for dual-triggered synergistic cancer therapy. Carbon. 2015;82:479–488. [Google Scholar]
- 20.Gary-Bobo M, Brevet D, Benkirane-Jessel N, et al. Hyaluronic acid-functionalized mesoporous silica nanoparticles for efficient photodynamic therapy of cancer cells. Photodiagnosis Photodyn Ther. 2012;9(3):256–260. doi: 10.1016/j.pdpdt.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Pitarresi G, Palumbo FS, Albanese A, Fiorica C, Picone P, Giammona G. Self-assembled amphiphilic hyaluronic acid graft copolymers for targeted release of antitumoral drug. J Drug Target. 2010;18(4):264–276. doi: 10.3109/10611860903434027. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y, Lee H, Kim YB, et al. Bioinspired surface immobilization of hyaluronic acid on monodisperse magnetite nanocrystals for targeted cancer imaging. Adv Mater. 2008;20(21):4154–4157. doi: 10.1002/adma.200800756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai J, Shah BP, Zhang Yixiao, Yang L, Lee K-B. Real-time monitoring of atp-responsive drug release using mesoporous-silica-coated multicolor upconversion nanoparticles. ACS Nano. 2015;9(5):5234–5245. doi: 10.1021/acsnano.5b00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou Z, He X, He D, et al. Programmed packaging of mesoporous silica nanocarriers for matrix metalloprotease 2-triggered tumor targeting and release. Biomaterials. 2015;58:35–45. doi: 10.1016/j.biomaterials.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 25.Gabizon A, Shmeeda H, Horowitz AT, Zalipsky S. Tumor cell targeting of liposome-entrapped drugs with phospholipid-anchored folic acid-PEG conjugates. Adv Drug Deliv Rev. 2004;56(8):1177–1192. doi: 10.1016/j.addr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y, Hashida M, Harashima H. Hyaluronic acid controls the uptake pathway and intracellular trafficking of an octaarginine-modified gene vector in CD44 positive- and CD44 negative-cells. Biomaterials. 2015;52:189–198. doi: 10.1016/j.biomaterials.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 27.Dai W, Jin W, Zhang J, et al. Spatiotemporally controlled co-delivery of anti-vasculature agent and cytotoxic drug by octreotide-modified stealth liposomes. Pharm Res. 2012;29(10):2902–2911. doi: 10.1007/s11095-012-0797-2. [DOI] [PubMed] [Google Scholar]
- 28.Chen M, Feng W, Lin S, He C, Gao Y, Wang H. Antitumor efficacy of a PLGA composite nanofiber embedded with doxorubicin@MSNs and hydroxycamptothecin@HANPs. RSC Adv. 2014;4(95):53344–53351. [Google Scholar]
- 29.Ma M, Chen H, Chen Y, et al. Hyaluronic acid-conjugated mesoporous silica nanoparticles: excellent colloidal dispersity in physiological fluids and targeting efficacy. J Mater Chem. 2012;22(12):5615–5621. [Google Scholar]
- 30.Zhang Y, Ang CY, Li M, et al. Polymer-coated hollow mesoporous silica nanoparticles for triple-responsive drug delivery. ACS Appl Mater Interfaces. 2015;7(32):18179–18187. doi: 10.1021/acsami.5b05893. [DOI] [PubMed] [Google Scholar]
- 31.Qiu K, He C, Feng W, et al. Doxorubicin-loaded electrospun poly(L-lactic acid)/mesoporous silica nanoparticles composite nanofibers for potential postsurgical cancer treatment. J Mater Chem B. 2013;1(36):4601–4611. doi: 10.1039/c3tb20636j. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Zhi Z, Jiang T, Zhang J, Wang Z, Wang S. Spherical mesoporous silica nanoparticles for loading and release of the poorly water-soluble drug telmisartan. J Control Release. 2010;145(3):257–263. doi: 10.1016/j.jconrel.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 33.He L, Huang Y, Zhu H, et al. Cancer-targeted monodisperse mesoporous silica nanoparticles as carrier of ruthenium polypyridyl complexes to enhance theranostic effects. Adv Funct Mater. 2014;24(19):2754–2763. [Google Scholar]
- 34.Mudakavi RJ, Raichur AM, Chakravortty D. Lipid coated mesoporous silica nanoparticles as an oral delivery system for targeting and treatment of intravacuolar Salmonella infections. RSC Adv. 2014;4(105):61160–61166. [Google Scholar]
- 35.Xiong Q, Zhang M, Zhang Z, Shen W, Liu L, Zhang Q. Anti-tumor drug delivery system based on cyclodextrin-containing pH-responsive star polymer: in vitro and in vivo evaluation. Int J Pharm. 2014;474(1–2):232–240. doi: 10.1016/j.ijpharm.2014.08.018. [DOI] [PubMed] [Google Scholar]