Abstract
To improve the oral bioavailability of cidofovir (CDV), a series of ether lipid ester prodrugs were synthesized and evaluated for activity against murine cytomegalovirus (MCMV) infection. Four of these analogs, hexadecyloxypropyl (HDP)-CDV, octadecyloxyethyl (ODE)-CDV, oleyloxyethyl (OLE)-CDV, and oleyloxypropyl (OLP)-CDV, were found to have greater activity than CDV against human CMV and MCMV in vitro. The efficacy of oral treatment with these compounds against MCMV infections in BALB/c mice was then determined. Treatment with HDP-CDV, ODE-CDV, OLE-CDV, or OLP-CDV at 2.0 to 6.7 mg/kg of body weight provided significant protection when daily treatments were initiated 24 to 48 h after viral inoculation. Additionally, HDP-CDV or ODE-CDV administered twice weekly or as a single dose of 1.25 to 10 mg/kg was effective in reducing mortality when treatment was initiated at 24 h, 48 h, or, in some cases, 72 h after viral inoculation. In animals treated daily with HDP-CDV or ODE-CDV, virus titers in lung, liver, spleen, kidney, pancreas, salivary gland, and blood were reduced 3 to 5 log10-fold, which was comparable to CDV given intraperitoneally. These results indicated that HDP-CDV or ODE-CDV given orally was as effective as parenteral CDV for the treatment of experimental MCMV infection and suggest that further evaluation for use in CMV infections in humans is warranted.
Cytomegalovirus (CMV) infections continue to be a major cause of morbidity in solid organ (35) and stem cell recipients (10, 12). The management of these infections has generally been through the use of prophylaxis or prevention therapy with ganciclovir (GCV) (15) or acyclovir (ACV) (3); however, the lack of activity when they are administered orally has limited their use. While the availability of oral ACV (valacyclovir) (29) and GCV (valganciclovir) (32) has broadened the range of choices for treatment or prevention of CMV infections, a number of problems, including neutropenia, nephrotoxicity, and the selection of drug-resistant mutants, remain (6, 8, 13, 27, 28, 39). Cidofovir (CDV) has greater activity against CMV in vitro and in vivo than any of the other licensed drugs (16, 22, 23, 40) and retains activity against many of the GCV- and foscarnet (PFA)-resistant mutants (4). However, CDV does not have activity when given orally and may also cause severe nephrotoxicity (25, 37). One approach to overcoming the limitation of the lack of activity of CDV given orally has been to prepare prodrugs of CDV that are bioavailable when administered orally. The synthesis of alkylglycerol phosphate or alkylpropyl phosphate esters of ACV, penciclovir, or GCV provided prodrugs that were active when given orally in animal models of herpes simplex virus, murine CMV (MCMV), and woodchuck hepatitis virus infections (17-19). Using similar methodology, a series of ether lipid ester analogs of CDV or cyclic CDV were prepared and evaluated for activity against CMV and poxviruses (4, 24).
It has been reported previously that this series of ether lipid ester prodrugs of CDV, particularly the hexadecyloxypropyl (HDP)-CDV and octadecyloxyethyl (ODE)-CDV analogs, exhibited potency that was multiple-log-enhanced compared with that of GCV and CDV for a variety of clinical and laboratory isolates of CMV (4). In addition, the analogs retained their activity against drug-resistant isolates with mutations in the UL97 or UL54 gene. It has also been reported that HDP-CDV and ODE-CDV had markedly enhanced activity in vitro against vaccinia and cowpox viruses (24) and were shown to have activity when administered orally in pharmacokinetic (9) and efficacy studies in mice (34).
Since there are no animal models that closely approximate human CMV (HCMV) disease, the surrogate viruses, MCMV, rat CMV (RCMV), and guinea pig CMV (GPCMV), have been used for preclinical evaluation of potential therapies for CMV infections (14, 22, 26, 41). We have used the murine model extensively for evaluation of new compounds and have reported previously that CDV has more activity than GCV (20, 21, 22).
The purpose of the experiments reported here was to compare the efficacies of HDP-CDV and ODE-CDV given orally with that of CDV given intraperitoneally (i.p.) for their ability to alter mortality and virus replication in target organs of mice infected with MCMV. Since CDV has been reported to have a long intracellular half-life (11) and can be efficacious when administered one to three times weekly (22, 33), we also determined the activities of these ether lipid esters when administered either once daily, twice weekly, or as a single dose.
MATERIALS AND METHODS
In vitro efficacy and toxicity.
The activities and toxicities of the four ether lipid esters of CDV in vitro were determined in primary murine embryo fibroblast (MEF) cells using standard plaque assay methods described previously (36).
Virus.
MCMV, strain Smith, was used both in vitro and in vivo.
Mice.
Female BALB/c mice, 3 weeks of age, were obtained from Charles River Laboratories, Raleigh, N.C. Mice were group housed in microisolator cages and utilized at a quantity of 15 mice per treatment group for statistical analysis. Mice were obtained, housed, utilized, and euthanized according to USDA and Association for Assessment and Accreditation of Laboratory Care regulatory policies. All animal procedures were approved by The University of Alabama at Birmingham Institutional Animal Care and Use Committee prior to initiation of the studies.
Antiviral compounds.
Cidofovir (Vistide; Gilead Pharmaceuticals, Foster City, Calif.) was diluted in sterile saline to yield the desired doses in 0.1-ml volumes. It was administered i.p. once daily for 5 days, two times weekly, or as a single dose, depending on the experimental protocol. HDP-CDV and ODE-CDV were synthesized, purified, and characterized as reported previously (4, 24) and provided as dry powders. OLE-CDV and OLP-CDV were synthesized by a similar methodology using oleyl bromide instead of hexadecyl bromide or octadecyl bromide. The structures of the compounds are shown in Fig. 1. The degree of purity was similar to that reported for HDP-CDV and ODE-CDV. The dry powders were weighed and dissolved in deionized water to yield the desired doses within 0.2-ml volumes for oral gavage. Each was administered orally as a single dose, twice weekly, or once daily for 5 days, depending on the experimental protocol. Uninfected mice served as toxicity controls for each compound and were treated similarly.
FIG. 1.
Structures of CDV, HDP-CDV, ODE-CDV, OLP-CDV, and OLE-CDV.
Experimental infections and viral pathogenesis.
MCMV infections were initiated by i.p. inoculation of BALB/c mice with an approximate 90% lethal dose and observed daily for 21 days. In certain experiments, samples of lung, liver, spleen, kidney, pancreas, salivary gland, and blood were obtained from three mice per treatment group or from control mice on day 1, 3, 5, 7, 10, 12, or 15 after MCMV infection. The samples were homogenized in medium (10%, wt/vol) and frozen at −70°C until they were assayed for virus (22).
Virus quantitation.
Tissue samples were thawed and assayed on MEF cells by plaque assay to determine MCMV titers (22). Briefly, samples of organ homogenates were diluted serially, and 0.2-ml volumes were placed into duplicate wells of 12-well plates containing MEF cell monolayers and incubated for 1 h. Medium containing 2% fetal bovine serum was added to each well, and the cultures were incubated for 7 days. The cultures were stained with neutral red (Gibco, Rockland, Md.) for approximately 6 h prior to enumeration of viral plaques.
Statistical evaluations.
Mortality rates were analyzed by Fisher's exact test, and mean days of death and virus titers in tissues were analyzed using the Mann-Whitney U rank sum test. A P value of ≤0.05 was considered significant.
RESULTS
In vitro activity of ether lipid esters of CDV.
It was reported previously that HDP-CDV and ODE-CDV were at least 100-fold more active than CDV against MCMV, RCMV, and GPCMV (4). For comparison purposes, these results, along with those for GCV and CDV, are included with those for the new analogs, oleyloxyethyl (OLE)-CDV and oleyloxypropyl (OLP)-CDV, in Table 1. In these studies, all four of the analogs were approximately 2- to 10-fold more active against MCMV than unmodified CDV. With the exception of OLP-CDV against RCMV, HDP-CDV, ODE-CDV, OLE-CDV, and OLP-CDV were 30- to 300-fold more active against RCMV and GPCMV than CDV.
TABLE 1.
In vitro activity of HDP-CDV, ODE-CDV, OLE-CDV or OLP-CDV against MCMV, RCMV, or GPCMV replication
| Compound | 50% Inhibitory concentration (μM)a against:
|
||
|---|---|---|---|
| MCMV | RCMV | GPCMV | |
| GCV | 4.3 ± 1.7 | 48.2 ± 1.1 | 239.0 ± 10.6 |
| CDV | 0.01 ± 0.01 | 0.3 ± 0.03 | 0.31 ± 0.003 |
| HDP-CDV | 0.0009 ± 0 | 0.004 ± 0.001 | 0.0009 ± 0.00007 |
| ODE-CDV | 0.001 ± 0 | 0.005 ± 0 | 0.0009 ± 0.00007 |
| OLE-CDV | 0.004 ± 0.004 | 0.008 ± 0.003 | 0.0013 ± 0.0003 |
| OLP-CDV | 0.004 ± 0.003 | 0.12 ± 0.13 | 0.007 ± 0.001 |
Values are expressed as means ± standard deviation from at least two plaque reduction assays.
Effect of daily treatment with HDP-CDV, ODE-CDV, OLE-CDV, or OLP-CDV on MCMV infections of mice.
To determine the abilities of these compounds to influence mortality rates in mice infected with MCMV, the compounds were dissolved in deionized water at doses of 20, 6.7, or 2 mg/kg of body weight and administered once daily for 5 consecutive days by oral gavage beginning 24, 48, or 72 h after viral inoculation. CDV was administered i.p. in similar doses for comparison, although it should be noted that the molecular weight of CDV is roughly 50% of that of the ether lipid conjugates of CDV. Toxicity was associated with the 20 mg/kg dose of HDP-CDV, ODE-CDV, OLE-CDV, or OLP-CDV, and some mortality and weight loss were observed (data not shown). At the 6.7 or 2.0 mg/kg dose, no toxicity was observed, and each compound significantly reduced final mortality rates when treatment was initiated at 24 to 48 h postinfection (Table 2). Only CDV at 6.7 mg/kg significantly reduced mortality rates when treatment was initiated at 72 h after viral inoculation (data not shown). Based on these data and other characteristics of the four analogs, HDP-CDV and ODE-CDV were selected for additional evaluation.
TABLE 2.
Effect of daily oral treatment with HDP-CDV, ODE-CDV, OLE-CDV, or OLP-CDV on mortality of BALB/c mice inoculated i.p. with MCMV
| Time of initiation and treatment (mg/kg)a | No. of mice died/total no. (%) | Mortality result P valueb | MDDc | MDD P valueb |
|---|---|---|---|---|
| 24 h | ||||
| i.p. saline placebo | 12/15 (80) | 6.3 | ||
| Oral water placebo | 8/15 (53) | 5.6 | ||
| CDV, 6.7 | 0/15 (0) | <0.001 | ||
| CDV, 2.0 | 0/15 (0) | <0.001 | ||
| HDP-CDV, 6.7 | 2/15 (13) | 0.02 | 3.5 | <0.05 |
| HDP-CDV, 2.0 | 3/15 (20) | NS | 4.0 | 0.07 |
| ODE-CDV, 6.7 | 0/15 (0) | <0.001 | ||
| ODE-CDV, 2.0 | 1/15 (7) | 0.01 | 5.0 | NS |
| OLE-CDV, 6.7 | 1/15 (7) | 0.01 | 6.0 | NS |
| OLE-CDV, 2.0 | 0/15 (0) | <0.001 | ||
| OLP-CDV, 6.7 | 0/15 (0) | <0.001 | ||
| OLP-CDV, 2.0 | 1/15 (7) | 0.01 | 4.0 | NS |
| 48 h | ||||
| i.p. saline placebo | 15/15 (100) | 5.1 | ||
| Oral water placebo | 15/15 (100) | <0.001 | 5.3 | |
| CDV, 6.7 | 0/15 (0) | <0.001 | ||
| CDV, 2.0 | 0/15 (0) | <0.001 | ||
| HDP-CDV, 6.7 | 0/15 (0) | <0.001 | ||
| HDP-CDV, 2.0 | 2/15 (13) | <0.001 | 6.0 | NS |
| ODE-CDV, 6.7 | 5/15 (33) | <0.001 | 10.2 | <0.001 |
| ODE-CDV, 2.0 | 0/15 (0) | <0.001 | ||
| OLE-CDV, 6.7 | 0/15 (0) | <0.001 | ||
| OLE-CDV, 2.0 | 3/15 (20) | <0.001 | 8.0 | 0.09 |
| OLP-CDV, 6.7 | 1/15 (7) | <0.001 | 8.0 | 0.06 |
| OLP-CDV, 2.0 | 2/16 (13) | <0.001 | 7.5 | NS |
Compounds were prepared daily in water and delivered orally in 0.2-ml doses, except CDV, which was prepared in sterile saline and delivered i.p. in 0.1-ml doses. Animals were treated once daily for 5 days beginning 24 or 48 h after viral inoculation.
P values were determined by comparing results for drug treatment groups with results for placebo control groups. NS, not significant compared to placebo controls.
MDD, mean day of death.
Effect of twice-weekly treatment with HDP-CDV or ODE-CDV on MCMV infections of mice.
CDV has also been reported to significantly reduce mortality of cowpox or vaccinia virus-infected mice with only one or two doses due to the long intracellular half-life of this drug (33). CDV, HDP-CDV, or ODE-CDV was given at 10, 5 (results not presented), 2.5, or 1.25 mg/kg twice weekly, 3 days apart beginning 24, 48, or 72 h after viral inoculation. Oral treatment with HDP-CDV or ODE-CDV provided significant protection from mortality when initiation of treatment at concentrations as low as 1.25 mg/kg was delayed until 24 to 48 h postinoculation (Table 3). Again, CDV given i.p. was the most active drug when treatment was delayed until 72 h postinfection.
TABLE 3.
Effect of twice-weekly oral treatment with HDP-CDV or ODE-CDV on mortality of BALB/c mice inoculated i.p. with MCMV
| Time of initiation and treatment (mg/kg)a | No. of mice died/total no. (%) | Mortality result P valueb | MDDc | MDD P valueb |
|---|---|---|---|---|
| 24 h | ||||
| Oral water placebo | 15/15 (100) | 4.5 | ||
| i.p. saline placebo | 15/15 (100) | 5.4 | ||
| CDV, 2.5 | 0/15 (0) | <0.001 | ||
| CDV, 1.25 | 1/15 (7) | <0.001 | 10.0 | 0.08 |
| HDP-CDV, 2.5 | 0/15 (0) | <0.001 | ||
| HDP-CDV, 1.25 | 3/15 (20) | <0.001 | 4.0 | NS |
| ODE-CDV, 2.5 | 4/15 (27) | <0.001 | 4.8 | NS |
| ODE-CDV, 1.25 | 1/15 (7) | <0.001 | 12.0 | 0.07 |
| 48 h | ||||
| Oral water placebo | 14/14 (100) | 4.8 | ||
| i.p. saline placebo | 15/15 (100) | 5.5 | ||
| CDV, 2.5 | 3/15 (20) | <0.001 | 4.7 | 0.07 |
| CDV, 1.25 | 1/15 (7) | <0.001 | 7.0 | 0.07 |
| HDP-CDV, 2.5 | 4/13 (31) | <0.001 | 4.8 | NS |
| HDP-CDV, 1.25 | 12/15 (80) | NS | 4.3 | NS |
| ODE-CDV, 2.5 | 3/14 (21) | <0.001 | 3.0 | <0.05 |
| ODE-CDV, 1.25 | 3/15 (20) | <0.001 | 4.0 | NS |
| 72 h | ||||
| Oral water placebo | 14/15 (93) | 5.1 | ||
| i.p. saline placebo | 15/15 (100) | 4.9 | ||
| CDV, 2.5 | 1/15 (7) | <0.001 | 7.0 | 0.06 |
| CDV, 1.25 | 4/15 (27) | <0.001 | 6.5 | <0.01 |
| HDP-CDV, 2.5 | 12/15 (80) | NS | 6.0 | <0.05 |
| HDP-CDV, 1.25 | 8/15 (53) | <0.01 | 6.8 | <0.05 |
| ODE-CDV, 2.5 | 11/15 (73) | <0.05 | 4.3 | NS |
| ODE-CDV, 1.25 | 15/15 (100) | NS | 6.5 | 0.001 |
CDV was prepared in sterile saline and delivered i.p. in 0.1-ml doses. HDP-CDV and ODE-CDV were prepared in deionized water and delivered orally in 0.2-ml doses. Animals were treated twice weekly beginning 24, 48, or 72 h after viral inoculation.
P values were determined by comparing results for drug treatment groups with results for placebo control groups. NS, not significant compared to placebo controls.
MDD, mean day of death.
Effect of single-dose treatment with HDP-CDV or ODE-CDV on MCMV infections of mice.
In the next experiment, CDV was given i.p., and HDP-CDV or ODE-CDV was given orally as a single dose of 30, 10, or 3 mg/kg beginning 24, 48, or 72 h after viral inoculation. Most of the treatment regimens used provided significant protection from mortality when initiated up to 48 h postinfection. When treatment was initiated at 72 h, little protection was observed. CDV significantly inhibited mortality rates when 30 mg/kg was given at 72 h after infection (Table 4).
TABLE 4.
Effect of single-dose oral treatment with HDP-CDV or ODE-CDV on mortality of BALB/c mice inoculated i.p. with MCMV
| Expt and time of initiation | Treatment (mg/kg)a | No. of mice died/total no. (%) | Mortality result P valueb | MDDc | MDD P valueb |
|---|---|---|---|---|---|
| 1 | |||||
| 24 h | Placebo | 14/15 (93) | 5.5 | ||
| HDP-CDV, 30 | 0/15 (0) | <0.001 | |||
| HDP-CDV, 10 | 0/15 (0) | <0.001 | |||
| HDP-CDV, 3 | 0/15 (0) | <0.001 | |||
| 48 h | Placebo | 12/15 (80) | 4.9 | ||
| HDP-CDV, 30 | 1/15 (7) | <0.001 | 2.0 | 0.05 | |
| HDP-CDV, 10 | 2/15 (13) | 0.001 | 3.0 | 0.01 | |
| HDP-CDV, 3 | 0/15 (0) | <0.001 | |||
| 72 h | Placebo | 14/15 (93) | 5.1 | ||
| HDP-CDV, 30 | 7/15 (47) | 0.01 | 5.9 | NS | |
| HDP-CDV, 10 | 10/15 (67) | NS | 6.8 | NS | |
| HDP-CDV, 3 | 13/15 (87) | NS | 5.6 | NS | |
| 2 | |||||
| 24 h | Placebo | 12/15 (80) | 5.1 | ||
| CDV, 30 | 0/15 (0) | <0.001 | |||
| ODE-CDV, 30 | 1/15 (7) | <0.01 | 2.0 | NS | |
| ODE-CDV, 10 | 0/15 (0) | <0.001 | |||
| ODE-CDV, 3 | 1/15 (7) | <0.001 | 3.0 | NS | |
| 48 h | Placebo | 15/15 (100) | 5.0 | NS | |
| CDV, 30 | 2/15 (13) | 0.001 | 9.5 | NS | |
| ODE-CDV, 30 | 15/15 (100) | NS | 7.0 | <0.001 | |
| ODE-CDV, 10 | 4/15 (27) | <0.001 | 3.8 | <0.01 | |
| ODE-CDV, 3 | 1/15 (7) | <0.001 | 5.0 | NS | |
| 72 h | Placebo | 8/12 (67) | 5.0 | ||
| CDV, 30 | 0/15 (0) | <0.001 | |||
| ODE-CDV, 30 | 11/14 (79) | NS | 7.2 | 0.01 | |
| ODE-CDV, 10 | 5/13 (38) | NS | 5.2 | NS | |
| ODE-CDV, 3 | 11/15 (73) | NS | 5.0 | NS |
CDV was prepared in sterile saline and delivered i.p. in 0.1-ml doses. HDP-CDV or ODE-CDV was prepared in deionized water and delivered orally in 0.2-ml doses. Animals were treated once beginning 24, 48, or 72 h after viral inoculation.
P values were determined by comparing results for drug treatment groups with results for placebo control groups. NS, not significant compared to placebo controls.
MDD, mean day of death.
Effect of CDV, HDP-CDV, or ODE-CDV on the pathogenesis of MCMV infections of mice.
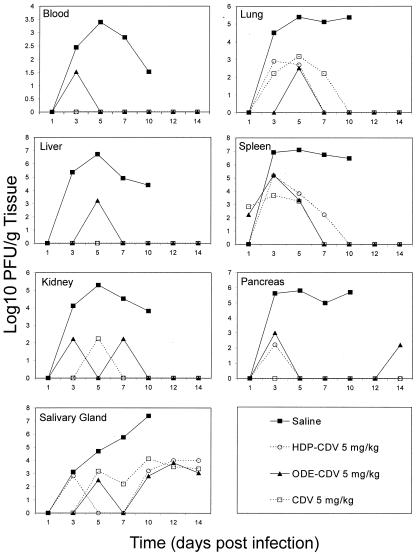
To compare the effect of treatment with oral HDP-CDV or ODE-CDV on the replication of MCMV in target organs of mice to that of treatment with i.p. CDV, animals were inoculated with MCMV and treated with CDV, HDP-CDV, or ODE-CDV at 5 mg/kg once daily for 5 days beginning 24 h after infection. On various days postinfection, animals were euthanized, and tissues were removed and assayed for MCMV. All of the treatment regimens resulted in significant reduction in mortality (P < 0.001) (data not presented) and 3 to 5 log10 decreases in virus titers in lung, liver, spleen, kidney, pancreas, salivary gland, and blood. The results observed with oral HDP-CDV or ODE-CDV indicated that they were at least as effective as i.p. CDV (Fig. 2).
FIG. 2.
Effect of daily oral treatment with HDP-CDV, ODE-CDV, or CDV on the pathogenesis of MCMV infection in mice. Treatment was initiated 24 h after viral inoculation and continued once daily for 5 consecutive days. Points represent the mean numbers of log10 PFU per gram of tissue.
DISCUSSION
Since HCMV does not generally replicate in tissues of nonhuman origin, there are few animal models for evaluation of new antivirals for CMV infections. There have been a number of models used in which immunocompromised mice have been implanted with CMV-infected cells (2, 31) or human tissue implants that could subsequently be infected with HCMV (5, 7, 23, 42). With the models in which human fetal tissue is implanted either into the eye or under the kidney capsule of SCID mice, the implants infected with HCMV and animals treated with experimental compounds have been particularly useful and appear to be predictive of the outcome in humans (23). However, the models are too expensive and labor intensive to use for screening new agents.
There are a number of natural CMV infections in animals, including mice (14), rats (40), and guinea pigs (26), that have been utilized for antiviral evaluation. We have utilized both acute lethal and chronic nonlethal MCMV-infected mice for evaluation of a number of antiviral agents, including ACV, GCV, PFA, and CDV (14, 20, 21, 22, 30, 36). In MCMV-infected mice, the virus replicates in lung, liver, spleen, kidney, intestine, pancreas, adrenals, and salivary glands and persists in most tissues for 1 to 2 months and in salivary glands for more than 3 months. This infection has many similarities to chronic CMV infection in humans and is an ideal model for evaluation of new antiviral agents. The approved drugs PFA, GCV, and CDV all had good activity in this infection model (22).
CDV is very active in this infection model and is approved for use in CMV retinitis in AIDS patients. The drug, however, has the major limitation of nephrotoxicity and is not bioavailable when administered orally, which makes long-term administration difficult. It was reported previously that a series of ether lipid esters of CDV had multiple-log increases in activity in vitro against MCMV and HCMV compared with the parent CDV or cyclic CDV (4). Similarly enhanced activities were also reported for vaccinia virus and cowpox virus (24), herpes simplex virus, Epstein-Barr virus, and human herpesvirus type 6 (43). The enhanced activity in vitro appears to be due to increased cellular uptake due to the lipid moiety. Intracellular levels of CDV are increased about 16-fold, and the long intracellular half-life of CDV is also manifested by HDP-CDV (1).
In pharmacokinetic studies in mice, HDP-CDV and ODE-CDV were shown to be bioavailable after oral administration. After oral administration of these prodrugs, plasma and tissue levels of CDV were higher and persisted longer than subcutaneously administered CDV. Of particular interest was the fact that the highest drug levels were in lung, liver, and spleen, but levels in kidney were considerably lower (9). These observations suggested that the ester analogs may be less nephrotoxic than parenteral CDV, since there is less drug accumulation in kidneys. In addition, the higher drug levels in lung and liver are encouraging, as these are tissues in which high levels of HCMV are found in infection in humans. However, it is possible that toxicity manifestations in these tissues may result from the increased drug levels.
A very similar set of results with these compounds against vaccinia and cowpox viruses has been reported previously, showing activity that is highly increased in vitro (24) and good when delivered orally in mice but not highly superior to that of CDV (34). Although in the poxvirus models it was possible to significantly reduce virus replication in liver, spleen, and kidney, virus replication was not reduced in lung. In contrast, in the MCMV infection, treatments with these same compounds at similar doses were highly effective in reducing replication in all tissues, including the lung. The difference seen in the lung between the two models is apparently due to the increased susceptibility of MCMV to CDV in vitro over that seen with vaccinia and cowpox virus (4, 24) and indicates that drug levels in tissues and virus susceptibility are both important variables that determine in vivo efficacy. Although it was not part of these studies, it would be of interest to determine if HDP-CDV and ODE-CDV have enhanced activity against CDV-resistant MCMV (38) in tissue culture cells and to correlate the in vitro activity with alteration of virus titers in tissues of mice infected with the resistant virus.
In the present studies, HDP-CDV and ODE-CDV given orally to mice infected with MCMV were highly effective in preventing mortality and reducing virus replication in all target organs tested. Administration of the drugs once daily, twice weekly, or as a single dose resulted in significant protection. Additionally, administration of the drugs could be delayed until 48 to 72 h postinfection and still significantly reduce mortality. It is interesting that, although the analogs are 10- to 100-fold more active than CDV in vitro, the level of protection obtained in vivo was very similar to that obtained with CDV given i.p. It should be pointed out, however, that when drug concentrations were compared on a molar basis, the ester analogs were found to be four to eight times as active as CDV due to the increased formula weight of the lipid. The possibility of being able to dose compounds such as these orally and only once or twice weekly would be a tremendous advantage in treatment of chronic viral infections such as CMV. This would greatly increase the flexibility of prophylactic, preemptive, or treatment regimens for these infections, particularly in transplant patients. Taking all of the experiments into account, there was no clear difference in the efficacies of HDP-CDV and ODE-CDV based on their ability to protect mice from mortality or reduce viral replication in target organs, and the two compounds were equally effective when treatment frequency was reduced or onset was delayed. The selection of one of these compounds to be developed for use in clinical studies will undoubtedly be made using additional criteria such as drug adsorption, distribution, metabolism, excretion, and toxicity.
We have also extended these studies to evaluation of the efficacies of HDP-CDV and ODE-CDV in two models of HCMV using SCID mice implanted either with human retinal tissue in the eye or with thymus and liver tissue under the kidney capsule. In both models, HDP-CDV and ODE-CDV given orally significantly reduced HCMV replication in HCMV-infected human implant tissue (5). The results obtained for mice infected with MCMV along with those obtained for both the HCMV-SCID-hu and orthopoxvirus-infected BALB/c mice indicate that these ether lipid ester analogs of CDV are extremely potent in vitro, have excellent bioavailability when given orally to mice, and are highly effective in these animal models. Oral administration of these analogs was at least as protective as parenteral CDV. These studies indicate that these new analogs need to be evaluated further to determine if they have a better toxicological profile than parenteral CDV, and if so, they need to be evaluated for use in CMV infections in humans.
Acknowledgments
These studies were supported by Public Health Services Contracts NO1-AI-15439 and NO1-AI-85347 from NIAID, NIH to the University of Alabama at Birmingham; grants Ey-11832 and AI-21696 to the San Diego Veterans Medical Research Federation; and Department of Defense Grant DAMD 17-01-2-0091 from the U.S. Army Medical Research and Material Command.
We thank Caroll Hartline and Bridgett Herrod for excellent technical assistance.
REFERENCES
- 1.Aldern, K. A., S. L. Ciesla, K. L. Winegarden, and K. Y. Hostetler. 2003. Increased antiviral activity of 1-O-hexadecyloxypropyl-[2-14C]cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol. Pharmacol. 63:678-681. [DOI] [PubMed] [Google Scholar]
- 2.Allen, L. B., S. X. Li, G. Arnett, B. Toyer, W. M. Shannon, and M. G. Hollingshead. 1992. Novel method for evaluating antiviral drugs against human cytomegalovirus in mice. Antimicrob. Agents Chemother. 36:206-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balfour, H. H., Jr., B. A. Chace, J. T. Stapleton, R. L. Simmons, and D. S. Fryd. 1989. A randomized, placebo-controlled trial of oral acyclovir for the prevention of cytomegalovirus disease in recipients of renal allografts. N. Engl. J. Med. 320:1381-1387. [DOI] [PubMed] [Google Scholar]
- 4.Beadle, J. R., C. Hartline, K. A. Aldern, N. Rodriguez, E. Harden, E. R. Kern, and K. Y. Hostetler. 2002. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob. Agents Chemother. 46:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bidanset, D. J., J. R. Beadle, W. B. Wan, K. Y. Hostetler, and E. R. Kern. 2004. Oral activity of ether lipid ester prodrugs of cidofovir against experimental human cytomegalovirus infections. J. Infect. Dis. 190:499-503. [DOI] [PubMed] [Google Scholar]
- 6.Boivin, G., C. Gilbert, A. Gaudreau, I. Greenfield, R. Sudlow, and N. A. Roberts. 2001. Rate of emergence of cytomegalovirus (CMV) mutations in leukocytes of patients with acquired immunodeficiency syndrome who are receiving valganciclovir as induction and maintenance therapy for CMV retinitis. J. Infect. Dis. 184:1598-1602. [DOI] [PubMed] [Google Scholar]
- 7.Brown, J. M., H. Kaneshima, and E. S. Mocarski. 1995. Dramatic interstrain differences in the replication of human cytomegalovirus in SCID-hu mice. J. Infect. Dis. 171:1599-1603. [DOI] [PubMed] [Google Scholar]
- 8.Chou, S., N. S. Lurain, K. D. Thompson, R. C. Miner, and W. L. Drew. 2003. Viral DNA polymerase mutations associated with drug resistance in human cytomegalovirus. J. Infect. Dis. 188:32-39. [DOI] [PubMed] [Google Scholar]
- 9.Ciesla, S. L., J. Trahan, W. B. Wan, J. R. Beadle, K. A. Aldern, G. R. Painter, and K. Y. Hostetler. 2003. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antivir. Res. 59:163-171. [DOI] [PubMed] [Google Scholar]
- 10.Crippa, F., L. Corey, E. L. Chuang, G. Sale, and M. Boeckh. 2001. Virological, clinical, and ophthalmologic features of cytomegalovirus retinitis after hematopoietic stem cell transplantation. Clin. Infect. Dis. 32:214-219. [DOI] [PubMed] [Google Scholar]
- 11.Cundy, K. C., Z. H. Li, M. J. Hitchcock, and W. A. Lee. 1996. Pharmacokinetics of cidofovir in monkeys. Evidence for a prolonged elimination phase representing phosphorylated drug. Drug Metab. Dispos. 24:738-744. [PubMed] [Google Scholar]
- 12.Enright, H., R. Haake, D. Weisdorf, N. Ramsay, P. McGlave, J. Kersey, W. Thomas, D. McKenzie, and W. Miller. 1993. Cytomegalovirus pneumonia after bone marrow transplantation. Risk factors and response to therapy. Transplantation 55:1339-1346. [DOI] [PubMed] [Google Scholar]
- 13.Erice, A., N. Borrell, W. Li, W. J. Miller, and H. H. Balfour, Jr. 1998. Ganciclovir susceptibilities and analysis of UL97 region in cytomegalovirus (CMV) isolates from bone marrow recipients with CMV disease after antiviral prophylaxis. J. Infect. Dis. 178:531-534. [DOI] [PubMed] [Google Scholar]
- 14.Glasgow, L. A., J. T. Richards, and E. R. Kern. 1982. Effect of acyclovir treatment on acute and chronic murine cytomegalovirus infection. Am. J. Med. 73:132-137. [DOI] [PubMed] [Google Scholar]
- 15.Goodrich, J. M., R. A. Bowden, L. Fisher, C. Keller, G. Schoch, and J. D. Meyers. 1993. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann. Intern. Med. 118:173-178. [DOI] [PubMed] [Google Scholar]
- 16.Hitchcock, M. J., H. S. Jaffe, J. C. Martin, and R. J. Stagg. 1996. Cidofovir, a new agent with potent anti-herpesvirus activity. Antivir. Chem. Chemother. 7:115-127. [Google Scholar]
- 17.Hostetler, K. Y., J. R. Beadle, G. D. Kini, M. F. Gardner, K. N. Wright, T. H. Wu, and B. A. Korba. 1997. Enhanced oral absorption and antiviral activity of 1-O-octadecyl-sn-glycero-3-phospho-acyclovir and related compounds in hepatitis B virus infection, in vitro. Biochem. Pharmacol. 53:1815-1822. [DOI] [PubMed] [Google Scholar]
- 18.Hostetler, K. Y., J. R. Beadle, W. E. Hornbuckle, C. A. Bellezza, I. A. Tochkov, P. J. Cote, J. L. Gerin, B. E. Korba, and B. C. Tennant. 2000. Antiviral activities of oral 1-O-hexadecylpropanediol-3-phosphoacyclovir and acyclovir in woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob. Agents Chemother. 44:1964-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hostetler, K. Y., R. J. Rybak, J. R. Beadle, M. F. Gardner, K. A. Aldern, K. N. Wright, and E. R. Kern. 2001. In vitro and in vivo activity of 1-O-hexadecylpropanediol-3-phospho-ganciclovir and 1-O-hexadecylpropanediol-3- phospho-penciclovir in cytomegalovirus and herpes simplex virus infections. Antivir. Chem. Chemother. 12:61-70. [DOI] [PubMed] [Google Scholar]
- 20.Kern, E. R. 1991. Value of animal models to evaluate agents with potential activity against human cytomegalovirus. Transplant Proc. 23:152-155. [PubMed] [Google Scholar]
- 21.Kern, E. R. 1997. Preclinical evaluation of antiviral agents, p. 79-111. In G. J. Galasso, R. J. Whitley, and T. C. Merigan (ed.), Antiviral agents and human viral diseases, 4th ed. Raven Press, New York, N.Y.
- 22.Kern, E. R. 1999. Animal models for cytomegalovirus infections: murine CMV, p. 927-934. In O. Zak, M. Sande, C. Carbon, R. Kaninsky, T. O'Reilly, and E. R. Kern (ed.), Handbook on animal models for antimicrobial therapy. Academic Press, London, England.
- 23.Kern, E. R., R. J. Rybak, C. B. Hartline, and D. J. Bidanset. 2001. Predictive efficacy of SCID-hu mouse models for treatment of human cytomegalovirus infections. Antivir. Chem. Chemother. 12(Suppl. 1):149-156. [PubMed] [Google Scholar]
- 24.Kern, E. R., C. Hartline, E. Harden, K. Keith, N. Rodriguez, J. R. Beadle, and K. Y. Hostetler. 2002. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 46:991-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lalezari, J. P., and B. D. Kuppermann. 1997. Clinical experience with cidofovir in the treatment of cytomegalovirus retinitis. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 14(Suppl. 1):S27-S31. [DOI] [PubMed] [Google Scholar]
- 26.Li, S. B., Z. H. Yang, J. S. Feng, C. K. Fong, H. L. Lucia, and G. D. Hsiung. 1990. Activity of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) against guinea pig cytomegalovirus infection in cultured cells and in guinea pigs. Antivir. Res. 13:237-252. [DOI] [PubMed] [Google Scholar]
- 27.Limaye, A. P., L. Corey, D. M. Koelle, C. L. Davis, and M. Boeckh. 2000. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet 356:645-649. [DOI] [PubMed] [Google Scholar]
- 28.Limaye, A. P., G. Raghu, D. M. Koelle, J. Ferrenberg, M. L. Huang, and M. Boeckh. 2002. High incidence of ganciclovir-resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy. J. Infect. Dis. 185:20-27. [DOI] [PubMed] [Google Scholar]
- 29.Lowance, D., H. H. Neumayer, C. M. Legendre, J. P. Squifflet, J. Kovarik, P. J. Brennan, D. Norman, R. Mendez, M. R. Keating, G. L. Coggon, A. Crisp, I. C. Lee, et al. 1999. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. N. Engl. J. Med. 340:1462-1470. [DOI] [PubMed] [Google Scholar]
- 30.Overall, J. C., Jr., E. R. Kern, and L. A. Glasgow. 1976. Effective antiviral chemotherapy in cytomegalovirus infection of mice. J. Infect. Dis. 133(Suppl.):A237-A244. [DOI] [PubMed] [Google Scholar]
- 31.Pari, G. S., D. Netski, S. St. Jeor, D. McCarthy, J. Smith, D. Georgio, and E. von Hofe. 1998. Generation of a nude mouse tumor model for in vivo replication of human cytomegalovirus. J. Infect. Dis. 177:523-528. [DOI] [PubMed] [Google Scholar]
- 32.Paya, C. V., J. A. Wilson, M. J. Espy, I. G. Sia, M. J. DeBernardi, T. F. Smith, R. Patel, G. Jenkins, W. S. Harmsen, D. J. Vanness, and R. H. Wiesner. 2002. Preemptive use of oral ganciclovir to prevent cytomegalovirus infection in liver transplant patients: a randomized, placebo-controlled trial. J. Infect. Dis. 185:854-860. [DOI] [PubMed] [Google Scholar]
- 33.Quenelle, D. C., D. J. Collins, and E. R. Kern. 2003. Efficacy of multiple- or single-dose cidofovir against vaccinia and cowpox virus infections in mice. Antimicrob. Agents Chemother. 47:3275-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quenelle, D. C., D. J. Collins, W. B. Wan, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2004. Oral treatment of cowpox and vaccinia virus infection in mice with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 48:404-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubin, R. H. 1990. Impact of cytomegalovirus infection on organ transplant recipients. Rev. Infect. Dis. 12(Suppl. 7):S754-S766. [DOI] [PubMed] [Google Scholar]
- 36.Rybak, R. J., J. Zemlicka, Y. L. Qiu, C. B. Hartline, and E. R. Kern. 1999. Effective treatment of murine cytomegalovirus infections with methylenecyclopropane analogues of nucleosides. Antivir. Res. 43:175-188. [DOI] [PubMed] [Google Scholar]
- 37.Safrin, S., J. Cherrington, and H. S. Jaffe. 1997. Clinical uses of cidofovir. Rev. Med. Virol. 7:145-156. [DOI] [PubMed] [Google Scholar]
- 38.Smee, D. F., B. B. Barnett, R. W. Sidwell, E. J. Reist, and A. Holý. 1995. Antiviral activities of nucleosides and nucleotides against wild-type and drug-resistant strains of murine cytomegaloviruses. Antivir. Res. 26:1-9. [DOI] [PubMed] [Google Scholar]
- 39.Smith, I. L., J. M. Cherrington, R. E. Jiles, M. D. Fuller, W. R. Freeman, and S. A. Spector. 1997. High-level resistance of cytomegalovirus to ganciclovir is associated with alterations in both the UL97 and DNA polymerase genes. J. Infect. Dis. 176:69-77. [DOI] [PubMed] [Google Scholar]
- 40.Snoeck, R., T. Sakuma, E. De Clercq, I. Rosenberg, and A. Holy. 1988. (S)-1-(3-Hydroxy-2-phosphonylmethoxypropyl)cytosine, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob. Agents Chemother. 32:1839-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stals, F. S., E. de Clercq, and C. A. Bruggeman. 1991. Comparative activity of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine and 9-(1,3-dihydroxy-2-propoxymethyl)guanine against rat cytomegalovirus infection in vitro and in vivo. Antimicrob. Agents Chemother. 35:2262-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weber, O., J. Reefschlager, H. Rubsamen-Waigmann, S. Raddatz, M. Hesseling, and D. Habich. 2000. A novel peptide aldehyde with activity against human cytomegalovirus in two different in vivo models. Antivir. Chem. Chemother. 11:51-59. [DOI] [PubMed] [Google Scholar]
- 43.Williams, S. L., C. B. Hartline, N. L. Kushner, E. A. Harden, J. R. Beadle, W. B. Wan, K. Y. Hostetler, and E. R. Kern. 2003. In vitro activity of alkoxyalkyl esters of cidofovir against replication of herpesvirus. Antivir. Res. 57:A61. [Google Scholar]