Abstract
Ruxolitinib (INCB018424) is the first potent, selective, oral inhibitor of JAK1 and 2 being developed for clinical use. Its major cellular and systemic effects are proliferation inhibition, apoptosis induction and reduction in cytokine plasma levels, all mediated by the drug's inhibition of JAKs' ability to phosphorylate STAT. In initial clinical trials of its use in myelofibrosis, ruxolitinib exhibited durable efficacy in reduction of splenomegaly and alleviation of constitutional symptoms. Patients also showed weight gain and improvement in general physical condition. The dose-limiting toxicity was thrombocytopenia. In preliminary findings of a Phase III trial in patients with primary, postpolycythemia-vera, or postessential- thrombocythemia myelofibrosis, administration at an initial dosage of 15 or 20 mg twice daily led to a spleen-volume response rate (≥35% reduction at 24 weeks) of 41.9 versus 0.7% for placebo (p < 0.0001); furthermore, 45.9% of the ruxolitinib recipients had ≥50% improvement in symptom score (on the modified Myelofibrosis Symptom Assessment Form version 2.0) versus 5.3% for placebo (p < 0.0001). Ruxolitinib recipients also showed improvement in parameters of quality of life.
Keywords: JAK2 inhibitor, JAK2V617F mutation, myelofibrosis, ruxolitinib
The incidence of primary myelofibrosis (PMF) ranges from 0.3 to 1.5 per 100,000 [1–6], and studies [7–13] show that by the end of the second decade after diagnosis, up to 30% of patients with either polycythemia vera (PV) or essential thrombocythemia (ET) develop transformation to secondary myelofibrosis (MF).
Lots of efforts have been made to elucidate the biomolecular pathophysiology underlying MF in all these forms and JAKs became the focus of research in the second half of the last decade. JAKs play critical roles in several important intracellular signaling pathways, including the JAK-signal transducer and activator of transcription (STAT) pathway triggered by cytokine and growth-factor receptors [14,15]. To date, four members of the JAK family have been identified: JAK1, JAK2, JAK3 and nonreceptor tyrosine kinase 2 (TYK2). Since the gain-of- function JAK2V617F mutation is found only in approximately 50% of MF patients, this mutation is not inherently necessary for MF development, but when present, it plays an important role in the molecular pathogenesis of MF. Some of the clinical features of the disease, such as anemia, splenomegaly, and also the risk of transformation to acute myeloid leukemia (AML), may be related to JAK2V617F mutational status or to the relative level of JAK2V617F - positive DNA compared with total JAK2 DNA (JAKV617F allele burden) [16–18]. In peripheral blood mononuclear cells of patients with MF, hyperactivation of JAK1 is also found and could be a consequence of high levels of circulating inflammatory cytokines [19]. An MPL515 mutation of the thrombopoietin receptor has the same consequences and it is found in 5% of MF patients [20,21]. Other mutations that can activate JAK2, such as LNK [22] and CBL [23], have been observed in some MF. Even without identified mutations, JAK2 activation has been demonstrated in the majority of MF patients.
The main debilitating factors in MF are severe splenomegaly, leukocytosis and thrombocytemia with predisposition to thrombotic events, as well as cytopenias and high levels of proinflammatory cytokines. In the majority of MF patients these are usually accompanied with constitutional symptoms such as fatigue, weight loss, low-grade fever and night sweats. To date, only allogeneic hematopoietic stem cell transplantation (allo-HSCT) is considered potentially curative in MF. However, because of high transplant-related mortality, as seen even with use of reduced-intensity conditioning, allo-HSCT is currently appropriate only for a small subset of patients (i.e., younger, sufficiently healthy patients with high-risk MF and for whom a suitable donor is available) [24]. Other treatment options are utilizied mainly to alleviate organomegaly and to achieve some level of control of leukocytosis or thrombocytosis (i.e., hydroxyurea, also known as hydroxycarbamide) and cytopenia (i.e., lenalidomide or low-dose thalidomide and prednisone). For patients with drug-refractory splenomegaly, splenectomy remains an option. However, procedure-related mortality remains approximately 9% [25]. Another possibility is spleen irradiation; however, if spleen size reduction is achieved, the effect is usually transient [26]. Palliation of constitutional symptoms remains challenging [27,28].
Overview of the market
Since the initial reports of JAK2V617F mutation in myeloproliferative neoplasms (MPNs) [29–32], several pharmaceutical companies have developed compounds with inhibitory properties against JAK family members. Four such drugs – ruxolitinib, TG101348, lestaurtinib and XL019 – were first tested in patients with intermediate or high-risk PMF or post-PV/ post-ET MF [33]. Other JAK inhibitors, such as SB1518, CYT387 and AZD148, are in development [34–36]. None have been marketed yet, but among them, ruxolitinib has advanced the farthest in its clinical development.
Ruxolitinib (INCB018424)
Ruxolitinib (formerly known as INCB018424) is a JAK1 and JAK2 inhibitor developed by Incyte Corporation (Wilmington, DE, USA). It is the first potent, selective, oral JAK1/JAK2 inhibitor being developed for clinical use. Incyte has the rights to its development and commercialization in the USA, while Novartis AG (Switzerland) has acquired these rights for territories outside the USA [101,102].
Chemistry
Ruxolitinib ([3R]-3-cyclopentyl-3-[4-(7H- pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]-propanenitrile) is an orally bioavailable cyclopentylpropionitrile derivative (Figure 1) that acts as an ATP-competitive inhibitor primarily against JAK1 and JAK2 [19,37]. In MF, its mechanism of action appears to be modulation of cytokine function, as achieved by inhibition of the JAK1/2-mediated downstream pathways [38].
Figure 1. Ruxolitinib.
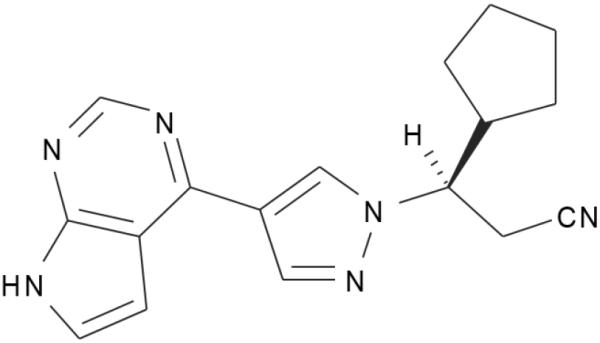
As a specific inhibitor of JAK1 and JAK2, the drug targets the ATP- binding pocket of each kinase, including the mutated JAK2V617F form.
Pharmacodynamics & preclinical studies
In preclinical studies, and specifically in `naked' kinase in vitro assay systems, ruxolitinib demonstrated inhibitory effects against JAK1 and JAK2 (with a half-maximal inhibitory concentration [IC50] of 3.3 and 2.8 nM, respectively), moderate inhibitory activity against TYK2 (with an IC50 of 19 nM), and minimal inhibitory activity against JAK3 (with an IC50 of 428 nM) or multiple other kinases [19,37]. In peripheral blood mononuclear cells, it inhibited the ability of IL-6 to induce STAT3 phosphorylation and the production of monocyte chemoattractant protein-1 [39]. It also inhibited the proliferation of factor-dependent cell progenitor (FDCP) cells and cells of the pro-B-cell Ba/F3 line expressing JAK2V617F (with an IC50 of 100–130 nM, the latter values seen in the context of whole-cell assays). The effect was absent in cell lines expressing activating mutations in the oncogene products BCR-ABL or c-Kit (IC50 >25 and >4 ∝M, respectively).
JAK2, following activation by erythropoietin, thrombopoietin and interleukin receptors, activates downstream pathways that control erythroid, myeloid and megakaryocytic development [40,41]. Ruxolitinib inhibits both mutant and wild-type JAK2. Correlation between inhibited cell proliferation and reduced intracellular levels of phosphorylated JAK2 and STAT5 in the Ba/F3 cell model was highly suggestive evidence that such inhibition might be a pharmacological mechanism of ruxolitinib. Indeed, ruxolitinib showed higher antiproliferative potency in cells from JAK2V617F - positive PV patients cultured in the absence of cytokine support (with an IC50 of 67 nM) than in cells from healthy donors (IC50 >400 nM) [42]. Moreover, the potency of ruxolitinib in Ba/F3 cells shifted more than fivefold when their wild- type endogenous JAKs were activated by the addition of IL-3 [42]. Ruxolitinib also inhibited the proliferation of erythroid progenitors sampled from PV patients and expanded ex vivo in serum-free media (IC50 of 60 nM) [42].
In a Ba/F3-EpoR-JAK2V617F cell line (exhibiting growth factor-independent JAK2 phosphorylation, engineered through expression of the erythropoietin receptor, EpoR), treatment with ruxolitinib resulted in a dose-dependent doubling of cells with depolarized mitochondria, suggesting that in this cell line, induction of apoptosis is an important basis of ruxolitinib effect [19]. In a mouse model of MPN involving implanted Ba/F3 cells expressing JAK2V617F, oral administration of ruxolitinib was well tolerated and markedly reduced the animals' splenomegaly [42]. In this model, selective JAK inhibition was found to eliminate neoplastic cells from the spleen, liver and bone marrow, normalize the histology of affected organs, and significantly prolong survival [42]. Measurement of the levels of IL-6 and TNF-α in plasma samples from mice inoculated with vehicle or ruxolitinib for 12 days showed significant suppression of IL-6 elevation and reduction of TNF-α levels to normal. Preclinical studies also showed that selective inhibition of JAK1/2 by ruxolitinib reduces the tumor burden of mice inoculated with JAK2V617F-expressing cells without causing anemia or lymphopenia [19].
Pharmacokinetics & metabolism
In healthy subjects receiving a single oral dose of ruxolitinib 25 mg, the plasma concentration profile of ruxolitinib showed a rapid absorption phase, attaining peak concentrations within 1 h after administration, and a monophasic or biphasic decline, with a mean observed terminal half-life of 2.32 h [43]. In patients with MF, absorption of 10–25 mg oral dose likewise was rapid, with a peak plasma concentration at 1–3 h after dosing, and decline was biphasic, with a terminal half-life of 2–3 h [103]. Drug exposure increased in a linear fashion with dosage, and did not show a plateau at doses of up to 200-mg once daily. More than 95% of administered drug was absorbed, and >99% of an administered dose was metabolized, predominantly in the liver [43]. Patients with impaired hepatic function may therefore require lengthened dosing intervals or lower total daily doses [44]. In 10-day multiple-dose studies in healthy volunteers, metabolite profiles were qualitatively and quantitatively similar on days 1 and 10, with no apparent accumulation of ruxolitinib or its metabolites [43].
The main route of metabolite excretion is in urine, with only a small percentage excreted in feces [43]. In patients with severe renal impairment (creatinine clearance <30 ml/min), ruxolitinib should therefore be administered with caution, and doses may need to be reduced to avoid adverse drug reactions. The same precautions should apply in patients with end- stage renal disease, as suggested by findings of minimal ruxolitinib clearance and high binding to plasma proteins in hemodialysis patients [45]. However, no correlation has been established between renal function and systemic exposure to ruxolitinib. Although ruxolitinib is a substrate of cytochrome P450 CYP(3A4), there has been no evidence of inhibition or induction of CYP enzymes [42], and no significant changes in ruxolitinib pharmacokinetics have been shown for its coadministration with P450 CYP(3A4) inducers (e.g., rifampin) [46] or with mild or moderate CYP(3A4) inhibitors (e.g., erythromycin) [47]. Nonetheless, in healthy subjects receiving ketoconazole, a strong CYP(3A4) inhibitor, at 200-mg twice daily for 4 days, the area under the curve of ruxolitinib increased by 91% and the drug's half-life was prolonged from 3.7 to 6.0 h [48]. It is possible that similar effects could be observed for other strong CYP(3A4) inhibitors (such as, but not limited to, itraconazole, posaconazole, voriconazole, clarithromycin, telithromycin, atazanavir, indinavir, nelfinavir, ritonavir, saquinavir and nefazodone). In healthy volunteers, administration with a high-fat meal reduced the maximum plasma concentration of a 25-mg dose of ruxolitinib by 24% but had little effect on its bioavailability, as measured by AUC [49]. The drug showed high plasma protein binding, at approximately 97%, and a relatively small volume of distribution, driving its short elimination half-life.
Safety & tolerability
In a pharmacokinetics/pharmacodynamics study of healthy persons dosed orally for 10 days, ruxolitinib was generally safe and well tolerated, with 25-mg twice daily and 100-mg once daily established as the maximum-tolerated dose (MTD) in these volunteers [49]. Grade 4 neutropenia with 12-day recovery occurred in one participant treated with 25-mg twice daily, suggesting myelosuppression at this dosage. During a median of more than 14.7 months of ruxolitinib therapy in a Phase I/II clinical trial, nonhematologic toxic effects considered to be at least possibly related to therapy occurred in less than 10% of 153 MF patients, and most were low grade (Table 1) [37]. The nonhematologic high grade (grade 3 or 4) adverse events were asthenia (2.0%), fatigue (1.3%), anxiety (1.3%), insomnia (1.3%) and fever (0.7%).
Table 1.
The most frequent nonhematologic toxic effects of ruxolitinib therapy in a Phase I/II clinical trial.
| Nonhematologic toxic effect | Occurrence (%) |
|---|---|
| Diarrhea | 5.9 |
| Fatigue | 4.3 |
| Headache | 3.3 |
| Peripheral edema | 2.6 |
| Pain in extremities | 2.6 |
| Urinary tract infection | 2.6 |
| Dizziness | 2.6 |
Among hematological toxicities, myelo- suppression was the most significant, as would be expected based on the mechanism of action of ruxolitinib (i.e., JAK1 and JAK2 inhibition). As a dose-limiting toxicity, thrombocytopenia occurred in 17 and 5% of patients as grade 3 and grade 4 adverse events, respectively. The incidence of thrombocytopenia was lower among patients who received 15-mg twice daily than among those who received 25-mg twice daily (at 3 vs 29%). Of note, patients who received 15-mg twice daily underwent subsequent dose increases to 20-mg twice daily (~25% of the total number of patients in this study) or 25-mg twice daily (~10% of patients). New-onset anemia occurred in 23% of the subgroup of patients who were transfusion-independent at baseline.
A total of two patients (1.3%) with a history of cardiopulmonary disease developed a clinical picture assessed by the investigator as systemic inflammatory response syndrome after abrupt cessation of ruxolitinib [103]; a systemic inflammatory response syndrome-like clinical picture has not been observed in larger clinical trials (described below). Among discontinuations considered to be possibly ruxolitinib related, one patient had intracranial hemorrhage without thrombocytopenia after 374 days of therapy on 25 mg twice daily and died; one patient progressed to chronic myelomonocytic leukemia after 147 days on 25-mg twice daily; and two patients developed grade 3 thrombocytopenia after 278 and 57 days on 50 mg [103]. After more than 25 months, overall survival was 84%. If only deaths during the study were considered, survival was 90% [37].
In the randomized, placebo-controlled, double-blinded Phase III Controlled Myelofibrosis Study with Oral JAK Inhibitor Treatment (COMFORT)-I clinical trial, the most common adverse events of any grade among the 155 MF patients treated with ruxolitinib were thrombocytopenia (34.2 vs 9.3% for placebo), anemia (31 vs 13.9%), fatigue (25.2 vs 33.8%), diarrhea (23.2 vs 21.2%), peripheral edema (18.7 vs 22.5%) and abdominal pain (10.3 vs 41.1%) [50]. Anemia and thrombocytopenia each led to the withdrawal of 0.6% of ruxolitinib recipients and 0.7% of placebo recipients. At the time of this writing, full safety data from the randomized, open-label, Phase III COMFORT-II clinical trial [104], comparing the efficacy and safety of ruxolitinib versus best available therapy, were not yet available.
Clinical efficacy
Phase I/II clinical trial of orally administered single-agent ruxolitinib therapy in patients with PMF or post-PV/post-ET MF
A total of 153 patients (53.0% with PMF, 31.8% with post-PV MF, and 15.2% with post-ET MF, all in advanced stage disease) were enrolled in a Phase I/II interventional open-label clinical trial that started in June 2007 [37,103,105]. Their median age was 65 years (range: 40–84 years), 63% were men, 86% had previously been treated for MF, and 82% were JAK2V617F -positive. The median JAKV617F allele burden was 84%. The response criteria were those of the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) [51]. A total of 4 weeks of daily oral therapy with ruxolitinib was considered to be one cycle.
In the first part of the study (conducted as a Phase I trial), the MTD of ruxolitinib was established to be 25-mg twice daily or 100- mg once daily. Thrombocytopenia was the dose-limiting toxic effect. In the second part of the study (Phase II), other dosing regimens were investigated, with the aim of identifying an effective dosing schedule with a reduced incidence of thrombocytopenia. All such regimens were scheduled twice daily and below the MTD (Table 2).
Table 2.
Twice daily-scheduled dosing regimens tested in second part of Phase I/II clinical trial.
| Tested regimens | Dosing schedules | Remarks |
|---|---|---|
| Dosing regimen 1 (regardless of Plt†) | 25 mg b.i.d. (induction: two cycles) + 10 mg b.i.d. (maintenance) | Fixed dosing regimen |
| Dosing regimen 2 (regardless of Plt†) | 10 mg b.i.d. (three cycles) | Possibility of dose escalation in case of suboptimal response (assuming toxicity absence) |
| Dosing regimen 3 (Plt†: 100–200 × 109/l) | Starting dose of 10 mg b.i.d. | Possibility of monthly 5 mg b.i.d. increments up to 25 mg b.i.d. in patients with inadequate response and acceptable toxicity |
| Dosing regimen 4 (Plt†: >200 × 109/l) | Starting dose of 15 mg b.i.d. |
Plt is the baseline platelet count.
b.i.d.: Twice daily.
Starting doses of 15-mg twice daily or 25-mg twice daily were identified as the most successful. Clinical improvement, as defined by reduction of palpable splenomegaly by ≥50% after three cycles, was documented in 52 and 49% of patients who received starting doses of 15-mg twice daily and 25-mg twice daily, respectively, and the response was maintained after 12 cycles in 73 and 78%, respectively, of these responders. Overall, the 15-mg twice-daily regimen appeared to be the more advantageous, because reduction in spleen size was comparable to that of recipients on the 25-mg twice-daily regime, but with a decreased incidence of grade 3 or 4 thrombocytopenia. Results were comparable among subgroups based on JAK2V617F mutation status or disease subtype (PMF, post-PV or post-ET MF). In patients on 15-mg twice daily, MRI studies after six therapy cycles showed a median 33% reduction in spleen volume, corresponding to a median 52% reduction in spleen length in matched patients. Among six patients with hepatomegaly, the liver volume had decreased after six cycles by 14%.
Patients on other ruxolitinib dosing regimens also showed clinical response, but in the course of treatment frequently required dose reductions because of thrombocytopenia. The exception was patients who received 25-mg once daily, because this group did not show clinical improvement to the level seen for other regimens. In the majority of patients, a reduction of spleen size was achieved within 2 months (i.e., was rapid). In all 16 and in all eight patients for whom data were available at 1.5 and 2 years, respectively, response to the drug was maintained.
Among recipients of 15-mg twice daily or 25-mg twice daily, the mean white cell count decreased from 29.8 to 16.0 × 109/l and remained stable through 1 year. Among the 17 patients with initially elevated platelet counts, a mean reduction of these counts from 728 × 109/l at baseline to 336 × 109/l after 3 months was observed, and in seven out of ten patients with normalized platelet counts, the platelet counts remained normal through 1 year. In the 25 patients with serial CD34+ cell counts, their mean values decreased from 583 to 398 cells/mm3 after 3 months. In the 27 patients who underwent 6-min walk tests, the mean distance after 1, 3 and 6 months was 34, 57 and 71 m, respectively. Overall, performance status improved over time, and these improvements were generally maintained. After two cycles of therapy, weight gain was observed, especially among patients in the lowest quartile of baseline BMI. Overall, the median weight gain after1 year was 9.4 kg in patients who received 15-mg twice daily and 7.1 kg in patients who received 25-mg twice daily.
Among biomolecular parameters associated with disease activity, JAK2V617F allele burden was found to be modestly decreased, with a mean maximal suppression of 13% from baseline. However, such data were available only for 34 patients. Reduction in plasma levels of certain proinflammatory cytokines (e.g., IL-6 and TNF-α) exhibited correlation with symptomatic improvements. Furthermore, plasma levels of leptin and erythropoietin increased. Constitutively phosphorylated STAT3 and/or STAT5 were observed at baseline, regardless of JAKV617F mutational status, and their reduction after treatment with ruxolitinib was dose and time dependent.
Phase III trials: COMFORT-I & COMFORT-II
COMFORT-I is an ongoing, randomized, double-blind, multicentric Phase III clinical trial in the USA, Canada and Australia, comparing the efficacy and safety of ruxolitinib with those of placebo [106]. COMFORT-II is an ongoing, randomized, open-label, multicentric Phase III clinical trial in Europe, comparing the efficacy, safety and tolerability of ruxolitinib with those of the best available oral and/or parenteral therapies, as selected by the investigator [107]. Over 200 patients with PMF, post-PV or post-ET MF are enrolled in each study. In both studies, the primary outcome measure is the proportion of patients achieving a ≥35% reduction from baseline spleen volume, as assessed by MRI or by CT scanning at 24 weeks (COMFORT-I) or 48 weeks (COMFORT-II), while the duration of a ≥35% reduction from baseline spleen volume is a secondary outcome.
Preliminary results from COMFORT-I [50] show a statistically significant reduction in spleen size in patients treated with ruxolitinib. For the primary end point, the response rate was 41.9% among recipients of ruxolitinib at a starting dosage of 15- or 20-mg twice daily (depending on baseline platelet count), and then optimized during treatment, compared with 0.7% for placebo (p < 0.0001). The proportion of patients with ≥50% improvement in total symptom score on the modified Myelofibrosis Symptom Assessment Form version 2.0 [52] was 45.9 versus 5.3% (p < 0.0001). Ruxolitinib recipients also showed improvement in quality of life, as measured by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ-C30) [53]. After a median 32.2 weeks of follow-up, median duration of response (reduction in splenic volume of ≥35%) had not been reached. At the time of this writing, efficacy data from the COMFORT-II trial [104] were not yet available.
Other clinical indications
In a Phase II clinical trial in patients with advanced PV or ET refractory or intolerant to hydroxyurea, 94% of PV patients and 61% of ET patients who received ruxolitinib achieved partial or complete remission [54]. Marked improvement of constitutional symptoms was noted. A Phase III clinical trial comparing ruxolitinib and best available therapy in these groups of patients (the RESPONSE trial) is ongoing [108]. Patients with other hematological malignancies marked by abnormal signaling activity in the JAK–STAT pathway might also benefit from ruxolitinib. One ongoing Phase II study is testing its benefit in patients with relapsed/refractory AML, acute lymphoblastic leukemia, myelodysplasia, chronic myelomonocytic leukemia or blastic-phase or tyrosine-kinase- refractory chronic myelogenous leukemia [109]. Another Phase I/II study is testing it in patients with refractory or relapsed AML or acute lymphoblastic leukemia [110]. A Phase I study is evaluating MTD, toxicity, pharmacokinetics and antitumor activity in children with relapsed or refractory solid tumors, leukemias or MPNs [111].
As it has shown capacities to reduce plasma levels of cytokines such as IL-6 and TNF-α[19], ruxolitinib has been evaluated in patients with highly active rheumatoid arthritis [33,55]. Topically administered ruxolitinib has also been evaluated for mild-to-moderate plaque psoriasis. In a 12-week Phase IIb double-blind, randomized, dose-ranging study conducted in 200 patients, topical application showed significant clinical activity (at day 84, the total lesion score for all dose groups was decreased by more than twofold over vehicle control) and was reported as safe and well tolerated [56].
Regulatory
In 2008, the US FDA granted orphan-drug designation to ruxolitinib for the treatment of MF [112]. In 2008 and 2009, the EMA of the EU granted it orphan-drug designation for the treatment of PMF and post-PV MF or post-ET MF (orphan decision number EU/3/08/572 and EU/3/09/620) [113]. The same designation was granted for treatment of PV and ET by both regulators [112,113].
Conclusion
While several new JAK2 inhibitors are in different stages of development for MPNs, ruxolitinib is furthest in development. This new approach to treatment has advantages including low toxicity, durable efficacy in reduction of splenomegaly and alleviation of constitutional symptoms; these effects are associated with a significant concomitant reduction in levels of circulating cytokines. In addition, ruxolitinib promotes weight gain and improvement in general physical condition. Although it did not produce substantial reduction in JAK2V617F allele burden and is not curative in MF, its positive effects suggest that it may have a notable role in the treatment of patients with symptomatic MF and poor quality of life. Owing to the still short follow-up of treated patients, its effects on survival, thrombotic events and transformation to AML remain to be seen.
Future perspective
Discovery of JAK2 mutations has dramatically changed the diagnostic approach to patients with MPNs. Concurrent with new insight into the pathophysiology of these malignant disorders, a new class of drugs, JAK 2 inhibitors, is being developed, with ruxolitinib being the first-in-class. Based on achievements in the field of small-molecule drugs, and on encouraging findings from clinical trials of ruxolitinib in particular, it seems highly probable that JAK2 inhibitors will soon become widely available for treatment of patients with MPNs. However, some questions remain to be fully answered in larger numbers of patients, including the issue of optimal timing, dosing schedule and duration of treatment in specific patient subgroups. For example, patients who are candidates for allogeneic stem cell transplantation might benefit from ruxolitinib by gains in overall performance status and reduction of splenomegaly, making them more suitable as transplant recipients. Combinations of JAK2 inhibitors with other drugs, such as histone deacetylase inhibitors, which are capable of reducing JAK2V617F allele burden [50], and are synergistic with JAK2 inhibitors, at least in vitro [51], also need to be further investigated.
EXECUTIVE SUMMARY.
Mechanism of action
Ruxolitinib is an ATP-competitive JAK1/2 inhibitor that downregulates the JAK-STAT pathway, inhibiting myeloproliferation, inducing apoptosis, and reducing numerous cytokine plasma levels. This inhibition occurs regardless of JAK2V617F mutational status.
Pharmacokinetic properties
After oral administration, ruxolitinib shows rapid absorption, with more than 95% of the administered dose absorbed. Plasma concentration then declines rapidly in a biphasic manner, with a terminal half-life of 2–3 h.
More than 99% of the drug is metabolized, predominantly in the liver, with no apparent accumulation of ruxolitinib or its metabolites. Excretion is primarily in urine.
Clinical efficacy
Significant (in up to 52% of patients) and durable (maintained after 12 cycles in up to 78% of patients) palpable spleen-size reduction by >50%, depending on dosing regimen.
Reduction in platelet (mean reduction from 728 to 336 × 109/l), white cell (mean white cell count decrease from 29.8 to 16.0 × 109/l) and CD34+ counts in peripheral blood (mean values decreased from 583 to 398 cells/mm3) after 3 months of treatment.
Alleviation of constitutional symptoms (e.g., fatigue, weight loss, low-grade fever and night sweats).
Improved physical condition and overall performance status.
Safety & tolerability
The main hematologic adverse event is myelosuppression (i.e., thrombocytopenia [dose-limiting and dose-dependent toxicity] and new-onset anemia [dose-dependent]).
Nonhematologic adverse events occur in less than 10% of treated patients, and few are high-grade (e.g., asthenia [2.0%], fatigue [1.3%], anxiety [1.3%] and insomnia [1.3%]).
Owing to the possible risk of a systemic inflammatory response-like syndrome after abrupt discontinuation of treatment, ruxolitinib should be used with caution in patients with a history of serious or active severe cardiopulmonary disease.
Drug interactions
Coadministration of ruxolitinib with cytochrome P450 CYP(3A4) inducers (e.g., rifampin), or with mild or moderate CYP(3A4) inhibitors (e.g., erythromycin) did not cause significant changes in ruxolitinib pharmacokinetics that would require dose adjustment. Nonetheless, ruxolitinib dose reductions are probably necessary when it is coadministered with a strong CYP(3A4) inhibitor (e.g., ketoconazole).
Dosage & administration
Regimens of ruxolitinib 15-mg twice daily or 25-mg twice daily per os were identified by a Phase II trial as the most appropriate.
Patients with impaired liver function may require longer dosing intervals.
In patients with severe renal impairment or end-stage renal disease, ruxolitinib should be administered with caution.
Acknowledgments
FINANCIAL & COMPETING INTERESTS DISCLOSURE S Verstovsek received research support from Incyte for the conduct of clinical studies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Footnotes
INFORMATION RESOURCES Incyte Corporation: www.incyte.com
Novartis AG: www.novartis.com
Bibliography
Papers of special note have been highlighted as:
n of interest
nn of considerable interest
- 1.Chaiter Y, Brenner B, Aghai E, Tatarsky I. High incidence of myeloproliferative disorders in Ashkenazi Jews in northern Israel. Leuk. Lymphoma. 1992;7(3):251–255. doi: 10.3109/10428199209053630. [DOI] [PubMed] [Google Scholar]
- 2.Dougan LE, Matthews ML, Armstrong BK. The effect of diagnostic review on the estimated incidence of lymphatic and hematopoietic neoplasms in Western Australia. Cancer. 1981;48(3):866–872. doi: 10.1002/1097-0142(19810801)48:3<866::aid-cncr2820480334>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Johansson P, Kutti J, Andreasson B, et al. Trends in the incidence of chronic Philadelphia chromosome negative (Ph-) myeloproliferative disorders in the city of Goteborg, Sweden, during 1983–99. J. Intern. Med. 2004;256(2):161–165. doi: 10.1111/j.1365-2796.2004.01357.x. [DOI] [PubMed] [Google Scholar]
- 4.Kutti J, Ridell B. Epidemiology of the myeloproliferative disorders: essential thrombocythaemia, polycythaemia vera and idiopathic myelofibrosis. Pathol. Biol. (Paris) 2001;49(2):164–166. doi: 10.1016/s0369-8114(00)00023-7. [DOI] [PubMed] [Google Scholar]
- 5.Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted county study, 1976–1995. Am. J. Hematol. 1999;61(1):10–15. doi: 10.1002/(sici)1096-8652(199905)61:1<10::aid-ajh3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Woodliff HJ, Dougan L. Myelofibrosis in Western Australia: an epidemiological study of 29 cases. Med. J. Aust. 1976;1(15):523–525. doi: 10.5694/j.1326-5377.1976.tb140817.x. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Larran A, Cervantes F, Bellosillo B, et al. Essential thrombocythemia in young individuals: frequency and risk factors for vascular events and evolution to myelofibrosis in 126 patients. Leukemia. 2007;21(6):1218–1223. doi: 10.1038/sj.leu.2404693. [DOI] [PubMed] [Google Scholar]
- 8.Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N. Engl. J. Med. 2005;353(1):33–45. doi: 10.1056/NEJMoa043800. [DOI] [PubMed] [Google Scholar]
- 9.Najean Y, Rain JD. Treatment of polycythemia vera: use of 32P alone or in combination with maintenance therapy using hydroxyurea in 461 patients greater than 65 years of age. The French Polycythemia Study Group. Blood. 1997;89(7):2319–2327. [PubMed] [Google Scholar]
- 10.Najean Y, Rain JD, Dresch C, et al. Risk of leukaemia, carcinoma, and myelofibrosis in 32P- or chemotherapy-treated patients with polycythaemia vera: a prospective analysis of 682 cases. The “French Cooperative Group for the study of polycythaemias”. Leuk. Lymphoma. 1996;22(Suppl. 1):111–119. doi: 10.3109/10428199609074368. [DOI] [PubMed] [Google Scholar]
- 11.Passamonti F, Rumi E, Arcaini L, et al. Prognostic factors for thrombosis, myelofibrosis, and leukemia in essential thrombocythemia: a study of 605 patients. Haematologica. 2008;93(11):1645–1651. doi: 10.3324/haematol.13346. [DOI] [PubMed] [Google Scholar]
- 12.Randi ML, Barbone E, Fabris F, Varotto L, Macri C, Girolami A. Post-polycythemia myeloid metaplasia: experience with a large cohort of patients. J. Med. 1994;25(6):363–369. [PubMed] [Google Scholar]
- 13.Wolanskyj AP, Schwager SM, Mcclure RF, Larson DR, Tefferi A. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo. Clin. Proc. 2006;81(2):159–166. doi: 10.4065/81.2.159. [DOI] [PubMed] [Google Scholar]
- 14.Ihle JN, Kerr IM. JAKs and STATs in signaling by the cytokine receptor superfamily. Trends. Genet. 1995;11(2):69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 15.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J. Cell. Sci. 2004;117(Part 8):1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 16.Barosi G, Bergamaschi G, Marchetti M, et al. JAK2 V617F mutational status predicts progression to large splenomegaly and leukemic transformation in primary myelofibrosis. Blood. 2007;110(12):4030–4036. doi: 10.1182/blood-2007-07-099184. [DOI] [PubMed] [Google Scholar]
- 17.Tefferi A, Lasho TL, Huang J, et al. Low JAK2V617F allele burden in primary myelofibrosis, compared to either a higher allele burden or unmutated status, is associated with inferior overall and leukemia-free survival. Leukemia. 2008;22(4):756–761. doi: 10.1038/sj.leu.2405097. [DOI] [PubMed] [Google Scholar]
- 18.Tefferi A, Lasho TL, Schwager SM, et al. The JAK2(V617F) tyrosine kinase mutation in myelofibrosis with myeloid metaplasia: lineage specificity and clinical correlates. Br. J. Haematol. 2005;131(3):320–328. doi: 10.1111/j.1365-2141.2005.05776.x. [DOI] [PubMed] [Google Scholar]
- 19.Quintas-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115(15):3109–3117. doi: 10.1182/blood-2009-04-214957. [DOI] [PMC free article] [PubMed] [Google Scholar]; nn Published report on the preclinical studies of ruxolitinib.
- 20.Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108(10):3472–3476. doi: 10.1182/blood-2006-04-018879. [DOI] [PubMed] [Google Scholar]
- 21.Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3(7):E270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh ST, Simonds EF, Jones C, et al. Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood. 2010;116(6):988–992. doi: 10.1182/blood-2010-02-270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grand FH, Hidalgo-Curtis CE, Ernst T, et al. Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood. 2009;113(24):6182–6192. doi: 10.1182/blood-2008-12-194548. [DOI] [PubMed] [Google Scholar]
- 24.Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J. Clin. Oncol. 2011;29(6):761–770. doi: 10.1200/JCO.2010.31.8436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tefferi A, Mesa RA, Nagorney DM, Schroeder G, Silverstein MN. Splenectomy in myelofibrosis with myeloid metaplasia: a single-institution experience with 223 patients. Blood. 2000;95(7):2226–2233. [PubMed] [Google Scholar]
- 26.Rambaldi A. Therapy of myelofibrosis (excluding JAK2 inhibitors) Int. J. Hematol. 2010;91(2):180–188. doi: 10.1007/s12185-010-0532-x. [DOI] [PubMed] [Google Scholar]
- 27.Arana-Yi C, Quintas-Cardama A, Giles F, et al. Advances in the therapy of chronic idiopathic myelofibrosis. Oncologist. 2006;11(8):929–943. doi: 10.1634/theoncologist.11-8-929. [DOI] [PubMed] [Google Scholar]
- 28.Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment) Blood. 2010;115(9):1703–1708. doi: 10.1182/blood-2009-09-245837. [DOI] [PubMed] [Google Scholar]
- 29.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 30.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 31.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005;352(17):1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 32.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Quintas-Cardama A, Kantarjian H, Cortes J, Verstovsek S. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat. Rev. Drug Discov. 2011;10(2):127–140. doi: 10.1038/nrd3264. [DOI] [PubMed] [Google Scholar]; n Excellent review of JAK inhibitors currently in different stages of development.
- 34.Hedvat M, Huszar D, Herrmann A, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16(6):487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyner JW, Bumm TG, Deininger J, et al. CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood. 2010;115(25):5232–5240. doi: 10.1182/blood-2009-05-223727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verstovsek S, Odenike O, Scott B, et al. Phase I dose-escalation trial of SB1518, a novel JAK2/FLT3 inhibitor, in acute and chronic myeloid diseases, including primary or post- essential thrombocythemia/polycythemia vera myelofibrosis. Blood. 2009;114(22):3905. [Google Scholar]
- 37.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N. Engl. J. Med. 2010;363(12):1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]; nn Published report on the Phase I/II clinical trial of ruxolitinib.
- 38.Tefferi A, Kantarjian HM, Pardanani AD, et al. The clinical phenotype of myelofibrosis encompasses a chronic inflammatory state that is favorably altered by INCB018424, a selective inhibitor of JAK1/2. The 50th ASH meeting, San Francisco, CA, USA. Blood. 2008;112(11):2804. Abstract. [Google Scholar]
- 39.Walker K. Inflammation research association - 15th international conference advances in asthma and COPD and other inflammatory diseases. IDrugs. 2008;11(12):863–865. [PubMed] [Google Scholar]
- 40.Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. JAK2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93(3):397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 41.Parganas E, Wang D, Stravopodis D, et al. JAK2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93(3):385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 42.Fridman J, Nussenzveig R, Liu P, et al. Discovery and preclinical characterization of INCB018424, a selective JAK2 inhibitor for the treatment of myeloproliferative disorders. The 49th ASH meeting; Atlanta, GA, USA. 2007. p. 3538. Blood. Abstract. [Google Scholar]
- 43.Shilling AD, Nedza FM, Emm T, et al. Metabolism, excretion, and pharmacokinetics of [14C]INCB018424, a selective Janus tyrosine kinase 1/2 inhibitor, in humans. Drug Metab. Dispos. 2010;38(11):2023–2031. doi: 10.1124/dmd.110.033787. [DOI] [PubMed] [Google Scholar]; nn Report on the pharmacokinetics of ruxolitinib.
- 44.Chen X, Shi J, Mcgee R, et al. The effect of various degrees of hepatic dysfunction on the pharmacokinetics of INCB018424. The 111th ASCPT meeting; Atlanta, GA, USA. 2010. pp. PIII–59. Clin. Pharmacol. Ther. Abstract. [Google Scholar]
- 45.Shi J, Chen X, Mcgee R, et al. Effects of various degrees of renal impairment and hemodialisis on the pharmacokinetics and pharmacodynamics of INCB018424. The 111th ASCPT meeting; Atlanta, GA, USA. 2010. pp. PII–53. Clin. Pharmacol. Ther. Abstract. [Google Scholar]
- 46.Shi J, Chen X, Yeleswaram S, et al. An open- label assessment of the effects of rifampin, a potent CYP3A4 inducer on the PK/PD of INCB018424 in healthy subjects. The 38th ACCP meeting; San Antonio, TX, USA. 2009. p. 9. J. Clin. Pharmacol. Abstract. [Google Scholar]
- 47.Shi J, Chen X, Yeleswaram S, et al. An open- label assessment of the effects of CYP3A4 inhibitors on the PK/PD of INCB018424 in healthy subjects. The 38th ACCP meeting; San Antonio, TX, USA. 2009. p. 10. J. Clin. Pharmacol. Abstract. [Google Scholar]
- 48.Shi JG, Chen X, Emm T, et al. The effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 phosphate) in healthy volunteers. J. Clin. Pharmacol. 2011 doi: 10.1177/0091270011405663. DOI:10.1177/0091270011405663. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 49.Shi JG, Chen X, Mcgee RF, et al. The pharmacokinetics, pharmacodynamics, and safety of orally dosed INCB018424 phosphate in healthy volunteers. J. Clin. Pharmacol. 2011 doi: 10.1177/0091270010389469. DOI: 10.1177/0091270010389469. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 50.Verstovsek S, Mesa R, Gotlib J, et al. Results of COMFORT-I, a randomized double-blind Phase III trial of JAK1/2 inhibitor INCB18424 (424) vs placebo (PB) for patients with myelofibrosis (MF). The 47th ASCO Meeting; Chicago, IL, USA. 2011. J. Clin. Oncol. Abstract 6500. [Google Scholar]
- 51.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- 52.Mesa RA, Schwager S, Radia D, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk. Res. 2009;33(9):1199–1203. doi: 10.1016/j.leukres.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of- life instrument for use in international clinical trials in oncology. J. Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 54.Verstovsek S, Passamonti F, Rambaldi A, et al. A Phase 2 study of INCB018424, an oral, selective JAK1/JAK2 inhibitor, in patients with advanced polycythemia vera (PV) and essential thrombocythemia (ET) refractory to hydroxyurea. Presented at: The 51st ASH Meeting; New Orleans, LA, USA. 2009. p. 311. Blood. Abstract. [Google Scholar]
- 55.Williams W, Scherle P, Shi J, et al. A randomized placebo-controlled study of INCB018424, a selective Janus kinase 1 & 2 (JAK1&2) inhibitor in rheumatoid arthritis (RA). Presented at: The 72nd ACR Meeting; San Francisco, CA, USA. 2008. Abstract. [Google Scholar]
- 56.Callis Duffin K, Luchi M, Fidelus-Gort R, et al. Novel mechanism for topical treatment of plaque psoriasis – results of a randomized, double blind, concentration ranging, vehicle controlled 12 week study with JAK 1/2 inhibitor INCB018424 cream. The 70th SID meeting; Atlanta, GA, USA. 2010. p. 261. J. Invest. Dermatol. Abstract. [Google Scholar]
Websites
- 101.Press release Incyte announces major collaboration and license agreement for two hematology-oncology programs. 2009 investor.incyte.com/phoenix.zhtml?c=69764&p=irol-newsArticle&ID=1359169&highlight=
- 102.Media releases Novartis gains rights to two oral targeted investigational therapies focusing on patients with life-threatening blood disorders and cancers. 2009 www.novartis.com/newsroom/media-releases/en/2009/1357027.shtml.
- 103.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N. Engl. J. Med. 2010;363(12):1117–1127. doi: 10.1056/NEJMoa1002028. Supplementary Appendix to. www.nejm.org/doi/suppl/10.1056/NEJMoa1002028/suppl_file/nejmoa1002028_appendix.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Media release Second Phase III study of Novartis JAK inhibitor INC424 meets primary end point in patients with myelofibrosis. 2011 www.novartis.com/newsroom/media-releases/en/2011/1496848.shtml.
- 105. ClinicalTrials.gov, NCT00509899. 2011 http://clinicaltrials.gov.
- 106. ClinicalTrials.gov, NCT00952289. 2011 http://clinicaltrials.gov.
- 107. ClinicalTrials.gov, NCT00934544. 2011 http://clinicaltrials.gov.
- 108. ClinicalTrials.gov, NCT01243944. 2011 http://clinicaltrials.gov.
- 109. ClinicalTrials.gov, NCT00674479. 2011 http://clinicaltrials.gov.
- 110. ClinicalTrials.gov, NCT01251965. 2011 http://clinicaltrials.gov.
- 111. ClinicalTrials.gov, NCT01164163. 2011 http://clinicaltrials.gov.
- 112.US FDA Orphan drug product designations and approvals search. 2011 www.accessdata.fda.gov/scripts/opdlisting/oopd/index.cfm.
- 113.European Medicines Agency Rare diseases designations. 2011 www.ema.europa.eu.


