Abstract
Empirical treatment is best guided by current surveillance of local resistance patterns. The goal of this study is to characterize the prevalence of antimicrobial nonsusceptibility within pneumococcal isolates from Canada. The Canadian Bacterial Surveillance Network is comprised of laboratories from across Canada. Laboratories collected a defined number of consecutive clinical and all sterile site isolates of S. pneumoniae in 2002. In vitro susceptibility testing was performed by broth microdilution with NCCLS guidelines. Rates of nonsusceptibility were compared to previously published reports from the same network. A total of 2,539 isolates were tested. Penicillin nonsusceptibility increased to 15% (8.5% intermediate, 6.5% resistant) compared to 12.4% in 2000 (P ≤ 0.025, χ2). Only 32 (1.3%) isolates had an amoxicillin MIC of ≥4 μg/ml and only 2 of 32 cerebrospinal fluid isolates had an intermediate susceptibility to ceftriaxone by meningeal interpretive criteria (MIC = 1 μg/ml). A total of 354 (13.9%) isolates were macrolide nonsusceptible (46.3% MLSB, 56.7% M phenotype), increasing from 11.4% in 2000 (P ≤ 0.0075, χ2). Only 13 (<1%) isolates had a telithromycin MIC of >1 μg/ml. Ciprofloxacin nonsusceptibility (defined as an MIC of ≥4 μg/ml) increased to 2.7% compared to 1.4% in 2000 (P ≤ 0.0025, χ2) and was primarily found in persons ≥18 years old (98.5%). Nonsusceptibility to penicillin, macrolides, and fluoroquinolones is increasing in Canada. Nonsusceptibility to amoxicillin and ceftriaxone remains uncommon. Newer antimicrobials such as telithromycin and respiratory fluoroquinolones have excellent in vitro activity.
Streptococcus pneumoniae is an important cause of both invasive and noninvasive infections in all age groups throughout the world (1, 2, 14). Early treatment with an antimicrobial agent effective against the organism allows the opportunity to reduce mortality and morbidity from these infections (10, 32). As a result, empirical treatment of S. pneumoniae infections is best guided by nonsusceptibility patterns. However, nonsusceptibility profiles within specific regions are constantly changing and, therefore, current surveillance data are required (5, 11, 23, 33). Most traditional first-line agents, such as penicillin, trimethoprim-sulfamethoxazole (TMP-SMX), and macrolides, are now associated with significant rates of nonsusceptibility (8, 13, 27, 32). Many practice guidelines are recommending newer antimicrobials with lower rates of nonsusceptibility, such as the respiratory fluoroquinolones, for the empirical treatment of community acquired pneumonia in individuals with comorbidities or those requiring hospitalization (3, 15, 34, 41).
Pneumococcal nonsusceptibility to the respiratory fluoroquinolones has been slowly increasing, and nonsusceptibility has been shown to develop during therapy, leading to treatment failure (5, 6, 9, 26, 42). Isolates that are nonsusceptible to amoxicillin and/or extended-spectrum cephalosporins have been identified in various surveillance programs in the United States and Europe (7, 16), further limiting the therapeutic options for serious pneumococcal infections. Nonsusceptibility to telithromycin, which was designed specifically to overcome resistance, has also been reported in Taiwan and Canada (17, 47, 49).
We present here the in vitro activity of commonly used antimicrobial agents in addition to new investigational agents against S. pneumoniae isolates from across Canada. The trends in nonsusceptibility are compared to previously published reports of nonsusceptibility rates from the same antimicrobial surveillance program.
MATERIALS AND METHODS
The Canadian Bacterial Surveillance Network is a volunteer group of private and hospital-affiliated laboratories from across Canada. These centers represent a sample of laboratories providing service to community and tertiary hospitals, as well as community clinics and doctor's offices. All 10 provinces and 1 territory are represented in the sample collection, with approximately one-half of isolates from the province of Ontario. Laboratories, based on their size and catchment area, were asked to collect either the first 20 (small laboratories) or 100 (larger laboratories servicing multiple sites) consecutive clinical isolates, followed by all sterile site isolates of S. pneumoniae in 2002. The date of collection, source of specimen, and patient age and gender were recorded on a standardized form. Duplicate isolates from the same patient were excluded. Isolates were transported on chocolate agar slants or swabs to a central laboratory. Upon receipt, the isolates were confirmed to be S. pneumoniae by standard methodology. After storage at −70°C, isolates were thawed and subcultured onto blood agar twice before susceptibility testing was performed. In vitro susceptibility testing was performed by broth microdilution according to National Committee for Clinical Laboratory Standards (NCCLS) guidelines (38, 39). The susceptibility interpretive criteria used were those published in NCCLS document M100-S14 (40). The nonsusceptible category was defined as isolates with MICs in the intermediate and resistant category. For the purpose of the present study, ciprofloxacin MICs of ≥4 μg/ml were used to define the nonsusceptible category. An MIC of ≥4 μg/ml was chosen because of the association of this degree of resistance with mutations in the quinolone resistance-determining regions of genes encoding DNA topoisomerase IV and DNA gyrase A (21, 43). The antimicrobial agents were supplied by their respective manufacturers or were purchased from Sigma (St. Louis, Mo.). Erythromycin-nonsusceptible isolates were further classified as having either the M or MLSB phenotype. Isolates that were erythromycin nonsusceptible and clindamycin susceptible were classified as having the M phenotype, and those that were not susceptible to both erythromycin and clindamycin were classified as having the MLSB phenotype (22, 30, 46). Multidrug resistance was defined as resistance (intermediate isolates were not included) to three or more of the following antimicrobial agents: penicillin, ceftriaxone, erythromycin, TMP-SMX, and ciprofloxacin. All isolates with reduced susceptibility to telithromycin and linezolid were retested to confirm the MIC.
Rates of nonsusceptibility were compared to previously published reports from the Canadian Bacterial Surveillance Network from 1994 to 1995, 1997 to 1998, and 2000 (5, 33, 45). From October 1994 to August 1995, 11.1% of the isolates tested were from sterile sites, 28.7% were from children ≤5 years old, and 28.3% were from adults older than 65 years of age. 1n 1997 and 1998, 38.1% of the isolates tested were from sterile sites, 31.3% were from children ≤5 years old, and 32.3% were from individuals older than 65 years of age. In 2000, 42% of isolates were from sterile sites, 26.7% were from children <5 years old, and 31.0% were from individuals older than 65.
Analysis was performed by using SAS version 8.2 (SAS Institute, Inc., Cary, N.C.). P values of <0.05 were considered statistically significant.
RESULTS
A total of 2,539 isolates were obtained from 63 microbiology laboratories from across Canada. Of these isolates, 78.5% were collected from hospital-based labs, and the remainder were from private laboratories. The isolates were cultured from various specimen sites: 990 (39.0%) were from the lower respiratory tract, 881 (34.7%) were from blood, 371 (14.6%) were from conjunctival swabs, 168 (6.6%) were from ear swabs, 32 (1.3%) were from cerebrospinal fluid, and the remaining 197 (7.3%) were from other sites. For isolates for which the age of the patient was known (99.3%), the age distribution consisted of 569 (22.6%) samples from patients ≤5 years of age, 158 (6.3%) from patients 6 to 18 years of age, 987 (39.2%) from patients 19 to 65 years of age, and 806 (32.0%) from patients older than 65 years of age.
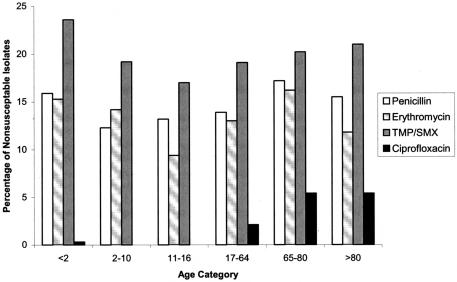
The in vitro susceptibility data for all antimicrobials tested are shown in Table 1 and Table 2. In this series the percentage of S. pneumoniae isolates that were penicillin nonsusceptible was 15.0%, with 8.5% of those isolates being in the intermediate category (MIC of 0.12 to 1 μg/ml) and 6.5% being resistant (MIC of ≥2 μg/ml). Of the 166 penicillin-resistant isolates 71.1% had an MIC of 2 μg/ml, with the remaining 38.9% having high-level resistance (MIC of ≥4 μg/ml). Penicillin-nonsusceptible strains were less likely to be isolated from sterile (12.4%) than from nonsterile (16.6%) sites (P = 0.005). The prevalence of nonsusceptibility in patients of different ages is demonstrated in Fig. 1. Penicillin nonsusceptibility was associated with macrolide nonsusceptibility, TMP-SMX nonsusceptibility, multidrug resistance (P ≤ 0.0001), and ciprofloxacin nonsusceptibility (P = 0.04).
TABLE 1.
In vitro activities of 17 antimicrobial agents tested against 2,539 isolates of S. pneumoniae collected from across Canada in 2002
| Antimicrobial agent (no. of isolates tested)a | MIC50 (μg/ml) | MIC90 (μg/ml) | Range | % of isolates per categoryb
|
|
|---|---|---|---|---|---|
| I | R | ||||
| Penicillin | <0.06 | 0.5 | ≤0.06-8 | 8.5 | 6.5 |
| Amoxicillin | <0.06 | 0.25 | ≤0.06-8 | 0.8 | 0.5 |
| Ceftriaxonec | |||||
| Meningeal (32) | ≤0.25 | ≤0.25 | ≤0.25-1 | 2 | 0 |
| Nonmeningeal (2,507) | ≤0.25 | ≤0.25 | ≤0.25-4 | 6.2 | 1.5 |
| Ciprofloxacin | 1.0 | 1.0 | ≤0.25-128 | NAd | 2.7 |
| Levofloxacin | 1.0 | 1.0 | ≤0.25-64.0 | 0.3 | 1.8 |
| Gatifloxacin | 0.25 | 0.25 | ≤0.06-32 | 0.4 | 1.6 |
| Moxifloxacin | 0.12 | 0.12 | ≤0.03-8 | 1.1 | 0.3 |
| Gemifloxacin | 0.015 | 0.03 | ≤0.008-1.0 | 0.8 | 0.9 |
| BMS-284756 | 0.03 | 0.06 | ≤0.008-1.0 | NA | NA |
| Tetracycline | ≤2.0 | 4.0 | ≤2.0-32 | 0.5 | 9.5 |
| TMP-SMX | ≤0.5 | 4.0 | ≤0.5-16 | 7.0 | 13.1 |
| Daptomycin (210) | ≤0.12 | 0.25 | ≤0.12-0.5 | NA | NA |
| Chloramphenicol | ≤4 | ≤4 | ≤4-16 | NA | 2.2 |
| Erythromycin | 0.12 | 4.0 | ≤0.012-64 | 0.2 | 13.8 |
| Clindamycin | ≤0.25 | ≤0.25 | ≤0.25-64 | 0.2 | 6.3 |
| Telithromycine | |||||
| MS (2,185) | ≤0.015 | ≤0.015 | ≤0.015-0.5 | 0 | 0 |
| MNS (354) | 0.06 | 0.5 | ≤0.015-2 | 0.3 | 0 |
| Linezolid | 1 | 1 | ≤0.25-4 | NA | 0.04 |
n = 2,539 isolates unless otherwise indicated.
I, intermediate; R, resistant.
The NCCLS susceptibility interpretive criteria for ceftriaxone for meningeal and nonmeningeal isolates of S. pneumoniae were applied.
NA, not applicable.
MS, macrolide susceptible; MNS, macrolide nonsusceptible.
TABLE 2.
MICs of 14 antimicrobial agents tested against 2,539 isolates of S. pneumoniae collected from across Canada in 2002
| Antimicrobial agent | No. of isolates inhibited by an MIC (μg/ml)a of:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.008 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | |
| Penicillin | 2,158* | 86 | 40 | 34 | 55 | 118 | 46 | 2 | |||||||
| Amoxicillin | 2,158* | 106 | 41 | 34 | 110 | 58 | 20 | 12 | |||||||
| Ceftriaxone | 2,302* | 42 | 157 | 34 | 4 | ||||||||||
| Ciprofloxacin | 7* | 664 | 1,634 | 166 | 14 | 11 | 10 | 19 | 13 | 1 | |||||
| Levofloxacin | 8* | 1,059 | 1,402 | 15 | 8 | 17 | 25 | 4 | 1 | ||||||
| Gatifloxacin | 7 | 549 | 1,883 | 45 | 5 | 10 | 26 | 12 | 1 | 1† | |||||
| Moxifloxacin | 13* | 458 | 1,938 | 74 | 6 | 15 | 27 | 7 | 1 | ||||||
| Gemifloxacin | 414* | 1,616 | 405 | 51 | 11 | 20 | 14 | 8 | |||||||
| Tetracyclin | 2,284* | 13 | 9 | 28 | 205† | ||||||||||
| TMP-SMX | 2,028* | 105 | 74 | 151 | 148 | 33† | |||||||||
| Daptomycinb | 130 | 76 | 4 | ||||||||||||
| Erythomycin | 2,177* | 7 | 4 | 9 | 40 | 57 | 52 | 27 | 27 | 138 | |||||
| Clindamycin | 2,375* | 4 | 4 | 5 | 5 | 12 | 48 | 27 | 59 | ||||||
| Chloramphenicol | 2,483* | 5 | 51† | ||||||||||||
| Telithromcyin | 2,258* | 67 | 72 | 27 | 55 | 47 | 12 | 1 | |||||||
| Linezolid | 43* | 545 | 1,802 | 149 | 1 | ||||||||||
*, MICs were less than or equal to the value given; †, MICs were greater than or equal to the value given.
Only 210 isolates were tested.
FIG.1.
Nonsusceptible isolates according to age category.
Of the 2539 isolates, 32 (1.3%) isolates had an amoxicillin MIC of ≥4 μg/ml. Two of these isolates had a ceftriaxone MIC of ≥4 μg/ml and all had a penicillin MIC of ≥2 μg/ml. The amoxicillin-nonsusceptible isolates were received from 19 different laboratories; 23 of the isolates were from Ontario, 5 were from Atlantic Canada and 4 were from Quebec. Thirty-two cerebrospinal S. pneumoniae isolates were received in 2002; only two of these had an intermediate susceptibility to ceftriaxone by meningeal interpretive criteria (MIC of 1 μg/ml).
A total of 354 (13.9%) isolates were macrolide nonsusceptible (erythromycin MIC of ≥0.5 μg/ml), 164 (46.3%) of which were clindamycin nonsusceptible (MLSB phenotype). The proportion of M versus MLSB phenotypes was similar among different age groups. The distribution of macrolide nonsusceptibility by age category is depicted in Fig. 1. There were 85 (24.2%) macrolide-nonsusceptible isolates from patients ≤5 years of age, 20 (5.7%) macrolide-nonsusceptible isolates from patients 6 to 18 years of age, 132 (37.5%) macrolide-nonsusceptible isolates from patients 19 to 65 years of age, and 115 (32.7%) macrolide-nonsusceptible isolates from patients older than 65 years of age. One hundred macrolide-nonsusceptible isolates were from sterile sites, of which thirty-eight (38%) had the M phenotype. Erythromycin nonsusceptibility was associated with penicillin nonsusceptibility, TMP-SMX nonsusceptibility, multidrug resistance (all P ≤ 0.001), and ciprofloxacin nonsusceptibility (P = 0.002).
There were 110 multidrug-resistant isolates identified. The most common pattern of multidrug resistance was nonsusceptibility to penicillin, erythromycin, and TMP-SMX (82.7%). Of the 110 multidrug-resistant isolates, 107 (97.3%) were erythromycin nonsusceptible, and 94 (85.5%) were TMP-SMX nonsusceptible.
Only 13 (0.55%) of the isolates had a telithromycin MIC of >1 μg/ml, and only 1 was telithromycin resistant. The isolate with a telithromycin MIC of 2 μg/ml was from the middle ear of a 1-year-old child from Quebec. The telithromycin MIC at which 50% of the isolates are inhibited (MIC50) and telithromycin MIC90 for macrolide-sensitive isolates were ≤0.015 μg/ml. For macrolide-nonsusceptible isolates, the telithromycin MIC50 and MIC90 were 0.06 and 0.5 μg/ml, respectively. Of 190 macrolide-resistant isolates with an M phenotype, 8 (4.2%) had a telithromycin MIC of ≥1 μg/ml compared to 5 of 164 (3.0%) of those with an MLSB phenotype (P = 0.77).
In the present study, the MIC50 and MIC90 for linezolid were both 1 μg/ml. There were 150 (5.9%) isolates with a linezolid MIC of ≥2 μg/ml. There was one isolate from the blood of a 49-year-old with a linezolid MIC of 4 μg/ml. Isolates with a linezolid MIC of ≥1 μg/ml were not more likely than other isolates to be nonsusceptible to TMP-SMX, ciprofloxacin, erythromycin, or penicillin (data not shown).
Sixty-eight (2.7%) isolates were not susceptible to ciprofloxacin. Sixty-seven (98.5%) of these were from persons ≥18 years old (see Fig. 1). Of the 990 (4.8%) specimens from the lower respiratory tract, 47 were not susceptible to ciprofloxacin compared to 22 of 1,554 other specimens (P < 0.001). MICs for other fluoroquinolones tested against ciprofloxacin nonsusceptible isolates are demonstrated in Table 3.
TABLE 3.
MICs for fluoroquinolones tested for isolates with a ciprofloxacin MIC of ≥4 μg/ml
| Antimicrobial agent | MIC90 (μg/ml) | No. of isolates inhibited by an MIC (μg/ml)a of:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | ||
| Ciprofloxacin | 64 | 14 | 11 | 10 | 19 | 13 | 1 | ||||||||
| Levofloxacin | 16 | 13 | 8 | 17 | 25 | 4 | 1 | ||||||||
| Gatifloxacin | 8 | 13 | 5 | 10 | 26 | 12 | 1 | 1 | |||||||
| Moxifloxacin | 4 | 2 | 11 | 5 | 15 | 27 | 7 | 1 | |||||||
| Gemifloxacin | 1 | 15 | 11 | 20 | 14 | 8 | |||||||||
NCCLS cutoffs for intermediate susceptibility are indicated in boldface.
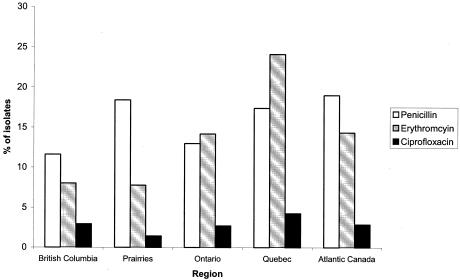
Isolates were received from all ten Canadian provinces and one territory. The distribution of nonsusceptibility by region is displayed in Fig. 2. The rate of penicillin nonsusceptibility was highest in Atlantic Canada and lowest in British Columbia with 19 and 12% of the isolates, respectively, being penicillin nonsusceptible. Erythromycin nonsusceptibility rates were highest in Quebec (24.1%) and lowest in the Quebec (8.0%) and Prairie (7.8%) provinces.
FIG. 2.
Regional variation in nonsusceptible patterns in S. pneumoniae in Canada in 2002. A total of 1,509 (55.5%) isolates were from Ontario, 424 (16.7%) were from the Prairies, 282 (11.1%) were from Quebec, 279 (11.0%) were from Atlantic Canada, and 137 (5.7%) were from British Columbia.
DISCUSSION
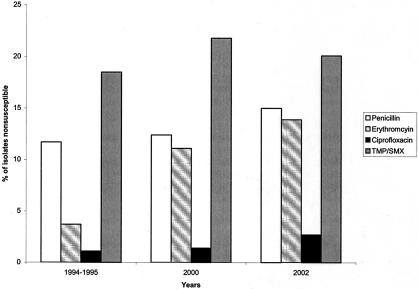
The prevalence of isolates that were nonsusceptible to penicillin increased to 15.0% in 2002 compared to previously published data from 2000 of 12.4% (P = 0.03) (Fig. 3). This increase occurred after a period of 6 years of stable rates of penicillin nonsusceptibility in isolates submitted to this surveillance network and is in keeping with results from surveillance studies from the United States and Europe over the same time period (16, 20, 25). Penicillin resistance in our sample of isolates has remained stable from 2000 to 2002 (6.5% versus 5.8%, P ≥ 0.05). There was a significant decrease in the proportion of penicillin resistant isolates from children <5 years of age from 34.1% of penicillin-resistant isolates in 2000 to 26.3% in 2002 (P ≤ 0.04). One explanation for this decrease may be the introduction of the pneumococcal conjugate vaccine in Canada in July 2001. Use of conjugate pneumococcal vaccine in children has been previously demonstrated to reduce the rates of invasive infection from penicillin nonsusceptible isolates in children and, to a lesser extent, in adults (51). Alternatively, this may be due to clonal dynamics or changes in the utilization of penicillins and aminopenicillin in different age groups (36, 37) Penicillin nonsusceptibility was associated with nonsusceptibility to erythromycin, TMP-SMX, and multidrug resistance. These associations are consistent with previous reports (16, 25, 36).
FIG. 3.
Nonsusceptibility among S. pneumoniae from 1994 to 1995, 2000, and 2002 to major antimicrobials.
Despite the increase in nonsusceptibility to penicillin, <2% of our isolates have penicillin MICs of ≥4 μg/ml, suggesting that empirical β-lactam therapy other than cefuroxime continues to be an acceptable choice for nonmeningeal pneumococcal disease in Canada (52).
Fewer than 2% of isolates exhibited high-level resistance to amoxicillin (MIC of ≥4 μg/ml). In contrast to studies from Europe identifying clones of amoxicillin-nonsusceptible isolates with penicillin MICs that are one or two dilutions lower then amoxicillin MICs (7), all of the amoxicillin-nonsusceptible isolates in our study had penicillin MICs that were equal to or higher than the amoxicillin MICs. The absence of high-level amoxicillin-resistant clones and continuing low levels of nonsusceptibility make amoxicillin an acceptable option as an antipneumococcal therapy in Canada.
The NCCLS has adopted interpretive criteria for ceftriaxone and cefotaxime for both nonmeningeal and meningeal isolates (40). Of the 32 cerebrospinal fluid isolates received in 2002, none were resistant to ceftriaxone based on the meningeal criteria. There were, however, 38 isolates from 2002 with ceftriaxone MICs of ≥2 μg/ml. The existence of such pneumococcal isolates in our study population, considered resistant by meningeal criteria, does lead to the possibility that resistant isolates might be found within the cerebrospinal fluid. This finding would continue to support the current recommendations for the use of the combination of a third generation cephalosporin and vancomycin as an initial empirical therapy for suspected bacterial meningitis (19).
The rate of macrolide nonsusceptibility increased from 3.7% in 1994 and 1995 to 11.1% in 2000 and to 13.9% in 2002. In the United States the prevalence of macrolide nonsusceptibility ranges from 28 to 30%, whereas in some Asian countries the resistance rates are up to 92% (16, 17, 20). The proportion of M versus MLSB phenotype in macrolide-resistant isolates was not different in surveillance data from 1994 to 1995, 2000, and 2002. This finding contrasts with pneumococcal surveillance data from the United States that implicates an increase of M phenotype isolates in children <5 years old, as the major reason for an increase in macrolide nonsusceptibility (12, 18). Regional variation in mechanisms responsible for erythromycin nonsusceptibility have been demonstrated throughout the world (29, 35). The exact reasons for the geographic variation are uncertain but are probably related to either antimicrobial use or clonal expansion.
Despite the increasing nonsusceptibility to macrolides, the ketolides maintain in vitro activity with an MIC50 and an MIC90 of ≤0.015 and 0.03 μg/ml, respectively. The activity of ketolides against macrolide-nonsusceptible strains was maintained whether they demonstrated the M or MLSB phenotype. The telithromycin MIC50 of macrolide-nonsusceptible isolates was, however, higher than the MIC50 of macrolide-susceptible isolates. There have been reports from Taiwan of isolates with increased MICs to telithromycin (17). A possible mechanism for ketolide nonsusceptibility may include the introduction of mutations outside of domain II and IV on the 23S ribosomal subunit (47).
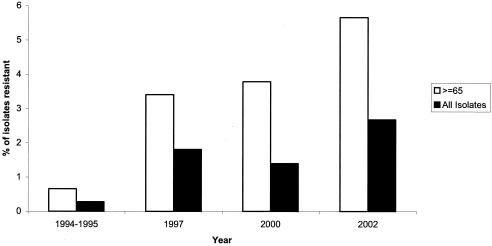
Of the isolates tested in our study, 2.7% demonstrated a ciprofloxacin MIC of ≥4 μg/ml. This prevalence is a significant increase from the 1.4% ciprofloxacin nonsusceptibility reported in 2000 (P ≤ 0.005). This increase is almost exclusively in adults older than 18 years, with the greatest increase in individuals older than 65 years. In this age group there was a marked increase from 0.7% ciprofloxacin nonsusceptibility in 1994 to 3.8% in 2000 and to 5.7% in our study from 2002 (Fig. 4). The prevalence of ciprofloxacin nonsusceptibility in individuals older than 65 years has been demonstrated previously and is not surprising since this demographic group has the highest proportion of risk factors for fluoroquinolone nonsusceptibility, such as chronic lung disease and institutionalization (24, 44). Recent reports of ciprofloxacin nonsusceptibility in hospitalized elderly patients in the United States demonstrated that in this population the nonsusceptibility rate was 3.5%, and specifically in a subset of patients from long-term care facilities the rates of nonsusceptibility may be as high as 8.7% (28). Based on this evidence we estimate the rate of ciprofloxacin nonsusceptibility in long-term care facilities in adults older than 65 years may now be >10% in our population. This specific group of patients is expected to grow as the population ages, which could further compound the problem of emerging nonsusceptibility to fluoroquinolones.
FIG. 4.
Trends in ciprofloxacin nonsusceptibility in all isolate and isolates from individuals >64 years of age.
Levofloxacin nonsusceptibility mirrors the ciprofloxacin nonsusceptibility, with a significant increase from 1.0% in 2000 to 2.17% in 2002. The increase is likely secondary to the accumulation of isolates with mutations in both parC and gyrA (4). Previous studies have demonstrated that levofloxacin-, moxifloxacin-, and gatifloxacin-susceptible isolates may already have a mutation within parC (5, 6, 31, 48, 50), which increases the likelihood of resistance developing to these agents either prior to or during therapy as a result of a subsequent mutation (6).
In conclusion, rates of nonsusceptibility to most antimicrobials continue to increase in Canadian isolates of S. pneumoniae. However, some older antimicrobials (amoxicillin and ceftriaxone), as well as newer agents (ketolides and respiratory fluoroquinolones) remain active against virtually all isolates and can continue to be recommended for empirical treatment of suspected pneumococcal infections.
Acknowledgments
The other members of the Canadian Bacterial Surveillance Network and their participating laboratories are as follows: S. Porter-Pong and A. Plevneshi, Toronto Medical Laboratories and Mount Sinai Hospital, Toronto, Ontario; H. R. Devlin, St. Michael's Hospital, Toronto, Ontario; R. G. Lewis, Cape Breton Regional Health Care Complex, Sydney, Nova Scotia; P. C. Kibsey, Victoria General Hospital, Victoria, British Columbia; J. Blondeau, Royal University Hospital, Saskatoon, Saskatchewan; G. K. Harding, St. Boniface General Hospital, Winnipeg, Manitoba; L. Thibault, Dr. Georges L. Dumont Hopital, Moncton, New Brunswick; F. Smaill, Hamilton Health Sciences Corp., Chedoke-McMaster, Hamilton, Ontario; M. Gourdeau and G. Murray, Hopital de l'Enfant-Jesus, Laval University, Quebec City, Quebec; S. Richardson and A. Matlow, Hospital for Sick Children, Toronto, Ontario; G. J. Hardy, Saint John Regional Hospital, Saint John, New Brunswick; P.R. Laberge, Centre Hospitalier Regional de Sept-Iles, Sept-Iles, Quebec; L. P. Abbott, Queen Elizabeth Hospital, Charlottetown, Prince Edward Island; M. Yorke, Westman Regional Laboratory, Brandon, Manitoba; N. Clerk and Z. Mooloo, William Osler Health Center, Etobicoke, Ontario; J. Downey and P. Da Camara, Toronto East General and Orthopedic Hospital, Inc., Toronto, Ontario; M. Bergeron, CHUQ-Ctr Hopital, Université Laval, Sainte-Foy, Québec; J. Hutchinson, Health Care Corp., St. John's, Newfoundland; S. Krajden, St. Joseph's Health Care Center, Toronto, Ontario; R. Price, Royal Victoria Hospital, Barrie, Ontario; J. F. Paradis, Hopital de Chicoutimi, Chicoutimi, Quebec; L. Bocci, Regional Hospital Centre, Bathurst, New Brunswick; M. Savard and David Thompson Regional Laboratories, Red Deer, Alberta; K. Ostrowska, Trillium Health Centre, Mississauga, Ontario; P. Pieroni, Provincial Laboratory, Regina, Saskatchewan; B. Mederski, North York General Hospital, Toronto, Ontario; C. Tremblay, Hotel Dieu de Quebec, Quebec City, Quebec; P. Leighton, Dr. Everett Chalmers Hospital, Fredericton, Nova Scotia; D. Yamamura and A. Sarabia, MDS Laboratories, Toronto; and D. Noria and A. Gelbloom, The Scarborough Hospital, Toronto.
REFERENCES
- 1.Anonymous. 1997. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. Recomm. Rep. 46:1-24. [PubMed] [Google Scholar]
- 2.Anonymous. 1999. Pneumococcal vaccines: World Health Organization position paper. Wkly. Epidemiol. Rec. 74:177-183. [PubMed] [Google Scholar]
- 3.Bartlett, J. G., S. F. Dowell, L. A. Mandell, T. M. File, Jr., D. M. Musher, and A. Fine. 2000. Practice guidelines for the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 31:347-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bast, D. J., D. E. Low, C. L. Duncan, L. Kilburn, L. A. Mandell, R. J. Davidson, and J. C. de Azavedo. 2000. Fluoroquinolone resistance in clinical isolates of Streptococcus pneumoniae: contributions of type II topoisomerase mutations and efflux to levels of resistance. Antimicrob. Agents Chemother. 44:3049-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, D., A. McGeer, J. C. de Azavedo, D. E. Low, and the Canadian Bacterial Surveillance Network. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N. Engl. J. Med. 341:233-239. [DOI] [PubMed] [Google Scholar]
- 6.Davidson, R., R. Cavalcanti, J. L. Brunton, D. J. Bast, J. C. de Azavedo, P. Kibsey, C. Fleming, and D. E. Low. 2002. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N. Engl. J. Med. 346:747-750. [DOI] [PubMed] [Google Scholar]
- 7.Doit, C., C. Loukil, F. Fitoussi, P. Geslin, and E. Bingen. 1999. Emergence in France of multiple clones of clinical Streptococcus pneumoniae isolates with high-level resistance to amoxicillin. Antimicrob. Agents Chemother. 43:1480-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowell, S. F., T. Smith, K. Leversedge, and J. Snitzer. 1999. Failure of treatment of pneumonia associated with highly resistant pneumococci in a child. Clin. Infect. Dis. 29:462-463. [DOI] [PubMed] [Google Scholar]
- 9.Empey, P. E., H. R. Jennings, A. C. Thornton, R. P. Rapp, and M. E. Evans. 2001. Levofloxacin failure in a patient with pneumococcal pneumonia. Ann. Pharmacother. 35:687-690. [DOI] [PubMed] [Google Scholar]
- 10.Feikin, D. R., A. Schuchat, M. Kolczak, N. L. Barrett, L. H. Harrison, L. Lefkowitz, A. McGeer, M. M. Farley, D. J. Vugia, C. Lexau, K. R. Stefonek, J. E. Patterson, and J. H. Jorgensen. 2000. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995-1997. Am. J. Public Health 90:223-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felmingham, D., R. R. Reinert, Y. Hirakata, and A. Rodloff. 2002. Increasing prevalence of antimicrobial resistance among isolates of Streptococcus pneumoniae from the PROTEKT surveillance study, and compatative in vitro activity of the ketolide, telithromycin. J. Antimicrob. Chemother. 50(Suppl. S)1:25-37. [DOI] [PubMed] [Google Scholar]
- 12.Gay, K., W. Baughman, Y. Miller, D. Jackson, C. G. Whitney, A. Schuchat, M. M. Farley, F. Tenover, and D. S. Stephens. 2000. The emergence of Streptococcus pneumoniae resistant to macrolide antimicrobial agents: a 6-year population-based assessment. J. Infect. Dis. 182:1417-1424. [DOI] [PubMed] [Google Scholar]
- 13.Gehanno, P., L. N′Guyen, M. Derriennic, F. Pichon, J. M. Goehrs, and P. Berche. 1998. Pathogens isolated during treatment failures in otitis. Pediatr. Infect. Dis. J. 17:885-890. [DOI] [PubMed] [Google Scholar]
- 14.Hausdorff, W. P., J. Bryant, P. R. Paradiso, and G. R. Siber. 2000. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use: part I. Clin. Infect. Dis. 30:100-121. [DOI] [PubMed] [Google Scholar]
- 15.Heffelfinger, J. D., S. F. Dowell, J. H. Jorgensen, K. P. Klugman, L. R. Mabry, D. M. Musher, J. F. Plouffe, A. Rakowsky, A. Schuchat, and C. G. Whitney. 2000. Management of community-acquired pneumonia in the era of pneumococcal resistance: a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Arch. Intern. Med. 160:1399-1408. [DOI] [PubMed] [Google Scholar]
- 16.Hoban, D., K. Waites, and D. Felmingham. 2003. Antimicrobial susceptibility of community-acquired respiratory tract pathogens in North America in 1999-2000: findings of the PROTEKT surveillance study. Diagn. Microbiol. Infect. Dis. 45:251-259. [DOI] [PubMed] [Google Scholar]
- 17.Hsueh, P. R., L. J. Teng, T. L. Wu, D. Yang, W. K. Huang, J. M. Shyr, Y. C. Chuang, J. H. Wan, J. J. Yan, J. J. Lu, J. J. Wu, W. C. Ko, F. Y. Chang, Y. C. Yang, Y. J. Lau, Y. C. Liu, C. M. Lee, H. S. Leu, C. Y. Liu, and K. T. Luh. 2003. Telithromycin- and fluoroquinolone-resistant Streptococcus pneumoniae in Taiwan with high prevalence of resistance to macrolides and beta-lactams: SMART program 2001 data. Antimicrob. Agents Chemother. 47:2145-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyde, T. B., K. Gay, D. S. Stephens, D. J. Vugia, M. Pass, S. Johnson, N. L. Barrett, W. Schaffner, P. R. Cieslak, P. S. Maupin, E. R. Zell, J. H. Jorgensen, R. R. Facklam, and C. G. Whitney. 2001. Macrolide resistance among invasive Streptococcus pneumoniae isolates. JAMA 286:1857-1862. [DOI] [PubMed] [Google Scholar]
- 19.Infectious Diseases and Immunization Committee, C. P. S. C. 2001. Therapy of suspected bacterial meningitis in Canadian children six weeks of age and older. Pediatr. Child Health 6:147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs, M. R., D. Felmingham, P. C. Appelbaum, and R. N. Gruneberg. 2003. The Alexander Project 1998-2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J. Antimicrob. Chemother. 52:229-246. [DOI] [PubMed] [Google Scholar]
- 21.Janoir, C., V. Zeller, M. D. Kitzis, N. J. Moreau, and L. Gutmann. 1996. High-level fluoroquinolone resistance in Streptococcus pneumoniae requires mutations in parC and gyrA. Antimicrob. Agents Chemother. 40:2760-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston, N. J., J. C. de Azavedo, J. D. Kellner, and D. E. Low. 1998. Prevalence and characterization of the mechanisms of macrolide, lincosamide, and streptogramin resistance in isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2425-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones, M. E., R. S. Blosser-Middleton, I. A. Critchley, J. A. Karlowsky, C. Thornsberry, and D. F. Sahm. 2003. In vitro susceptibility of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis: a European multicenter study during 2000-2001. Clin. Microbiol. Infect. 9:590-599. [DOI] [PubMed] [Google Scholar]
- 24.Jones, R. N., D. J. Biedenbach, and M. L. Beach. 2003. Influence of patient age on the susceptibility patterns of Streptococcus pneumoniae isolates in North America (2000-2001): report from the SENTRY Antimicrobial Surveillance Program. Diagn. Microbiol. Infect. Dis. 46:77-80. [DOI] [PubMed] [Google Scholar]
- 25.Karlowsky, J. A., C. Thornsberry, M. E. Jones, A. T. Evangelista, I. A. Critchley, and D. F. Sahm. 2003. Factors associated with relative rates of antimicrobial resistance among Streptococcus pneumoniae in the United States: results from the TRUST Surveillance Program (1998-2002). Clin. Infect. Dis. 36:963-970. [DOI] [PubMed] [Google Scholar]
- 26.Kays, M. B., D. W. Smith, M. E. Wack, and G. A. Denys. 2002. Levofloxacin treatment failure in a patient with fluoroquinolone-resistant Streptococcus pneumoniae pneumonia. Pharmacotherapy 22:395-399. [DOI] [PubMed] [Google Scholar]
- 27.Kays, M. B., M. F. Wack, D. W. Smith, and G. A. Denys. 2002. Azithromycin treatment failure in community-acquired pneumonia caused by Streptococcus pneumoniae resistant to macrolides by a 23S rRNA mutation. Diagn. Microbiol. Infect. Dis. 43:163-165. [DOI] [PubMed] [Google Scholar]
- 28.Kupronis, B. A., C. L. Richards, and C. G. Whitney. 2003. Invasive pneumococcal disease in older adults residing in long-term care facilities and in the community. J. Am. Geriatr. Soc. 51:1520-1525. [DOI] [PubMed] [Google Scholar]
- 29.Lagrou, K., W. E. Peetermans, J. Verhaegen, S. Van Lierde, L. Verbist, and J. Van Eldere. 2000. Macrolide resistance in Belgian Streptococcus pneumoniae. J. Antimicrob. Chemother. 45:119-121. [DOI] [PubMed] [Google Scholar]
- 30.Leclercq, R., and P. Courvalin. 1991. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob. Agents Chemother. 35:1267-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim, S., D. Bast, A. McGeer, J. de Azavedo, and D. E. Low. 2003. Antimicrobial susceptibility breakpoints and first-step parC mutations in Streptococcus pneumoniae: redefining fluoroquinolone resistance. Emerg. Infect. Dis. 9:833-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lonks, J. R., J. Garau, L. Gomez, M. Xercavins, D. E. Ochoa, I. F. Gareen, P. T. Reiss, and A. A. Medeiros. 2002. Failure of macrolide antibiotic treatment in patients with bacteremia due to erythromycin-resistant Streptococcus pneumoniae. Clin. Infect. Dis. 35:556-564. [DOI] [PubMed] [Google Scholar]
- 33.Low, D. E., J. de Azavedo, C. A. Weiss, T. Mazzulli, M. Kuhn, D. Church, K. Forward, G. Zhanel, A. E. Simor, Canadian Bacterial Surveillance Network, and A. McGeer. 2002. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in Canada during 2000. Antimicrob. Agents Chemother. 46:1295-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandell, L. A., T. J. Marrie, R. F. Grossman, A. W. Chow, and R. H. Hyland. 2000. Canadian guidelines for the initial management of community-acquired pneumonia: an evidence-based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. Clin. Infect. Dis. 31:383-421. [DOI] [PubMed] [Google Scholar]
- 35.Marchese, A., E. Tonoli, E. A. Debbia, and G. C. Schito. 1999. Macrolide resistance mechanisms and expression of phenotypes among Streptococcus pneumoniae circulating in Italy. J. Antimicrob. Chemother. 44:461-464. [DOI] [PubMed] [Google Scholar]
- 36.McCormick, A. W., C. G. Whitney, M. M. Farley, R. Lynfield, L. H. Harrison, N. M. Bennett, W. Schaffner, A. Reingold, J. Hadler, P. Cieslak, M. H. Samore, and M. Lipsitch. 2003. Geographic diversity and temporal trends of antimicrobial resistance in Streptococcus pneumoniae in the United States. Nat. Med. 9:424-430. [DOI] [PubMed] [Google Scholar]
- 37.McGeer, A., and D. E. Low. 2003. Is resistance futile? Nat. Med. 9:390-392. [DOI] [PubMed] [Google Scholar]
- 38.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 39.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial disk susceptibility tests, 7th ed. Approved standard M2-A7. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 40.National Committee for Clinical Laboratory Standards. 2004. Performance standards for antimicrobial susceptibility testing. Fourteenth informational supplement M100-14. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 41.Niederman, M. S., L. A. Mandell, A. Anzueto, J. B. Bass, W. A. Broughton, G. D. Campbell, N. Dean, T. File, M. J. Fine, P. A. Gross, F. Martinez, T. J. Marrie, J. F. Plouffe, J. Ramirez, G. A. Sarosi, A. Torres, R. Wilson, and V. L. Yu. 2001. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am. J. Respir. Crit. Care Med. 163:1730-1754. [DOI] [PubMed] [Google Scholar]
- 42.Perez-Trallero, E., J. M. Marimon, L. Iglesias, and J. Larruskain. 2003. Fluoroquinolone and macrolide treatment failure in pneumococcal pneumonia and selection of multidrug-resistant isolates. Emerg. Infect. Dis. 9:1159-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richardson, D. C., D. Bast, A. McGeer, and D. E. Low. 2001. Evaluation of susceptibility testing to detect fluoroquinolone resistance mechanisms in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:1911-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahm, D. F., D. E. Peterson, I. A. Critchley, and C. Thornsberry. 2000. Analysis of ciprofloxacin activity against Streptococcus pneumoniae after 10 years of use in the United States. Antimicrob. Agents Chemother. 44:2521-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simor, A. E., M. Louie, The Canadian Bacterial Surveillance Network, and D. E. Low. 1996. Canadian national survey of prevalence of antimicrobial resistance among clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 40:2190-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutcliffe, J., A. Tait-Kamradt, and L. Wondrack. 1996. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob. Agents Chemother. 40:1817-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tait-Kamradt, A., T. Davies, P. C. Appelbaum, F. Depardieu, P. Courvalin, J. Petitpas, L. Wondrack, A. Walker, M. R. Jacobs, and J. Sutcliffe. 2000. Two new mechanisms of macrolide resistance in clinical strains of Streptococcus pneumoniae from Eastern Europe and North America. Antimicrob. Agents Chemother. 44:3395-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urban, C., N. Rahman, X. Zhao, N. Mariano, S. Segal-Maurer, K. Drlica, and J. J. Rahal. 2001. Fluoroquinolone-resistant Streptococcus pneumoniae associated with levofloxacin therapy. J. Infect. Dis. 184:794-798. [DOI] [PubMed] [Google Scholar]
- 49.Walsh, F., J. Willcock, and S. Amyes. 2003. High-level telithromycin resistance in laboratory-generated mutants of Streptococcus pneumoniae. J. Antimicrob. Chemother. 52:345-353. [DOI] [PubMed] [Google Scholar]
- 50.Weiss, K., C. Restieri, M. Laverdiere, A. McGeer, R. J. Davidson, L. Kilburn, D. J. Bast, J. de Azavedo, and D. E. Low. 2001. A nosocomial outbreak of fluoroquinolone-resistant Streptococcus pneumoniae. Clin. Infect. Dis. 33:517-522. [DOI] [PubMed] [Google Scholar]
- 51.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, and A. Schuchat. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737-1746. [DOI] [PubMed] [Google Scholar]
- 52.Yu, V. L., C C. Chiou, C. Feldman, A. Ortqvist, J. Rello, A. J. Morris, L. M. Baddour, C. M. Luna, D. R. Snydman, M. Ip, W. C. Ko, M. B. Chedid, A. Andremont, and K. P. Klugman. 2003. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin. Infect. Dis. 37:230-237. [DOI] [PubMed] [Google Scholar]