Abstract
Zebrafish are increasingly used in auditory studies, in part due to the development of several transgenic lines that express hair cell-specific fluorescent proteins. However, it is largely unknown how transgene expression influences auditory phenotype. We previously observed reduced auditory sensitivity in adult Brn3c:mGFP transgenic zebrafish, which express membrane-bound green fluorescent protein (GFP) in sensory hair cells. Here, we examine the auditory sensitivity of zebrafish from multiple transgenic and background strains. We recorded auditory evoked potentials in adult animals and observed significantly higher auditory thresholds in three lines that express hair cell-specific GFP. There was no obvious correlation between hair cell density and auditory thresholds, suggesting that reduced sensitivity was not due to a reduction in hair cell density. FM1-43 uptake was reduced in Brn3c:mGFP fish but not in other lines, suggesting that a mechanotransduction defect may be responsible for the auditory phenotype in Brn3c animals, but that alternate mechanisms underlie the increased AEP thresholds in other lines. We found reduced prepulse inhibition (a measure of auditory-evoked behavior) in larval Brn3c animals, suggesting that auditory defects develop early in this line. We also found significant differences in auditory sensitivity between adults of different background strains, akin to strain differences observed in mouse models of auditory function. Our results suggest that researchers should exercise caution when selecting an appropriate zebrafish transgenic or background strain for auditory studies.
Keywords: Zebrafish, fluorescent protein, hearing, hair cell, transgenic
1. Introduction
Transgenic organisms that express fluorescent proteins (FP), most often variants of green fluorescent protein (GFP), are widely used to study auditory development and function and to understand the causes and consequences of ototoxic insults (e.g., McDermott et al., 2010; Sweet et al., 2011; Coffin et al., 2013; Huang et al., 2013; Sheets et al., 2014). For example, the transcription factor Atoh1 is sufficient for hair cell specification and Atoh1-GFP constructs have been used as markers of hair cell development or as drivers of hair cell regeneration (Zheng and Gao, 2000; Woods et al., 2004; Jones et al., 2006; Lin et al., 2011; Lewis et al., 2012). In the lateral line system in fishes, GFP has been used as a sensory hair cell marker for a range of studies, including research on hair cell death and regeneration (Moon et al., 2011; Namdaran et al., 2012; Coffin et al., 2013). Studies in larval zebrafish engineered to express cytoplasmic or organelle-specific modified FPs demonstrate sequential calcium-dependent signaling events in toxin-treated sensory hair cells, further demonstrating the power of FP reporters for auditory research (Esterberg et al., 2013, 2014). Collectively, these studies show the importance of fluorescent protein expression systems for research on the auditory periphery.
Fluorescent protein expression is generally considered benign (Heim et al., 1994). However, GFP expression in vitro can cause side effects including altered cell physiology and apoptosis (Liu et al., 1999; Zhang et al., 2003; Baens et al., 2006; Coumans et al., 2014). Similarly, GFP expression in transgenic mice is associated with multiple pathologies, including heart dysfunction and neuronal toxicity (Huang et al., 2000; Krestel et al., 2004). We previously observed that adult transgenic Brn3c:mGFP zebrafish, which express membrane-bound GFP in hair cells, had elevated auditory thresholds compared to wildtype controls (Uribe et al. 2013a, and unpublished observations). These findings are significant because zebrafish are an important model for investigating the genetics and development of the inner ear, ototoxicity studies, and hair cell regeneration (Whitfield, 2002; Goodrich, 2005; Nicolson, 2005; Brignull et al., 2009; Coffin et al., 2014).
Zebrafish have both an inner ear and a mechanosensory lateral line system. Hair cells in both systems are homologous to mammalian hair cells and show similar responses to ototoxic damage (Popper and Fay, 1999; Harris et al., 2003; Coffin et al., 2004; Ou et al., 2010). Lateral line hair cells act as low-frequency detectors of near-field vibratory stimuli, while inner ear hair cells detect both near-field and far-field acoustic stimuli and vestibular cues (Kalmijin, 1988; Fay and Simmons, 1999; McHenry and Van Netten, 2007). Hair cells in the adult zebrafish inner ear can detect sound frequencies up to 4000 Hz, while larvae are sensitive to frequencies up to 1200 Hz (Wang et al., 2015). Larvae may not be sensitive to higher frequencies due to the relatively late development of peripheral auditory structures such as Weberian ossicles, although the upper limit of larval zebrafish hearing has not been thoroughly explored (Higgs et al., 2002; Zeddies and Fay, 2005; Bhandiwad et al., 2013; Lu and DeSmidt, 2013).
Here we investigate whether several transgenic or background zebrafish lines used in hearing studies have altered inner ear morphology or auditory sensitivity. We show that hair cell-specific GFP expression is associated with significantly reduced auditory sensitivity in the adults of three different transgenic zebrafish lines. We also show significant differences in adult auditory sensitivity across background strains. Our data suggest that the choice of transgenic or background line may have significant consequences for the experimental outcome and limit comparisons across studies using different strains.
2. Materials and Methods
2. 1 Zebrafish husbandry and lines
Adult zebrafish (Danio rerio) were maintained in the zebrafish facilities of Washington State University Vancouver or Western Kentucky University. The adult zebrafish used in this study were at least 6 months old with a total length of 29–55 mm and were a mix of males and females. All larval zebrafish used for mechanotransduction or acoustic startle assays were 5–7 days post-fertilization (dpf) (sex not yet determined) and were the products of natural pairwise or group mating in the animal care facilities at Washington State University Vancouver. All experiments were approved by Institutional Animal Care and Use Committees from the institutions where the experiments were performed (Washington State University, University of Washington, or Western Kentucky University).
We tested four non-transgenic zebrafish lines: *AB, WIK, Tupfel long fin (TL), and a line of unknown genetic background obtained from a Florida zebrafish breeder, which we designate here as “outbred”. The outbred strain was used as a proxy for true “wild” zebrafish, and because fish from this breeder have been previously used for auditory studies (Schuck and Smith, 2009; Schuck et al., 2011). *AB and TL fish (purchased from ZIRC) were maintained in the Coffin Laboratory at Washington State University Vancouver as inbred lines. WIK fish were a kind gift from A. Nechiporuk at Oregon Health & Science University, also maintained as an inbred line. Outbred fish were purchased from a commercial supplier (Segrest Farms, Gibsonton, FL) and housed in the Animal Care Facility at Western Kentucky University.
Six transgenic lines were used for larval or adult experiments. Four of these were created on *AB backgrounds (Myo6, Bcl2, NeuroD, α-tubulin) and one on a TL background (ET4). The sixth line (Brn3c) is on a mixed genetic background. Tg(pou4f3:GAP-GFP) (Brn3c) fish express membrane-bound GFP under control of a brn3c (pou4f3) enhancer, created on a TL background, then outcrossed to Tübingen (Tu), and then later crossed to *AB for at least two generations before incrossing (Xiao et al., 2005; T. Linbo, personal communication). Tg(myo6b:EGFP-Bcl2) fish (Bcl2) express the pro-survival protein Bcl2 fused to the C-terminal of EGFP, driven by the myo6b promoter and created on the *AB background. The Brn3c and Bcl2 lines primarily express GFP in sensory hair cells of the inner ear and lateral line, with some expression in the eye and optic tectum in Brn3c fish. Hair cells in Brn3c fish express GFP in the plasma membrane, while in hair cells of Bcl2 fish, GFP is found in the smooth ER, mitochondrial membranes, nuclear envelope, and cytosol (Coffin et al., 2013). Tg(myo6b:EGFP) fish, created on a *AB background, are identical to the Bcl2 line, except that the GFP is not fused to a second protein; this line was created as a control for the Bcl2 line and expresses cytoplasmic GFP (Suli et al., 2014; A. Coffin, unpublished data). ET4 fish, a kind gift from A.J. Hudspeth at the Rockefeller University, were created on a TL background, arose from an enhancer trap screen, and express EGFP in hair cell cytoplasm (Parinov et al., 2004).
Tg(α-tubulin:tdTomato) X Tg(h2afv:GFP) fish are a cross between two transgenic lines, designated here as “α-tubulin” for identification. The first line, created on a *AB background, expresses tdTomato (a derivative of DsRed) under the control of the α-tubulin promoter, leading to FP signal in the cytosol of hair cells and some neurons (Ma and Raible, 2009; and T. Schilling, personal communication). The second line expresses a histone (H2AZ)-GFP fusion protein in all cell nuclei (Pauls et al., 2001). The original genetic background is unknown but it has been outcrossed to *AB, then crossed again to the α-tubulin:tdTomato fish for a primarily *AB background. Tg(NeuroD:tagRFP) fish (NeuroD) express cytoplasmic tagRFP under control of the NeuroD promoter and were created on a *AB background (McGraw et al., 2012). These fish express the transgene in dorsal root ganglion neurons and a subset of other neurons, but not in hair cells, serving as a control for hair cell-specific transgene expression. Bcl2, Myo6, and NeuroD fish were all created on an inbred *AB background maintained at the University of Washington so the genetic background of these lines is highly similar. Similarly, the Brn3c and α-tubulin fish were crossed into the same *AB background as these other three lines. All transgenic lines were raised in the Coffin Lab at Washington State University. Fish sizes for each line are shown in Table 1 and images of all transgenic lines are shown in Figure 1. For ease of visualization we have elected to primarily show larval stages.
Table 1.
Sizes of adult animals from each fish line. Length was measured as the total length (snout to tip of tail, T.L.). Data are presented as the size range, followed by the average. Fish were all between 12 and 22 months of age.
| Strain | *AB | α-tubulin | NeuroD | Brn3c | Bcl2 | WIK | Myo6 | ET4 | TL |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| T.L Min-Max | 30–46 | 34–43 | 32–38 | 30–40 | 29–40 | 33–41 | 35–40 | 52–58 | 44–55 |
| Ave (mm) | 36.8 | 38.73 | 35.38 | 36.35 | 35.5 | 36.2 | 38.3 | 54.2 | 48.3 |
|
| |||||||||
| Wt. Min-Max | 0.28–1.05 | 0.43–0.95 | 0.25–0.58 | 0.27–0.89 | 0.2–0.94 | 0.14–0.7 | 0.45–0.7 | 0.66–0.81 | 0.27–0.75 |
| Ave (g) | 0.56 | 0.64 | 0.41 | 0.49 | 0.49 | 0.42 | 0.59 | 0.75 | 0.50 |
Fig. 1.
Transgene expression profiles for zebrafish lines used in this study. (A) Brightfield image of 5 dpf *AB zebrafish head. The otoliths of the inner ear are clearly visible. (B) *AB larva stained with the vital dye DASPEI, showing the location of lateral line neuromasts (yellow dots). (C) Lateral view of a Brn3c:mGFP fish shows GFP expression in lateral line neuromasts, inner ear epithelia, and retinal tectum. (D) GFP expression in the myo6:EGFP-Bcl2 larva is localized to lateral line neuromasts and inner ear epithelia. Note that the heart also expresses GFP as a transgenesis marker, but the heart GFP is not fused to Bcl2. (E) Myo6:EGFP larvae express bright cytoplasmic GFP in lateral line and inner ear hair cells. (F) ET4 larvae express GFP in all hair cells. (G) The α-tubulin:tdTomato larva expresses the red fluorescent protein tdTomato in all hair cells, and shows diffuse expression in multiple brain regions. (H) Lateral view of NeuroD:tagRFP transgenic fish shows RFP expressed in multiple brain regions as well as the pancreas, but not in the inner ear or lateral line. Scale bar in A equals 250 μm and applies to all larval head images (A–H). (I) Lateral view of Brn3c:mGFP adult with neuromasts visible as rows of fluorescent dots around the eye and on the gill cover. The brain and inner ear fluorescence is obscured by skin pigmentation. The scale bar in G is 1 mm.
2.2 Auditory evoked potentials
To measure adult hearing thresholds, we performed auditory evoked potential (AEP) recordings (Corwin et al., 1982, Kenyon et al., 1998) from 6–13 fish from each zebrafish line, except *AB (n=22) and ET4 (n=4). Individual fish were anaesthetized with 100 ppm MS-222 (Sigma-Aldrich, St. Louis, MO), restrained in a mesh harness, and suspended under water in a 19 L vessel. Each fish was positioned so that the top of its head was approximately 6 cm below the surface and 22 cm above the underwater speaker. Three stainless steel subdermal electrodes (27 gauge, Rochester Electro-Medical, Inc., Lutz, FL) were inserted approximately 2 mm subdermally into the fish to record AEPs. The reference electrode was inserted into the medial dorsal surface of the head anterior to and between the eyes. The recording electrode was placed along the dorsal midline approximately halfway between the anterior edge of the dorsal fin and the posterior edge of the operculae, directly over the brainstem. The ground electrode was placed in the tail musculature.
A TDT System III physiology apparatus (Tucker-Davis Technologies Inc., Alachua, FL) operating SigGen and BioSig software was used to present sound stimuli (tone pips) and collect AEP waveforms. Auditory stimuli were passed through a P1000 power amplifier (Hafler, Port Coquitlam, BC) connected to a University Sound UW-30 underwater speaker (Electro-Voice, Fairport, NY) (Smith et al., 2004a,b; Smith et al., 2006; Sun et al., 2011). Auditory thresholds were determined at 8 frequencies for each fish (100, 250, 400, 600, 800, 1000, 1500 and 3000 Hz). The sound pressure levels of each presented frequency were confirmed using a calibrated underwater hydrophone (calibration sensitivity of −195 dB re 1 V/mPa, GRAS Type 10CT, Denmark), placed proximately to the fish harnessing apparatus. Auditory thresholds were determined in 5 dB increments by visual inspection of AEP waveforms, using the procedure previously described by Smith et al. (2004b, 2006). AEP threshold data were analyzed by 2-way ANOVA using frequency and fish line as independent variables.
2.3 Acoustic startle response
After confirming GFP expression at 3 days post-fertilization (dpf), embryos were transported from Washington State University to the University of Washington where they were kept in incubators at 28°C until testing at 6–7 dpf.
Experimental procedures for behavioral testing were conducted as in Bhandiwad et al. (2013). Cohorts of 24 fish were placed in individual wells of a 96-well plate mounted onto a vertically oriented Brüel-Kjær Type 4810 shaker (Nærum, Denmark). This setup allows for acoustic/vibratory stimuli to be delivered primarily in the dorsoventral axis of the fish. The experimental apparatus was housed inside a sound attenuation chamber (Industrial Acoustics, North Aurora, IL) on a vibration-isolation air table to minimize external vibratory noise. A custom MATLAB script was used to relay the stimulus signal to a Brüel & Kjær Type 2710 amplifier via a Tucker Davis Technologies (TDT) System III device. Stimulus generation, capture, and TDT System III were controlled using Matlab and ActiveX software (Microsoft Corp., Redmond, WA). Behavioral responses were recorded using a Photron Fastcam 1024PCI (Photron USA, Inc., San Diego, CA) at 1000 fps (512 × 512 pixel resolution) synchronized to the vibratory stimulus via a TTL pulse triggered through the TDT System III.
For the acoustic startle threshold experiments, each replicate (defined as one plate containing 24 fish arranged in a 6×4 array) consisted of stimuli at frequencies of 90, 190, and 310 Hz, and at a sound level of 14 dB to −10 dB re. 1 m/s2 (varied in steps of 6 dB). That is, each plate of fish was presented with 35 stimuli presented in a repeated measures design. These frequencies were chosen because 5 dpf zebrafish are highly sensitive to frequencies in the 90–310 Hz range (Bhandiwad et al., 2013), so it was assumed that any potential strain-specific differences would most likely be apparent at these frequencies. These trials were separated by a randomized inter-trial interval of 70 ± 10 s in order to reduce habituation. The behavioral responses were measured for 50 ms after stimulus onset. For each trial, responses for each fish were coded binomially (1 for response, 0 for non-response). If no fish responded to a particular stimulus, the response was coded as having a threshold of 20 dB, one step (6 dB) above the highest presented stimulus level. The binomial response data collected from each plate were analyzed using a curve-fitting procedure. For each frequency, binomial responses at each stimulus level were averaged and converted to a response percentage. Thresholds for each frequency were determined by fitting the response percentages with a Weibull cumulative function using a maximum likelihood method. Thresholds were interpolated from the fitted function and were defined as the stimulus level at which the startle response could be reliably elicited > 5% of the time.
The experimental procedure for the prepulse inhibition (PPI) experiments was similar to the startle response experiments except that a frequency of 820 Hz at 20 dB re: 1 m/s2 was used as a universal startle-stimulus (Bhandiwad et al., 2013). The number of frequencies was the same as in the startle trials (90, 190, 310 Hz). Each replicate consisted of 20 trials, with four sound levels for each frequency presented in random order. These sound levels were empirically determined as the four largest sub-startle threshold levels for each frequency. A PPI trial consisted of a 100 ms randomized prepulse stimulus with a 24 ms ramp time followed by the startle stimulus. The inter-pulse interval, or the time between the end of the prepulse tone and the beginning of the startle tone, was 70 ms, which we determined previously (Bhandiwad et al., 2013). Each PPI-startle stimulus presentation (trial) was preceded by a no prepulse ‘catch’ trial in order to determine baseline startle response probability. The catch trial also controlled for possible habituation to the stimuli. The PPI effect was calculated as the difference between the percent response to the prepulse trial and the mean response probability of the catch trials immediately preceding and following the prepulse trial. For all prepulse experiments, plates of fish were presented with no more than 20 total (prepulse and catch) stimuli to minimize habituation. After 20 presentations, fish were replaced with naïve fish from the same cohort. Thus, for each dataset we used a total of 48 fish from the same clutch.
In the PPI experiments, binomial response data were converted into response percentage, as in the case of the startle experiments. However, this response percentage was subtracted from the mean of the paired no-prepulse catch trials before and after stimulus presentation. This yielded a difference in startle probability from the expected value. In cases where the difference was negative, the difference was set to zero. This difference was then fitted to a cumulative Weibull distribution. The threshold for the inhibition of the startle response was determined for each prepulse frequency tested and was defined as the stimulus level that elicited a 5% reduction of the probability of startle response between PPI trials and the paired catch trials.
For both startle response and PPI experiments, data were analyzed using the Friedman test with frequency and fish line as independent variables, followed by post-hoc analysis using a Wilcoxon rank-sum test.
2.4 Inner ear analysis
We quantified hair cell density in inner ear end organs to ask if cell number was correlated with differences in auditory evoked potentials (Smith et al., 2006; Oxman et al., 2007). After euthanizing adults in an ice-water bath as per American Veterinary Medical Association (AVMA) recommendation, the lower jaw was removed and the bony capsule surrounding the inner ear was punctured, and the head was fixed in 4% paraformaldehyde for either 4 hours at room temperature or overnight at 4 °C. Heads were rinsed 3 times in 0.1 M phosphate-buffered saline (PBS, pH 7.4, Life Technologies, Eugene, OR) and the ears were isolated from the bony capsule with fine forceps, the otoliths were removed, and the inner ear epithelia (saccule, utricle, and lagena) were carefully dissected out and trimmed.
To visualize filamentous actin in hair bundles, epithelia were incubated for 30 minutes at room temperature in Alexa Fluor® 488 Oregon Green or 568 Rhodamine Phalloidin (Life Technologies) diluted 1:100 in PBS. We used two different fluorophores to provide contrast with the transgene expressed by each line (e.g., if a transgenic line expressed GFP in hair cells, rhodamine phalloidin was used for contrast). Epithelia were rinsed once in PBS and mounted on a glass slide with coverslip using Fluoromount-G (Southern Biotech, Birmingham, AL).
Hair bundles were imaged on a Leica SP8 confocal microscope (Leica Microsystems, Buffalo Grove, IL) using a 20x objective lens and 3x digital zoom. Total saccular length was determined for each sample and five images were taken along the midline at 5, 25, 50, 75, and 90% positions from the rostral end of the saccule, based on a similar sampling strategy by Smith et al. (2006) and Oxman et al. (2007). Four images were taken for each utricle, with one in the extrastriolar region (centered along the rostral-caudal and dorsal-lateral axes) and three locations within the striolar region. Likewise, three representative striolar regions were chosen for the lagena. Hair bundle density counts were performed using ImageJ with the cell counter plugin (v.1.47; National Institutes of Health, Bethesda, MD). All bundles were counted within a 2500 μm2 region centered on each image. Bundle count data were analyzed separately for each epithelium using a 2-way ANOVA with fish line and epithelial region as independent variables. Posthoc analysis used a Bonferroni test to correct for multiple pairwise comparisons, comparing each transgenic line to the background strain for that line. For comparison of wildtype strains, *AB were selected as the comparison strain. GraphPad Prism (v. 6, San Diego, CA) was used for statistical analysis.
2.5 Mechanotransduction assay
We performed a vital dye uptake assay to ask if fluorescent protein expression altered mechanotransduction channel function. This assay was performed in larval zebrafish because their hair cells are functionally mature by 5 dpf and are easily viewed in vivo (Murakami et al., 2003; Santos et al., 2006). Larvae were immersed in 3 μM FM1-43 (Life Technologies) in E2 embryo medium (EM) for 30 seconds (Seiler and Nicolson, 1999; Westerfield, 2000; Gale et al., 2001; Meyers et al., 2003). As a positive control, a higher concentration of CaCl2 was used as a mechanotransduction channel blocker (Ricci and Fettiplace, 1998; Ricci et al., 1998; Coffin et al., 2009). *AB wildtype larvae were treated for 10 minutes in calcium-supplemented EM for a final concentration of 2100 μM CaCl2, then incubated in FM1-43 for 30 s with the same calcium concentration. All FM1-43 treatments were followed by four consecutive 30 s EM washes, anesthesia with buffered 0.001% MS-222, and immediate imaging using a Leica SP8 confocal microscope.
Fish were placed in the center of a raised triple coverslip and larvae were carefully rotated on their side, exposing the MI1 neuromast (located posterior to the eye; Raible and Kruse, 2000). A 40X water immersion lens was used to image neuromasts. FM1-43 fluorescence was excited using a 488 nm laser and emissions were collected between 595–738 nm. Z-sections of each neuromast were taken at 0.5 μm intervals to a total depth of approximately 20 μm. All neuromasts for a given experiment were measured with the same gain and laser intensity settings. Maximum projection images were created from each image stack. To quantify fluorescence, mean background fluorescence from a non-neuromast region was subtracted from mean FM1-43 intracellular fluorescence to generate standardized mean FM1-43 fluorescence for each MI1 neuromast. FM1-43 fluorescence was normalized to *AB (wildtype) neuromast fluorescence from that experimental day to control for FM1-43 uptake variation between experiments. Normalized fluorescent intensity was analyzed in Prism by 1- way ANOVA with fish line as the independent variable, followed by Bonferroni-corrected pairwise comparisons.
3. Results
3. 1 Auditory evoked potentials
In a previous study, we found that adult transgenic Brn3c zebrafish that express GFP in sensory hair cells exhibited reduced auditory sensitivity compared to wildtype animals (Uribe et al. 2013a and unpublished data). Based on this observation, we investigated whether several transgenic zebrafish used in auditory research have altered hearing. These transgenic lines are shown in Figure 1. Brn3c, Myo6, and ET4 fish all express GFP in inner ear and lateral line hair cells; Myo6 and ET4 fish express cytoplasmic GFP, while in Brn3c fish, GFP is expressed at the plasma membrane. Bcl2 fish express a GFP-Bcl2 fusion protein in hair cells, which is expressed in the cytosol and also localizes to the membrane of several organelles. Both α-tubulin and NeuroD fish express variations of red fluorescent protein; α-tubulin fish express cytoplasmic tdTomato in hair cells (as well as GFP in all cell nuclei), while NeuroD fish express cytoplasmic tagRFP in a subset of neurons, but not in hair cells. All lines except ET4 are on a primarily *AB background, while ET4 is on a TL background.
Here we show that adult Brn3c fish had elevated hearing thresholds that were at approximately 130 dB (re 1 μPa) from 100 to 1600 Hz, significantly higher than any wildtype strain within this same frequency range (Fig. 2A–B). Bcl2 fish also had high thresholds at all frequencies up to 1600 Hz (Fig. 2A). Interestingly, we observed lower AEP thresholds (increased auditory sensitivity) in α-tubulin and Myo6 fish. AEP thresholds in α-tubulin fish ranged between 98 to 124 dB (re 1 μPa), while thresholds in Myo6 fish ranged from 89 to 109 dB, with both fish lines showing maximum sensitivity in the 400 to 1000 Hz frequency range, similar to thresholds of non-transgenic animals (Fig. 2A–B). NeuroD fish also had lower AEP thresholds ranging from 99 to 118 dB (re 1 μPa), with substantial overlap seen in the NeuroD and α-tubulin audiograms (Fig. 2A). ET4 fish had significantly elevated thresholds compared to wildtype TL fish, with the highest thresholds in the low-frequency range (100–400 Hz) (Fig. 2C). Statistics for all comparisons are given in Table 2.
Fig. 2.
AEP hearing thresholds vary depending on transgene expression and background strain. (A) Comparison of *AB controls to transgenic lines on primarily *AB backgrounds shows that two fish lines expressing hair cell GFP (Brn3c, Bcl2) have significantly increased auditory thresholds (F1,7=91.11, p<0.01; F1,7=24.74, p<0.01, for Brn3c and Bcl2, respectively). NeuroD fish that express red fluorescent protein in neurons, but not fluorescent protein in hair cells, have significantly decreased thresholds (F1,7=11.88, p<0.01), as do fish expressing cytoplasmic GFP under the control of the myo6b promoter (F1,7=62.70, p<0.01). α-tubulin fish, which express tRFP in hair cells, have similar thresholds as *AB wildtype fish (F1,7=3.27, p=0.72). (B) Analysis of non-transgenic animals shows that *AB and TL strains exhibit increased auditory thresholds at all frequencies compared to WIK and outbred wildtype fish (See Table 2). WIK and outbred animals have largely similar audiograms except at 400 and 1600 Hz. (C) ET4 transgenic fish, which express hair cell-specific GFP, have elevated thresholds relative to the TL background strain (F1,7=10.66, p<0.01). N=4–13 for all treatment categories except for *AB (n=22). The complete statistical analysis is shown in Table 2.
Table 2.
Statistical results of two-way ANOVAs with zebrafish strain and frequency as main effects and auditory evoked potential (AEP) hearing threshold as the dependent variable. Each ANOVA compared the AEP thresholds of each of the following strains to those of *AB zebrafish, except for ET4, which was compared to its genetic background TL. Symbols next to strains denote whether the strain had thresholds that were generally greater (+) or less than (−) those of AB zebrafish or TL zebrafish in the case of ET4. Significant p-values are bolded.
| Source (df) | Strain (1) | Frequency (7) | Strain*Freq (7) | |||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
|
| ||||||
| α-tubulin (−) | 3.27 | 0.72 | 6.29 | <0.01 | 0.70 | 0.67 |
|
| ||||||
| Bcl2 (+) | 24.74 | <0.01 | 1.52 | 0.16 | 0.57 | 0.78 |
|
| ||||||
| Brn3c (+) | 91.11 | <0.01 | 0.91 | 0.50 | 1.19 | 0.31 |
|
| ||||||
| NeuroD (−) | 11.88 | <0.01 | 4.16 | <0.01 | 0.55 | 0.80 |
|
| ||||||
| Myo6 (−) | 62.70 | <0.01 | 4.30 | <0.01 | 1.81 | 0.09 |
|
| ||||||
| WIK (−) | 28.68 | <0.01 | 4.81 | <0.01 | 0.97 | 0.46 |
|
| ||||||
| Outbred (−) | 26.22 | <0.01 | 4.62 | <0.01 | 3.34 | <0.01 |
|
| ||||||
| TL (+) | 11.15 | <0.01 | 0.99 | 0.44 | 1.06 | 0.39 |
|
| ||||||
| ET4 (+) | 10.66 | <0.01 | 1.07 | 0.39 | 0.52 | 0.82 |
We also examined AEP thresholds in four non-transgenic lines to assess the effect of genetic background on auditory sensitivity (Fig. 2B). We found that adult WIK fish exhibited auditory thresholds that ranged from 90 to 117 dB (re 1 μPa) with the lowest thresholds observed from 400 to 1500 Hz. WIK thresholds were similar to those from our outbred control strain across much of the audiogram. Surprisingly, we found that *AB fish had relatively poorer auditory sensitivity than WIK or outbred fish, with *AB thresholds ranging from 115 to 122 dB (re 1 μPa) (see Table 2). The *AB fish also did not exhibit a bandpass auditory filter or U-shaped audiogram with best sensitivity from 400 to 1500 Hz, as seen in WIK and outbred fish, but instead had a relatively flatter audiogram across the frequencies tested (Fig. 2B). TL fish had the highest thresholds of any background strain, with thresholds above 115 dB for all frequencies tested. These data show significant differences in auditory sensitivity across wildtype backgrounds and FP expression patterns, and demonstrate significantly reduced hearing ability in some commonly used fish lines that express hair cell-specific GFP.
3.2 Larval auditory responses
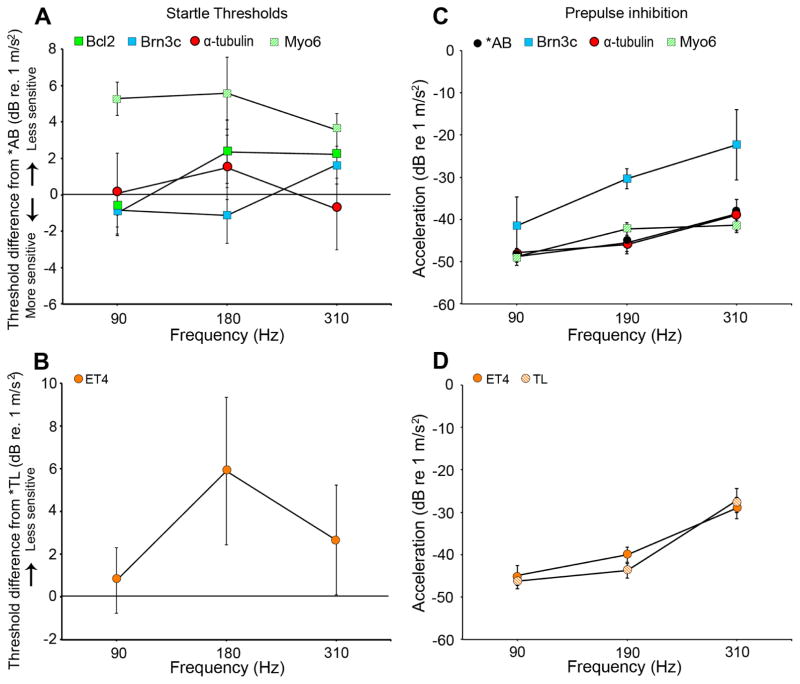
Given the striking auditory sensitivity differences in adults, we next asked if larval fish show similar differences in auditory sensitivity. Adult zebrafish have specialized adaptations (e.g., Weberian ossicles) that allow them to detect sound pressure, but larval zebrafish (5–7 dpf) lack such adaptations and are primarily sensitive to acoustic particle acceleration at this developmental stage. We first performed startle response assays with larval zebrafish to assess their response to vibrational stimuli. Startle responses were analyzed as relative threshold differences from non-transgenic age-matched larvae (*AB or TL, as appropriate). Null results from the startle response assay indicate that there were no behavioral differences in response to high intensity auditory stimuli between transgenic fish and matched controls. Figure 3A shows the startle response thresholds for transgenic lines on a predominantly *AB background. Myo6 fish have significantly less sensitive startle thresholds than *AB (χ2=6.23, p=0.01), while all other lines are not significantly different from the background strain. There is also no difference in startle responses between ET4 fish and the TL background strain (Fig. 3B, χ2=1.67, p>0.05).
Fig. 3.
Larval Brn3c and Myo6 transgenics exhibit reduced auditory function. (A) Startle response data show that most transgenics do not have altered vibrational sensitivity compared to their *AB counterparts, although Myo6 fish are significantly less sensitive (χ2 = 6.23, p = 0.01). The “zero” line represent *AB thresholds. (B) ET4 fish do not have significantly different startle response thresholds from TL background fish (χ2 = 1.67, p > 0.05), with TL thresholds represented as the “zero” line. (C) Prepulse inhibition measurement reveals that Brn3c transgenics have less sensitive hearing compared to *AB controls (χ2 = 5.15, p = 0.02). (D) There is no difference in PPI threshold between ET4 and TL fish (χ2 = 0.01, p > 0.05). N=7 for startle response experiments, n=6–9 for PPI experiments, where each experiment used 24 fish. Bars indicate ± S.E.
We then used a more sensitive prepulse inhibition (PPI) assay to assess the auditory thresholds of larvae (6–7 dpf) to prepulse tone stimuli (90–310 Hz) that inhibit the acoustic startle response (Bhandiwad et al., 2013). The sound levels of these stimuli are below the startle threshold, allowing us to assess the complete hearing range of larval zebrafish. We observed significantly higher PPI thresholds in Brn3c larvae compared to age-matched *AB fish (Fig. 3C, χ2=5.15, p=0.02). We did not detect auditory threshold differences between the other transgenic lines with a predominantly *AB background as compared to *AB controls, nor did we see a difference between ET4 and TL larvae. These data suggest that the underlying cause of threshold differences in adults may manifest early in development for Brn3c transgenic fish, but that elevated thresholds in other lines are not apparent in larvae.
3.3 Hair cell quantification
Increased auditory threshold shifts are correlated with reduced hair cell density in fish sensory epithelia (Smith et al., 2006; Uribe et al., 2013a). We therefore examined hair cell density in phalloidin-labeled inner ears from adult transgenic and non-transgenic lines. In the saccule, which is the primary auditory organ in zebrafish, Brn3c fish have significantly reduced hair cell density in the 5% position (rostral, high-frequency end; Smith et al., 2011), while Myo6 fish have significantly greater hair cell density at the 90% position (caudal, low-frequency region) when compared to the *AB background strain (Fig. 4B, see figure legend for statistical values). Brn3c fish have poor AEP thresholds, while Myo6 fish have relatively good hearing, suggesting a possible (albeit limited) effect of bundle density. However, Bcl2 fish, a transgenic line with higher AEP thresholds, had hair cell densities comparable to the *AB background strain. ET4 fish, with poor AEP sensitivity, did not have altered hair cell density compared to its TL control, nor was there a difference in saccular bundle density between wildtype strains (Fig. 4).
Fig. 4.
Hair bundle density in the zebrafish saccule. (A) Schematic of the saccular epithelium showing the location of our five sampling regions along the rostral-caudal axis. The confocal image on the right is an example of a phalloidin-labeled saccule. All boxes are 50 X 50 μm and are drawn to scale. V=ventral, R=rostral. (B) Comparison of bundle density in *AB fish and transgenic lines on a *AB background. Hair bundle density significantly differs by genotype and saccular location (2-way ANOVA, F5,225=6.88, p<0.001; and F4,225=79.06, p<0.001, respectively). Bonferroni-corrected posthoc tests were then used to compare each transgenic line to the *AB wildtype fish, where **p<0.01 indicates significant differences from *AB at that saccular location. (C) There is no significant difference in saccular hair bundle density between wildtype strains (2-way ANOVA, F2,96=2.65, p=0.07). (D) Saccular bundle density does not differ between transgenic ET4 fish and wildtype TL fish (2-way ANOVA, F1,31=0.08, p=0.78). Data are presented as mean ± S.E. N=4–12 fish per group.
In the utricle, only Brn3c fish had fewer hair cells in any of the four sampling regions compared to its *AB background, and this was only in region four (Fig. 5B). There was no difference in utricular bundle density between background strains, nor between ET4 and TL fish (Fig. 5C–D). In the lagena, Bcl2 fish had increased hair cell bundle density compared to *AB controls in region three only, while WIK fish had greater lagenar bundle density in region three than either of the other wildtype strains (Fig. 6, see figure legend for details). Thus, there is little relationship between hair cell density and auditory sensitivity in any of the three end organs.
Fig. 5.
Hair bundle density in the utricle. (A) Schematic of the utricle showing one sampling location in the extrastriolar region (box 1) and three locations in the striolar region (boxes 2–4, striolar region in light yellow). The phalloidin-labeled confocal image on the right depicts a striolar location. All boxes are 50 × 50 μm and are drawn to scale. (B) For lines made on a *AB background, bundle density is significantly different between genotypes and utricular locations (2-way ANOVA, F5,189=8.36, p<0.001; and F3,189=89.39, p<0.001, respectively). Posthoc comparisons of transgenic lines to *AB wildtype fish show a significant difference between *AB and Brn3c fish at utricular position 4 (*p<0.05). (C) There is no significant difference in utricular hair bundle density between background strains (2-way ANOVA, F2,59=2.64, p=0.08 for background as a main effect). (D) Utricular hair bundle density does not differ between ET4 fish and the TL background strain on which they were created (2-way ANOVA, F1,24=0.21, p=0.65). Data are presented as mean ± S.E. N=4–15 fish per group.
Fig. 6.
Hair bundle density in the lagena. (A) Lagena schematic showing the three sampling locations, with an example of location 2 shown in the confocal image. All boxes are 50 × 50 μm and are drawn to scale. (B) For *AB and transgenic lines created on the *AB background, bundle density is significantly different between genotypes and sampling regions (2-way ANOVA, F5,160=8.91, p<0.001; and F2,160=43.02, p<0.001, respectively). In sampling region 3, bundle density is significantly higher in Bcl2 fish than in *AB fish (Bonferroni-corrected posthoc, ***p<0.001). (C) There is a significant difference in lagenar bundle density between wildtype lines (2-way ANOVA, F2,68=9.08, p<0.001 for genotype main effect), with WIK fish having higher density in region 3 than AB fish (*p<0.05). (D) There is no difference in bundle density between ET4 and TL fish (2-way ANOVA, F1,24=0.40, p=0.53 for genotype main effect). Data are presented as mean ± S.E. N=8–17 fish per genotype.
3.4 Mechanotransduction assay
Reduced auditory sensitivity can occur due to mechanotransduction defects, or deficits further downstream such as synaptic dysfunction (e.g., Söllner et al., 2004; Trapani and Nicolson, 2011). The vital dye FM1-43 enters hair cells via the mechanotransduction channel, and therefore dye uptake is considered a proxy for channel function (Meyers et al., 2003; Park et al., 2013; Uribe et al., 2013b). We assessed mechanotransduction by measuring the uptake of fluorescent FM1-43 in MI1 neuromasts of 5–6 dpf larvae, selecting a subset of lines that showed either reduced AEP sensitivity (Brn3c, ET4) or increased sensitivity (Myo6). FM 1-43 fluorescence was significantly reduced in lateral line hair cells of Brn3c larvae, but not in hair cells of ET4 fish, which also have significantly increased AEP thresholds as adults (Fig. 7). There was also no difference in FM 1-43 fluorescence between background strains (*AB vs. TL). High extracellular calcium reduces the open probability of the transduction channel and serves here as a positive control (Eatock, 2000; Coffin et al., 2009). FM1-43 fluorescence was reduced by 70% in the presence of high calcium, demonstrating that our assay is sensitive to mechanotransduction perturbation. These data suggest that the auditory dysfunction we observed in Brn3c fish may be caused by altered mechanotransduction, but that mechanotransduction is not reduced in other fish lines with high AEP thresholds.
Fig. 7.
Transgene expression affects mechanotransduction in Brn3c larvae. We used uptake of FM1-43 by hair cells as a proxy for transduction channel function. (A–C) Confocal images of FM1-43 labeling of the MI1 neuromast in (A) *AB fish, (B) Brn3c fish, and (C) ET4 fish. The scale bar in A = 10 μm and applies to all panels. (D) Quantification of mean fluorescence (arbitrary units) in the MI1 neuromast shows a significant effect of genotype/treatment (1-way ANOVA, F5=24.72, p<0.001), due to a significant reduction in FM fluorescence in the high calcium (**p<0.001) and Brn3c (**p<0.01) groups (Tukey’s multiple comparison test). Data are presented as mean ± S.E., and mean fluorescence is normalized to fluorescence in *AB controls from the same experiment. High extracellular calcium, which reduces the open probability of the transduction channel, was used as a control. N= 10–11 fish per treatment.
4. Discussion
We demonstrate that adults of different transgenic and wildtype zebrafish lines have significant differences in auditory sensitivity. In our study, three adult transgenic zebrafish lines expressing hair cell-specific GFP (Brn3c, Bcl2, ET4) were less sensitive to auditory stimuli, although a similar line, Myo6, showed increased sensitivity. In the Brn3c line, which has the highest AEP thresholds of any line we tested, reduced auditory sensitivity is detectable in larvae, suggesting that the cause of this defect manifests early in development. In general, larval zebrafish have reduced auditory sensitivity compared to adults due to the delayed development of Weberian ossicles – modified vertebrae that couple the swim bladder to the inner ear and allow for sound pressure detection (Haddon and Lewis, 1996; Fay and Simmons, 1999; Higgs et al., 2003). Given the absence of Weberian ossicles, our larval assays employed particle motion stimuli, rather than the sound pressure stimuli used for the AEPs, so we cannot directly compare between adult and larval studies. However, the increased auditory sensitivity gained by Weberian ossicle development means that functional differences are more likely to be detected in adult animals, which is consistent with our data. Adult zebrafish are used in a variety of auditory studies, including research on hair cell death and putative mechanisms of regeneration (Bang et al., 2002; Schuck et al., 2011; Liang et al., 2012, Burns et al., 2013; Uribe et al., 2013a). In particular, a recent study demonstrated that biophysical hair cell properties are mature in zebrafish over two months old, suggesting that adult animals are preferable for some auditory physiology experiments (Olt et al., 2014). Our data show that care is needed in selecting a fish line for auditory studies and in comparing results between fish lines, particularly for research using adult animals.
There are several possible explanations for these functional differences. Fluorescent reporter expression could modulate hair cell survival. Long-term GFP expression in vitro and in vivo can alter metabolic functions, affect ubiquitin targeting, and activate apoptosis (Liu et al., 1999; Zhang et al., 2003; Baens et al., 2006; Agbulut et al., 2007; Taghizadeh and Sherley, 2008; Koike et al., 2012; Coumans et al., 2014). Increased hearing thresholds are associated with reduced hair cell density in vertebrates, including fishes (Coffin et al., 2012; Uribe et al., 2013a; Smith, 2016). It is therefore possible that GFP expression causes hair cell death, resulting in poorer auditory sensitivity in adults. However, an apoptotic mechanism is not supported by our data, as only the Brn3c transgenic fish exhibit reduced hair cell numbers, and this occurs only in one region of the saccule and utricle (compared to the *AB background). Furthermore, Myo6 fish have better auditory sensitivity than their *AB counterparts, but only have increased hair cell density in one high-frequency region of the saccule, despite having reduced AEP thresholds at several frequencies.
In Brn3c transgenic larvae, FM 1-43 uptake was reduced by 50%, suggesting a mechanotransduction deficit. A previous study showed that enhanced GFP (EGFP) can bind to the actin-binding site of myosin and disrupt muscle contraction (Agbulut et al., 2007). Hair bundles are particularly rich in myosin proteins and mutations in a number of different myosin genes cause deafness in humans and animal models (Avraham et al., 1995; Friedman et al., 1999; Ernest et al., 2000). Thus, fluorescent protein expression could interfere with the interaction of actin-myosin molecules and could modulate stereocilia dynamics, including the set point of the transduction apparatus (Seiler et al., 2004; Nayak et al., 2007). However, we did not observe a defect in FM 1-43 uptake in ET4 fish, another transgenic line with hair cell-specific GFP and reduced AEP thresholds. It is likely that alternative mechanisms are responsible for the auditory defects seen in other lines, and possible that multiple mechanisms underlie the threshold increase in Brn3c fish. Membrane-bound GFP may alter the biophysical properties of the plasma membrane, thereby affecting membrane depolarization in response to mechanotransduction channel opening, which would not be detectable with our FM 1-43 assay. Bcl2 fish, which express a GFP-Bcl2 fusion protein in the cytosol and organelle membranes (smooth ER, mitochondria), also have increased AEP thresholds when compared to wildtype *AB fish. Previous research in the larval zebrafish lateral line demonstrates that hair cell responses to chemical toxins are partially determined by changes in mitochondrial membrane potential and calcium flux between ER and mitochondria (Owens et al., 2007; Esterberg et al., 2013, 2014). Therefore, GFP targeted to intracellular membranes could also influence organelle function. Future work is needed to assess hair cell physiology in these fish lines.
Mechanotransduction-independent mechanisms could also be affected by FP localization. For example, Brn3c fish express membrane targeted GFP tagged to GAP43, a modulator of GTP-ase activity, and in Bcl2 fish, GFP is attached to the pro-survival protein Bcl2. Interestingly, these two transgenics exhibit the highest AEP thresholds. These data suggest that the proteins fused to GFP could perturb signal transduction pathways at a variety of cellular locations and that GFP expression alone may not be responsible for the altered phenotype. Furthermore, compartmental localization may be significant in instances where GFP is fused to another protein. The α-tubulin transgenic expresses GFP fused with histone in the nuclei of hair cells and has hearing thresholds that are not significantly different than its primarily *AB background. Our results with the Brn3c, Bcl2 and α-tubulin transgenics suggest a complex interplay may occur between the compartmental localization of a fluorophore and the activity of its fused proteins. Furthermore, ET4 fish, which express cytoplasmic GFP, have greatly elevated AEP thresholds, even when compared to the relatively poor hearing of the TL background strain. We therefore propose that a single mechanism may not be sufficient to explain the auditory heterogeneity between adult fish strains.
To our knowledge, all of the transgenic lines we have examined were created by random insertion of one or more transgene copies into the DNA backbone, so transgene copy number and insertional location may have a significant effect if the transgene modifies or disrupts expression of a gene important for auditory function. It is possible that elevated thresholds in the Brn3c, ET4, or Bcl2 lines result from a position effect that disrupts an important auditory gene, rather than from FP expression per se. Future genetic mapping work is needed to test this hypothesis.
Interestingly, we see considerable variation in AEP thresholds between non-transgenic (wildtype) zebrafish lines. Variation in startle responses, but not prepulse inhibition, was identified in larval *AB, WIK, TL, and Tu wildtype lines (Burgess and Granato, 2007), but we are not aware of reported variation in adult zebrafish strains. Heterogeneity in auditory function is also seen in mouse strains, although this variation most commonly manifests as variable-onset age-related hearing loss (Zheng et al., 1999; Kane et al., 2012). For example, in an AEP study of inbred mice from the Jackson Laboratory, 20% (16/80) of lines showed increased thresholds by three months of age (Zheng et al., 1999). In mammals, age-related hearing loss usually occurs first at the high frequencies, while we see the greatest elevation in thresholds in the center of the zebrafish audiogram (400–1600 Hz). While a recent study suggested that zebrafish show signs of age-related hearing loss (Wang et al., 2015), fish continually produce hair cells throughout life, and the extent of possible age effects are not understood (Lombarte and Popper, 1994; Lanford et al., 1996). We therefore consider it unlikely that the auditory variability in our study results from age-related hearing loss. Given these differences in wildtype hearing, it is likely that some of the differences between transgenic lines may be attributed to background strain. For example, ET4 transgenic fish have significantly elevated thresholds, and the TL strain on which they were created also shows relative poor AEP sensitivity. However, comparison of Bcl2 vs. *AB fish, both from the same genetic stock and maintained in the same lab, demonstrates that differences in adult auditory function are not solely due to background strain.
The Brn3c line represents an excellent resource to further tease apart the relative influence of genetic background on AEP sensitivity. Brn3c fish incorporate elements of TL and Tu strains on a primarily *AB background, and these two strains could significantly alter the relative contributions of the transgene or the *AB strain. Evaluation of hybrids between *AB, TL and Tu could clarify the effects of these background strains on the phenotype in Brn3c transgenic fish. Familial effects may also play an important role in hearing sensitivity. For example, our Myo6 line, on an *AB background, exhibits improved hearing relative to *AB controls. The Myo6 fish were established with sibling founders and have been separated from the *AB wildtype fish for approximately eight generations. If over succeeding generations of inbreeding, the background and transgenic lines develop distinct familial auditory traits, this could explain the different phenotypes. Future studies comparing fish from different familial origins and transgenic vs. non-transgenic siblings could resolve this issue. Differences between wildtype strains are still important to note, and additional work is needed to determine the genetic modifers responsible for strain-specific auditory differences in zebrafish.
In summary, we demonstrate significant differences in auditory thresholds between different adult transgenic and wildtype zebrafish lines. Brn3c, Bcl2, and ET4 fish, all of which express hair cell-specific GFP, have relatively poor hearing, while NeuroD and α-tubulin animals had sensitivity similar to non-transgenic WIK fish and to an outbred wildtype control line. Myo6 fish have relatively low AEP thresholds, suggesting that this line may be useful for auditory studies that require FP expression for hair cell visualization. *AB and TL adults, both common laboratory strains, had increased thresholds when compared to other wildtype lines. Our findings suggest that care is required when selecting a zebrafish line for auditory studies.
Highlights.
Adult zebrafish strains have differing auditory sensitivity.
Sensitivity differences also correlate with fluorescent protein expression.
Larval Brn3c:mGFP fish have reduced auditory-evoked behavior and a potential defect in hair cell transduction.
Hair cell density does not differ between strains.
Caution is warranted when selecting a zebrafish line for auditory research.
Acknowledgments
We thank Christopher Riso, Alex Young, and Victoria Peters for fish husbandry assistance and Lauren Hayashi for help with experiments. We also thank A. Nechiporuk for WIK fish and A.J. Hudspeth for ET4 fish.
This research was supported by the National Institute of General Medical Sciences of the National Institutes of Health award 8P20GM103436-12 to M.E.S., by the National Institute on Deafness and Other Communication Disorders award 5R03DC011344 to A.B.C, and by Washington State University Vancouver start-up funds to A.B.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
J. David Monroe, Email: jerry.monroe@wku.edu.
Dustin P. Manning, Email: dustin.manning@email.wsu.edu.
Phillip M. Uribe, Email: uribe@vetmed.wsu.edu.
Ashwin Bhandiwad, Email: ashwin.bhandiwad@mail.nih.gov.
Joseph A. Sisneros, Email: sisneros@uw.edu.
Michael E. Smith, Email: michael.smith1@wku.edu.
Allison B. Coffin, Email: Allison.coffin@wsu.edu.
References
- Agbulut O, Huet A, Niederländer N, Puceat M, Menasché P, Coirault C. Green fluorescent protein impairs actin-myosin interactions by binding to the actin-binding site of myosin. J Biol Chem. 2007;282(14):10465–10471. doi: 10.1074/jbc.M610418200. [DOI] [PubMed] [Google Scholar]
- Avraham KB, Hasson T, Steel KP, Kingsley DM, Russell LB, Mooseker MS, Copeland NG, Jenkins NA. The mouse Snell’s waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat Genet. 1995;11(4):369–375. doi: 10.1038/ng1295-369. [DOI] [PubMed] [Google Scholar]
- Baens M, Noels H, Broeckx V, Hagens S, Fevery S, Billiau AD, Vankelecom H, Marynen P. The dark side of EGFP: defective polyubiquitination. PLoS One. 2006;1:e54. doi: 10.1371/journal.pone.0000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang PI, Yelick PC, Malicki JJ, Sewell WF. High-throughput behavioral screening method for detecting auditory response defects in zebrafish. J Neurosci Methods. 2002;118(2):117–187. doi: 10.1016/s0165-0270(02)00118-8. [DOI] [PubMed] [Google Scholar]
- Bhandiwad AA, Zeddies DG, Raible DW, Rubel EW, Sisneros JA. Auditory sensitivity of larval zebrafish (Danio rerio) measured using a behavioral prepulse inhibition assay. J Exp Biol. 2013;216(18):3504–3513. doi: 10.1242/jeb.087635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignull HR, Raible DW, Stone JS. Feathers and fins: non-mammalian models for hair cell regeneration. Brain Res. 2009;1277:12–23. doi: 10.1016/j.brainres.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. J Neurosci. 2007;27(18):4984–4994. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, Collado MS, Oliver ER, Corwin JT. Specializations of intercellular junctions are associated with the presence and absence of hair cell regeneration in ears from six vertebrate classes. J Comp Neurol. 2013;521(6):1430–1448. doi: 10.1002/cne.23250. [DOI] [PubMed] [Google Scholar]
- Coffin AB, Kelley MW, Manley GA, Popper AN. Evolution of sensory hair cells. In: Manley, et al., editors. Evolution of the Auditory System. New York: Springer-Verlag; 2004. [Google Scholar]
- Coffin AB, Reinhart KE, Owens KN, Raible DW, Rubel EW. Extracellular divalent cations modulate aminoglycoside-induced hair cell death in the zebrafish lateral line. Hear Res. 2009;253(1–2):42–51. doi: 10.1016/j.heares.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Mohr RA, Sisneros JA. Saccular-specific hair cell addition correlates with reproductive state-dependent changes in the auditory saccular sensitivity of a vocal fish. J Neurosci. 2012;32:1366–1376. doi: 10.1523/JNEUROSCI.4928-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Rubel EW, Raible DW. Bax, Bcl2, and p53 differentially regulate neomycin and gentamicin-induced hair cell death in the zebrafish lateral line. J Assoc Res Otolaryngol. 2013;14(5):645–659. doi: 10.1007/s10162-013-0404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Brignull H, Raible DW, Rubel EW. Hearing loss, protection, and regeneration in the larval zebrafish lateral line. In: Coombs S, Bleckmann H, Fay RR, Popper AN, editors. The Lateral Line. New York: Springer; 2014. pp. 314–347. [Google Scholar]
- Corwin JT, Bullock TH, Schweitzer J. The auditory brainstem response in five vertebrate classes. Electroencephalogr Clin Neurophysiol. 1982;54:629–641. doi: 10.1016/0013-4694(82)90117-1. [DOI] [PubMed] [Google Scholar]
- Coumans JV, Gau D, Poljak A, Wasinger V, Roy P, Moens P. Green fluorescent protein expression triggers proteome changes in breast cancer cells. Exp Cell Res. 2014;320(1):33–45. doi: 10.1016/j.yexcr.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA. Adaptation in hair cells. Annu Rev Neurosci. 2000;23:285–314. doi: 10.1146/annurev.neuro.23.1.285. [DOI] [PubMed] [Google Scholar]
- Ernest S, Rauch GJ, Haffter P, Geisler R, Petit C, Nicolson T. Mariner is defective in myosin VIIA: a zebrafish model for human hereditary deafness. Hum Mol Genet. 2000;9(14):2189–2196. doi: 10.1093/hmg/9.14.2189. [DOI] [PubMed] [Google Scholar]
- Esterberg R, Hailey DW, Coffin AB, Raible DW, Rubel EW. Disruption of intracellular calcium regulation is integral to aminoglycoside-induced hair cell death. J Neurosci. 2013;33(17):7513–7525. doi: 10.1523/JNEUROSCI.4559-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterberg R, Hailey DW, Rubel EW, Raible DW. ER-mitochondrial calcium flow underlies vulnerability of mechanosensory hair cells to damage. J Neurosci. 2014;34(29):9703–9719. doi: 10.1523/JNEUROSCI.0281-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay RR, Simmons AM. The sense of hearing in fishes and amphibians. In: Fay RR, Popper AN, editors. Comparative Hearing: Fish and Amphibians. New York: Springer; 1999. pp. 269–318. [Google Scholar]
- Friedman TB, Sellers JR, Avraham KB. Unconventional myosins and the genetics of hearing loss. Am J Med Genet. 1999;89(3):147–157. doi: 10.1002/(sici)1096-8628(19990924)89:3<147::aid-ajmg5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM1–43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J Neurosci. 2001;21(18):7013–7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV. Hear, hear for the zebrafish. Neuron. 2005;45(1):3–5. doi: 10.1016/j.neuron.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Haddon C, Lewis J. Early ear development in the embryo of the zebrafish, Danio rerio. J Comp Neurol. 1996;365(1):113–128. doi: 10.1002/(SICI)1096-9861(19960129)365:1<113::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio) J Assoc Res Otolaryngol. 2003;4:219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim R, Prasher DC, Tsien RY. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc Natl Acad Sci USA. 1994;91(26):12501–12504. doi: 10.1073/pnas.91.26.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs DM, Souza MJ, Wilkins HR, Presson JC, Popper AN. Age- and size-related changes in the inner ear and hearing ability of the adult zebrafish (Danio rerio) J Assoc Res Otolaryngol. 2002;3(2):174–184. doi: 10.1007/s101620020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs DM, Rollo AK, Souza MJ, Popper AN. Development of form and function in peripheral auditory structures of the zebrafish (Danio rerio) J Acoust Soc Am. 2003;113(2):1145–1154. doi: 10.1121/1.1536185. [DOI] [PubMed] [Google Scholar]
- Huang M, Kantardzhieva A, Scheffer D, Liberman MC, Chen ZY. Hair cell overexpression of Islet1 reduced age-related and noise-induced hearing loss. J Neurosci. 2013;33(38):15086–15094. doi: 10.1523/JNEUROSCI.1489-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WY, Aramburu J, Douglas PS, Izumo S. Transgenic expression of green fluorescence protein can cause dilated cardiomyopathy. Nat Med. 2000;6(5):482–483. doi: 10.1038/74914. [DOI] [PubMed] [Google Scholar]
- Jones JM, Montcouquiol M, Dabdoub A, Woods C, Kelley MW. Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J Neurosci. 2006;26(2):550–558. doi: 10.1523/JNEUROSCI.3859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmijn AJ. Hydrodynamic and acoustic field detection. In: Atema J, Fay RR, Popper AN, Tavolga WN, editors. Sensory Biology of Aquatic Animals. New York: Springer; 1988. pp. 83–130. [Google Scholar]
- Kane KL, Longo-Guess CM, Gagnon LH, Ding D, Salvi RJ, Johnson KR. Genetic background effects on age-related hearing loss associated with Cdh23 variants in mice. Hear Res. 2012;283(1–2):80–88. doi: 10.1016/j.heares.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon TN, Ladich F, Yan HY. A comparative study of hearing ability in fishes: the auditory brainstem response approach. J Comp Physiol A. 1998;182:307–318. doi: 10.1007/s003590050181. [DOI] [PubMed] [Google Scholar]
- Koike M, Yutoku Y, Koike A. Ku80 attentuates cytotoxicity induced by green fluorescent protein transduction independently of non-homologous end joining. FEBS Open Bio. 2012;3:46–50. doi: 10.1016/j.fob.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krestel HE, Mihaljevic AL, Hoffman DA, Schneider A. Neuronal co-expression of EGFP and beta-galactosidase in mice causes neuropathology and premature death. Neurobiol Dis. 2004;17(2):310–318. doi: 10.1016/j.nbd.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Presson JC, Popper AN. Cell proliferation and hair cell addition in the ear of the goldfish, Carassius auratus. Hear Res. 1996;100(1–2):1–9. doi: 10.1016/0378-5955(96)00110-4. [DOI] [PubMed] [Google Scholar]
- Lewis RM, Hume CR, Stone JS. Atoh1 expression and function during auditory hair cell regeneration in post-hatch chickens. Hear Res. 2012;289(1–2):74–85. doi: 10.1016/j.heares.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Wang D, Renaud G, Wolfsberg TG, Wilson AF, Burgess SM. The stat3- socs3a pathway is a key regulator of hair cell regeneration in zebrafish. J Neurosci. 2012;32(31):10662–10673. doi: 10.1523/JNEUROSCI.5785-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin V, Golub JS, Nguyen TB, Hume CR, Osterle EC, Stone JS. Inhibition of notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricle. J Neurosci. 2011;31(43):15329–15339. doi: 10.1523/JNEUROSCI.2057-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HS, Jan MS, Chou CK, Chen PH, Ke NJ. Is green fluorescent protein toxic to the living cells? Biochem Biophys Res Commun. 1999;260(3):712–717. doi: 10.1006/bbrc.1999.0954. [DOI] [PubMed] [Google Scholar]
- Lombarte A, Popper AN. Quantitative analyses of postembryonic hair cell addition in the otolithic endorgans of the inner ear of the European hake, Merluccius merluccius (Gadiformes, Teleostei) J Comp Neurol. 1994;345(3):419–428. doi: 10.1002/cne.903450308. [DOI] [PubMed] [Google Scholar]
- Lu Z, DeSmidt AA. Early development of hearing in zebrafish. J Assoc Res Otolaryngol. 2013;14(4):509–521. doi: 10.1007/s10162-013-0386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma EY, Raible DW. Signaling pathways regulating zebrafish lateral line development. Curr Biol. 2009;19(9):R381–386. doi: 10.1016/j.cub.2009.03.057. [DOI] [PubMed] [Google Scholar]
- McDermott BM, Jr, Asai Y, Baucom JM, Jani SD, Castellanos Y, Gomez G, McClintock JM, Starr CJ, Hudspeth AJ. Transgenic labeling of hair cells in the zebrafish acousticolateralis system. Gene Expr Patterns. 2010;10(2–3):113–118. doi: 10.1016/j.gep.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw FD, Snelson CD, Prendergast A, Suli A, Raible DW. Postyembryonic neuronal addition in zebrafish dorsal root ganglia is regulated by Notch signaling. Neural Dev. 2012;7:23. doi: 10.1186/1749-8104-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry MJ, van Netten SM. The flexural stiffness of superficial neuromasts in the zebrafish (Danio rerio) lateral line. J Exp Biol. 2007;210(23):4244–4253. doi: 10.1242/jeb.009290. [DOI] [PubMed] [Google Scholar]
- Meyers JR, MacDonald RB, Duggan A, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23(10):4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon IS, So JH, Jung YM, Lee WS, Kim EY, Choi JH, Kim CH, Choi JY. Fucoidan promotes mechanosensory hair cell regeneration following amino glycoside- induced cell death. Hear Res. 2011;282(1–2):236–242. doi: 10.1016/j.heares.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Murakami SL, Cunningham LL, Werner LA, Bauer E, Pujol R, Raible DW, Rubel EW. Developmental differences in susceptibility to neomycin-induced hair cell death in the lateral line neuromasts of zebrafish (Danio rerio) Hear Res. 2003;186(1–2):47–56. doi: 10.1016/s0378-5955(03)00259-4. [DOI] [PubMed] [Google Scholar]
- Namdaran P, Reinhart KE, Owens KN, Raible DW, Rubel EW. Identification of modulators of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2012;32(10):3516–3528. doi: 10.1523/JNEUROSCI.3905-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak GD, Ratnayaka HS, Goodyear RJ, Richardson GP. Development of the hair bundle and mechanotransduction. Int J Dev Biol. 2007;51(6–7):597–608. doi: 10.1387/ijdb.072392gn. [DOI] [PubMed] [Google Scholar]
- Nicolson T. The genetics of hearing and balance in zebrafish. Annu Rev Genet. 2005;39:9–22. doi: 10.1146/annurev.genet.39.073003.105049. [DOI] [PubMed] [Google Scholar]
- Olt J, Johnson SL, Marcotti W. In vivo and in vitro biophysical properties of hair cells from the lateral line and inner ear of developing and adult zebrafish. J Physiol. 2014;592(10):2041–2058. doi: 10.1113/jphysiol.2013.265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HC, Santos F, Raible DW, Simon JA, Rubel EW. Drug screening for hearing loss: using the zebrafish lateral line to screen for drugs that prevent and cause hearing loss. Drug Discov Today. 2010;15(7–8):265–271. doi: 10.1016/j.drudis.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens KN, Cunningham DE, MacDonald G, Rubel EW, Raible DW, Pujol R. Ultrastructural analysis of aminoglycoside-induced hair cell death in the zebrafish lateral line reveals an early mitochondrial response. J Comp Neurol. 2007;502:522–543. doi: 10.1002/cne.21345. [DOI] [PubMed] [Google Scholar]
- Oxman DS, Barnett-Johnson R, Smith ME, Coffin AB, Miller DD, Josephson R, Popper AN. The effect of vaterite deposition on otolith morphology, sound reception and inner ear sensory epithelia in hatchery-reared chinook salmon (Oncorhynchus tshawytscha) Can J Fish Aquatic Sci. 2007;64:1469–1478. [Google Scholar]
- Pauls S, Geldmacher-Voss B, Campos-Ortega JA. A zebrafish histone variant H2A.F/Z and a transgenic H2A.F/Z:GFP fusion protein for in vivo studies of embryonic development. Dev Genes Evol. 2001;211:603–610. doi: 10.1007/s00427-001-0196-x. [DOI] [PubMed] [Google Scholar]
- Parinov S, Kondrichin I, Korzh V, Emelyanov A. Tol2 transposon-mediated enhancer trap to identify developmentally regulated zebrafish genes in vivo. Dev Dyn. 2004;231(2):449–459. doi: 10.1002/dvdy.20157. [DOI] [PubMed] [Google Scholar]
- Park S, Lee JH, Cho HJ, Lee KY, Kim MO, Yun BW, Ryoo Z. Tmie is required for gentamicin uptake by the hair cells of mice. Comp Med. 2013;63(2):136–142. [PMC free article] [PubMed] [Google Scholar]
- Popper AN, Fay RR. The auditory periphery in fishes. In: Fay RR, Popper AN, editors. Comparative Hearing: Fish and Amphibians. New York: Springer; 1999. pp. 43–100. [Google Scholar]
- Raible DW, Kruse GJ. Organization of the lateral line system in embryonic zebrafish. J Comp Neurol. 2000;421(2):189–198. [PubMed] [Google Scholar]
- Ricci AJ, Fettiplace R. Calcium permeation of the turtle hair cell mechanotransducer channel and its relation to the composition of endolymph. J Physiol. 1998;506(1):159–173. doi: 10.1111/j.1469-7793.1998.159bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Wu YC, Fettiplace R. The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J Neurosci. 1998;18(20):8261–8277. doi: 10.1523/JNEUROSCI.18-20-08261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos F, MacDonald G, Rubel EW, Raible DW. Lateral line hair cell maturation is a determinant of aminoglycoside susceptibility in zebrafish (Danio rerio) Hear Res. 2006;213(1–2):25–33. doi: 10.1016/j.heares.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Schuck JB, Smith ME. Cell proliferation follows acoustically-induced hair cell bundle loss in the zebrafish saccule. Hear Res. 2009;253(1–2):67–76. doi: 10.1016/j.heares.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck JB, Sun H, Penberthy WT, Cooper NG, Li X, Smith ME. Transcriptomic analysis of the zebrafish inner ear points to growth hormone mediated regeneration following acoustic trauma. BMC Neurosci. 2011;12:88. doi: 10.1186/1471-2202-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler C, Nicolson T. Defective calmodulin-dependent rapid apical endocytosis in zebrafish sensory hair cell mutants. J Neurobiol. 1999;41(3):424–434. [PubMed] [Google Scholar]
- Seiler C, Ben-David O, Sidi S, Hendrich O, Rusch A, Burnside B, Avraham KB, Nicolson T. Myosin VI is required for structural integrity of the apical surface of sensory hair cells in zebrafish. Dev Biol. 2004;272(2):328–338. doi: 10.1016/j.ydbio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Sheets L, Hagen MW, Nicolson T. Characterization of ribeye subunits in zebrafish hair cells reveals that exogenous ribeye B-domain and CtBP1 localize to the basal ends of synaptic ribbons. PLoS One. 2014;9(9):e107256. doi: 10.1371/journal.pone.0107256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME. The relationship between hair cell loss and hearing loss in fishes. In: Popper AN, Hawkins A, editors. The Effects of Noise on Aquatic Life II. New York: Springer-Verlag; 2016. pp. 1079–1086. [Google Scholar]
- Smith ME, Kane AS, Popper AN. Noise-induced stress response and hearing loss in goldfish (Carassius auratus) J Exp Biol. 2004a;207:427–435. doi: 10.1242/jeb.00755. [DOI] [PubMed] [Google Scholar]
- Smith ME, Kane AS, Popper AN. Acoustical stress and hearing sensitivity in fishes: does the linear threshold hypothesis hold water? J Exp Biol. 2004b;207:3591–3602. doi: 10.1242/jeb.01188. [DOI] [PubMed] [Google Scholar]
- Smith ME, Coffin AB, Miller DL, Popper AN. Anatomical and functional recovery of the goldfish (Carassius auratus) ear following noise exposure. J Exp Biol. 2006;209:4193–4202. doi: 10.1242/jeb.02490. [DOI] [PubMed] [Google Scholar]
- Smith ME, Schuck JB, Gilley RR, Rogers BD. Structural and functional effects of acoustic exposure in goldfish: evidence for tonotopy in the teleost saccule. BMC Neurosci. 2011:12. doi: 10.1186/1471-2202-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner C, Rauch GJ, Siemens J, Geisler R, Schuster SC, Müller U, Nicolson T, Tübingen Screen Consortium, 2004. Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature. 2000;428:955–959. doi: 10.1038/nature02484. [DOI] [PubMed] [Google Scholar]
- Suli A, Guler AD, Raible DW, Kimelman D. A targeted gene expression system using the tryptophan repressor in zebrafish shows no silencing in subsequent generations. Development. 2014;141(5):1167–1174. doi: 10.1242/dev.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Lin CH, Smith ME. Growth hormone promotes hair cell regeneration in the zebrafish (Danio rerio) inner ear following acoustic trauma. PLoS One. 2011;6(11):e28372. doi: 10.1371/journal.pone.0028372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet EM, Vemaraju S, Riley BB. Sox2 and Fgf interact with Atoh1 to promote sensory competence throughout the zebrafish inner ear. Dev Biol. 2011;358(1):113–121. doi: 10.1016/j.ydBio.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghizadeh RR, Sherley JL. CFP and YFP, but not GFP, provide stable fluorescent marking of rat hepatic adult stem cells. J Biomed Biotechnol. 2008;2008:453590. doi: 10.1155/2008/453590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani JG, Nicolson T. Mechanism of spontaneous activity in afferent neurons of the zebrafish lateral-line organ. J Neurosci. 2011;31(5):1614–1623. doi: 10.1523/JNEUROSCI.3369-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe PM, Sun H, Wang K, Asuncion JD, Wang Q, Chen CW, Steyger PS, Smith ME, Matsui JI. Aminoglycoside-induced hair cell death of inner ear organs causes functional deficits in adult zebrafish (Danio rerio) PLoS One. 2013a;8(3):e58755. doi: 10.1371/journal.pone.0058755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe PM, Asuncion JD, Matsui JI. Ethanol affects the development of sensory hair cells in larval zebrafish (Danio rerio) PLoS One. 2013b;8(12):e83039. doi: 10.1371/journal.pone.0083039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Song Q, Yu D, Yang G, Xia L, Su K, Shi H, Wang J, Yin S. Ontogenetic development of the auditory sensory organ in zebrafish (Danio rerio): changes in hearing sensitivity and related morphology. Sci Rep. 2015;5:15943. doi: 10.1038/srep15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for Laboratory Use of Zebrafish (Danio rerio) 4. University of Oregon Press; Eugene, OR: 2000. [Google Scholar]
- Whitfield TT. Zebrafish as a model for hearing and deafness. J Neurobiol. 2002;53(2):157–171. doi: 10.1002/neu.10123. [DOI] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7(12):1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Xiao T, Roeser T, Staub W, Baier H. A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development. 2005;132:2955–2967. doi: 10.1242/dev.01861. [DOI] [PubMed] [Google Scholar]
- Zeddies DG, Fay RR. Development of the acoustically evoked behavioral response in zebrafish to pure tones. J Exp Biol. 2005;208(7):1363–1372. doi: 10.1242/jeb.01534. [DOI] [PubMed] [Google Scholar]
- Zhang F, Hackett NR, Lam G, Cheng J, Pergolizzi R, Luo L, Shmelkov SV, Edelberg J, Crystal RG, Rafii S. Green fluorescent protein selectively induces HSP70-mediated up- regulation of COX-2 expression in endothelial cells. Blood. 2003;102(6):2115–21121. doi: 10.1182/blood-2003-01-0049. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao W-Q. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130(1–2):94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]