SUMMARY
Signaling through the IL-17 receptor (IL-17R) is required to prevent oropharyngeal candidiasis (OPC) in mice and humans. However, the IL-17-responsive cell type(s) that mediate protection are unknown. Using radiation chimeras we were able to rule out a requirement for IL-17RA in the hematopoietic compartment. We saw remarkable concordance of IL-17-controlled gene expression in C. albicans-infected human oral epithelial cells (OECs) and in tongue tissue from mice with OPC. To interrogate the role of the IL-17R in OECs, we generated mice with conditional deletion of IL-17RA in superficial oral and esophageal epithelial cells (Il17raΔK13). Following oral Candida infection, Il17raΔK13 mice exhibited fungal loads and weight loss indistinguishable from Il17ra−/− mice. Susceptibility in Il17raΔK13 mice correlated with expression of the antimicrobial peptide β-defensin 3 (BD3, Defb3). Consistently, Defb3−/− mice were susceptible to OPC. Thus, OECs dominantly control IL-17R-dependent responses to OPC through regulation of BD3expression.
ETOC Blurb
IL-17 receptor signaling is required to prevent oropharyngeal candidiasis (“oral thrush”) in both mice and humans. Conti et al. demonstrate in mice that IL-17R-dependent anti-fungal responses in superficial oral epithelial cells (OECs) are critical for protection. Moreover, OECs dominantly control IL-17R-dependent responses through production of the antimicrobial peptide β-defensin 3.
INTRODUCTION
Fungal infections are an increasing threat (Brown et al., 2012). To date, there are no licensed vaccines to any fungal pathogens, and antifungal medications are costly, toxic and increasingly ineffective due to drug resistance. Oropharyngeal candidiasis (OPC, thrush) is an opportunistic infection caused by the commensal fungus Candida albicans. Susceptibility is associated with T cell deficiency, as most HIV/AIDS patients experience recurrent OPC. Thrush is also associated with, denture use, salivary gland defects (e.g., Sjögren’s syndrome) and immunosuppressive regimens associated with cancer, transplantation or autoimmunity. Oral thrush also causes nutritional deficits in infants and a failure to thrive (Fidel, 2011; Glocker and Grimbacher, 2010).
IL-17 (IL-17A) is a vital mediator of antifungal immunity. IL-17 is expressed by lymphocytes, most notably CD4+ “Th17” cells, but also CD8+ T cells and various innate immune populations such as γδ-T cells, NKT cells, ‘natural’ Th17 cells and innate lymphoid 3 (ILC3) cells (Cua and Tato, 2010). Some data suggest that IL-17 may be expressed by myeloid cells, though this remains controversial (Huppler et al., 2015; Taylor et al., 2014). Humans with defects in the IL-17R signaling pathway show remarkably restricted disease susceptibility, with high sensitivity to chronic mucosal candidiasis (CMC) and S. aureus infections, but typically not to other infections (Milner and Holland, 2013). Like immunocompetent humans, WT mice are resistant to OPC (Kamai et al., 2001). However, Il17ra−/−, Il17rc−/− Il23−/− Act1−/− and Rorc−/− mice are all susceptible (Conti et al., 2014; Conti et al., 2009; Ferreira et al., 2014; Ho et al., 2010), pointing to a role for the IL-17/Th17 pathway in OPC.
The oral mucosa is poorly understood with respect to immune function. A key constituent of oral immunity is the oral epithelial cell (OEC), which mediates early recognition of and response to microbes (Moyes et al., 2015). Like other mucosae, the oral epithelium constitutes a physical barrier to restrict pathogen entry. OECs interact with C. albicans through multiple receptors, including HER2/Neu and EGFR (Zhu et al., 2012). OECs also sense the dimorphic transition from the commensal yeast to virulent hyphae by inducing distinct downstream signaling pathways (Moyes et al., 2016). In response to C. albicans, OECs express innate cytokines and antimicrobial effectors, including IL-1β, CCL20, G-CSF and β-defensins. Within one day of infection, IL-17 is highly expressed in lymphocytes (Conti et al., 2014; Hernández-Santos et al., 2013). In mice, which do not harbor C. albicans as a commensal microbe and thus are immunologically naïve to this organism, IL-17 is produced upon first encounter by innate lymphocytes, specifically γδ-T cells and CD4+TCRβ+ ‘natural’ Th17 populations (Conti et al., 2014; Huppler et al., 2015). In recall settings, IL-17 is additionally made by conventional Th17 cells, which augment the innate Type 17 response to improve C. albicans clearance (Bär et al., 2012; Hernández-Santos et al., 2013).
IL-17 signaling induces a panel of antifungal target genes that collectively control C. albicans from the oral cavity. Downstream gene products include neutrophil-recruiting factors (CXC chemokines, G-CSF), myeloid and lymphoid chemoattractants (CCL20, MCP1), and antimicrobial peptides (AMPs: β-defensins, lipocalin-2, S100A8/9) (Conti and Gaffen, 2015). While IL-17-dependent signals are essential for effective immunity to OPC, the nature of the responding cell type(s) is less clear. OECs do not express IL-17 (Huppler et al., 2015) but do express the IL-17R (Gaffen et al., 2014). The oral mucosa is enriched for hematopoietic cells during infection, which also express high levels of IL-17RA (Ishigame et al., 2009; Ye et al., 2001). In this regard, a recent study reported that IL-17RA signaling on NK cells mediates immunity to bloodstream C. albicans infections (Bar et al., 2014). Additionally, saliva is an important component of immune control of C. albicans, and salivary gland epithelial cells also respond to IL-17 to produce salivary AMPs (Conti et al., 2011).
The objective of this study was to identify the key IL-17-responding cell(s) in OPC. We approached this question with radiation chimeras and by creating CRE-expressing transgenic mice with specificity for suprabasal oral/esophageal epithelium. Whereas IL-17RA-mediated responses in the hematopoietic compartment were dispensable for immunity to OPC, a deficiency of IL-17RA in OECs rendered mice almost as susceptible as a complete Il17ra knockout. Gene profiling showed close parallels between human OECs infected in vitro with C. albicans and genes induced in murine oral tissue during OPC. In contrast to Il17ra−/− mice, regulation of neutrophil-specific genes was largely intact in mice with the conditionally targeted Il17ra allele, whereas expression of β-defensin 3 (BD3) was markedly impaired. Mice lacking BD3 were just as susceptible to OPC as Il17ra−/− mice. Thus, the oral epithelial cell is the dominant IL-17-responsive cell type in controlling immunity to oral mucosal candidiasis.
RESULTS
IL-17RA on hematopoietic cells is dispensable for protection from OPC
We evaluated the contribution of IL-17RA in hematopoietic cells in OPC using an adoptive transfer approach. Femoral bone marrow (BM) cells (103) from Il17ra−/− (CD45.2+) and WT (CD45.1+) mice were transferred into lethally irradiated Il17ra−/− or WT recipients. After 8 weeks, reconstitution was confirmed by flow cytometry of peripheral blood (data not shown). Successfully reconstituted mice were subjected to OPC by sublingual incubation with a cotton ball soaked in 107 CFU/ml C. albicans yeast (Solis and Filler, 2012). After 5 d, oral fungal loads were assessed by CFU enumeration, which has a limit of detection (LOD) of ~30 CFU/g (Whibley et al., 2016). WT mice reconstituted with WT or Il17ra−/− BM were resistant to infection, with a negligible fungal load (CFU<2), indicating that IL-17RA expression on hematopoietic cells is not required for fungal clearance (Fig 1A). As previously shown, Il17ra−/− mice were susceptible to OPC (Conti et al., 2009), displaying a mean fungal burden of 1847 CFU/g. Similarly, Il17ra−/− mice reconstituted with WT or Il17ra−/− bone marrow exhibited fungal loads that were indistinguishable from Il17ra−/− mice (3635 and 2153 CFU/g, respectively). Consistently, WT recipients returned to starting weight by day 5, regardless of BM source. In contrast, Il17ra−/− mice experienced progressive weight loss, even when reconstituted with WT BM (Fig 1B).
Figure 1. IL-17RA expressed on hematopoietic cells is dispensable for protection against oral candidiasis.
Reciprocal adoptive transfers of femoral BM were performed in WT or Il17ra−/− mice followed by oral infection with C. albicans. A. After 5 d, fungal loads were assessed by CFU enumeration of tongue homogenates. Bars show geometric mean. Each point is 1 mouse. ***P<0.01 by ANOVA and t-test with Mann-Whitney correction. B. Weight was assessed daily, and is shown as mean % of starting weight. C. Defb3 was evaluated on d 5 by qPCR. Data relative to Gapdh + SEM. ***P<0.01 by ANOVA and post-hoc Tukey’s test. N.S., not significant. All experiments were performed at least twice.
Exposure to C. albicans activates a reproducible gene profile in mouse oral tissue, which peaks at day 2 when fungal loads are highest. Many of these genes are impaired in Il17ra−/− mice, such as Defb3 (encoding β-defensin 3, BD3) (Conti et al., 2009; Hernández-Santos et al., 2013). In keeping with their fungal loads, WT mice receiving WT or Il17ra−/− bone marrow induced Defb3 efficiently. However, induction was lower in Il17ra−/− recipients reconstituted with WT bone marrow. Thus, IL-17RA is required exclusively in non-hematopoietic cells.
C. albicans induces an antifungal gene response in human OECs
Signals induced in OECs upon early exposure to C. albicans help distinguish between commensal colonization and pathogenic invasive infections (Moyes et al., 2015). In an effort to understand how IL-17 signaling impacts the response of human OECs to C. albicans infection, OKF6/TERT-2 oral keratinocytes were treated with IL-17A (50 ng/mL) and a suboptimal dose of TNFα (0.5 ng/mL) for 24 h. Cells were then infected with 1×107 C. albicans yeast cells for 5 h and subjected to RNA-Seq. 358 genes exhibited a minimum 2-fold change in expression (P<0.05) between the TNF/IL-17A-treated and untreated OKF6/TERT-2 monolayers (Fig 2A, Table S1). Many of the genes exhibiting TNF/IL-17-sensitive expression in OECs have mouse homologues that are IL-17-dependent in OPC (Conti et al., 2009). These included chemokines, cytokines, transcriptional or post-transcriptional immune regulators, AMPs and other factors associated with inflammation.
Figure 2. C. albicans induces an antifungal gene response in human OECs that parallels the IL-17-dependent response to murine OPC.
A. OKF6/TERT2 human OECs were treated with IL-17+TNFα for 24 h. Cells were exposed to C. albicans yeast for 5 h and subjected to RNA-Seq. Data show normalized RPKM values of genes whose orthologues were previously shown to be induced in an IL-17R-dependent manner in murine OPC (Conti et al., 2009). Yellow = high and blue = low relative expression. B. Ingenuity Pathway Analysis of RNA-Seq data, showing C. albicans-exposed OKF6/TERT2 cells (untreated relative to IL-17+TNFα-treated) compared to murine OPC (C. albicans-infected WT tongue relative to uninfected). Data indicate z-scores of predicted regulation by infection. Red = predicted activation. Blue = predicted repression. White = no predicted regulation.
Insights into pathogenesis can be obtained by inferring signal transduction pathways based on downstream gene expression changes, a particularly useful way to compare species where not all genes have direct homologues (Liu et al., 2015). Accordingly, we compared the human OEC RNA-Seq dataset to an RNA-Seq profile of WT and Il17ra−/− mice 24 h after oral infection (Tables S1–S2). Data were analyzed using the Upstream Regulator Analytic from Ingenuity Pathway Analysis software, which assesses the overlap between empirical data and a curated database of target genes for each of several hundred known regulatory proteins. A number of signaling pathways were identified common to both datasets, including TNF and IL-17 based pathways, TLR pathways, NF-κB activation, EGFR signaling, among others (Fig 2B). Thus, despite the substantial differences between the experimental systems (human vs. mouse, culture monolayers vs. tissue, cytokine treatment vs. genetic deletion), data confirm that IL-17 signaling in human OECs parallels IL-17-dependent gene expression during OPC.
Generation of Cre-expressing transgenic mouse with specificity for oral epithelium
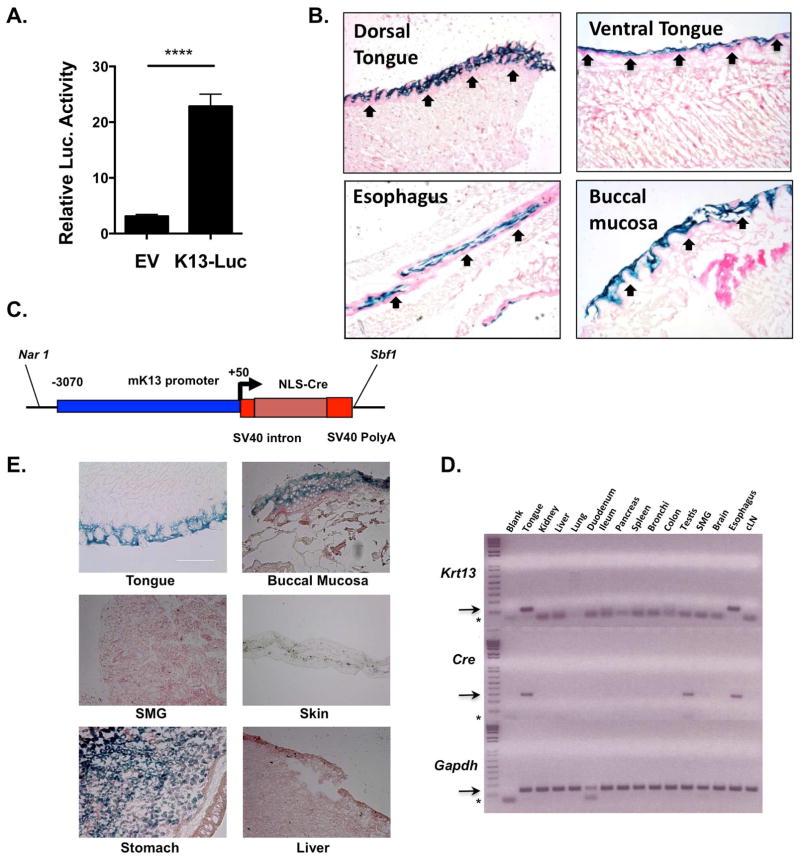
To date, there are surprisingly few tools available to interrogate activity in oral epithelium. The oral mucosa consists of morphologically discrete epithelial layers with keratinization patterns distinct from skin (Presland and Dale, 2000). Whereas K14 and K5 are expressed in the basal stem cells of oral keratinized and non-keratinized epithelia, K13 is expressed superficially in the differentiated suprabasal layers, representing the first line of contact to oral microbes. To identify K13 regulatory sequences, we cloned the Krt13 putative proximal promoter (−3070 to +50 relative to the transcriptional start site) upstream of Luciferase. This construct exhibited potent activity in the murine oral keratinocyte (IMOK) cell line (Fig 3A) (Parikh et al., 2008). To evaluate the Krt13 promoter in vivo, we created transgenic mice expressing LacZ driven by the K13 promoter (K13LacZ). Oral tissues were analyzed for β-galactosidase activity by Xgal staining, including esophagus, buccal mucosa, and dorsal and ventral tongue. K13-driven LacZ activity was restricted to the differentiated suprabasal layers of these tissues, with no discernible expression in the basal or sub-basal layers (Fig 3B). There was no LacZ activity in gut, fore-stomach or GI tract (data not shown). Therefore, the K13 promoter shows specificity for expression in OECs, consistent with its known expression pattern.
Figure 3. The Keratin 13 promoter shows specific activity in oral epithelium.
A. The K13 promoter in the pGL3-Luc vector was transfected into oral epithelial IMOK cells, and Luc activity assessed after 24 h. Data show mean + SEM. ***P<0.01 by student’s t-Test. EV, empty vector. B. K13LacZ mice were analyzed for β-galactosidase activity by Xgal staining. C. K13-Cre cassette used to create K13CRE mice. D. Indicated tissues from K13CRE founder #176 were analyzed for Krt13 and Cre by qPCR. SMG, submandibular salivary gland; cLN, cervical lymph node. Arrow = specific PCR product. * = unincorporated PCR primers. E. Tissues from K13CRE-Rosa26LacZ mice were stained with Xgal to assess β-galactosidase activity. Data are from 2–4 independent experiments.
We created transgenic mice expressing the K13 promoter upstream of a Cre recombinase cassette (Fig 3C). Four PCR+ founders were identified, and tissues were evaluated for coincident Cre and Krt13 mRNA expression in tongue and esophageal tissue (Fig 3D). The line where Cre expression most closely paralleled the known expression pattern of Krt13 (and K13-LacZ mice) was selected for all subsequent experiments. Progeny from Cre-expressing lines were then crossed to Gt(Rosa)26tm1Sor reporter mice (K13CRERosa26LacZ). LacZ staining of sections from the K13CRE-Rosa26LacZ mice showed strong Cre expression and activity in oral tissues such as the tongue and buccal mucosa, as well as the upper GI tract including stomach (Fig 3E) and the vaginal epithelium (not shown). Little or no LacZ was detected in skin, submandibular salivary gland or hematopoietic organs such as spleen and cervical lymph nodes (cLN).
IL-17RA expressed by OECs contributes to protection from oral candidiasis
To determine the extent to which IL-17RA expression in oral epithelium is required for immunity to OPC, K13Cre mice were crossed to recently-developed Il17rafl/fl mice (Kumar et al., 2016) (Il17rafl/flK13Cre+, also termed Il17raΔK13). To assess efficiency of receptor deletion, we evaluated IL-17RA in the oral cavity by IHC. Tongue from Il17rafl/flK13Cre− littermates (also termed WT) but not Il17ra−/− controls stained for IL-17RA throughout the tissue (Fig 4A). In Il17raΔK13 mice, IL-17RA was effectively deleted in the suprabasal layers (BL) of the oral epithelium but not other areas of the tissue (Fig 4A). To assess the consequence of OEC-specific deletion, Il17raΔK13 and controls were subjected to OPC and fungal loads assessed over 14 days. All infected animals had high C. albicans fungal burdens for the first 2 d p.i.. Il17ra−/− mice maintained high fungal loads through day 14. Il17raΔK13 mice showed a similar susceptibility profile as Il17ra−/− mice. Specifically, fungal counts on days 3–4 were statistically indistinguishable from Il17ra−/− mice (Fig 4B). Both Il17raΔK13 and Il17ra−/− mice lost weight progressively, whereas Il17rafl/flK13Cre− littermates returned to their starting weights (Fig 4B).
Figure 4. IL-17RA in oral epithelial cells is necessary for immunity to OPC.
A. Expression of IL-17RA was assessed by IHC. BL, suprabasal layer (n=3). B. Indicated mice were subjected to OPC as in Fig 1. Left: Mean fungal loads + SD at the indicated days post infection (left). Il17ra−/− (green, n= 3–6), Cre+ (blue, n=3–7), Cre− (black, n= 3–4). Right: Mean % weight change relative to day 0 + SD. Data are from 2–4 experiments per time point. C. Saliva was incubated with C. albicans cells for 1 h at 37°C and CFU was assessed by plating. Data show mean + SEM. Experiment was done twice.
The composition of saliva also influences oral C. albicans colonization. We previously found that saliva from Il17ra−/− mice exhibits a modestly impaired ability to kill C. albicans in vitro (Conti et al., 2009). AMPs in saliva such as defensins are derived from salivary glands but are also shed from the oral epithelium. To determine whether IL-17RA-dependent factors from OECs contributed to salivary candidacidal function, mice were administered carbachol to induce salivation, saliva was incubated with 106 C. albicans cells for 1 h, and cultures plated for colony enumeration. Saliva from sham-infected mice exhibited similar baseline candidacidal activities (Fig 4C). After infection, saliva from Il17raΔK13 mice and Il17rafl/flK13Cre− controls maintained similar levels of Candida-killing capacity, while Il17ra−/− saliva showed a trend to reduced killing efficiency. Consequently, the increased susceptibility of Il17raΔK13 mice to OPC does not appear to be due to defects in salivary function.
OEC-specific IL-17RA regulation of neutrophils
IL-17RA induces expression of neutrophil-related chemokines and growth factors during OPC, such as CXC chemokines and G-CSF (Conti et al., 2009; Huppler et al., 2014). Here, we evaluated PMN recruitment to the supra-and sub-basal layers of epithelial tissue. Immediately after infection (day 1), there was significantly reduced neutrophil infiltration to the supra-basal region in Il17ra−/− mice compared to Il17rafl/flK13Cre− (WT) controls. Surprisingly, however, there was no difference in PMN numbers in Il17raΔK13 mice in this area compared to WT (Fig 5A, B). At later times (days 3–5), PMN numbers were elevated in both Il17ra−/− and Il17raΔK13 supra-basal tissue compared to WT, which probably reflects the ongoing C. albicans infection in knockout but not WT mice (Fig 5A, B). In sub-basal layers, there was no difference in PMN counts in any of the mice (Fig 5A,B). However, although neutrophil levels are elevated, they never reach the same frequency as in WT mice. Therefore, OEC-specific deletion of IL-17RA does not cause impaired local recruitment of PMNs to the superficial epithelial layer, though a complete Il17RA-deficiency does (Conti et al., 2009). As an additional assessment of pathology, we quantified tissue hyperplasia, measured as the distance between the outermost keratinized portion of the epithelium and stem cells of the basal layer. In line with the sustained levels of PMNs in the Il17raΔK13 mice, the supra-basal epithelium was hyperplastic compared to Il17rafl/flK13Cre− controls (Fig 5C–D). This finding indicates that IL-17RA signaling in OECs is not required for cellular proliferation.
Figure 5. Tissue changes in Il17raK13 compared to Il17ra−/− mice following C. albicans infection.
A. 20X images from H&E-stained tongue sections from the indicated C. albicans-infected mice at days 1 or 5. Black arrow indicates division between supra- and sub-basal layers. White arrows indicate PMNs. Scale bar is 100 μM. B. Quantification of PMNs in H&E images (n=4 mice/group, minimum of 6 sections per mouse). *P<0.05 WT (Cre−) vs. Il17ra−/− ‡ P<0.05 Il17raK13 vs. WT (Cre−) by ANOVA with Kruskal-Wallis correction. Data indicate means + SEM. C. Hyperplasia is indicated as thickening of epithelium between basal cell layer to superficial keratinized layer. Arrows indicate distance measured in microns using ImageJ. D. Quantification of hyperplasia by blinded assessors (n=4 mice/group, minimum 4 sections per mouse). * P<0.05 compared to WT. ‡P<0.05 compared to Il17raΔK13 by ANOVA. Data indicate means + SEM. E. MPO staining in infected mice. Arrows indicate areas of high MPO staining. Experiments were performed on sections from 3–5 mice per strain. All experiments were done twice.
We next asked whether the neutrophils migrating to the superficial epithelium functioned normal by staining tissue for myeloperoxidase (MPO). Neutrophils from all mice stained positive for MPO, indicating that the neutrophils within the tissue appear functional (Fig 5E). Collectively, these data confirm and extend our previous findings that IL-17RA regulates PMN migration, but indicate surprisingly that the neutrophil defect is less marked in Il17raΔK13 mice compared to Il17ra−/− mice. Accordingly, the high susceptibility of Il17raΔK13 mice to OPC is likely not due to neutrophil defects.
OPC-induced gene profiles in Il17raΔK13 mice: Role for β defensin 3
To gain an unbiased assessment of genes regulated by IL-17RA within OECs, we performed RNA-Seq analysis of tongue tissue from Il17rafl/flK13Cre−, Il17raΔK13 and Il17ra−/− mice 1 day after oral C. albicans infection (see Fig 2). A panel of genes emerged that were expressed in Il17rafl/flK13Cre− littermate controls (WT) upon infection (i.e., that were different from sham-infected mice) that were differentially regulated in both Il17raΔK13 and Il17ra−/− mice after infection (Fig 6A, Fig S1). Notably, the majority of the gene changes that are absent in the Il17ra−/− mice also absent in the Il17raΔK13 mouse. These overlapping genes included known IL-17RA targets important in antifungal defense, including AMPs (Defb3, S100A9) and chemokines (Cxcl1, Cxcl5) (Tables S1, S2). These data confirmed that the OEC-specific deletion of IL-17RA is concordant with the full knockout with respect to early antifungal responses. However, some genes are also regulated by other IL-17RA-expressing tissues.
Figure 6. C. albicans-induced gene expression changes require epithelial IL-17RA and demonstrate a role for BD3.
A. RNA-seq was performed 24 h after infection (n=3). Heatmap shows genes that are differentially expressed after infection of WT mice for 24 h and whose regulation is altered in infected Il17ra−/− or Il17raK13 mice. Values = log (base 2) fold change. Red bar indicates genes whose C. albicans-induced regulation required IL-17RA in oral epithelium. B. Tongue RNA from C. albicans-infected mice was assessed by qPCR (n= 6–9). Bars indicate mean + SEM. *P<0.05 by ANOVA and student’s t-test. C. Tongue sections were stained with α-BD3 Abs or 2° Ab. Arrows indicate suprabasal areas (n=3). D. TR146 cells were incubated with IL-17 for 20 h ± IL-17A. BD2 in supernatants was assessed by ELISA. Bars indicate mean + SEM. ** P<0.01. E. TR146 cells were infected with C. albicans yeast at an MOI of 0.01 ± IL-17A (200 ng/ml) for 24 h. Secreted LDH was assessed in triplicate. F. Indicated mice were subjected to OPC and oral fungal loads assessed after 5 d. Bars indicate geometric mean. **P<0.01. **** P<0.0001. All experiments were done at least twice. See also Fig S1 and Table S1.
To understand the kinetics of the OEC-dependent gene expression, we investigated the profile of a subset of genes identified by RNASeq over 5 days. We first focused on neutrophil-associated genes including S100A9, chemokines (Cxcl1, also known as KC; Cxcl2, Cxcl5) and G-CSF (Csf3). Although all these genes were reduced in Il17raΔK13 mice on day 1 (Fig 6A, B), expression recovered by days 2–3, consistent with the elevated levels of neutrophils observed in these mice compared to the Il17ra−/− mice (Fig 5). WT mice also showed reduced expression of these genes, presumably because they have cleared the infection and the overall immune response has returned to baseline. Lipocalin-2 (Lcn2), while not required for the response to OPC (Ferreira et al., 2014), serves as a sensitive measure of IL-17 signaling activity in OPC and other settings (Shen et al., 2006). There was impaired expression of Lcn2 in both Il17raΔK13 mice and Il17ra−/− mice at day 1, the only time point when this gene was high in WT mice (Fig 6A,B).
β-Defensins exhibit potent anti-Candida activity. Defb1 was impaired at day 1 in Il17ra−/− mice, consistent with its known role in immunity to OPC (Tomalka et al., 2015), and here we demonstrate an IL-17-dependent activation of this factor. However, Defb1 was not altered in Il17raΔK13 mice. Defb2 and Defb4 were not altered at any time points (not shown). In contrast, Defb3 expression correlated well with OPC susceptibility in Il17raΔK13 mice, as its expression was markedly impaired at days 1–3 in Il17raΔK13 and Il17ra−/− mice compared to WT (Fig 6A,B). To determine whether BD3 protein was reduced in situ, we performed IHC with anti-BD3 Abs on Il17rafl/flK13Cre−, Il17raΔK13 and Il17ra−/− tongue sections after OPC (Fig 6C). BD3 was expressed in the superficial tongue and the OECs in Il17rafl/flK13Cre− mice, but was absent in both the Il17raΔK13 and Il17ra−/− OECs. We next confirmed that human OECs stimulated with IL-17 induced secretion of BD2, the human orthologue of murine BD3 (Fig 6D). Since clinical blockade of IL-17 is associated with impaired epithelial repair in the gut (Whibley and Gaffen, 2015), we asked whether IL-17 can protect from cell damage induced by C. albicans. However, IL-17 did not alter the cytotoxicity arising from C. albicans infection of cultured OECs, measured by secretion of lactate dehydrogenase (Fig 6E). Finally, mice lacking BD3 (Defb3−/−) were just as susceptible to OPC as Il17ra−/− mice (Fig 6F). Thus, OEC-derived BD3 appears to account for the high susceptibility of Il17raΔK13 mice to OPC.
DISCUSSION
Our understanding of fungal pathogens has historically lagged behind that of bacteria or viruses (Brown et al., 2012), but recent discoveries in studies of C. albicans have dramatically advanced the field. These insights include (i) the identification of C-type lectin receptors as key pattern recognition receptors for fungi, and (ii) the recognition that IL-17 signaling is central to antifungal immunity (Hernández-Santos and Gaffen, 2012). Mice lacking IL-17RA or IL-17RC are susceptible to OPC and dermal candidiasis (Conti et al., 2009; Conti et al., 2015; Ho et al., 2010; Kagami et al., 2010). This finding was upheld in humans with IL-17R mutations (Ling et al., 2015; Puel et al., 2011). Our bioinformatics data provide additional evidence that the murine response to oral C. albicans is concordant with human OECs (Fig 2). Not only are there conspicuous similarities in individual genes or their orthologues (β-defensin 2/3, chemokines, lipocalin-2 (Jia et al., 2000)), at a global level the pathways induced by C. albicans show strong parallels.
Nonetheless, it has not been obvious in which tissue(s) or cell types IL-17 signaling is required. Mesenchymal and epithelial cell types are generally considered the main responders to this cytokine. Their sensitivity is thought to be due to high expression of IL-17RC on these cell types, whereas this subunit is expressed at low or undetectable levels in hematopoietic cells (Ishigame et al., 2009; Kuestner et al., 2007). IL-17RA is expressed more broadly than IL-17RC, with particularly high levels in hematopoietic cells (Ishigame et al., 2009; Yao et al., 1995; Ye et al., 2001). Here, we found that the hematopoietic compartment was dispensable for immunity to OPC (Fig 1), similar to the tissue-dependence of IL-17RA in a murine arthritis model (Lubberts et al., 2005). However, this contrasts with a report that NK cells are the essential IL-17-responsive cell type in systemic candidiasis (Bar et al., 2014).
Although OECs comprise only a fraction of total tongue tissue, they are the first cells to encounter C. albicans in the mouth. Until now, genetic tools to study oral tissue have been limited. Epithelial tissues are classified by differentiation-specific expression of keratins, cytoskeletal proteins that provide structural integrity and function as a protective barrier. In oral and buccal epithelia, Keratins (K)-13 and -4 are expressed in superficial and intermediate tissue layers (Presland and Dale, 2000). We show that mice expressing Cre under control of the K13 promoter exhibit efficient and specific conditional deletion within these tissues (Figs 2–3). The creation of this K13CRE line will be valuable for many areas of inquiry, such as other oral/esophageal/vaginal infections, oral cancer, and wound healing in non-keratinized tissues.
There is an intriguing concordance between K13 expression and sites of C. albicans infections, including oral and esophageal mucosa and vaginal epithelium (data not shown). To date, the participation of IL-17 in vaginal candidiasis remains controversial, but this mouse system will be useful for probing the role of IL-17 in this tissue (Ibrahim et al., 2013; Pietrella et al., 2011; Yano et al., 2012). It is unlikely that K13 acts as a receptor for C. albicans, though this possibility cannot be formally excluded. Rather, epithelial cells within non-keratinized layers have a rapid turnover (~4 days), compared to ~2 weeks in skin. These sites are also thinner and more permeable than skin, and thus may be more amenable to fungal invasion. Recent data showed that a C. albicans-derived toxin termed Candidalysin is required to establish pathogenic infections of the oral mucosa (Moyes et al., 2016). Conceivably, the cellular receptor for Candidalysin is more highly expressed in Krt13+ OECs.
Our data with Il17raΔK13 mice suggested that the bulk of IL-17RA-driven immunity in the context of OPC is from OECs (Fig 4). While there was not a statistically significant difference in fungal burdens between Il17raΔK13 and Il17ra−/− mice, there was a consistent trend to lower fungal counts in the Il17raΔK13 animals, suggesting there may be contributions of IL-17RA in other cells. This idea is supported by the global analysis of the gene differences between Il17raΔK13 and Il17ra−/− mice during infection. Although expression of many genes was conserved during OPC on day 1, there were notable differences (Fig S1, 6B). Genes associated with neutrophils are a case in point; many of the factors that regulate neutrophil chemotaxis (Cxcl1), expansion (Csf3) or function neutrophils (S100A9) were more severely impaired in the total knockout compared to the OEC-specific deletion. These data are consistent with histological analyses, which demonstrated reduced recruitment of PMNs to Il17ra−/− suprabasal oral tissue compared to comparable sites in Il17raΔK13 mice (Fig 5). In contrast, expression of Defb3 was markedly reduced in Il17raΔK13 mice, correlating well with fungal loads (Fig 6). These data collectively imply that IL-17RA-driven expression of neutrophil-attractive factors is driven by OECs at early time points (day 1) but by other cells at subsequent times (days 3–5).
The nature of other IL-17RA-responsive cells is an intriguing question. We speculate that mesenchymal cells (e.g., muscle or fibroblastic cells) within the oral mucosa may be involved. We base this hypothesis on a study in a murine psoriasis model that showed a similar dichotomy in IL-17-dependent functions by cell type. In a murine psoriasis model, a deficiency in Act1 (a key adaptor downstream of IL-17RA) in dermal keratinocytes impairs neutrophilic abscess formation, whereas Act1 deleted only dermal fibroblasts impacts keratinocyte hyperproliferation (Ha et al., 2014). Notably, mice or humans lacking Act1 are also susceptible to OPC (Boisson et al., 2013; Ferreira et al., 2014). Although we did not see an obvious contribution of saliva in the Il17raΔK13 mice (Fig 4), this assay is not very sensitive, so we cannot rule out a role for IL-17RA in salivary glands.
IL-17 regulates expression of β-defensins at mucosal surfaces (Aujla et al., 2007; Kao et al., 2004). IL-17 is implicated in regulation of murine BD3 and its human homologue BD2 (Figs 1, 5–6, (Conti et al., 2009; Jia et al., 2000; Simpson-Abelson et al., 2015)). The importance of BD3 was confirmed by the observation that Defb3−/− mice showed the same susceptibility to OPC as Il17ra−/− animals. BD3 exerts direct antifungal activity on C. albicans (Edgerton et al., 2000; Joly et al., 2004). Additionally, BD3 binds to CCR6, potentially allowing it to serve as a chemoattractant for IL-17-expressing cells that express this receptor (Yamazaki et al., 2008). CCL20 is another ligand for CCR6 that is induced during OPC by IL-17RA signaling (Fig 6). Intriguingly, CCL20 also exhibits candidacidal activity (Yang et al., 2003). BD1 is implicated in OPC (Tomalka et al., 2015), and our data indicate this is also IL-17RA-dependent. Thus, genes expressed in OPC have the capacity to function as AMPs and chemoattractants, establishing a feed-forward amplification loop that controls fungal infections.
Most data indicate that IL-17A is the dominant cytokine in OPC, but other Type 17 cytokines contribute. Patients with autoimmune polyendocrinopathy syndrome (AIRE deficiency) produce neutralizing Abs against IL-17A, IL-17F and IL-22, correlating with CMC (Kisand et al., 2010; Puel et al., 2010). Il17rc−/− mice (which respond only to IL-17A and 17F) phenocopied Il17ra−/− mice (Ho et al., 2010), later confirmed in humans (Ling et al., 2015). Il17a−/− but not Il17f−/− mice are susceptible to OPC, but dual blockade of IL-17A and IL-17F increases susceptibility over anti-IL-17A treatment alone (Gladiator et al., 2013; Whibley et al., 2016). Th17 cells also make IL-22, and Il22−/− mice are moderately susceptible to OPC (Conti et al., 2009). IL-25 (IL-17E) has not been evaluated in OPC, to our knowledge, but there is no change in Il17e expression in mice upon oral infection with C. albicans (Whibley et al., 2016). Thus, there appears to be cooperative activity of Type 17 cytokines in immunity to oral C. albicans infections.
In summary, this and the accompanying report by Chen et al. (Chen et al., 2016) provide insight into the tissue selective role by which IL-17R-dependent immunity to mucosal pathogens is controlled. Drugs that target IL-17 and Th17-related pathways are now approved for moderate-severe plaque psoriasis (Sanford and McKeage, 2015); inevitably, as their clinical use increases, so does the potential for opportunistic mucosal infections. Ultimately, studies like these may inform the development of better-targeted therapeutics for human disease.
EXPERIMENTAL PROCEDURES
Cell culture
OKF6/TERT2 cells (Dickson et al., 2000) were cultured in Serum-Free Fibroblast media, 25 μg/ml bovine pituitary extract and 2 ug/ml EGF (Life Technologies, Grand Island NY). Cells were treated with TNFα (0.5 ng/mL) and IL-17A (50–200 ng/mL) (R&D Systems) for 24 h and then infected with 1×107 C. albicans yeast for 5 h. TR146 cells were from ECACC (Salisbury, United Kingdom) and cultured in DMEM-F12/15% FBS.
K13 promoter
The Krt13 promoter (−3090 to +40) was obtained by PCR from BAC clone RP23-10A2 and cloned into the pGL3-basic vector (Promega). IMOK cells (Parikh et al., 2008) were transfected with the reporter with FuGENE 6, normalized to a pCMV-LacZ control. Function was assessed with the Luciferase Assay system (Promega), and β-galactosidase with the Galacton Plus kit (Life Technologies).
Mice
Il17ra−/− mice and anti-IL-17RA Abs were a gift from Amgen (Seattle WA). Il17rafl/fl mice were made as described (Kumar et al., 2016). Defb3−/− mice were from the MMMRC (UC Davis, CA). K13LacZ transgenic mice were created a K13-LacZ cassette at the Roswell Park Cancer Institute (RPCI) Transgenic Core Facility. Oral tissue sections were stained with Xgal to detect β-galactosidase activity. K13CRE transgenic mice were created with the Krt13-NLS-Cre by the Pittsburgh Transgenic and Gene Targeting Core Facility, and founders identified by PCR. C57BL/6 and Gt(Rosa)26tm1Sor mice were from The Jackson Laboratory (Bar Harbor ME). Experiments were performed in accordance with IACUC protocols approved by the University of Pittsburgh, the State University of New York at Buffalo or RPCI.
Oral Candidiasis
OPC was performed by sublingual inoculation with a cotton ball saturated in C. albicans (CAF2-1) for 75 min (Solis and Filler, 2012). Tongue homogenates were dissociated on a GentleMACS (Miltenyi Biotec, Cambridge MA) and serial dilutions plated on YPD+Amp. All efforts were made to minimize suffering, in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH.
Salivary assays
Saliva was collected after carbachol injection (100 ul, 10 μg/ml) and used immediately (Conti et al., 2009). 90 ul of saliva was incubated with 104 cells of C. albicans for 1 h at 37°C, plated in triplicate and assayed after 2 d for CFU enumeration on YPD-Amp.
Immunohistochemistry, ELISA, LDH assays
Cryosections were stained with α-IL-17RA mAbs (Amgen), α-MPO mAbs (R&D Systems) or anti-rat BD3 Abs (Santa Cruz Biotechnology) using the BioLegend IHC Protocol for Frozen Tissue or Immunocruz LSAB Staining System. Image analysis was performed by 2 independent assessors in a blinded fashion. BD2 ELISAs were performed with Abs from Peprotech. LDH assays were performed with a CytoTox 96 Assay System (Promega).
RNA-Seq and qPCR
RNA-Seq mouse libraries were prepared with total RNA using a Qubit 2.0 fluorometer (Thermo Fisher) and Agilent Bioanalyzer TapeStation 2200 (Agilent Technologies). For OKF6-TERT2 in vitro infections, RNA-seq libraries (non-strand specific, paired-end) were prepared with the TruSeq RNA kit (Illumina). 100 nucleotides were determined from each end of cDNA fragments using the HiSeq platform. Single reads were aligned to the UCSC mouse or human reference genomes (mm10, GRCm38.75; Ensembl GRCh38) using TopHat2. Differential gene expression was assessed using DESeq (Bioconductor). Pairwise differential expression was quantified with Cuffdiff. Cufflinks was used to determine FPKM levels for each gene from the STAR alignment and was used as input for Cuffdiff. Read counts were then normalized across all samples and differentially expressed genes were determined by adjusted P-value with a threshold of 0.05.
For real-time PCR, RNA was isolated from tongue with RNeasy Mini Kits (QIAGEN), and cDNA generated with Superscript III First Strand Kits (Invitrogen). Real-time qPCR using SYBR Green FastMix ROX (Quanta Biosciences) was performed using a 7300 Real time instrument (Applied Biosystems) and normalized to GAPDH. Primers were from Superarray Biosciences or QuantiTect Primer Assays (QIAGEN).
Data Access
Upon acceptance, raw sequencing reads will be submitted to the NCBI Sequence Read Archive (SRA; http://www.ncbi.nlh/nih.gov/sra) and processed gene expression data will submitted to the NCBI Gene Expression Omnibus (GEO). The RNA-seq data for the in vitro infections has been deposited under accession code SRP077728.
Supplementary Material
HIGHLIGHTS.
Human and murine oral epithelial cells show concordant responses to C. albicans
IL-17R signaling in the oral epithelium is necessary for defense against candidiasis
IL-17R signaling in oral epithelial cells is a major driver of β-defensin expression
β-defensin 3-deficient mice are susceptible to oral candidiasis
Acknowledgments
SLG was supported by the NIH (AI107825, DE022550, DE023815). HRC was supported by F32-DE023293 and the University of Toledo. VMB and SGF were supported by U19AI110820. JKK was supported by DE022550 and R37-HL079142. AGH was supported by DE018279 and a Dept. of Veterans Affairs Merit award I01BX002607. JRN was supported by the Medical Research Council (MR/M011372/1) and Biotechnology & Biological Sciences Research Council (BB/N014677/1). This work is solely the responsibility of the authors and does not necessarily reflect the views of the NIH or other funding bodies. We thank W Horne for assistance with RNA-Seq. We thank Amgen for Il17ra−/− mice and anti-IL-17RA Abs, and MJ McGeachy and PS Biswas for helpful suggestions and critical reading.
ABBREVIATIONS
- AMP
antimicrobial peptide
- BD
β-defensin
- CMC
chronic mucosal candidiasis
- K13
keratin 13
- LDH
lactate dehydrogenase
- OEC
oral epithelial cell
- OPC
oropharyngeal candidiasis
Footnotes
CONTRIBUTIONS
Conceptualization: SLG, HRC, SS, SGF, VMB; Methodology: SLG, SS, JKK; Formal Analysis: VMB; Investigation: HRC, EEC, JPH, BGM, BMC, MRH, LB, NS, JAC, AHV, SD, AGH, AVG, JPR, DLS, SLG and SS; Resources: JKK, JRN; Writing – Original draft: SLG, HRC, VMB; Writing – Review and Editing: JKK, JRN, SGF, SS; Supervision: HRC, SLG; Funding Acquisition: SLG, HRC, VMB, SGF, JKK.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19:377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bär E, Gladiator A, Bastidas S, Roschitzki B, Acha-Orbea H, Oxenius A, LeibundGut-Landmann S. A novel Th cell epitope of Candida albicans mediates protection from fungal infection. J Immunol. 2012;188:5636–5643. doi: 10.4049/jimmunol.1200594. [DOI] [PubMed] [Google Scholar]
- Bar E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity. 2014;40:117–127. doi: 10.1016/j.immuni.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Boisson B, Wang C, Pedergnana V, Wu L, Cypowyj S, Rybojad M, Belkadi A, Picard C, Abel L, Fieschi C, et al. A biallelic ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity. 2013;39:676–686. doi: 10.1016/j.immuni.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- Chen K, Trevejo-Nunez G, Way E, Elsegeiny W, Ricks D, Garg A, Wang T, Gaffen SL, Kolls J. Epithelial IL-17R signaling is required for mucosal chemokine gradients and pulmonary host defense against K. pneumoniae. Cell Host Microbe. 2016 doi: 10.1016/j.chom.2016.10.003. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti H, Baker O, Freeman A, Jang W, Li R, Holland S, Edgerton M, Gaffen S. New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol. 2011;4:448–455. doi: 10.1038/mi.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti H, Peterson A, Huppler A, Brane L, Hernández-Santos N, Whibley N, Garg A, Simpson-Abelson M, Gibson G, Mamo A, et al. Oral-resident, Candida albicans infections. J Exp Med. 2014;211:2075–2084. doi: 10.1084/jem.20130877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti H, Shen F, Nayyar N, Stocum E, JNS, Lindemann M, HoA, Hai J, Yu J, Jung J, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Gaffen SL. IL-17-Mediated Immunity to the Opportunistic Fungal Pathogen Candida albicans. J Immunol. 2015;195:780–788. doi: 10.4049/jimmunol.1500909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Whibley N, Coleman B, Garg A, Jaycox J, Gaffen S. Signaling through IL-17C/IL-17RE is dispensable for immunity to systemic, oral and cutaneous candidiasis. PLoS One. 2015;10:e0122807. doi: 10.1371/journal.pone.0122807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton M, Koshlukova SE, Araujo MW, Patel RC, Dong J, Bruenn JA. Salivary histatin 5 and human neutrophil defensin 1 kill Candida albicans via shared pathways. Antimicrob Agents Chemother. 2000;44:3310–3316. doi: 10.1128/aac.44.12.3310-3316.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MC, Whibley N, Mamo AJ, Siebenlist U, Chan YR, Gaffen SL. Interleukin-17-induced protein lipocalin 2 is dispensable for immunity to oral candidiasis. Infect Immun. 2014;82:1030–1035. doi: 10.1128/IAI.01389-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidel PL., Jr Candida-Host Interactions in HIV Disease: Implications for Oropharyngeal Candidiasis. Adv Dent Res. 2011;23:45–49. doi: 10.1177/0022034511399284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL, Jain R, Garg A, Cua D. IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladiator A, Wangler N, Trautwein-Weidner K, Leibundgut-Landmann S. Cutting Edge: IL-17-Secreting Innate Lymphoid Cells Are Essential for Host Defense against Fungal Infection. J Immunol. 2013;190:521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- Glocker E, Grimbacher B. Chronic mucocutaneous candidiasis and congenital susceptibility to Candida. Curr Opin Allergy Clin Immunol. 2010;10:542–550. doi: 10.1097/ACI.0b013e32833fd74f. [DOI] [PubMed] [Google Scholar]
- Ha HL, Wang H, Pisitkun P, Kim JC, Tassi I, Tang W, Morasso MI, Udey MC, Siebenlist U. IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms. Proc Natl Acad Sci U S A. 2014;111:E3422–3431. doi: 10.1073/pnas.1400513111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Santos N, Gaffen SL. Th17 cells in immunity to Candida albicans. Cell Host Microbe. 2012;11:425–435. doi: 10.1016/j.chom.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Santos N, Huppler AR, Peterson AC, Khader SA, KCM, Gaffen SL. Th17 cells confer long term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol. 2013;6:900–910. doi: 10.1038/mi.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A, Shen F, Conti H, Patel N, Childs E, Peterson A, Hernandez-Santos N, Kolls J, Kane L, Ouyang W, et al. IL-17RC is required for immune signaling via an extended SEF/IL-17R signaling domain in the cytoplasmic tail. J Immunol. 2010;185:1063–1070. doi: 10.4049/jimmunol.0903739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppler AR, AHV, HRC, Gaffen SL. Neutrophils do not express IL-17A in the context of acute oropharyngeal candidiasis. Pathogens. 2015;4:559–572. doi: 10.3390/pathogens4030559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppler AR, Conti HR, Hernandez-Santos N, PSB, Darville T, Gaffen SL. Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis. J Immunol. 2014;192:1745–1752. doi: 10.4049/jimmunol.1302265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, Luo G, Gebremariam T, Lee H, Schmidt CS, Hennessey JP, Jr, French SW, Yeaman MR, Filler SG, Edwards JE., Jr NDV-3 protects mice from vulvovaginal candidiasis through T- and B-cell immune response. Vaccine. 2013;31:5549–5556. doi: 10.1016/j.vaccine.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Jia HP, Wowk SA, Schutte BC, Lee SK, Vivado A, Tack BF, Bevins CL, McCray PB., Jr A novel murine β-defensin expressed in tongue, esophagus, and trachea. J Biol Chem. 2000;275:33314–33320. doi: 10.1074/jbc.M006603200. [DOI] [PubMed] [Google Scholar]
- Joly S, Maze C, McCray PB, Jr, Guthmiller JM. Human β-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol. 2004;42:1024–1029. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol. 2010;185:5453–5462. doi: 10.4049/jimmunol.1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamai Y, Kubota M, Kamai Y, Hosokawa T, Fukuoka T, Filler S. New model of oropharyngeal candidiasis in mice. Anti-microb Agents Chemo. 2001;45:3195–3197. doi: 10.1128/AAC.45.11.3195-3197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-κB signaling pathways. J Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuestner R, Taft D, Haran A, Brandt C, Brender T, Lum K, Harder B, Okada S, Osatrander C, Kreindler J, et al. Identification of the IL-17 receptor related molecule, IL-17RC, as the receptor for IL-17F. J Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Monin L, Castillo P, Elsegeiny W, Horne W, Eddens T, Vikram A, Good M, Schoenborn A, Bibby K, et al. Intestinal Interleukin-17 receptor signaling mediates reciprocal control of the gut microbiota and autoimmune inflammation. Immunity. 2016;44:659–671. doi: 10.1016/j.immuni.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y, Cypowyj S, Aytekin C, Galicchio M, Camcioglu Y, Nepesov S, Ikinciogullari A, Dogu F, Belkadi A, Levy R, et al. Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J Exp Med. 2015;212:619–631. doi: 10.1084/jem.20141065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shetty AC, Schwartz JA, Bradford LL, Xu W, Phan QT, Kumari P, Mahurkar A, Mitchell AP, Ravel J, et al. New signaling pathways govern the host response to C. albicans infection in various niches. Genome Res. 2015;25:679–689. doi: 10.1101/gr.187427.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubberts E, Schwarzenberger P, Huang W, Schurr JR, Peschon JJ, van den Berg WB, Kolls JK. Requirement of IL-17 receptor signaling in radiation-resistant cells in the joint for full progression of destructive synovitis. J Immunol. 2005;175:3360–3368. doi: 10.4049/jimmunol.175.5.3360. [DOI] [PubMed] [Google Scholar]
- Milner J, Holland S. The cup runneth over: lessons from the ever-expanding pool of primary immunodeficiency diseases. Nat Rev Immunol. 2013;13:635–648. doi: 10.1038/nri3493. [DOI] [PubMed] [Google Scholar]
- Moyes D, Wilson D, Richardson J, SM, Tang S, JW, Hofs S, Gratacap R, Robbins J, Manohursingh R, et al. Candidalysin: A fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–68. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes DL, Richardson JP, Naglik JR. Candida albicans-epithelial interactions and pathogenicity mechanisms: Scratching the surface. Virulence. 2015;6:338–346. doi: 10.1080/21505594.2015.1012981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh N, Nagarajan P, Sei-ichi M, Sinha S, Garrett-Sinha LA. Isolation and characterization of an immortalized oral keratinocyte cell line of mouse origin. Arch Oral Biol. 2008;53:1091–1100. doi: 10.1016/j.archoralbio.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrella D, Rachini A, Pines M, Pandey N, Mosci P, Bistoni F, d’Enfert C, Vecchiarelli A. Th17 cells and IL-17 in protective immunity to vaginal candidiasis. PLoS One. 2011;6:e22770. doi: 10.1371/journal.pone.0022770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presland R, Dale B. Epithelial structural proteins of the skin and oral cavity: Function in health and disease. Crit Rev Oral Biol Med. 2000;11:383–408. doi: 10.1177/10454411000110040101. [DOI] [PubMed] [Google Scholar]
- Puel A, Cypowji S, Bustamante J, Wright J, Liu L, Lim H, Migaud M, Israel L, Chrabieh M, Audry M, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford M, McKeage K. Secukinumab: first global approval. Drugs. 2015;75:329–338. doi: 10.1007/s40265-015-0359-0. [DOI] [PubMed] [Google Scholar]
- Shen F, Hu Z, Goswami J, Gaffen SL. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138–24148. doi: 10.1074/jbc.M604597200. [DOI] [PubMed] [Google Scholar]
- Simpson-Abelson MR, Childs EE, Ferreira MC, Bishu S, Conti HR, Gaffen SL. C/EBPβ Promotes Immunity to Oral Candidiasis through Regulation of β-Defensins. PLoS One. 2015;10:e0136538. doi: 10.1371/journal.pone.0136538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis NV, Filler SG. Mouse model of oropharyngeal candidiasis. Nat Protoc. 2012;7:637–642. doi: 10.1038/nprot.2012.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PR, Leal SM, Jr, Sun Y, Pearlman E. Aspergillus and Fusarium corneal infections are regulated by Th17 cells and IL-17-producing neutrophils. J Immunol. 2014;192:3319–3327. doi: 10.4049/jimmunol.1302235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalka J, Azodi E, Narra HP, Patel K, O’Neill S, Cardwell C, Hall BA, Wilson JM, Hise AG. β-Defensin 1 plays a role in acute mucosal defense against Candida albicans. J Immunol. 2015;194:1788–1795. doi: 10.4049/jimmunol.1203239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whibley N, Gaffen SL. Gut-Busters: IL-17 Ain’t Afraid of No IL-23. Immunity. 2015;43:620–622. doi: 10.1016/j.immuni.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whibley N, Tritto E, Traggiai E, Kolbinger F, Moulin P, Brees D, Coleman BM, Mamo A, Garg A, Jaycox JR, et al. Antibody blockade of IL-17-family cytokines in immunity to acute murine oral mucosal candidiasis. J Leukoc Biol. 2016;99:1153–1164. doi: 10.1189/jlb.4A0915-428R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Chen Q, Hoover D, Staley P, Tucker K, Lubkowski J, Oppenheim J. Many chemokines including CCL20/MIP-3α display antimicrobial activity. J Leukoc Biol. 2003;74:448–455. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- Yano J, Noverr MC, Fidel PL., Jr Cytokines in the host response to Candida vaginitis: Identifying a role for non-classical immune mediators, S100 alarmins. Cytokine. 2012;58:118–128. doi: 10.1016/j.cyto.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Phan QT, Boontheung P, Solis NV, Loo JA, Filler SG. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc Natl Acad Sci U S A. 2012;109:14194–14199. doi: 10.1073/pnas.1117676109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.