Abstract
Purpose
Evaluate the relationship between body mass index (BMI), overweight status (OW), or obesity (OB) and ambulatory status in a predominantly Hispanic population of children with spinal dysraphism (SD).
Methods
Retrospective data were extracted from records of 272 children and youth aged 0–24 years with a diagnosis of SD. Body mass index (BMI) and OW/OB rates were calculated for children 0–3 years, 4–11 years, and adolescents older than 11.
Results
Ethnicity was predominantly Hispanic (65.4%). No difference in mean BMI or OW/OB rate was found between ambulation groups (p=.20; p=.72). Mean BMI and OW/OB rate increased with increasing age in all groups (p<.001; p=.02). Forty-four percent of patients were OW/OB, which was greater among Hispanics (48.2%) compared with non-Hispanics [(35.2%), p=.03]. Female gender was a risk factor for increased BMI among Hispanics (p=.00).
Conclusion
Despite no difference in ambulatory status, increasing BMI and OW/OB are associated with Hispanic ethnicity and increasing age.
Keywords: Children, spinal dysraphism, spina bifida, Hispanic, overweight, obesity, body mass index (BMI), age, ambulation
Childhood obesity has been called one of the greatest health problems of the 21st century.1 Estimates suggest that the prevalence of overweight and obesity (OW/OB) are two to three times higher in children with disabilities compared with age-matched peers.2–4 Spinal dysraphism (SD) is one of the most common complex congenital abnormality compatible with life, and is one of the most common causes of serious locomotor disability in children.5 The prevalence of obesity in young people with SD has been previously estimated to be between 28–50% in children, and 34–64% in young people.6 Cross-sectional studies of people with SD reveal the incidence of obesity increases with age.7 Life expectancy for children with disabilities has vastly increased in recent years,2,3 with at least 75% surviving well into adulthood.8 This advance has made healthy lifestyle maintenance, medical management, and quality-of-life improvement priorities in this population.
People with physical disabilities spend less time performing physical activities than their able-bodied peers,9 thus are at greater risk of the comorbid sequela associated with a sedentary lifestyle. It has been proposed that when walking becomes too difficult, SD patients usually choose to use a wheelchair as their primary mode of mobility because the advantage of walking does not justify the energy expenditure.10 A multitude of factors have been identified that may affect the ambulatory status of children with SD, although the relationship between obesity and ambulatory status has not been well elucidated. Seitzberg et al. found that the majority of children with myelomeningocele who were walking freely at ages of five to eight years kept walking as adults.11 Similarly, an age of four years has been identified as the age that children with SD commonly start to lose lean muscle, and gain fat.12
Spinal dysraphism disproportionately affects Hispanic populations in the United States (U.S.), with Hispanics having the greatest prevalence among all ethnicities.13,14 Hispanics are the nation’s largest and fastest growing minority group, with the state of California having the largest total Hispanic population of any state within the U.S.15,16 Hispanics also have the highest prevalence of obesity among ethnic groups of able-bodied U.S. children and adolescents.17 Despite the potential greater risk of obesity in Hispanic children with SD, to our knowledge no study has evaluated the prevalence of overweight status or obesity (OW/OB), nor the association between OW/OB, age, and ambulatory status in this group. Here, we investigate the relationship between OW/OB, body mass index (BMI), age, and ambulatory status among Hispanic and non-Hispanic children followed in a multi-disciplinary spinal defects clinic located near the California-Mexico border. We sought to test the hypotheses that increased BMI and OW/OB is associated with a downward trend in ambulatory status and that Hispanic ethnicity is a risk factor for OW/OB in children with SD.
Methods
Patients
With institutional review board (IRB) approval, we reviewed a SD database which included a consecutive series of children with SD born in the period 1989–2013, and treated in the multidisciplinary clinic for patients with spinal defects at Rady Children’s Hospital San Diego, California. Inclusion criterion was the presence of a SD diagnosis; including those with myelomeningocele, lipomyelomeningocele, meningocele, spinal cord lipoma requiring neurosurgical intervention, caudal regression syndrome, dermal sinus tract, and tethered cord. Exclusion criteria included children with a history of spinal cord injury, and all other non-spinal dysraphic neuromotor conditions. Of the 299 patients identified, 272 met inclusion criteria and 27 patients were excluded secondary to spinal cord injury diagnosis.
Data collection
The electronic medical record and archived records from Rady Children’s Hospital were used for data collection. Information was collected from the pediatric multidisciplinary spinal defects clinical documentation of the disciplines: Urology, Orthopedic Surgery, Neurosurgery, Physiatry, Social Work, and Occupational/Physical Therapy.
Measures
Body weight in kilograms (kg), height in centimeters (cm), ambulatory status, and age in years were extracted from spinal defects clinical documentation. Body height (crown to heal) was measured by a specialized nurse, either supine, or in the standing position in those children who were able to do so. Body mass index (BMI) was calculated by weight in kg divided by the square of height in meters. Overweight was defined as 85–95th percentile, and obese as ≥ 95th percentile, of the sex- and age-specific BMI charts maintained by the Center for Disease Control (CDC) for patients 2–18 years old.18 For patients older than 18 years, overweight was defined as a BMI ≥ 25 and obese as a BMI ≥ 30 (following CDC guidelines).18 Lesion level and lesion type were extracted from neurosurgical records. Lesion level was categorized as cervical, thoracic, lumbar, lumbosacral, and sacral. Lesion type was categorized as myelomeningocele, lipomyelomeningocele, meningocele, lipomeningocele, dermal sinus tract, tethered cord, and caudal regression syndrome. Ambulatory status was defined as ambulatory, ambulatory requiring assistive devices, or limited to wheelchair use. Ambulatory status was obtained from Physiatry documentation of patient or parent/care giver report of ambulatory status during the majority of daytime hours. A standardized assessment of ambulation was not used, as the goal was to capture how the patient was actually ambulating during the majority of the day rather than potential ambulatory ability, which may not be sustained throughout the day. Demographic data were extracted from billing records.
Statistical analysis
The variables analyzed in the current study were selected from a larger database containing bowel and bladder management, ventriculoperitoneal shunt status, and other clinical history and included: age, gender, Hispanic or non-Hispanic ethnicity, spinal lesion type, spinal lesion level, BMI, presence of OW/OB, and ambulatory status. All lesion types except myelomeningocele and lipomyelomeningocele were grouped as “other” for statistical purposes due to low frequency. Similarly, cervical and lumbosacral lesion levels were grouped as “other” secondary to low frequency. Descriptive statistics were used to determine ambulatory status, mean BMI, and OW/OB rates among three different age groups: 0–3 years, 4–10 years, and 11–24 years. We also examined ambulatory status, mean BMI, and OW/OB rates for subgroups defined by lesion level, and lesion type. An independent samples t-test was used to identify differences in mean BMI among Hispanic and non-Hispanics. A Pearson chi-square analysis was performed to assess differences in OW/OB rates among Hispanic and non-Hispanics. Analysis of variance (ANOVA) was used to determine differences in mean BMI and obesity among ambulatory groups, lesion level groups, and age groups; this was done both for all patients, and by Hispanic and non-Hispanic ethnicity. A multiple comparisons ANOVA accounting for both ethnicity, and age groups was performed to determine differences in BMI. Finally, a multivariate linear regression analysis was performed to identify significant predictors of BMI. SPSS software (version 22) was used for all analyses.19 A p value of < .05 was considered significant.
Results
Entire population
Analyses were conducted on 272 patients younger than one year up to 24 years and are stratified by Hispanic or non-Hispanic ethnicity in Table 1. Median age was 13 years, with 9.9% falling into the 0–3 year age group, 31.2% in the 4–10 year group, and 58.8% in the 11–24 year group. Females constituted 49.6% of the study population. Ethnicity was predominantly Hispanic 65.4% (n=178) compared with 34.6% non-Hispanics (n=94). Neurologic level of involvement in our population included: thoracic 15.4% (n=42), lumbar 42.6% (n= 116), sacral 13.9% (n= 38), and not identified in 22.7% (n=62). Cervical and lumbosacral levels were grouped as “other” in 5.1% (n=14), secondary to low frequency. Lipomyelomeningocele was identified in 11.7% (n=32), myelomeningocele in 68% (n=185), and the remainder of spinal lesion types were grouped as “other” secondary to low frequency, totaling 20.2% (n=55). Twenty-eight percent of patients were ambulatory (n=77), 33.4% required assisted device (n=91), 34.5% were limited to wheelchair use (n=94), and 3.6% were too young to assess (n=10). Sixteen patients (5.8%) did not have documentation of either height or weight and therefore, were not included in BMI or OW/OB analyses. The mean weight was 45.3kg (SD 27.4), mean height was 133.5cm (SD 30.5), and mean BMI was 21.9 (SD 7.2). Forty-four percent of all patients were either overweight or obese (n=113), and within this group the majority met criteria for obesity (28.5%, n=73), Table 1.
Table 1.
Patient Demographics and Characteristics
| Variable | All Patients n = 272 |
Hispanic n = 178 (65.4%) |
Non-Hispanic n =94 (34.5%) |
p value |
|---|---|---|---|---|
| Mean age, years (SD) | 12.60 (6.5) | 13.20 (6.6) | 11.6 (6.3) | .06a |
| Age groups, n (%) | NS | |||
| 0–3 years | 27 (9.9) | 16 (8.9) | 11 (11.7) | |
| 4–10 years | 85 (31.2) | 53 (29.77) | 32 (34.04) | |
| 11–24 years | 160 (58.8) | 109 (61.23) | 51 (54.25) | |
| Gender, n (%) | NS | |||
| Female | 135 (49.6) | 88 (49.4) | 47 (50.0) | |
| Male | 137 (50.3) | 90 (50.5) | 47 (50.0) | |
| Neuro. lesion level, n (%) | NS | |||
| Thoracic | 42 (15.4) | 27 (15.1) | 15 (15.9) | |
| Lumbar | 116 (42.6) | 74 (41.5) | 42 (44.6) | |
| Sacral | 38 (13.9) | 27 (15.1) | 11 (11.7) | |
| Other | 14 (5.1) | 8 (4.4) | 6 (6.3) | |
| Missing | 62 (22.7) | 42 (23.5) | 20 (21.2) | |
| Neuro. lesion type, n (%) | NS | |||
| Lipomyelomeningocele | 32 (11.7) | 19 (10.6) | 13 (13.8) | |
| Myelomeningocele | 185 (68.0) | 121 (67.9) | 64 (68.0) | |
| Other | 55 (20.2) | 38 (21.3) | 17 (18.0) | |
| Ambulatory Status, n (%) | NS | |||
| Ambulatory | 77 (28.3) | 52 (29.2) | 25 (26.5) | |
| Assisted device | 91 (33.4) | 56 (31.4) | 35 (37.2) | |
| Wheel chair | 94 (34.5) | 66 (37.0) | 28 (29.7) | |
| Too young to assess | 10 (3.6) | 4 (2.2) | 6 (6.3) | |
| Mean BMI (SD) | 21.96 (7.2) | 22.87 (7.9) | 20.24 (6.6) | .01a |
| Obese, n (%) | 73 (28.5) | 54 (30.3) | 19 (21.5) | .08b |
| Overweight or Obese, n (%) | 113 (43.7) | 81 (48.2) | 31 (35.2) | .046b |
Abbreviations: BMI, body mass index; NS, not significant; SD, standard deviation.
T-test p value
Pearson χ2 test p value
No difference in mean BMI was found among ambulatory status groups (p=.20), nor among spinal lesion level groups (p=.63). Similarly, no difference in OW/OB rates were found among ambulatory status groups (p=.72). When assessing BMI by age groups, the mean BMI for ages 0–3 was 17.3, 18.4 in ages 4–10, and 24.6 in ages 11–24, with the older group having a significantly higher BMI than the two younger groups (p<.001). Overweight/obesity rate also increased with increasing age groups (p=.02).
Ethnic group comparisons
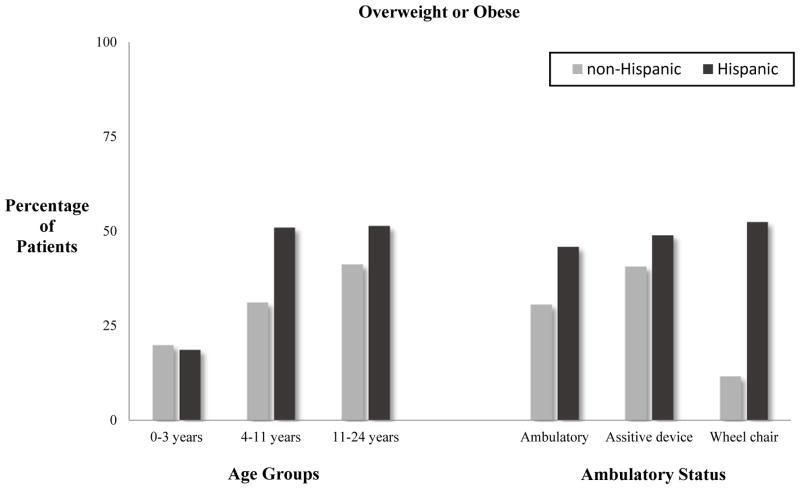
When comparing Hispanics with non-Hispanics, there was no difference in spinal lesion level (p=.78), spinal lesion type (p=.39), or ambulatory status (p=.29), Table 1. Hispanics were found to have a significantly higher BMI than non-Hispanics, with mean Hispanic BMI of 23, and mean BMI of 20 among non-Hispanics (p=.01), Table 1. Female gender was found to be a risk factor for increased BMI among Hispanic patients only (p=.00). Similarly, the prevalence of OW/OB was revealed to be greater among Hispanics, 48.2% (n=81), compared with non-Hispanics, 35.2%, [(p=.046) Table 1]. No difference in OW/OB was found between male and female gender for both Hispanics (p=.35), as well as non-Hispanics (p=.26). The older age groups (ages 11–24 years) had a significantly higher mean BMI than the two younger groups for both Hispanics (p<.001) and non-Hispanics (p<.001), Table 2. When assessing OW/OB rates by age group and ethnicity, only Hispanics were found to have increasing OW/OB rates with each increase in age group (p=.046), Figure 1. Overweight and obesity was not found to differ among age groups in the non-Hispanic population (p=0.37), but did trend towards significant with respect to ambulatory status (p=.08), Figure 1. On univariate ANOVA, a statistically significant association was found between age and ethnicity, although this relationship was extremely weak (p=.01; adjusted R squared=.02). On multivariate linear regression analysis (F=30.3; p< .001), both age (p<.001), and ethnicity (p=.04), were revealed as significant predictors of an elevated mean BMI, with age having a greater contribution than ethnicity.
Table 2.
Mean Body Mass Index by Ethnicity
| Hispanic n= 168 |
Non-Hispanic n=88 |
|||
|---|---|---|---|---|
|
| ||||
| Mean BMI (SD) | P value | Mean BMI (SD) | P value | |
|
| ||||
| Age group, years | .00a | .00a | ||
| 0–3 | 18.0 (2.6) | 16.2 (2.1) | ||
| 4–10 | 19.8 (7.1) | 17.5 (3.9) | ||
| 11–24 | 25.4 (8.1) | 23.0 (7.6) | ||
| Gender | .00b | .11b | ||
| Female | 24.7 (8.5) | 21.3 (7.4) | ||
| Male | 20.9 (6.9) | 18.9 (5.6) | ||
| Neuro. lesion level | .33a | .61a | ||
| Lumbar | 23.2 (8.6) | 21.5 (7.6) | ||
| Sacral | 20.8 (5.0) | 22.1 (5.4) | ||
| Thoracic | 23.8 (7.5) | 19.5 (7.7) | ||
| Neuro. lesion type | ||||
| Lipomyelo. | 21.4 (7.1) | .24a | 19.2 (5.9) | .22a |
| Myelo. | 22.4 (7.5) | 21.1 (7.1) | ||
| Other | 24.8 (9.4) | 17.4 (4.4) | ||
| Ambulatory status | .21a | .44a | ||
| Ambulatory | 21.5 (5.6) | 20.1 (5.7) | ||
| Assist device | 22.9 (8.2) | 19.7 (4.9) | ||
| Wheel chair | 24.3 (9.4) | 22.0 (9.4) | ||
Abbreviations: ANOVA, Analysis of variance; BMI, body mass index; SD, standard deviation.
ANOVA test p value
T-test p value
Figure 1.
Relationship of Age and Ambulatory Status to Overweight/Obesity Status
Discussion
This study documents obesity rates among children and youth with SD that are comparable with the general population, and to our knowledge is the first study in the U.S. that documents a greater prevalence of OW/OB among Hispanic children with SD compared with non-Hispanic counterparts. We did not identify a significant relationship between ambulatory status and OW/OB or BMI in either Hispanic or non-Hispanic patients, although OW/OB trended towards significance in the non-Hispanic group, which may reflect a lack of power to detect. Despite no difference in ambulatory status, both increasing age, and Hispanic ethnicity, were found to contribute to increasing BMI and OW/OB which has not been previously reported in such a large cohort of children with disabilities.
Obesity and BMI
Obesity in children with SD can increase difficulties with bladder catheterization, and other self-care activities; increase preventable secondary medical conditions such as pressure sores, and metabolic syndrome; and increase social isolation and depression, all of which can lead to decreased quality of life.6, 19,20 The CDC uses BMI as a proxy for body fat and considers a child between the ages of two and 18 years to be overweight if between 85–95th percentile, and obese if above 95th percentile.18 Body mass index (BMI) standards for able-bodied children have not been validated for children with disabilities, thus no standard definitions of obesity exist for children with SD.20 Obesity in young people with SD has been assessed by a variety of methods7,10,12,21 and has been previously estimated by BMI to have a prevalence between 28–50% in children, and 34–64% in young people.6 We also used BMI as a proxy for body fat secondary to availability of information, although it must be noted that BMI may underestimate the true extent of obesity in this population20 secondary to lower limb atrophy and vertebral anomalies.12 Similar to previous findings, we found 43.7% of all patients to meet criteria for overweight or obesity,6 with the majority meeting criteria for obesity (28.5%). As predicted, increased BMI and OW/OB prevalence was significantly greater in Hispanic children than in non-Hispanic children. Female gender was found to be a risk factor for increased BMI among Hispanic children.
Age
Preschool-age is a critical period of development that affects growth patterns and associated health outcomes into adulthood.22 Longitudinal research has shown that children who are overweight by the age of two years are already at greater risk for becoming overweight adults compared with non-overweight peers.23 Cross-sectional studies of people with SD reveal an increased incidence of obesity with age, and that among non-ambulatory children with SD, obesity rates become greater than those in the general population during pre-school years.7 Similarly, Shepherd et al. evaluated body composition in 59 Australian children with myelomeningocele, and found that before the age of four years, body composition was similar to that of reference children, but that after the age of four years, body cell mass and lean mass fail to grow and the deficit in lean tissue becomes compensated for by increased adipose tissue.12 We found a significantly higher mean BMI in older children (ages 11–24 years) in both Hispanics and non-Hispanics, when compared with the two younger age groups (0–3 and 4–10 years), with age having greater contribution that ethnicity. This is similar to previous general population findings, however has not been shown in such a large cohort of children with disabilities, or while controlling for ethnicity. When looking at OW/OB among age groups in all patients, a significant increase in OW/OB rate was found with increased age group, although it appeared only Hispanic children significantly contributed to this finding. The finding that BMI increased more significantly than OW/OB rate among age groups appears to further elucidate the imprecision of using BMI as a proxy for body composition in this population. The observation of increased obesity with age has previously been deemed a consequence of mobility limitations as well as metabolic alterations, including higher body fat percentage and lower resting energy expenditure that increase with age.5,12,24 Weight loss interventions targeting younger children appear to yield better long-term weight outcomes, relative to older children and adolescents,25 which further highlights critical early developmental years. These findings all support the need for awareness and assessment of weight issues in SD as early as possible during development.
Ambulatory status
The obesity epidemic is related in part to declining rates of physical activity in the U.S.26 Hispanics and African Americans, women (of all ages), and people with disabilities, are a few sub-populations that the CDC has identified to be at risk for lower rates of regular physical activity.27 Children with SD may have a variety of challenges such as weakness, cognitive impairments, orthopedic deformities, psychosocial issues, and bowel and bladder problems, that make them at risk for developing an inactive lifestyle.24,28 The cause of obesity in the SD population is multifactorial, although non-ambulatory status and sedentary lifestyle are commonly implicated. Additional interest in ambulatory potential and other quality-of-life measures in patients with SD has increased in conjunction with increasing life expectancy,2,3,4 as quality of life has repeatedly been found to be lower in non-ambulatory subsets.4,8 The most commonly identified single factor affecting ambulatory status in those with SD is level of neural involvement,10,29 with few studies finding an association between obesity and ambulatory status.10,30 Asher and Olson10 found that transitions in ambulatory status in patients with SD were usually downward and were related to lack of motivation, musculoskeletal deformity, and obesity. In addition, patients with myelomeningocele who are functional ambulators have been found to be more physically active during the day, and have less body fat than less ambulatory people.30 Conversely, obesity is also thought to contribute to a sedentary lifestyle in SD children,21 thus the relationship between obesity and decreased ambulatory status is likely bi-directional.
Compared with healthy peers, young people with disabilities are restricted in the performance of daily activities;30 therefore, when the energy expenditure of walking becomes too great, SD patients usually resort to use of a wheelchair.10 Rimmer et al. refer to this as a “disability-associated low energy expenditure deconditioning syndrome” in which a sedentary lifestyle in people with disabilities creates a vicious cycle of further deconditioning, disability, and disease risk.9 We, however, did not reproduce a significant relationship between increasing BMI and decreasing ambulatory status. This finding may be due to several factors: the underestimation of overweight/obesity when using BMI in this population, potential inaccurate measures of true height in those who are unable to stand or have physical deformities, potential inaccuracy of reported ambulatory status/lack of validated measure use, as well as the cross-sectional assignment of ambulatory status rather than longitudinal data.
Hispanic ethnicity
The prevalence of SD has been shown to vary by ethnicity, with continued highest rates in the U.S. among Hispanic births.14 Childhood obesity is more prevalent among minority and low-income families,17 which is a critical public health concern due to fast growing minority groups in the U.S., and high costs associated with treating obesity-related diseases.31 Childhood obesity among U.S. minority groups may be further compounded when coupled with disability. Despite the phenomenon’s importance, there are few data regarding the prevalence of obesity among Hispanic children with SD. One study from Mexico City assessing weight-based diagnoses among 66 Mexican children with SD found that 56% of patients were overweight or obese, and that risk increased at age six and again at age 11.32 Despite no differences in ambulatory status, our results revealed Hispanic ethnicity as a significant contributor to increased mean BMI and OW/OB, with Hispanic females possibly being at greater risk, which to our knowledge, is the first time this has been reported in a U.S. series of children with SD.
Members of ethnic minority groups demonstrate poorer weight loss outcomes than non-Hispanic Whites,33 which is partly due to social and environmental barriers to weight loss. Hispanics are more likely to underestimate their weight status,34 eat high-fat/low-fruit and vegetable diets,35 live in poverty, lack health insurance, and have limited opportunities for physical activity compared with White peers.31,36 Immigrant Hispanics may also face barriers related to acculturation, language, and immigration status, which can hinder weight loss strategies.37 Skill-building, culturally tailored interventions involving parent–child dyads have proven effective in weight reduction among Hispanic American pre-school aged children.38 Flores et al. identified culturally specific themes deemed to be critical to weight loss success by parents of obese Hispanic children, which included: focus on whole-family approaches to nutritional and physical activity change, whole-body physical activities that children enjoy, low-cost exercise programs that involve minimal time investment, and healthy but flavorful modifications of traditional Latino meal and beverage items.39 These results also suggest that a “one-size-fits-all” approach to weight management for overweight children may not be successful for Hispanic and other ethnic minorities.
Future opportunities
Despite multiple reports of increased prevalence of obesity in SD populations it has been recently found that height and weight are not routinely recorded for children attending an outpatient spina bifida clinic.40 This report also found that in those who did have a BMI in the overweight/obese range, weight management was mentioned in the notes less than 25% of the time.40 Weight management education was not assessed in the current study, but we agree that important health promotion opportunities are likely being missed in this population. Clinicians should focus on discussing the individual child’s obesity risk factors, the negative sequela of obesity, and provide frequent assessment of weight-related issues. Recent scientific information suggests that the most effective interventions to address excess weight and obesity in children should include behavioral modification and education to improve eating habits and increase energy expenditure.41,42 The current study also highlights the need for culturally specific considerations, including educating Hispanic families about potential greater obesity risk; in addition to providing ethnically specific weight loss strategies that are sensitive to language preferences, traditional diets, and household finances; and encourage participation of the entire family.
In addition, our results further support the previous recommendations to institute nutritional and physical activity programs during pre-pubertal years to prevent the excess weight gain in children with SD that otherwise seems to develop with age.10,12 It is important to note that the physical limitations of those with SD make physical activity difficult, and feasible and effective types of exercise are not well defined. Adaptive physical educational opportunities that are age-appropriate and logistically feasible, such as at the school or local community centers, can help to address this need.
Study limitations
Limitations of the current study include the retrospective nature of the review, lack of longitudinal data, and the limited sample of patients assessed. This sample of patients was assessed at a regional referral center, with a high Hispanic population, and may not reflect all patients with SD. A subset ethnicity analysis was not performed among the non-Hispanic group, and it is therefore unclear whether findings are representative of predominantly White or African American populations. Assessing body composition, particularly body fat percentage, should be included in future analyses of obesity rates in youth with SD, especially those evaluating the association of obesity and ambulatory potential. We also did not have complete capture of all variable data due to incomplete medical records (in particular, level of lesion, which was similar between comparison groups and unlikely to affect the analysis). Additionally, only subjective ambulatory status data were included, whereas validated measures of ambulatory status obtained at multiple time points may be more accurate. This study also only assessed specific variables of interest, and was not intended to be an all-inclusive assessment of risk factors for non-ambulatory status or elevated BMI among children with SD.
Conclusion
Overweight status and obesity is a multi-factorial issue in children with spinal dysraphism and may be increased in those with Hispanic ethnicity. In children with spinal dysraphism, body mass index and overweight/obesity rates increase with age, and pre-pubertal years may be critical in halting progression to adulthood obesity and associated deleterious effects. Clinicians who work with spinal dysraphism children should be encouraged to assess, monitor, and discuss weight-related issues with patients and their families with particular attention to ethnic and cultural needs. Further studies to examine the association between body composition and ambulatory status may provide insight into physical activity barriers and improving quality of life in children and youth with spinal dysraphism.
Acknowledgments
Funding: This project was partially funded through a Patient-Centered Outcomes Research Institute (PCORI) Pipeline-to-Proposal Award, administered on behalf of PCORI by Colorado Foundation for Public Health and the Environment. The content is solely the responsibility of the authors and does not necessarily represent the official views of PCORI or the Colorado Foundation for Public Health and the Environment. This project was also supported in part by grant number K99/R00 HS022404 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Footnotes
Financial disclosure: The authors have no financial relationships relevant to this article to declare.
Conflict of interest: The authors have no conflicts of interest to declare.
Contributor Information
Michelle L. McDonald, Department of Urology, University of California San Diego Health Care System, San Diego, California.
Andy Huang, School of Medicine, University of California San Diego, San Diego, California.
James A. Proudfoot, Clinical and Translational Research Institute, University of California San Diego, San Diego, California.
Joan T. Le, Division of Rehabilitation Medicine, University of California San Diego Health Sciences at Rady Children’s Hospital, San Diego, California.
George J. Chiang, Department of Surgery/Pediatric Urology at the University of California San Diego, San Diego, California.
Ruth A. Bush, Hahn School of Nursing and Health Sciences at the University of San Diego, San Diego, California.
References
- 1.World Health Organization (WHO) Childhood overweight and obesity. Geneva, Switzerland: World Health Organization; 2015. Available at http://www.who.int/dietphysicalactivity/childhood/en/index.html. [Google Scholar]
- 2.Rimmer JH, Rowland JL, Yamaki K. Obesity and secondary conditions in adolescents with disabilities: addressing the needs of an underserved population. J Adolesc Health. 2007 Sep;41(3):224–9. doi: 10.1016/j.jadohealth.2007.05.005. http://dx.doi.org/10.1016/j.jadohealth.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Reinehr T, Dobe M, Winkel K, et al. Obesity in disabled children and adolescents: an overlooked group of patients. Dtsch Ärztebl Int. 2010 Apr;107(15):268–75. doi: 10.3238/arztebl.2010.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocaue B, Bishop E, Scogin M, et al. Assessing health-related quality of life in children with spina bifida. J Neurosurg Pediatr. 2015 Feb;15(2):144–9. doi: 10.3171/2014.10.PEDS1441. [DOI] [PubMed] [Google Scholar]
- 5.Liptak G. What is Spina Bifida? Arlington, VA: Spina Bifida Association; 2014. Available at http://www.spinabifidaassociation.org/site/c.evKRI7OXIoJ8H/b.8277225/k.5A79/What_is_Spina_Bifida.htm. [Google Scholar]
- 6.Dosa NP, Foley JT, Echrich M, et al. Obesity across the lifespan among people with spina bifida. Disabil Rehabil. 2009 Mar;31(11):914–20. doi: 10.1080/09638280802356476. [DOI] [PubMed] [Google Scholar]
- 7.Roberts D, Shepherd RW, Shepherd K. Anthropometry and obesity in myelomeningocele. Error! Hyperlink reference not valid. 1991 Apr;27(2):83–90. doi: 10.1111/j.1440-1754.1991.tb00358.x. http://dx.doi.org/10.1111/j.1440-1754.1991.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 8.Buffart LM, van den Berg-Emons RJ, van Meeteren J, et al. Lifestyle, participation, and health-related quality of life in adolescents and young adults with myelomeningocele. Dev Med Child Neurol. 2009 Nov;51(11):886–94. doi: 10.1111/j.1469-8749.2009.03293. [DOI] [PubMed] [Google Scholar]
- 9.Rimmer JH, Schiller W, Chen MD. Effects of disability-associated low energy expenditure deconditioning syndrome. Error! Hyperlink reference not valid. 2012 Jan;40(1):22–9. doi: 10.1097/JES.0b013e31823b8b82. [DOI] [PubMed] [Google Scholar]
- 10.Asher M, Olson J. Factors affecting the ambulatory status of patients with spina bifida cystica. Error! Hyperlink reference not valid. 1983 Mar;65(3):350–6. [PubMed] [Google Scholar]
- 11.Seitzberg A, Lind M, Biering-Sørensen F. Ambulation in adults with myelomeningocele. Is it possible to predict the level of ambulation in early life? Childs Nerv Syst. 2008 Feb;24(2):231–7. doi: 10.1007/s00381-007-0450-2. http://dx.doi.org/10.1007/s00381-007-0450-2. [DOI] [PubMed] [Google Scholar]
- 12.Shepherd K, Roberts D, Golding S, et al. Body composition in myelomeningocele. Am J Clin Nutr. 1991 Jan;53(1):1–6. doi: 10.1093/ajcn/53.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Kshettry VR, Kelly ML, Rosenbaum BP, et al. Myelomeningocele: surgical trends and predictors of outcome in the United States, 1988–2010. J Neurosurg Pediatr. 2014 Jun;13(6):666–78. doi: 10.3171/2014.3.PEDS13597. [DOI] [PubMed] [Google Scholar]
- 14.Williams LJ, Rasmussen SA, Flores A, et al. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995–2002. Pediatrics. 2005 Sep;116(3):580–6. doi: 10.1542/peds.2005-0592. http://dx.doi.org/10.1542/peds.2005-0592. [DOI] [PubMed] [Google Scholar]
- 15.United States Census Bureau. State and County Quickfacts: California. Suitland, MD: 2013. Available at http://www.census.gov/quickfacts/table/RHI725213/06,00. [Google Scholar]
- 16.Pew Research Center. Demographic profile of Hispanics in California, 2011. Washington, DC: 2015. Available at http://www.pewhispanic.org/states/state/ca/ [Google Scholar]
- 17.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012 Feb;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Rep. 2010 Jun 25;(25):1–5. [PubMed] [Google Scholar]
- 19.IBM Corp. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp; Released 2013. [Google Scholar]
- 20.Simeonsson RJ, McMillen JS, Huntington GS. Secondary conditions in children with disabilities: spina bifida as a case example. Ment Retard Dev Disabil Res Rev. 2002;8(3):198–205. doi: 10.1002/mrdd.10038. [DOI] [PubMed] [Google Scholar]
- 21.Short KR, Frimberger D. A Review of the potential for cardiometabolic dysfunction in youth with spina bifida and the role for physical activity and structured exercise. Int J Pediatr. 2012 Apr;2012:1–11. doi: 10.1155/2012/541363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Berg-Emons HJ, Bussmann JB, Meyerink HJ, et al. Body fat, fitness and level of everyday physical activity in adolescents and young adults with meningomyelocele. J Rehabil Med. 2003 Nov;35(6):271–5. doi: 10.1080/16501970310012400. [DOI] [PubMed] [Google Scholar]
- 23.Barker DJ, Osmond C, Forsén TJ, et al. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005 Oct;353(17):1802–1809. doi: 10.1056/NEJMoa044160. http://dx.doi.org/10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 24.Freedman DS, Kettel L, Serdula MK, et al. The relation of childhood BMI to adult adiposity: the Bogalusa Heart Study. Pediatrics. 2005 Jan;115(1):22–7. doi: 10.1542/peds.2004-0220. [DOI] [PubMed] [Google Scholar]
- 25.Crytzer TM, Dicianno BE, Kapoor R. Physical activity, exercise, and health-related measures of fitness in adults with spina bifida: a review of the literature. PM&R. 2013 Dec;5(12):1051–62. doi: 10.1016/j.pmrj.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Cheng JK, Wen X, Coletti KD, et al. 2-Year BMI changes of children referred for multidisciplinary weight management. Int J Pediatr. 2014 Jan;2014:1–7. doi: 10.1155/2014/152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control. Obesity among adults in the United States—no change since 2003–2004. Atlanta, GA: Center for Disease Control; 2007. Available at http://www.cdc.gov/nchs/pressroom/07newsreleases/obesity.htm. [Google Scholar]
- 28.U.S. Department of Health and Human Services. Healthy People 2010: understanding and improving health. 2. Washington, DC: U.S. Government Printing Office; 2000. Available at: http://www.healthypeople.gov/2010/document/pdf/uih/2010uih.pdf. [Google Scholar]
- 29.van den Berg-Emons HJ, Bussmann JB, Brobbel AS, et al. Everyday physical activity in adolescents and young adults with meningomyelocele as measured with a novel activity monitor. J Pediatr. 2001 Dec;139(6):880–6. doi: 10.1067/mpd.2001.119991. http://dx.doi.org/10.1067/mpd.2001.119991. [DOI] [PubMed] [Google Scholar]
- 30.Hoffer M, Feiwell E, Perry J, et al. Functional ambulation in patients with myelomeningocele. Error! Hyperlink reference not valid. 1973 Jan;55(1):137–48. [PubMed] [Google Scholar]
- 31.Buffart LM, Roebroeck ME, Rol M, et al. Triad of physical activity, aerobic fitness and obesity in adolescents and young adults with myelomeningocele. J Rehabil Med. 2008 Jan;40(1):70–5. doi: 10.2340/16501977-0135. [DOI] [PubMed] [Google Scholar]
- 32.Rosas L, Thiyagarajan S, Goldstein BA, et al. The effectiveness of two community-based weight loss strategies among obese, low-income US Latinos. Error! Hyperlink reference not valid. 2015 Apr;115(4):537–550. doi: 10.1016/j.jand.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vega-Sánchez R, Gómez-Aguilar M, Haua K, et al. Weight-based nutritional diagnosis of Mexican children and adolescents with neuromotor disabilities. Error! Hyperlink reference not valid. 2012 Jul;5(218):1–7. doi: 10.1186/1756-0500-5-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo DC, Sa J. A meta-analysis of psycho-behavioral obesity interventions among US multiethnic and minority adults. Prev Med. 2008 Dec;47(6):573–82. doi: 10.1016/j.ypmed.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Johnson WG, Bluestein BB, Dasilva F, et al. Perceptions of overweight in US and global cultures. Eat Behav. 2015 Apr;17:125–9. doi: 10.1016/j.eatbeh.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Treviño RP, Marshall RM, Hale DE, et al. Diabetes risk factors in low-income Mexican-American children. Diabetes Care. 1999 Feb;22(2):202–7. doi: 10.2337/diacare.22.2.202. http://dx.doi.org/10.2337/diacare.22.2.202. [DOI] [PubMed] [Google Scholar]
- 37.Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010 Feb;140(2):304–10. doi: 10.3945/jn.109.112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Timmins CL. The impact of language barriers on the health care of Latinos in the United States: A review of the literature and guidelines for practice. J Midwifery Womens Health. 2002 Mar;47(2):80–96. doi: 10.1016/s1526-9523(02)00218-0. [DOI] [PubMed] [Google Scholar]
- 39.Barkin SL, Gesell SB, Po’e EK, et al. Culturally tailored, family-centered, behavioral obesity intervention for Latino-American preschool-aged children. Pediatrics. 2012 Sep;130(3):445–56. doi: 10.1542/peds.2011-3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flores G, Maldonado J, Durán P. Making tortillas without lard: Latino parents’ perspectives on healthy eating, physical activity, and weight-management strategies for overweight Latino children. J Acad Nutr Diet. 2012 Jan;112(1):81–9. doi: 10.1016/j.jada.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 41.McPherson AC, Swift JA, Yung E, et al. The assessment of weight status in children and young people attending a spina bifida outpatient clinic: a retrospective medical record review. Disabil Rehabil. 2013 Mar;35(25):2123–31. doi: 10.3109/09638288.2013.771705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mellin LM, Silnkard LA, Irwin CE. Adolescent obesity intervention: validation of the SHAPEDOWN program. J Am Diet Assoc. 1987 Mar;87(3):333–8. [PubMed] [Google Scholar]
- 43.Block P, Skeels SE, Keys CB, et al. Shake-It-Up: health promotion and capacity building for people with spinal cord injuries and related neurological disabilities. Disabil Rehabil. 2005 Feb 18;27(4):185–90. doi: 10.1080/09638280400019583. http://dx.doi.org/10.1080/09638280400019583. [DOI] [PubMed] [Google Scholar]