Abstract
The contribution of microRNAs to the regulation of mRNA expression during physiological and developmental processes are well-recognized. These roles are being expanded by recent observations that emphasize the capability of miRNA to participate in inter-cellular signaling and communication. Several factors support a functional role for miRNA as mediators of cell-to-cell signaling. miRNA are able to exist within the extracellular milieu or circulation, and their stability and integrity maintained through association with binding proteins or lipoproteins, or through encapsulation within cell-derived membrane vesicles. Furthermore, miRNA can retain functionality and regulate target gene expression following their uptake by recipient cells. In this overview, we review specific examples that will highlight the potential of miRNA to serve as paracrine signaling mediators in metabolic diseases and cancers. Elucidating the mechanisms involved in inter-cellular communication involving miRNA will provide new insights into disease pathogenesis and potential therapeutic opportunities.
Keywords: miRNAs, extracellular vesicles, cell-cell communication, cancer microenvironment, metabolic disorders
The functions of miRNAs
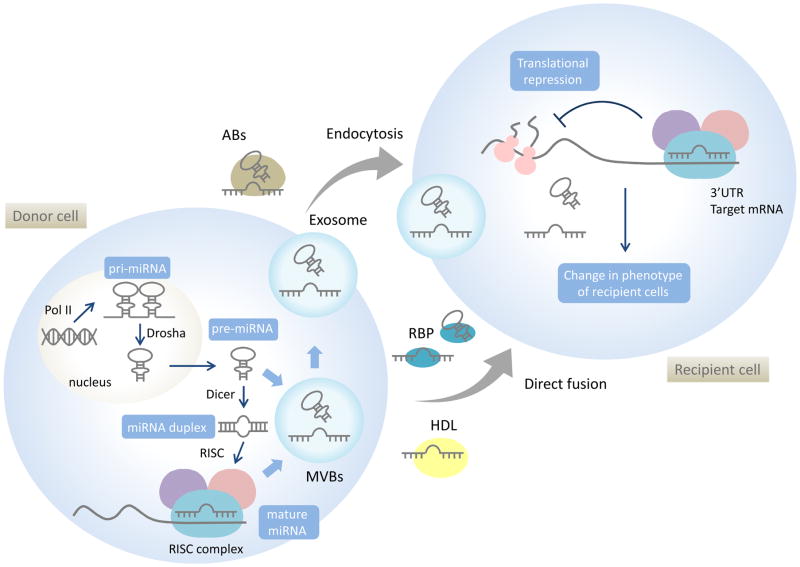
MicroRNAs (miRNAs) are small non-coding RNA of 18–25 nucleotides (nt) that can negatively regulate gene expression through either post-transcriptional degradation or translational repression. A single miRNA can target a broad range of mRNAs with nearly complementary sequences, and thereby has the capacity to have a broad impact on gene expression [1]. In humans, the miRBase database identifies 2,588 mature miRNAs (http://www.mirbase.org/) [2]. Many of these miRNA are also highly conserved across many species [3]. MiRNAs are initially transcribed as primary-miRNA (pri-miRNA) with a characteristic stem-loop structure. The stem-loop structure of pri-miRNAs is cleaved by the enzyme Drosha within the nucleus and results in precursor miRNA (pre-miRNA). Pre-miRNAs are then exported from nucleus into the cytoplasm by exportin 5 and processed by Dicer, an RNase III enzyme, to generate mature strands. The mature miRNA strand is incorporated into an Argonaute-containing RNA-induced silencing complex (RISC). The RISC can bind to a perfect or a nearly perfect complementary sequence within a target mRNA and the sequence is cleaved by the miRNA-RISC complex. In addition, miRNA can also induce protein translational repression of the target genes [4] (Figure. 1).
Figure 1. miRNA biogenesis, cellular release and paracrine cell-to-cell communication.
MiRNAs are typically transcribed by polymerase II (Pol II) as primary miRNA (pri-miRNA). Pri-miRNAs are cleaved by RNase III-type enzyme Drosha to produce hairpin-structured precursors (pre-miRNAs). Pre-miRNAs are transported to cytoplasm, the Dicer complex removes the loop region from pre-miRNAs to generate an imperfect duplex miRNA. Mature miRNA is bound by Argonaute to form a RNA-induced silencing complex (RISC). In the cytoplasm, pre-miRNAs or mature miRNAs can also incorporate into multivesicular bodies (MVBs). miRNA can be released from cells through release of exosomes derived from MVB’s, microvesicles derived from plasma membranes or within apoptotic bodies. They can also be associated and released with RNA-binding protein complexes (RBP) or high density lipoproteins (HDL). Extracellular miRNAs can be transferred to recipient cells and bind to their target messenger RNAs (mRNAs) to repress their translation or induce their degradation.
MiRNAs can contribute to diverse physiological roles and developmental processes and can also contribute to the pathobiology of diseases such as cancer [5] or metabolic disease [6]. The extent of their combinatorial impact is emphasized by the ability of each miRNA to bind several target sequences in several different mRNAs, and for a single mRNA to be regulated by multiple miRNAs thereby providing the capacity to modulate gene regulatory networks. While the intracellular roles of miRNA are well-established, emerging data indicates that miRNA may also function in inter-cellular or cross-organ communications, resulting from the ability of miRNA to transfer to recipient cells from donor cells through circulating blood, lymph and extracellular fluids [7]. The presence of miRNA in the extracellular environment further supports the hypotheses that cells selectively release miRNAs which mediate cell–cell signaling via paracrine or even endocrine routes [8].
Mechanisms of cellular release and uptake of miRNAs
The cellular release of miRNA has been proposed to occur through several different pathways. Active secretion can occur with miRNA-binding proteins (RBPs) such as Argonaute2 (AGO2), and high density lipoprotein (HDL) [9, 10], as well as through cellular release of membrane-bound extracellular vesicles (EV). Intact and functional miRNA can exist within the extracellular space or even within the circulation. Retention of functional capacity is essential for a role in intercellular communication. Indeed, miRNAs can remain stable in the circulation even in the presence of conditions that can degrade most RNAs [11]. Protection from degradation may be related to either the association of miRNA with RBPs, or their sequestration within HDLs or within EV in which a lipid bilayer can provide protection from ribonucleases [8]. Emerging evidence indicates the presence of selective release of miRNA within EV and thereby supporting a regulated process that may have physiological relevance. In addition, passive release of miRNA can also occur from cells in response to injury, chronic inflammation, or cell apoptosis or necrosis [11]. The contributions of miRNA as effectors of intercellular signaling in these settings are unknown.
EV represents a heterogeneous group of vesicles such as exosomes, microvesicles (MVs), or apoptotic bodies (ABs) [8, 12]. Much attention has focused on exosomes, a type of EV that is defined by their biogenesis that involves the formation of intralumenal vesicles (ILVs) within multivesicular bodies (MVBs) during maturation of endosomes. Some MVBs are fated for degradation in lysosomes, whereas others can fuse with the plasma membrane, leading to the secretion of ILVs as exosomes [13]. The biogenesis of exosomes was first suggested to be associated with the endosomal sorting complex required for transport (ESCRT) [14]. ESCRT-related proteins are conserved from yeast to mammals [15], and have been shown to be recruited to the cytosolic sides of MVBs to form exosomes [16]. However, exosome formation has been noted to occur independent of ESCRT, involving a process that is dependent on molecules such as ceramide or tetraspanins [17]. In this way, several proteins can contribute to exosome production and release, while precise mechanisms for exosome release as signaling effectors have not yet been clearly elucidated.
Many RNAs, including miRNA that are found within EVs have been noted to be enriched with respect to their originating cells [8], suggesting that RNA molecules can be selectively incorporated into EVs. Some differences in RNA content have been demonstrated with different types of EVs, with ABs primarily containing rRNA, whereas MVs or exosomes containing mRNA and miRNA, but little rRNA. In addition to the potential presence of selective pathways of enrichment that are yet to be defined, these may reflect differences in biogenesis. The nomenclature used for EV has been applied inconsistently, and the separative processes used in many studies have not specifically separated out a unique population of vesicles. MiRNA can be found within many different types of EV, and thus we have used the term EV to refer to the results of these studies even when the original publications may have referred to exosomes, exosome-like vesicles, microvesicles etc.
MiRNAs that are released from cells packaged within EV such as exosomes may be taken up by nearby cells, or transported to cells through the blood or lymph system. Recent reports have suggested that EV delivery of miRNAs to recipient cells can occur [18] and that these miRNAs can function in transcriptional regulation in recipient cells [19, 20]. However, the primary mechanism by which EV selectively interact with target cells has not been elucidated. Mechanisms of uptake of EV could involve endocytosis or direct fusion with cellular membranes [13, 21]. Uptake could involve EV surface proteins such as tetraspanins and lectins as well as integrins, proteoglycans, or lectin receptor proteins on recipient cells surface. miRNAs could also be released into the extracellular environment or circulation by endocytosis of vesicles and taken up into recipient cells by binding to receptors of cellular membrane recognizing miRNA-RNA-binding protein complex [22]. Heparan sulfate proteoglycans (HSPGs) have been reported to serve as receptors of cancer cell-derived EV in glioblastoma patients [23]. In addition, miRNA associated with HDL have also been reported to be capable of uptake by recipient cells. Within the circulation, many miRNAs are detected present in association with Ago2 protein and are not associated with vesicles [9]. The mechanisms by which these miRNA are released or how selectivity of uptake by recipient cells occur remains obscure. In some cases, these may be released from cells in a non-specific manner. Thus, for the purposes of this overview, we have focused only on studies that refer to miRNA within EV as inter-cellular mediators.
MiRNAs involved in Cell-to-Cell Communication in metabolic disease
Many studies of miRNAs have been reported in obesity and metabolic disease which are serious health issues in developed countries [24]. Early reports identified the expression of many different miRNAs associated with human obesity. These have been confirmed in both independent human studies as well as in rodent models of obesity/Insulin resistance (IR) [25]. More recently, miRNAs that are specific for metabolic diseases have been identified, and their function and target pathways elucidated. Indeed, miRNAs have been demonstrated to be important regulators of a number of critical metabolic functions, such as insulin secretion in the pancreas [26], lipid and glucose metabolism in the liver [27], and leptin signaling in the hypothalamus [28]. Unlike the role of secreted proteins such as cytokines and proteins, the role of miRNAs in intercellular communication in these conditions remains undefined. Examples of such roles for miRNAs in metabolic disorders are provided in Table 1 and illustrated in Figure 2.
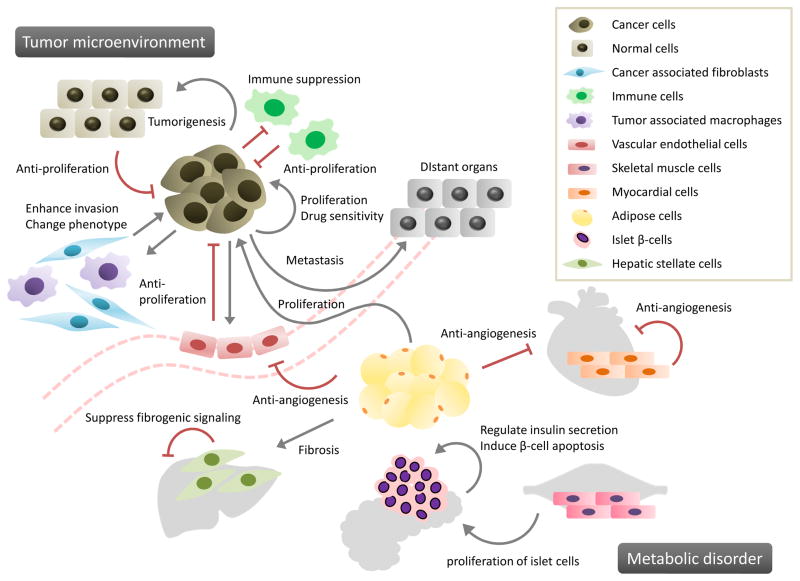
Figure 2. Involvement of paracrine miRNA signaling in cancers and metabolic disorders.
MiRNAs can contribute to cell-to-cell communication in several physiological and pathophysiological processes associated with cancers and metabolic disorders. Selected examples of paracrine miRNA signaling between donor and recipient cells are illustrated, and further elaborated in Tables 1 and 2. Gray arrows indicate supportive effects whereas red bars indicate suppressive effects.
Cardiovascular events
Early in the course of diabetes, high blood glucose levels can lead to endothelial dysfunction and microvascular dysfunction [29]. Deregulated myocardial angiogenesis is a major manifestation of diabetes-caused ischemic cardiovascular disease [30]. The contribution of paracrine miRNA is this setting is illustrated by studies in the Goto-Kakizaki rat model of type 2 diabetes, where EV derived from myocytes were noted to be enriched in miR-320. This miRNA can functionally down-regulate target genes such as insulin-like growth factor-1 (IGF1), Hsp20 and Ets2 in recipient cardiac endothelial cells, whereas its over-expression can inhibit cell migration and tube formation [31].
In another setting, EV has been identified from supernatants of isolated adipocytes [32] or adipose stromal cells (ADSCs) [33] in culture. Adipocyte EV has been linked to lipid metabolism and obesity-related IR. Similarly, EV secreted by ADSCs has been implicated in angiogenesis, immunomodulation and tumor development [34], and can promote vascular endothelial cells (ECs) migration and proliferation [35]. These EV mRNAs and miRNAs could target several pathways such as angiogenesis, cellular transport, or apoptosis [36]. In contrast, reduced levels of vascular endothelial growth factor (VEGF), Matrix metalloproteinase-2 (MMP-2) and especially miR-126 can impair the angiogenic potential of EV in obesity [37]. Alterations in miR-126 levels can deregulate Sprouty-related, EVH1 domain-containing 1 (Spred1) expression and inhibit the Erk1/2 mitogen-activated protein kinase (MAPK) pathway in endothelial cells and impaired angiogenic ability. In addition, miR-126 is reduced in patients with coronary atherosclerosis [38] and inversely correlated with patients with high LDL levels [39], underscoring its potential importance in maintaining vascular homeostasis across multiple tissues.
Non-alcoholic fatty liver disease (NAFLD)
NAFLD is the hepatic manifestation of the metabolic syndrome and defined by the accumulation of fat in the liver in patients who do not consume excessive alcohol. NAFLD can manifest as simple steatosis alone, or as nonalcoholic steatohepatitis (NASH), a progressive liver disease characterized by inflammatory changes and fibrosis in addition to steatosis. 5–10% of patients diagnosed with NAFLD will develop NASH and of these upto 30% of these may develop cirrhosis [40]. We have reported miRNA profiling studies in experimental NASH. The significance of deregulated miRNA expression has been investigated in several liver diseases. Amongst these, miR-122 is a liver specific miRNA that is highly enriched within hepatocytes. MiR-122 has a central role in maintaining hepatocyte function and homeostasis with the capacity to regulate various genes involved in hepatic cholesterol and lipid metabolism. A reduction in hepatic miR-122 expression was reported in NASH [41], and a deletion of miR-122 in mice leads to the development of HCC [42]. Circulating miR-122 and miR-34a may represent novel, noninvasive biomarkers of diagnosis and histological disease severity in patients with NAFLD as well as chronic hepatitis C [43]. Additionally, a role for miR-122 has also been implicated in EV-mediated HCV transmission [44]. Even though several NAFLD associated miRNA have been reported, their participation in cell-to-cell communication is unknown. An interesting study reported that EV derived from obese but not from lean visceral adipose tissue can induce transforming growth factor-β (TGF-β) signaling pathway deregulation following their uptake in HepG2 cells [45]. These observations suggest the possibility of similar processes occurring in NAFLD [46]. Some of the effects of TGF-β on stimulating pathways of fibrogenesis in activated HSCs are mediated via connective tissue growth factor 2 (CCN2) [47]. EV released by quiescent but not activated HSC contain high levels of Twist1, which can be transferred via EV to modulate expression of miR-214 and downstream CCN2 in recipient cells [48, 49].
Insulin resistance and β-cell dysfunction
Diabetes mellitus is a common metabolic disorder characterized by dysfunction of insulin-secreting pancreatic β-cells. MiRNAs have been identified as important determinants of the functional integrity of pancreatic β-cells, and implicated in the regulation of β-cell differentiation and other activities such as proliferation, survival, insulin biosynthesis and secretion [50]. Release of EVs has been reported from pancreatic islets as well as β-cell lines. Although the functional role of β-cell EVs is just beginning to be elucidated, there is evidence supporting the participation of EVs in the crosstalk with ECs or lymphocytes [51, 52]. Thus, EVs from human islets contain miRNAs (such as miR-27b, miR-126, miR-130 and miR-296) involved in β-cell function, insulin secretion and angiogenesis. These EVs can be taken up by human islet ECs resulting in insulin mRNA expression, protection from apoptosis and enhancement of angiogenesis [51]. Exposure of MIN6B1 murine β-cells to inflammatory cytokines alters the release of several microRNAs, which, in contrast to EV from similar cells not exposed to cytokines, can induce apoptosis in cytokine-untreated MIN6B1 cells. Down-regulation of the miRNA-mediating silencing protein Ago2 in recipient cells prevents apoptosis induced by EV derived from cytokine-treated cells. These data implicate the transfer of EV miRNAs as a novel cell-to-cell communication mechanism that can regulate the activity of pancreatic β-cells [53].
Another example of inter-cellular miRNA signaling was provided by studies of cross-talk between EV released from skeletal muscle and beta cells. Islets from mice fed on high palmitate diets (HPD) were larger and had altered expression of genes involved in development, such as Ptch1. MiR-16 was increased within vesicles obtained from skeletal muscle cells of HPD, and could regulate gene expression of several developmental genes such as Ptch1 following their uptake by MIN6B1 cells in vitro, thus recapitulating the in vivo observations and suggesting that the release of EV from muscles can modulate beta-cell mass in lipid-induced insulin-resistance mice [54].
Metabolic diseases and cancer
Obesity has been causally linked to an increased risk of several different types of cancer [55], and adipose tissue plays a role in development of some tumors. EVs derived from pre-adipocytes (3T3-L1) can promote tumorigenesis in breast cancer cells. An anti-tumor compound, shikonin, increased levels of miR-140 in 3T3-L1-derived EVs and impacted ductal carcinoma cells in situ through the SOX9 signaling pathway [56]. Adipocytes can promote migration and EMT seen in breast cancers, and ADSCs can behave similarly [57]. Human ADSC derived EV can promote breast cancer cell migration and proliferation through the Wnt pathway [33]. In glioblastoma, ADSC-derived EVs stimulate cells to enter S and G2/M phase and enhance cancer cell proliferation, whereas MSC-derived EV may inhibit cancer cell proliferation and induce apoptosis [58].
MiRNAs in Cell-to-Cell Communication in Cancers
The involvement of miRNAs in cancer-related processes has been extensively evaluated, and the role of cancer-related miRNA in tumor metabolism and progression has been recognized [59]. Cancer-related miRNAs have been detected not only in cancer cells but also in cancer-derived EVs [60]. Inter-cellular signaling involving miRNA has defined roles within the tumor microenvironment (TME) or pre-metastatic niche [61–64], and examples are provided in Table 2 and illustrated in Figure 2. Release of miRNA within EV from non-tumoral, stromal, immune, or ECs within the local microenvironment have been implicated in driving tumor growth, spread and metastases.
Cancer proliferation
Autonomous proliferation, resistance to apoptosis and immortalization are hallmarks of cancer. Cancer-related miRNA contribute to bidirectional signaling between normal and cancer cells that regulate cell growth and cell death and support unrestrained cancer cell proliferation. For example, cancer cells can release miRNAs such as miR-21 that can repress tumor suppressors such as PTEN and PDCD4 [65]. MiR-21 has been identified in EVs derived from breast cancer [66] and from glioblastoma cells [10]. The serum EV miR-224 level is significantly higher in patients with HCC than those with chronic hepatitis B or liver cirrhosis [67]. MiR-224 is a master regulator of cell cycle progression, and overexpression results in G1/S checkpoint release followed by accelerated cell growth. Enforced expression of miR-224 increases the growth rate of normal cholangiocytes, cholangiocarcinoma cell lines, and HCC cell lines [68]. TGF-β activated kinase-1 (TAK1) is an essential inhibitor of hepatocarcinogenesis, and its absence in vivo is associated with the spontaneous development of HCC related to aberrant responses to inflammatory and stress signaling [69]. TAK1 is the most likely candidate pathway that could be modulated by miRNAs and a biologically plausible target for intercellular modulation. HCC cell-derived EVs can modulate TAK1 expression and associate signaling and enhance transformed cell growth in recipient HCC cells [70]. Release of EV associated miRNAs from non-malignant cells can also contribute to tumorigenesis. Thus, ectopic expression of miR-409 in normal prostate fibroblasts conferred a cancer-associated stroma-like phenotype. The release of miR-409 via EVs promoted tumor induction and epithelial-to-mesenchymal transition (EMT) in vitro and in vivo through miR-409 dependent repression of tumor suppressors such as Ras suppressor 1(RUS1) and stromal antigen 2 (STAG2) [71].
Cancer microenvironment
Inter-cellular signaling is essential in defining tumor development, growth and progression within the tumor microenvironment. Within the tumor microenvironment, stromal cells such as cancer associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), pericytes, ECs, and infiltrating immune cells can signal amongst each other and with normal or transformed cells through the release of miRNA and protein mediators [72]. The transfer of miRNA within EV is a potent mechanism that can support the survival of cancer cells, or promote angiogenesis [73–77]. An example of the interactions is highlighted in studies in chronic lymphocytic leukemia (CLL). CLL-derived EV is enriched in miR-146a and several proteins, and following their uptake by ECs and MSCs can induce an inflammatory phenotype which resembles the phenotype of CAFs. ECs which incorporated CLL-derived EVs increased angiogenesis, and co-injection of these EVs and CLL cells promotes tumor growth in immunodeficient mice [78]. Similarly, estrogen receptor (ER) repression was observed in ER-positive breast cancer cells, MCF-7/ltE2-, treated with conditioned media from CAFs derived from ER-negative breast cancer cells. EV miR-221/222 derived from these CAFs was transferred to MCF-7/ltE2- and ER repression could be rescued by knockdown of miR-221/222 [79]. A miRNA signature indicative of hyperactive MAPK signaling is associated with reduced recurrence-free and overall survival [80]. CAFs-secreted miRNAs may contribute to MAPK-induced ER repression to drive the ER-negative phenotype in breast cancer cells. Immune cells are also prominent within the tumor environment, and immune escape by tumors can involve secretion of EVs by cancer cells with resultant suppression of anti-tumor immune responses [81]. In most cases, such suppressive responses are mediated by proteins within and on the surface of EVs, although there are reports of lung cancer cell derived EV miR-21 and miR-29a binding to Toll-like receptors on immune cells and resulting in release of pro-metastatic inflammatory cytokines [82].
Cancer migration and metastasis
Cancer cells can modify their surrounding microenvironment to promote invasiveness and to prepare other organs and distant sites for metastasis [66, 83–85]. MiR-105, which is characteristically expressed and secreted by metastatic breast cancer cells, is a potent regulator of migration through targeting the tight junction protein ZO-1. In endothelial monolayers, EV miR-105 efficiently destroys tight junctions and the integrity of these natural barriers against metastasis. Overexpression of miR-105 in non-metastatic cancer cells induces metastasis and vascular permeability in distant organs, whereas inhibition of miR-105 in highly metastatic tumors alleviates these effects [84]. Some miRNAs involved in the formation of the pre-metastatic niche have been identified [86, 87]. In renal cancer stem cells, several miRNAs found in EVs have been implicated in the formation of the lung pre-metastatic niche and can lead to tumor invasion and metastasis, in addition to inducing angiogenesis [86]. Moreover, TAMs can regulate the invasiveness of breast cancer cells through exosome-mediated delivery of oncogenic miRNAs. EV miR-223 associated with IL-4-activated macrophages was significantly elevated during co-cultivation of TAM with breast cancer cells, whereas inhibition of miR-223 decreased cell invasiveness [88]. MiR-181c promotes the destruction of blood brain barrier through the abnormal localization of actin via the down regulation of its target gene, PDPK1. Systemic injection of brain metastatic cancer cell-derived EVs containing miR-181c promote brain metastasis of breast cancer cells and are preferentially incorporated into the brain in vivo [89].
Anti-tumorigenesis
Thus far, we have described miRNAs dependent signaling related to cancer proliferation and progression. Consistent with the bidirectional effect of paracrine signaling between cancer and non-cancerous cells, it could be expected that EV miRNAs derived from normal cells could serve to maintain normalcy through tumor-suppressive effects. Supportive evidence for this arises from observations in several settings showing anti-tumor effects from delivery of miRNA. Through transfer of miR-142 and miR-223, human macrophages can post-transcriptionally regulate endogenous proteins such as insulin-like growth factor-1 receptor (IGF1R), to functionally inhibit HCC cell proliferation [61]. Transfer of miR-16 into PNT-2 prostate epithelial cells reduces cell proliferation [90]. Similarly, miR-143 can inhibit cell proliferation in prostate cancer cells through the suppression of Kirsten rat sarcoma viral oncogene homolog (K-RAS) [91]. Furthermore, normal ECs can transfer miRNA such as miR-503 to tumor cells through EVs, with effects on proliferation and invasion through inhibition of cyclin D2 and cyclin D3 in breast cancer cells [62].
Anti-tumor effects can also be enhanced by exogenous agents. Thus, the anti-angiogenic effects of docosahexaenoic acid (DHA), a natural compound with potential for use as a preventative agent or as an adjuvant to breast cancer therapy could be mediated through EV miRNAs. DHA alters the secretion and miRNA content of EVs released by breast cancer cells, which can be taken up by endothelial cells resulting in a decrease of pro-angiogenic target genes such as plasminogen activator (PLAU), angiomotin like-1 (AMOTL1) and neuropilin 1 (NRP1) and inhibition of tube formation [92].
Drug sensitivity
Acquired resistance to therapy is a major limitation to the treatment of cancer. Chemoresistance in MCF-7 breast cancer cells has been associated with miR-100, miR-222 and miR-30a which are abundant in EVs from these cells and which, when transferred to drug sensitive cells, can alter cell cycle progression and apoptosis pathways that decrease drug susceptibility [93]. EV mediated transfer of a drug-resistant phenotype from resistant to sensitive breast cancer cells could potentially be related to intercellular transfer of specific miRNAs. Overexpression of miR-142-3p suppresses migration and invasion in HCC cells, and this miRNA can negatively regulate RAS-related C3 botulinus toxin substrate 1 (RAC1) in HCCs [94]. Transfer of miR-142-3p from TAMs to HCC through EV in response to propofol can inhibit HCC invasion, effects that are reversed by down-regulating miR-142-3p. In addition, plasma EV from tumor-bearing mice exposed to propofol can suppress tumor growth [95]. Thus, EV mediated miRNA signaling can contribute to the cancer phenotype and sensitivity to drugs.
Induction of dormancy
Breast cancer patients can develop metastatic disease even after 10–20 years following resection of the primary tumor [96]. These patients are asymptomatic because the disseminated cells appear to become dormant and are undetectable. Bone marrow mesenchymal stem cell derived EVs (BM-MSCs) can enable breast cancer cells to maintain dormancy for decades in the bone marrow in these cases. MiR-23b within EVs from BM-MSCs is suggested to suppress MARCS, which encodes myristoylated alanine-rich C kinase substrate and promotes cell cycling and motility [97].
Conclusions and future perspectives
The discoveries that small RNAs were conserved in several species and that miRNAs could post-transcriptionally regulate mRNA expression have provided new insights into cellular regulation of gene expression. Recent studies indicate a role for miRNA in paracrine signaling, and further extend the potential impact of these small RNA in modulating normal or pathological processes. Although the precise contributions of cell-to-cell communication by miRNA remain to be conclusively established, the potential for miRNAs to serve as mediators of paracrine signaling is conceptually exciting for several reasons. First, circulating extracellular miRNAs associated with EV, RNA binding proteins or HDL may avoid degradation and retain the ability to exert a functional effect at distant sites. Second, the impact of a single miRNA is broad because of the potential to target multiple pathways through effects on several different genes. Because EVs have the ability to transfer molecules other than miRNAs, the combinational effects of miRNAs with other molecules packaged within EV provides exciting possibilities for therapeutic applications, while avoiding immune responses related to endogenously administered artificial nucleic acids such as siRNAs. Of note however, is that the processes by which miRNAs mediate cell-cell signaling in vertebrates have not been elucidated. To date, signaling by extracellular miRNA trafficking has been consistently shown only in cultured cells and for selected miRNAs. Most EVs do not contain miRNA. The concentration of miRNAs in body fluids is far lower than in cells of origin and within the local microenvironment [98], and direct evidence of a distant functional effect remains to be convincingly demonstrated. Nevertheless, the considerable excitement related to the emerging data of cell-to-cell transfer of miRNA, as well as to recent reports demonstrating cross-kingdom transfer of miRNA, is likely to be translated into specific studies to evaluate the impact of inter-cellular miRNA communication in both normal physiology and in disease processes.
Table 1.
MiRNAs in Cell-to-Cell Communication: Metabolic disease
| function | Secretion cells | Target cells | miRNA | miRNA targets | References |
|---|---|---|---|---|---|
| Cardiovascular event | |||||
| Anti-angiogenic regulation | Diabetic cardiomyocytes | mouse cardiac ECs | miR-320 | IGF-1, Hsp20, Ets2 | 30 |
| Angiogenesis | ADSCs | ECs | miR-126 | MAPK pathway Spred1 Erk1/2 |
36 |
|
| |||||
| Nonalcoholic fatty liver disease: NAFLD | |||||
| Dysregulation of TGF-β pathway members Pathogenesis of NAFLD. |
Adipocytes | HCCs HSCs |
obese individual’s exosome: miR148b, miR-4269 miR-23b, miR-4429 |
TGF-b pathway | 44, 45 |
| Suppression of fibrogenic signaling | HSCs | HSCs | Twist1 | miR-214, CCN2 | 47, 48 |
|
| |||||
| Insulin resistance and β-cell dysfunction | |||||
| Insulin secretion and angiogenesis | Human islet cells | Human islet ECs | miR-27b, miR-126, miR-130, miR-296 | 50 | |
| Induce apoptosis | MIN6B1 Rat insulinoma INS-1 derived 832/13 cells |
β-cells | miRNAs (after cytokine treatment) | 52 | |
| Proliferation of islet cells | Quadriceps cells (C57BL/6 mice with high palmitate diets) | MIN6B1 | miR-16 | Ptch1 | 53 |
ADSCs: adipose stromal cells, ECs: Endothelial cells, HSCs: hepatic stellate cells, MIN6B1: murine insulin-secreting cells
Table 2.
MiRNAs in Cell-to-Cell Communication: Cancer
| Function | Secretion cells | Target cells | miRNA | miRNA targets | References |
|---|---|---|---|---|---|
| Cancer proliferation | |||||
| Suppress anti-tumor genes | BCCs | miR-21 | PTEN | 65 | |
| Suppress anti-tumor genes | Glioblastoma malignant ascites | miR-21 | PDCD4 | 10 | |
| Cell cycle progression | HCCs (serum of HCC patients) | HCCs, CCAs nomal cholangiocytes | miR-224 | G1/S checkpoint | 66, 67 |
| Tumor proliferation | HCCs | HCCs | EV miRNAs | TAK1 | 69 |
| Tumorigenesis, EMT | Stromal fibroblasts | PCCs | miR-409 | RUS1, STAG2 | 70 |
| Tumorigenesis | Mouse preadipocyte treated with Shikonin | BCCs | miR-140 | SOX2/SOX9 | 33 |
|
| |||||
| Cancer microenvironment | |||||
| ECs migration and tube formation | Leukemia cells | HUVECs | miR-92a | integrin α5 | 72 |
| Angiogenesis | Metastatic BCCs | HUVECs | miR-210 | Ephrin-A3 | 73 |
| Angiogenesis | Leukemia cells | HUVECs | miR-210 | Ephrin-A3 | 74 |
| Angiogenesis | Colorectal cancer cells | HUVECs | miR-92a | Dickkopf-3 | 75 |
| ECs migration and angiogenesis | Melanoma | HUVECs | miR-9 | The JAK-STAT pathway SOCS5 | 76 |
| Angiogenesis Tumor proliferation |
chronic lymphocytic leukemia cells | HMEC-1 | miR-146a | 77 | |
| MAPK-induced ER repression | CAFs | ER-positive BCCs | miR-221/222 | MAPK | 78, 79 |
| Immune suppression | HEK-293 | Murine macrophage | miR-21, miR-29a | Directly bind Toll-like receptor | 81 |
| ECs migration and tube formation | Leukemia cells | HUVECs | miR-92a | integrin α5 | 72 |
| Angiogenesis | Metastatic BCCs | HUVECs | miR-210 | Ephrin-A3 | 73 |
|
| |||||
| Cancer invasion and metastasis | |||||
| Increase invasive ability | Metastatic BCCs | Non-invasive mammary ECs | miR-10b | HOXD10 and KLF4 | 82 |
| Promote cell migration | Monocytic leukemia cells | HMEC-1 | miR-150 | c-Myb | 23 |
| Disrupt endothelial barriers | Metastatic BCCs | HMVECs | miR-105 | ZO-1 | 83 |
| Transfer metastasis capability | Metastatic BCCs | Non-metastatic BCCs | miR-200 family | Zeb2, Sec23a | 84 |
| Metastasis | Colorectal cancer | miR-21 | PDCD4 | 64 | |
| Premetastatic niche formation | Renal cancer stem cells | Lung cells (in vivo) | miR-200c, miR-92, miR-141, miR-29a, miR-650, miR-151 | 85 | |
| Premetastatic niche formation | Metastatic rat adenocarcinoma | Lymph node stroma cells, lung fibroblasts | miR-494, miR-542-3p | Cadherin-17 | 86 |
| Regulate and promote invasiveness | TAMs(IL-4 activated M2 macrophage) | BCCs | miR-223 | MEF2C | 87 |
| Impair blood-brain barrier | ECs | Metastatic BCCs | miR-181c | PDPK1 | 88 |
|
| |||||
| Anti-tumorigenesis | |||||
| Inhibit cell growth | Human macrophages | HCCs | miR-142, miR-223 | stathmin-1 IGF1R |
60 |
| Inhibit cell growth | Prostate ECs | PCCs | miR-143 | KRAS | 90 |
| Inhibit cell growth and invasion | ECs | BCCs | miR-503 | CCND2, CCND3 | 61 |
| Anti-angiogenic action | BCCs (after treatment with DHA) | ECs | miR-23b, miR-320b | PLAU, AMOTL1, NRP1 and ETS2 | 91 |
|
| |||||
| Drug sensitivity | |||||
| Acquire drug-resistance | Drug-resistant BCCs | Drug-sensitive BCCs | miR-100, miR-222, miR-30a | 92 | |
| Increase chemo- sensitivity | TAMs | Hepa1-6 cells (tumor-bearing mice) | miR-142-3p | RAC1 | 93, 94 |
|
| |||||
| Induction of dormancy | |||||
| Induce dormancy | BM-MSCs | Bone marrow-metastatic Human BCCs |
miR-23b | MARCKS | 96 |
BCCs: breast cancer cells, HCCs: hepatocellular carcinoma cells, CCAs: cholangiocarcinoma cells, PCCs: prostate cancer cells, EMT: epithelial-to-mesenchymal transition, HUVECs: human umbilical cord vein endothelial cells, HMECs: human endothelial cells, CAFs: cancer associated fibroblasts, MSCs: mesenchymal stem cells, TAMs: tumor-associated macrophages, BM-MSCs: bone marrow mesenchymal stem cells
Research Agenda.
The mechanisms by which miRNA are sequestered and are selectively enriched within extracellular vesicles need to be elucidated.
Studies are needed to show the direct impact of extracellular RNA in functional effects such as modulation of gene expression following their uptake by recipient cells.
Studies of therapeutic targeting of paracrine miRNA signaling in conditions where intercellular communication contributes to disease such as cancers or metabolic diseases such as NAFLD.
Practice points.
miRNA can serve as paracrine signaling mediators between different types of cells
Circulating miRNA that are selectively released into the extracellular milieu in pathophysiological processes and are detectable in the circulation may represent potential biomarkers associated with disease processes.
Acknowledgments
Financial Support: Supported in part by Grant UH3TR000884 from the National Institutes of Health
Footnotes
Disclosures: There are no potential conflicts relevant to the manuscript
Conflict of Interest
There are no conflicts of interest relevant to the manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths-Jones S, Grocock RJ, van Dongen S, et al. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34(Database issue):D140–4. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berezikov E, Guryev V, van de Belt J, et al. Phylogenetic shadowing and computational identification of human microRNA genes. Cell. 2005;120(1):21–4. doi: 10.1016/j.cell.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 4.Roberts TC. The microRNA Machinery. Adv Exp Med Biol. 2015;887:15–30. doi: 10.1007/978-3-319-22380-3_2. [DOI] [PubMed] [Google Scholar]
- 5.Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol. 2012;6(6):590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukong TN, Momen-Heravi F, Kodys K, et al. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog. 2014;10(10):e1004424. doi: 10.1371/journal.ppat.1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber JA, Baxter DH, Zhang S, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56(11):1733–41. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 9.Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108(12):5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vickers KC, Palmisano BT, Shoucri BM, et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13(4):423–33. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zernecke A, Bidzhekov K, Noels H, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12- dependent vascular protection. Sci Signal. 2009;2(100):ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 12.Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–6. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Prime Rep. 2011;3(15) doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2011;106(2):145–55. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 15.Roxrud I, Stenmark H, Malerød L. ESCRT & Co. Biol Cell. 2010;102(5):293–318. doi: 10.1042/BC20090161. [DOI] [PubMed] [Google Scholar]
- 16.Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–25. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Andreu Z, Yáñez-Mó M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. doi: 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu G, Drescher KM, Chen XM. Exosomal miRNAs: Biological properties and therapeutic potential. Front Genet. 2012;3:56. doi: 10.3389/fgene.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *19.Kosaka N, Iguchi H, Yoshioka Y, et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285(23):17442–52. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Liu D, Chen X, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39(1):133–44. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Tian T, Zhu YL, Zhou YY, et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem. 2014;289(32):22258–67. doi: 10.1074/jbc.M114.588046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christianson HC, Svensson KJ, van Kuppevelt TH, et al. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110(43):17380–5. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Deiuliis JA. MicroRNAs as regulators of metabolic disease: pathophysiologic significance and emerging role as biomarkers and therapeutics. Int J Obes (Lond) 2016;40(1):88–101. doi: 10.1038/ijo.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klöting N, Berthold S, Kovacs P, et al. MicroRNA expression in human omental and subcutaneous adipose tissue. PLoS One. 2009;4(3):e4699. doi: 10.1371/journal.pone.0004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plaisance V, Waeber G, Regazzi R, et al. Role of microRNAs in islet beta-cell compensation and failure during diabetes. J Diabetes Res. 2014;2014:618652. doi: 10.1155/2014/618652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu W, Cao H, Ye C, et al. Hepatic miR-378 targets p110α and controls glucose and lipid homeostasis by modulating hepatic insulin signalling. Nat Commun. 2014;5:5684. doi: 10.1038/ncomms6684. [DOI] [PubMed] [Google Scholar]
- 28.Derghal A, Djelloul M, Airault C, et al. Leptin is required for hypothalamic regulation of miRNAs targeting POMC 3′UTR. Front Cell Neurosci. 2015;9:172. doi: 10.3389/fncel.2015.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagami H, Kaneda Y, Ogihara T, Morishita R. Endothelial dysfunction in hyperglycemia as a trigger of atherosclerosis. Curr Diabetes Rev. 2005;1(1):59–63. doi: 10.2174/1573399052952550. [DOI] [PubMed] [Google Scholar]
- 30.Costa PZ, Soares R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci. 2013;92(22):1037–45. doi: 10.1016/j.lfs.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Huang W, Liu G, et al. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol. 2014;74:139–50. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koeck ES, Iordanskaia T, Sevilla S, et al. Adipocyte exosomes induce transforming growth factor beta pathway dysregulation in hepatocytes: a novel paradigm for obesity-related liver disease. J Surg Res. 2014;192(2):268–75. doi: 10.1016/j.jss.2014.06.050. [DOI] [PubMed] [Google Scholar]
- 33.Lin R, Wang S, Zhao RC. Exosomes from human adipose derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013;383(1–2):13–20. doi: 10.1007/s11010-013-1746-z. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Yu M, Tian W. Physiological and pathological impact of exosomes of adipose tissue. Cell Prolif. 2016;49(1):3–13. doi: 10.1111/cpr.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopatina T, Bruno S, Tetta C, et al. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal. 2014;12:26. doi: 10.1186/1478-811X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eirin A, Riester SM, Zhu XY, et al. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551(1):55–64. doi: 10.1016/j.gene.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Togliatto G, Dentelli P, Gili M, et al. Obesity reduces the pro-angiogenic potential of adipose tissue stem cell-derived extracellular vesicles (EVs) by impairing miR-126 content: impact on clinical applications. Int J Obes (Lond) 2016;40(1):102–11. doi: 10.1038/ijo.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fichtlscherer S, De Rosa S, Fox H, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107(5):677–84. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 39.Sun X, Zhang M, Sanagawa A, et al. Circulating microRNA-126 in patients with coronary artery disease: correlation with LDL cholesterol. Thromb J. 2012;10(1):16. doi: 10.1186/1477-9560-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–97. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai WC, Hsu SD, Hsu CS, et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest. 2012;122(8):2884–97. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu SH, Wang B, Kota J, et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. 2012;122(8):2871–83. doi: 10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cermelli S, Ruggieri A, Marrero JA, et al. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS ONE. 2011;6(8):e23937. doi: 10.1371/journal.pone.0023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bukong TN, Momen-Heravi F, Kodys K, et al. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog. 2014;10(10):e1004424. doi: 10.1371/journal.ppat.1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koeck ES, Iordanskaia T, Sevilla S, et al. Adipocyte exosomes induce transforming growth factor beta pathway dysregulation in hepatocytes: a novel paradigm for obesity-related liver disease. J Surg Res. 2014;192(2):268–75. doi: 10.1016/j.jss.2014.06.050. [DOI] [PubMed] [Google Scholar]
- *46.Ferrante SC, Nadler EP, Pillai DK, et al. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease. Pediatr Res. 2015;77(3):447–54. doi: 10.1038/pr.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang G, Brigstock DR. Regulation of hepatic stellate cells by connective tissue growth factor. Front Biosci (Landmark Ed) 2012;17:2495–507. doi: 10.2741/4067. [DOI] [PubMed] [Google Scholar]
- 48.Chen L, Charrier A, Zhou Y, et al. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2014;59(3):1118–29. doi: 10.1002/hep.26768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen L, Chen R, Kemper S, et al. Suppression of fibrogenic signaling in hepatic stellate cells by Twist1-dependent microRNA-214 expression: Role of exosomes in horizontal transfer of Twist1. Am J Physiol Gastrointest Liver Physiol. 2015;309(6):G491–9. doi: 10.1152/ajpgi.00140.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *50.Eliasson L, Esguerra JL. Role of non-coding RNAs in pancreatic beta-cell development and physiology. Acta Physiol (Oxf) 2014;211(2):273–84. doi: 10.1111/apha.12285. [DOI] [PubMed] [Google Scholar]
- 51.Figliolini F, Cantaluppi V, De Lena M, et al. Isolation, characterization and potential role in beta cell-endothelium cross-talk of extracellular vesicles released from human pancreatic islets. PLoS One. 2014;9(7):e102521. doi: 10.1371/journal.pone.0102521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheng H, Hassanali S, Nugent C, et al. Insulinoma-released exosomes or microparticles are immunostimulatory and can activate autoreactive T cells spontaneously developed in nonobese diabetic mice. J Immunol. 2011;187(4):1591–600. doi: 10.4049/jimmunol.1100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *53.Guay C, Menoud V, Rome S, et al. Horizontal transfer of exosomal microRNAs transduce apoptotic signals between pancreatic beta-cells. Cell Commun Signal. 2015;13:17. doi: 10.1186/s12964-015-0097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jalabert A, Vial G, Guay C, et al. Exosome-like vesicles released from lipid-induced insulin-resistant muscles modulate gene expression and proliferation of beta recipient cells in mice. Diabetologia. 2016;59(5):1049–58. doi: 10.1007/s00125-016-3882-y. [DOI] [PubMed] [Google Scholar]
- 55.Arnold M, Leitzmann M, Freisling H, et al. Obesity and cancer: An update of the global impact. Cancer Epidemiol. 2016;41:8–15. doi: 10.1016/j.canep.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Gernapudi R, Yao Y, Zhang Y, et al. Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res Treat. 2015;150(3):685–95. doi: 10.1007/s10549-015-3326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamat P, Schweizer R, Kaenel P, et al. Human Adipose-Derived Mesenchymal Stromal Cells May Promote Breast Cancer Progression and Metastatic Spread. Plast Reconstr Surg. 2015;136(1):76–84. doi: 10.1097/PRS.0000000000001321. [DOI] [PubMed] [Google Scholar]
- 58.Del Fattore A, Luciano R, Saracino R, et al. Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opin Biol Ther. 2015;15(4):495–504. doi: 10.1517/14712598.2015.997706. [DOI] [PubMed] [Google Scholar]
- *59.Kosaka N, Iguchi H, Yoshioka Y, et al. Competitive interactions of cancer cells and normal cells via secretory microRNAs. J Biol Chem. 2012;287(2):1397–405. doi: 10.1074/jbc.M111.288662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kosaka N, Yoshioka Y, Hagiwara K, et al. Trash or treasure: extracellular microRNAs and cell-to-cell communication. Front Genet. 2013;4(173) doi: 10.3389/fgene.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aucher A, Rudnicka D, Davis DM. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibits proliferation. J Immunol. 2013;191(12):6250–60. doi: 10.4049/jimmunol.1301728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bovy N, Blomme B, Frères P, et al. Endothelial exosomes contribute to the antitumor response during breast cancer neo adjuvant chemotherapy via microRNA transfer. Oncotarget. 2015;6(12):10253–66. doi: 10.18632/oncotarget.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *63.Nishida-Aoki N, Ochiya T. Interactions between cancer cells and normal cells via miRNAs in extracellular vesicles. Cell Mol Life Sci. 2015;72(10):1849–61. doi: 10.1007/s00018-014-1811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *64.Yoshioka Y, Katsuda T, Ochiya T. Circulating microRNAs as Hormones: Intercellular and Inter-organ Conveyors of Epigenetic Information? EXS. 2015;106:255–67. doi: 10.1007/978-3-0348-0955-9_12. [DOI] [PubMed] [Google Scholar]
- 65.Asangani IA, Rasheed SA, Nikolova DA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27(15):2128–36. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- *66.Pigati L, Yaddanapudi SC, Iyengar R, et al. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS ONE. 2010;5(10):e13515. doi: 10.1371/journal.pone.0013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sohn W, Kim J, Kang SH, et al. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med. 2015;18(47):e184. doi: 10.1038/emm.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.An F, Olaru AV, Mezey E, et al. MicroRNA-224 Induces G1/S Checkpoint Release in Liver Cancer. J Clin Med. 2015;4(9):1713–28. doi: 10.3390/jcm4091713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inokuchi S, Aoyama T, Miura K, et al. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci U S A. 2010;107(2):844–9. doi: 10.1073/pnas.0909781107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *70.Kogure T, Lin WL, Yan IK, et al. Inter cellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54(4):1237–48. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Josson S, Gururajan M, Sung SY, et al. Stromal fibroblast-derived miR-409 promotes epithelial-to-mesenchymal transition and prostate tumorigenesis. Oncogene. 2015;34(21):2690–9. doi: 10.1038/onc.2014.212. [DOI] [PubMed] [Google Scholar]
- 72.Kuninty PR, Schnittert J, Storm G, Prakash J. MicroRNA Targeting to Modulate Tumor Microenvironment. Front Oncol. 2016;6:3. doi: 10.3389/fonc.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32(22):2747–55. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 74.Kosaka N, Iguchi H, Hagiwara K, et al. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem. 2013;288(15):10849–59. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tadokoro H, Umezu T, Ohyashiki K, et al. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem. 2013;288(48):34343–51. doi: 10.1074/jbc.M113.480822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamada N, Nakagawa Y, Tsujimura N, et al. Role of intracellular and extracellular microRNA-92a in colorectal cancer. Transl Oncol. 2013;6(4):482–92. doi: 10.1593/tlo.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhuang G, Wu X, Jiang Z, et al. Tumoursecreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31(17):3513–23. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paggetti J, Haderk F, Seiffert M, et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood. 2015;126(9):1106–17. doi: 10.1182/blood-2014-12-618025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shah SH, Miller P, Garcia-Contreras M, et al. Hierarchical paracrine interaction of breast cancer associated fibroblasts with cancer cells via hMAPK-microRNAs to drive ER-negative breast cancer phenotype. Cancer Biol Ther. 2015;16(11):1671–81. doi: 10.1080/15384047.2015.1071742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller P, Clarke J, Koru-Sengul T, et al. A novel MAPK-microRNA signature is predictive of hormone-therapy resistance and poor outcome in ER-positive breast cancer. Clin Cancer Res. 2015;21(2):373–85. doi: 10.1158/1078-0432.CCR-14-2053. [DOI] [PubMed] [Google Scholar]
- 81.Clayton A. Cancer cells use exosomes as tools to manipulate immunity and the microenvironment. Oncoimmunology. 2012;1(1):78–80. doi: 10.4161/onci.1.1.17826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fabbri M, Paone A, Calore F, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2015;109(31):E2110–6. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singh R, Pochampally R, Watabe K, et al. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer. 2014;13:256. doi: 10.1186/1476-4598-13-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou W, Fong MY, Min Y, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–15. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Le MT, Hamar P, Guo C, et al. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest. 2014;124(12):5109–28. doi: 10.1172/JCI75695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grange C, Tapparo M, Collino F, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71(15):5346–56. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 87.Rana S, Malinowska K, Zoller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia. 2013;15(3):281–95. doi: 10.1593/neo.122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang M, Chen J, Su F, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tominaga N, Kosaka N, Ono M, et al. Brain metastatic cancer cells release microRNA- 181c-containing extracellular vesicles capable of destructing blood–brain barrier. Nat Commun. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takeshita F, Patrawala L, Osaki M, et al. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via down regulation of multiple cell-cycle genes. Mol Ther. 2010;18(1):181–7. doi: 10.1038/mt.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu B, Niu X, Zhang X, et al. miR-143 decreases prostate cancer cells proliferation and migration and enhances their sensitivity to docetaxel through suppression of KRAS. Mol Cell Biochem. 2011;350(1–2):207–13. doi: 10.1007/s11010-010-0700-6. [DOI] [PubMed] [Google Scholar]
- 92.Hannafon BN, Carpenter KJ, Berry WL, et al. Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA) Mol Cancer. 2015;14:133. doi: 10.1186/s12943-015-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen WX, Liu XM, Lv MM, et al. Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS ONE. 2014;9(4):e95240. doi: 10.1371/journal.pone.0095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu L, Cai C, Wang X, et al. MicroRNA-142-3p, a new regulator of RAC1, suppresses the migration and invasion of hepatocellular carcinoma cells. FEBS Lett. 2011;585(9):1322–30. doi: 10.1016/j.febslet.2011.03.067. [DOI] [PubMed] [Google Scholar]
- 95.Zhang J, Shan WF, Jin TT, et al. Propofol exerts anti-hepatocellular carcinoma by microvesicle-mediated transfer of miR-142-3p from macrophage to cancer cells. J Transl Med. 2014;12:279. doi: 10.1186/s12967-014-0279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karrison TG, Ferguson DJ, Meier P. Dormancy of mammary carcinoma after mastectomy. J Natl Cancer Inst. 1999;91(1):80–5. doi: 10.1093/jnci/91.1.80. [DOI] [PubMed] [Google Scholar]
- 97.Ono M, Kosaka N, Tominaga N, et al. Exosomes from bone marrow mesenchymal stem cells contains a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7(332):ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 98.Williams Z, Ben-Dov IZ, Elias R, et al. Comprehensive profiling of circulating microRNA via small RNA sequencing of cDNA libraries reveals biomarker potential and limitations. Proc Natl Acad Sci U S A. 2013;110(11):4255–60. doi: 10.1073/pnas.1214046110. [DOI] [PMC free article] [PubMed] [Google Scholar]